by
Françoise Corbineau & Daniel Côme
Université Pierre et Marie Curie
Physiologie Végétale Appliquée
Tour 53, ler étage, 4, place Jussieu
75252 Paris Cedex 05, France
INTRODUCTION
Terminalia superba and Terminalia ivorensis are among the most important of the some 200 species belonging to the genus Terminalia (Combretaceae). Thanks to the qualities of these two timber species, native to West and Central Africa, a number of important plantation programmes have been started within their natural range, in Congo and in Côte d'Ivoire: and introduction trials have been established elsewhere, especially in Latin American countries. While seeds of Terminalia superba do not pose any problems in seed germination (Roederer, 1988), those of Terminalia ivorensis germinate with great difficulties. A study was hence undertaken on the germination of seeds of this latter species.
The work reported upon took place in 1987 and 1988, with the following objectives:
MATERIAL AND METHODS
Plant material
Tests were undertaken on 4 seedlots supplied by CTFT (now CIRAD-Forêt), Nogent-sur-Marne
(France). The seedlots (No 86/5722N, 86/5723N, 86/5725N and 86/5729N) were collected from 4
different trees in January 1986 in the Assuefri area (Côte d'Ivoire). When received at the laboratory the
weight of the dewinged seeds ranged from 95 to 230 mg and their mean moisture content, determined
after drying at 105°C, was of the order of 9.5% of the dry weight.
Other seedlots were collected from one and the same tree in the Yapo area (Côte d'Ivoire) on 16, 20, 22 and 29 December 1987, on 2, 12, 21 and 28 January 1988 and on 2 and 10 February 1988, to determine the influence of the development stage of the seed on their germination capacity. The seeds were dewinged and despatched as soon as they were collected and were put in germination test on receipt at the laboratory.
Germination tests
Germination tests were undertaken at different temperatures ranging from 5 to 40°C, in darkness. The
complete seeds or the extracted embryos were placed into 10 cm diameter Petri dishes, on cotton
humidified with deionised water or a gibberellin (GA3) 10-3M solution. Each test included 50 seeds or 30
embryos distributed into 2 Petri dishes. Germination was regularly observed during 30 to 60 days. An
embryo or a seed was considered having germinated when the radicle had reached a length of a few mm
(for an embryo), or had broken through the seedcoats.
Seed pretreatments
The complete seeds received different pretreatments aimed at facilitating their germination. All seeds
were soaked during 3 hours in concentrated sulphuric acid, and abundantly rinsed in running water for
15 to 20 minutes. Part of the seeds were then soaked during 24 hours in a cellulase (which is an enzyme
which degrades cellulose and thus the cell walls) solution, at a concentration of 1.25 g 1-1 and at 25°C.
After washing in deionised water, the seeds pretreated with sulphuric acid only or with both sulphuric
acid and cellulase were then kept at 30°C for 5 days, in the presence of a GA3 10-3M solution; and then
put to germinate, at the same temperature, on cotton humidified with deionised water.
Drying of the pretreated seeds
Part of the seeds pretreated with sulphuric acid, cellulase and GA3, were dried at room temperature
(20+ -2°C) during 2 days. Their mean moisture content was then found to be between 9 and 10% of the
dry weight. They were then put to germinate on cotton humidified with deionised water.
RESULTS
Germination of seeds collected in 1986
No seeds of seedlots 86/5722N, 86/5723N and 86/5725N germinated in water, at any of the
temperatures used (5–40°C). Seeds of seedlot 86/5729N germinated slowly at 30°C, but the germination
was only 30% after 2 months (Figure 1); they germinated with even more difficulty at 25 and 35°C, and
did not germinate at all below 25°C (results not presented).
The inability of the un-cut seeds to germinate is mainly due to the thick and hard seedcoats, as extracted embryos germinated much more easily (Figure 1 and Table 1).
Figure 1. Germination of un-cut seeds (1) and of extracted embroys (2) of seedlot 86/5729N, at 30°C in water.
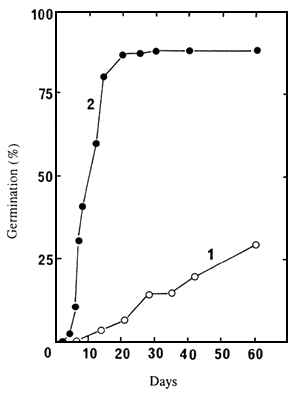
Table 1. Germination percentages obtained after 60 days at 30°C, in water, with un-cut seeds and extracted embryos of seedlots 86/5722N, 86/5723N, 86/5725N and 86/5729N.
| Seedlot | Germination (%) un-cut seed | Germination (%) extracted embryos |
| 86/5722N | 0 | 60 |
| 86/5723N | 0 | 56 |
| 86/5725N | 0 | 70 |
| 86/5729N | 30 | 89 |
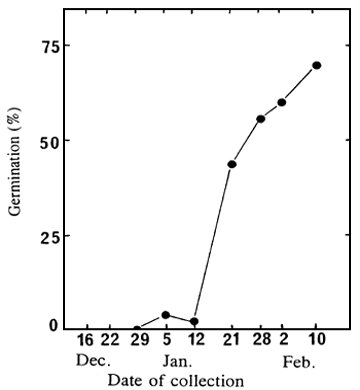
Influence of the development stage of the seeds
Extracted embryos of seeds collected from 16 December 1987 to 10 February 1988 were put to
germinate in water, at 30°C. Figure 2A shows results after 60 days; and Figure 2B presents the
percentage of well developed embryos at the time of collection.
Figure 2. Variation, according to the date of collection, of the germination ability of un-cut seeds and extracted embryos (A), and of the percentage of full seeds (B).
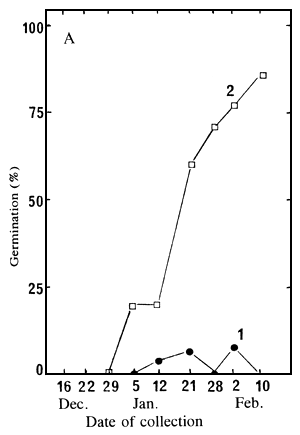 | 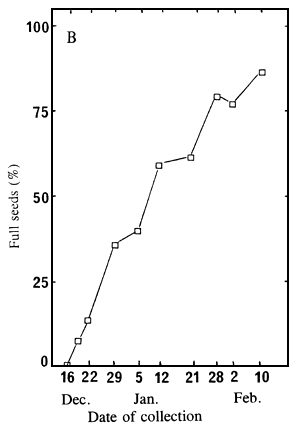 |
| A: Germination percentages of un-cut seeds (1) and extracted embryos (2), after 60 days at 30°C, in water; | B: Percentage of seeds having a well developed embryo. |
Germination of un-cut seeds is extremely low whichever the date of collection; however, the extracted embryos began to germinate in seedlots collected on and after 5 January, and their germination consistently improved until the last collection (Figure 2A). Figures 2A and 2B clearly show that embryos germinate easily only when they are well developed within the seeds. To obtain seeds with good germination ability the collection must take place when the seeds have reached full maturity. However, it should be noted that even mature seeds germinate with great difficulty if un-cut.
Effect of gibberellin
This study was carried out on seedlots 86/5722N, 86/5725N and 86/5729N. Un-cut seeds were put to
germinate at 30°C in continuous contact with a GA3 10-3M solution. Figure 3 shows that GA3 strongly
stimulates germination. Seeds of seedlots 86/5722N and 86/5725N did not germinate at all in water, but
respectively 70% and 54% of seeds of these seedlots were able to germinate in 7 weeks in presence of
GA3. Those of seedlot 86/5729N also germinated much better (82% after 7 weeks) in the presence of
GA3 than in water only (Figure 1).
A similar study, conducted using seeds collected between 16 December 1987 and 10 February 1988, showed that GA3 also facilitates the germination of immature seeds (Figure 4), but only when the embryos are already fairly well developed (Figure 2B).
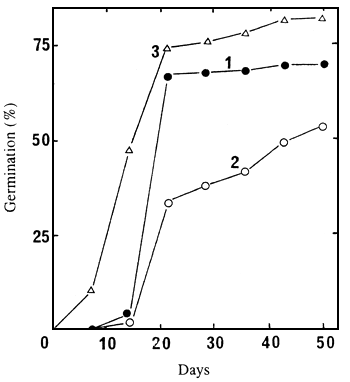 |  | |
| Figure 3. Germination of un-cut seeds of seedlots 86/5722N (1), 86/5725N (2), and 86/5729N (3), at 30°C, in the presence of GA3 10-3M. | Figure 4. Variation, according to the date of collection, of the germination percentage of uncut seeds obtained after 60 days at 30°C in the presence of GA3 10-3M. | |
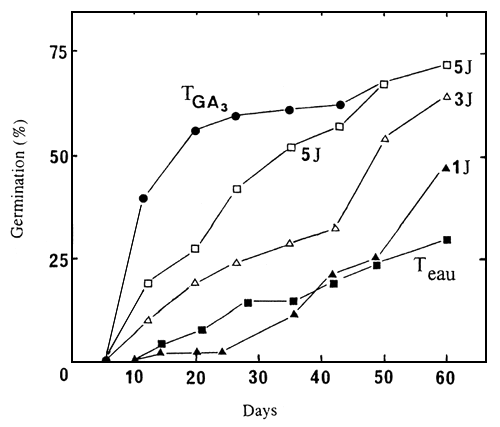
To determine the effects of length of time of treatment with GA3 seeds of seedlot 86/5729N were soaked on a GA3 10-3M solution for 1, 3 and 5 days, and then put to germinate in water. Seeds put to germinate continuously in water, or in presence of GA3 103M, served as controls. A treatment of at least 5 days by GA3 was necessary to obtain, after 50 or 60 days, the same germination percentage as for seedlots which were in continuous contact with this growth regulator (Figure 5). With the other seedlots (86/5722N and 86/5725N), the same treatment was not sufficient to obtain a significant stimulation of germination (results not presented).
Figure 5. Germination of un-cut seeds of seedlot 86/5729N, at 30°C, put in contact with GA3 10-3M during 1, 3 and 5 days, then transferred to water. In controls, seeds were put to germinate continuously in water (Twat), or in a GA3 10-3M solution (TGA3).
Effect of a treatment by sulphuric acid and cellulase
As the inability to germinate seemed to result largely from obstruction by the seedcoat, a pretreatment by
concentrated sulphuric acid and by cellulase (1.25 gl-1) was tested. Seeds pretreated during 3 hours in
sulphuric acid, with or without soaking for 24 hours in a cellulase solution, were put to germinate at
30°C in water, or in a GA3 10-3M solution. Seeds similarly pretreated were put in contact with a GA3 103M
solution for 5 days, and then dried for 2 days before being put to germinate in water at 30°C. Figure
6 and Table 2 synthesise the results obtained with seedlots 86/5722N, 86/5725N and 86/5729N.
Figure 6. Germination at 30°C, of seeds of seedlot 86/5729N, to which different pretreatments were applied, and which were put to germinate in water or in a GA3 10-3M solution: A = seeds pretreated 3 hours with concentrated sulphuric acid; B = seeds pretreated 3 hours with concentrated sulphuric acid, then for 24 hours with cellulase (1.25 g l-1). T = control with no pretreatment and put to germinate in water. 1 = pretreated seeds put to germinate in water; 2 = pretreated seeds put to germinate in the presence of GA3; 3 = pretreated seeds soaked for 5 days in GA3 before being put to germinate in water; 4 = pretreated seeds soaked for 5 days in GA3 and then dried, before being put to germinate in water.
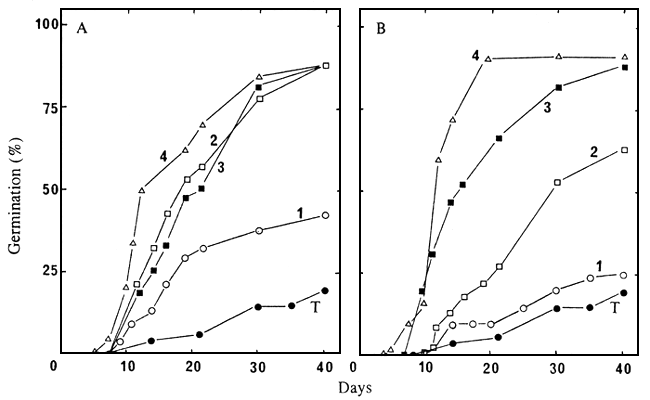
Table 2. Effects of different pretreatments on germination percentages obtained after 30 days at 30°C, in water or in a GA3 10-3M solution, with seeds of seedlots 86/5722N, 86/5723N, 86/5725N and 86/5729N. The results show the mean of two observations. Pretreatments were 3 hours with concentrated sulphuric acid, 24 hours with cellulase (1.25 g l-1) and 5 days in GA3 10-3M, with or without drying. Control seeds received no pretreatment.
| Pretreatment | Medium | Germination (%) Seedlot 86/5722N | Germination (%) Seedlot 86/5725N | Germination (%) Seedlot 86/5729N |
| None (control seeds) | water | 0 | 0 | 15 |
| H2SO4 | water | 8 | 20 | 38 |
| H2SO4 | GA3 | 9 | 55 | 78 |
| H2SO4 + cellulase | GA3 | 6 | 5 | 20 |
| H2SO4 + cellulase | water | 18 | 42 | 52 |
| H2SO4 + GA3 | water | 12 | 56 | 82 |
| H2SO4 + GA3 + cellulase | water | 40 | 36 | 82 |
| H2SO4 + GA3 + drying | water | 22 | 49 | 84 |
| H2SO4 + GA3 + cellulase + drying | water | 66 | 58 | 91 |
When seeds were put to germinate in water, pretreatment by sulphuric acid only had minor positive effects. These were not enhanced when the pretreatment by sulphuric acid was followed by soaking in cellulase. On the other hand, seeds pretreated with sulphuric acid only, or with sulphuric acid and cellulase, germinated much better when put in a GA3 10-3M solution, however, cellulase did not seem to have any particular effect. The stimulating effect of gibberellin was very significant even when this growth regulator was applied only for 5 days after pretreatment by sulphuric acid and cellulase. The best results were obtained with seeds which were dried after the successive pretreatments by sulphuric acid, cellulase and gibberellin, and were then put to germinate in water.
DISCUSSION AND CONCLUSIONS
Seeds of Terminalia ivorensis germinate with great difficulty. This inability to germinate is caused by the seedcoat, most probably because this is thick and lignified. Extracted embryos germinate much better than un-cut seeds. In species growing under a warm climate (Côme and Corbineau, 1992), such as Cedrela odorata (Côme et al., 1985), Mangifera indica (Corbineau et al., 1986), Symphonia globulifera (Corbineau and Côme, 1986a), Shorea roxburghii (Corbineau and Côme, 1986b) or Hopea odorata (Corbineau and Côme, 1986b, 1988), the optimal germination temperature is generally relatively high (25–30°C). This is also the case for Terminalia superba (Roederer, 1988).
However, the extracted embryos of Terminalia ivorensis germinate well only if they have reached a proper stage of development prior to collection. To obtain seeds with good germination ability, it is thus necessary to ensure that the seeds are completely mature at the time of collection. This was well demonstrated by the study of the germination of embryos extracted from seeds at different stages of their development. The seedlots in which the extracted embryos did not germinate totally were certainly collected too early.
GA3 at high concentration (10-3M) significantly facilitates the germination of complete seeds, but this stimulating effect is possible only if the seeds are sufficiently mature, i.e. if the embryo has reached its maximal development. A treatment by GA3 is difficult to utilise in field conditions as it is efficient only if it is applied for a long enough time.
Scarification for 3 hours in sulphuric acid is not sufficient to ensure a satisfactory germination of the seeds, but it will have positive effects if complemented by soaking for 24 hours in a cellulase solution (1.25 g l-1). After such a combined treatment, the use of GA3 10-3M is very efficient, even if applied for 5 days only.
To obtain good results, the following treatment could be envisaged: soak for 3 hours in concentrated sulphuric acid, rinse in water, soak for 24 hours in a cellulase solution, rinse in water, soak for 5 days in a GA3 solution. After such a treatment, the seeds may even be dried at room temperature without negative effects on germination. However, it will be necessary to check whether the positive effects of such a pretreatment are maintained, after drying, also during seed storage.
The treatment described is tedious for large scale application. It could however be very useful in the utilization of research quantities of precious material, such as seeds collected during exploration of the natural range (always costly, often not repeatable); or seeds produced by controlled crossing.
References
Côme D. & Corbineau F. 1992 Les semences et le froid. Dans Les végétaux et le froid, Hermann, Paris, 401–461.
Corbineau F. & Côme D. 1986a Experiments on the storage of seeds and seedlings of Symphonia globulifera L. f. (Guttiferae). Seed Sci. & Technol., 14, 585–591.
Corbineau F. & Côme D. 1986b Experiments on germination and storage of the seeds of two dipterocarps: Shorea roxburghii and Hopea odorata. The Malaysian Forester, 49, 4, 371–381.
Corbineau F. & Côme D. 1988 Storage of recalcitrant seeds of four tropical species. Seed Sci. & Technol., 16, 97–103.
Corbineau F., Defresne S. & Côme D. 1985 Quelques caractéristiques de la germination des graines et de la croissance des plantules de Cedrela odorata L. (Méliacées). Bois et Forêts des Tropiques, 207, 17–22.
Corbineau F., Kanté M. & Côme D. 1986 Seed germination and seedling development in the mango (Mangifera indica L.). Tree Physiology, 1, 151–160.
Roederer Y. 1988 Etude de la germination des semences de Terminalia superba Engler et Diels et de sa variabilité. Diplôme d'Etudes Doctorales, Université Pierre et Marie Curie, Paris, 100 p.
Forest Genetic Resources Information no. 21. FAO, Rome (1993)
Manuscript received April 1993