by
C.E. Harwood1; M.W. Haines2 & E.R. Williams1
1 CSIRO Division of Forestry, PO Box 4008 QVT,
Canberra ACT 2600, Australia
2 Conservation Commission of the Northern Territory
PO Box 496, Palmerston 0831, Australia
ABSTRACT
Early growth of a seedling seed orchard of Acacia crassicarpa, laid out initially as a provenance/progeny trial of 170 open pollinated families from natural provenances in Papua New Guinea (PNG) and Queensland, is reported. Fifteen months after planting, mean height for PNG families was 5.24 m, bole length to first fork 2.02 m and dbh 5.10 cm. The corresponding means for Queensland families were 3.33, 1.60 and 2.89. Variation among local provenances within PNG was small: all PNG families came from a small and relatively uniform geographic area which may be considered a single region of provenance. There were, however, statistically significant differences between families within PNG local provenances. The results obtained were in agreement with previous provenance studies on the species. Because of their poor performance, it is anticipated that all Queensland families will be removed from the orchard before flowering commences.
INTRODUCTION
The best-performing natural provenances of several fast-growing tropical acacia species are located in remote areas of northern Australia, Papua New Guinea (PNG) and Indonesia, making seed collection from natural populations difficult and expensive (Gunn and Midgley 1991). Planted stands representing a broad genetic base of one or more superior natural provenances therefore provide an attractive option for production of seed of good genetic quality at lower cost. Selective thinning of such stands may enable genetic improvement in selected economic traits such as vigour and tree form (Zobel and Talbert 1984). The seed produced may be used directly for operational plantings, or may be used to provide part or all of the genetic base for tree improvement programs elsewhere.
Recognizing the increasing international demand for high-quality seed of certain tropical acacias and eucalypts, CSIRO's Australian Tree Seed Centre (ATSC) has since 1988 established over 30 ha of seed production areas in northern Australia, in collaboration with the Queensland Forest Service, the Conservation Commission of the Northern Territory and Melville Forest Products Ltd (Harwood et al. 1993). The species planted include Acacia aulacocarpa, A. auriculiformis, A. crassicarpa, A. mangium, Eucalyptus pellita, E. urophylla and Casuarina junghuhniana. As part of this program a 2.1 ha planting of Acacia crassicarpa was established at Melville Island, north of Darwin in Australia's Northern Territory.
The primary aim of the planting was the convenient and relatively cheap production of substantial quantities of seed of good genetic quality from broad genetic bases of the best provenances of the species. The planting is hereafter referred to as a seedling seed orchard (SSO), because although the stand is comprised of the progenies of unselected open-pollinated families from natural stands, family pedigrees have been retained (cf. Snyder 1972), and, as discussed below, heavy thinning within families has been carried out and some inferior provenances and families will be removed during subsequent thinnings.
Isozyme studies have shown that A. crassicarpa is primarily outcrossing in natural stands (Moran et al. 1989).
MATERIALS AND METHODS
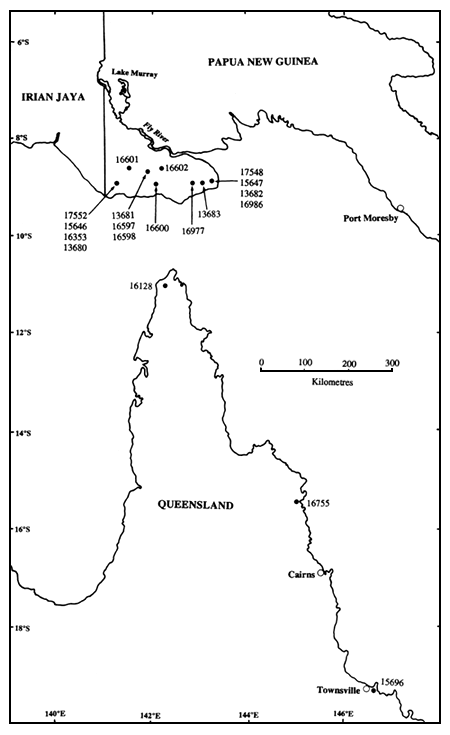
The geographic locations of the CSIRO seedlots contributing to the SSO are shown in Figure 1. Each CSIRO seedlot number represents several single-tree seedlots collected within a local provenance (here defined arbitrarily as an area up to about 5 × 5 km for purposes of further discussion - see Turnbull and Griffin 1986). Single-tree seedlots, hereafter referred to as families, were collected from trees at least 100 m apart from one another to minimise relatedness between families (the trees were in A. crassicarpa stands, i.e. they were not isolated individuals). Table 1 lists the CSIRO seedlots, and families per seedlot, included in the planting. The seedlots span most of the natural range of the species, except for occurrences in Irian Jaya (Gunn and Midgley 1991). The three CSIRO seedlots from Queensland are separated from one another by several hundred kilometres. In the southern part of Western Province, PNG, the species has a widespread distribution, and sampling strategy is constrained primarily by travel logistics in unroaded country. It will be seen that a number of the CSIRO seedlots from PNG sample either the same local provenance (e.g. Oriomo, Gubam-Bimadebun), or immediately adjacent geographic locations.
Plants were raised in plastic, book-type containers, of 200 cm3 soil volume, using a sand, loam and peat-moss potting mix which incorporated a slow release fertilizer. They were inoculated in the nursery with an appropriate Rhizobium strain for the species.
The planting site at Yapilika, Melville Island (11°34 S, 130°34 E, 20 m.a.s.l.) has a mean annual rainfall of about 1750 mm with a four-month dry season, and a mean annual temperature of 24°C. The soil type is an infertile laterite-derived fine sand with a pH of about 5. The site was ploughed prior to planting, and each plant received 229 g of complete fertilizer (6:12:8 NPK, plus zinc and copper), developed for operational plantings of Pinus caribaea) applied by hand shortly after planting.
The planting, involving 24 seedlings of each of 170 families, used six complete replicates of 170 plots, each family being represented in each replicate by a four-tree line plot. The six replicates were contiguous, and plots were laid out in an alpha-design with row-column properties (Williams and Matheson 1993), using the design-generation package “ALPHA +” prepared by E.R. Williams. The precision of comparisons between different CSIRO seedlots was maximised within rows and columns using an option in ALPHA +. Spacing was 3 m between rows, and 1.5 m between trees within rows, giving a plot size of 3 × 6 m. Two perimeter rows of surplus stock were planted around the stand at the same spacing as an external buffer.
Growth was assessed in May 1993, 15 months after planting. Height and bole length to the first major fork were measured with height poles. Forking was deemed to have occurred where a leading branch was more than half the diameter of the main stem. Diameter at breast height over bark (dbh) was measured with templates graduated in 1 cm increments. Where plants forked below breast height, the diameters dbh1 and dbh2 of both stems were measured and an equivalent single stem diameter, d, calculated as follows:
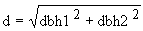
Table 1. CSIRO seedlots and families of Acacia crassicarpa included in the seed orchard, and mean height, bole length and Dbh of CSIRO seedlots at 16 months.
| CSIRO | Location | Lat. | Long. | Alt. | No. | Ht. | B.L. | Dbh | |
| Seedlot | oS | oE | (m) | fams | (m) | (m) | (cm) | ||
| Papua New Guinea | |||||||||
| 17552 | Bensbach | 8 53 | 141 17 | 25 | 16 | 5.19 | 1.99 | 5.13 | |
| 15646 | Wemenever | 8 56 | 141 17 | 20 | 8 | 5.38 | 1.96 | 5.26 | |
| 16353 | N.E. of Balamuk | 8 49 | 141 20 | 20 | 2 | 5.38 | 1.69 | 5.42 | |
| 13680 | Wemenever | 8 51 | 141 26 | 30 | 15 | 5.27 | 1.84 | 5.23 | |
| 16601 | Konifok-Serisa | 8 34 | 141 27 | 25 | 2 | 5.15 | 1.75 | 4.96 | |
| 13681 | Mata | 8 40 | 141 45 | 30 | 8 | 5.14 | 1.75 | 4.96 | |
| 16597 | Gubam-Bimadebun | 8 37 | 141 55 | 25 | 21 | 5.27 | 2.10 | 5.12 | |
| 16598 | Gubam-Bimadebun | 8 37 | 141 55 | 25 | 11 | 5.28 | 2.02 | 5.23 | |
| 16600 | Mai Kussa | 8 52 | 142 03 | 15 | 3 | 4.97 | 1.98 | 4.81 | |
| 16602 | Dimisisi | 8 31 | 142 13 | 50 | 7 | 5.07 | 1.65 | 4.96 | |
| 16977 | Wipim | 8 49 | 142 48 | 45 | 18 | 5.24 | 2.18 | 4.96 | |
| 13683 | Woroi-Wipim | 8 49 | 143 00 | 20 | 15 | 5.25 | 2.28 | 5.04 | |
| 17548 | Oriomo Old Zip | 8 48 | 143 06 | 20 | 4 | 5.29 | 2.36 | 4.85 | |
| 15647 | Oriomo | 8 50 | 143 08 | 10 | 2 | 5.10 | 1.87 | 5.17 | |
| 13682 | Oriomo | 8 50 | 143 10 | 20 | 11 | 5.30 | 1.87 | 5.16 | |
| 16986 | Oriomo DPI | 8 51 | 143 11 | 35 | 7 | 5.31 | 2.17 | 5.11 | |
| Mean of 150 PNG families | 5.24 | 2.02 | 5.10 | ||||||
| Queensland | |||||||||
| 15696 | Townsville | 19 23 | 147 01 | 15 | 6 | 2.80 | 1.57 | 1.93 | |
| 16128 | Jardine R | 11 02 | 142 22 | 20 | 11 | 3.46 | 1.60 | 3.27 | |
| 16755 | Parish of Annan | 15 36 | 145 19 | 80 | 3 | 3.95 | 1.66 | 3.49 | |
| Mean of 20 Queensland families | 3.33 | 1.60 | 2.89 | ||||||
no. fams. = number of families per CSIRO seedlot,
Ht. = height
B.L. = bole length
Dbh = diameter at breast height over bark
Figure 1. Geographic locations of CSIRO seedlots included in the seed orchard
Very few plants had more than two stems at breast height; only the two largest stems were measured on these plants. Plot means for the three traits were calculated and analysis of variance was performed on plot mean data using the statistical package GENSTAT 5.2. An initial analysis used a linear model in which replicates, regions (PNG and Queensland), CSIRO seedlots within regions, and families within CSIRO seedlots were estimated as fixed effects. A subsequent analysis was carried out on families from the PNG region. This excluded all Queensland seedlots, and used a similar linear model but with the region term omitted.
RESULTS AND DISCUSSION
Rapid early growth of A. crassicarpa was recorded, exceeding that of A. auriculiformis and A. mangium planted in adjacent trials at the same time. Survival averaged 94.4 per cent over the whole planting and exceeded 92 per cent for all CSIRO seedlots. CSIRO seedlot means for height, bole length and dbh are presented in Table 1. All PNG seedlots grew much faster than any of the three from Queensland (P<.001). Better performance of PNG provenances, relative to Queensland provenances has also been reported in species/provenance trials in Thailand (Chittachumnonk and Sirilak 1991) and Hainan Island, China (Yang and Zeng 1991).
Variation among PNG seedlots was not significant for height, but was significant (P<0.05) for bole length and dbh (Table 2). Between-seedlot differences were much smaller than the difference between the two regions. There was no obvious geographic trend in the variation between PNG seedlots, there being above-average and below-average seedlots from within the eastern, central and western parts of the collection area (Table 1). This finding is in agreement with the results of the trials cited above, and those of Sim and Gan (1991) in Sabah, in which differences between PNG local provenances have been small. PNG seedlots tested to date are all from a relatively small area of low relief some 200 km by 50 km in extent (Figure 1, Gunn and Midgley 1991), so the lack of variation among these seedlots is not surprising. The area of occurrence of A. crassicarpa in PNG and adjacent Irian Jaya could be considered as a single region of provenance (Turnbull and Griffin 1986), although it has been subdivided into smaller regions of provenance by Harwood et al. (1993) for the management of genetic resources of other acacia species and Eucalyptus pellita. The main environmental trend which might be expected to exert selection pressure resulting in differentiation of populations within the region is one of decreasing length of dry season, and increasing rainfall and relative humidity, from south to north (Vercoe and McDonald 1991).
The SSO was selectively thinned after assessment, retaining the best tree in each four-tree line plot, selected by eye for form (straight, single stemmed trees preferred) and vigour (the tree demonstrating the best growth retained if it had a substantial size advantage over the best-formed tree). This thinning reduced stand density to about 550 stems per hectare, and aimed to promote development of broad, heavy crowns for maximum seed production. This thinning also ensures that adjacent retained trees are unrelated, thus reducing inbreeding in the SSO. Assuming that a second assessment confirms the poorer growth of Queensland provenances, all Queensland trees (20 families) will be removed from the SSO in a second thinning, before flowering commences. It is expected that substantial seed production will commence in 1995. Options for further thinning, to reduce stand density to a desired level of about 150 stems per hectare for operational seed production, include partial or complete removal of inferior PNG families. At this stage it appears unlikely that any of the PNG local “provenances” (comprised of up to four CSIRO seedlots) will be removed completely, as all of them have one or more “good” families with mean height, diameter and/or bole length in excess of the overall mean for all PNG families. Variation between families within CSIRO seedlots is highly significant for all traits.
Table 2. Summary analysis of variance tables for height, bole length and Dbh (Queensland seedlots excluded from analysis)
| (a) Height | |||||
| df | ss | ms | F | P | |
| Replicates stratum | 5 | 6.9426 | 1.3885 | 5.61 | |
| Replicates.units stratum CSIRO seedlots | 15 | 5.7081 | 0.3805 | 1.54 | 0.086 |
| Families within seedlots | 134 | 184.1608 | 0.5036 | 2.03 | <.001 |
| Residual | 744(1) | 184.1608 | 0.2475 | ||
| Total | 898(1) | 264.2462 | |||
| (b) Bole length | |||||
| df | ss | ms | F | P | |
| Replicates stratum | 5 | 10.432 | 2.086 | 1.88 | |
| Replicates.units stratum CSIRO seedlots | 15 | 29.542 | 1.969 | 1.78 | 0.034 |
| Families within seedlots | 134 | 206.534 | 1.541 | 1.39 | 0.005 |
| Residual | 744(1) | 825.473 | 1.110 | ||
| Total | 898(1) | 1071.941 | |||
| (c) Diameter (Dbh) | |||||
| df | ss | ms | F | P | |
| Replicates stratum | 5 | 14.3765 | 2.875 | 6.45 | |
| Replicates. units stratum CSIRO seedlots | 15 | 12.7875 | 0.8535 | 1.91 | 0.019 |
| Families within seedlots | 134 | 107.0839 | 0.799 | 1.79 | < 0.001 |
| Residual | 744(1) | 331.8379 | 0.446 | ||
| Total | 898(1) | 465.7881 |
df = degrees of freedom
ss = sum of squares
ms = mean squares
F = F-ratio
P = probability of such an F-ratio occurring by chance
(1) = one plot with no trees - i.e. missing value
Design and management of this SSO illustrates several general points in the planning of first-generation SSOs of species for which limited provenance information is available (Nanson 1972). Review of available information prior to planting had suggested that the performance of Queensland provenances was substantially poorer than PNG provenances. Inclusion of a small number (20 out of a total of 170) families from three widely-separated Queensland provenances has enabled this assumption to be confirmed for the Melville Island environment without compromising the primary role of the stand as a SSO of the best provenances: the Queensland families can be completely removed without creating large physical gaps in the SSO. Major differences in growth and flowering phenology between A. crassicarpa local “provenances” within PNG were not anticipated, so an orchard representing all PNG provenances was considered feasible. All available PNG families were included to maximise the representation of PNG genetic material, rather than limiting families used per local “provenance” to some arbitrary number such as ten or 20. Available resources did not permit the planting of separate stands representing subdivisions of the PNG genetic material, which would have been valuable for management of coancestry in subsequent generations. However, it would be possible to reconstruct genetically separate sublines by vegetative propagation of outstanding individual trees in the SSO, using propagation techniques developed for A. auriculiformis and A. mangium (Carron and Aken 1992). Mixed-model statistical analyses in which rows and columns were estimated as random effects using the REML option in GENSTAT 5.2 (Williams and Matheson 1993) substantially reduced the residual mean square relative to that in the randomised complete block model reported here, and will be presented in a later publication.
ACKNOWLEDGEMENTS
ATSC's seed production program has been made possible by funding from the Australian International Development Assistance Bureau. Support of ATSC's operations provided by FAO's Forest Resources Division has contributed to the assembling of the genetic resources used. Melville Forest Products Ltd made land and equipment available for the trial. The contributions of staff of the Conservation Commission of the Northern Territory, in particular Beau Robertson, at all stages of the project, are gratefully acknowledged. The PNG seedlots used in the planting were collected in collaboration with the Papua New Guinea Department of Forests, who have also established seed production areas of the species locally. Peter Dart (Department of Agriculture, University of Queensland) provided the Rhizobium inoculum. We thank Ken Eldridge for helpful comments on an earlier draft of this paper.
References
Carron, L.T. & Aken, K.M. eds. 1992 Breeding technologies for tropical acacias. ACIAR Proceedings No 37
Chittachumnonk, P. & Sirilak, S. 1991 Performance of Acacia species in Thailand. pp 153–158 in Turnbull, J.W. ed. Advances in Tropical Acacia Research. ACIAR Proceedings No. 35.
Gunn, B.V. & Midgley, S.J. 1991 Exploring and accessing the genetic resources of four selected tropical acacias. pp. 57–63 in Turnbull, J.W. ed. Advances in Tropical Acacia Research. ACIAR Proceedings No. 35.
Harwood, C.E., Applegate, G.B., Robson, K.J. & Haines, M.W. 1993 Establishment and management of seed production areas of tropical tree species in northern Australia. Paper presented to ASEAN-Canada Forest Tree Seed Centre international conference on genetic conservation and production of tropical tree seed, Chiang Mai, June 14–16 1993. (in press)
Moran, G.F., Muona, O. & Bell, J.C. Breeding systems and genetic diversity in Acacia auriculiformis and A. crassicarpa. Biotropica 21, 250–256.
Nanson. 1972 The provenance seedling seed orchard. Silvae Genetica 21, 243–249.
Sim, B.L. & Gan, E. 1991 Performance of Acacia species on four sites of Sabah Forest Industries. pp. 159–165 in Turnbull, J.W. ed. Advances in Tropical Acacia Research. ACIAR Proceedings No. 35.
Snyder, E.B. 1972 Glossary for forest tree improvement workers. Southern Forest Experiment Station, United States Department of Agriculture.
Turnbull, J.W. & Griffin, A.R. 1986 The concept of provenance and its relationship to infraspecific classification in forest trees. pp. 157–189 in B.T. Styles ed. Infraspecific Classification of Wild and Cultivated Plants. Clarendon Press, Oxford.
Vercoe, T.K. & McDonald, M.W. E. pellita F. Muell. and Acacia seed collection in New Guinea, September-October 1990. FAO Forest Genetic Resources Information 19, 38–42.
Williams, E.R. and Matheson, A.C. 1993 Design and analysis of forestry field trials. ACIAR/CSIRO, Australia. (in press).
Yang Minquan & Zeng Yutian. 1991 Results from a four-year-old tropical Acacia species/provenance trial on Hainan Island, China. pp. 170–172 in Turnbull, J.W. ed. Advances in Tropical Acacia Research. ACIAR Proceedings No. 35.
Zobel, B.J. & Talbert, J.T. 1984 Applied Forest Tree Improvement. John Wiley and Sons, New York.
Forest Genetic Resources Information no. 21. FAO, Rome (1993)
Manuscript received June 1993