Plates 1 and 2 (pp. 8 & 9)
The absence of active virological research in Africa restricts our knowledge of viral infection to those detectable by externally visible, or microscopically recognisable virus-induced pathological changes. The only confirmed case of viral infection in African fish is Lymphocystis, in cichlids from the East African lakes (Paperna, 1973) which is manifested by readily detected, macroscopic dermal pustules (pox-like infection). A case of mortality, with characteristic abdominal dropsy of carp, in Kajansi farm, Uganda, could have been associated with viral (Rhabdovirus carpio) aetiology. The rhabdovirus-caused carp disease (SVC) occurs predominantly in coldwater regions, although, in cell culture the virus was found to grow in temperatures up to 31°C (Fijan et al., 1971).
Recently, a whirling viral disease of tilapia fry has been reported by Avtalion & Shlapobersky (1994) to cause mortalities of laboratory reared (less than one month old) Oreochromis aureus, O. niloticus, Sarotherodon galilaeus and tilapia hybrids including red tilapia. Affected fish were dark skinned, anorexic and showed characteristic whirling behaviour prior to death. Symptoms first occurred in 4–8 day old fish, death appeared first in 6–8 day old fish and subsequently, another wave in 14–18 day old fish. Hexagonal particles, iridovirus-like, of about 100 nm, were demonstrated within inclusions in the endoplasmic reticulum in cells from the brain tissue.
Herpes-like infections of nuclei and cytoplasm of haematopoietic kidney cells were demonstrated in pond-reared goldfish from Israel, which were also showing severe infection of the same tissue by gram negative bacteria (apparently Aeromonas salmonicida atypica).
Tissue cultures of cells from African cichlids (of Oreochromis spp. and their hybrids), used for detecting viruses, have been established in USA (endothelial cell line from O. mossambicus bulbus arteriosus [TmB] - Lewis & Marks, 1985) and in Taiwan (from ‘Tilapia’ - O. niloticus x mossambicus ovaries [TO-2] - Chen et al., 1983a and kidneys [TK-1] - Chen et al., 1983b).
A birnavirus (reminiscent of European eel virus [EVE], related to the AB strain of IPNV) was isolated (on tilapia derived cell lines and on CHSE-214 continuous salmonid derived cell line) from O. mossambicus (Hedrick et al., 1983, Chen et al., 1985). EVE viruses were incriminated in the aetiology of eel branchionephritis (Chen et al., 1985).
The highly contagious herpes virus, causing disease in cultivated American catfish (CCV), in the Southern USA, proved to be transmissible and pathogenic to Clarias batranchus (Galla & Hartmann, 1974) and therefore comprises a potential epizootic risk to catfish (siluroids) if introduced into Africa with its American catfish host.
Cusak and Cone (1986) reviewed studies suggesting that parasitic crustaceans can be vectors of viral infections of fish. Ahne (1985) transmitted SVC virus to carp via Argulus foliaceus and leeches, acting as mechanical vectors.
Species affected
A variety of marine and freshwater fish; in Africa known only from cichlids; including
species of Tilapia, Oreochromis and Haplochromis.
Geographic range
In cichlid fish in Lakes Victoria (Nyanza) (Oreochromis variabilis and Haplochromis spp.),
in Lake George (H. elegans) and L. Kitangiri (Tilapia amphimelas and O. esculentus) in
East Africa.
Description taxonomy and diagnosis
Infection manifested in one to numerous dermal clusters of rounded pustules or wart-like
growths. Histological sections reveal aggregates of grossly hypertrophic cells (in cichlids
200–330 μm in diameter), enclosed within a thick hyaline (eosinophilic) wall and an
extremely large nucleus and nucleolus. Cytoplasm contained basophilic (DNA)
inclusions, (numerous, small-rounded in cichlids) and vacuoles (Paperna, 1973).
Lymphocystis viruses are large (160–300 nm), icosahedral, DNA viruses (iridovirus-like)
replicating within the cytoplasmic inclusions and are released into the cytoplasm to form
regular arrays (Paperna et al., 1987).
Life history and biology
Viral particles are released to the water when infected cells eventually degenerate, burst
or are sloughed off (Paperna et al., 1987). Infection was experimentally transmitted
among fish of the same or closely related species through an inoculum of viral material,
or immersion in water contaminated with the contents of lesions (Cook, 1972; Vaughan,
1979). The virus was grown in a cell line that had been established from fins and caudal
tissue of the same host fish species (Walker & Hill, 1980).
Pathology
Even if it spreads throughout the fish skin (not in cichlids), infection is rarely fatal and
eventually (within 20 days to 4 months) regresses (Cook, 1972; Paperna et al., 1982).
Tissue response around the nodules is very restricted. Gradual regression of infection
was found to coincide with evident degeneration of lymphocystis infected cells, while
fractionation of the hyaline capsule was followed by subsequent invasion of the lumen
by macrophages and lymphocytes. The regression of infection and absence of recurrent
infection suggests acquired resistance (Cook, 1972; Roberts, 1976; Paperna et al., 1982,
1987).
Epizootiology
Natural infection is low, in 4 out of 265 (1.5%) O. variabilis and 8 of 98 (8%) Haplochromis
sp. in Northern Lake Victoria, and in 1 out of 95 (1%) H. elegans in Lake George
(Paperna, 1973). In culture installations infection may become epizootic (Paperna et al.,
1982).
Control
Unknown.
REFERENCES
Ahne, W., 1985. Argulus foliaceus L. and Piscicola geometra L. as mechanical vector of spring viraemia of carp virus (SVCV). J. Fish Dis., 8: 241–242.
Avtalion, R.R. & Shlapobersky, M., 1994. A whirling viral disease of tilapia larvae. Israeli J. Aquacult. (Bamidgeh), 46: 102–104.
Chen, S.N., Chi, S.C., Ueno, Y. & Kou, G.H., 1983a. A cell line derived from tilapia ovary. Fish Pathol., 18: 13–18.
Chen, S.N., Kou, G.H., Hedrick, R.P. & Fryer, J.L., 1985. The occurrence of viral infections of fish in Taiwan. In: Ellis, A.E. (ed.) Fish & Shellfish Pathology, 33: 313–319.
Chen, S.N., Ueno, Y., Wen, S.C. & Kou, G.H., 1983b. Establishment of cell line from kidney of tilapia. Bull. Eur. Ass. Fish Pathol., 4: 1–4.
Cook, D.W., 1972. Experimental infection studies with lymphocystis virus from Atlantic croacker. Proc. 3d Ann. Workshop World Maricult. Soc., pp. 329–335.
Cusack, R. & Cone, D.K., 1986. A review of parasites as vectors of viral and bacterial diseases of fishes. J. Fish Dis., 9: 169–171.
Fijan, N. Petrinec, Z., Sulimanovic, D. & Swillenberg, L.O., 1971. Isolation of viral causative agent from the acute form of infectious dropsy of carp. Veterinaarski Archiv., 41: 125–138.
Galla J.D. & Hartmann J.X., 1974. Extension of the host range of channel catfish virus CCV to the walking catfish Clarias batranchus (Linnaeus) Florida Scientist, n.v. 31.
Hedrick, R.P., Fryer, J.L., Chen, S.N. & Kou, G.H., 1983. Characteristics of four birnaviruses isolated from fish in Taiwan. Fish Pathol., 18: 91–97.
Lewis D.H. & Marks, J.E., 1985. Microcultures of Sarotherodon mossambicus (Peters) cells: their use in detecting fish viruses. J. Fish Dis., 8: 477–478.
Paperna, I., 1973. Lymphocystis in fish from East African Lakes J.Wildl. Dis., 9: 331–335.
Paperna, I. Sabnai, I. & Colorni, A., 1982. An outbreak of lymphocystis in Sparus auratus L. in the Gulf of Aqaba, Red Sea. J. Fish Dis., 5: 433–437.
Paperna I., Ventura, T.M. & Alves de Matos, A.P., 1987. Lymphocystis infection in snakeskin gourami, Trichogaster pectoralis (Regan), (Anabantidae). J. Fish Dis., 10: 11–19.
Roberts, R.J., 1976. Experimental pathogenesis of lymphocystis in the plaice (Pleuronectes platessa). In: Page L.A. (ed.) Wildlife Diseases, pp. 431–441.
Vaughan, G.E., 1979., Comparative vulnerability of bluegills with and without lymphocystis disease to predation by largemouth bass. Prog. Fish Cult., 41: 163–164.
Walker D.P. & Hill, B.J., 1980. Studies on culture, assay of infectivity and some in vitro properties of Lymphocystis disease. J. Gen, Virol., 51: 385–395.
ILLUSTRATIONS
Plate 1. Viral infections: a,b. Transmission electron microscopic (TEM) view of Herpes-like infection in gold fish, naked (80–100 nm in size) and coated viral particles (106–153 nm) formed in the nucleus (N) and spread into the cytoplasm (Cy). c,d. Viral particles (size about 100 nm) recovered from tilapia fry affected by whirling disease (by TEM). e. Hypertrophic virus infected endothelial cell in the lamina propria of gourami (Trichogaster trichopterus) gut (Histology by light microscopy [LM]). f–h. TEM view of virus infected endothelial cell from the gourami; the corona-like particles are 111–141 nm in size. A. host-cell peripheral cytoplasmic layer; C, debris filled interior space of the cell; V, virus forming layer.
Plate 2. Lymphocystis and other iridovirus-like infections: a. Tilapia amphimelas (50 mm long) from L. Kitangiri, Kenya, infected with lymphocystis (arrow). b. Tail of Haplochromis sp. from L. Victoria (Entebbe), with cluster of lymphocystis cells. c,d. Histological sections of a cluster of hypertrophic lymphocystis cells from L. Victoria Haplochromis sp. (cells' diam. 0.25–0.3 mm). e. Histological section (in LM) of an hypertrophic lymphocystic cell (size 0.13–0.16 mm) from gourami, virus particles are visible as fine dots. f–h. Lymphocystis virus particles (166–216 nm wide) from gourami, in active (g,h) and aged (f) cells. i,j. Iridovirus-like particles (size 141–193 nm) from viscera of gourami.
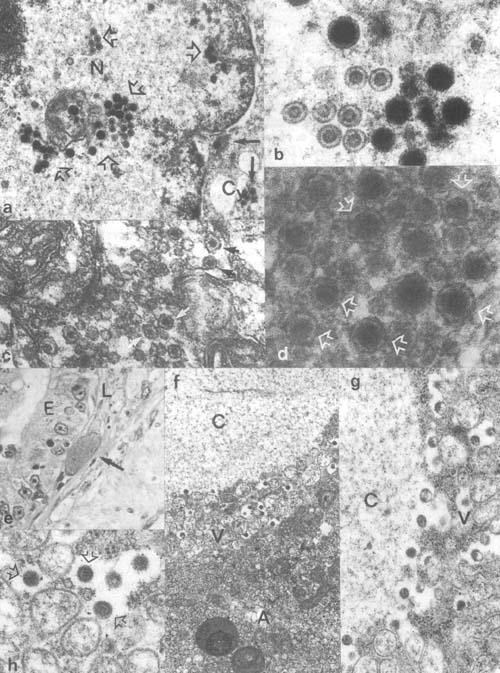
Plate 1. Viral infections (legend p. 7)
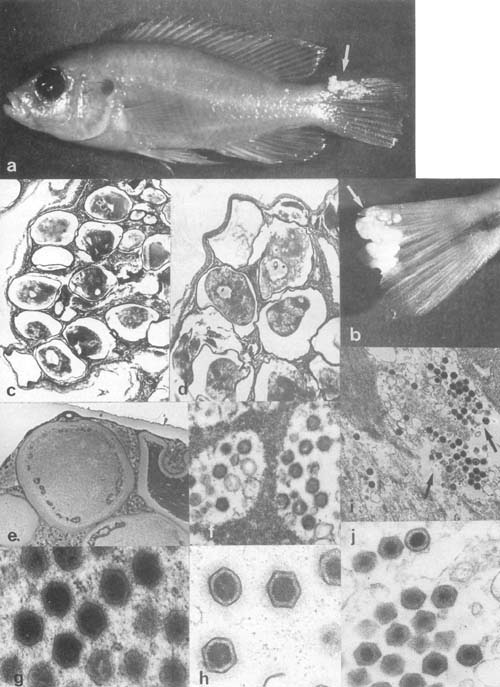
Plate 2. Lymphocystis and other iridovirus-like infections (legend p. 7)