Plates 3. to 5. (pp. 26–28).
“Bacterial diseases are responsible for heavy mortality in both wild and cultured fish. The actual role of these micro-organisms may vary from that of a primary pathogen to that of an opportunist invader of a host rendered moribund by some other disease process” (Richards & Roberts, 1978). The non-specific nature of diseases induced by non-fastidious and opportunistic bacterial organisms makes them unpredictable and complicates their differential diagnosis. Many of these organisms are a usual component of the bacterial flora in aquatic habitats, particularly eutrophic systems. Stressors, often inevitable in most culture systems, predispose fish to bacterial-born diseases (Snieszko, 1974). The course of events from stress to predisposition to infection, include physiological changes (described as a general alarm response syndrome) the consequences of which are to enforce barriers, normally preventing entry of bacteria to fish inner systems and at the same time incapacitating fish defence responses and immune reactions (Mazeaud et al., 1977; Barton & Iwama, 1991).
Our knowledge of bacterial infections and diseases occurring in African fish, is comprised almost entirely of presumptive diagnoses of pathological or clinical conditions. Most data on African fish are derived from studies on tilapia cultured in Israel and on introduced fish (mainly tilapia) elsewhere, outside Africa. Many of these reported infections are non-specific and common to other non-African fish cultured in these systems. However, it is not certain if they are relevant to African fisheries.
Species affected
Streptococcus-related diseases occur in many cultured marine and freshwater fish.
Several streptococcal species are involved, and the relationship between aetiological
agents, isolated from the different fish has not been fully determined. In lsrael,
Streptococcosis affects Oreochromis spp. as well as grey mullets (Mugil cephalus and
Liza ramada) and silver carp, but not common carp (Hubbert, 1989). Streptococcosis
also affected coldwater reared rainbow trout (Eldar et al., 1994).
Geographic range
Streptococcus-related diseases were first reported from Oreochromis spp. and
Sarotherodon spp., kept in Japan (Kitao et al., 1981). Outbreaks, caused by Beta and
Gama-haemolytic strains, in Japan (Miyazaki et al., 1984) as well as in Taiwan (Tung
Ming-Chen et al., 1985) have similar clinical and gross pathological signs to the
streptococcal meningoencephalitis found in cultured tilapia hybrids, in lsrael (Hubbert,
1989). Isolates from Japan and Taiwan were shown to be conspecific with Israeli isolates
(Eldar et al., 1994).
Diagnosis
In acute infection, signs develop within 3–4 days, gross ascites occurs, as well as dermal
lesions. Exophthalmus is characteristic of the chronic stage, which may be clinically
manifested or subclinical. The chronic condition does not occur in silver carp. Clinical
symptoms are characteristically of the neuro-motor type; erratic swimming, swimming
in circles and lack of control of the pectoral fins, leaving fish in an erect position.
Bacteria are readily isolated from the brain, optic nerve and fatty tissue around the eyes, but only from the viscera in a late chronic condition.
Isolated bacteria were gram-positive, non-sporulating, facultatively anaerobic chains forming cocci and catalase negative. They were able to grow at pH 9.6, but not at 10°C nor at 45°C, nor in the presence of 40% (v/v) bile salts or the presence of 6.5% NaCI (w/v). Deoxyribonucleic acid base composition (G+C% = 37%), deoxyribonucleic acid-deoxyribonucleic acid hybridisations, biochemical and serological studies indicate that these strains constitute two distinct species of streptococci; S. difficile (Manitol +ve) and S. shiloi (manitol -ve). Both strains were isolated from cultured tilapia (Oreochromis hybrids), in Israel. Isolates from Israeli grey mullet and tilapia from Japan were identified as S. difficile, while isolates from Taiwanese tilapia and trout corresponded to S. shiloi (Eldar et al., 1994).
Pathology
The brain seems to be the primary site of infection. In the acute state the ascitis fluid is
fibrinous and contains bacteria-laden macrophages, all vital organs become heavily
infected and mortality becomes massive (50–60% of the stock). Epizootic infection, in a
variety of estuarine fish, caused clinical signs of erratic swimming, whirling motion and
gross pathological signs of haemmorhagic lesions on the body, exophthalamus, corneal
opacity, some macroscopic changes in the liver and spleen, and ascites (dropsy) with
mucoid inflammation of the gut.
Histopathological changes in the heart result from acute pericarditis, with aggregation of bacteria-laden macrophages and accumulation of fibrinous exudate in the outer layer of the heart muscle. In the brain, meningoencephalitis is evident by accumulation of bacteria-laden macrophages in the meninges. In the liver, multiple necrotic areas develop, holding bacteria-laden macrophages and sometimes with a formation of granulomata. Proliferative changes have also been reported to occur in the spleen, with the kidney containing free bacteria, and granulomatous inflammation also occurring in the ovaries and the testes (Miyazaki et al., 1984; Hubbert, 1989).
Epizootiology
Case reports suggest stress-related epizootiology and pathogenesis. Handling
promotes a transition from the chronic state to outbreaks of acute disease and mass
mortality.
Variation in the pathological description between case reports from Japan, Taiwan and Israel, may be due to the stage of pathogenesis, which is both environmental and handling stress-mediated, rather than aetiological, although, there were differences in the representation of species in the different countries (see above).
Conspecificity between isolates from different fish species (see above, Eldar et al., 1994) suggests interspecific cross transmission (Hubbert, 1989).
Experience with disease incidences, in farmed fish, suggests that S. shiloi epizootiology is linked with cold water conditions (in reared trout) and therefore is the aetiological origin for streptococosis in overwintering (cold stressed) tilapia, while other species are the aetiological agents of the other infections not related to cold stress (Bejerano, Y., Nir David Laboratories for Fish Diseases, pers. comm.). Circumstantial evidence suggests water, rather than feed, as the aetiological origin (Hubbert, 1989).
In Japan, streptococci strains isolated from fish were widespread in the environment, both in mud samples and in water (Kitao et al., 1981). Food has been implicated as a source of infection in maricultured fish (Taniguchi, 1982), but it is unlikely to be relevant in cases of tilapia fed on dry and pelleted feeds (Hubbert, 1989).
Species affected and geographic range
Halophilic vibrios were isolated from visceral organs of diseased tilapia in Israel (Hubbert,
1989) and Japan (Sakata & Hattori, 1988).
Diagnosis
Affected Oreochromis niloticus in Japan and tilapia hybrids in Israel, showed
characteristic haemorrhages around the base of the fins, prostration in the swimming
movement and stiffness of the muscles. Gills may have purple colouration. The gut
appears to be filled with gas and mucus material. Isolates taken from the blood, livers
or kidneys and gut contents of clinical cases often contain pure culture of the aetiological
agent. Several halophilic Vibrio were isolated from these fish. V. vulnificus, were
distinguished from other vibrios by their ability to ferment lactose. They were also positive
for fermentation of galactose, cellobiose, and salicin, decarboxylation of lysine and
ornitine, grew at 42°C and were negative for arginine dihydrolase, Vogues proskauer
test and sucrose fermentation. Japanese strains were pathogenic to both tilapia and
carp (Sakata & Hattori, 1988). The aetiological agent in Israel was Vibrio parahemoliticus,
identified with the aid of API (France) 20E and 20NE (Hubbert, 1989).
Pathology
Infection with V. parahemoliticus in overwintering tilapia, in Israeli farms, gives rise to
fulminating septicaemia which is rapidly fatal to most fish, usually within 48–72 hours,
following handling. Experimentally, 10/4 cells and higher caused 100% mortality
(Hubbert, 1989). Vibriosis of tilapia farmed in Japan, occurred as a chronic condition and
resulted in a gradual death of 10–20% of the pond fish (Skata & Hattori, 1988).
Pathological changes in acute conditions are mainly haemorrhagies in visceral organs,
sometimes with haemorrhagic ascitic fluid, and distended guts with gases and mucous
fluid. In chronic vibriosis necrotic nodules occur in the spleen, but thus far has not been
reported from infections in tilapia. Vibrio may also be found in the blood of clinically
unaffected overwintering fish.
Epizootiology
Vibriosis is a stress-mediated disease. In Japan, this disease occurs spontaneously
(Sakata & Hattori, 1988), while in Israel, it occurs as a direct result of handling
overwintering fish (Hubbert, 1989).
Vibrio vulnificus is ubiquitous in the haline environment. Clinical isolates, from various fish and from the environment, may differ slightly, only strains isolated from eels were reported to be pathogenic to eels (Muroga et al., 1976).
Species affected and geographical range
Outbreak of disease, with the aetiological agent identified as Pasteurella multocida,
occurred in tilapia hybrids from a fish farm on the shore of L. Kinneret, Israel (Nizan &
Hammerschlag, 1993). Bipolar, Pasteurella-like bacteria were found in histological
sections of tilapia hybrids obtained from numerous other farms in the Jordan valley, some
with histories of massive mortalities, as well as in moribund commercially reared
scalaries (Pterophyllum scalare) in the same region.
Diagnosis
Gross pathological signs in affected fish, were white nodules of varied sizes in all visceral
organs. Isolates from spleen and kidney nodules yielded bacterial growth on brain and
heart infusion +ve yeasts, and Rimmler-Shotts agar. Bacteria were short rods, non-motile,
Gram negative, bipolar, Catalase +ve, Oxidaze weakly +ve. Colonies were
grey/white-yellow, producing acid on triple sugar iron agar. API-20NE tests for reduction
of nitrates to nitrites, Indole and acidification of glucose were positive; all other tests were
negative.
Pathology
Histopathology is very characteristic; variable sized granulomata with a necrotic core
and peripheral layers of macrophages and also, in the later stage, fibroblasts assembling
into a fibrous capsule. Free bacteria aggregate in the periphery and inside the nodule.
Many macrophages contain ingested bacteria. Necrotic nodules persist for a long time
after the systemic bacterial infection subsides.
Epizootiology
Pasteurellosis, occurred in stored overwintering tilapia, at 15–17°C ambient water
temperatures and the abundance of nodules in fish examined from many different farms
in Israel suggests the pathogen is widely distributed.
Several species of Pasteurella were incriminated as the causative agent of morbid diseases in striped bass (Morone saxatilis), white perch (M. americanus) and grey mullet in marine and estuarine habitats, in the USA (Lewis et al., 1970; Paperna & Zwerner, 1976), also in cultured yellowtail and red sea bream in Japan (Kusuda et al., 1978; Yasunaga et al., 1983) and lately in sea cultured European sea bass (Dicentrarchus labrax) and bass (Morone) hybrids in Israel.
A relationship between aetiological agents from tilapia and those from the European sea bass and bass hybrids has yet to be established. P. multocida is a known pathogen of poultry (Bredy & Botzler, 1989), which suggests, if both piscine and avian agents are the same, that chicken manure used to fertilise ponds may be the aetiological source (Nizan & Hammerschlag, 1993).
Species affected and geographical range
Haemorrhagic septicaemia, due to Aeromonas hydrophila and sometimes other gram
negatives (Edwardsiella tarda), has been reported from farmed tilapia in the Mombasa
region of Kenya (Roberts & Sommerville, 1982). Septicaemia, caused by A. hydrophila,
was reported from farmed O. niloticus in Egypt, while the bacterium was also isolated
from the same fish species in the Nile (Amin et al., 1985; Faisal et al., 1984). A variety
of gram negative bacteria, predominantly Aeromonas hydrophila (in 36% of cases) but
also other facultative pathogenic genera or species (Edwardsiella sp. and Yershinia
enterocolitica), were isolated from moribund cultivated tilapia (Oreochromis
mossambicus-hornorum hybrids and O. niloticus) in the lvory coast (J-P. Coquelet,
unpublished report).
A. hydrophila and Pseudomonas putida were isolated from moribund, septicaemic Anguilla mossambica reared in Grahamstown, South Africa (Jackson, 1978).
Aeromonas and Pseudomonas spp. and unidentified gram-negatives were reported to be the cause of high mortalities in reared O. mossambicus, O. niloticus and Tilapia zillii, in the Philippines (Lio-Po et al., 1983) Haemorrhagic septicaemia has been reported from pond cultured tilapia (O. niloticus) in Japan, involving Pseudomonas fluorescens (Miyazaki et al., 1984) and Edwardsiella tarda (Kitao et al., 1980; Miyashita, 1984). The latter, as well as causing acute infections in overwintering farmed tilapia, in Israel, cause a characteristic chronic clinical condition (Paperna, 1984).
Mortality of carp at Kajansi farm, Uganda, caused by extreme dropsy and furunculosis-like lesions in Synodontis afrofisheri in the Volta lake, Ghana, provide further documented evidence of clinical conditions with presumptive bacterial aetiology.
Diagnosis
External and internal gross pathological signs exhibit a similar pattern in all acute
systemic, gram negative, bacterial infections and are therefore not very different from
that observed in acute vibriosis (3.1.2). Routine standard methodologies are available
(Bullock, et al. 1971; Lewis, 1973) for isolation, culture and differential diagnosis of
Aeromonas and other gram-negative facultative bacteria. For biochemical profiles for
the diagnosis of E. tarda see Darunee et al. (1984) and Wakabayashi & Eugusa (1973)
and for Pseudomonas fluorescens see Miyashita (1984).
Pathology
Acute clinical conditions, associated with systemic infections, resulted in mortalities
within 24–48 hours. Tank-reared, two-week-old fry died at a rate of 15% daily. Affected
fish were heavily pigmented, with petechial haemorrhages. In more chronic-type clinical
conditions, eroded fins occur as well as skin lesions and sluggish swimming (Roberts &
Sommerville, 1982; Lio-Po et al., 1983). Internally, the liver is usually pale and there may
be focal haemorrhages over the visceral and peritoneal surfaces. Some reports (Faisal
et al., 1989) also note swelling and haemorrhaging of the spleen and the kidneys and
accumulation of ascitic fluid. Histological changes become distinct only if clinical
conditions are prolonged, being mainly focal cellular necrosis in the liver and
haemopoietic cells, heart and skeletal muscles. Strands of fibrin and bacteria aggregate
at the periphery of the necrotic areas. Cellular inflammatory infiltrate, when present,
consists of macrophages with ingested bacteria or melanosomes (Roberts &
Sommerville, 1982).
In chronic septicaemia caused by Pseudomonas spp. in Japan (Miyazaki et al., 1984) and unidentified gram-negatives (presumably pseudomonads) in overwintering tilapia in Israel and southern Africa (Paperna, 1984), white nodules occurred in the spleen, liver, and kidneys. The Japanese fish had exophthalmia with corneal opacity and an inflamed swimbladder which contained milky fluid. Histology revealed granulomata of epitheloid and encapsulated necrotic lesions. Fibrin strands, bacterial aggregates and infiltrating phagocytic cells, with ingested bacteria, precipitated in the periphery of the granulomata. Large abcesses contained cellular infiltrate of necrotic phagocytes with ingested bacteria. Inflammatory foci also occurred on the gills in the Japanese fish and the swimbladder contents consisted of neutrophil infiltrate and bacteria. Follow up of the overwintering fish showed perpetuation of a granulomatous process even after complete disappearance of the bacteria. With the rise in water temperature, lesions regressed into fibroblast encapsulated necrotic foci.
Epizootiology
Generally, fish are predisposed to systemic gram-negative facultative bacterial infections
by handling trauma or adverse growth conditions such as inadequate feeding, poor water
conditions, overstocking and, outside the tropical region, low temperatures (Roberts &
Sommerville, 1982; Paperna, 1984). Stress-mediated infections, due to handling, usually
lead to acute haemorrhagic septicaemia. In overwintering tilapia farmed outside the
tropical regions a chronic condition with visceral granuloma develops, resulting at times
in losses of up to 98% of the overwintering stock (Paperna, 1984).
Wounds, caused by tags, were the cause of bacterial septicaemia in tilapia brood stocks. A. hydrophila infected lesions, caused by attached epistiliid sessilian ciliates, are known as “red sore” disease (Esch et al., 1976; Overstreet & Howse, 1977).
Some strains of A. hydrophila, E. tarda or species of Pseudomonas are aetiological causes for systemic infection in diverse species of fish (carp, eels and tilapia) (Miyazaki et al., 1984; Darunee et al., 1984; Faisal et al., 1989). Interspecific differences in vulnerability to these facultative bacterial infections reflects the degree of compatibility between fish and their environment (e.g. carp vs. tilapia in overwintering storage) rather than an innate tolerance or susceptibility to a specific bacterial pathogen.
Species affected and geographical range
Myxobacterial skin lesions and gill rot in Africa have been reported from eels reared in
South Africa (Jackson, 1978) and cultured tilapia (Oreochromis niloticus) in Kenya
(Roberts & Sommerville, 1982) and in Egypt (Amin et al., 1988) and outside Africa in
cultured tilapia and their hybrids in Israel. In Southeast Asia catfish of the genus Clarias
develop myxobacterial skin lesions on the back (“saddle back”) and around the mouth
(Lio-Po et al., 1983).
Diagnosis
Myxobacteria of the genus Flexibacter (Cytophaga), including F. columnaris, the
commonest aetiological agent of fin rot and skin lesions (“saddle back”) of freshwater
fish, are readily recognised in direct microscopic examination of a squash of an affected
tissue by their long thin (filamentous) structure. Myxobacteria are isolated on Cytophaga
agar medium (Ancker & Ordal, 1959; Fijan, 1969), re-cultured on Nutrient, 5% sheep
blood, MacConkey and SS agar (Difco) (Amin et al., 1988). Methods of characterisation
of pure isolates were described by Cruickshank et al., (1975).
Pathology
Myxobacterial gill infections, reported from O. niloticus tilapia in Egypt (Amin et al., 1988),
included both acute and chronic clinical conditions. These data were similar to
pathological data on myxobacterial infections in other fish (carp, trout) elsewhere
(Richards and Roberts, 1978). Snieszko (1981) differentiated between the acute necrotic
disease caused by Flexibacter columnaris and the chronic proliferative condition caused
by other Flexibacter or Cytophaga spp. There is also evidence for systemic
myxobacterial infections in fish (Richards & Roberts, 1978).
Gill lesions in the acute clinical condition (usually caused by F. columnaris) are necrotic and often rapidly expanding, and death is more rapid. On the skin, acute lesions are often confined to the head and back (“saddle back”) (Roberts & Sommerville, 1982). Such lesions are white or yellow with a reddish zone of hyperaemia around the periphery and comprise of bacterial cells and necrotic tissue covering haemorrhagic ulcers. Histology reveals epidermal spongiosis and a subsequent necrosis which extends into the dermis. Chronic myxobacterial infections cause extensive hyperplasia in the gills, with resulting fusion of the lamellae and sometimes also with proliferation of mucus glands and chloride cells. Proliferation in the skin occurs at the tips of fins and at skin folds.
Other pathological manifestations of myxobacterial infection reported elsewhere, which might also be relevant to African fish are as follows (see Richards & Roberts, 1978):
Fin rot condition has been reported only from fish outside Africa (Richards & Roberts, 1978). It induces a severe epidermal and dermal oedema, a fibrinous exudate overlaid with cytophaga bacteria, and cellular exudates, with subsequent sloughing and progressive erosion of the fins. Myxobacteria may be displaced by secondary opportunistic saprophytic bacteria.
Cotton wool disease, affecting tropical aquarium fish, is suggested to result from irritation of gill lamellae, producing catarrhal exudate over the gills which serves as an attractive substance for cytophaga proliferation (Cawley, MSc. thesis, 1975, quoted by Richards & Roberts, 1978).
Epizootiology
Myxobacteria are ubiquitous opportunists of the aquatic habitat. Many, particularly those
causing gill and skin rot conditions, will colonise only damaged or ulcerating integument,
necrotic tissue or irritated mucus excreting epithelium (cotton wool disease). Acute gill
and skin infections are most often associated with handling, or mechanical damage of
skin and gills in hauling and sorting. Adverse ambient conditions - low temperatures,
excessive organic load, ammonia or nitrites, are important contributory factors in such
situations. The same environmental parameters, as well as other adverse growing
conditions (overcrowding and inadequate feeding or nutrition), predispose fish to chronic
myxobacterial diseases. Dispersed toxic substances may play an important role in the
aetiology of chronic myxobacterial infections (tail rot etc.).
Antibiotic therapy by use of medicated feeds appears to be a feasible undertaking, as long as the infected fish are still willing to eat.
Drug sensitivity tests of the pathogen targeted for treatment must be repeatedly performed to ensure efficacy of antibiotics added to feeds. Application of medicated feeds, as a non-specific prophylactic measure, is economically wasteful, harmful to the environment (damaging nitrification processes and primary production) and promotes drug resistance amongst pathogens in the habitat.
In the case of streptococcal infection, use of medicated feeds in polyculture is not cost-effective as carp, which always comprise the greater portion of the pond biomass, are refractory to infection.
Vaccination has been repeatedly considered as a potential solution and some research is in progress. There are, however, serious doubts as to how effective this vaccination may be and whether it will be economically worthwhile. The feasibility of vaccination use will largely depend on the degree and durability of protection afforded by immersion vaccination or via feeds. Specific immune response has been induced in tilapia to various antigens (Sailendri & Muthukkaruppan, 1975) and to some bacterial infections. Elevation of antibody titers to a level of 100% protection to challenges during two to five weeks was obtained in Nile tilapia (O. niloticus) following injection of formalin-killed A. hydrophila and Freunds complement adjuvant vaccines (Ruangapan et al., 1986). Attempts to vaccinate reared tilapia (O. niloticus) fingerlings with lyophilized Aeromonas hydrophila in the Ivory Coast were, however, unsatisfactory (J-P. Coquelet, unpublished report).
Serum agglutinating antibody titer of tilapia hybrids rose following injection with a V. parahemoliticus bacterin both with and without Freunds complement adjuvant, while titers for naturally occurring antibodies in the overwintering fish were usually low. Immunisation trials by immersion showed significant efficacy in protecting tilapia from a challenge for about 60 days (Hubbert, 1989). Neither significant elevation in agglutinin titers nor protection against challenge was obtained in Nile tilapia immunised with formalin-killed Edwardsiella tarda, using the hyperosmotic infiltration method (Loi-Po & Wakabayashi, 1986). lida et al. (1982) and Kusuda & Tagaki (1983) found that vaccination using toxoid, rather than bacterin, elicited a greater antibody response and they recommended the use of detoxified endotoxin in streptococcal infections of yellowtail.
There is a serious risk of introducing bacterial infections into Africa (notably Streptococcus, Pasteurella and mycobacteria; see 3.2), which are prevalent in countries outside Africa specialising in tilapia culture, with genetically improved culture seed - breeders, fry and apparently also eggs. Only an adequately enforced ban on such imports will secure African habitats from these infections.
Species affected and geographical range
Reported from many marine and freshwater fish species and also fish families occurring
in Africa (Cichlidae, Characidae) (Nigrelli & Vogel, 1963; Noga et al. 1990). There are
several case reports from African cichlid species; from Sarotherodon andersonii and T.
sparmanii in the Okavango swamps in Botswana (Roberts & Matthiesen 1979 report,
quoted in Roberts & Sommerville, 1982), farmed O. niloticus in Kenya (Roberts &
Sommerville 1982) and also from aquarium reared Haplochromis multicolor,
Hemichromis bimaculatus (Nigrelli & Vogel, 1963) and Oreochromis mossambicus
(Noga et al., 1990).
Mycobacterial infections seen in grey mullet (Mugilidae) entering inland waters in Israel (Paperna, et al., 1994) are likely to be of marine origin. Mycobacterial infections have also been reported from ornamental cyprinids, and exotic tropical fish reared in Malaysia (Shamsudin et al., 1990). Mycobacterium spp. isolated from fish are also infectious to humans (Nigrelli & Vogel, 1963; Anon., 1992).
Nocardiasis has not yet been reported from African fish, although, it has been reported in tropical fish from South America (Hyphessobrycon innesi - Valdez & Conroy, 1963) and Southeast Asia (Gourami, Trichogaster trichopterus - Paperna, unpublished).
Diagnosis
In piscine tuberculosis white nodules occur in all visceral organs, but mainly in the spleen,
which also usually becomes enlarged. Similar granulomatous nodules occur in the
viscera of fish with nocardial infection. Skin lesions occur only in some cases of
tuberculosis, but are characteristic to nocaridian infections. Positive identification of
mycobacteria is by acid fast positive staining with Ziehl-Nielson (or other modifications
for acid fast staining). Some Nocardia are acid fast positive. Both Mycobacteria and
Nocardia are gram-positive but the latter stain more distinctly than the former.
Mycobacteria are short rods, but may be filamentous or branching and therefore
confused with acid fast positive Nocardia, which is a pleomorphic actinomycete forming
both hyphae, bacilli and cocci. Identification to species requires culture and biochemical
characterisation; mycobacteria are fastidious and need special media, whereas
Nocardia grow well on nutrient agar. Molecular biology methodology has recently been
introduced for differential diagnosis of piscine mycobacteria (Knibb et al., 1993).
Pathology
Spleen and kidneys usually contained the highest concentration of mycobacterial
tuberculi. Visceral granulomatous lesions were comprised of either epitheloid, with a
core of degenerating cells, or necrotic nodules enclosed in epitheloid gradually replaced
by encapsulating fibroblasts; the cores contained acid fast staining bacteria. Systemic
granulomata of Nocardia demonstrate similar configurations.
The mycobacterial infection reported from O. mossambicus, by Noga et al. (1990), was atypical. The multiple skin lesions were comprised of single nodules or aggregates of small nodules of vacuolated inflammatory cells. Dermal aggregates extended into the epithelial layer. Lymphocytes as well as pigment cells were aggregating around the lesions, resulting in heavy melanization of the skin. Visceral lesions were small and contained mostly pigmented and non pigmented chronic inflammatory cells.
In nocardial infections, deep, ulcerating, sometimes haemorrhagic lesions develop in the skin, from which nocardial bacteria are readily isolated.
Epizootiology
Infection, in cultured marine fish, may reach epizootic proportions (Colorni, 1992; Anon.,
1992). In culture installations, infection spreads apparently via necrophagy of diseased
fish. Feeding on trash fish is a potential source for introduction of mycobacterial infection
into farmed stocks. Transovarian transmission was demonstrated only in viviparous fish
(Nigrelli & Vogel, 1963). There is no supporting evidence for transmission via processed
feeds. The data are, however, too sporadic for a comprehensive epizootiological
evaluation.
Control
Both the relatively high tolerance to antibiotics, demonstrated from in vitro drug sensitivity
tests, and the granulomatous nature of the pathological process, contribute to the
experienced low efficacy of applied drugs for cure of both piscine tuberculosis (Colorni,
1992) and nocardial infections. Avoiding feeding of cultured fish with untreated marine
products seems to be an important precautionary measure.
Species affected and geographical range
Occurs in both freshwater and marine fish and is widespread in all cichlid species in
southern Africa, Kenya and Israel, in the common carp (“Mucophilus” agent) and in the
Mediterranean and Red Sea grey mullets (Mugilidae) (Paperna & Sabnai, 1980; Paperna
et al., 1981; Paperna & Alves de Matos, 1984).
Diagnosis
Epitheliocystis is chlamydial organisms invading and causing gross hypertrophy of
integumental epithelial cells, mostly of the gills; lining and respiratory cells as well as
mucus and chloride cells. Infected cells grow into transparent (up to 100 × 55 μm in size)
bodies with fine granular contents. Transmission electron microscopy reveals
pleomorphic (round, rods and cocci) prokaryotic organisms bound with a trilaminated
membrane and containing a nucleoid. Epitheliocystis organisms thus far have not been
cultured in vitro.
Life history and biology
Invaded epithelial cells grow gradually into a grossly hypertrophic body. Organisms are
secluded within an inclusion which expands with the increase in the bacterial mass. The
border of the inclusion is lined by deformed host cell organelles: a network of
microtubules or microfibrils or residue of mucus droplets. Through successive binary
divisions, or budding, round chlamydia-like organisms (averaging 0.7μm in diameter)
form branching chains which further transform into rickettsia-like rods (1–2μm long),
single or attached into chains, which finally split into cocci (0.5 × 0.3μm in size) with
condensed cytoplasm, which are the presumed dispersing infective stages. The latter
are released into the water with the collapse of the host cell. This stage is structurally,
as well as functionally, reminiscent of the chlamydial elementary bodies (Paperna &
Sabnai, 1980; Paperna et al. 1981). Epitheliocystis from different fish host species may
vary in the morphology of individual stages and demonstrate additional stages with
distinct morphology and division patterns (in carp - Paperna & Alves de Matos, 1984).
Pathology
In benign infections, tissue changes are limited to the formation of a thin capsular
structure around the hypertrophic cell, the respiratory capillaries may send extensions
to the surface of the infected cell. The “proliferative” condition results in massive epithelial
hyperplasia which may embed the hypertrophic infected cell as well as part or all of the
gill lamellae of the filament. In mullets and carp, the mere overload of the gills with
hypertrophic cells caused severe erosion of the gill architecture, which interferes with
the respiratory function (Paperna, 1977; Paperna & Sabnai, 1980).
Epizootiology
Infection spreads and accelerates into severe hyperinfection very quickly in fry and
fingerlings of grey mullet and carp held in overcrowded tanks, but thus far not in young
tilapia, which maintain benign infection even in adverse growth conditions.
After the climax, in surviving carp and grey mullets (and marine spariids) infection spontaneously subsides to become benign and low level (Paperna & Sabnai, 1980).
Control
Applications of antibiotics have, thus far, proved to be ineffective. The proliferative
condition may be mediated through immediate reduction of stocking densities.
REFERENCES
Amin, N.E., Abdallah, I., Faisal, M., Easa, M. El-S., Alaway, T. & Alyan, S.A., 1988. Columnaris infection among cultured Nile tilapia Oreochromis niloticus. Antonie van Leeuveen., 54: 509–520.
Amin, N.E., Abdallah, I., Ellawy, T. & Ahmed, S.M., 1985. Motile aeromonas septicaemia among T. nilotica (Sarotherodon niloticus) in Upper Egypt. Fish pathol., 20: 93–97.
Anacker, R.L. & Ordal, E.J., 1959. Studies of the myxobacterium Chondrococcus columnaris. I. Serological typing. J. Bacteriol., 78: 25–32.
Anon., 1992. Bacteria problem closes US farm. Fish Farming internat., 19: No. 6 (June).
Barton, B. A. & Iwama, G.K., 1991. Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annual Rev. Fish Dis., 1: 3–26.
Bredy J.P. & Botzler, R.G., 1989. The effect of six environmental variables on Pasteurella multocida population in water. J. Wildl. Dis., 25: 232–239.
Bullock G.L., Conroy, D.A. & Snieszko, S.F., 1971. Bacterial diseases of Fishes. Book 2A. In: Snieszko, S.F. & Axelrod, H.R. (eds.) Diseases of Fishes. T.F.H. publ. Inc. Neptune City, N.J., 151pp.
Colorni, A., 1992. A systemic mycobacteriosis in the european sea bass Dicentrarchus labrax cultured in Eilat (Red Sea). Israeli J. Aquacult. (Bamidgeh), 44: 75–81.
Cruickshank, R., Duguid, J.P., Marmion, B.P. & Swain, R.H., 1975. Text book of Medical Microbiology. 12 edn. Churchill Livington, Edinb., Lond., N.Y.
Darunee S-O, Muroga, K. & Nakai, T., 1984. A case of Edwardsiella tarda infection in cultured coloured carp Cyprinus carpio. Fish Pathol., 19: 197–199.
Eldar, A., Bejerano, J. & Bercovier, H., 1994. Streptococcus shiloi and Streptococcus difficile: two new streptococcal species causing a meningoencephalitis in fish. Curr. Microbiol., 28: 139–143.
Esch, G.H., Hazen., T.C., Dimock, R.V. & Gibbons, J.W., 1976. Thermal effluent and the epizootiology of the ciliate Epistilis and the bacterium Aeromonas in association with centrarchid fish. Trans. Am. Microsc. Soc., 95: 687–693.
Faisal, M., Abdelhamid, H.S., Torky, H., Soliman, M.K. & Abu Elwafaa, N., 1984. Distribution of Aeromonas hydrophila in organs and blood of naturally and experimentally infected Oreochromis niloticus. J. Egypt. Vet. Med. Assoc., 44: 11–20.
Faisal, M., Popp, W. & Refai, M., 1989. Aeromonas hydrophila -bediingte septikamie bei der niletilapia Oreochromis niloticus. Berl. Munch. Tieartl. Wschr., 102: 1087–1093.
Fijan, N., 1969. Antibiotic additive for the isolation of Chondrococcus columnaris from fish. Appl. Microbiol., 17: 333–339.
Hubbert, R.M., 1989. Bacterial diseases in warmwater aquaculture. In: Shilo, M. & Sarig, S. (eds.) Fish Culture in Warm Water Systems: Problems and Trends. CRC Press, Boca Raton, Florida. pp. 179–194.
Iida, T., Wakabayashi, H. & Yoshida, T., 1982. Vaccination for control of streptococcal disease in cultured yellowtail. Fish. Pathol. 16: 201–206.
Jackson, P.B.N., 1978. Health and disease in intensive aquaculture. J. S.A. Vet. Ass., 49: 57–59.
Kitao, T., Aoki, T. & Sakoh, R., 1981. Epizootic in cultured freshwater fish caused by beta-hemolytic streptococcus species. Fish Pathol., 15: 301–307.
Kitao, T., Aoki, T., Tawara, K., Kumada, K., Shiomitsu, K. & Fukudome, M., 1980. On an edwardsiellosis in tilapia. Abstract, Annual meeting Jap. Soc. Sci. Fish. (April), p.82.
Knibb, W., Colorni, A., Anakaoua, M., Lindell, D., Diamant, A. & Gordin, H., 1993. Detection and identification of a pathogenic marine mycobacterium from the European seabass Dicentrarchus labrax using polymerase chain reaction and direct sequencing of 16S rDNA sequences. Molecul. Mar. Biol. Biothechnol. 2: 225–232.
Kusuda, R., Kawai, K. & Masui, T., 1978. Etiological studies on bacterial pseudotuberculosis in cultured yellowtail with Pasteurella piscicida as a causative agent -II. On the serological properties. Fish Pathol., 13: 79–83.
Kusuda, R. & Tagaki, S., 1983. Antibody production against Streptococcus spp. in naturally infected yellowtail Seriola quinqueradiata. Rep. USA Mar. Biol. Inst. Kochi. Univ. Japan, 5:21.
Lewis, D., Grumble, L.C., McConnel, S. & Flowers, A.I., 1970. Pastorella-like bacteria from a epizootic in menhaden and grey mullet in Galveston Bay. J. Wildl. Dis., 6: 160–162.
Lewis, D.H., 1973. Predominant aerobic bacteria of fish and shellfish. Texas A&M University, 102 pp.
Lio-Po G. & Wakabayashi H., 1986. Immuno-response in tilapia Sarotherodon niloticus vaccinated with Edwardsiella tarda by hyperosmotic infiltration method. Veterin. Imm. Immunopath., 12: 351–357.
Lio-Po, G.D., Pascual, J.P. & Santos, J.G., 1983. Philippines. Fish Quarantine and fish diseases in southeast Asia, report of a workshop held in Jakarta, Indonesia, 7–10 Dec. 1982. Ottawa Ont. IDRC (79pp.), 35–46.
Mazeaud, M.M., Maseaud, F. & Donaldson, E.M., 1977. Primary and secondary effects of stress in fish: some new data with general review. Trans. Am. Fish. Soc., 106: 201–212.
Miyashita, T., 1984. Pseudomonas fluorescens and Edwardsiella tarda isolated from diseased tilapia. Fish Pathol., 19: 45–50.
Miyazaki, T., Kubota, S.K. & Miyashita, T., 1984. A histopathological study of Pseudomonas fluorescens infection in tilapia. Fish Pathol., 19: 161–166.
Muroga K, Yo, Y. & Nishibuchi, M., 1976. Pathogenic Vibrio isolated from cultured eels -I. Characteristics and taxonomic status. Fish Pathol., 11: 141–145.
Nigrelli, R.F. & Vogel, H., 1963. Spontaneous tuberculosis in fishes and in other cold blooded vertebrates with special reference to Mycobacterium fortuitum Cruz from fish and human lesions. Zoologica, 48: 131–144.
Nizan, S. & Hammerschlag E., 1993. First report of pasteurellosis in freshwater hybrid tilapia (Oreochromis aureus x O.niloticus) in Israel. Bull. Eur. Ass. Fish Pathol., 13: 179–180.
Noga, E.J., Wright, J.F. & Pasarell, L., 1990. Some unusual features of Mycobacteriosis in the cichlid fish Oreochromis mossambicus. J. Comp. Pathol., 102: 335–344.
Overstreet, R.M. & Howse, H.D., 1977. Some parasites and diseases of estuarine fishes in polluted habitats of Mississippi. Ann. N.Y. Acad. Sci., 298: 427–462.
Paperna, I., 1977. Epitheliocystis infection in wild and cultured sea bream (Sparus auratus, Sparidae) and grey mullets (Liza ramada Mugilidae). Aquaculture, 10: 169–176.
Paperna, I., 1984. Winter diseases of cultured tilapia. In: Acuigrup Spain (ed.) Fish Diseases. Fourth COPRAQ Session. Editora ATP. Madrid, pp. 139–147.
Paperna, I. & Alves de Matos, A.P., 1984. The developmental cycle of epitheliocystis in carp, Cyprinus carpio L. J. Fish Dis., 7: 137–147.
Paperna, I., Markus, E.E., & Gerasi, L., 1994. Natural reservoirs for pathogens of maricultured fishes. Sixth internat. Colloq. Pathol. Mar. Aquacult. Montpellier, France - Abstract (unpublished).
Paperna, I. & Sabnai, I., 1980. Epitheliocystis disease in Fishes. In: Ahne, W. (ed.) Fish Diseases, Third COPRAQ-Session, Springer-Verlag Berlin, Heidelberg 228–234.
Paperna, I., Sabnai, I. & Zachary, A., 1981. Ultrastructural studies in piscine epitheliocystis: evidence for a pleomorphic developmental cycle. J. Fish Dis., 4: 459–472.
Paperna, I. & Zwerner, D.E., 1976. Parasites and diseases of striped bass, Morone saxatilis (Walbaum) from the lower Chesapeake bay. J. Fish Biol., 9: 267–287.
Richards, R.H. & Roberts, R.J., 1978. The bacteriology of teleosts. In: Roberts, R.J. (ed.) Fish Pathology. Bailliere, Tindall, London. pp. 183–204.
Roberts, R.J. & Sommerville, C., 1982. Diseases of tilapias. In: Pullin R.S.V. & Lowe-McConnel R.H. (eds.) The Biology and Culture of Tilapia. ICLARM conference proceeding, Manila, Philippines. pp. 247–263.
Ruangpan L., Kitao, T. & Yoshida, T., 1986. Protective efficacy of Aeromonas hydrophila vaccines in nile tilapia. Veterin. Imm. Immunopath., 12: 345–350.
Sailendri, K. & Muthukkaruppan, V.R., 1975. The immune response of the teleost, Tilapia mossambica to soluble and cellular antigens. J. Exp. Zool., 191: 371–382.
Sakata T. & Hattori, M., 1988. Characteristics of Vibrio vulnificus isolated from diseased tilapia. Fish Pathol., 23: 33–40.
Shamsudin, M.H., Tajima, K., Kimura, T., Shariff, M. & Anderson, I.G., 1990. Characterization of the causative organisms of ornamental fish mycobacteriosis in Malaysia. Fish Pathol., 25: 1–6.
Snieszko, S.F., 1974. The effect of environmental stress on outbreaks of infectious diseases of fishes. J. Fish Biol., 6: 197–208.
Snieszko, S.F., 1981. Bacterial gill diseases of freshwater fish. Fish Wildl. Ser. Fish Disease Leaflet, 62: 11pp.
Taniguchi, M., 1982. Experiments on preoral inoculation via food to induce yellowtail streptococcosis. Bull. Jap. Soc. Sci. Fish., 48: 1721–1723.
Tung Ming-Chen, Shin-Chu Chen & Shin-Shyong Tsai, 1985. General septicaemia of streptococcal infection in cage cultured tilapia, Tilapia mossambica, in southern Taiwan. CAO Fisheries series No. 4, Fish Disease Research (VII) 12: 95–105.
Valdez, I. & Conroy, D.A., 1963. The study of a tuberculosis -like condition in neon tetras (Hyphessobrycon innesi). 2. Characteristics of the bacterium isolated. Microbiol. Espan., 16: 249–253.
Wakabayashi, H. & Eugusa, S., 1973. Edwardsiella tarda (Paracolobacterium anguillimortiferum) associated with pond-cultured eel disease. Bull. Jap. Soc. Sci. Fish., 39: 931–936.
Yasunaga, N., Hatai, K. & Tsukahara, J., 1983. Pasteurella piscicida from an epizootic of cultured red sea bream. Fish Pathol., 18: 107–110.
ILLUSTRATIONS
Plate 3. Bacterial infections: a,b. Dropsy in carp from Kajansi farm, Uganda (× 0.8). c. Exophthalmus and cornea hyperplasia in eyes of Oreochromis sp. from Kajansi farm, Uganda (× 2.5). d. Ulcerating skin lesions in Synodontis afrofisheri from north Lake Victoria (× 0.8). e. Vibrio parahemoliticus (arrow) in kidney blood of farmed tilapia hybrid in Israel. f. V. parahemoliticus (arrow) in macrophage aggregate in spleen of a fish of the same group as e. g. Inflammatory lesions in winter stored tilapia hybrid with Pseudomonas sp. as presumptive causative agent. h–g. Histopathological process in tilapia hybrid spleen (h,j) and liver (i) due to pasteurellosis, from granulomatous lesion to liquifactious necrosis.
Plate 4. Bacterial infections continued: a-g. Pasteurellosis in cichlid fishes: a. Hepatocyte degeneration in tilapia-hybrid liver. b. degeneration and death of cells invaded by the bacteria; c,d. the resulting disintegration of the tissue due to infection (same fish). e–g. Pasteurellosis in angel fish (Pterophyllum scalare), showing nodular, bacteria loaded (arrows) lesions (abcesses) with gradually disintegrating macrophage-like cells. h,i. Epitheloid granulomatous lesions with acid fast positive bacteria (Mycobacteria) (arrows) in estuarine grey mullets from Israel (E, epitheloid cells; F, loose outer and, Fi, dense inner layers of fibroblasts; L, surrounding spleen tissue).
Plate 5. Epitheliocystis: a,b. Hypertrophic epitheliocystis infected cells on gills of Oreochromis aureus, Israel. c. Low mag. transmission electron microscopic (TEM) view of hypertrophic epitheliocystis infected cells (approx. 27 μm in diam) in gills of carp, Israel. d. Hyperinfected gill of Mugil cephalus, Israel. e–h. TEM view of epitheliocystis organisms: e. dividing round bodies (0.7 μm in diam.) and f,h. rods or elongate bodies (1–2 μm long) (same arrows), and cocci (elementary bodies) (0.5 × 0.3 μm) (open arrows) of Oreochromis Epitheliocystis; g, round bodies of carp epitheliocystis (0.4–0.8μm in diam) (W- host cell wall).
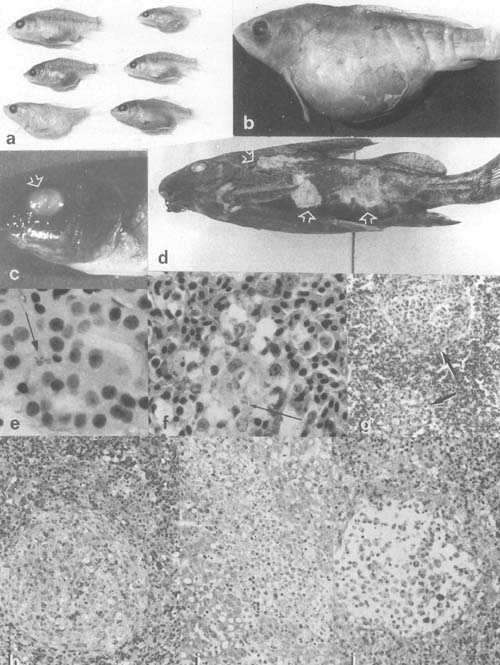
Plate 3. Bacterial infections (legend p. 25)
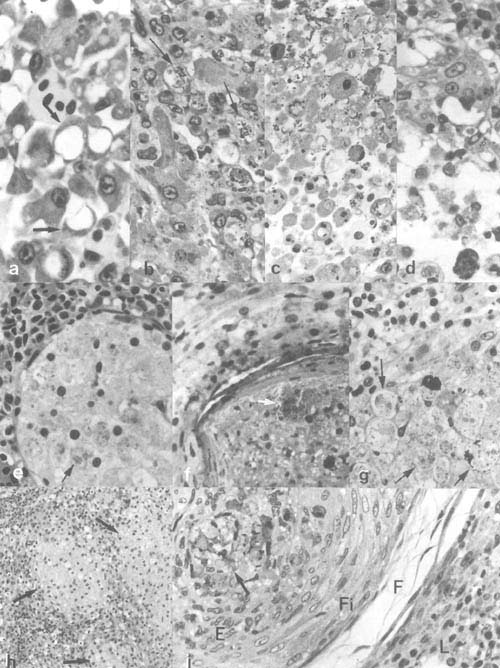
Plate 4. Bacterial infections continued (legend p. 25)
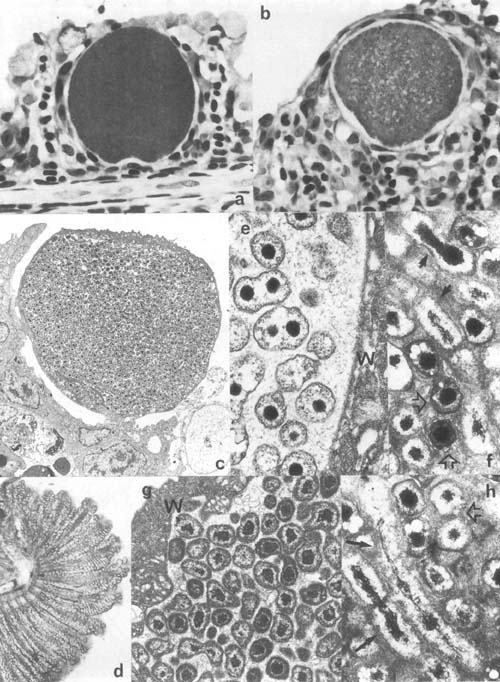
Plate 5. Epitheliocystis (legend p. 25)