3.1. Description of the genus and species
3.2. Habitat
3.3. Natural food and feeding
3.4. Natural reproduction
3.5. Oocyte development
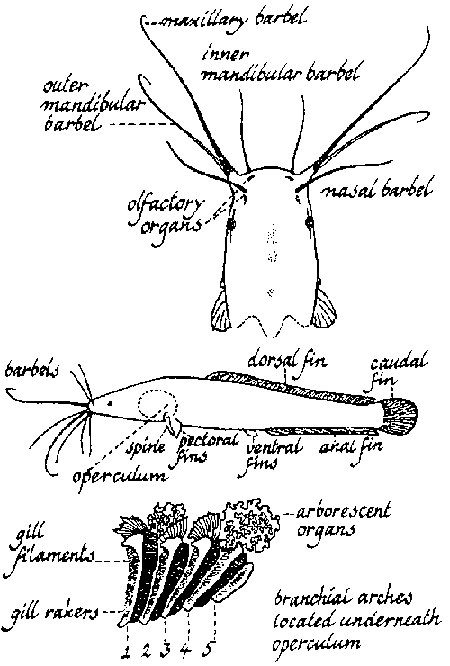
The catfish genus can be defined as displaying an eel shape, having an elongated cylindrical body with dorsal and anal fins being extremely long (nearly reaching or reaching the caudal fin) with both fins containing only soft fin rays (Figure 3). The outer pectoral ray is in the form of a spine and the pelvic fin normally has six soft trays. The head is flattened, highly ossified, the skull bones (above and on the sides) forming a casque and the body is covered with a smooth, scaleless skin. The skin is generally darkly pigmented on the dorsal and lateral parts of the body. The colour is uniformly marbled and changes from greyish olive to blackish according to the substrate. On exposure to light the skin colour generally becomes lighter.
They have four pairs of unbranched barbels, one nasal, one maxillar (longest and most mobile) on the vomer and two mandibulars (inner and outer) on the jaw. Tooth plates are present on the jaws as well as on the vomer. The major function of the barbels is for prey detection.
A supra-branchial or accessory respiratory organ, composed of a paired pear-shaped air-chamber containing two arborescent structures is generally present. These arborescent or cauliflower-like structures located on the fourth branchial arcs, are supported by cartilage and covered by highly vascularised tissue which can absorb oxygen directly from the atmosphere (Moussa, 1956). Since the air chamber communicates directly with the pharynx and the gill-chamber, this accessory air breathing organ allows the fish to survive out of water for many hours or for many weeks in muddy marshes.
Figure 3. Morphological characteristics of C. gariepinus.
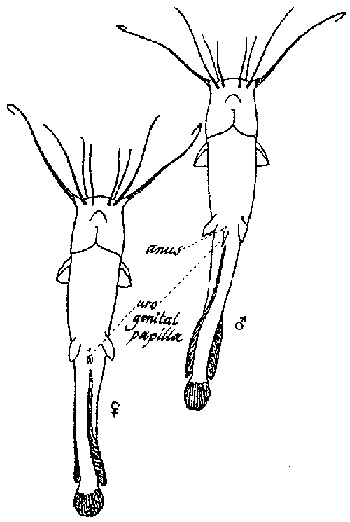
The male and females of C. gariepinus can be easily recognized as the male has a distinct sexual papilla, located just behind the anus. This sexual papilla is absent in females (Figure 4).
Figure 4. Sexual characteristics of C. gariepinus
Clarias spp. inhabit calm waters from lakes, streams, rivers, swamps to floodplains, many of which are subject to seasonal drying. The most common habitats frequented are floodplain swamps and pools in which the catfish can survive during the dry seasons due to the presence of their accessory air breathing organs (Bruton, 1979a; Clay, 1979).
Although numerous studies on the food composition of C. gariepinus have been carried out, a consistent pattern has not emerged and they are generally classified as omnivores or predators. Micha (1973) examined catfishes from the River Ubangui (Central African Republic) and found that C. lazera (= C. gariepinus) fed mainly on aquatic insects, fish and higher plant debris. They have been found to feed on terrestrial insects, molluscs and fruits.
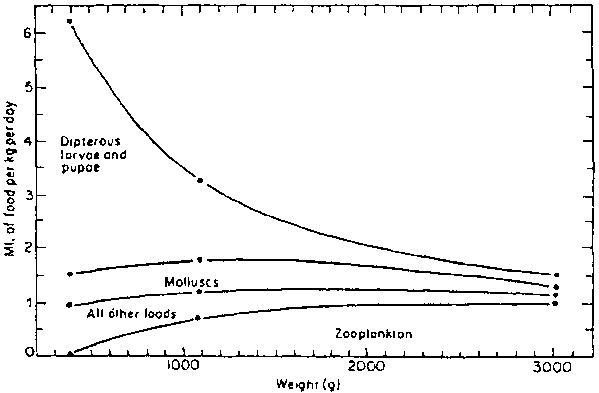
Similarly, Bruton (1979b) found that catfish in Lake Sibaya (South Africa) fed mainly on fish or crustacea, and that terrestrial and aquatic insects were an important part of the diet of juvenile and adult fish which inhabit shallow areas. However, molluscs, diatoms, arachnids, and plant debris were the minor food items consumed in this lake. Munro (1967) studied the feeding habits of C. gariepinus in Lake McIIwaine (Zimbabwe) and found that feed composition changed as fish became larger; Diptera, particularly chironomid pupae, predominating in the diet of the smaller fish and becoming progressively less important with increasing size. Zooplankton became more important with increasing fish size and predominated in the diet of the largest fish. Most of the minor food groups also showed a progressive increase or decrease in importance in relation to increasing size (Figure 5). The greater importance of zooplankton in the diet of large fish was believed to be due to the increased gape and number of gill rakers of the larger fish (Jubb, 1961; Groenewald, 1964); presumably resulting in more efficient filter feeding.
Figure 5. Apparent changes in the composition of the mean daily ration of C. gariepinus in relation to increasing size. Source: Munro, 1967.
Spataru et al. (1987) studied the feeding habits of C. gariepinus in Lake Kinneret (Israel) and found that preyed fish were the most abundant food component (81%) and constituted the highest biomass.
In conclusion, we can consider C. gariepinus as a slow-moving omnivorous predatory fish which feeds on a variety of food items from microscopic zooplankton to fish half its length or 10% of its own body weight.
In order to feed on this wide variety of food organisms in different situations C. gariepinus is equipped with a wide array of anatomical adaptations for feeding under low visibility (Bruton, 1979b) including:
· A wide mouth capable of considerable vertical displacement for engulfing large prey or large volumes of water during filter feeding.Slow, methodical searching is the normal predatory tactic of C. gariepinus, with catfish grasping their prey by suction; a negative pressure (suction) being created by a sudden increase of the bucco-pharyngeal chamber.· A broad band of recurved teeth on the jaws and pharyngeal teeth preventing prey from escaping.
· An abundant network of sensory organs on the body, head, lips and circumoral barbels. These barbels are extensively used for prey detection and fixation. Hecht and Applebaum (1988) found that C. gariepinus with barbels were 22.6% more efficient at catching prey than those without. This would indicate that tactile behaviour is important in the prey catching processes.
· A wide, rounded, caudal fin, typical of fish which ambush their prey.
· Long gill rakers on the five branchial arches.
· A short and dilatable oesophagus which opens into a distinct muscular stomach (mechanical digestion) and a simple thin walled intestine.
An important aspect of predation by C. gariepinus is their ability to switch feeding from one type of prey to another. In Lake Sibaya (South Africa), catfish ignore (or cannot catch) fish prey during daylight and feed mainly on invertebrates, which are abundant and relatively easy to catch. By contrast, at night, when fish prey become more vulnerable, they switch their feeding habits to fish prey (Bruton, 1979b). In general, fish prey provides far more energy per unit weight than other prey items. However, switching feeding habits relies on the existence of at least two alternate abundant preys.
C. gariepinus shows a seasonal gonadal maturation which is usually associated with the rainy season. The maturation processes of C. gariepinus are influenced by annual changes in water temperature and photoperiodicity and the final triggering of spawning is caused by a rise in water level due to rainfall (de Graaf et al., 1995).
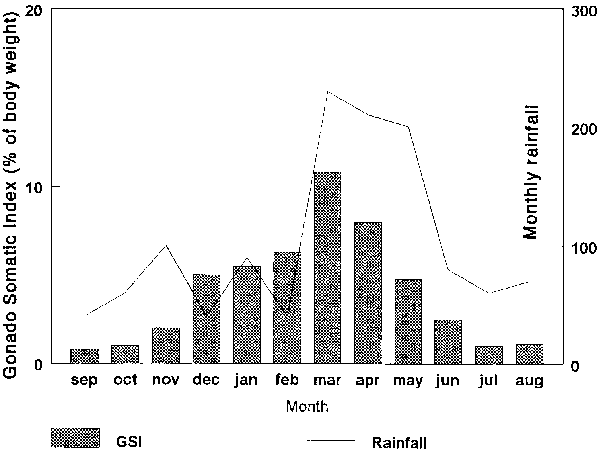
An example of the maturation and spawning cycle of C. gariepinus in Lake Victoria (Kenya) is presented in Figure 6; reproduction starting in March just after the start of the first heavy rains (as indicated by the decrease in the Gonado Somatic Index1, G.S.I.). Natural reproduction is generally completed in July with the G.S.I. remaining low until November, thereafter the oocytes gradually maturing and becoming ripe again in March.
1 GSI = {weight ovary/total weight}*100
Figure 6. The Gonado Somatic Index (% of body weight) of C. Mossambicus (= C. gariepinus) and the monthly registered rainfall (mm), Nyanza Gulf, Lake Victoria, Kenya. (After Owiti and Dadzie, 1989).
Spawning usually takes place at night in the shallow inundated areas of rivers, lakes and streams. Courtship is usually preceded by highly aggressive encounters between males. Courtship and mating takes place in shallow waters between isolated pairs of males and females. The mating posture, a form of amplexus (the male lies in a U-shape curved around the head of the female) is held for several seconds (see Figure 7). A batch of milt and eggs is released followed by a vigorous swish of the female’s tail to distribute the eggs over a wide area. The pair usually rest after mating (from a few seconds to up to several minutes) and then usually resume mating.
Figure 7. The courtship ritual of C. Gariepinus. Source: Bruton, 1979a.
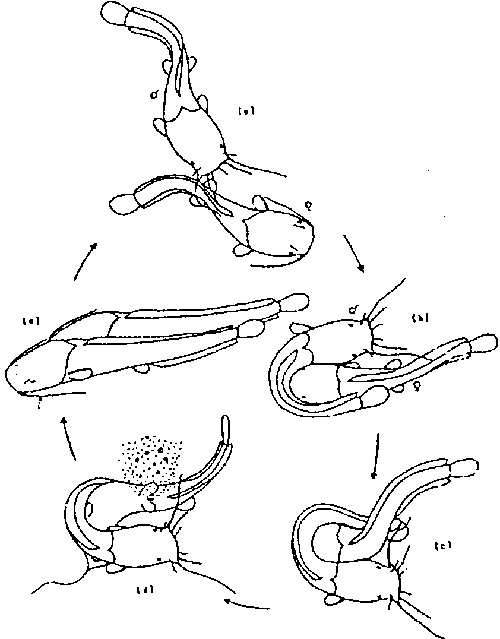
There is no parental care for ensuring the survival of the catfish offspring except by the careful choice of a suitable site. Development of eggs and larvae is rapid and the larvae are usually capable of swimming within 48-72 hours after fertilization at 23-28oC.
The development of the oocytes of the African catfish is mostly related to temperature (as is common with a large number of fish species). Within the development of the oocyte six chronological stages2 can be seen (Owiti and Dadzie, 1989; see Figure 8).
2 The photographs are provided by the Department of Inland Fisheries and Aquaculture, Wageningen, the NetherlandsFigure 8. Histological section of an ovary of C. gariepinus, with the different stages of oocyte development.
Stage 1, Immature virgin:
Macroscopic description: The ovary is colourless to translucent brown, lanceolate and lobular in appearance, occupying the posterior quarter of the body cavity. In fish larger than 10 cm the ovary can be distinguished from the testis due to its smoothness in contrast to the serrated edges of the testis.
Histological description: Pre-vitellogenesis3 stage or primary oocytes. The oocyte are small (7-10 micron) and contain no yolk. The number of primary oocytes increases through mitotic division.
3 Vitellogenesis is the process of yolk formationStage 2, Developing virgin:
Macroscopic description: The ovary is translucent, brown in colour and occupies about one third of the length of the peritoneal cavity. Individual oocytes are visible with the naked eye as tiny specks.
Histological description: Pre-vitellogenesis stage or primary oocytes. The oocyte are small (7-10 micron) and do not yet contain yolk. The number of primary oocytes increases through mitotic division and at the end of this stage the oocyte increase its size to approximately 200 micron.
Stage 3, Ripening:
Macroscopic description: The ovary is opaque, brownish-green in colour, occupying about one half the length of the ventral cavity. Eggs are visible as yellowish-green or brownish-yellow granules and blood capillaries visible around the ovary.
Histological description: Endogenous vitellogenesis stage. Within this stage the yolk of the oocyte (the future reserve/feed for the hatched larvae) is formed. The origin of the yolk in this stage is the oocyte itself.
Stage 4, Maturing or ripe:
Macroscopic description: The ovary is large, opaque, and brown-green in colour. The eggs are yolk laden and clearly visible to the naked eye. The ovary occupies four-fifths of the peritoneal cavity. A highly developed capillary network is visible. Eggs ooze out freely with pressure on the belly.
Histological description: Exogenous vitellogenesis. The oocyte increases to its final size of 1000-1200 micron (1-1.2 mm). During this phase yolk formation in the oocyte increases and the origin of the proteins needed for this process is outside the oocyte (the liver). A large nucleus (0.2 mm) is clearly visible a little outside of the centre of the oocyte (See Figure 9). The oocytes at this stage are also called “ripe eggs”. They remain in this stage until environmental factors (rainfall and water level rise or a hormonal injection) stimulate their ovulation.
Stage 5, Running or spawning:
Macroscopic description: The eggs are translucent, flat, with cytoplasm concentrated at the animal pole and visible as a reddish brown spherical cap (Figure 10). This aspect is quite distinct from the round eggs present in the ovaries before reproduction/hypophysation (Figure 9).
Figure 9. Ripe oocytes of C. gariepinus with a visible nucleus.
Figure 10. Ovulated eggs of C. gariepinus (Hogendoorn and Vismans, 1980)
Stage 6, Spent:
Macroscopic description: The ovary is flaccid, flabby and bloodshot with thick, whitish tough walls. The genital aperture of the female looks inflamed. Some translucent and opaque (residual) eggs visible to the naked eye.
Development from stages 1 to 4 is related to temperature (and of course age when first maturation is considered). Development from stages 4 to 5 is triggered by environmental stimuli or can be provoked by hormonal injections (see Figure 11). This development process will take place once the water temperature is 20-22oC or higher. After ovulation of the “ripe eggs” the majority of the oocytes found in the ovary consists again of stage 1 oocytes, the cycle is repeated and after approximately six weeks a new batch of “ripe eggs” is ready for ovulation.
Figure 11. Pathway of oocyte development of C. gariepinus