SIEBE C. VAN DE GEIJN
Research Institute for Agrobiology and Soil Fertility (AB-DLO), Wageningen, The Netherlands
JAN GOUDRIAAN
Wageningen Agricultural University, Dept. Theoretical Production Ecology,
Wageningen, The Netherlands
Water use and water-use efficiency
Response at the level of stomata
Effects at the level of the leaf
Effects at the plant and canopy level
Effects over the growing season
Effects on the regional vegetation-atmosphere water vapour exchange
Concluding remarks
Acknowledgements
References
The projected climatic effects of the continuously increasing concentrations of CO2 and other radiatively active trace gasses in the atmosphere have caused concern over the last decades and increasingly attracted scientists' and policy makers' attention. The expected changes at a global level will be reflected in changed weather conditions in the growing season at regional and local levels that directly affect agriculture and natural vegetation. The exchange of water and energy is determined both by the climate and by the gas exchange properties of the vegetation. Feedback on land cover, changed vegetation properties and local and regional climate is therefore of prime importance.
So far, the General Circulation Models (GCMs) are not able to give accurate predictions of climate change with great geographical detail. Also, feedback processes by the biosphere are still poorly represented in the models. These shortcomings limit predictions at the regional level that are needed in impact studies for agricultural production. Qualitative changes in potential production may well be estimated on the basis of the scenarios, but quantitative changes in the actual production, including the limitations caused by the changed variability of weather conditions (daily and seasonal amplitudes of temperatures, seasonal patterns in precipitation, cloudiness) remain difficult to estimate.
In addition to changes in precipitation and total water availability for irrigation, that directly affect agricultural production, changes in the pattern of water use by crop plants throughout the season may affect the outcome. Of special concern is the change in the physiological functioning of the vegetation as a consequence of the changed atmospheric composition. Most plants react to the changed atmospheric CO2 concentration with changed stomatal response, and not only is growth affected but also the transpiration. The complex nature of the physiological response in interaction with micrometeorological processes at the leaf and canopy level requires further attention.
This paper gives an indication of the state of the art regarding the effects of elevated CO2 on stomatal behaviour, transpiration, water-use efficiency and total water-use. It briefly touches upon the interaction between vegetation and atmosphere at a larger scale. Aspects related to these effects at the level characteristic for a doubling of the present atmospheric CO2 concentration [CO2] are discussed. Changes in atmospheric concentration will not occur overnight but are expected to lag behind relative to changes in greenhouse gas concentration. Therefore uncertainties remain not only on the expected time course of modifications and adaptations, but also on the level at which new threats and opportunities arise in future conditions.
DEFINITIONS
It should be realized that various expressions for water-use efficiency are used (Figure 5.1), and that the physiological and agronomic definitions may differ. Time-averaged water use and water-use efficiency (seasonal or periodic whole-plant transpiration rate (WPTR), and water-use efficiency (WUE)) are often confounded with instantaneous transpiration rate and transpiration efficiency (ITE) (Morison, 1993; Eamus, 1991). The latter expression is best described as the ratio between the instantaneous CO2 fixation (actual net photosynthesis) and water loss by transpiration (both measured with a leaf or plant chamber). Water-use efficiency is the ratio of the net gain in dry matter over a given period, divided by the water loss (from the vegetation alone or from soil and vegetation together) over the same period. Mostly the ITE, normally only measured during the light period, is higher than WUE, both because of variations in ITE over day and season and because of carbon losses by respiration in the dark. WUE seems to be the more relevant parameter to the question of impact on agricultural production.
INTERACTIONS WITH EXPERIMENTAL CONDITIONS
Most available data on water use and water-use efficiency have been collected from single plants grown in pots or hydroponically, and using porometer measurements supplemented with periodic weight determinations. Values for water-use efficiency and total water use in (semi-) field conditions over the full season, or an extended period, are scarce. Moreover, a complication for the correct estimation of the effect of elevated [CO2] and temperature changes on transpiration is that almost all experiments in this field have been performed in environmentally controlled and generally well mixed and ventilated experimental set-ups (enclosures or Open-Top Chambers (OTCs)), where the indirect effects may not show up so prominently as in a real and outside future climate (Unsworth et al., 1984; Leuning and Foster, 1990). Similar interpretation problems have been met in the evaluation of air pollution research.
Figure 5.1. Different expressions for the relation water use - carbon assimilation and growth
STOMATAL DENSITY
The plant leaves lose water primarily by evaporation through the stomata. The stomatal density depends upon plant species, and can be related to the plant-ecotype (between 300 and 800 stomata/mm (Rowland-Bamford et al., 1990; Woodward, 1987, 1993; Kimball et al., 1986)). Woodward (1987) correlated the decrease in the stomatal density over time, observed in herbarium leaves collected over the last centuries, with the rising CO2 concentrations and concluded from the shift in d 13C (Woodward, 1993) that the water-use efficiency concomitantly has improved. The nitrogen content in the leaves had dropped, in line with most data from elevated CO2 experiments (Penuelas and Matamala, 1990). Experimentally, an increase in [CO2] up to about 310 m l/l decreased the stomatal density, but sometimes no effect is found above this [CO2] (Woodward and Bazzaz, 1988). This matter is still under dispute (Körner, 1988; Woodward, 1993) although such a correlation has also been confirmed for palaeo-records (Van der Burgh et al., 1993). Among species large differences in response of stomatal density to elevated [CO2] seem to exist. Experiments with a range of CO2 concentrations (160-900 m l/l) (Rowland-Bamford et al., 1990; O'Leary and Knecht, 1981) showed an increase of stomatal density of rice and bean leaves, with a differential effect at abaxial (increasing) and adaxial sides. At subambient CO2 concentrations stomatal density dropped. This is in contrast with the findings of Oberbauer et al. (1985) for tropical trees. The relative effect of changes in stomatal density and decreasing stomatal aperture at elevated [CO2] for the water relations, however, have not been evaluated. A gradual change in [CO2] over the next century may lead to a natural selection that favours cultivars having a lower stomatal density, especially for water-limited growth conditions. It should, however, be realized that other environmental factors like salt stress can also modify stomatal density (Rozema et al., 1991a). Whatever the net effect, the resulting stomatal conductance is primarily determined by stomatal functioning, and much less by density.
STOMATAL FUNCTIONING
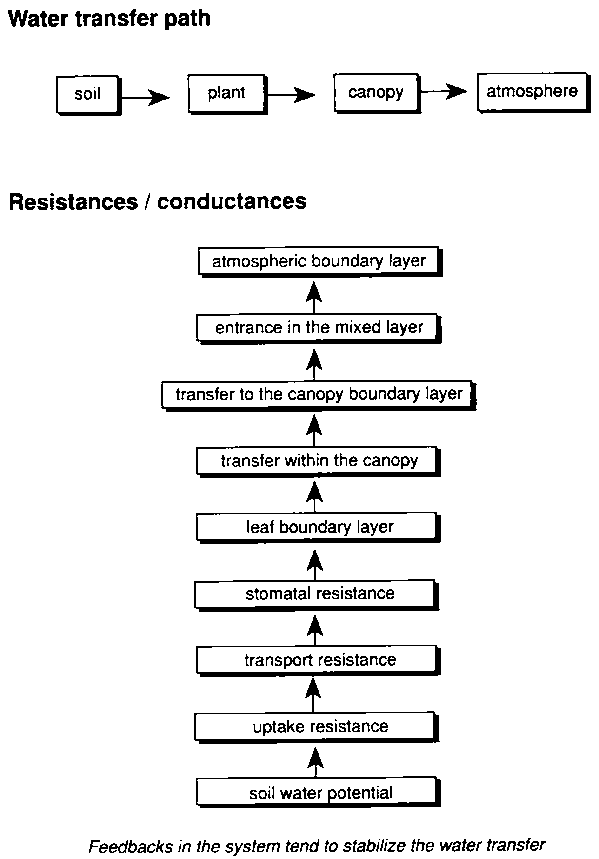
In the pathway from the stomatal cavity to the leaf surface, and from ambient air to the photosynthetic machinery in the mesophyll, the stomata are a major resistance for gas transport between the leaf and the surrounding air. A change in gas exchange resistance of the stomatal pores therefore affects the entrance of CO2 and even more the exit of water vapour (Figure 5.2). The opening status of the stomata is a compromise between water loss and uptake of CO2 from ambient air (Farquhar et al., 1980; Mott, 1990; Wolfe, 1994; Stanghellini and Bunce, 1994; Leuning, 1995). In line with this proposition, stomatal response to elevated concentrations of CO2 (Ca) in general is reflected in partial stomatal closure. The mechanism behind this stomatal closure is not yet clear (Mott, 1990; Wolfe, 1994). The observations are in line with the notion that plants tend to regulate the internal CO2 concentration (Ci) in the substomatal cavity such that for a given water vapour deficit there is a constant ratio (Ci/Ca) with the atmospheric concentration (Mott, 1990; Goudriaan and Unsworth, 1990). Such a regulation would directly lead to the partial closure at elevated CO2, as observed in many studies using porometers (Tyree and Alexander, 1993; Morison and Gifford, 1983; Morison, 1987). Jackson et al. (1994) measured photosynthesis and water relations of native grassland species and calculated Ci/Ca for C3 and C4 plants. They confirmed the conservation of the value, with only a small (not significant) tendency to rise with increasing [CO2]. The rate of photosynthesis and therefore the required supply of carbon dioxide is directly coupled to light intensity. In line with this, the conductance of stomata is also highly correlated to light (Leuning, 1995). This relation can be modified by environmental conditions like drought or stress by air pollution. The ratio Ci/Ca in stationary conditions is about two-thirds for C3 but about one-third for C4 plants. The lower value for C4 plants reflects the higher affinity for CO2 of the C4. photosynthetic pathway, and reflects the more efficient water use for these plants (Goudriaan and Unsworth, 1990; Kimball et al., 1993; Kimball et al., 1995).
ACCLIMATION OF STOMATAL MOVEMENT
Little is known about the acclimation of the stomatal movement to long-term exposure to elevated [CO2]. Some studies suggest that the lower stomatal conductance not only persists over extended exposure periods to elevated [CO2], but is also conserved after subsequent lowering of the [CO2] (Gorissen, pers. comm.). For glasshouse horticulture it was reported that the stomatal conductance of high-[CO2] tomato plants was less sensitive to short-term [CO2] fluctuations, and higher than that of plants grown at ambient concentrations and measured at double present [CO2] (Stanghellini and Bunce, 1994). This may indicate that the sensitivity of stomatal movement of high [CO2] plants to changes in [CO2] is reduced, but still a lower stomatal conductivity exists when compared to leaves growing and measured in present [CO2] conditions.
The partial closure of stomata is reflected in the reduced conductance at the leaf level (Atkinson et al., 1991; Sionit et al., 1984). At a CO2 concentration double the present, conductance is reduced by 30-40%, although large differences among species exist (Hendrey et al., 1993; Morison, 1987).
LEAF TEMPERATURE AND VAPOUR PRESSURE DEFICIT
Water loss by transpiration is not only affected by the conductivity of the stomata, but also by the driving forces for exchange of the water vapour from the leaf surface to the surrounding atmosphere. Therefore the gradient in partial pressure of the water vapour at the leaf surface is also of importance (McNaughton and Jarvis, 1991). All other factors being equal, the existing vapour pressure deficit (VPD) between stomatal cavity and surrounding air (Figures 5.2 and 5.3), the boundary layer, will increase at a reduced transpiration rate, and feed back to stimulate transpiration. The reduced transpiration will cool the leafless, and consequently a rise in temperature in the stomatal cavity and at the leaf surface may occur. Thus, in addition to the global greenhouse effect on air temperature, the temperature at the leaf surface may rise by 0.5 to 1.5 °C (Idso et al., 1987; Morison, 1987; Kimball et al., 1995; van de Geijn et al., 1993). In their FACE experiments (Free Air Carbon dioxide Enrichment) in Arizona, Kimball et al. (1995) measured an average rise in canopy temperature of 0.56°C over the growing season. Such a higher leaf temperature may also have important consequences for the longevity and photosynthetic capacity of the individual leaves and at the canopy level, as ageing may be accelerated (Kimball et al., 1995; Chaudhury et al., 1989; Ellis et al., 1990; Kuiper, 1993). It is presently not known how different crops respond to the increase in leaf and canopy temperature.
The mostly negative feedback on water vapour exchange will at least partly counteract the effect of the reduced stomatal conductance even without rise in ambient temperature at the global scale. A rise of ambient growing season temperatures due to the greenhouse effect will also tend to stimulate transpiration. Depending on changes in air humidity it may affect VPD at the leaf surface (Figure 5.3) determining the fluxes of sensible and latent heat (Massman and Ham, 1994) and thereby the energy balance. Increased water availability may, however, offset the relative warming at the leaf surface, especially if, through preceding water saving and lower instantaneous requirements, partial stomatal closure alleviates water stress during periods of high water requirements in the diurnal sequence (Tyree and Alexander, 1993). It requires detailed microclimatological data and complex calculations to integrate these various mechanisms in operation at the leaf level (Jacobs and de Bruin, 1992).
Figure 5.3. Stepwise transfer of water from the root environment to the atmosphere
LEAF AREA AND WEIGHT
Changes in area of individual leaves also have a direct effect on water loss at a per leaf basis. In general a substantial increase in individual leaf weight is found. This is primarily due to an increased leaf thickness and additional accumulation of non-structural carbohydrates. Leith et al. (1986) found for soybeans that a small but significant increase in leaf area with CO2 concentration may be expected (about 5% increase with a doubling [CO2]). This is consistent with trends reported in other papers (Rozema, 1993; Stanghellini and Bunce, 1994). In general, however, weight gain through increased leaf thickness is more important than that by the increase in leaf area. The relative gain in leaf area may also depend on growing conditions such as water shortage.
Aspects of the water economy at the leaf level also apply at the canopy level. Moreover, light distribution in the canopy, leaf age, humidity gradients and coupling with the atmosphere for different canopy layers and canopy structures all have their effect. The direct effects on vegetation properties discussed here are linked to the change in CO2 concentration and temperature, but indirect effects of a climate change related with changes in ambient temperature and air humidity, coupling to the lower atmosphere and albedo changes, may even dominate (Figure 5.3). Mismanagement or over-exploitation of natural resources may lead to changes in the vegetation and even to desertification (Breman, 1992), and via a positive feedback aggravate changes in local and regional climate as studied in the EU-EFEDA-programme (European Field Experiment in Desertification-threatened Areas (Bolle et al., 1993)). This field of research is still under development and the subject of international programmes like the IGBP-BAHC (International Geosphere-Biosphere Programme - Biospheric Aspects of the Hydrological Cycle (IGBP, 1993)).
LEAF AREA INDEX
The leaf area index (leaf surface area per unit soil surface area) of a crop with adequate water supply at elevated [CO2] increases, especially early in the season, as a result of earlier and more rapid leaf production in the vegetative growth phase (Ackerly et al., 1992; Grashoff et al., 1995; Morison and Gifford, 1984a,b). This applies especially for indeterminate growing species and under non-limiting supply of nutrients. Early development of the canopy will lead to an earlier full ground cover, and may thus limit water loss from direct soil evaporation. Depending on the local precipitation and available soil water reserves, such an early enhanced canopy development may also be favourable for the full utilization of water resources (Chaudhury et al., 1990a,b). The higher transpiration early in the season may also lead to an earlier depletion of water reserves in the soil (Morison and Gifford, 1984a,b).
In their FACE experiments Hendrey et al. (1993) found that for cotton leaf area increased by 25% in dry plots, but by only 11% in irrigated plots as compared to the respective controls. Such enhancements in leaf area and biomass formation in dry conditions could improve the duration of the vegetative soil cover, and help counteract erosion and other forms of land degradation.
PLANT WATER STATUS
It has been shown that plant water status is generally improved at elevated CO2. Part of the effect can be ascribed to a reduced transpiration demand (per unit leaf area), and therefore a partial alleviation of the water stress (Paez et al., 1984). Also for plants with a normal water supply, pre-dawn and midday water potentials have been found to be less negative in high [CO2] (Clifford et al., 1993; Jackson et al., 1994). Even in saline conditions the effect of partial stomatal closure is reflected in a higher salt tolerance (Rozema et al., 1991b; Bowman and Strain, 1987). As a consequence, the restriction of photosynthesis under low water supply may be less severe, or be delayed.
CHANGES IN THE ROOTING PATTERN
Several studies have reported an increased input of carbon in below-ground processes (van de Geijn and van Veen, 1993; Rogers et al., 1994). An important aspect to be considered in reviewing the literature at this point is that most experiments concern plant growth over a short period and with a soil volume limited by pot or container size. In a study with container-grown grasses and clover Nijs et al. (1989) showed that in a rapidly developing terminal drought period water-use efficiency is about doubled, and stress is developing later under elevated [CO2]. These conditions are thus improving the possibility to escape drought stress. Even though the trend is clear, elevated [CO2] plants grown with restricted soil volume may not take full advantage of a larger root system. In (semi-) field experiments (Chaudhury et al., 1990b; Rogers et al., 1994) the larger root system indeed leads to a better exploration of the soil volume, and an earlier, or higher, rooting density at the larger depths. Experiments with potted plants can therefore not be expected to show the full extent to which elevated [CO2] plants may profit in a (transient) drought period. The observation of Morison and Gifford (1984a,b) that the pattern of depletion of soil water reserves is rather similar may partly be an underestimation of the real moisture availability in field conditions because of a better access to deeper soil layers. It has also been observed that a faster recovery of the crop is possible when grown at elevated [CO2] (Bhattacharya et al., 1990). The explanation of this observation has not yet been given, but could be related to deeper and more extensive rooting patterns.
TEMPERATURE EFFECTS
The importance of temperature at the canopy level is two-fold. Firstly higher temperatures increase transpiration by changing the VPD at the leaf surface, and secondly the higher canopy temperature may lead to an accelerated ageing of the foliage, and a shortening of the growing season, or, for example, grain-filling period. The latter type of effect is discussed extensively in crop growth modelling studies that describe changes in crop productivity due to climate change (Ellis et al., 1990; Leuning et al., 1993; Grashoff et al., 1994; Van Keulen and Seligman, 1987; Acock and Acock, 1993; Kenny et al., 1993).
On theoretical grounds it can be expected that transpiration losses will increase with higher air temperatures. It is likely that the evaporative demand as determined by the vapour pressure deficit would increase by about 5 to 6% per degree warming (McKenney and Rosenberg, 1993). The overall effect of a higher temperature alone is an intensification of the hydrological cycle. It should be realized that the combined result of a higher ambient temperature, leading to a higher evaporative demand, and partial stomatal closure, counteracting this, could be overridden by changes in other environmental and atmospheric conditions like soil water availability, precipitation patterns, cloudiness and air humidity.
The effects of elevated CO2 alone on transpiration integrated over the season show that any of the cases (higher water use; no change; lower water use) can be found. Especially in the case of non-limiting water supply a higher water use can be expected even with a lower stomatal conductance as leaf area will increase with about the same factor as total biomass. This will compensate for the reduction in water exchange per leaf area. This applies especially in early season. In conditions with transient water shortage, profit from partial stomatal closure will be highest, as both the depletion of soil water reserves is delayed and plant sensitivity to midday water shortage (suppressed photosynthesis) is lessened.
In conditions with adequate water supply, water use over the whole season, and especially in periods in the season with a closed canopy (e.g., with leaf area index over 3), is little different if at all (Kimball et al., 1995; Dijkstra et al., 1993). Figure 5.4 shows some results of gas exchange measurements with wheat and faba bean grown for the whole season at two CO2 concentrations (Dijkstra et al., 1993). ITE and WUE (24h basis) are both higher (about 40-50%) at elevated [CO2], but transpiration per unit soil surface is not changed significantly (CET-24h Table 5.1). Here treatment temperatures have been kept unchanged, tracking outside conditions. The improvement in seasonal water-use efficiency (SWUE) ranges from 23% (wheat) to 54% (faba bean), similar to the results reported by Dijkstra et al. (1993; Table 5.1).
Similarly, Baker and Allen (1993) presented results of canopy gas exchange for soybean and citrus at a range of temperatures (Table 5.2). Although day time water-use efficiency (DWUE) fell with temperature, they measured at 800 m l/l [CO2] a doubling (soybean) or more of the DWUE, independent of temperature (28-35°C). Seasonal water-use efficiency varies with crop and growth conditions (Table 5.3).
Figure 5.4. Effect of ambient ( )
and elevated (
)
and elevated (![]() )
[CO2] on CET (Canopy Evapotranspiration rate, mmol/m2/s)
on spring wheat (1992)
)
[CO2] on CET (Canopy Evapotranspiration rate, mmol/m2/s)
on spring wheat (1992)
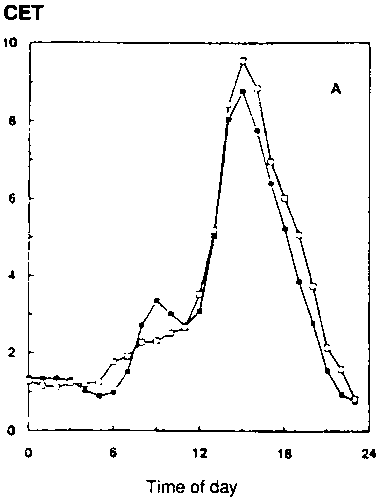
Figure 5.4. Effect of ambient ( )
and elevated (
)
and elevated (![]() )
[CO2] on CET (Canopy Evapotranspiration rate, mmol/m2/s)
on faba bean (1993) on Julian day 188
)
[CO2] on CET (Canopy Evapotranspiration rate, mmol/m2/s)
on faba bean (1993) on Julian day 188
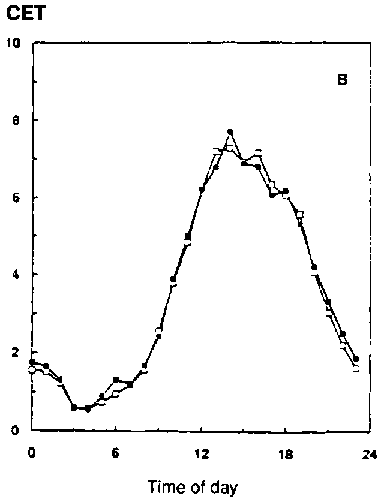
Figure 5.4. Effect of ambient ( )
and elevated (
)
and elevated (![]() )
[CO2] on CCER (Canopy Carbon Exchange Rate, m mol/m2/s)
on spring wheat (1992)
)
[CO2] on CCER (Canopy Carbon Exchange Rate, m mol/m2/s)
on spring wheat (1992)
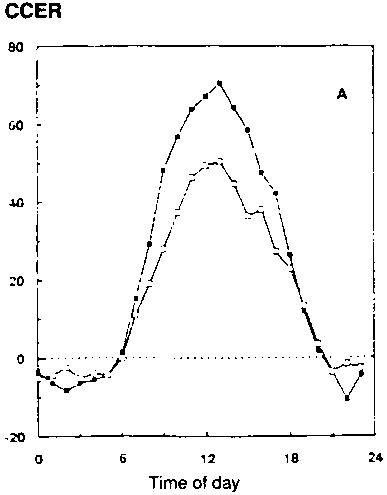
Figure 5.4. Effect of ambient ( )
and elevated (
)
and elevated (![]() )
[CO2] on CCER (Canopy Carbon Exchange Rate, m mol/m2/s)
on faba bean (1993) on Julian day 188
)
[CO2] on CCER (Canopy Carbon Exchange Rate, m mol/m2/s)
on faba bean (1993) on Julian day 188
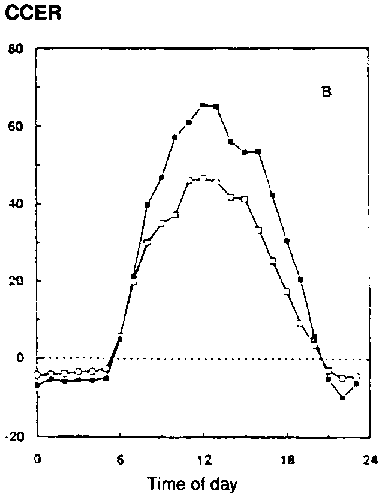
Water-use efficiency rises especially in conditions with water limitations (Chaudhury et al., 1990a). In their study of the potential changes of productivity of cool-season legumes, Grashoff et al. (1994) concluded that for rainfed faba bean crops in the Netherlands, Syria and Israel productivity would increase, especially under water limitation. Faba bean is a special case, because crop development rate is accelerated by drought. A small increase in temperature (1.7°C) would decrease yield, but the lower water requirement at higher [CO2] (1.7°C and 460 m l/l for the climate in 2030) more than compensates for this (Table 5.4), raising yields from 2.8 to 4.7 t/ha (Syria), 3.9 to 5.0 t/ha (Israel) and 5.1 to 5.7 t/ha (Netherlands). In fully irrigated conditions yields would be higher, but the relative CO2 effect would be lower. Interestingly the standard deviation of the predicted yield (>10 year simulated average) shows a tendency to decrease in the changed climate scenario (Table 5.4).
Table 5.1. Effect of [CO2] on full canopy gas exchange parameters for spring wheat (1991) and faba bean (1992) both measured on Julian day 188. Data modified from Dijkstra et al. (1993)
|
|
Spring wheat |
Faba bean | ||||
|
[CO2] m mol/mol |
350 |
700 |
ratio |
350 |
700 |
ratio |
|
CCERmax 1 |
50.2 |
70.4 |
1.40 |
45.6 |
66.8 |
1.47 |
|
CDR 2 |
-3.8 |
-6.0 |
1.59 |
-3.85 |
-6.50 |
1.69 |
|
CCER light period 7 |
67.5 |
95.6 |
1.42 |
67.7 |
100.7 |
1.49 |
|
CDR dark period 7 |
-5.4 |
-8.6 |
1.59 |
-5.5 |
-9.3 |
1.69 |
|
Net CCER shoot-24h 7 |
62.1 |
87.0 |
1.40 |
62.2 |
91.4 |
1.47 |
|
CETmax 3 |
9.55 |
8.77 |
0.92 |
7.29 |
7.71 |
1.06 |
|
CET-24h 4 |
5.24 |
4.90 |
0.94 |
5.50 |
5.60 |
1.02 |
|
CCERmax/CETmax 5 |
5.26 |
8.03 |
1.53 |
6.26 |
8.66 |
1.39 |
|
CCER-24h/CET-24h 6 |
11.85 |
17.75 |
1.49 |
11.31 |
16.32 |
1.44 |
1 CCERmax = maximum canopy CO2 exchange rate on a day (m mol/m2/s)
2 CDR = mean canopy dark (night) respiration rate (m mol/m2/s)
3 CETmax = maximum canopy evapotranspiration rate on a day (mmol/m2/s)
4 CET-24h = integrated canopy evapotranspiration (mm/d)
5 Or maximum ITE in mmol CO2/mol H20
6 Or daily WUE in g CO2/kg H2O
7 g CO2/m2/d
Table 5.2. Daytime canopy water-use efficiency (D-WUE: photosynthesis per unit water transpired: mmol CO2/mol H2O) (data from Baker and Allen, 1993)
|
|
[CO2] m mol/mol |
Temp. (°C) |
DWUE |
High/low ratio |
|
Soybean |
330
|
28 |
2.95 |
|
|
31 |
2.63 |
|
||
|
33 |
2.37 |
|
||
|
35 |
2.33 |
|
||
|
800 |
28 |
6.00 |
2.03 |
|
|
31 |
5.11 |
1.94 |
||
|
33 |
4.95 |
2.09 |
||
|
35 |
4.71 |
2.02 |
||
|
Citrus |
330 |
25 |
2.30 |
|
|
34 |
1.25 |
|
||
|
840 |
25 |
5.40 |
2.34 |
|
|
34 |
3.52 |
2.81 |
Table 5.3. Seasonal crop water-use efficiency ((C)WUE in g DM/kg water) (data from Morison, 1993 and ref. therein)
|
|
Ambient [CO2] |
Double [CO2] |
Ratio |
|
|
Sorghum |
3.08 |
4.13 |
1.34 |
|
|
Wheat well-watered |
5.1 |
6.3 |
1.23 |
|
|
Wheat water shortage |
6.2 |
8.9 |
1.43 |
|
|
Wheat |
2.62 |
3.45 |
1.31 |
|
|
Wheat well-watered |
1.58 |
2.14 |
1.35 |
grain only |
|
Wheat water shortage |
1.27 |
1.86 |
1.46 |
grain only |
|
Faba beans |
4.91 |
7.82 |
1.54 |
|
|
Water hyacinth |
1.4 |
2.6 |
1.85 |
|
Table 5.4. Average simulated seed yield in t/ha of rainfed and fully irrigated faba bean crops in Wageningen (Netherlands), Tel Hadya (Syria) and Migda (Israel) under present and changed climate conditions (year 2030: + 1.7°C and [CO2] at 460 m mol/mol; year 2080: +3.0°C and [CO2] at 700 m mol/mol; no precipitation change). Modified from Grashoff et al. (1994)
|
Location and Scenario |
Rainfed |
Fully irrigated |
|||||
|
yield |
change (%) |
sd (%) |
yield |
change (%) |
sd (%) |
||
|
Wageningen |
|||||||
|
|
Current climate |
5.1 |
- |
32 |
6.1 |
- |
9 |
|
|
Year 2030 |
5.7 |
12 |
29 |
6.4 |
5 |
7 |
|
|
Year 2080 |
6.7 |
31 |
22 |
7.2 |
18 |
6 |
|
Tel Hadya |
|||||||
|
|
Current climate |
2.8 |
- |
41 |
6.9 |
- |
11 |
|
|
Year 2030 |
4.7 |
68 |
35 |
8.0 |
16 |
8 |
|
|
Year 2080 |
7.4 |
164 |
22 |
9.5 |
38 |
6 |
|
Migda |
|||||||
|
|
Current climate |
3.9 |
- |
39 |
6.4 |
- |
9 |
|
|
Year 2030 |
5.0 |
28 |
41 |
7.2 |
12 |
10 |
|
|
Year 2080 |
6.7 |
72 |
35 |
8.5 |
33 |
11 |
One of the more complex aspects of the estimation of changes in the water use of arable crops is the extrapolation from leaf and canopy to the field and region. This has long been recognized by the international scientific community, and has given rise to the establishment of several large-scale measuring campaigns (HAPEX: Hydrologic Atmospheric Pilot Experiment (Sahel and Spain), GEWEX: Global Energy and Water Cycle Experiment (World Climate Research Programme)) and to the start of the IGBP-BAHC programme (IGBP, 1993). A major part of the efforts will go into the development of the SVAT-models (Soil-Vegetation-Atmosphere-Transfer models). The extrapolation from the stomatal and leaf level to the region have been the subject of experimental and theoretical studies (McNaughton, 1994; McNaughton and Jarvis, 1991; Jacobs and de Bruin, 1992; Jacobs, 1994; Hollinger et al., 1994; Baldocchi, 1994a,b). In general the structure of the canopy determines to a large extent the transfer of heat and water vapour to the lower atmosphere. Most models to date neglect or oversimplify the feedback between the vegetated surface and the lower atmosphere, the planetary boundary layer (PBL). Jacobs (1994) showed in a study using coupled models (one-dimensional model for the PBL coupled to a modified Penman-Monteith big-leaf model) that in such a model the sensitivity of regional transpiration to changes in surface resistance is reduced (about halved) but that the sensitivity to changes in albedo is increased by between 25 and 250% relative to the values obtained without PBL feedback.
In addition to the sensitivity of stomata to photosynthesis and [CO2], Jacobs (1994) introduced an additional correlative relationship between the humidity deficit at the leaf surface and the Ci/Ca ratio. The changes in transpiration caused by changes in surface resistance are damped. However, within the canopy the changes in the specific humidity lead to a positive feedback, e.g., the CO2 effects on surface resistance are enhanced. The inclusion of PBL feedback and stomatal response to humidity deficit leads in his study to an estimated overall decrease of the regional transpiration by 10 to 30%. Modifying factors are surface roughness determined by the vegetation type, temperature and air humidity. In particular, information about the last factor, extrapolated from regionalized GCM output, is virtually absent or highly speculative.
Arable crops, especially in the temperate regions in general, have a low surface roughness, and in that case the transpiration is primarily determined by the radiation energy. This is also true for pastures. In rangelands with sparse higher vegetation the higher roughness leads to a stronger coupling, a situation comparable to forests and mixed vegetation (McNaughton and Jarvis ,1991; Hollinger et al., 1994; McNaughton, 1994).
Present knowledge does not allow a firm statement to be made concerning a future situation with respect to water-limited agricultural production. At a regional production level the available quantitative data from regionalized GCM runs are insufficient to be used as direct inputs for crop growth models. At a global level the simultaneous changes in rainfall pattern, air humidity and possible shifts in vegetation zones add to uncertainty, as many and often non-linear feedbacks are expected to operate.
The limited amount of available experimental data has as a trend that water use per unit soil surface area will change little (-10 to +10%). As, however, the general trend towards an improved water-use efficiency is clear, the productivity per unit of available water is expected to rise by 20-40%, probably much less than the value (100%) calculated from a reduced stomatal conductivity and an increased photosynthesis. Some studies show that in situations with marginal water availability the threshold for a successful crop may shift to lower values (Chaudhury et al., 1990,b; Clifford et al., 1993; Grashoff et al., 1994). Whether at otherwise unchanged water availability this would open up possibilities to reverse existing trends towards desertification in certain areas is very questionable, as apart from precipitation falling short to maintain existing vegetation, other aspects like over-exploitation might dominate. It should, however, be emphasized that present knowledge on feedback among vegetation characteristics, gas exchange and albedo and the regional climate is insufficient to draw firm conclusions.
Some related problems have not been touched upon. For example, changes in precipitation distribution throughout the season may lead to shifts in the accessibility of fields for farm operations in early spring or late autumn. In addition they may cause changes in soil moisture and thereby modify the rate of mineralization of nutrients (nitrogen, phosphorus). Such interactions, combined with changes in (air and soil) temperatures, may markedly change soil fertility and thereby local farming systems and crop productivity. Depending on the aim of the study, such impacts have to be considered more or less relevant to the present subject.
In many studies the impact of climate change on crop growth and yield is analysed using crop simulation models (Van Keulen and Seligman, 1987; Kenny et al., 1993; Acock and Acock, 1993; Grashoff et al., 1994, 1995). A proper analysis of the performance of such models should be made to verify their reliability in the projected conditions of changed atmospheric composition and changed climate. The absolute values of the regionally and globally aggregated predicted crop yields, often calculated using a proportionality factor for the CO2 response, are at present probably less reliable than the predicted sensitivity of the yield to various climate and management factors (see chapter of Tinker et al.).
The above considerations relate to a projected world, changed primarily in terms of climate and atmospheric composition. It should be emphasized that at many points agricultural practice is very dynamic, and will respond to changed conditions by adaptation (McKenney et al., 1992). Crop and cultivar choice will, in most instances and in the most productive areas, change over time and gradually incorporate the traits necessary for adapted performance, or change to better adapted species. A rising temperature will for most current cultivars, for instance, accelerate crop development. This would in itself lead to a reduced water use over the shortened growth period, but also to a loss of potential yield. One may argue that farmers will repair such a loss of production potential by a proper choice of adapted cultivars or crop species, unless temperatures exceed the appropriate temperature window (Behl et al., 1993).
These developments require, however, that new technologies and genetic resources be practically and economically accessible for all farmers, a situation that is not reached at present for farmers in arid and semi-arid regions in developing countries where the risks but probably also opportunities for agricultural production may be greatest.
Part of this work was done with support from the European Union, under contract EV5V-CT920169 (CROPCHANGE), and the Dutch National Programme on Global Air Pollution and Climate Change. The additional support from FAO is gratefully acknowledged.
Ackerly, D.D., Coleman, J.S., Morse, S.R. and Bazzaz, F.A. 1992. CO2 and temperature effects on leaf area production in two annual plant species. Ecology 73: 1260-1269.
Acock, B. and Acock, M.C. 1993. Modelling approaches for predicting crop ecosystem responses to climate change. In: International Crop Science I. D.R. Buxton et al. (eds.). Crop Science Society of America, Madison, WI. pp. 299-306.
Atkinson, C.J., Wookey, PA. and Mansfield, T.A. 1991. Atmospheric pollution and the sensitivity of stomata on barley leaves to absisic acid and carbon dioxide. New Phytol. 117: 535-541.
Baker, J.T. and Allen, L.H. Jr. 1993. Contrasting crop species responses to CO2 and temperature: rice, soybean and citrus. Vegetatio 104/105: 239-260.
Baldocchi, D.D. 1994a. A comparative study of mass and energy exchange over a closed C3 (wheat) and an open C4 (corn) canopy. I: The partitioning of available energy into latent and sensible heat exchange. Agric. For. Meteorol. 67: 191-220.
Baldocchi, D.D. 1994b. A comparative study of mass and energy exchange over a closed C3 (wheat) and an open C4 (corn) canopy. II: Canopy CO2 exchange and water-use efficiency. Agric. For. Meteorol. 67: 291-321.
Behl, R.K., Nainawatee H.S. and Singh, K.P 1993. High temperature tolerance in wheat. In: International Crop Science I. D.R. Buxton et al. (eds.). Crop Science Society of America, Madison, WI. pp 349-355.
Bhattacharya, N.C., Hileman, D.R., Ghosh, P.P. and Musser, R.L. 1990. Interaction of enriched CO2 and water stress on the physiology of and biomass production in sweet potato grown in open-top chambers. Plant Cell Environ. 13: 933-940.
Bolle, H.-J., André, J.-C., Arrue, J.L., Barth, H.K., Bessemoulin, P., Brasa, A., De Bruin, H.A.R., Cruces, J., Dugdale, G., Engman, E.T., Evans, D.L., Fantechi, R., Fiedler, F., Van de Griend, A., Imeson, A.C., Jochum, A., Kabat, P., Kratzsch, T., Lagouarde, J.-P, Langer, I., Llamas, R., Lopez-Baeza, E., Melia Miralles, J., Muniosguren, L.S., Nerry, F., Noilhan, J., Oliver, H.R., Roth, R., Saatchi, S.S., Sanchez Diaz, J., De Santa Olalla, M., Shuttleworth, W.J., Søgaard, H., Stricker, H., Thornes, J., Vauclin, M. and Wickland, D. 1993. EFEDA: European field experiment in a desertification-threatened area. Ann. Geophysicae 11: 173-189.
Bowman, W.D. and Strain, B.R. 1987. Interaction between CO2 enrichment and salinity stress in the C4 non-halophyte Andropogon glomeratus (Walter) BSP. Plant Cell Environ. 10: 267-270.
Breman, H. 1992. Desertification control, the West African case; prevention is better than cure. Biotropica 24: 328-334.
Chaudhury, U.N., Kanemasu, E.T. and Kirkham, M.B. 1989. Effect of elevated levels of CO2 on winter wheat under two moisture regimes. Report No. 50: Response of Vegetation to Carbon Dioxide. US DOE, Washington DC. 49 p.
Chaudhury, U.N., Kirkham, M.B. and Kanemasu, E.T. 1990a. Carbon dioxide and water level effects on yield and water use of winter wheat. Agron. J. 82: 637-641.
Chaudhury, U.N., Kirkham, M.B. and Kanemasu, E.T. 1990b. Root growth of winter wheat under elevated carbon dioxide and drought. Crop Sci. 30: 853-857.
Clifford, S.C, Stronach, I.M., Mohamed, A.D., Azam-Ali, S.N. and Crout, N.M. 1993. The effects of elevated atmospheric carbon dioxide and water stress on light interception, dry matter production and yield in stands of groundnut (Arachis hypogaea L.). J. Exp. Bot. 44: 1763-1770.
Dijkstra, P., Schapendonk, A.H.C.M. and Groenwold, J. 1993. Effects of CO2 enrichment on canopy photosynthesis, carbon economy and productivity of wheat and faba bean under field conditions. In: Climate Change: Crops and Terrestrial Ecosystems. S.C. van de Geijn, J. Goudriaan and F. Berendse (eds.). Agrobiol. Themas 9. AB-DLO, Wageningen. pp. 23-41.
Eamus, D. 1991. The interaction of rising CO2 and temperatures with water-use efficiency. Plant Cell Environ. 14: 843-852.
Ellis, R.H., Hadley, P., Roberts, E.H. and Summerfield, R.J. 1990. Quantitative relations between temperature and crop development and growth. In: Climate Change and Genetic Resources. M. Jackson, B.V. Ford-Lloyd and M.L. Parry (eds.). Belhaven Press, London, pp. 85-115.
Farquhar, G.D., Schulze, E.-D. and Küppers, M. 1980. Responses to humidity by stomata of Nicotiana glauca L. and Corylus avellana L. are consistent with the optimization of carbon dioxide uptake with respect to water loss. Austr. J. Plant Physiol. 7: 315-327.
Goudriaan, J. and Unsworth, M.H. 1990. Implications of increasing carbon dioxide and climate change for agricultural productivity and water resources. In: Impact of Carbon Dioxide, Trace Gases, and Climate Change on Global Agriculture. ASA Spec. Pub No. 53. pp. 111-130.
Grashoff, C., Rabbinge, R. and Nonhebel, S. 1994. Potential effects of global climate change on cool season food legume productivity. In: Expanding the Production and Use of Cool Season Food Legumes. F.J. Muehlbauer and W.J. Kaiser (eds.). Kluwer Academic, Dordrecht, pp. 159-174.
Grashoff, C., Dijkstra, P., Nonhebel, S., Schapendonk, A.H.C.M. and van de Geijn, S.C. 1995. Effects of climate change on productivity of cereals and legumes; model evaluation of observed year-to-year variability of the CO2 response. Global Change Biology 1 (6): 417-428.
Hendrey, G.R., Lewin, K.F. and Nagy, J. 1993. Free air carbon dioxide enrichment: Development, progress, results. Vegetatio 104/105: 17-31.
Hollinger, D.Y., Kelliher, F.M., Schulze, E.-D. and Köstner, B.M.M. 1994. Coupling of tree transpiration to atmospheric turbulence. Nature 371: 60-62.
Idso, S.B., Kimball, B.A. and Mauney, J.R. 1987. Atmospheric carbon dioxide enrichment effects on cotton midday foliage temperature: Implications for plant water-use efficiency. Agron. J. 79: 667-672.
IGBP [International Geosphere-Biosphere Programme]. 1993. Biospheric Aspects of the Hydrological Cycle (BAHC). The Operational Plan. IGBP Report No. 27. Stockholm, Sweden.
Jackson, R.B., Sala, O.E., Field, C.B. and Mooney, H.A. 1994. CO2 alters water use, carbon gain, and yield for the dominant species in a natural grassland. Oecologia 98: 257-262.
Jacobs, C.M.J. 1994. Direct Impact of Atmospheric CO2 Enrichment on Regional Transpiration. Thesis. Wageningen Agricultural University. 179 p.
Jacobs, C.M.J. and de Bruin, H.A.R. 1992. The sensitivity of regional transpiration to land-surface characteristics: significance of feedback. J. Climate 5: 683-698.
Kenny, G.J., Harrison, P.A., Olesen, J.E. and Parry, M.J. 1993. The effects of climate change on land suitability of grain maize, winter wheat and cauliflower in Europe. Eur. J. of Agronomy 2: 325-338.
Kimball, B.A., Mauney, J.R., Radin, J.W., Nakayama, F.S., Idso, S.B., Hendrix, D.L., Akey, D.H., Allen, S.G., Anderson, M.G. and Hatung, W. 1986. Effects of increasing atmospheric CO2 on the growth, water relations, and physiology of plants grown under optimal and limiting levels of water and nitrogen. In: Response of Vegetation to Carbon Dioxide. Report No. 039. US DOE, Carbon Dioxide Research Division, and USDA-ARS, Washington DC.
Kimball, B.A., Mauney, J.R., Nakayama, F.S. and Idso, S.B. 1993. Effects of increasing atmospheric CO2 on vegetation. Vegetatio 104/105: 65-75.
Kimball, B.A., Pinter, P.J. Jr., Garcia, R.L., LaMorte, R.L., Wall, G.W., Hunsaker, D.J., Wechsung, G., Wechsung, F. and Kartschall, Th. 1995. Productivity and water use of wheat under free-air CO2 enrichment. Global Change Biology 1 (6): 429-442.
Körner, C. 1988. Does global increase of CO2 alter stomatal density? Flora 181: 253-257.
Kuiper, P.J.C. 1993. Diverse influences of small temperature increases on crop performance. In: International Crop Science I. D.R. Buxton et al. (eds.). Crop Science Society of America, Madison, WI. pp. 309-313.
Leith, J.H., Reynolds, J.F. and Rogers, H. 1986. Estimation of leaf area of soybeans grown under elevated carbon dioxide levels. Field Crops Res. 13: 193-203.
Leuning, R. 1995. A critical appraisal of a combined stomatal-photosynthetic model for C3 plants. Plant Cell Environ. 18: 339-355.
Leuning, R. and Foster, L.J. 1990. Estimation of transpiration by single trees: comparison of a ventilated chamber, leaf energy budgets and a combination equation. Agric. For. Meteorol. 51: 63-68.
Leuning, R., Wang, Y.P, de Pury, D., Denmead, O.T., Dunin, F.X., Condon, A.G., Nonhebel, S. and Goudriaan, J. 1993. Growth and water use of wheat under present and future levels of CO2. J. Agric. Meteorol. 48: 807-810.
Massman, W.J. and Ham, J.M. 1994. An evaluation of a surface energy balance method for partitioning ET data into plant and soil components for a surface with partial canopy cover. Agric. For. Meteorol. 67: 253-267.
McKenney, M.S. and Rosenberg, N.J. 1993. Sensitivity of some potential evapotranspiration estimation methods to climate change. Agric. For. Meteorol. 64:81-110.
McKenney, M.S., Easterling, W.E. and Rosenberg, N.J. 1992. Simulation of crop productivity and responses to climate change in the year 2030: The role of future technologies, adjustments and adaptations. Agric. For. Meteorol. 59: 103-127.
McNaughton, K.G. 1994. Effective stomatal and boundary-layer resistances of heterogeneous surfaces. Plant Cell Environ. 17: 1061-1068.
McNaughton, K.G. and Jarvis, P.G. 1991. Effects of spatial scale on stomatal control of transpiration. Agric. For. Meteorol. 54: 279-301.
Morison, J.I.L. 1987. Intercellular CO2 concentration and stomatal response to CO2. In: Stomatal Function. E. Zeiger, G.D. Farquhar and I.R. Cowan (eds.). Stanford Univ. Press, California. pp. 229-251.
Morison, J.I.L. 1993. Response of plants to CO2 under water limited conditions. Vegetatio 104/105: 193-209.
Morison, J.I.L. and Gifford, R.M. 1983. Stomatal sensitivity to carbon dioxide and humidity. Plant Physiol. 71: 789-796.
Morison, J.I.L. and Gifford, R.M. 1984a. Plant growth and water use with limited water supply in high CO2 concentrations. I. Leaf area, water use and transpiration. Aust. J. Plant Physiol. 11: 361-374.
Morison, J.I.L. and Gifford, R.M. 1984b. Plant growth and water use with limited water supply in high CO2 concentrations. II. Plant dry weight, partitioning and water-use efficiency. Aust. J. Plant Physiol. 11: 375-384.
Mott, K.A. 1990. Sensing of atmospheric CO2 by plants. Plant, Cell Environ. 13: 731-737.
Nijs, I., Impens, I. and Behaeghe, T. 1989. Effects of long-term elevated atmospheric CO2 concentration on Lolium perenne and Trifolium repens canopies in the course of a terminal drought stress period. Can. J. Bot. 67: 2720-2725.
Oberbauer, S.O., Strain, B.R. and Fetcher, N. 1985. Effect of CO2-enrichment on seedling physiology and growth of two tropical tree species. Physiol. Plant. 65: 352-364.
O'Leary, J.W. and Knecht, G.N. 1981. Elevated CO2 concentrations increase stomate numbers in Phaseolus vulgaris leaves. Bot. Gaz. 142: 436-441.
Paez, A., Hellmers, H. and Strain, B.R. 1984. Carbon dioxide enrichment and water stress interaction on growth of two tomato cultivars. J. Agric. Sci. (Camb.) 102: 687-693.
Penuelas, J. and Matamala, R. 1990. Changes in N and S leaf content, stomatal density and specific leaf area of 14 plant species during the last three centuries of CO2 increase. J. Exp. Bot. 41: 1119-1124.
Rogers, H.H., Runion, G.B. and Krupa, S.V. 1994. Plant responses to atmospheric CO2 enrichment with emphasis on roots and the rhizosphere. Environ. Pollution 83: 155-189.
Rowland-Bamford, A.J., Nordenbrock, C., Baker, J.T., Bowes, G. and Allen, L.H. Jr. 1990. Changes in stomatal density in rice grown under various CO2 regimes with natural solar irradiance. Envir. Exp. Bot. 30: 175-180.
Rozema, J. 1993. Plant responses to atmospheric carbon dioxide enrichment: interactions with some soil and atmospheric conditions. Vegetatio 104/105: 173-190.
Rozema, J., Lenssen, G.M., Arp, W.J. and van de Staay, J.W.M. 1991a. Global change, the impact of the greenhouse effect (atmospheric CO2 enrichment) and the increased UV-B radiation on terrestrial plants. In: Ecological Responses to Environmental Stresses. J. Rozema and J.A.C. Verkleij (eds.). Kluwer Academic, Dordrecht. pp. 220-231.
Rozema, J., Dorel, R, Janissen, R., Lenssen, G., Broekman, R., Arp, W. and Drake, B.G. 1991b. Effect of elevated atmospheric CO2 on growth, photosynthesis and water relations of salt marsh grass species. Aqu. Bot. 39: 45-55.
Sionit, N., Rogers, H.H., Bingham, G.E. and Strain, B.R. 1984. Photosynthesis and stomatal conductance with CO2-enrichment of container- and field-grown soybeans. Agron. J. 76: 447-451.
Stanghellini, C. and Bunce, J.A. 1994. Response of photosynthesis and conductance to light, CO2, temperature and humidity in tomato plants acclimated to ambient and elevated CO2 Photosynthetica 29: 487-497.
Tyree, M.T. and Alexander, J.D. 1993. Plant water relations and the effects of elevated CO2: a review and suggestions for future research. Vegetatio 104/105: 47-62.
Unsworth, M.H., Heagle, A.S. and Heck, W.W. 1984. Gas exchange in open top field chambers. I. Measurement and analysis of atmospheric resistances. Atmosph. Environ. 18: 373-380.
van de Geijn, S.C. and van Veen, J.A. 1993. Implications of increased carbon dioxide levels for carbon input and turnover in soils. Vegetatio 104/105: 283-292.
van de Geijn, S.C., Goudriaan, J., van der Eerden, L.J. and Rozema, J. 1993. Problems and approaches to integrating the concurrent impacts of elevated CO2, temperature, UV-B radiation and ozone on crop production. In: International Crop Science I. D.R. Buxton et al. (eds.). Crop Science Society of America, Madison, WI. pp. 333-338.
van der Burgh, J., Visscher, H., Dilcher, D.L. and Kurschner, W.M. 1993. Paleoatmospheric signatures in Neogene fossil leaves. Science 260: 1788-1790.
van Keulen, H. and Seligman, N.G. 1987. Simulation of Water Use, Nitrogen Nutrition and Growth of a Spring Wheat Crop. Simulation Monographs, Pudoc, Wageningen, Netherlands.
Wolfe, D.W. 1994. Physiological and growth responses to atmospheric carbon dioxide concentration. In: Handbook of Plant and Crop Physiology. M. Pessarakli (ed.). Marcel Dekker, New York. pp. 223-242.
Woodward, F.I. 1987. Stomatal numbers are sensitive to increases in CO2 from pre-industrial levels. Nature 327: 617-618.
Woodward, F.I. 1993. Plant responses to past concentrations of CO2. Vegetatio 104/105: 145-155.
Woodward, F.I. and Bazzaz, F.A. 1988. The responses of stomatal density to CO2 partial pressure. J. Exp. Bot. 39: 1771-1781.