A study of aerobic degradation was conducted with two soils, a loam and a sandy loam, at a nominal dose rate of 1 mg/kg of [14C]tebufenozide. The soil samples were incubated in the dark at 25°C for one year, in bottles with traps for CO2 and volatile organic compounds. The three labels were used (Reynolds, 1992e).
Duplicate samples were taken on days 0, 1, 3, 7, 14, 30, 60, 90, 120, 180, 270, and 365 and analysed by TLC and/or HPLC. The identities of the parent compound and major metabolites were confirmed by using a second chromatographic technique and by mass spectrometry. The average recovery of 14C was >94%.
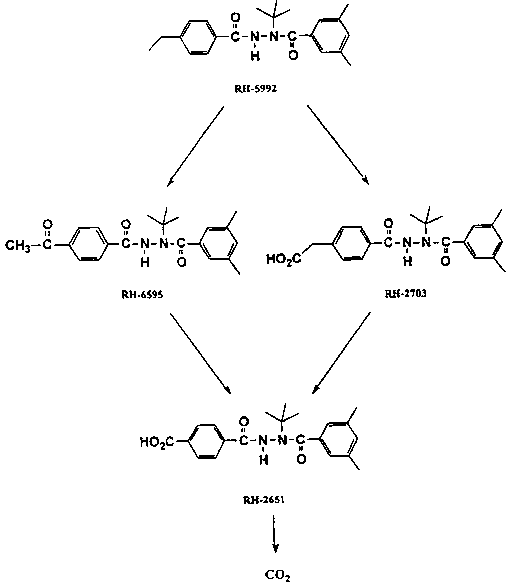
Three degradation produces were observed in addition to the parent compound and CO2, the ketone RH-6595 and the carboxylic acids RH-2651 and RH-2703. All three are products of oxidation of the ethyl group on the A-ring of the molecule. Five other products were detected but not identified; none of them accounted for more than 5.5% of the applied activity in any sample.
The nature and amounts of the products from the three different labels were very similar in each soil, but the rates of degradation in the two soils were very different. In the loam soil (California), only 7-9% of the parent compound remained by day 365 and 54-62% of the total applied radioactivity had been converted to 14CO2 by the end of the study. The calculated half-life of tebufenozide was 105 days. In the sandy loam soil (New Jersey) the rate of degradation was much slower; 61-71% of the parent compound remained by the end of the study and only 2-5% of the initial tebufenozide was mineralized: its calculated half-life was 704 days. It was suggested that this soil may have been atypical, but there was no explanation for the low rate of degradation.
Soil-bound residues increased gradually from all three labels as the study progressed to 12.3-16.6% in the sandy loam and 22.4-26.9% in the loam. They were characterized by various methods. Mild acidic extraction solubilized 2.6-7.0% of the total applied 14C and acid hydrolysis released about 2.9-7.1%. The remainder was fractionated into humic and fulvic acids and humin, with most of the activity recovered from the humic and fulvic acid fractions. The proposed degradation pathways of tebufenozide in soil are shown in Figure 8. The half-life observed in the New Jersey soil was inconsistent with all other reported information on soil degradation.
Figure 8. Proposed degradation pathways of tebufenozide in soil.
The degradation of tebufenozide was also investigated under aerobic conditions in four German soils, a low organic sand (Speyer 2.1), a high organic sand (Speyer 2.2), a sandy loam (Speyer 2.3) and a loamy sand (SLV). Soil samples dosed with [14C]tebufenozide at a rate of either 0.193 kg ai/ha or 0.257 mg ai/kg of dry soil were incubated at 20 ± 2°C in the dark for 120 days at 40% maximum water capacity. The degradation of tebufenozide was continuous in all four soils, with half-lives between 27.8 and 31.5 days and disappearance times for 90% of the initial concentration from 92.2 to 104.5 days (Schanne 1995a). The characteristics of the soils are shown below.
|
|
Low org. sand |
High org. sand |
Sandy loam |
Loamy sand |
|
Organic C, % |
0.98 |
2.50 |
1.11 |
1.07 |
|
Clay, % |
3.25 |
4.25 |
9.44 |
10.16 |
|
Silt, % |
9.46 |
9.16 |
27.71 |
35.23 |
|
Sand, % |
87.28 |
86.59 |
62.81 |
54.6 |
|
Cation exchange capacity, meq/100g |
3.76 |
10.26 |
9.47 |
5.33 |
|
Water capacity, g/100 g dry soil |
21.0 |
41.3 |
31.4 |
34.7 |
|
Microbial biomass, mg/100 g dry |
start 12.6 |
start 21.1 |
start 19.4 |
start 12.6 |
|
soil |
final 7.8 |
final 31.8 |
final 10.4 |
final 22.5 |
|
pH |
5.5 |
5.5 |
1.1 |
1.0 |
The DT-50 and DT-90 values calculated for the four soils are shown below.
|
|
DT-50, days |
DT-90, days |
|
Low organic sand |
31.1 |
103.2 |
|
High organic sand |
28.2 |
93.6 |
|
Sandy loam |
27.8 |
92.2 |
|
Loamy sand |
31.5 |
104.5 |
Carbon dioxide and non-extractable residues reached a maximum of 38% and 42.8% of the applied radioactivity respectively, indicating that these are the major terminal residues. At day 120, up to 12% of the applied activity was associated with the humin fraction of the soil organic matter and up to 31% with humic and fulvic acids. The degradation products RH-6595 (ketone), RH-2651 and RH-2703 (carboxylic acids) were again identified.
Photodegradation on soil. Sandy loam soil treated with 10 mg/kg of A-ring-labelled tebufenozide was maintained at 25°C and irradiated with a xenon arc lamp for 30 days with a 12-hour light/dark cycle. The study was designed to trap any volatile materials and minimize thermal decomposition (Reynolds, 1992d).
Samples were analysed after 0, 3, 7, 21 and 30 days of irradiation. They were extracted with acidified acetonitrile/water, partitioned with methylene chloride and analysed by TLC or HPLC with radiometric detection. The half-life of tebufenozide was calculate to be 98 days under the test conditions.
A total of seven photoproducts were detected, none accounting for more than 5.3% of the applied 14C in any sample. The most abundant was the ketone RH-6595; the B-ring aldehyde RH-0970 was also identified.
Adsorption/desorption was studied in five different soil types, clay, loam, loamy sand, sandy clay loam and loamy sand, with [14C]tebufenozide labelled in the A-ring. The soil pH ranged from 5.6 to 7.8 and the organic matter content from 0.8% to 3.6% (Hawkins, 1992).
Replicate experiments were conducted with each of the five soils at four aqueous concentrations from 0.0516 to 0.755 mg/kg in 0.01 M calcium chloride. In each experiment, the soil and aqueous solution of the test compound were equilibrated for 24 hours, and the phases then separated and radioassayed. The soil was desorbed twice with fresh calcium chloride solution for 24 hours with radioassay after each desorption. All equilibrations were carried out at 25 ± 1°C.
The average KOC for the soils was 572, 928 and 1168 for adsorption, first desorption and second desorption respectively. On this basis the potential mobility would be classified as low.
A laboratory column leaching study was carried out in accordance with the BBA guideline. Three soils were selected: a sand with low organic matter (0.7%), a sand with high organic matter (2.29%), and a sandy loam with an organic matter content of 1.34% (Knoch, 1993). After sieving, the soils were packed in glass columns of 5 cm diameter and 40 cm length to produce a soil depth of 30 cm. Formulated tebufenozide was added at 37.91 m g of ai to each soil column to simulate the application of 0.192 kg ai/ha. Leaching was by application of an artificial rainfall of 393 ml within 48 hours (simulating a rainfall of 200 mm). The leachate was collected from the first and second 24 hour periods and analysed.
The tebufenozide in the leachate from the sandy soil after 48 h was <2% of that applied. In sandy loam soil the amount of tebufenozide in the first 24 h leachate was <2% of that applied, and over the entire period 0.95 mg was recovered in the leachate, 2.5% of the added tebufenozide. In the loamy sand column no significant amounts of tebufenozide were observed in the leachate during the first 24h. By the end of the leaching period the leachate from one of the duplicate columns contained 5.5% of the applied tebufenozide and that from the second column contained none.
A column leaching study was carried out with aged residues in clay loam, sand, sandy loam and loam. Radiolabelled tebufenozide was mixed with the soils at a nominal rate of 1 mg/kg and the mixtures aged for 30 days, yielding the residues shown below (Reynolds, 1992b).
|
Compound |
% of applied 14C |
|||
|
|
Clay loam |
Sand |
Sandy loam |
Loam |
|
Tebufenozide |
52.3 |
71.4 |
52.7 |
66.4 |
|
RH-6595 |
4.11 |
3.05 |
3.65 |
2.38 |
|
RH-2651 |
5.98 |
2.06 |
15.22 |
0.18 |
|
RH-2703 |
1.21 |
4.87 |
2.62 |
0.95 |
Duplicate glass columns were filled to a height of 30 cm with each of the sieved soils. The corresponding soil with the aged residue was applied to the top of each column and the columns were eluted with 0.01 M calcium chloride solution. The columns were divided into 6-cm sections, and the eluates, soil sections and dose plugs were radioassayed. The distribution of the radioactivity is shown in Table 16.
Table 16. Distribution of radioactivity in aged soil columns (Reynolds, 1992).
|
Sample |
% of 14C recovered from column |
|||
|
Clay loam |
Sand |
Sandy loam |
Loam |
|
|
Treated plug |
52.0 |
46.9 |
60.2 |
91.8 |
|
Segment 1 (0-6 cm) |
25.2 |
3.36 |
3.85 |
5.53 |
|
Segment 2 |
11.6 |
4.81 |
3.50 |
1.58 |
|
Segment 3 |
2.44 |
5.66 |
3.84 |
0.22 |
|
Segment 4 |
0.71 |
6.55 |
3.5 |
0.10 |
|
Segment 5 |
0.27 |
6.96 |
2.27 |
0.02 |
|
Eluate |
7.76 |
25.8 |
22.9 |
0.77 |
Analysis of the eluates indicated that the two carboxylic acids were mobile in all four soils, and the parent tebufenozide was somewhat mobile in sand.
A similar study was carried out with a sandy soil with a low organic matter content. The soil was treated with [14C]tebufenozide and aged in air for 40 days before leaching. After ageing, 17.5% of the radioactivity was unextractable, 11.3% had been converted to carbon dioxide and 0.3% had been volatilized. The remainder of the extractable applied 14C was associated with tebufenozide (46.7%), RH-6595 (6.6%), RH-2651 (9.1%), RH-2703 (4.6%), and two unknowns present at 1.6% and 5.2 %.
After one day of leaching the highest level of radioactivity in the eluate was 0.3%, and after the second day the average activity was 6.9% of that applied. Analysis of the eluates showed that no tebufenozide or ketone (RH 6595) was present in the leachate, whose activity was due to the presence of two carboxylic acids (Schanne, 1995b).
Field dissipation studies were carried out in the USA, Canada, Japan and Germany to determine the persistence and mobility of tebufenozide and its soil degradation products.
In two trials in California, the test material was applied directly to bare sandy soils with low organic matter at a rate of 1.12 kg ai/ha. Under these conditions, residues of tebufenozide and its products were found only in the top 30 cm of the soil: no downward movement of the compounds was observed. The degradation of tebufenozide at the two sites was similar. The half-lives determined from the exponential decline were 53 and 39 days. The trials demonstrated that even when tebufenozide was applied at the maximum rate and to unprotected soil it did not persist and residue levels were below 0.01 mg/kg after one year or less (Hawkins, 1993).
Two similar studies were conducted at two other US locations. The soil at a New York site was a sandy loam at the top, becoming a loam below 15 cm and a silt loam below 60 cm; the soil at a Washington site was a low-organic sand. Each site received four treatments 14 days apart of 0.56 kg ai/ha giving a total of 2.24 kg ai/ha. All treatments were made to bare soil using the 2SC formulation of tebufenozide. Residues of tebufenozide and its degradation products were found only in the top 45 cm. The half-lives determined from the exponential decline were 52 and 31 days at New York and Washington respectively. This study also shows that tebufenozide residues are near or below 0.01 mg/kg after a year or less (Hawkins, 1994).
Field dissipation studies were conducted at two sites in Japan, with cultivated loam soils of high (6.49%) and low (1.45%) contents. At each site tebufenozide was applied three times at one-week intervals at a rate of 0.4 kg ai/ha. Soil from the top 10 cm was sampled on days 0, 7, 14, 30, 55 or 60, and 90 and analysed for tebufenozide and its degradation products. The half-life of tebufenozide was 6 days in the soil with high organic matter and 19 days in that with low organic matter (Yajima, 1992).
Other field dissipation studies were carried out at four different Japanese sites under paddy conditions, with tebufenozide applied at a rate of 0.3 kg ai/ha. Soil from the top 10 cm was sampled according to a regular schedule for a total of 240-365 days. The half-lives in the four soils ranged from 4.2 to 30 days (Chong, 1992).
The dissipation of tebufenozide under field conditions was also investigated at four locations in Germany. The test substance was applied at a rate of 0.192 kg ai/ha to bare soil and samples were collected from 0-10 cm, 10-20 and 20-40 cm depths before and after application, and approximately 1 week, 2 weeks, and 1, 2, 3, and 5 months after application. The soils were a loamy sand, two sandy loams and a sandy silt loam. Soil samples were analysed for residues of tebufenozide and its products, with an LOD for each of the analytes of 0.01 mg/kg. The DT50 and DT90 values for tebufenozide in each soil are shown below (Sochor and Holzwarth, 1995).
|
Disappearance times of tebufenozide, days |
||||
|
|
Loamy sand |
Sandy loam |
Sandy loam |
Sandy silt loam |
|
DT 50 |
108 |
10 |
13 |
5 |
|
DT 90 |
968 |
112 |
43 |
137 |
Three dissipation studies were conducted in different regions of Canada in orchard soils. The soils at the three sites were characterized as loamy fine sand, silty loam, and very fine sand. In all the trials the test compound was applied four times at a rate of 0.28 kg ai/ha. Samples taken at intervals 0 to 368 days were divided into 0-7.5 cm, 7.5-25 cm, 25-50 cm and 50-60 cm sections for analysis (MacLeod, 1995a,b.c).
The first study, with loamy fine sand, was in a mature orchard of malus trees with a ground cover of bunch-type grass. The concentration of tebufenozide shortly after application was low, the maximum concentration being reached in the top 7.5 cm on day 122, probably owing to test material moving from the vegetation into the soil (MacLeod, 1995a).
The trial with silty loam was adjacent to an apple orchard. The concentration of tebufenozide in the top 7.5 cm after the last application was 0.29 mg/kg and was below the limit of determination (0.02 mg/kg) in the sample taken 284 days after the last application. The half-life of tebufenozide was 75 days.
The third trail was in an area typical of the fruit growing region. Applications were directly on to the bare soil surface. Residues of tebufenozide on day 0 averaged 1.29 mg/kg in the top 7.5 cm and decreased to 0.12 mg/kg in the sample taken 368 days after the last treatment. The calculated half-life was 135 days.