Plant metabolism was studied in apples, grapes, rice, and sugar beet. All plants except apples, were treated with tebufenozide with all three radiolabels, apples only with material labelled in the A-ring.
Grapes. Grape vines (mature Concord) were treated once with [14C]tebufenozide applied as a 10% EC formulation at a nominal rate of 1.2 kg ai/ha. Application was with a backpack compressed air sprayer. Samples of leaves and fruit were collected 0, 15 and 31 (harvest) days after treatment. Leaves were taken only to determine the total radioactivity, not for metabolite identification (Hawkins et al., 1991).
Samples were extracted with aqueous methanol which extracted more than 98% of the 14C of all three labels.
Residues in the extract were partitioned between ethyl acetate and water. The ethyl acetate fractions were analysed by HPLC and TLC. Only one radioactive component was observed and confirmed to be parent tebufenozide, which is evidently not metabolized by grapes. The three labels gave similar results, which are shown in Table 9.
Table 9. Residues in grapes and leaves after application of [14C]tebufenozide to vines (Hawkins, 1991).
|
Sample |
14C, mg/kg as tebufenozide, at intervals (days) |
||||||||
|
A-ring label |
B-ring label |
t-butyl label |
|||||||
|
0 |
15 |
31 |
0 |
15 |
31 |
0 |
15 |
31 |
|
|
Leaves |
187.3 |
41.4 |
93.4 |
136 |
39.3 |
36.4 |
229.4 |
119 |
115 |
|
Grapes |
1.95 |
0.72 |
1.0 |
0.35 |
0.69 |
1.27 |
3.15 |
1.64 |
2.45 |
Rice. The metabolism of tebufenozide in rice was studied in a field experiment in California. Four 7.2 m2 plots were lined with plastic, filled with 45 cm of a sandy loam soil and flood-irrigated after planting until final harvest. Tebufenozide, labelled in all three positions, was applied three months after planting at a nominal rate of 1.2 kg ai/ha, higher than the expected use rate. One plot served as a control. Straw, grain, paddy soil and paddy water samples were collected before and immediately after application, then at 15 days, 30 days, and at mature harvest after 64 days (Randazzo, 1992).
Straw and grain samples were chopped in a blender and ground to a fine powder in a mill. Mature grain was hulled before processing. The total radioactivity in the rice samples was determined by combustion analysis. The results are given in Table 10.
Table 10. Total radioactivity in rice treated with [14C]tebufenozide (Randazzo, 1992).
|
Sample |
Days after treatment |
14C, mg/kg as tebufenozide |
||
|
A-ring label |
B-ring label |
t-butyl label |
||
|
Straw |
15 |
25.23 |
38.16 |
30.0 |
|
Straw |
30 |
36.59 |
27.1 |
37.46 |
|
Straw |
64 |
62.3 |
68.32 |
23.68 |
|
Immature grain |
15 |
3.06 |
3.0 |
3.11 |
|
Immature grain |
30 |
2.28 |
3.18 |
2.6 |
|
Mature grain (hulled) |
64 |
0.33 |
0.40 |
0.29 |
|
Hulls |
64 |
7.03 |
13.86 |
11.19 |
The residues in the straw, grain and hull samples were extracted with acidified aqueous acetonitrile and partitioned into organic and aqueous fractions. Most of the activity was found in the organic fraction, which was analysed after solid-phase extraction by TLC and HPLC with radiometric detection. The average recoveries were 80.6-99.1% from straw, 92-105% from grain, and 97% from hulls. The main residue in all the samples was found to be the parent compound. Four metabolites were isolated, none of which accounted for more than 10% of the sample activity. The metabolites were identified by mass spectrometry. The proposed metabolic pathway is shown in Figure 5. The metabolic profile in the final harvest samples is shown in Table 11.
Table 11. Residues in rice at harvest after treatment with [14C]tebufenozide.
|
Sample |
Compound |
% of A-ring label |
total recovered B-ring label |
radioactivity t-butyl label |
|
Straw |
Tebufenozide |
78.7 |
77.9 |
71.9 |
|
|
RH-0970 |
1.2 |
1.2 |
1.1 |
|
|
RH-6595 |
1.1 |
1.3 |
1.3 |
|
|
RH-1788 |
1.8 |
2.0 |
2.8 |
|
|
RH-9886 |
1.3 |
1.2 |
1.5 |
|
Hulls |
Tebufenozide |
58.2 |
63.0 |
62.6 |
|
|
RH-0970 |
1.0 |
1.2 |
1.6 |
|
|
RH-6595 |
4.8 |
3.6 |
3.9 |
|
|
RH-1788 |
9.4 |
6.6 |
6.9 |
|
|
RH-9886 |
1.1 |
1.2 |
1.3 |
|
Grain |
Tebufenozide |
51.7 |
49.5 |
52.0 |
|
|
RH-0970 |
1.5 |
1.4 |
1.5 |
|
|
RH-6595 |
4.6 |
4.8 |
4.8 |
|
|
RH-1788 |
8.8 |
9.4 |
9.8 |
|
|
RH-9886 |
1.2 |
1.1 |
1.2 |
The post-extraction solids contained 9.5-17% of the total activity. The activity from one straw and one grain sample was released by mild basic hydrolysis and found to represent mainly the parent compound, with small amounts of the metabolites found in the primary extraction.
Sugar beet. Metabolism in sugar beet was studied in a field experiment in California. The three 14C-labelled versions of tebufenozide were each isotopically diluted with the corresponding 14C-labelled or unlabelled compound.
Each labelled tebufenozide was used as a 5% EC to treat a separate plot of sugar beet at a nominal rate of 2.24 kg ai/ha. One plot was untreated as a control. Sugar beet tops and roots were harvested at 0, 30, 61, and 120 days after treatment. The samples taken at day 0 were not analysed.
Root samples were rinsed with water, air-dried and homogenized in liquid nitrogen. Leaf samples were homogenized without rinsing. The total radioactivity in the samples, determined by combustion radioanalysis and expressed as mg/kg tebufenozide equivalent, are shown in Table 12. The residue levels in the control samples were all 0.01 mg/kg (Wu, 1993a).
Table 12. Residues in sugar beet after treatment with [14C]tebufenozide (Wu, 1993a).
|
Sample |
Days after treatment |
14C, mg/kg as tebufenozide |
||
|
A-ring label |
B-ring label |
t-butyl label |
||
|
Tops |
30 |
2.75 |
4.09 |
2.63 |
|
|
61 |
0.76 |
1.13 |
0.96 |
|
|
120 |
0.44 |
0.27 |
0.56 |
|
Roots |
30 |
0.40 |
0.84 |
0.44 |
|
|
61 |
0.35 |
0.66 |
0.57 |
|
|
120 |
0.16 |
0.23 |
0.13 |
Most of the residue, 38-59% in the final harvest beet tops and 59-83% in the roots, was found to be the parent compound, confirmed by isolation and mass spectrometry. In addition twelve metabolites were isolated and identified, none of which accounted for more than 10% of the total activity. Four of them (RH-2703, RH-2631, RH-0126, and RH-0897) were also found as conjugates in small quantities. The proposed metabolic pathways are shown in Figure 6, and the distribution of metabolites with the B-ring label in the final harvest samples is shown in Table 13.
Figure 5. Proposed metabolic pathways of tebufenozide in rice.
Table 13. Distribution of radioactivity in sugar beet treated with tebufenozide labelled in the B-ring (Wu, 1993a).
|
Compounds |
% of total radioactivity |
|
|
Tops |
Roots |
|
|
Tebufenozide |
41.4 |
66.6 |
|
RH-6595 |
0.65 |
2.8 |
|
RH-9886 |
0.05 |
0.06 |
|
RH-112652 |
0.04 |
- |
|
RH-1788 |
1.7 |
0.8 |
|
RH-2703 |
2.6 |
0.8 |
|
RH-2703 conj. |
2.3 |
0.73 |
|
RH-9871 |
0.1 |
0.01 |
|
RH-2631 |
3.4 |
- |
|
RH-2631 conj. |
0.68 |
0.16 |
|
RH-0282 |
2.3 |
2.8 |
|
RH-0126 |
1.96 |
0.86 |
|
RH-0126 conj. |
3.6 |
- |
|
RH-0875 |
0.7 |
0.09 |
|
RH-0897 |
3.5 |
1.0 |
|
RH-0897 conj. |
6.5 |
2.5 |
|
RH-120898 |
. |
2.2 |
The main residue found in the tops and roots was the parent compound. Metabolism via oxidation of the alkyl substituents on both aromatic rings produced metabolites with varied sites and degrees of oxidation. None of the metabolites exceeded 10% of the total residue. No differences were observed between the products with the three radiolabels.
Apples. Tebufenozide can be applied to apples at various times during the whole growing season from bloom until shortly before harvest. Metabolism was studied in a field trial simulating early-season and mid-season applications at high rates, with tebufenozide uniformly labelled with 14C in the A-ring. One apple tree was treated twice with a 35-day interval at 1.12 kg ai/ha giving a total of 2.24 kg ai/ha. Foliage was sampled after the first treatment, and fruit and foliage were both sampled before and immediately after, 29 days after, and 68 days after the second treatment when the fruit was ripe at the final harvest (Wu, 1993b).
Figure 6. Proposed metabolic pathways of tebufenozide in sugar beet.
Fruit and foliage samples were homogenized and the total radioactive residues determined by combustion radioanalysis. The results are given in Table 14.
Table 14. Residues in apple foliage and fruit after application of [14C]tebufenozide (Wu, 1993b).
|
Sample |
14C, mg/kg as tebufenozide |
|
|
Fruit |
Foliage |
|
|
Post-treatment 1 |
- |
106 |
|
Pre-treatment 2 |
1.34 |
23 |
|
Post-treatment 2 |
5.34 |
188 |
|
29 days after 2 |
0.32 |
48 |
|
Final harvest (68 days after 2) |
0.21 |
27 |
The metabolitic profiles of the fruit samples at both 29 and 68 days after the second treatment were determined by analysis of the organic extracts by TLC and HPLC with radiometric detection. Residues left in the aqueous layers (12-13% of the total) were hydrolyzed by treatment with cellulase and determined similarly. The main residue was found to be the parent compound. Four metabolites were isolated and identified, none of which represented more than about 6% of the sample activity. One metabolite, RH-1788, was found in both free and conjugated forms. RH-0282 and RH-2778 were found only as conjugates. The metabolic profiles in the fruit samples are shown in Table 15.
Table 15. Metabolite profiles in apple samples after application of [14C]tebufenozide.
|
Compound |
14C at 29 days |
14C at 68 days (harvest) |
||
|
% of total |
mg/kg as tebufenozide |
% of total |
mg/kg as tebuconozide |
|
|
tebufenozide |
71.2 |
0.22 |
77.26 |
0.165 |
|
RH-1788 |
4.96 |
0.021 |
2.49 |
0.008 |
|
RH-1788 conj. |
1.52 |
|
1.47 |
|
|
RH-9886 |
0.21 |
0.001 |
0.20 |
0.0000 |
|
RH-0282 conj. |
6.02 |
0.02 |
4.32 |
0.009 |
|
RH-2778 conj. |
2.46 |
0.008 |
2.71 |
0.006 |
|
Total |
86.37 |
0.27 |
88.45 |
0.188 |
The metabolic profile in the foliage sample from the final harvest was also determined. The residue was almost entirely parent tebufenozide (>93%). The only metabolite identified was RH-0282, present in both free and conjugated forms at levels of 0.24 and 0.52%, respectively, of the total activity. At least 13 unknowns were also present in the foliage, none accounting for more than 1.88% of the total activity.
The proposed metabolic pathways for tebufenozide in apples are shown in Figure 7.
Figure 7. Proposed metabolic pathways of tebufenozide in apples.
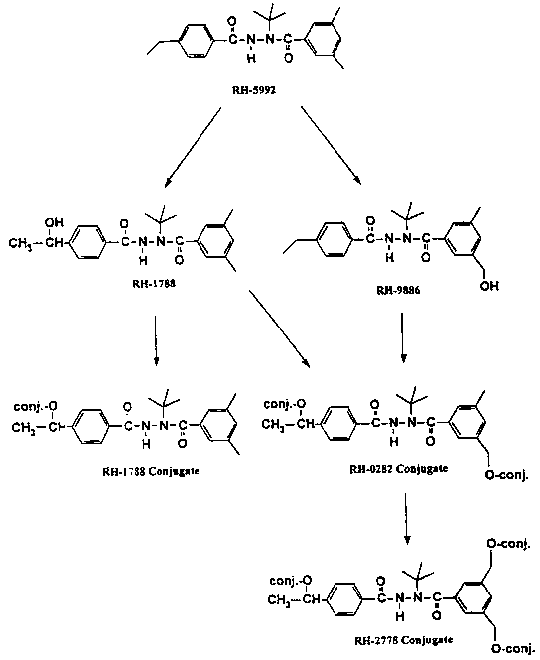
Note: "conj." marks possible locations of conjugates.