5.1 Introduction
5.2 Biophotolysis of water by microalgae and cyanobacteria
5.3 Hydrogen from organic compounds
5.4 Enhancement of hydrogen-producing capabilities through genetic engineering
5.5 Research and development on biological hydrogen production
5.6 Future prospects
References
Hydrogen gas is seen as a future energy carrier by virtue of the fact that it is renewable, does not evolve the "greenhouse gas" CO2 in combustion, liberates large amounts of energy per unit weight in combustion, and is easily converted to electricity by fuel cells. Biological hydrogen production has several advantages over hydrogen production by photoelectrochemical or thermochemical processes. Biological hydrogen production by photosynthetic microorganisms for example, requires the use of a simple solar reactor such as a transparent closed box, with low energy requirements. Electrochemical hydrogen production via solar battery-based water splitting on the hand, requires the use of solar batteries with high energy requirements.
Low conversion efficiencies of biological systems can be compensated for, by low energy requirements and reduced initial investment costs. Moreover, in laboratory experiments, a light energy conversion efficiency as high as 7% has been obtained using a photoheterotrophic process (Fig. 5-1). The basic characteristics of biological hydrogen production and experiments designed to improve the feasibility of biological hydrogen production, particularly through the use of photosynthetic microorganisms, are described in this Chapter. Though not described in the text of this Chapter, progress has also been made in research on anaerobic fermenters (see, for example, ref. 1).
5.2.1 Hydrogenase-dependent hydrogen production
5.2.2 Nitrogenase-dependent hydrogen production
Microalgae are primitive microscopic plants living in aqueous environments. Cyanobacteria, formerly known as blue-green algae, are now recognized as bacteria since the anatomical characteristics of their cells are prokaryotic (bacterial type). Miroalgae and Cyanobacteria along with higher plants, are capable of oxygenic photosynthesis according to the following reaction:
CO2 + H2O ® 6
[CH2O] + O2.
organic compounds
Photosynthesis consists of two processes: light energy conversion to biochemical energy by a photochemical reaction, and CO2 reduction to organic compounds such as sugar phosphates, through the use of this biochemical energy by Calvin-cycle enzymes. Under certain conditions, however, instead of reducing CO2, a few groups of microalgae and Cyanobacteria consume biochemical energy to produce molecular hydrogen (Fig. 5-2; 5-3). Hydrogenase and nitrogenase enzymes are both capable of hydrogen production.
Gaffron and Rubin (3) reported that a green alga, Scenedesmus, produced molecular hydrogen under light conditions after being kept under anaerobic and dark conditions.
Figure 5.1 - Components of a hydrogen production system
A 25 to 30% sugar concentration was obtained regardless of the sugar concentration of the initial saccharified solution. Table 3-8 shows the results of the RO membrane operation using filtrate derived from UF membrane-treated low-concentration saccharified solutions.
Figure 5.2 - Hydrogenase-mediated hydrogen production
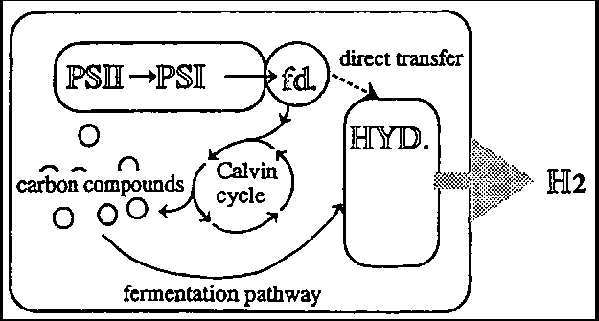
Figure 5.3 Nitrogenase-mediated hydrogen production in heterocystous cyanobacteria
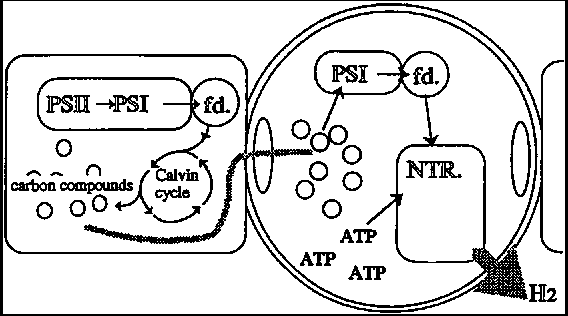
Hydrogenase, the enzyme responsible for this hydrogen production, catalyses the following reaction:
(2H+ + 2Xreduced ® 6 H2 + 2Xoxidized)
The electron carrier, X, is thought to be ferredoxin. Since ferredoxin is reduced with water as an electron donor by the photochemical reaction, green alga are theoretically water-splitting microorganisms.
Following Gaffron and Rubin's work, basic studies on the mechanisms involved in hydrogen production have determined that the reducing power (electron donation) of hydrogenase does not always come from water, but may sometimes originate intracellularly from organic compounds such as starch. The contribution of the decomposition of organic compounds to hydrogen production, is dependent on the algal species concerned, and on culture conditions. Even when organic compounds are involved in hydrogen production, an electron source can be derived from water, since organic compounds are synthesized by oxygenic photosynthesis. The reason for hydrogenase inactivity in green algae under normal photosynthetic growth conditions is unclear. Hydrogenase is thought to become active in order to excrete excess reducing power under specific conditions, such as anaerobic conditions.
The oil crisis in 1973 prompted research on biological hydrogen production, including photosynthetic production, as part of the search for alternative energy technologies. Green algae were known as light-dependent, water-splitting catalysts, but the characteristics of their hydrogen production were not practical for exploitation. Hydrogenase is too oxygen-labile for sustainable hydrogen production: light-dependent hydrogen production ceases within a few to several tens of minutes since photosynthetically produced oxygen inhibits or inactivates hydrogenases. A continuous gas flow system designed to maintain low oxygen concentrations within the reaction vessel, was employed in basic studies (4), but has not been found practically applicable.
Greenbaum and co-workers reported very high (10 to 20%) efficiencies of light conversion to hydrogen, based on PAR (photosynthetically active radiation which includes light energy of 400-700nm in wavelength). These authors recently reported what may represent a "short circuit" of photosynthesis, whereby hydrogen production and CO2 fixation occurred by a single photosystem (photosystem II only) of a Chlamydomonas mutant (5).
Green algae are applicable in another method of hydrogen production. The work of Gaffron and Rubin (3) demonstrated that Scenedesmus produced hydrogen gas not only under light conditions, but also produced it fermentatively under dark anaerobic conditions, with intracellular starch as a reducing source. Although the rate of fermentative hydrogen production per unit of dry cell weight, was less than that obtained through light-dependent hydrogen production, hydrogen production was sustainable due to the absence of oxygen. On the basis of experiments conducted on fermentative hydrogen production under dark conditions, Miura and Miyamoto's group (6) proposed hydrogen production in a light/dark cycle. According to their proposal, CO2 is reduced to starch by photosynthesis in the daytime (under light conditions) and the starch thus formed, is decomposed to hydrogen gas and organic acids and/or alcohols under anaerobic conditions during nighttime (under dark conditions). The technological merits of this proposal include the fact that oxygen-inactivation of hydrogenase can be prevented through maintenance of green algae under anaerobic conditions, nighttime hours are used effectively, temporal separation of hydrogen and oxygen production does not require gas separation for simultaneous water-splitting, and organic acids and alcohols can be converted to hydrogen gas by photosynthetic bacteria under light conditions (Section 5.3). A pilot plant using a combined system of green algae and photosynthetic bacteria was operated within a power plant of Kansai Electric Power Co. Ltd. (Nankoh, Osaka, Japan). Miyamoto and co-workers (7) recently proposed chemical digestion of algal biomass as a means of producing substrates for photosynthetic bacteria, thus improving the yield of starch degradation.
Asada and Kawamura (8) determined that cyanobacteria also produce hydrogen gas auto-fermentatively under dark and anaerobic conditions. Spirulina species were demonstrated to have the highest activity among cyanobacteria tested. The nature of the electron carrier for hydrogenase in cyanobacteria is still unclear. Hydrogenases have been purified and partially characterized in a few cyanobacteria and microalgae (9).
Benemann and Weare (10) demonstrated that a nitrogen-fixing cyanobacterium, Anabaena cylindrica, produced hydrogen and oxygen gas simultaneously in an argon atmosphere for several hours. Nitrogenase is responsible for nitrogen-fixation (11) and is distributed mainly among prokaryotes, including cyanobacteria, but does not occur in eukaryotes, under which microalgae are classified. Molecular nitrogen is reduced to ammonium with consumption of reducing power (e' mediated by ferredoxin) and ATP. The reaction is substantially irreversible and produces ammonia:
N2 + 6H1+ + 6e- ® 2HN312ATP12(ADP+Pi)
However, nitrogenase catalyzes proton reduction in the absence of nitrogen gas (i.e. in an argon atmosphere).
2H+ + 2e- ® H24ATP4(ADP+Pi)
Hydrogen production catalyzed by nitrogenase occurs as a side reaction at a rate of one-third to one-fourth that of nitrogen-fixation, even in a 100% nitrogen gas atmosphere.
Nitrogenase itself is extremely oxygen-labile. Unlike in the case of hydrogenase, however, cyanobacteria have developed mechanisms for protecting nitrogenase from oxygen gas and supplying it with energy (ATP) and reducing power. The most successful mechanism is the localization of nitrogenase in the heterocysts of filamentous cyanobacteria (Fig. 5-3). Vegetative cells (ordinary cells) in filamentous cyanobacteria carry out oxygenic photosynthesis. Organic compounds produced by CO; reduction are transferred into heterocysts and are decomposed to provide nitrogenase with reducing power. ATP can be provided by PSI-dependent and anoxygenic photosynthesis within heterocysts. Investigations into prolongation and optimization of hydrogen production, (12, 13) revealed that the hydrogen-producing activity of cyanobacteria was stimulated by nitrogen starvation.
The presence and physiological roles of hydrogenases in nitrogen-fixing cyanobacteria remains controversial, but 'uptake' hydrogenase appears to consume and re-use hydrogen gas, resulting in a decrease in net hydrogen production. Asada and Kawamura (14) reported aerobic hydrogen production by a nitrogen-fixing Anabaena sp., believed to be an uptake hydrogenase-deficient strain. After being cultured for 12 days, the strain accumulated approximately 10% hydrogen and 70% oxygen gas within the gas phase of the vessel, by the nitrogenase side reaction, even in the presence of air.
Miyamoto et al. (15) conducted outdoor experiments on hydrogen production by Anabaena cylindrica, in California. A nitrogen-starved culture of the cyanobacteria was continuously sparged by argon-based gas, while the hydrogen content of the effluent gas was measured. The average conversion efficiency over a period of one month (combustion energy of hydrogen gas produced by cyanobacteria/incident solar energy into the photo-bioreactor area) was approximately 0.2%.
Mitsui and co-workers extensively screened cyanobacteria for their hydrogen-producing ability, and tested Miami BG-7, one of the most potent hydrogen-producing cyanobacteria, in an outdoor culture (16). These workers also isolated a unicellular aerobic nitrogen fixer, Synechococcus sp. Miami BG043511, and with the use of synchronous culture techniques, discovered a new mechanism for protecting and driving oxygen-labile nitrogenase in non-heterocystous and oxygen-evolving cells (17). This strain is also a potent hydrogen-producer, having an estimated conversion efficiency of 3.5% based on PAR using an artificial light source (18).
5.3.1 Hydrogen production by photosynthetic bacteria
5.3.2 Combined photosynthetic and anaerobic and bacterial hydrogen production
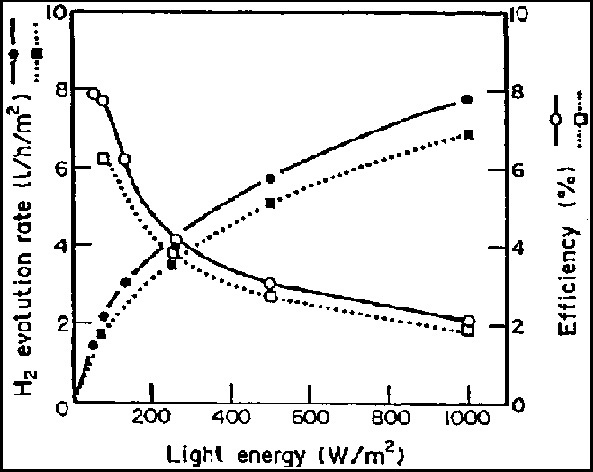
Photosynthetic bacteria undergo anoxygenic photosynthesis with organic compounds or reduced sulfur compounds as electron donors. Some non-sulfur photosynthetic bacteria are potent hydrogen producers, utilizing organic acids such as lactic, succinic and butyric acids, or alcohols as electron donors. Since light energy is not required for water oxidation, the efficiency of light energy conversion to hydrogen gas by photosynthetic bacteria, is in principle much higher than that by cyanobacteria. Hydrogen production by photosynthetic bacteria is mediated by nitrogenase activity, although hydrogenases may be active for both hydrogen production and hydrogen uptake under some conditions. Miyake and Kawamura demonstrated a maximum energy conversion efficiency (combustion energy of hydrogen gas produced/incident light energy) of 6 to 8% using Rhodobacter sp. in laboratory experiments (Fig. 5-4).
Figure 5.4 - Effect of light intensity on the rate and efficiency of hydrogen production in Rhodobacter species (Miyake and Kawamura, 1987)
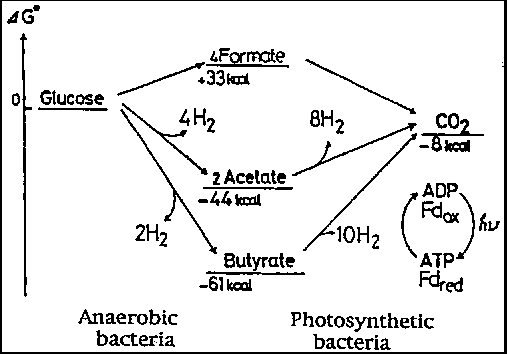
Anaerobic bacteria metabolize sugars to produce hydrogen gas and organic acids, but are incapable of further breaking down the organic acids formed. Miyake et al. (20) proposed the combined use of photosynthetic and anaerobic bacteria for the conversion of organic acids to hydrogen. Theoretically, one mole of glucose can be converted to 12 moles of hydrogen (Fig. 5-5) through the use of photosynthetic bacteria capable of capturing light energy in such a combined system. From a practical point of view, organic wastes frequently contain sugar or sugar polymers. It is not however easy to obtain organic wastes containing organic acids as the main components. The combined use of photosynthetic and anaerobic bacteria should potentially increase the likelihood of their application in photobiological hydrogen production.
Figure 5.5 - Free energy changes in hydrogen-producing reactions by anaerobic an photosynthetic bacteria (Miyake, et al., 1984
Although genetic studies on photosynthetic microorganisms have markedly increased in recent times, relatively few genetic engineering studies have focused on altering the characteristics of these microorganisms, particularly with respect to enhancing the hydrogen-producing capabilities of photosynthetic bacteria and cyanobacteria. As described in Section 5.3.2., some nitrogen-fixing cyanobacteria are potential candidates for practical hydrogen production. Hydrogen production by nitrogenase is, however, an energy-consuming process due to hydrolysis of many ATP molecules. On the other hand, hydrogenase-dependent hydrogen production by cyanobacteria and green algae is "economic" in that there are no ATP requirements. This mechanism of hydrogen production is not however sustainable under light conditions. Water-splitting by hydrogenase is potentially an ideal hydrogen-producing system. Asada and co-workers (21, 22) attempted to overexpress hydrogenase from Clostridium pasteurianum in a cyanobacterium, Synechococcus PCC7942, by developing a genetic engineering system for cyanobacteria. These workers also demonstrated that clostridial hydrogenase protein, when electro-induced into cyanobacterial cells is active in producing hydrogen by receiving electrons produced by photosystems (23).
Another strategy being examined is the enhancement of hydrogen-producing capabilities of photosynthetic bacteria. In nitrogenase-mediated hydrogen-producing reactions, a considerable amount of light energy which is converted to biochemical energy by the photosystem, is lost through various biochemical processes. Control of the photosystem at an appropriate level for nitrogenase activity, would result in reduced energy losses, and thus improved light energy conversion. To this end, with the objective of utilizing genetic engineering techniques in controlling the photosystem level in the potent hydrogen-producing photosynthetic bacteria Rhodobacter sphaeroides RV, the puf operon encoding photoreaction center and light-harvesting proteins was isolated and characterized (24).
As explained in the introduction to this chapter, biological hydrogen production is now receiving much attention as an environmentally acceptable technology. Although a few research groups are active in basic or applied fields of hydrogen production, recent world-wide environmental problems have prompted the formation of national projects for biological hydrogen production. The German Federal Ministry for Research and Technology funded a biological hydrogen production project (1989-1994), in which universities undertook basic research. In Japan, the Ministry of International Trade and Industry is promoting a project for biological hydrogen production by environmentally acceptable technology (1991-1998) through the Research Institute of Innovative Technology for the Earth (RITE), with financial support of the New Energy and Industrial Development Organization (NEDO). The project includes development of total technologies for biological hydrogen production, the screening and breeding of microorganisms, and basic research and development of photobioreactors and anaerobic bioreactors.
The Hydrogen Committee of the International Energy Agency (IEA, under the auspices of the OECD) has rearranged Annex Committees for hydrogen technologies. The target of Annex 10 is the photoproduction of hydrogen. This consists of three subtasks: i) photoelectrochemical hydrogen production, ii) photobiological hydrogen production, and iii) standardization. The three-year plan (1995-1997) aims to establish a closely collaborative world-wide research network to promote hydrogen production technologies.
Biological hydrogen production is the most challenging area of biotechnology with respect to environmental problems. The future of biological hydrogen production depends not only on research advances, i.e. improvement in efficiency through genetically engineering microorganisms and/or the development of bioreactors, but also on economic considerations (the cost of fossil fuels), social acceptance, and the development of hydrogen energy systems.
|
1. |
Ueno, Y. et al, J. Ferment. Bioeng., 79, 395-397 (1995). |
|
|
|
|
2. |
Miyake, J., SEN-I Gakkaishi [in Japanese], 48, 33-37 (1992). |
|
|
|
|
3. |
Gaffron, H. and Rubin, J.,J. Gen. Physiol, 26, 219-240 (1942). |
|
|
|
|
4. |
Greenbaum, E., Biophysical J., 54, 365-368 (1988). |
|
|
|
|
5. |
Greenbaum, E. et al., Nature, 376, 438-441 (1995). |
|
|
|
|
6. |
Miura, Y. et al., Agr. Biol. Chem, 50, 2837-2844 (1986). |
|
|
|
|
7. |
Ike, A. et al., J. Marine Biotech., 4, 47-51 (1996). |
|
|
|
|
8. |
Asada, Y. and Kawamura, S., J. Ferment. Tech., 64, 553-556 (1986). |
|
|
|
|
9. |
Schulz, R., J. Marine Biotech., 4, 16-22 (1996). |
|
|
|
|
10. |
Benemann, J.R. and Weare, N.M., Science, 184, 174-175 (1974). |
|
|
|
|
11. |
Miyake, J. et al., In "Biomass Handbook" Eds. Kitani, O. and Hall, C.W., 362-370 (1989) Gorton and Breach Science Publishers, New York. |
|
|
|
|
12. |
Weissman, J.C. and Benemann, J.R., Appl. Environ. Microbiol., 33, 123-131 (1977). |
|
|
|
|
13. |
Miyamoto, K. et al., Appl. Environ. Microbiol., 28, 440-446 (1979). |
|
|
|
|
14. |
Asada, Y. and Kawamura, S.,Appl. Environ. Microbiol., 51, 1063-1066 (1986). |
|
|
|
|
15. |
Miyamoto, K. et al., J. Ferment. Technol, 52, 287-293 (1979). |
|
|
|
|
16. |
Phlips, E.J. and Mitsui, A., In "Advance in Photosynthetic Research" Ed. Sysbesma, C., Vol. 2 801-804 (1984) Martinus Nijhoff/Dr. W. Junk Publishers, Hague. |
|
|
|
|
17. |
Mitsui, A. et al., Nature, 323, 720-722 (1986). |
|
|
|
|
18. |
Kumazawa, S. and Mitsui, A., Biotech. and Bioeng., 44, 854-858 (1994). |
|
|
|
|
19. |
Miyake, J. and Kawamura, S., Int. J. Hydrogen Energy, 39, 147-149 (1987). |
|
|
|
|
20. |
Miyake, J. et al., J. Ferment. Technol, 62, 531-535 (1984). |
|
|
|
|
21. |
Miyake, M. et al., J. Marine Biotech., 4, 61-63 (1996). |
|
|
|
|
22. |
Aoyama, K. et al., J. Marine Biotech., 4, 64-67 (1996). |
|
|
|
|
23. |
Miyake, M. and Asada, Y., In "Abstracts of 4th Int. Conference on the Mol. Biol. Hydrogenases" 104-105 (1994). |
|
|
|
|
24. |
Nagamine, Y. et al., J. Marine Biotech., 4, 34-37 (1996). |