A.K. FOK, M.S. UENO and R.D. ALLEN
Pacific Biomedical Research Center and Department of Microbiology
University of Hawaii
Honolulu, Hawaii 96822, USA
Results
Summary
Acknowledgements
References
Food or digestive vacuoles (DVs) in Paramecium have been the subject of investigations for more than a century. Because of their large size and rapid formation, these DVs can be readily studied using light microscopic techniques.1 In fact, phagosomal acidification was first observed in this cell type as early as 1893.2 However, not until the last ten to fifteen years have we come to appreciate the true complexity of the phagosome-lysosome system in this cell. Notwithstanding these complexities, there are major advantages of using this cell as a model to study the control of intracellular digestion. In Paramecium the duration of the digestive cycle is relatively short, and the digestive processes are sufficiently synchronous and separated in time to permit perturbation of individual processes in situ and dissection of these processes via a pulse-chase protocol. Thus, by following a number of parameters such as vacuolar pH, vacuole size, acid phosphatase activity and thin-section and freeze-fracture morphology we have been able to distinguish at least four digestive processes that occur during a digestive cycle.3 These four processes can each be subdivided into at least four steps as outlined in Table 1.
These processes are facilitated in the vacuoles by the sequential addition and/or retrieval of at least three separate morphologically and functionally distinct pools of membrane-bound vesicles. This results in the formation of four morphologically distinct stages of the vacuole, DV-I to DV-IV, with each stage having its own characteristics.4 The discoidal vesicles provide membranes for vacuole formation. The acidosomes are responsible for DV acidification, which kills the ingested microorganisms, denaturing their proteins and preparing the vacuoles for lysosomal fusion. The lysosomes contain a complement of acid hydrolases capable of degrading most of the ingested macromolecules. The degraded products are transported from the vacuole to the cytosol to be used for the cell's growth. Finally, following defecation of the undigestible vacuolar contents from the DV-IV at the cytoproct, the spent DV-IV membranes are retrieved and the resultant vesicles are transported along microtubular ribbons back to the oral region where they are reused in forming new DVs.3
Having obtained a relatively complete picture of the number of processes and steps involved in the digestive cycle, we have now turned our attention to the study of the regulation of these processes, in particular to whether these processes have to take place in a sequential order and how each step or process affects subsequent ones. We used (1) a pulse-chase protocol together with exposing these labelled cells before the onset of each of actin filaments, (2) ionophores that dissipate pH gradients, (3) weak bases that elevate vacuolar and vesicular pH and (4) trifluoperazine, a calmodulin antagonist. These cells were then analysed using acid phosphatase cytochemistry, monoclonal antibodies specific for different membrane pools, and lysosome morphometry, as well as the acidification and degradation assays developed for these purposes. Thus, while the above perturbants may exert additional inhibitory effects on cellular processes not measured in this study, the totality of the experimental data appears to support the idea that the digestive processes behave as a chain reaction where each event exerts some control over the subsequent digestive steps. In this report we attempt to provide a brief summary of our findings on the regulatory mechanisms of the phagosome-lysosome system. The results are divided into three sections, each describing the effects of one of the first three digestive processes on the subsequent process or processes.
DV formation
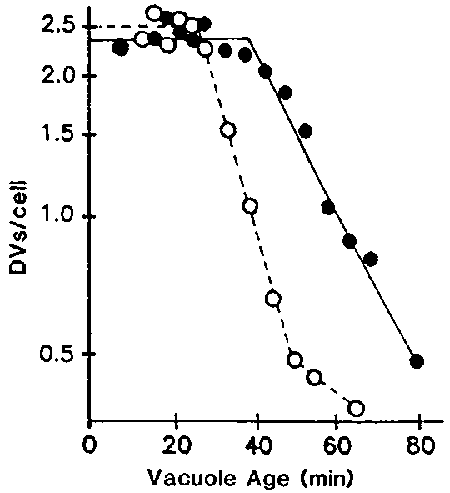
Vacuole formation is the first process in a digestive cycle and obviously is also the fuss, and perhaps the most crucial, regulatory process in a digestive cycle. When DV formation is blocked, all subsequent processes are eliminated. However, when DV formation and the subsequent processes are allowed to proceed normally, we find that the formation rate determines, to a large extent, the defecation rate.5 This conclusion is derived from studying the formation rate when cells were pulsed continuously with latex beads. During such a continuous pulse, the accumulation of labelled DVs could be divided into two periods: an initial period of linear but rapid increase in DV number followed by a second period after the mean number of labelled DVs/cell had reached a plateau that was maintained for the remainder of the pulse (Figure 1). The presence of this plateau indicates that either Paramecium is quickly saturated with DVs after feeding so that no new DVs are formed and no labelled DVs are ingested, or that the rates of formation and defecation are equal. To distinguish between these two possibilities, cells were pulsed with fluorescent beads for one hour and divided into two portions. One portion of the cells was kept in the initial pulse, which showed no net change in DV numbers for the remainder of the pulse, and the second portion was washed briefly to remove the fluorescent beads and fed again with a second label of nonfluorescent beads. In the latter group, the loss of the older fluorescent DVs was linear at a rate identical to their original formation rate during the beginning of the first pulse. The rate of DVs formation after exposure to the second label was also linear and rapid, but when exposed to the larger bead size used in this experiment, the DVs/cell value plateaued at a higher steady level. Since new DVs are continuously being formed while old DVs are continuously being defecated, to attain a steady level of labelled DVs, the defecation rate has to equal the DV formation rate. Not only are the rates of formation and defecation equal, but we also find that the defecation rate appears to be governed by the formation rate. This is confirmed by comparing the formation and defecation rates when cells were pulsed with decreasing bead concentrations. Under these conditions, the DV formation rates were lowered and lower plateau levels of labelled DVs were obtained. For each bead concentration, the defecation rate was reduced to the same extent as the formation rate (Figure 2). These findings suggest that (1) the formation process is the key step in regulating digestion and (2) the defecation rate is governed by the formation rate.
DV acidification effects of blocking acidosome-DV fusion on vacuolar acidifications as well as the lysosome fusion-digestion and defecation processes
As a nascent DV grows in size, acidosomes6,7 bind specifically to its surface. These acidosomes, which are located mostly in the cytopharynx area, are irregularly shaped electron translucent vesicles. They are acid phosphatase negative7 and have a distinctive freeze-fracture morphology8 and deep-etched appearance (unpublished observation). Microfilaments are found in regions where acidosomes bind to the nascent DV and to the very young DV. These microfilaments apparently play an important role in the acidosome-DV fusion, since perturbation by cytochalasin B (CB) results in a block in acidosome DV fusion.7,9
When acidosome-DV fusion was blocked by CB, DV acidification as well as the subsequent third10 and fourth processes of the digestive cycle became severely affected. Acidification of DVs, the pH was measured with indicactor-dye-stained yeast cells, was almost abolished.7 Lysosome-DV binding, as determined by measuring the number of bound lysosomes per m m of cross-sectioned DV-membrane profile (Figure 3), and lysosome-DV fusion, as determined by the appearance of phagolysosomal membrane antigens in the DV membrane (Figure 4) and acid phosphatase in the lumen, were greatly reduced (Figure 5). In our numerous pulse-chase studies we have rarely, if ever, observed lysosomes bound to the newly formed DVs in unperturbed cells, but numerous lysosomes accumulate around the normally acidified DV-II. This resulting lysosome layer bound to the DV-II often becomes highly conspicuous.7 When cells were exposed to 0.3 mM CB, lysosome binding to DVs was practically eliminated; only one 20-min-old DV in the treated cells had associated lysosomes; 1.4 lysosomes/m m of DV-membrane profile. The other eight DVs had none or just a few scattered lysosomes associated with their membranes10 (Figure 3).
To measure the lysosome-DV fusion rate, we used two monoclonal antibodies specific for the phagolysosomal (DV-III) membrane10,11 (Figure 4) jointly with acid phosphatase cytochemistry (Figure 5). In control cells chased after a 3-min pulse in latex beads, the DV-III membrane antigens were not expressed on young DVs. As these DVs aged, they showed increasing binding to these antibodies so that all 20-min-old DVs were positive. The lysosome-DV fusion rates for both control experiments were 5.1%/min as calculated from the linear portion of the curve (Figure 4). In CB-treated cells only 20% of the labelled DVs expressed the phagolysosomal membrane antigens by 20 min. although this had increased to 40% by 45 min. making the lysosome-DV fusion rate only 1.3%/min. This represented a 75% inhibition when compared with the control rate. This inhibition was readily reversible; the removal of CB by washing resulted in normal rates of fusion after a lag of about 15 min. When cells with 3-min-old DVs in which acidification was nearing completion but the lysosome fusion step had not yet begun were exposed to CB, CB did not inhibit the fusion rates but did reduce the maximal extent of fusion to 78% of that in the control cells (Figure 4).
Using acid phosphatase cytochemistry12,13 as a lysosome-DV fusion assay, results obtained from four separate experiments were all similar to those obtained with monoclonal antibodies (Figure 5). When acidosome-DV fusion was blocked by CB, less than 5% of the labelled 15-min-old DVs were acid phosphatase positive. With prolonged exposure to CB, only 15% of the labelled DVs became positive, giving a lysosome-DV fusion rate (0.65%/min) of less than 8% of the control rate. Again, the inhibitory effect of CB was readily reversible; 40% of the DVs became acid phosphatase positive 20 min after a wash, as compared with 50% in the control (Figure 5). Proteolysis of phagocytosed FITC-albumin in CB-treated cells was also reduced to a similar amount (unpublished results). As regards the fourth process, DVs in CB-labelled DVs in untreated cells were not ingested for at least 45 min. whereas labelled DVs in untreated cells could be defecated any time after 20 minutes.14
These results demonstrate that when acidosome-DV fusion is blocked, all subsequent processes are essentially blocked: vacuoles will not be acidified and will not acquire the lysosomal membrane nor the lysosomal acid phosphatase. Finally, proteolysis is essentially prevented and vacuole defecation is greatly delayed.
Effects of inhibition of vacuolar acidification on the lysosome fusion-digestion and defecation processes
NH4Cl has been shown to raise the pH of the endocytic compartments.15 We have used yeast labelled with fluorescein isothiocyanate to show that the vacuolar pH was elevated from 3 to about 5.8 in the presence of 30 mM NH4Cl (unpublished). Though vacuolar acidification was inhibited, this concentration of NH4Cl had no observable inhibitory effect on acidosome-DV fusion. The net effect of this pH elevation that we observed is an inhibition of lysosome-DV fusion. When measured by the Gomori method,13 lysosome-DV fusion was seen to be inhibited by 40% (Figure 6) and proteolysis of phagocytosed FIT-albumin was inhibited by 72% (Figure 7,) with about half of this latter inhibition being attributable to the effects of NH4Cl on proteolysis itself independent of its effects on vacuolar acidification and lysosome-DV fusion.16 Under this treatment the defecation process was also slowed down (unpublished results).
Lysosome fusion-digestion, lysosome-DV fusion
The extent of lysosome-DV fusion will indicate the percentage of DVs converted to phagolysosomes, thus regulating the efficiency of proteolysis. However, it appears that once the acidosomes fuse with the DVs, lysosomes are able to recognize and bind to these DVs.10 Thus, when cells were exposed to either NH4Cl, monensin or FCCP after the acidification process had commenced, little inhibition of lysosome-DV fusion per se was noted.
Lysosomal proteolysis
When cells were pulsed with undigestible materials such as latex beads in axenic medium, the duration of the processing period was 28 min and t½ for defecation was 37 min. However, when cells were pulsed with beads and digestible material such as albumin, vacuolar acidification and lysosome-DV fusion were normal but the retrieval of acid phosphatase was delayed (Figure 8), and the duration of the processing period was extended to 43 min and t½ for defecation was 60 min (Figure 9).17 These results show that the fourth process of defecation is influenced by the duration and efficiency of proteolysis, both of which are influenced by the phagocytosed materials contained within the DVs.
These results show that in Paramecium a chain of events takes place whereby each event exerts some control over the subsequent digestive processes. Thus, when DV formation is blocked, the acidification, lysosome fusion-digestion end defecation processes will not take place. When acidosome-DV fusion is blocked by CB, vacuolar acidification fails to occur. Under the last condition, lysosome-DV binding and fusion as well as proteolysis are inhibited by 90% and the DVs will not begin to be defecated for at least 50 min. whereas labelled DVs from untreated cells start to be defecated after 20 min. When the decrease in vacuolar pH is inhibited by NH4Cl inhibiting acidosome-DV fusion, the secondary effect is a much lower reduction in the lysosome-DV fusion rate than when acidosome-DV fusion is prevented. As any reduction in the rate of lysosome-DV fusion will result in a slower rate of phagosomes being converted to phagolysosomes, proteolysis in these cells was found to be reduced. When the rate of proteolysis is reduced and its completion delayed, retrieval of lysosomal membrane and enzyme will be delayed, which will result in an extension of the digestive period and/or a slower rate of defecation. This results in defecation commencing at a later time. Thus, the overall effect is that the time required to complete a vacuole cycle is dependent on a number of factors operating in a sequential manner on the vacuole. When one of these factors is compromised, the resulting effects will be propagated along the chain of steps. This leads to an extended life of the vacuole as well as a decreased membrane recycling rate.
We thank Ms. Mari Moore for her data for Figures 8 and 9. This research was supported in part by grants from the National Science Foundation, and the MBRS, MARC and RCMI programs of the National Institutes of Health, USA.
1. MAST, S.O. 1947. Biol. Bull. 92: 31-72.
2. METCHNIKOFF, E. 1893. Lectures on the Comparative Pathology of Inflammation. Kegan, Paul, Trench, Truber and Co., London.
3. FOK, A.K. and R.D. ALLEN. 1987. In Paramecium. H.D. Gortz, Ed.: 301-324. Springer-Verlag, Berlin.
4. ALLEN, R.D. and A.K. FOK. 1984. Eur. J. Cell Biol. 35: 149-155.
5. FOK, A.K., B.C. SISON, Jr, M.S. UENO and R.D. ALLEN. 1988. J. Cell Sci. 90: 517-524.
6. ALLEN, R.D. and A.K. FOK. 1983. Eur. J. Cell Biol. 29: 150-158.
7. ALLEN, R.D. and A.K. FOK. 1983. J. Cell Biol. 97: 566-570.
8. ALLEN, R.D. and A.K. FOK. 1983. Eur. J. Cell Biol. 29: 159-165.
9. ALLEN, R.D. and A.K. FOK. 1985. Eur. J. Cell Biol. 37: 35-43.
10. FOK, A.K., M.S. UENO, E.A. AZADA and R.D. ALLEN. 1987. Eur. J. Cell Biol. 43: 412-420.
11. FOK, A.K., M.S. UENO and R.D. ALLEN. 1986. Eur. J. Cell Biol. 40: 1-8.
12. BARKA, T. and P.J. ANDERSON. 1962. J. Histochem. Cytochem. 10: 741-753.
13. GOMORI, G. 1952. Microscopic Histochemistry, Principles and Practice. University of Chicago Press, Chicago.
14. FOK, A.K., S.S.K. LEUNG, D.P. CHUN and R.D. ALLEN. 1985. Eur. J. Cell Biol. 37: 27-34.
15. MAXFIELD, F.R. 1982. J. Cell Biol. 95: 676-681.
16. FOK, A.K. and M.S. UENO. 1987. Eur. J. Cell Biol. 45: 145-150.
17. FOK, A.K. and M.S. UENO. In preparation.
Table 1. The phagosome-lysosome system in Paramecium
|
Four processes |
DV age (min) |
Membrane structure |
pH | |
|
Formation and release of DVs |
|
|
| |
|
|
a. old DV membranes recycle back to cytopharynx |
|
|
|
|
|
b. particles captured by oral and somatic cilia |
0-6 |
discoidal vesicle |
7 |
|
|
c. discoidal vesicles fuse with cytopharynx |
|
|
|
|
|
d. a newly formed DV is released (DV-I) |
|
|
|
|
Acidification-condensation |
|
|
| |
|
|
a. acidosomes bind to the forming DV |
|
|
|
|
|
b. acidosomes fuse with DV-I |
4-10 |
acidosome |
3 |
|
|
c. DV condenses, fission of membrane tubules |
|
|
|
|
|
d. vacuolar pH drops from 7 to 3 (DV-II) |
|
|
|
|
Lysosome fusion-digestion |
|
|
| |
|
|
a. lysosomes bind to acidified DV-II |
|
|
|
|
|
b. lysosomes fuse with DV-II, forming a DV-III |
8-20 |
lysosomes |
3-7 |
|
|
c. proteolysis and DV pH returns to 7 |
|
|
|
|
|
d. lyso. membrane & acid phosphatase retrieved |
|
|
|
|
Defecation |
|
|
| |
|
|
a. lyso. membrane and enzyme retrieval completed |
|
|
|
|
|
b. egestion-competent DV-IV moves to cytoproct |
>20 |
? |
? |
|
|
c. DV and plasma membrane fuse at cytoproct |
|
|
|
|
|
d. retrieval of spent DV membrane |
|
|
|
Figure 1. Cells were pulsed continuously with fluorescent beads of 0.06 m m ( ) or washed with axenic medium for 3 min after 60 min of pulse (top arrow). Immediately after the wash (bottom arrow), cells were pulsed with 0.3 m m non-fluorescent beads and were scored for both fluorescent (
) or washed with axenic medium for 3 min after 60 min of pulse (top arrow). Immediately after the wash (bottom arrow), cells were pulsed with 0.3 m m non-fluorescent beads and were scored for both fluorescent ( ) and non-fluorescent (
) and non-fluorescent (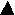 ) bead-labelled DVs.
) bead-labelled DVs.
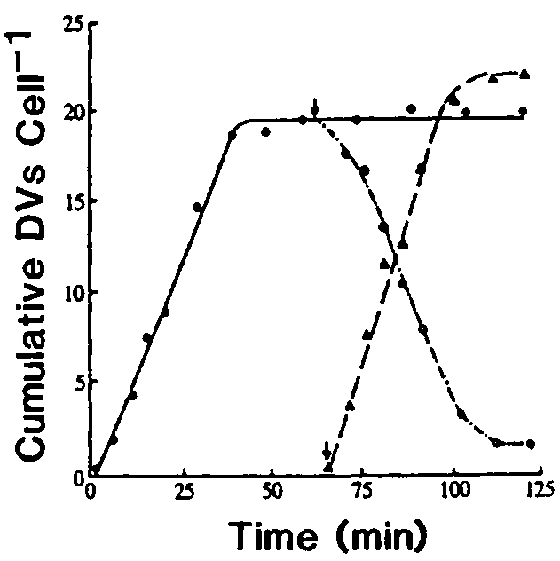
Figure 2. Cells were pulsed with varying concentrations of fluorescent beads of 0.26 m m in diameter for two hours and were fixed at various times during the pulse. After washing, fluorescent DVs in 2 100 cells were scored for each time point. For cells pulsed with the lowest two concentrations of beads, lucifer yellow was added to aid in the visualization of the labelled DVs.
Figure 3. Binding of lysosomes to the DVs in DV-age- and acidification-dependent. Because lysosomes in Paramecium contain a distinctive glycocalyx in their luminal surface and characteristic paracrystalline inclusions, they can be recognized without acid phosphatase cytochemistry. Micrographs used to determine the number of lysosomes per m m length of cross-sectioned DV-membrane profile were randomly selected. In untreated cells, values shown by the closed circles represent the exact DV age, whereas the open circles represent the mean of DV age in cells that were pulsed for 3 min. Cells treated with CB ( , 0.3 mM) were not chased to remove excess latex beads. Each value represents measurements obtained from I to 10 micrographs of various magnifications.
, 0.3 mM) were not chased to remove excess latex beads. Each value represents measurements obtained from I to 10 micrographs of various magnifications.
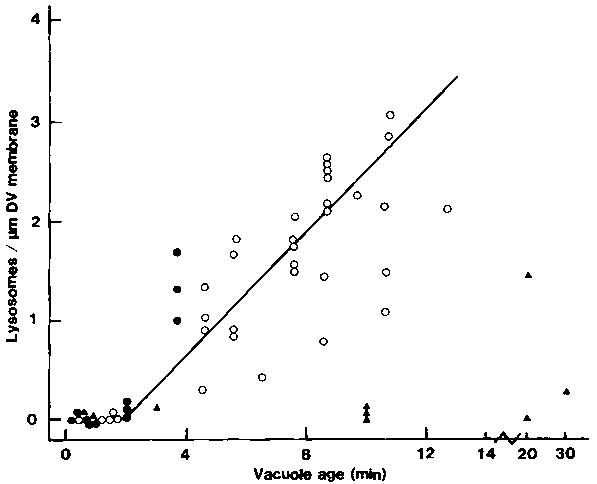
Figure Effects of CB on the rate of lysosome-DV fusion. To
mark the age of the DVs, cells were pulsed with nonfluorescent latex beads for
3 min and chased in fresh axenic medium. Cells were fixed in 4% formalin in
phosphate-buffered saline and acetone permeabilized before being incubated in
either of the two phagolysosome membrane-specific monoclonal antibodies (189D1G12
and 320E7, as represented by the open and closed symbols, respectively), followed
by the FITC-labelled second antibody. For each time, labelled DVs in 2 100 cells
were scored for positive fluorescence and an average value for the percentage
of fluorescence-positive DVs/cell was thus obtained. CB (0.3 mM) was added to
cells with 15-s-old ( ,
, ) or 3-min-old
(
) or 3-min-old
( ,
, ) vacuoles. To test for reversibility
of the CB effects, aliquots of cells (exposed to CB when their DVs were 15-s-old)
were washed (arrow) after 15 min of CB exposure (
) vacuoles. To test for reversibility
of the CB effects, aliquots of cells (exposed to CB when their DVs were 15-s-old)
were washed (arrow) after 15 min of CB exposure ( ,
, ).
Control cells (
).
Control cells (![]() ,
, )
were exposed to the same amount of DMSO (0.2% v/v) as the experimental cells
but not to CB.
)
were exposed to the same amount of DMSO (0.2% v/v) as the experimental cells
but not to CB.
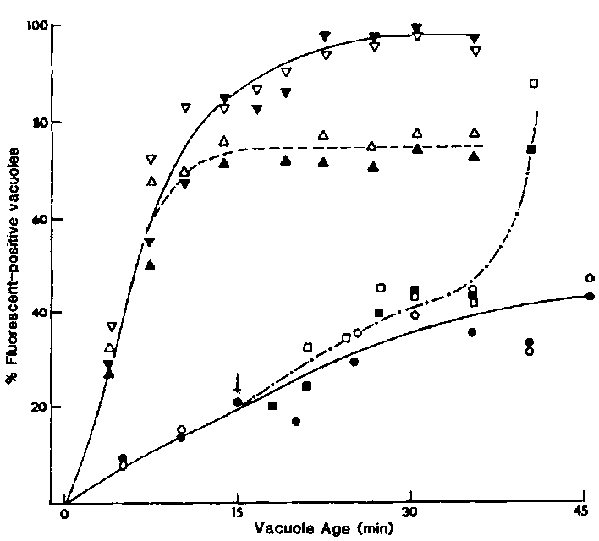
Figure 6. Effects of NH4Cl on the rate of lysosome-DV
fusion when vacuolar acidification was inhibited. Cells were pulsed with small
fluorescent beads for 3 min and then chased. NH4Cl (30 mM) was added
to cells containing 0-min-old DVs ( ). Cells with 3-min-old
DVs (
). Cells with 3-min-old
DVs ( ) were also exposed to this weak base for the purpose
of differentiating their effects on lysosome-DV fusion independent of the DV
acidification process, which was well along at this vacuole age. Vacuoles in
control cells (
) were also exposed to this weak base for the purpose
of differentiating their effects on lysosome-DV fusion independent of the DV
acidification process, which was well along at this vacuole age. Vacuoles in
control cells ( ) were not exposed to NH4Cl.
Cells were fixed in 5% formalin in 0.05 M cacodylate buffer at pH 7.2, washed
in acetate buffer at pH 5.0, and incubated in the Gomori medium13
at 37 DC for 10 min. For each time, 200 fluorescent DVs were each evaluated
for acid phosphatase activity.
) were not exposed to NH4Cl.
Cells were fixed in 5% formalin in 0.05 M cacodylate buffer at pH 7.2, washed
in acetate buffer at pH 5.0, and incubated in the Gomori medium13
at 37 DC for 10 min. For each time, 200 fluorescent DVs were each evaluated
for acid phosphatase activity.
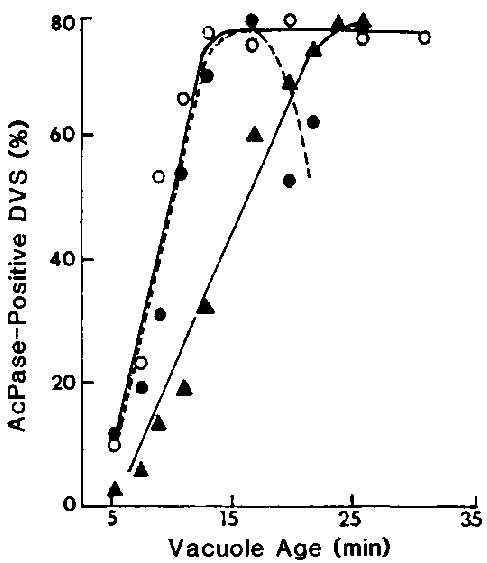
Figure 7. Effects of NH4Cl (30 mM) on the rate of FITC-albumin proteolysis. With ( ) or without (
) or without ( ) this weak base, cells were pulsed with a mixture of latex beads and FITC-albumin from 0 to 3 min and chased. Cells were also treated with NH4Cl at 6 min (
) this weak base, cells were pulsed with a mixture of latex beads and FITC-albumin from 0 to 3 min and chased. Cells were also treated with NH4Cl at 6 min ( ); at this age NH4Cl at 30 mM has been shown to exert no inhibitory effect10 on the lysosome-DV fusion rate. For each time, TCA-soluble fluorescence is expressed in arbitrary units/mg protein.
); at this age NH4Cl at 30 mM has been shown to exert no inhibitory effect10 on the lysosome-DV fusion rate. For each time, TCA-soluble fluorescence is expressed in arbitrary units/mg protein.
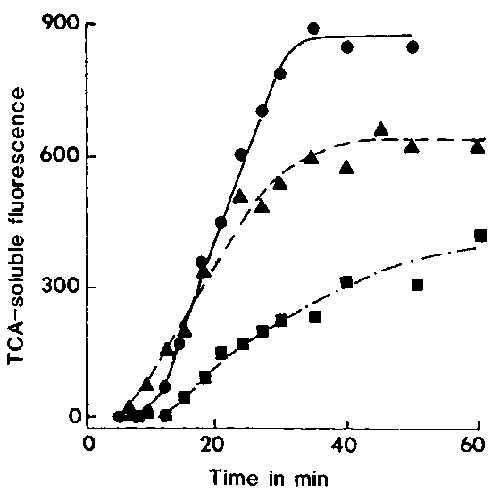
Figure 8. The rate of acid phosphatase retrieval depends on the contents of the vacuoles. Cells were pulsed with fluorescent latex beads with ( ) or without (
) or without ( ) albumin for 3 min and were chased in axenic medium. For each time, 75-225 fluorescent DVs were evaluated for this enzyme activity.
) albumin for 3 min and were chased in axenic medium. For each time, 75-225 fluorescent DVs were evaluated for this enzyme activity.
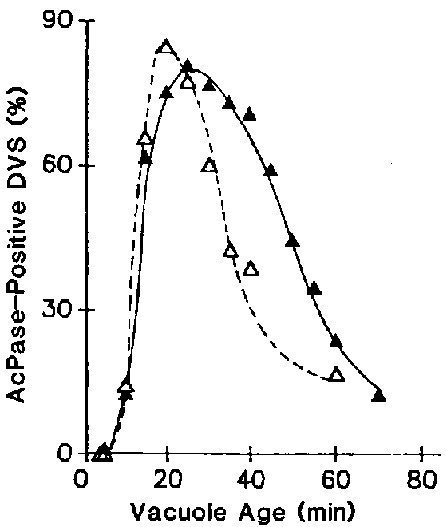
Figure 9. The duration of the processing period (which is defined as the period of time when the DVs are not defecation competent) and the rate Or defecation are dependent on the contents of the vacuoles. Cells were pulsed with latex beads with ( ) or without (
) or without ( ) albumin for 3 min and were chased in axenic medium. Labelled vacuoles were scored for the determination of the processing period and the rate of defecation as described previously.16
) albumin for 3 min and were chased in axenic medium. Labelled vacuoles were scored for the determination of the processing period and the rate of defecation as described previously.16