J.W. SLOT, H.W. SCHUURMAN and H.J. GEUZE
Laboratory of Cell Biology, Medical Faculty
University of Utrecht
The Netherlands
References
Receptor-mediated endocytosis is studied extensively in liver parenchymal cells.1,2 At the sinusoidal, i.e., basolateral cell surface, different receptors and their ligands are trapped in coated pits that pinch-off from the plasma membrane. The vesicles formed lose their clathrin coat and coalesce into a system of anastomosing tubules and connected vesicles, the endocytotic or endosomal compartment. We described this tubulo-vesicular system in relation to the distribution of a receptor that is unique for liver cells, the asialoglycoprotein receptor (ASGPR).3 This receptor binds galactose terminal glycoproteins, which after internalization are mainly destined for lysosomal degradation. This fate awaits many exogenous products that are taken up by adsorptive endocytosis. Endocytotic products accumulate in endosomal vacuoles.4,3 While the vacuoles grow larger, they show increasing numbers of internal vesicles. The vacuoles remain connected to tubular elements. In well-differentiated hepatic parenchymal cells, the endosomal tubules have predominantly a peripheral location and are particularly abundant in the basolateral cell corners. It is here that small endosomal vacuoles seem to arise. In less polarized cells, such as the cultured hepatoma cell lines Hep-G2 (human) and H4S (rat), the endosomal elements occupy less characteristic positions in the cells. In these cells the endosomal tubules and those of the trans-Golgi reticulum (TGR)5 are easily mixed up when observed by electron microscopy (EM). Therefore, in immunocytochemical studies endocytosed markers like cationized ferritin or specific ligands, tagged to electron-dense markers for EM detection, are required to distinguish endosomes from TGR. The elements of the TGR, on the other hand, can be recognized by marking endogenous secretory proteins, such as albumin in liver cells.
Significant differences exist in the molecular composition of the plasma membrane and of the lysosomal membrane. Intensive membrane remodelling along the prelysosomal route seems the obvious way to maintain this difference in spite of a conspicuous membrane flow resulting from the vectorial transport of exogenous material to lysosomes. The intermediate composition of endosomes that we observed for several membrane proteins illustrates that such membrane remodelling indeed takes place (Table 1). In several studies we investigated these changes in the endosomal compartment by observing individual membrane proteins after immunogold labelling in thin cryosections.
Immunocytochemistry showed that the membrane of coated pits is, to some extent, different from the rest of the plasma membrane. Pits were enriched in ligand-receptor complexes. The pits appeared to be non-selective for different receptors. The ASGPR occurred together with the mannose 6-phosphate receptor (MPR), which recognizes the phosphomannosyl residues on lysosomal enzymes. These two receptors also co-localized to coated pits with the receptor for polymeric IgA (IgAR).6 Recently, careful analysis of endosomal elements in cell fractions demonstrated that the transferrin receptor (TFR) system entered Hep-G2 cells together with the ASGPR system.7 Hence, at least 8 different compounds (4 receptors + 4 ligands) enter the same elements of the endosomes. Of only the ligands of ASGPR and MPR, the main destination is the lysosomal compartment. TFR with ligand and ASGPR recycle to the cell surface. The MPR is also known to be absent from lysosomes.8,9 The IgAR with covalently linked ligand is directed to the bile capillary (apical) cell membrane, where it is cleaved from the membrane and secreted. By comparing the distribution of these different molecules in immunodouble-labelled sections, we studied the sites of selective removal of the ASGPR, MPR and the IgAR-ligand complex in liver cells.
Within the peripheral endosomal tubular system we found microdomains enriched in IgAR and ASGPR, indicating a very early sorting of the two receptor systems. This is in agreement with results demonstrating that segregation of endocytosed IgA and ASGPR ligand occurs during the very first minutes after uptake.1
We observed accumulation of ASGPR ligand in the endosome vacuoles, whereas ASGPR was present mainly in the tubules.3 Therefore, we called this tubulo-vesicular compartment CURL, i.e., compartment of uncoupling receptor and ligand.3 Recently, the distribution of ASGPR in endosomes was studied in more detail.10 It was shown that the larger the endosomal vacuoles were, the less ASGPR was present in their limiting membranes (Figures 1, 2). ASGPR seemed to accumulate in the tubules. It was proposed that the ASGPR and ligand dissociate in the acidic environment inside the endosomal vacuoles)11,12 and that the receptor molecules then migrate laterally into the tubules. Since receptor negative vacuoles are often seen in continuity with receptor enriched tubules (Figure 2), we propose an efficient block occurs at the junction of tubules and vacuoles that prevents the receptor from moving back into the vacuoles and the ligand from moving in the reverse direction.
Besides the sorting of IgAR and ASGPR in peripheral early endosomes, we studied the intracellular pathways of two other membrane proteins in endosomes, which occurred predominantly in the deeper (late) endosomes, namely the MPR and a 120-kD lysosomal glycoprotein, LGP120.13 MPR enters the endosomes by coated vesicles together with ASGPR at a concentration that is almost proportional to the concentration of both receptors at the sinusoidal cell surface.6 Nevertheless, quantitation of the immunolabelling in Hep-G2 cells demonstrated that MPR is 5 times more abundant in endosomes than ASGPR relative to their densities at the plasma membrane (Table 1). Most likely this is due to two phenomena. First, MPR is segregated at a later stage from the endosomes than ASGPR. This fits well with the impression that late endosomal vacuoles (large vacuoles with many internal vesicles) were still labelled for MPR. Second, there may occur an additional supply of more MPR molecules to endosomes via another route than coated pits. A significant pool of cellular MPR is localized in the TGR of Hep G29 and H4S cells (Figure 3). In the TGR the receptor and lysosomal enzymes seem to accumulate in characteristic electron-dense, clathrin-coated buds and vesicles. Similar vesicles are often observed adjacent to endosomal vacuoles. This suggests a direct transport of MPR from TGR to endosomes, by-passing the plasma membrane. In the endosomes, MPR is usually enriched in tubules attached to the vacuoles, which indicates that MPR, like ASGPR, is recaptured from the lysosomal route by lateral migration from the vacuoles into the tubules. This would finally lead to the absence of MPR from lysosomes.
It was therefore of interest to compare the MPR distribution with that of the typical lysosomal membrane protein LGP120, which we found at least as abundant in endosomes as in lysosomes of H4S cells (Table 1). This is in agreement with observations of other cell types.14 LGP120 occurred in vacuoles, but not in tubules of endosomes. The cell surface was virtually devoid of LGP120, whereas the Golgi complex and TGR showed less than 5% of the total cellular LGP120 labelling.
We offered H4S cells cationized ferritin (CF) for 5,10,30 or 60 min prior to fixation, prepared cryosections and studied the distribution of MPR and LGP120 by immunolocalization. At each time interval we counted the number of CF containing vacuoles, (i.e., endosomal vacuoles and lysosomes), labelled for MPR and LGP120 (Figure 4). The complete data are reported elsewhere.15 Apparently, CF is taken up in a very short living category of vacuoles in which neither MPR nor LGP120 was detectable. Then a rapidly growing fraction of the CF-positive vacuoles became labelled for MPR, which peaks at 10 min. The increase of MPR labelling during the first minutes is in agreement with the above and suggested additional direct transport of this receptor from TGR to endosomes. Furthermore, the main removal of MPR from the endosomal vacuoles seems to take place after 10 min. The ASGPR, on the other hand, has been reported to be segregated from the degradative route within the first few minutes after endocytosis.7 This is consistent with our impression that MPR, in contrast to ASGPR, occurs in relatively late endosomal vacuoles. The LGP120+ fraction of CF=containing vacuoles increases more slowly than those with MPR. Increasing percentages of 50, 80 and 90% of the MPR+ CF vacuoles were also labelled for LGP120 at 10, 30 and 60 min. respectively (not shown in Figure 4). Conversely, the percentage of LGP120+ CF vesicles that labelled also for MPR decreased according to 75, 50 and 30% at 10, 30 and 60 min. respectively. Since MPR-/LGP120+ vesicles can probably be defined as lysosomal structures, the latter figures give an impression of the rate of uptake of CF in lysosomes. After 1 h approximately two thirds of the CF containing structures are lysosomes.
About half of the LGP120 labelling is located in the endosomal vacuoles (Table 1). Only a very little LGP120 is found in the Golgi area, which is consistent with a proposed fast transport of newly synthesized LGP120 to lysosomes.16 It also suggests that the protein does not recycle in circuits in which Golgi elements or TGR participate, as is probably the case for ASGPR and MPR.9,17,18 Therefore, the inflow of LGP120 into endosomes from the Golgi complex or TGR, either via the cell surface or directly by vesicles, is probably less significant than for the other receptor molecules that we described. Instead, the high concentration of LGP120 in the late vacuoles, is likely to be a result of recycling molecules from the lysosomes back into the endosomal vacuoles although there is no direct evidence for such a recycling route. Small irregular membranous structures rich in LGP120 may function as intermediates. They are found to contain approximately 10% of cellular LGP120 labelling. That with time an increasing percentage of CF positive vesicles marked for MPR also contained LGP120 demonstrates that the bulk of LGP120 enters the endosomes at a later stage than MPR. However, some MPR+/LGP120+ vacuoles were reached by CF very soon after uptake. Therefore, it is likely that LGP120 is added gradually along a relatively long stretch of the endocytotic route.
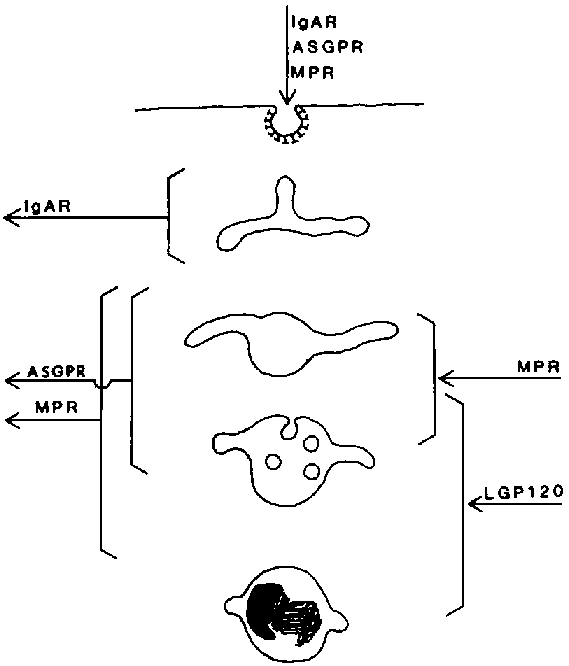
Our observations are summarized in Figure 5. For three receptors and a major lysosomal membrane protein we describe the sites of entry into and segregation from endosomes in hepatocytes. Our observations are in agreement with a gradual transformation of the endosomal membrane from a plasma membrane-like composition immediately after endocytosis into one that resembles more closely the lysosomal membrane.
1. COURTOY, P.J., J. QUINTART, J.N. LIMET, C. de ROE and P. BAUDHUIN. 1985. In Endocytosis. I. Pastan and M.C. Willingham, Eds.: 163-188. Plenum Press, New York.
2. GEUZE, H.J., J.A. VANDERDONK, C.F. SIMMONS, J.W. SLOT, G.J, STROUS and A.L. SCHWARTZ. 1986. Int. Rev. Exptl. Pathol. 29: 113-171.
3. GEUZE, M.J., J.W. SLOT, G.J. STROUS, H.F. LODISH and A.L. SCHWARTZ. 1983. Cell 32: 277-287.
4. WALL, D A., G. WILSON and A.L. HUBBARD. 1980. Cell 21: 79-93.
5. GRIFFITHS, G. and K. SIMONS. 1986. Science 234: 438-442.
6. GEUZE, H.J., J.W. SLOT, G.J. STROUS, J. PEPPARD, K. VONFIGURA, A. HASILIK and A.L. SCHWARTZ. 1984b. Cell 37: 195-204.
7. STOORVOGEL, W., H.J. GEUZE and G.J. STROUS. 1987. J. Cell Biol. 104: 1261-1268
8. SAHAGIAN, G.G. and E.F. NEUFELD. 1983. J. Biol. Chem. 258: 7121-7128.
9. GEUZE, H.J., J.W. SLOT, G.J. STROUS, A. HASILIK and K. VONFIGURA. 1985. J. Cell Biol. 101: 2253-2262.
10. GEUZE, H.J., J.W. SLOT and A.L. SCHWARTZ. 1987. J. Cell Biol. 104: 1715-1723.
11. TYCKO, B. and F.R. MAXFIELD. 1982. Cell 28: 643-651.
12. SCHWARTZ, A.L., G.J. STROUS J.W. SLOT and H.J. GEUZE. 1985. EMBO J. 4: 899-904.
13. LEWIS, V., S.A. GREEN, M. MARSH, P. VIHKO, A. HELENIUS and I. MELLMAN. 1985. J. Cell Biol. 100: 1839-1847.
14. GRIFFITHS, G., B. HOFLACK, K. SIMONS, I. MELLMAN and S. KORNFELD. 1988. Cell 52: 329-341.
15. GEUZE, H.J., W. STOORVOGEL, G.J. STROUS, J.W. SLOT, J.E. BLEEKEMOLEN and I. MELLMAN. In press. J. Cell Biol.
16. GREEN, S.A., K.P. ZIMMER, G. GRIFFITHS and 1. MELLMAN. 1987. J. Cell Biol. 105: 1227-1240.
17. GEUZE, H.J., J.W. SLOT, G.J. STROUS, J.P. LUZIO and A.L. SCHWARTZ. 1984a. EMBO J. 3: 2677-2685.
18. ZIJDERVELD-BLEEKEMOLEN, J.E., J.E. SCHWARTZ, A.L. SLOT, J.W. STROUS and H.J. GEUZE. 1987. J. Cell Biol. 104: 1647-1654
Table 1. Immunogold labelling in cryosections of hepatoma cell lines (Hep-G2 and H4S) for ASGPR, MPR and LGP120 in plasma membrane (PM), endosomes (End) and lysosomes (Lys).
|
|
HepG2 |
H4S(3) | ||||||
|
|
ASGPR(1) |
MPR(2) |
MPR |
LGP120 | ||||
|
|
a |
b |
a |
b |
a |
b |
a |
b |
|
PM: |
51 |
(39) |
18 |
(9) |
16 |
(10) |
1 |
(1) |
|
End: |
48 |
(37) |
82 |
(42) |
84 |
(53) |
58 |
(50) |
|
Lys: |
1 |
(1) |
- |
(-) |
- |
(-) |
41 |
(36) |
a: relative distribution of gold particles over the three compartments mentioned.
b: percentage of particles of total cellular labelling.
From: (1)Zijderveld-Bleekemolen et al., 1987. (2)Schuurman, Slot, Bleekemolen and Geuze (in reparation). (3)Geuze et al., in press.
Figure 4. Percentage of cationized ferritin (CF) containing vacuoles in H4S cells, in which mannose 6-phosphate receptor (MPR) (MPR+), LGP120 (LGP120+) or none of these molecules (MPR-/LGP120-) were detected by immunogold labelling after incubating the cells for various periods with CF (Geuze et al., in press)
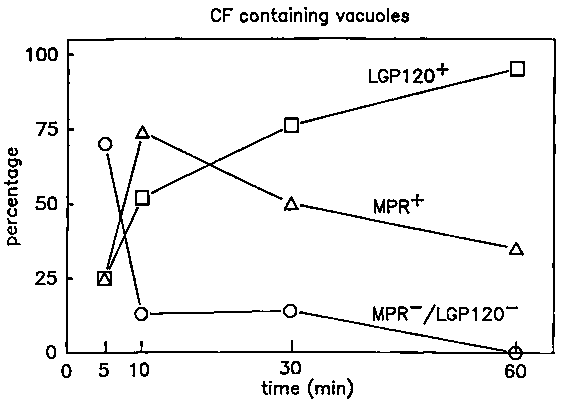
Figure 5. Impression of membrane remodelling in the endosomes of liver cells. The arrows indicate at which level inflow and segregation of typical endosomal membrane proteins occur.