2.1 Physical properties
2.2 Physico-chemical properties
2.3 Chemical properties
2.1.1 Structure
2.1.2 Soil water relationships
i. Formation of structure
ii. Soil structure data
Virgin ferralsols have excellent physical conditions which favour root penetration and provide ample space for air and water; these advantageous properties are particularly well expressed in the oxic horizons and the overlying humus layers.
When clearing land in ferralsols which were under natural vegetation for long periods, soil management practices do not have to create a suitable soil structure for cultivated plants, but rather to preserve it against deterioration. In most cases the ferralsols, before being brought into cultivation, have already acquired a distinctive pedological arrangement of soil particles as a result of the activity of living organisms, the channelling by roots, and, probably to a less extent, by seasonal wetting and drying.
The concept of soil structure is a complex one, and the understanding of the processes which are responsible for its stability may help to select adequate conservation methods. A preliminary remark may be useful at this point; this discussion does not deal with the cementation of soil constituents into irreversibly indurated concretions as ironstone or ferruginous gravel. 1/
1/ This induration process may eventually occur in ferralsols with impeded drainage, but it is felt that only limited areas which suffer from this kind of phenomena are considered for agriculture.The present review only considers those aggregates which slake in water or can be broken by pressure between the fingers or by hand. Their stability depends on the properties of the mineral and the organic parts of the soil and this chapter is divided accordingly.
a. Mineral soil constituents
In order to destroy or to build structure, rearrangement of soil particles is necessary, and this implies movement of some fractions. The more easily clay is dispersed, the more mobile it is and the more rapid the structure is deteriorated under the influence of water.
It has already been said that by definition the dominant clay mineral in ferralsols is kaolinite, combined with various amounts of iron and aluminium oxides. Kaolinite in ferralsols is usually flocculated and the clay micelles tend to stick together.. This is due to the low electric charge of this mineral, especially in acid conditions. There are only weak repulsive forces between particles and they easily cluster into aggregates which do not release much movable clay. The colloidal mineral fraction is not readily mobilized by rainwater as to move into small pores where it may clog available space for water and air.
There are other consequences of the low electric charge of kaolinite clays: only a thin layer of cation is absorbed by this colloid, and there is consequently little swelling or shrinking of the soil upon wetting and drying. In this perspective, swelling is compared with the osmotic uptake of water by the counterion cloud and considered as osmotic swelling. The weak extensibility of the soil material excludes the possibility of building internal pressures and the subsequent closing of pore space; for the same reasons only few cracks develop upon drying.
That there is practically no swelling or shrinking of soil at variable moisture contents in ferralsol was shown by CHANDLER and SILVA (1960), who found that the pore space calculated from bulk density measurements on dry clods was almost equal to the porosity filled by water when the soil was completely saturated (62.3 versus 62.5 percent for a Catalina clay in Puerto Rico, a Humic Ferralsol).
This may be a disadvantage in some subsoil horizons however; one of the mechanisms by which roots penetrate into the soil is based on the shrinking of the soil mass and the concomitant creation of pore space around the root tips by the withdrawal of water during evapotranspiration. This process is probably not contributing actively in the development of rooting systems in oxic horizons.
The clay fraction of well drained ferralsols usually contains besides kaolinite appreciable amounts of ironoxides that are present in various forms of crystallization and hydration. They are usually associated with aluminum hydroxides. Both are thought to have a strong influence on the aggregation of soil particles. It is common experience that the removal of iron and aluminum oxides by chemical methods in the laboratory yields large amounts of clay-size particles, which are released by the Al or Fe cements. The processes involved in this binding of particles are not completely understood, but they may include among other phenomena chemical bonds, hydrogen bridges, or electrical attraction between negatively charged kaolinite crystals and positively charged precipitates of sesquioxides. It is not the purpose of this review to discuss the validity of the hypothesis which have been brought forward on the influence of iron oxides and aluminum in these soils. At any rate, the role of aluminum in precipitating clays is well known and the common acid conditions of ferralsols are favourable for a strong influence of aluminum on the soil suspension.
There have not been many experiments with lime on the stability of structure in ferralsols. As a rule, there is no need for adding calcium ions to the exchange complex in order to prevent the dispersion of clay, as the counterion cloud around the platelets, which is necessary for maintaining dispersion, is almost non existent. The primary purpose of liming ferralsols is not to protect the structure: SCHUFFELEN and MIDDELBURG (1954) have even observed deleterious effects of lime on the stability of the structure in soils with acric properties. Clays would be peptized at neutral pH conditions by the adsorption of hydroxyl ions, and permeability considerably reduced.
b. Organic matter
There is no doubt that organic matter as a whole contributes substantially in maintaining the soil structure in ferralsols. In the laboratory the removal of organic substances by chemical methods causes the breakdown of aggregates and the release of a considerable amount of clay.
Not all organic soil constituents are equally beneficial for the maintenance of acquired structural features however. Many soil investigators have found that structural stability is primarily dependent on the amount of non-humified organic matter, especially those fractions which are produced by the early decomposition of fresh residues (COMBEAU, 1965).
These compounds are very shortlived and the action on structural stability depends on the biological activity of the soil. COMBEAU (1965) found that the stability decreased with increasing moisture content and air humidity, but improved at high temperature.
The more decomposed forms of humus have either no influence on structural stability, or induce dispersion of clay. It is common experience that the largest proportion of water dispersable clay is found in the surface horizons. The humus which is extractable by pyrophosphate at pH 10, or perhaps the fulvic acid part of it, could be responsible for the degradation of structure after cultivation, especially in light textured ferralsols.
If the stability of aggregates in ferralsols is to be held at its original level, there should be a permanent intensive biological activity in the surface layers, either by the supply of fresh organic matter or by the active decay of roots. This makes soil structure which is conditioned by organic matter rather temporary. Moreover, its stabilization is locally confined to places where fresh organic matter is decaying, or where recent roots are decomposing. RUSSEL's conclusions (1971) may be particularly valid for the management of ferralsols: "the cultivation can rapidly undo an important part of the structure produced by the roots of the previous crop, by breaking up the existing system of stabilized channels. Thus the technique based on minimum tillage should be of particular value for perennial crops, or when pastures or grass leys are to be converted to arable crops".
As far as one can judge from present experience, ferralsols do not suffer from structural imperfections as primary limiting factor. In most cases nutrient shortage, inadequate water supply, or deseases reduce yields before structure deterioration becomes a major problem. This is especially true in extensive agricultural systems, where little fertilizers and pesticides are used.
The same situation may not apply to intensive cropping systems, where the conservation of structure may be as necessary as the supply of nutrients by fertilizers and the protection of crops against pests. Few examples are known however of such undertakings which could support any firmly based management recommendations. It seems that light textured ferralsols suffer more from structure deterioration than clayey soil, which often contain considerable amounts of iron oxides. In sandy ferralsols, experiments in Brazil showed that 4 years cropping reduced significantly the percentage of aggregates of more than 2 mm size, but that the deterioration was the same, regardless of lime and fertilizer treatment (PRATT, 1965). It is assumed that the reduction of structural stability is correlated with the decomposition of organic matter, or with the decrease of microbiological activity.
In ferralsols, high percentages of clay correlate with appreciable amounts of iron oxides, and stronger adherence between particles to form stable aggregates. Sandy topsoils may either fall into single grained loose materials, or become extremely hard and massive upon drying. At present no satisfactory explanation for these phenomena can be given however.
a. Shape and size of aggregates
aa. Macrostructure
When observed in the field, most oxic horizons present a structure the components of which are fine to very fine crumbs which may combine into weak to very weak subangular blocky peds.
This is particularly noticeable when the study of the structure in the field is done in two ways. The first is to begin with the smallest individual particles and try to determine how they build up the aggregates; the second is to take large fragments out of the soil and see how they break into smaller units. This second method discloses the existence and the forms of surfaces of weakness within the soil mass, which depend essentially on the action of internal pressure induced by swelling and shrinking.
Practically all well drained oxic horizons exhibit, after crushing, a very fine porous crumb structure (less then 1 mm) the aggregates of which do not display well defined shapes. They consist chiefly of primary aggregates built up of individual sand part idea, and held together by crystallized or amorphous clay-size substances. In the most typical ferralsols these primary aggregates do not build up other aggregates of different shape; they may pile up and form bigger crumbs, which, however, rarely exceed 0.6 cm in size; as a result, the overall appearance of typical oxic horizons is massive.
When the same oxic materials are examined by the second method of appraising soil structure, it is practically impossible to find, within a large fragment, surfaces of weakness having a constant orientation; when a piece of soil is broken into two parts by pulling it apart cautiously with both hands, no preferential surfaces of least resistance can be recognized and it can be fractured along arbitrary planes. Proceeding in this manner it is possible to make fragments of any desired shape, bigger however than the crumbs resulting from crushing. This is especially true for subsoil oxic horizons.
Ferralsols thus have a vary open structure; the presence of some blocky aggregates that are usually weakly developed is indicative of younger types of ferralsols, which may have a greater mineral reserve than the typical ones.
In typical oxic horizons, the aggregate surfaces, if any appear upon breaking large clods, are not covered by clay skins which may seal inner pore space and make it unaccessible to roots. In younger less weathered layers, few patchy, usually thin, argillans may occur.
Surface horizons, where biological activity is higher, display usually structural aggregates that are formed by the microfauna; coarse pores are evidently more frequent than in the subsoil.
The structures which were described above are undoubtedly favourable for rooting, as far as the mechanisms of root penetration are concerned. They are typical for the oxic horizons and the topsoil of ferralsols. Strictly speaking they do not need to be intensively or deeply plowed, or reworked for the preparation of seed-beds; there is no reasons to dig large planting pits for tree crops either, if the objective of these practices were only to create pore space in virgin ferralsols.
Not all horizons in a ferralsol are typical oxic horizons however. In the lower part of the profile, materials with strongly developed blocky structure or which have kept the original rock structure may be present. The latter are usually mottled by yellow or read streaks on a greyish matrix, and the colour pattern follows more or less the spatial arrangement of grains as it existed in the parent rock. Such horizons, though potentially better provided by nutrient releasing minerals, are often more closely packed and seldom possess the open structure of the oxic horizons. When planting trees, these horizons should be opened by digging large pits in order to improve root growth; if the weathering rook (saprolite) is overlain by a stone-line, in which the gravel or rock fragments form a barrier, the planting boles should, if possible, at least touch the saprolitic material. The feasibility of such practices depend of course on the thickness of the horizons above the stone-line.
The formation of soil structure may also be influenced by the movement of the water table. Groundwater displaces iron by reducing it into the ferrous stage; air, on the contrary, fixes it as ferric oxides in preferential sites, for example along root channels or in other large pores. The consistence of the soil materials which are impregnated by iron oxides becomes firmer and causes soil structure and consistence to vary; some aggregation occurs which often coincides with colour patterns, commonly known as gley or mottling. Such colour, consistence and structure variations cannot always be clearly distinguished from cementation effects which may be related to plinthite. At any rate, if such structures are present in the main rooting zone of the crops to be grown, the soils should receive special management Land of this type certainly should be avoided where it is anticipated that plant roots will have to develop into the mottled horizon. It may eventually be used for crops which are produced during the dry season if it is expected that soil moisture will remain close enough to the root zone, or when irrigation and drainage control the depth of the water table. The limiting factor in these cases is not the structure itself however, but poor aeration caused by ground-water. If artificial drainage is envisaged, special attention should be paid to the possible induration of plinthite into ironstone after repeated wetting and drying.
ab. Microstructure
The binding of clay particles to form very small aggregates in ferralsols is particularly pronounced. It can be referred to as microaggregation, which in the earlier literature was known as the formation of pseudo-sand and pseudo-silt. By this process soils with very high clay contents (> 60%) feel loamy in the field, and actually behave mechanically as medium or even light textured soils. The effect of micro-aggregation is well illustrated by the data listed in table 3 (AHN, 1972), which give the aggregate and particle-size distribution of some ferralsols of Ghana.
The mean size of the micropeds is in the coarse silt and fine sand fraction. As pointed, out by AHN, they have considerable stability in the field, as they resist four hours end-over-end shaking in the laboratory. It can also be seen from table 3 that the A horizons have some water dispersable clay, which is not found, in the oxic B, thus indicating the action of organic matter on dispersion.
In some cases this strong aggregation makes it necessary to use special analytical procedures when clayey ferralsols which are rich in iron oxides are tested chemically for available nutrients. MOURA (1968) observed that after grinding oxic horizons developed from basic rocks, the usual extractants withdraw more anions and cations than when the unground fine earth is used for the analysis. It is assumed that the interior of the peds is only slowly accessible for the extracting solutions. Some erroneous conclusions regarding fertility levels may be drawn from data obtained by rapid chemical methods which do not take microaggregation into account.
b. Aggregate stability
There are several methods to measure aggregate stability of soils. One of them has been proposed by French pedologists who use an index of structural instability S1 which is calculated, by the following equation (HENIN et al. (1955):
where
L = maximum percentage of clay and silt (0-20 m) which is released, by the soil during wet sieving of the fine earth (passing 2 mm sieve). There are three pre-treatments of the sample: air dried; treated with alcohol, and treated with benzene. L. is the largest figure obtained by one of the treatments.For medium textured ferralsols which contain between 13 and 45% 0-20 micron fraction, the percentage of stable aggregates is related to clay and organic matter content, according to the following equation (COMBEAU and MONNIER, 1961):A = arithmetic mean of percentages of aggregates larger than 200 microns that remain on the 200 micron sieve after the standard wet sieving procedure.
CS = 0.9 times the percentage of coarse sand (larger than 200 microns).
(A-CS) alcohol = 3.59 + 0.984 (% 0-20 m)Table 3 MECHANICAL ANALYSIS OF FERRALSOLS AFTER DIFFERENT TREATMENTS (AHN, 1972) 1/(A-CS) benzene = 11.43 (% C) - 16.4
1/ All samples were shaken end-over-end for four hours.Structural stability also varies during the year. The lowest stability (highest S1) is observed during the rainy season; the proportion of stable aggregates is the highest at the end of the dry season. The amplitude of these seasonal variations may be of the order of half the mean annual value (COMBEAU and QUANTIN, 1963: COMBEAU, 1965).
2/ Profile 0.1 over granite, Suko series.
3/ Profile LB.2 over phyllites, Yago series.
Continuous cropping of ferralsols results in an increase of the instability of soil structure, as shown by COMBEAU and QUANTIN (1963): S1 was 0.40 under a virgin savanna vegetation; it reached 1.0 after four years cropping and 1.7 after eight years. The soils which were examined contained 15-20% clay. It may be recalled that an index of 1 means that one of the treatments produces almost as much clay plus silt as there are aggregates left on the 200 micron sieve.
Heavy textured ferralsols, that are rich in iron are commonly the most stable. ESCOLAR and LOPEZ (1968) found the following aggregate distribution in the topsoil of a Catalina clay, a Humic Ferralsol of Puerto Rico, (table 4), containing 18% free iron.
Table 4 AGGREGATE DISTRIBUTION IN A CATALINA CLAY (HUMIC FERRALSOL)
|
Size |
>5 mm |
5-3 mm |
3-2 mm |
2-1 mm |
|
Distribution % |
43.9 |
22.5 |
10.5 |
12.7 |
|
Percent water stable aggregates |
|
90.2 |
85.3 |
71.7 |
COMBEAU and MONNIER (1961) found the following percentages of water stable aggregates larger than 0.2 mm , after pretreatments of air, alcohol and benzene in subsoil samples from a profile developed on weathering products of basalts: 77.1(alcohol); 73.2(air) and 0.3(benzene). These figures illustrate the high stability of ironoxide rich ferralsols.
As a rule the larger aggregates in the topsoil horizons are more stable than equal-size peds in the subsoil. On the contrary, small aggregates in subsoils are more resistent than their homologues in the surface horizons. Organic matter seems to improve the stability of larger aggregates in the topsoil.
Findings of SMITH and CERNUDA (1951) showed that acric ferralsols are physically among the most stable soils. Subsoil aggregates of 1 g., wetted in partial vacuum to avoid slaking by air-trapping, were only destroyed after the impact of 400 falling water drops (or 400 times 5.6 ergs of energy per milligram of soil), which was about 20 times the energy required for peds isolated from other kinds of soil.
It may be pointed out that burning of forest soils in French Guiana resulted in an increase of the percentage of waterstable aggregates (TURENNE, 1969).
c. Pore size distribution
The porosity of oxic horizons tends to be high. For example, BENNEMA, JONGERIUS and LEMOS (1970) measured the pore volume of subsurface horizons in ferralsols by optical methods and found that it was greater than in argilluvic horizons of similar texture.
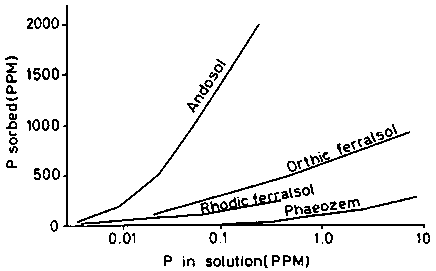
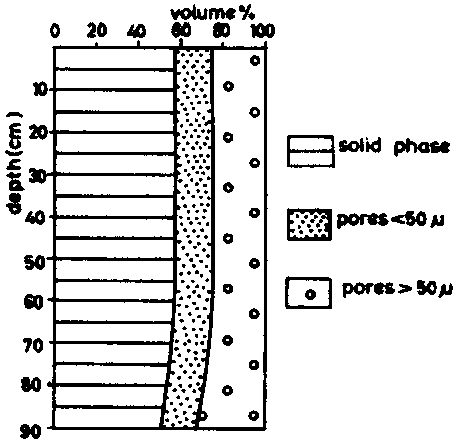
The pore-size distribution of a Latosol Roxo of Brazil (Rhodic Ferralsol), is given in table 5, taken from determinations by MOURA (1968). The first site had been cleared two months before sampling, the second had been cultivated for 15 years. The data are illustrated in figure 6a (site l) and 6b (site II).
It is obvious that the non-capillary pores (pores larger than 50 m) are considerably reduced by cropping; the volume which corresponds to pores of less than 50 microns remains practically unchanged. The cropping of this land which is rich in clay has mainly affected the permeability of the ferralsol, without changing significantly the available water retention characteristics.
MEDINA and GROHMANN (1966) found for medium and light textured soils in the campo cerrado of Brazil the following pore-size distributions (table 6).
The amount of total pores is almost the same in both soils. In the ferralic arencsol (P 854), the proportion of macropores of more than 50 m diameter is considerably higher however. The sandiest soils are likely to be the most easily leached.
There are practical consequences of the behaviour of ferralsols with respect to pore-size distribution. Medium and fine textured soils may be compacted by trampling by animals, by pressure of heavy machinery, or rolling. As seen in the previous examples, this compaction, affects mainly the larger pores. Even after heavy loads of cattle on a Humic Ferralsol in Puerto Rico (Catalina clay) for 18 months, the volume of large pores was not reduced to figures lower than 8.8 volume percent in the first 7.5 cm of soil (CHANDLER and SILVA 1960). The available water holding capacity (between 1/2 and 15 atmospheres) was more than doubled (6.5 to 14%), resulting in an increase of 5.6 mm of water stored in the upper 7.5 cm.
Table 5 PORE SIZE DISTRIBUTION IB VOLUME PERCENTAGE IN A LATOSOL ROXO BRAZIL (MOURA, 1968)
Table 6 PORE SIZE DISTRIBUTION OF A FERRALSOL AND A FERRALIC ARENOSOL IN THE CAMPO CERRADO OF BRAZIL (MEDINA AND GROHMANN, 1966)
|
Profile |
Depth cm |
Clay % (weight) |
Pore-size distribution (volume %) |
Total |
Macropores > 50 m |
Micropores < 50 m |
|
Ferralsol (P 850) |
0-13 |
24.0 |
46.4 |
19.4 |
27.0 |
|
13-41 |
25.5 |
46.5 |
16.5 |
30.0 |
|
|
41-87 |
27.0 |
43.4 |
10.1 |
33.3 |
|
|
87-120 |
32.0 |
46.8 |
16.0 |
30.8 |
|
|
Ferralic |
0-23 |
13.1 |
42.3 |
25.1 |
17.2 |
|
Arenosol |
23-62 |
14.0 |
43.1 |
25.0 |
18.1 |
|
(p 854) |
62-120 |
15.0 |
47.3 |
27.7 |
19.6 |
These data are shown in figure 6 (c) and 6 (d).
Figure 6 (a) Latosol Roxo, recently cleared
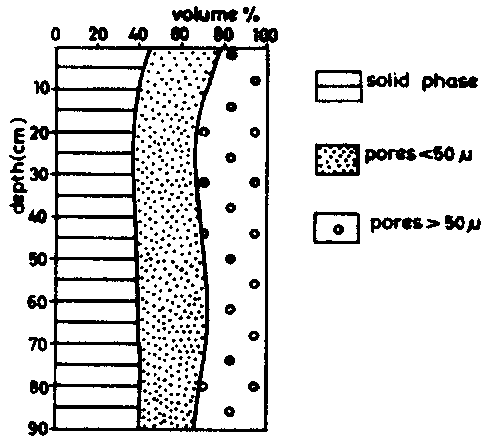
Figure 6 (b) id., after 15 years cropping
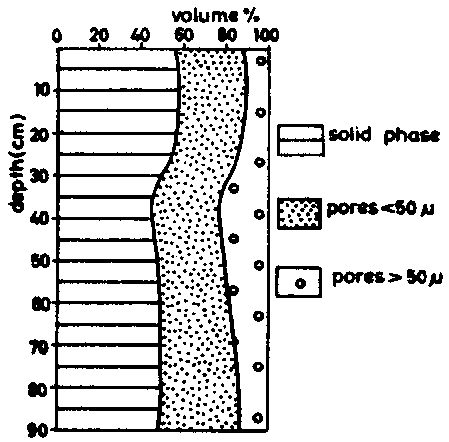
Figure 6 (c) medium textured ferralsol
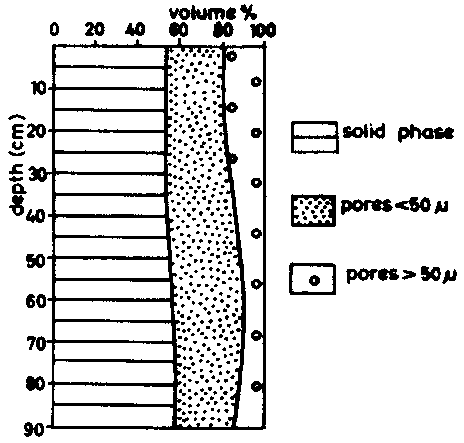
Figure 6 (d) Ferralic Arenosol
d. Bulk densities
Ferralsols which are clayey have low bulk densities in the oxic horizons and in the topsoil. This is partly due to the high microaggregation, and the considerable amount of total pore space.
Light textured and medium textured ferralsols have somewhat higher bulk densities; the lowest value occur in the horizons which contain much organic matter.
The range of bulk densities observed in oxic horizons varies between 1 and 1.5 approximately; the relation between the sand content and the bulk density in oxic horizons is given by the following equation: bulk density (g./cm3) = 1.03 + 0.004 percentage sand (r = 0.727). In the surface horizons no significant correlation with sand or organic carbon content could be found.
Bulk density is mathematically related to total pore volume. Root development is strongly dependent on pore size distribution, and several authors have studied the influence of bulk density on root elongation.
TROUSE and BAVER (1962) observed a serious reduction of root development in Low Humic Latosols (Ferralsol) at densities of 1.35 g /cm. They observed daily growth of sugar cane roots of 20 mm at bulk densities of 1.04 which was reduced to less than 8 mm. for d = 1.38. At this value the roots developed mainly through fracture planes between aggregates.
i. Permanent wilting point
ii. Field capacity
iii. Available water
iv. Infiltration, water movement
The percentage of water held in ferralsols at 15 bars (pF = 4.2) is closely related to the clay content. As a rule the correlation between the clay percentage and the moisture held at wilting point is greater in subsoil horizons than in the A horizons. Organic matter seems to interfere with the moisture holding characteristics of the clay, but its influence is not always clear,
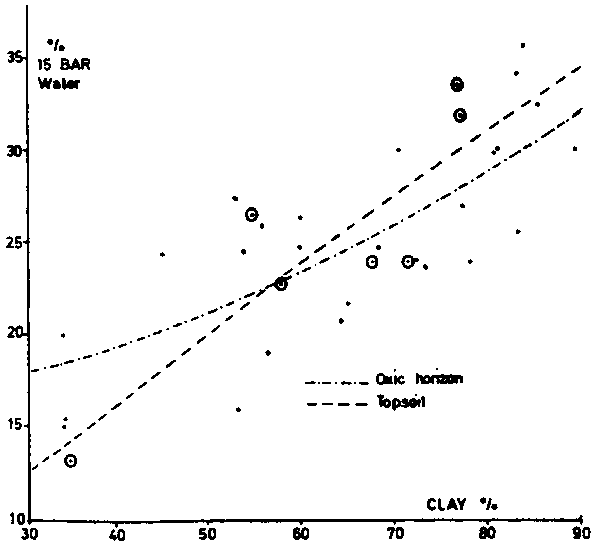
Soil compaction and plowing do not change significantly the moisture retention characteristics at high tensions. The water hold at 15 bar can be approximated in oxic horizons by the following equation:
15-bar water % = 10 + 0.234 clay % (with r = 0.645 at n = 24);In the horizons overlying the oxic horizon this percentage corresponds to
1.3 + 0.375 clay % (with r = 0.867 at n = 8).The quadratic regressions for the oxic horizons are:
15-bar water = water = 15.7 + 0.024 Cl + 0.0017 Cl2where Cl is the clay percentage; in the A1 horizons this equation is:
15-bar water = - 6 + 0.448 Cl - 0.0065 Cl2.These equations are shown in figure 7.
Figure 7 - Water content at 15 bar tension
The organic matter apparently reduces the forces by which the clay holds water at low water contents, particularly in soils that are medium textured. Therefore at equal field capacities, the amount of water available to plants may be greater in horizons which are rich in humus.
There is no good agreement in the literature regarding the tension at which water is held by ferralsols at their field capacity. It is commonly accepted that a ferralsol which has been fully saturated with water reaches a water tension of about 1/3 atmosphere (pF 2.54) after two days free draining. Some report much lower tensions of 1/15 and 1/20 bar in sandy and clayey ferralsols however. Nevertheless 1/3 atmosphere is usually taken as the conventional limit.
It is assumed that at this point the gravitational movement of water is markedly reduced, leaving only the pores which are smaller than 8.6 microns filled with water. The water which is held at less than 1/3 atmosphere drains out of the profile in less than two days. As long as it percolates through horizons which are located within the reach of the roots, it is of course still available to plants. Under tropical climates the field capacity has only a reduced practical significance, especially in rainfed agriculture.
The factors which determine the total amount of water held by the ferralsols at 1/3 atmosphere depends mainly on the pore-size distribution. It is therefore essentially determined by the structure of the soil. Texture may also play a role in as much as fine silt (2-20 m) tends to increase the field capacity; fine sand, where it contains partly weathered minerals may also substantially raise the water content held at this tension (AHN, 1972).
Weathering processes in oxic horizons have usually produced maximum amounts of clay. Typical oxisols are moreover exceptionally low in silt (2-20 m). Silt/clay ratios are often less than 0.15. This causes strong water retention at wilting point, and reduces the storage capacity at field capacity: hence the narrow range of available water which is normally observed in oxic horizons. As a rule of thumb it is commonly accepted that they cannot store more than 10 mm of rain per 10 centimeter of soil depth between the critical tensions of 1/3 atmosphere (field capacity) and 15 bars (permanent wilting point).
This low figures make ferralsols with deep oxic horizons particularly sensitive to drought especially for crops with shallow rooting habits; drought hazards are also severe in profiles which either contain stone-lines which reduce the soil volume, or where chemical conditions (as Ca deficiencies) are such that they drastically restrict root growth.
In some undeep ferralsols the lower parts of the profiles which have not reached extreme weathering may contain more silt and less clay and the available water content may therefore be larger. This important property may partly explain, in addition to the release of nutrients by weathering processes in deeper layers, the higher plant production potential of ferralsols with saprolite at shallow depth, especially for deep rooting crops.
Figure 8a - Soil moisture balance diagrams (Thornthwaite and Mather - 1955) - soil depth 50 cm.
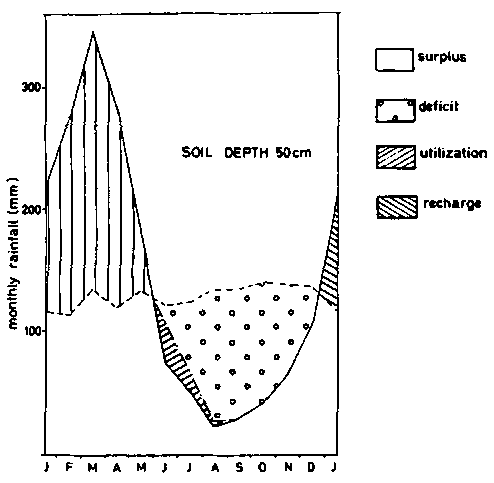
Figure 8b - Soil moisture balance diagrams (Thornthwaite and Mather - 1955) - soil depth 100 cm.
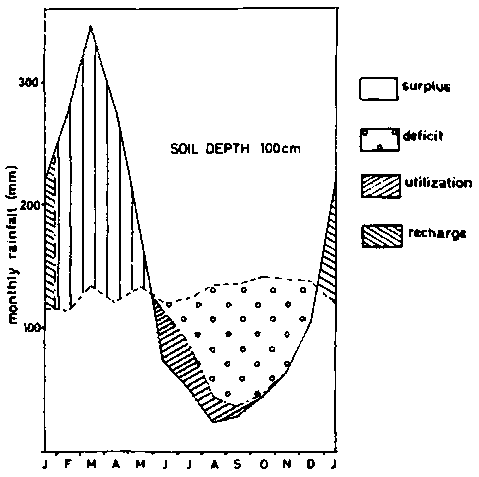
Figure 8c - Soil moisture balance diagrams (Thornthwaite and Mather - 1955) - soil depth 150 cm.
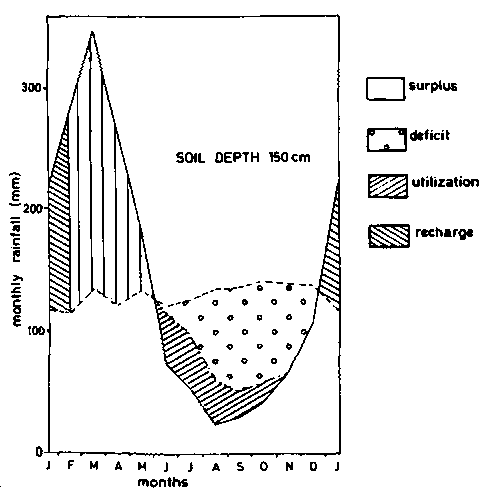
Figure 8d - Soil moisture balance diagrams (Thornthwaite and Mather - 1955) - soil depth 300 cm.
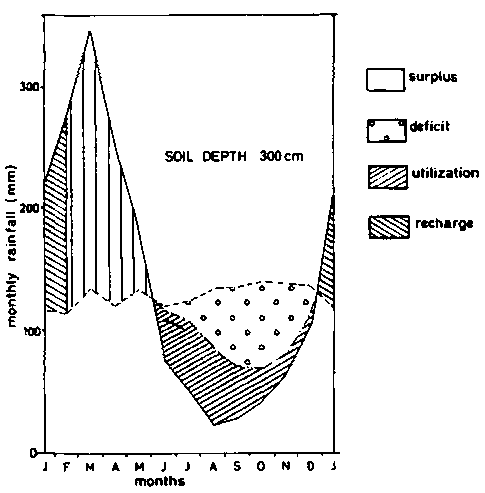
As pointed, out earlier, agriculture on ferralsols is narrowly rain dependent. An example of a water balance diagram according to THORNTWAITE and MATHER (1955) is given in figure 8 for different soil depths, based on calculations by BARREIRA PEREIRA and DE SOUZA RODRIGUES (1971) for soils in the Amazon. Taking 10 mm of rainfall per 10 cm of soil, the water deficit for profiles that are 50, 100, 150 and 300 cm deep, would be respectively 482, 432, 382 and 278 mm per year, on the assumption that the plants cannot take water from underlying horizons. Actual evapo-transpiration calculated for the whole year in the same soils were respectively 1063, 1113, 1163 and 1267 mm. The advantages of having large rooting volumes are obvious.
Ferralsols of Puerto Rico have infiltration rates which vary between 8.5 and 15 cm /hour (dry infiltration rates with wet buffer compartment), according to LOPEZ and BONNET (1968); other reports on ferralsols mention 8 cm /hour after one hour of continuous flooding.
Movement of water from moist to dry areas within a profile is as important for adequate water supply to plants as is the amount of available water itself. Usually a distinction is made between water flow at saturation, and movement under stress during dry periods; water may also be translocated as vapour, and transferred from warm to cool areas in the soil by a distillation process.
Ferralsols release most of their water at tensions below 1 bar. When they dewater, the conductivity for moisture decreases abruptly; the capillary conductivity is strongly water content dependent: the drier the soil, the slowest the transfer of water towards the roots. Laboratory experiments by SHARMA and UEHARA (1968) have shown that the decrease in conductivity is steep, and that it falls below 10-5 cm /sec. at tensions of 60 cm of water, During dry periods the roots which are living in ferralsols have to grow towards moist spots in the profile, rather than to expect any supply from water transfer within the soil.
In this respect most ferralsols behave as sandy soils. There is one difference however for medium and clayey textured oxic horizons: these are physiologically dry at considerably higher water contents than sands. The water which is present in the microaggregates is available as a source for vapour which may move to the rooting zone by distillation effects, and be condensed in cooler areas, which management techniques, such as mulching, should try to create at the vicinity of the cultivated plants.
Water loss by evaporation from ferralsols is apparently not restricted to the first few centimeters of the topsoil. WALTON (1962) contends that in hot climates dry soil does not form a protective barrier and that evaporation from deep layers is not negligible. He found that water was lost from soil horizons between 30 and 60 cm depth, even when the surface layers had dried completely.
2.2.1 Ion exchange reactions
2.2.2 Base saturation
The exchange of cations and anions between the solid and the liquid phase in oxic horizons and in the overlying topsoils is mainly conditioned by the type and amount of clay minerals, oxides and organic matter. Since oxic horizons are generally low in silt, the contribution of this particle-size fraction to exchange reactions is negligible; moreover, the small quantities of micas which might be present do not participate significantly in the possible exchange or fixation of NH4+ by K+ ions.
There are by definition no or only traces of 2 : 1 silicate-clay minerals as montmorillonites, illites, etc. in oxic horizons. The finest soil particles are dominantly composed of kaolinite, goethite, gibbsite and various amounts of other iron and aluminum oxides. The crystals are generally covered by coatings: the cations and anions in the soil solution are said not to be in direct contact with the bare clay minerals, but rather with micelles covered by oxides and organic matter. The presence of organic compounds, which are active ion exchangers makes it convenient to speak of an exchange complex. Its electric charges and the bonding energies for ions result from various processes which have been divided by soil chemists into several classes.
The first one, known as permanent charge, is caused by the isomorphic substitution of Si4+ by Al3+, or Al3+ by Mg++ in the crystal lattice of the clays. There are practically no substitutions in kaolinite however, and consequently, very little permanent negative charge sites in oxic horizons. This permanent charge, however small, is independent of the pH of the soil solution, and the cations which neutralize it can be exchanged at any time, without modification of the permanent cation exchange capacity. Among these exchangeable cations Ca, Mg, K, Na and Al are the most common.
The second kind of bonding energies for ions is due to the dissociation of H+ from active molecules located at the border of the exchange complex, creating negative sites, or to the protonation into OH2+, giving positive charges. Protons (H+) may for example be released by acid groups at the broken edges of clay particles, or by carboxyl or phenol groups in the organic matter, or by aluminum and iron hydroxides. The dissociation of H+ creates vacancies which may be filled by metallic ions. It is strongest at high concentrations of OH- in the soil solution and is therefore called the pH-dependent part of the cation exchange capacity which increases with raising pH. At low pil values this type of cation exchange capacity may completely disappear.
In some highly weathered soils which are rich in sesquioxides, for example those derived from basalts and ultrabasic rocks, positive charges may develop by protonation of hydroxyl groups. This may eventually produce an anion adsorption capacity. The anion bonding is not exclusively active at low pH values however and the real nature of this positive charges in the exchange complex is still to be more closely investigated. According to ATKINSON et al. (1967) the zero point of charge of goethite is at about pH 7.5, and that of hematite somewhere above 8.5.
The brief outline on ion exchange phenomena is illustrated in figure 9; it is thought to be a useful model for understanding the practical implications of a part of the chemistry of the soil, without resort to theories which are more rigorous, but become forbiddingly complex.
Fig. 9(a), (b) and (c) represent the exchange components of different soil samples; (a) and (b) have permanent charges which remain unchanged in the pH range set out on the X-axis of the diagrams; in addition of this constant negative surface charge, a pH-dependent variable charge develops with increasing pH. It is shown in figures (a), (b) and (c) as open triangles placed on top of the rectangular representation of the permanent charge; at the bottom of the same figures the decreasing importance of positive charges, when passing to alkaline conditions, is illustrated by a line which reaches the X-axis at pH 8.
The resulting net charge is represented by a heavy line. It is obtained by the algebraic summation of the components. Where it crosses the X-axis, there is no net charge on the exchange complex, and the point is the zero point of charge (ZPC).
When comparing a soil which possesses some permanent charge for example (b), with a soil devoid of it, (c), a displacement of the ZPC from low pH to higher values is observed. In example (c) the soil will have no cation exchange capacity at pH lower than 6, but rather a strong affinity for anions. It may fix phosphates tightly. It will have a higher pH in KCl solutions than in water, and probably belong to the Acric Ferralsols.
Figure 9 - Ion exchange components in ferralsols
Each component of the exchange complex is supposedly associated. with a specific part of the soil material. The permanent charge is by definition located in the crystal lattice of silicate clay minerals; the negative pH-dependent component operates from broken edges of clays and oxides, and at sites originating in the organic matter. Positive charges are produced by sesquioxides. Opposed charges can apparently exist simultaneously in the same exchange complex. They do not necessarily neutralize each other neatly, and a rigid framework of specific exchange sites of opposite signs is possible.
The way by which management practices may act upon the exchange characteristics are illustrated in figure 9 (d). The soil in this example has a moderate permanent charge. There is only little organic matter, and consequently the dissociation of H+ which produces additional sites for binding metallic cations is very reduced; line (a) indicates this stage. If the humus content were higher (i.e. resulting from erosion control), the pH dependent charge component could be as high as b. The soil contains also considerable amounts of oxides which generate positive charges at pH's lower than 6. Line a , which quantifies these sites may be moved into position by adding phosphates, silicates, or organic matter which neutralize the positive charges.
The resulting net capacity of the soil to retain ions may be modified by (1) adding organic matter to the soil or (2) applying fertilizers which reduce the quantity of positive charges. By using both methods the dimensions of the net resulting capacity are changed from the lower values shown by the full heavy line to higher ones illustrated by the broken line. Theoretically, if the soil had been maintained at pH 5, it could have passed from acric properties dominated by positive charges to conditions which permit cations to be retained by the exchange complex.
An increase of the CEC could also have been achieved by raising the pH with lime as shown by the dotted line (3). This is only true however if Ca were not specifically absorbed by the oxides, in such a way that it became unavailable, blocked exchange sites, and thus reduced the effective cation exchange capacity. The latter phenomenon is particularly active in soils that have oxidic or ferritic mineralogy. Liming of acric soils, where the ZPC coincides with a high pH, may have adverse effects on soil structure SCHUFFELEN and MIDDELBURG (1954) report that the soil colloids with considerable exchange alkalinity (or substantial positive charges) absorb OH ions at neutral pH conditions, and are thus peptized. The dispersion of clays by liming acric soils may cause a drastic decrease in soil permeability, and depress yields.
Some ion exchange components in ferralsols of Brazil are given in table 7 for surface horizons (0-15 cm).
PRATT and ALVAHYDO (1966) found that the ratio of pH dependent to permanent cation exchange capacity (CEC) was 3.0 to 5.3 For Red Latosols (Orthic Ferralsols), 1.6. to 4.3 for Yellow Latosols (Xanthic Ferralsols). In humic ferralsols, where the organic matter content is high, the ratio may be greater. In ferralsols which intergrade to less weathered materials the permanent charge may become important.
The cation exchange capacity in ferralsols strongly depends on the organic matter content and the pH of the soil. Clay minerals quantitatively contribute very little to the total CEC. In a kaolinitic material at pH 7, 30% clay may only provide sites for absorbing ± 3 meq per 100 g soil, whilst 1% organic carbon in fresh organic matter may bond up to 4.5 meq. Under such pH conditions 60 percent of the CEC originates from the organic matter. Under normal field pH this proportion may be considerably modified however.
Table 7 ION EXCHANGE COMPONENTS IN FERRALSOLS OF BRAZIL (PRATT AND ALVAHYDO, 1966)
|
Soil
|
pH paste
|
clay
|
meq./100 g |
||||
|
Permanent |
pH 7 |
Soil pH |
pH-dep. CEC/ |
||||
|
RYL |
1 |
4.3 |
Kaolinite |
1.3 |
3.5 |
2.0 |
1.7 |
|
RYL |
2 |
4.0 |
Kaolinite, |
1.1 |
4.3 |
1.5 |
2.9 |
|
RYL |
8 |
5.5 |
- |
2.3 |
8.0 |
4.8 |
2.5 |
|
RYL |
9 |
4.6 |
- |
2.8 |
7.7 |
2.1 |
1.6 |
|
RYL |
11 |
4.3 |
Kaolinite, |
3.1 |
7.5 |
4.5 |
1.4 |
|
TR |
16 |
4.2 |
Oxides, |
1.6 |
7.5 |
1.4 |
3.7 |
|
TR |
18 |
4.2 |
Oxides, |
0.9 |
5.7 |
0.8 |
5.3 |
|
TR |
20 |
4.1 |
- |
2.2 |
9.0 |
2.2 |
3.0 |
1/ KCl acidity at zero base saturationThe cation exchange capacity varies after clearing land. Data on virgin forest soils and three months after clearing and burning, gave the following equations for CEC, calculated by TURENNE (1969) from analytical data obtained with NH4OAc at pH 7:
Virgin Soil: CEC = 1.72 C % + 0.178 (Clay + fine silt) - 2.86This author concludes that the clearing of the land, and the exposure to direct sunlight cause a very rapid decline of the exchange capacity (pH 7) of the organic matter. The factors in TURENNE's equation for the contribution of organic carbon to the CEC of the soil are surprisingly low in the burned soil, and the addition of charcoal to the soil may partly explain the results of his calculations.Cleared, Burned: CEC = 0.358 C % + 0.203 (Clay + fine silt) - 0.585
GREENLAND (1972) states that the organic matter normally carries about 200 meq of carboxyl groups per 100 g carbon. Their degree of dissociation, generating sites for cation retention, decreases with pH, the pka, values ranging from 4 to 6. Thus between pH 4 and 6 half of the carboxyl groups carry negatively charged exchange sites. With these constants, 2% organic carbon would be able to retain 2 meq per 100 g of soil at pH 5.
The contribution of organic matter to the cation exchange capacity is strongly pH dependent. ABRUÑA and VICENTE (1955) estimated that at pH 7 it would account for approximately 150 meq /100 g organic matter, or 260 meq /100 g C. The titration curves of extracted organic matter indicate a high buffer capacity above pH 7, and an almost four times reduction of the retention capacity at pH 4.5, to ± 65 meq per 100 g C. The formation of poorly soluble humates was suggested as a possible explanation of the buffering power.
ABRUÑA and VICENTE-CHANDLER (1955) noted strong differences in exchange properties of the various portions of the organic matter in ferralsols. The fraction which can be extracted by flotation has an extremely high capacity (over 700 meq /100 g C); the most easily oxidizable fractions thus appear to be the most active in cation retention. The part of the humus which is difficult to destroy by oxidation has on the contrary no or very little base exchange capacity, and seems to be tightly held by mineral soil colloids.
The net negative charge on the exchange complex is neutralized by cations the most important of which are Ca, Mg, K, Na and Al. Three parameters are commonly used to estimate their quantities and availability, the cation exchange capacity (CEC), the sum of bases (S) and the base saturation (V %). The experience has shown that it is important to distinguish some critical levels with respect to soil fertility in ferralsols, and several class limits with agronomic significance have been proposed.
When the sum of bases (Ca, Mg, K, Na) plus the aluminum extracted by a normal solution of KCl is less than 1 meq /100 g of clay, it is considered that the soil at the field pH has almost no cation exchange capacity. It is then meaningless to calculate base saturation. These soils, Acric Ferralsols, require special practices for fertilization, especially for phosphorus and calcium. There are only few examples however where they have economically been put into crop production and most land of this kind lies idle.
Non-acric soils are usefully subdivided into subtrophic and dystrophic groups. Thirty-five percent base saturation (sum of bases x 100/CEC at pH 7) is the limit which is commonly used to separate them.
The depth to which the saturation percentage is to be considered should not be limited to the surface horizons, but should also include the subsoil. Subsoil acidity is detrimental to rootgrowth and it restricts the volume from which plants can extract water. In the dystrophic types it is worthwhile to distinguish aluminum dominated saturations. When the aluminum occupies more than 50% of the permanent charge, the uptake of nutrients by crops may be severely hindered, and productivity markedly depressed. This is shown in table 8 taken from experiments conducted on ferralsols developed from basic rocks in Paraná (OLMOS et al. 1971). Aluminum saturation is expressed as (Al x 100) / (sum of bases + Al).
Table 8 TYPE OF BASE SATURATION IN B HORIZONS OF FERRALSOLS AND YIELDS OF CORN (OLMOS ET AL. 1971)
|
Type of saturation in B - horizon
|
Yields of corn Kg/ha |
|
|
without fertilizers |
with fertilizers |
|
|
Dystrophic, Al-dominated |
1 004 |
1 829 |
|
Dystrophic, not Al-dominated |
1 657 |
4 205 |
|
Eutrophic |
2 282 |
5 198 |
2.3.1 Nitrogen and organic matter levels
2.3.2 Phosphorus
2.3.3 Potassium
2.3.4 Calcium, aluminum, pH and liming
2.3.5 Sulphur and minor elements
i. Nitrogen and organic matter levels
ii. Organic matter changes and nitrogen supply
The roles of organic matter, nitrogen and living organisms in soils are closely interrelated. Organic matter regulates the nitrogen economy and in this respect it either acts as a source or a sink. It contains bioactive substances which stimulate or retard plant growth; it may favour the development of the microfauna and the microflora and control the activity of pathologic organisms.
The crop production in ferralsols which do not receive fertilizers is strongly dependent on the natural supplies of nitrogen. These come essentially from the decomposition of soil organic matter. This nitrogen pool has to be replenished, either by fallows, by crop residues, green manures or fertilizers in order to have it operating on a sustained basis.
Although modern agriculture in ferralsols is economically not feasible without high yields, which cannot be obtained permanently from natural sources of nitrogen only, it is useful for good management to understand the mechanisms of soil organic matter changes which provide the nitrogen to the crops in the less intensive agricultural systems.
BARTHOLOMEW (1972), and GREENLAND (1972) have presented excellent reviews on this subject. The concept which they have used are based on a mathematical model which relates the changes in N content of the soil to the algebraic sum of the gains originating from the plant residues and the losses caused by humus decomposition.
The primary differential equation reads as follows:
where N is the total nitrogen content, t is the time, A is the annual rate of addition, k is a decomposition constant defining the fraction of the total N which is released by the soil organic matter.
When management practices are constant over a long period of time during which k and A remain unchanged, the soil reaches an equilibrium level NE at the moment that the additions (A) are outbalanced by the losses (kN). Under such conditions NE equals A/k and the NE content depends both on the rate of addition and decomposition. At NE the amount supplied by the soil to the crop cannot exceed the amount of N that is imported into the soil-plant system; if no fertilization is applied, crops have to live on outside natural N sources which are scarce, and would only permit yields of 600-1200 kg/ha of corn, or 400-800 kg/ha of wheat, provided no other limiting factors are restricting plant growth (BARTHOLOMEW, 1972).
It should be pointed out that the equation NE = A/k is only a rough approximation of the complex processes which take place in the soil organic matter. It is only applicable when A and k are considered over a large number of years at seasonally comparable periods or as annual averages. Obviously A and k change markedly from one season to another in the course of one year and induce fluctuations in the nitrogen content. The larger the amplitude of the seasonal variations, the higher the potential of the soil to supply or to bind nitrogen, provided NE itself is not too small.
A and k are not identical in all soils, even under similar climatic conditions. The fraction of soil matter which is decomposed per unit time, or k, is related to texture and mineralogy. For example VERDADE (1969) reports that clayey soils in São Paulo have usually double the N content of sandy soils, GREENLAND (1972) states that a large percentage of hydrous oxides leads to lower k values, and consequently to higher N contents. Many tropical soils contain larger amounts of organic matter than soils in temperate regions; this may be due to strong linkage of humus with hydrous aluminum and iron oxides, which protect it against decomposition. The contamination by volcanic ash and the presence of allophane may lead to similar results.
Red soils usually contain more organic matter than yellow soils, even though the latter look darker than the former. SYS (1971) compared a series of soils of Katanga (Zaire) and found the amounts which are given in table 9.
Table 9 ORGANIC CARBON CONTENT (TONS PER HECTARE) IN TOP 50 CM IN RED AND YELLOW SOILS OF KATANGA (ZAIRE)
|
Parent material
|
Tons organic carbon per hectare |
|
|
red soils |
yellow soils |
|
|
Clay on limestone |
79 |
65 |
|
Clay on slates |
71 |
55 |
|
Sandy clay on conglomerate |
61 |
47 |
Table 10 C, N CONTENTS AND C/N RATIOS IN SAVANNA AND RAINFOREST SOILS IN ZAIRE (FRANKART, I960)
|
|
Forest zone |
Savanna area |
||||
|
Rain forest |
Fallow |
Crop |
Savanna |
Fallow |
Crop |
|
|
C/N in A1 |
8.6 |
9.0 |
8.6 |
13.3 |
13.7 |
12.4 |
|
C/Tons/ha/meter |
86 |
88 |
83 |
107 |
121 |
85 |
|
N/Tons/ha/meter |
10.1 |
11.3 |
10.6 |
8.9 |
8.5 |
7.1 |
Table 11 PH AND BASE SATURATION IN FOREST AND SAVANNA SOILS IN N-E ZAIRE (FRANKART, 1960)
|
Area
|
pH in |
Base saturation in |
||||
|
A1 |
A3 |
C |
A1 |
A3 |
C |
|
|
Forest |
6.6 |
5.6 |
5.3 |
91 |
47 |
27 |
|
Savanna |
5.4 |
5.2 |
5.3 |
23 |
13 |
19 |
SAUNDER and GRANT (1962) contend that organic matter levels can be maintained under cropland for as long as high yields of adapted crops can be obtained by adequate mineral soil fertility and sufficient water supply, and provided plant residues are returned to the soil. Crops with fibrous root systems would be best. Rapid decline in organic matter level will however occur when poor yields contribute little in the way of roots or aerial organic materials.
The ability of the soil organic matter to undergo changes is more important for crop production than is its absolute amount. This capacity depends on the internal potential of the humus, and on external factors. The latter may be conditioned by plowing, mulching, exposure to sunlight, drought and moisture. The decomposition rates of organic matter and the release of nitrogen may also be modified by the chemical characteristics of the soil environment.
Organic matter in freshly cleared land is reported to behave differently than humus from old croplands in its ability to supply N. This, at least, is what is generally advanced, as an explanation for the lack of response to N fertilizers of recently opened fields. Examples of experiments that confirm these views are given below (MASCARENHAS, 1957).
Table 12 YIELDS OF SOYA IN KG/HA, IN LATOSOL ROXO (RHODIC FERRALSOL) OF PH 4.8 AND 5.5, UNDER "CERRADO" SAVANNA (BRAZIL)
|
Treatment
|
recently cleared |
cultivated land |
||
|
not limed |
limed |
not limed |
limed |
|
|
O |
1.354 |
1.288 |
1.497 |
1.764 |
|
PK |
2.042 |
2.653 |
819 |
1.563 |
|
PK + inoc. 1/ |
1.747 |
2.424 |
1.507 |
2.431 |
|
N1PK |
2.188 |
2.344 |
1.264 |
2.049 |
|
N1PK + inoc. |
1.879 |
2.288 |
1.118 |
1.840 |
|
N2PK |
2.087 |
2.729 |
1.257 |
1.882 |
1/ inoc. = inoculumMcCLUNG et al. (1962) had observed similar reactions on dark red latosols (Orthic Ferralsols), cropped with cotton after cerrado vegetation; he estimated that the supply of N from the soil itself would probably be shortlived, MIKKELSEN et al. (1963) even related depressing effect of N fertilization in land which had been opened from natural pastures on latosols (pH 4,9), to stalk breakage and lodging, due to the excessive amounts of N released by fast mineralization.
Although liming generally increased yields in recently openedacid soils, it decreases the response to N (see table 12). An additional example of this can be drawn from an experiment conducted by MASCARENHAS et al. (1967) with beans on a Dark Red Latosol (pH 4,0) in São Paulo State of Brazil (table 13).
Table 13 YIELDS OF BEANS (PHASEOLUS VULGARIS) IN KG/HA ON DARK RED LATOSOLS (ORTHIC FERRALSOLS) OF BRAZIL
|
Nitrogen level
|
Yields during |
|||
|
First year |
Second year |
|||
|
unlimed |
limed |
unlimed |
limed |
|
|
N0 |
283 |
334 |
445 |
564 |
|
N1 |
269 |
373 |
491 |
751 |
|
N2 |
260 |
378 |
465 |
761 |
Not all freshly formed organic matter acts as a nitrogen source for plant nutrition. Smith and ABRUNA (1955) observed that truncated soils, from which the first 90 cm had been removed, could accumulate annually in the top 30 cm under good tropical kudzu and molasses grass (Pueraria phaseoloides Benth and Melinis minutiflora) 336 kg of N per hectare. This nitrogen however, although it corresponded to approximately 75% of the nitrogen content of normal cropped land, was a poor nutrient supplier, as was indicated by subsequent yields. SMITH and ABRUNA (op. cit.) suggest that most N was tied up in the processes of forming soil organic matter, and they point out that the results of their experiments are consistent with the concept of a stable soil organic matter level, or equilibrium level, towards which a soil has a strong tendency to develop.
a. Gains in nitrogen
The nitrogen pool of the soil has to be replenished periodically in order to balance the losses. There are different natural sources: plant residues, biologic fixation by microorganisms, and the nitrogen contained in rainfall. Obviously, there are no soil-bound limiting factors which are exclusive for ferralsols, although the efficiency of nitrogen fixation is for a great deal dependent on soil properties.
The vegetation itself is a valuable reservoir of nitrogen. Generally applicable data, which give an estimate of the order of magnitude of such possible supplies, have been published by NYE and GREENLAND (1960). BARTHOLOMEW et al. (1953) tabulated the nutrient content of forest fallows, from which figure 10 has been adapted.
The decomposition of litter in a typical forest site at Yangambi rapidly supplies nitrogen and other nutrients. Estimates of the rate of release are illustrated in figure 11.
According to KASS (1970) the annual gains in nitrogen of soil-plant systems in tropical environments may be estimated as follows:
Figure 10 - Nutrient accumulation in a secondary forest in Yangambi (Zaïre), according . to BARTHOLOMEW et al. (1953). SANCHEZ, 1972.
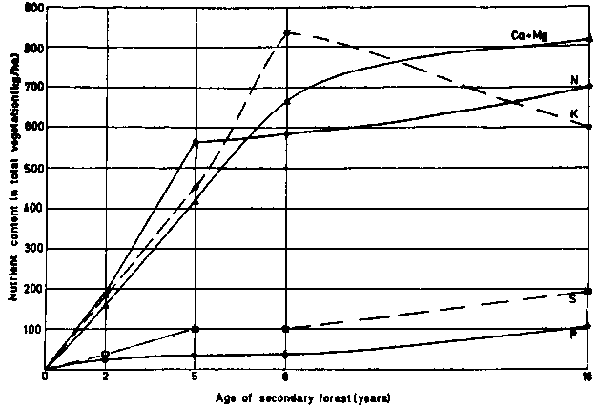
Figure 11 - Litter decomposition rates in Yangambi (Zaïre); according to BARTHOLOMEW et al. (1953), SANCHEZ, 1972.
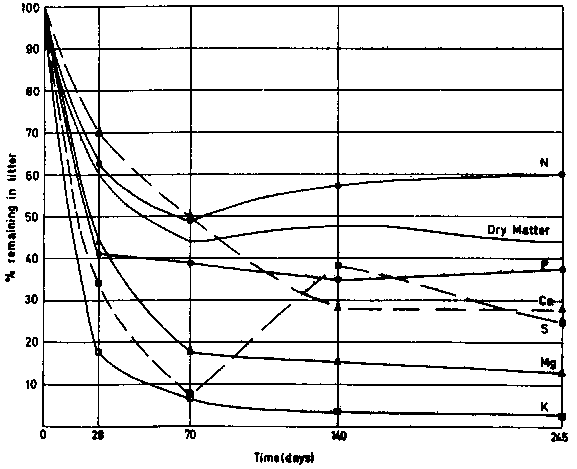
Table 14 GAINS OF NITROGEN IS KG/HA/YEAR IN TROPICAL SOIL-PLANT SYSTEMS (L = LOW ESTIMATE, H = HIGH ESTIMATE), KASS (1970)
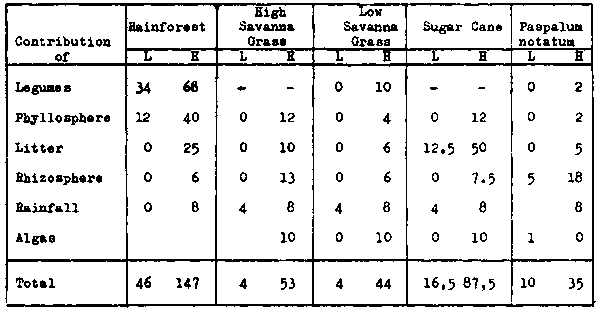
Among the processes which are responsible for the addition of nitrogen to the soil-plant system, the fixation by the legume symbiosis, and by free-living bacteria have been studied most thoroughly. These investigations are briefly discussed in the following sections.
Symbiotic fixation
The Rhizobium-legume symbiosis is not the only microbiological process for fixing atmospheric nitrogen. Other plants as Casuerina, Coriaria, Discaria, and Myrica occur in the tropics and produce nodules which fix nitrogen. Podocarpus also is an active nitrogen fixer when living in mycorrhizal association. Blue green algas and other plant species may also contribute in symbiosis to the nitrogen economy, but the applications to soil management are mainly limited to the restoration of soil fertility during long fallows or in forestry programmes. The legumes remain the most frequently used symbiotic host plants in agriculture.
Several general laws regarding the symbiotic fixation of nitrogen that are common to all soils are worth remembering. BARTHOLOMEW (1972) points out that nitrogen fixation processes are growth related: it means that nitrogen is fixed and used only to the extent that it is needed for the development of the bacteria and the host plant. The fixation for example is retarded by the presence of available inorganic nitrogen in the soil: in fact fixation only works when it has to cope with a nitrogen deficit: some bacteria are inactive at SOBS time and nodulation of roots is not always a good guide for estimating the fixation intensity.
Most legumes require optimum soil conditions for sustained high yields, and the symbiosis is only achieved when bacterial strains specifically related to the host plant occur. Phaseolus, Alfalfa, and soybeans are examples of such crops. They cannot achieve efficient nitrogen fixation in most ferralsols unless the soil chemical characteristics have been throughly improved.
In an experiment conducted on Latosol Roxo in São Paulo, a Rhodic Ferralsols, MASCARENHAS (1967) observed that nodulation of soya was increased by liming (3t dolomitic limestone/ha). Applications of nitrogen reduced nodulation especially at high fertilizer rates. In recently cleared land which was included in this experiment, nodulation was more abundant after inoculation, but the yields were not changed. Rhizobium was reported to be inefficient in increasing yields when sufficient N is supplied by the soil itself, as was most likely in a freshly opened field.
In old cropland, spontaneous nodulation often masks the effects of the inoculum on the number of nodules; the yields however respond to inoculation, when no chemical N is added to the soil: this would indicate that good nodulators may be poor nitrogen fixers. MASCARENHAS experiments are summarized in table 12.
Not all Rhizobia are as exacting to calcium supply and host specificity. The Vigna group would have less rigorous requirements. This strain is commonly occurring in moat soils of Central Africa (LAUDELOUT, 1961), and it is not necessary to inoculate the cultivated host plants accommodated to the specificity of the bacteria, nor to apply line even to acid soils. Stylosanthes is also a legume which is particularly well suited for optimum efficiency in soils with a low base saturation. It even seems to be very sensitive to overliming. PRATT (1966) found reduced growth of Stylosanthes after liming a soil (pH: 4,5) high in organic matter, which may have affected the manganese availability. DOBEREINER (1970) states that a soil pH > 5 is detrimental to growth and N fixation in Stylosanthes.
Nodulation of Phaseolus may be improved by green manures. MIYASAKA et al. (1966) report that the incorporation of plant material, especially grasses, into a Latosol Roxo resulted in more abundant nodulation without having significant effects on the yields of beans.
As pointed, out earlier the nitrogen fixation is growth related. Therefore the amounts of N which are fixed may vary from a few kilograms to 500 kg N/ha in large yields of soybeans (BARTHOLOMEW, 1972). The latter have probably not been obtained in ferralsols.
It is generally accepted that a legume may fix 1 mg of N per 15 mg of carbon assimilated by higher plants (or 67 mg N per G of C). The benefits for the cultivated associated crops, or for the soil depend on the use which is made of the legume.
Fixation by free-living organisms
The extent of nitrogen fixation by non-symbiotic microorganisms is determined by factors that are soil-related. Oxygen supply is of considerable importance: cell-free extracts of organisms fix only nitrogen under anaerobic conditions (STEWART, 1969); Azotobacter, although at is an obligate aerobe, fixes N more efficiently at reduced oxygen pressures (PARKER, 1954): in most laboratory experiments anaerobic fixation is usually four times as important as aerobic fixation.
Most nitrogen fixing bacteria in acid tropical soils are heterotrophs: they need an organic substrate for energy supply and growth. In the case of nitrogen fixation, even though it is an exothermic process, external sources of organic carbon compounds are necessary in order to form the aminoacids. Moreover the Azotobacterioeae are specific in their requirements, and cannot use complex carbohydrates such as cellulose. They need the association of cellulolytic organisms (JENSEN, 1965). Most experiments on soils have shown that with increasing organic carbon supplies the nitrogen fixation by heterotrophic organisms is stimulated. At any rate, high energy phosphate supply is always needed (STEWART, 1966).
Most organisms have exacting pH requirements. Azotobacter will not fix nitrogen below pH 6.0 (STEWART, 1966). Azotobacter spp. tend to disappear almost completely in soils of pH < 5.0 (CARNEIRO, 1968). Beyerinckia and Derxia, on the other hand, only occur in acid soils. The optimal soil pH for Beyerinckia would be 5.5-5.9: this organism would not survive outside the pH-range of 4.0-7.4 (BECKING, 1961).
Not the overall soil pH has to be taken into account however. In ferralsolic areas with a pronounced dry season, termites may form microenvironments of higher pH by the concentration of bases in the mounds and nests. BOYER (1971) mentions greater numbers of Beyerinckia and Azotobacter chroococum in samples from termite mounds built by Bellicositermes than in the surrounding topsoils.
Clostridium, Pseudomonas and Bacillus are found over a wider pH range. It is not established whether the hydrogen ion concentration is directly involved, or if aluminum or manganese activities, or nutrient deficiencies are responsible for the reduced fixation when pH conditions become marginal. At any rate, molybdenum and iron are components of the nitrogenase enzyme complex (BURRIS, 1969). Since fixation is growth-related adequate supplies of K and P are necessary. PRAMANIK and HISRA (1955) found a significant increase of Azotobacter after P fertilization.
It is generally assumed that little N-fixation will occur when sufficient amounts of either ammonium or nitrates are present in the soil. Urea has been reported to retard fixation markedly, and N2O is a specific competitive inhibitor (STEWART, 1966). It is not possible however to set critical levels only on the basis' of the actual amounts of N in the soil. Clearly more complex C:N:P:S ratios are involved in regulating the nitrogen fixation. BREMNER and SHAW (1958), and GREENLAND (1962) found that when the C:N ratio in the soil is less than 5, no fixation of nitrogen may be expected.
Among the climatic factors, the soil moisture content which may be seasonally deficient may reduce cell growth and nitrogen fixation may. Beyerinckia is particularly sensitive to drought.
Under normal field conditions temperature is seldom a limiting factor. Optimums for growth are 26-33° C for Beyerinckia fluminensis. 25-37° C for Derxia gummosa, 29-37° C for Azotobacter paspali (DOBEREINER, 1969).
There are some special environments in tropical agriculture which influence microbiological activity that are worth mentioning: in paddy soils algae may be the major contributors to N fixation. Blue green algae only fix nitrogen at pH's between 6 and 9, although some have been found to operate at pH 4.
Another specific ecosystem is the litter, and ether plant residues which may form substrates for nitrogen fixation by heterotrophs. Not all residues are adequate however. The efficiency of mulches for tropical crops depends much on their C/N ratio. Data of BARTHOLOMEW et al. (1953) show that no gains from fixation should be expected with ratios of less than 20; where forest mulches produce gains for the ecosystem, they are of the order of 1-2 mg N per Kg of plant material (1-2 ppm) in 35 weeks.
Some tropical crops such as sugarcane produce huge amounts of organic matter: the high frequency of Beyerinckia in such fields, may enhance nitrogen fixation KASS and DROSDOFF (1970) calculated however that the gains would not be more than one ppm of N per year, or 2-3 kg/ha/year. The Azotobacteriaceas in addition have to live in association with celluloses decomposers, or with photosynthetic organisms in order to obtain the simple carbohydrates which they need for N-fixation.
The rhizosphere is a suitable environment for the development of microorganisms, by the exudates and the presence of decaying plant tissues. This area however is not particularly abundant in nitrogen fixing species, except for the rhizoplan of sugar cane, rice and some tropical grasses described by Brazilian investigators. The number of microcolonies never exceed 105 per gram soil however. Such low populations may increase the yields of associated crops, but it is not demonstrated yet if these increments are due to better nitrogen supplies, or to production of growth stimulating agents (BROWN et al. 1964, MISHUTIN, 1967). Whether Azotobacter may control pathogenic organisms is still under discussion.
Stimulating effects of higher plants on asymbiotic N fixation appear to be specific. Azotobacter paspali only develop faster when they are associated with tetraploid forms of Paspalum notatum (DOBEREINER, 1970). Some plants on the ether hand, reduce the bacterial development: Melinis Minutiflora depresses Beyerinckia occurrence (DOBEREINER, ivid.).
KASS, DROSDOFF and ALEXANDER (1971) observed that the association of Azotobacter paspali only increases the N content of the roots, and not the areal parts of the grass. Since all fixation ceased when soils were incubated in the dark, the activity of a photosynthetic microorganism may be involved. The authors estimated on the basis of the greenhouse experiments that the nitrogen gains by Paspalum notatum are probably between 10 and 20 kg/N/ha/year.
To summarize, N fixation by free living organisms is a process which needs considerable amounts of organic matter to be efficient. According to DOBEREINER (1969) free living organisms would be able to fix between 12 and 30 mg. N per g of carbon source. In the absence of nitrogen in the soil, for ten kilograms of N fixed per hectare, the bacteria would have used between 330 and 830 kg of carbon, all other conditions being optimized.
Non symbiotic fixation in the field is a process by which nitrogen is brought into the soil-plant system mainly in order to restore equilibrium between C and N as determined by prevailing ecological conditions. The estimates by KASS and DROSDOFF (1970) of the amounts that are gained are rather low. They are only effective when environmental conditions are such that organic fitter and living organisms act as nitrogen fixers, and net as immediate sources of nutritional nitrogen to plants which are cultivated simultaneously.
In the field there are seldom situations whereby the soil organic system releases N to plants, and at the same time operates as a nitrogen fixer. Both processes seem to be mutually exclusive. Therefore they are interesting For management operations which use fallows, or long resting periods during which high equilibrium NE levels can be achieved.
In intensive agriculture where such long resting periods are not economic, they are not considered as primary sources , of N for crop production. This is particularly true For cropland. Pastures and livestock production in extensive system may be partly supported by biological nitrogen fixation; in intensive management its role is rather minor.
b. Release of nitrogen
Ammonification and nitrification
Successive wetting and drying produces increased mineralization of organic matter in practically all soils. Ferralsols exhibit the same phenomena. In areas which are subject to repeated drying and which are exposed to high temperatures in the surface horizons, the stimulation of organic matter decomposition may bring about a nitrate flush at the beginning of the rainy season. Clearing forest land often produces the same effects.
The amount of N released depends on the temperature, the length of the dry period, the organic carbon content, the C/N ratio of the organic matter, and the kind of soil (AGARWAL, SINGH and KANCHIRO, 1971). The process may in part be purely physical and chemical as far as the production of ammonium is concerned, but nitrification is essentially biological.
Ammonification is not narrowly controlled by ecological conditions, and occurs in a wide range of temperatures and pH environments. Even between 50°-70° C it produces high amounts of NH4+, provided sufficient moisture is present. Ammonification continues up to moisture tensions of pF 5.6 (DOMMERGUES, 1962).
Nitrification from ammonium is achieved by two autotrophic obligate-aerobe microorganisms: Nitrosomonas, which produces nitrites (NO2-) and Nitrobacter which finalizes the oxydation into nitrates (NO3-). Their activity is highest between pH 7 and 9. The minimum pH is flexible and varies between 7 and 5 (DOMMERGUES, 1970).
Nitrobacter is sensitive to temperature and is not active at more than 40° C; its efficiency is lowered by excessive concentrations of NH3 in the soil. As a rule nitrification is diminished by impeded drainage and lack of oxygen due to waterlogging. At the dry end of the soil moisture range, nitrification is stopped at pF 4.5-4.2. Therefore high temperatures, above 40° C and low water contents seen to favour the accumulation of ammonium or nitrites.
Grassland soils contain usually less nitrifying bacteria than forest soils. MEIKLEJOHN (1962) found that nitrite oxidizers were almost absent in savanna soils even after the start of the rainy season. The lack of available N is often due to the absence of bacteria able to oxidize nitrite to nitrate. The growth of the nitrifying bacteria may be suppressed by toxins secreted by some tropical grasses (BOUGHEY et al. 1964); grassroots are more capable to absorb N as ammonium directly and may therefore become more competitive to other plants for obtaining nitrogen nutrients.
Volatilization of nitrogen
Denitrification is probably not a major cause of nitrogen losses in well drained ferralsols, since it requires dominant anaerobic conditions, pH's well above 5, sufficient nitrates, and a good supply of organic matter. The optimal pH for denitrification is between 7 and 8.6, which reflects neutral to alkaline conditions which seldom occur in ferralsols. In some special habitats as irrigated rice paddies high pH's may exist and special management practices should then be selected in order to avoid N-losses. Some of these are the non-utilization of nitrate fertilizers or the use of denitrification-inhibitors.
GREENLAND (1962) conducted incubation experiments in the laboratory with samples taken from freely drained upper slope soils of Ghana at pH's ranging from 6 to 7. For water contents of 160% of the waterholding capacity the tests showed that very rapid losses of N could occur; most took place within three days of incubation. Under field conditions however it is unlikely that denitrification would cause appreciable nitrogen losses, except when soils are flooded and oxygen pressures are reduced within the soil mass. Soils may eventually be saturated with water after heavy rainstorms: but this condition in well drained ferralsols would not last much more than one day.
The amounts of nitrogen which are lost from tropical soils by ammonium volatilization are the highest from soils with a low cation exchange capacity. The release is the slowest in acid soils (CORNFORTH and DAVIS, 1968); sandy soils which warm up more quickly may suffer heavier ammonium losses than clayey soils. When large amounts of readily decomposable organic matter are added, the production of ammonium and its accumulation in the topsoil may inhibit the nitrification of nitrites. Possible losses of N2 which are formed by the decomposition of ammoniumnitrites have been suggested as a possible explanation (ibid.). The soil mixtures which were used for the laboratory and greenhouse tests contained 0.225 and 0.45% N; this high percentage was reached by adding plant materials having a C/N ratio, of 9. Such amounts of organic material in cultivated soils are rather unfrequent.
Leaching of nitrogen
Most nitrogen losses in ferralsols have been attributed to leaching.
Lysimeter experiments conducted by KUPPER et al. (1953) showed that nitrates, applied on the soil surface at a rate of 30 kg N/ha, are completely leached after six months (or 962 mm of rain) at more than 45 cm depth in clayey soils; the same effect is obtained after two months in sandy soils. The columns used in these tests did not carry any vegetation.
Normally ammonium does not percolate through the soil fast enough so that it can escape nitrification. In KUPPER's experiments it was completed after two months in the light textured soils, and after four months in the clayey materials.
In most soils which are not covered by vegetation all applied nitrogen will be leached from the first 50 cm after 1 000 mm of rain: in light textured ferralsols it is estimated that about 750 mm would be sufficient.
The vegetation offers the most efficient way for reducing nitrogen losses due to leaching. At any rate in humic climates they will always remain considerable. VICENTE-CHANDLER at al. (1964) found that intensively managed forage crops only recovered 55, 54, 48 and 30% of the nitrogen applied to Humic Ferralsols, at rates of respectively 200, 400, 600 and 800 kg N/ha as ammoniumsulphate.
i. Forms of phosphorus
ii. Phosphorus adsorption, fixation and residual effects
It is generally accepted that with increasing weathering the percentage of total P in the soil decreases. Moreover, an important part of the phosphorus which remains in the profile is occluded by sesquioxydes during the weathering process, and becomes practically unavailable for plant nutrition.
There is also a gradual decline in the proportion of the "active" mineral phosphorus, as slowly available aluminum, iron and calcium phosphates. The rest of P, which occurs as organic compounds and in solution, is present in greatly varying amounts among the ferralsols.
The distribution of the various soil phosphorus components in an Orthic Ferralsol, under tropical savanna, is illustrated in fig. 12. The profile in this example is an intergrade to younger less weathered soils, and it contains more KCl extractable aluminum than the medal concept would normally accept. Some chlorite is present in the clay fraction. It can be seen that below 40 cm depth the amount of inorganic P is constant; only the organic P varies. In the top layers, approximately 75% of the total P is organic. It is assumed that the plants have in part extracted P from the mineral pool, and preserved it from further losses by tying it up in the organic matter. The original data for figure 12 are given in table 15.
Not all ferralsols show a distribution of phosphorous similar to the given example however. The topsoil of rhodic ferralsols may contain as much as 1000 ppm of total P: the orthic and xanthic types normally have not more than 400 ppm, as was reported by JORGE and VALADARES (1969) in Brazil. In these investigations it was found that only 5-15% is in the organic form.
WESTIN and DE BRITO (1969) determined between 18 and 284 ppm of total P, with values of 20-40 for the percentages of organic-P on the total P content in ferralsols of Venezuela.
Low percentages of total phosphorus in parent materials of ferralsols are often responsible for poor soil fertility. The original P content places a ceiling on the soil potential, not only for crop production, but also for organic matter accumulation and nitrogen fixation. Since there are only few external sources of phosphorus, the amounts of total P in soils parallel those of the parent rocks. Acid igneous rocks contain usually less than 800 ppm, whereas basic rocks may have as much as 2700. Sedimentary rocks range between 250 and 750 ppm. Basic rocks are therefore in a better position For maintaining soil fertility, by the more intensive organic matter turnover which reciprocally keeps phosphates for longer time in more available forms.
Fig. 12 - Distribution of phosphorus in a ferralsols (BENAVIDES, 1963)
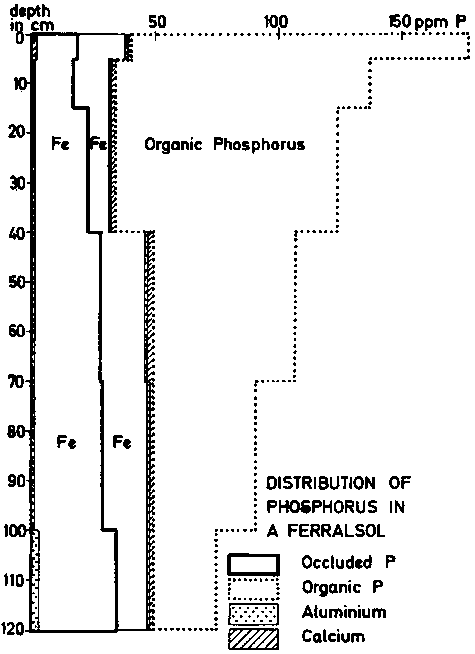
Table 15 FORMS OF PHOSPHORUS IN A FERRALSOL IN PARTS PER MILLION (BENAVIDES, 1963)
Low Al-P amounts are indicative of strong weathering under well drained conditions; the tie-up of phosphorus in occluded form would be the most intensive in climatic regimes having a dry season. Impeded drainage would somewhat raise the aluminum phosphorous level (WESTIN and DE BRITO, 1969).
ENWEZOR and ALBOORE (1966) found in the surface horizons in Nigeria organic/total P percentages between 17 and 29% in savanna areas and 72% for forest soils. The total phosphorus content in the upper horizons in forest soils was almost twice as much as under savanna. The natural grassland vegetation has apparently only a low efficiency in recycling phosphorus. The forest is more active in absorbing P by roots in the subsoil and, unless time is a limiting factor, is more capable to restore P levels in the surface layers.
Since a substantial part of the phosphorus components in ferralsols are organic, the agricultural practices which favour the decomposition of humus result in an accelerated release of P to the soil solution. Liming may have such effects. When they are combined with the neutralization of exchangeable aluminum, lime significantly increases the amount of available phosphorus, provided the organic P-pool is sufficiently large. In highly weathered soils of the tropics, and where the economic resources of the farmers cannot support the expenditures for purchasing chemical fertilizers, the phosphorus management is largely a soil organic matter management.
C:N:P ratios in ferralsols have not yielded significant correlation with the mineralization of P from organic sources, and further research on this subject is needed.
The capacity of soils to fix phosphorus was estimated by CATANI and GLORIA (1964) using p32. As in many other investigations it was found that there is a close relationship with texture (figure 13).
Other factors are also important. Within soils of equal mineralogy the amount of P retained by soil samples is correlated with the free-iron content, with the clay percentage and with the extractable aluminum. As a rule, the more crystalline the exchange complex, the smaller the fixation capacity; in ferralsols of comparable pH, the strongest fixation will be found in soils intergrading to rejuvenated profiles, in which the KCl extractable aluminum content and the permanent charge are higher than modal. Contamination with volcanic ash may also raise the P-fixation power of ferralsols.
FOX et al. (1968) ranked the fixation capacities of the most common components of clays as follows: amorphous hydrated oxides > gibbsite > kaolin > goethite ³ montmerillonite.
The fixation intensity due to the action of oxides may be lowered by blocking the bonding sites with organic matter, as explained in the chapter dealing with the charge characteristics of the exchange complex; conversely, application of phosphates may increase the net cation exchange capacity. Significant response to much treatments will only be obtained in soils that are particularly rich in iron oxides, especially of oxidic or ferritic mineralogy.
Fig. 13 Linear regression of phosphate fixation as a function of clay percentage
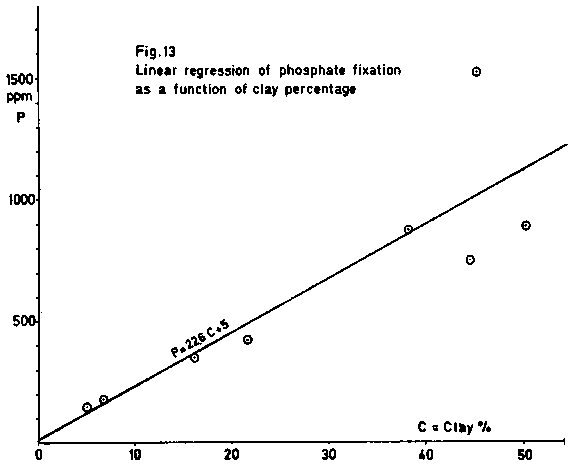
The concept of fixation is not well defined. Much fixed phosphorus remains slowly available for plant nutrition, and residual effects of fertilizers applications are common in ferralsols. It is seldom known how long they last however. LAUDELOUT (1959) observed that freshly precipitated iron and aluminum phosphates are as available to plants as the phosphorus from the fertilizer itself.
Evidences of residual effects of phosphates in humic ferralsols with acric properties in Brasilia were obtained by IRI Research Institute (1967), from which report figure 14 has been reproduced. The soybean yields obtained one year after the application of superphosphate significantly correlated with the amounts of P2O5.
According to FOX (1973); phosphate adsorption values can be converted into phosphate fertilizer requirements. Adsorption isotherms describe the equilibria between adsorbed P and the activity of P in the soil solution. Examples of such curves, given by FOX (ibid.) are illustrated in figure 15. Assuming that corn requires 0.06 ppm P in the soil solution the orthic ferralsol would need approximately 200 ppm P adsorbed on the exchange complex, Ferralsols are not the strongest phosphate fixers.
Fig. 14 - Residual effect of phosphorus on yields, costs and returns from soya on Humic Ferralsol at Brasilia (IRI Research Institute, 1967)
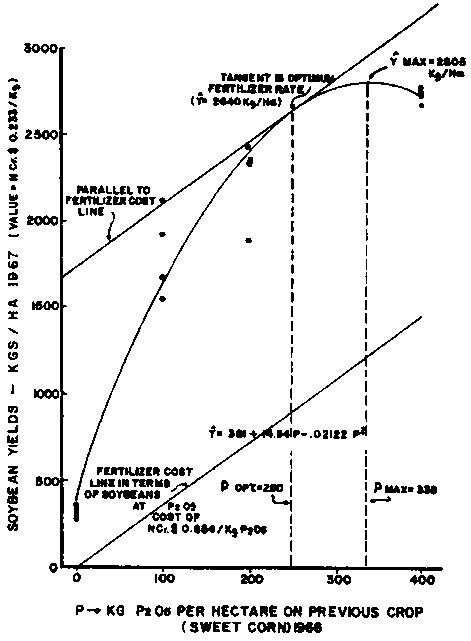
Phosphate adsorption by plants is conditioned by other nutrients. Transport in the plant depends on the availability of magnesium, and is important especially for the production of seeds which are rich in oil. Magnesium deficient ferralsols will often not respond to phosphate fertilization, if the unbalanced cationic nutrition is not corrected simultaneously.
Oxic horizons by definition contain only traces of weatherable minerals. Consequently they are poor in potassium included in the crystal lattice of soil particles. The bulk of potassium is essentially present in solution or it is adsorbed on the exchange complex.
Another kind of K-reservoir exists in the soil organic matter and the vegetation. The order of magnitude of the latter can be estimated from that data in table 1, page 14.
Since there are only limited amounts of 2:1 minerals in the clay fraction, little K-fixation is normally occurring in oxic horizons. However the horizon which contain an appreciable quantity of chloritized expansible layer silicates or which have a sizeable amount of amorphous materials may not release potassium with chemical extractants as readily as may be expected from exchange reactions. In such cases the potassium removed by crops frequently exceeds the quantities which are determined by chemical laboratory analysis as soluble or in exchangeable form (OLIVEIRA et al. 1971).
The activity of potassium in the soil solution depends on the percentage K saturation and the nature of the exchange complex. Adsorption isotherms which express the relation between absorbed K and K in solution at given ionic concentrations, have been presented by FOX (1973). They indicate that in ferralsols only low K saturations on the exchange complex are necessary in order to achieve adequate potassium intensities in the soil water for uptake by plant roots, and that at this level the capacity of the mineral soil to maintain continuous replenishment of K in the soil solution from exchange sites is rather limited.
They also imply that potassium ions are not tightly held by ferralsols and that K is subject to leaching. As compared to Ca and Mg, losses by lixiviation are not so severe however. The behaviour of potassium is somewhat similar to the leaching of nitrogen, although K shortage usually appears earlier in crop rotations than the potassium deficiency.
Potassium supply to the soil solution is not biologically controlled as it is the case for nitrogen. After a period of dryness, the heavy rain does not produce a potassium "flush", comparable to the surge in nitrates due to accelerated nitrification. Therefore losses are less important. The most effective way to prevent potassium leaching in ferralsols, is to have crop roots developing as early as possible in the growing season into the deeper layers, from which it may be adsorbed and recycled in the vegetation. This recovery of potassium by plant roots is probably more efficient in tropical climates, where periods of rainfall and plant growth coincide, than in temperate or mediterranean climates, where the highest precipitation occurs in the cropless winter season.
According to MIDDELBURG (1950) and SCHUFFELEN and MIDDELBURG (1953), the leaching of cations in soils with low base exchange capacity, results in an increase of the ratio of monovalent to divalent cations absorbed on the exchange sites. The leaching in acid ferralsols is therefore achieved mostly at the expense of calcium and magnesium. This may explain why potassium deficiencies are rather late in becoming limiting in the sequence of nutritional disorders which affect crop production in ferralsols. Potassium furthermore seldom appears as a single factor in causing reduction of yields, and equilibrium levels between Ca:Mg:K in the soil solution determine cationic uptake by plants. Mg and K antagonism are well known. The optimum ratios in exchangeable bases will be discussed in later chapters on diagnostic techniques for fertilizer requirements.
i. Calcium deficiencies
ii. Exchangeable aluminum, aluminum in solution
iii. pH and liming
With increasing leaching many ferralsols become depleted of available calcium. At low base exchange capacities, the strong dilution progresses at the expense of bivalent rather than monovalent cations. It is mainly under savanna vegetation, which has only a weak capacity to recycle nutrients into the surface horizons, that calcium deficiencies are most likely to occur.
There may be other reasons for having calcium deficient crops in acric, iron rich ferralsols. Specific adsorption may take place, and calcium may be fixed and made only slowly available to plants. In such cases the tie-up of calcium also causes a reduction of the cation exchange capacity, due to blocking of available sites.
Examples of calcium shortage in soils are known from Latin America. Many soils of Central Brazil which occur under savanna vegetation (cerrado) have only traces of exchangeable calcium (de FREITAS, McCLUNG and LOTT, 1960). The roots of most crops do not penetrate into layers which lack or are extremely poor in calcium (PEARSON, 1966). The soil volume which can be explored by the rooting system is consequently reduced.
Due to the low mobility of calcium in soils and plants, increased efficiency of liming can be achieved by the incorporation of the amendment into the subsoil. MIKKELSEN et al. (1963) at Orlândia reports that plowing down half of the lime (2 tons of 27% CaOO and 19% MgO) to 25 cm depth produced 80% mere seed cotton than disking in the entire two tons at 10 cm depth only. In the same experiment it was noted that two tons of lime applied half by plowing to 25 cm and half by disking at 10 cm depth produced 95% of the yield obtained by four tons of lime disked into the surface ton centimeters only.
As leaching becomes more intensive the amounts of Ca, Mg and K on the exchange complex and in the soil solution decrease. The soil may adjust to the new concentrations of cations by changing the activities of H+ and Al.
In ferralsols which have a permanent charge (by definition not higher than 10 meq /100 g of clay in the oxic horizon) it is commonly assumed that the permanent exchange sites which become available are readily occupied by exchangeable aluminum, which produces acidity by hydrolysis. If aluminum occupies more than 60 percent of the exchange complex (which is active at the pH of the soil), large quantities of Al occur in the soil solution (NYE et al. 1961).
In ferralsols which have a low permanent charge, or a high ratio pH-dependent to permanent charge, as it is the case in most typical profiles, the leaching of cations and the dilution of the soil water causes the effective exchange capacity to decline. The soil acts as a weak acid and, since both the CEC and the exchangeable bases decreases simultaneously, there is practically no modification of the base saturation. There are no exchange sites to be occupied by aluminum. The pH of the soil is then primarily defined by the ionization constants of the acidic groups: as a rule, organic compounds develop more acid conditions than sesquioxides and kaolinite. Since aluminum does not participate significantly in the exchange reactions, toxicities originating from this cation are not frequently encountered; high acidity rather causes manganese concentrations to become increasingly detrimental to plant growth, especially in soil layers which are low in organic matter.
The critical pH below which aluminum begins to play a role in exchange reactions is usually 5.2 in ferralsols. Changes in pH therefore control the effects of aluminum ions in the soil solution. These may be achieved by liming or other chemical amendments. Flooding of ferralsols, and reduction of iron, also causes the pH to raise, and subsequently reduces the activity of aluminum (CIAT, 1972).
As the cation exchange capacity of ferralsols is predominantly pH-dependent, there is no consistent relationship between the saturation percentage calculated on the basis of the cation exchange capacity determined at pH 7, and the pH of the soil suspension. A low soil pH either indicates a weak base saturation in soils having a permanent charge, or a small amount of exchangeable cations in a horizon which is rich in organic matter. In both oases it denotes soils which are chemically poor. The opposite is not always true. A pH close to six may be obtained in poor soils having a very small or no CEC, especially in subsurface horizons.
The purposes of liming may vary considerably from one case to another; one may apply calcium only as a nutrient, in order to correct deficiency symptoms in crops. The objective of applying lime may be to neutralize the aluminum which is present in ferralsols having a permanent charge, and depress its detrimental effects on plant development. In other instances, manganese toxicities may be controlled by lime applications.
One of the purposes may be to raise the pH of the soil to optimum levels for cultivated plant species, or reach values which are adequate for phosphorus availability. The efficiency of nitrogen fixation is also strongly influenced by soil pH, aluminum and manganese activities in the soil solution. In soils that are not oxidic or ferritic, liming may increase the capacity of the soil to retain cations. On the other hand calcium contents usually enhance mineralization of soil organic matter, and indirectly act on the nitrogen supplying power of the surface horizons.
i. Sulphur
ii. Minor elements
OLSON and ENGELSTAD (1972) estimate that the highly weathered soils of the tropics only contain 100 ppm of S. In surface horizons most of it would be in the organic form. Sulphur deficiencies have most frequently been observed in savanna soils. McCLUNG et al. (1959) reports that 75% of the sulphur contained in gramineae may be volatilized by burning. Severe nutritional disorders occur when less than 3 ppm of S is extracted by neutral NH4OAc, or if the organic sulphur content does not reach 35 ppm.
Lack of sulphur becomes only evident during intensive farming. MIKKELSEN et al. (1963) estimate that 30 kg S/ha may satisfy the needs of most crops, and that usually 40 kg of gypsum/ha, or superphosphates which contain this sulphate are sufficient to correct the sulphur shortage.
McCLUNG and QUINN (1959) assume that sulphur deficiencies are often induced by the formation of organic matter after substantial nitrogen fertilization. Any practice which increases the nitrogen in the soil, and contributes to organic matter accumulation would at the same time sequester sulphur, and make it temporarily unavailable to plants. Normal C:H:S ratios in the soil would be about 10:8:1.
Grasses are stronger competitors for sulphur than legumes. In pastures the latter suffer more from lack of S than the former, and may completely disappear. Without augmented sulfur supply to grass/legume associations, the grass may become completely dominant (McCLUNG and QUINN, 1959).
Minor elements are seldom limiting factors in crop production under extensive systems of agriculture in tropical areas where traditional yields are low (DROSDOFF, 1972). With more intensive management, the demand for micronutrients by plants increases beyond the supply levels of virgin soils however. This is especially true of acid soils which are under continuous cultivation and receive large amounts of nitrogen and phosphorus fertilizers. Boron, zinc and molybdenum are usually the most deficient, particularly when liming and high pH's may have reduced the availability of boron and zinc in the soil.
Excessive amounts of oligoelements in acid soils of the tropics are related to the desaturation of the exchange complex which may either be due to leaching, the utilization of acidifying fertilizers, or to the lack of organic matter which may control their solubility. Manganese, aluminum and iron are the most common cations which are discussed in toxicity cases.
a. Boron
Highly leached acid soils are generally low in available boron, and most of it is in organic form, which can be supplied to plants after the decomposition of humus.
MARTIN (1969) found boron deficiency symptoms in oil palm on soils which contained less than 0.2 ppm of B; they are easily corrected by applications of borates at a rate of 50 g per tree (or 7-8 kg/ha).
Fertilized and limed humic ferralsols with acric properties at Brasilia responded not significantly to applications of boron with corn, cotton and soya as test crops (BRITTO et al. 1971) Borax at a rate of 10 kg/ha was used in this experiment.
b. Iron (CIAT, 1972)
The iron content in the soil solution of flooded ferralsols increases considerably when reducing conditions are maintained for considerable time during irrigation. CIAT (1972) in rice experiments on ferralsols found that the Fe concentration remains low for several weeks before rising sharply to a maximum of 300-350 ppm. After the peak the high pH depresses the solubility of about 150-200 ppm, the Eh decreases at about 90 mV and the pH equilibrates at a value of 6.5
Rice may suffer from Fe toxicity when the soil solution concentration is above 300 ppm; at lower levels the deposition of iron oxides as coatings on roots is reported to prevent the uptake of nutrients, especially P, in flooded previously well drained ferralsols, and to cause oranging and early senescence of lower leaves.
The excessive amounts of iron in the flooded rice paddies can be avoided by intermittent or rotational irrigation. The correction may also be achieved by improving the internal drainage, often at the expense however of stronger leaching of nutrients. Preflooding which has the advantage of passing the Fe peak before the early stages of the growth of the rice, has the disadvantage of more important P fixation by the soil.
c. Manganese
The uptake of manganese by plants in well drained ferralsols is dependent on the pH conditions of the soil, its calcium and iron content, and the amount of organic matter. Crop species are also important.
Most ferralsols develop excessive manganese contents at low pH and weak base saturation. ABRUÑA et al. (1970) found that ferralsols in Puerto Rico with a base saturation of 32-40% gave: 2380 ppm Mn in tobacco, leaves; levels of 3000 are considered toxic; at a saturation of 95% this amount was reduced to 580 ppm. The soils had an initial pH of 4.4, contained 200 ppm of exchangeable, and 2000 ppm of easily reducible manganese, PRATT (1966) found that a pH of 6 is completely effective in eliminating toxicity levels in ferralsols of Brazil.
The critical pH depends on the soil organic matter content. MEDCALF (1956) found that mulching has a depressive effect on the uptake of Mn by coffee.
BOYER (1956) reported manganese deficiencies in groundnuts on light textured ferralsols in the République Centrafricaine, when the total manganese content was below 60 ppm.
d. Molybdenum
Molybdenum is present in soils as molybdate anions, which are nearly immobile and in this respect are very similar to PO4. In acid soils it occurs adsorbed or included in crystals of hydrous iron and aluminum oxides; when adsorbed it is able to react with other anions; it may also be fixed as insoluble salts. A part of it is present in the organic matter (SAUCHELLI, 1969).
The availability of Mo increases with pH, even above neutrality; in soils which only contain marginal amounts of micronutrients the addition of lime may correct the deficiency of molybdenum, but also reduce the availability of manganese and zinc below critical levels (DROSDOFF, 1972).
Molybdenum is essential in nitrogen fixation reaction.
NEWTON and SAID (1957) mention molybdenum deficiencies in ferralsols of Java. Applications of 1 kg/ha of sodium molybdate increased yields of groundnuts.
DE FREITAS, McCLUNG and LOTT (1960) observed response to applications of molybdenum on recently cleared ferralsols after savanna vegetation with soybeans only in the absence of liming. Increased yields were then obtained by 0.3 kg sodium molybdate per hectare. MASCARENHAS et al. (1967) did not detect minor element deficiencies in newly opened fields which were limed and fertilized on Latosol Roxo after cerrado vegetation. The test crop was also soya.
IRI (1968) considers that molybdenum is probably needed for obtaining adequate production of Stylosanthes gracilis on savanna soils of Brasilia. CHACON (1968) found better response of soya and groundnuts to molybdenum when soils were not limed in the Eastern plains of Venezuela.
e. Zinc
The solubility of zinc in the soil decreases with rising pH; at pH above 6.5, only small amounts of available zinc are present in weakly buffered ferralsols. The critical pH level is difficult to define however. DROSDOFF (1972) mentions zinc deficiency symptoms both in light textured ferralsols at pH below 5, and in clayey ferralsols with a pH above 6. Both the total and the available zinc are important in evaluating optimum conditions for plant uptake of this element.
Some zinc deficiencies in ferralsols would be due to overliming. MOITY (1954) reports a soil pH above 6.5 as inducing zinc deficiencies in bananas.
The solubility of zinc nitrate is very high, and would be mainly responsible for leaching losses in most ferralsols, which could cause depletion of exchangeable zinc under acid conditions.
According to STANTON and BURGER (1967) well crystallized iron oxides, for example goethite, could fix zinc under slowly available forms.
IRI (1969) observed that corn develops extreme foliar chlorosis and fails to develop normally when zinc is not applied to sweet corn on fertilized and limed ferralsols at Brasilia, in fields which were newly opened after savanna vegetation.
In light textured soils the application of five kg/ha of Zn SO4 banded adjacent to the row with other fertilizers was the most efficient way to correct the nutrient deficiency. 25 kg broadcast was also effective (IGUE and GALLO, 1960).
Humic ferralsols with acric conditions at Brasilia, when fertilized and limed, respond strongly to applications of zinc both with corn, cotton and soya as test crops (BRITTO et al. 1971). Micronutrients alone, nor macro-nutrients alone had any effect on corn. Ten kilos zinc sulphate per hectare was enough to correct the deficiency.
Fig. 15 - Phosphate sorption by ferralsols of tropical America (adapted from FOX, 1973)