Introduction
Why cool fish with ice?
Why not use other cooling substances?
The manufacture of ice
Ice is ice
Block ice or small ice?
Flake ice or tube ice?
Why not sea water ice?
Which ice is best?
This Advisory Note summarizes the principal methods of manufacturing ice and the advantages and disadvantages of the main types produced; some reference is made to costs.
Ice as a cooling medium for fish has a great deal in its favour; it has a very large cooling capacity for a given weight or volume; it is harmless, portable, and cheap. It is especially valuable for preserving fish since very rapid cooling is possible through intimate contact between fish and small pieces of ice. Ice keeps the chilled fish cold, moist and glossy and prevents the dehydration that could accompany other methods of cooling, such as refrigerated storage without ice. White fish such as cod, caught in Arctic water and preserved in ample crushed ice soon after catching, will keep in first-class condition for five or six days and will remain edible for up to 15 days.
Ice is its own thermostat and, since fish is mainly water, ice maintains fish at a temperature just slightly above the point at which the fish would begin to freeze; the point of equilibrium for sea fish, iced soon after catching, is near to 31°F since the mixture usually includes some salt and blood.
First of all, the difference in temperature between such substances as solid carbon dioxide or liquid nitrogen and the fish to be cooled is very great. This means that fish in close contact with solid carbon dioxide, for example, can rapidly become partially frozen, while fish can be seriously damaged by sudden immersion in very low temperature liquids. These substances are used for cooling fish other than by direct contact, but the power required to manufacture them, and hence the cost, is much greater than for a quantity of ice having the same cooling capacity. Five times the power is needed to make solid carbon dioxide, twenty times as much power to make liquid nitrogen. In Britain, solid carbon dioxide costs about twenty times as much as ice for the same cooling capacity.
Ice that is to be used for cooling fish must be made from water that is fit to drink.
Whatever method of manufacture is used, ice making requires from 2 to 5 horse-power for every ton made each day; in other words, to produce a ton of ice may require anything from 30 to 80 kilowatt hours of electricity. Because of the fairly high power requirements, manufacture of ice in quantity on board ship is impracticable except on fairly large vessels. Generous use of insulation will assist in keeping down production costs for all methods of manufacture.
Ice is produced in two main forms, as blocks and as small ice:
Block ice manufacture
The traditional method is to immerse cans filled with water in a bath of low temperature brine. Size of block may range from 55 lb to 300 lb or more; 3 cwt is considered to be the largest size of block a man can conveniently handle. A thickness of at least 7 inches is desirable if the block is to remain stable when being moved about on edge.
Very large blocks are usually made only in large installations that operate continuously, since the freezing time can be 18 hours or more. Losses during distribution in warm climates are less with large blocks since they take much longer to thaw. Blocks are harvested by immersing the cans in warm water or brine to release the ice; a 24-hour cycle has been found to be most practicable in large installations.
There have been few improvements in the efficiency of the brine-freezing method in recent years; experiments have shown that some improvement in performance can be gained by increasing brine velocity up to about 35 ft/minute after which there is no worthwhile decrease in freezing time.
Careful control of brine temperature is necessary to avoid production of cracked blocks; too cold a brine for freezing or too warm a brine for thawing can cause trouble. In very large plants the power required for ice production can be as low as 2 horse-power for a ton of ice a day but to this figure must be added the power needs of block crushers, harvesting cranes and other auxiliaries for fair comparison to be made with other methods of manufacture. Two men can handle up to 500 tons of ice a day in a modern automatic plant.
Block ice can be made more rapidly by a direct evaporation system known as the rapid ice method; a primary refrigerant is circulated through a jacket round the cans of water and also through needles, or pipes, passing through the centres of the cans. Ice forms simultaneously at the outside and at the centre of the can; the greatest distance the unfrozen water can be from a refrigerated surface is then rarely more than 2 to 3 inches.
This method enables block ice to be produced much more quickly, and there is a considerable saving in weight of plant and in factory space required. Blocks are removed from the moulds by gravity after a hot gas defrost, thus dispensing with the need for thawing tanks and overhead crane. Unskilled labour can operate the plant, and the ice can be delivered at a low temperature. Power requirements for freezing are usually slightly higher than for the brine method because of the employment of a lower operating temperature.
Small ice
The term small ice is used here to describe the many kinds of ice made in small pieces under a host of brand names. The range includes snow or powder ice, flake ice, cube ice, scale ice, plate ice, tube ice, ribbon ice and many others.
All of the methods are based upon the formation of a fairly thin skin of ice on a smooth refrigerated surface, and the removal of that skin either by mechanical action or by warming of the surface. Freezing time varies as the square of the thickness of the ice layer; in theory it is therefore more economical to harvest the ice in thin layers. If heat is used to remove the ice, some of this advantage is lost; mechanical harvesting should be less wasteful.
The two most important types of small ice may be classified roughly as (i) flakes, which are smooth pieces that are either flat or slightly curved, depending upon whether they have been removed from a flat plate or from a cylinder of fairly large diameter, and (ii) cylinders, which are pieces cut from long, hollow tubes of ice formed inside refrigerated pipes of small diameter.
Flake ice is normally removed by a wedging action from the drum on which it has been frozen; the cutter may rotate on the inside of a vertical stationary drum or the drum may rotate horizontally against a fixed knife; there are also other variations. No heating is employed to remove the ice.
Cylindrical or tube ice is removed by hot gas defrosting from the inside of the tube in which it is formed, usually while the ice cylinder still has a hollow core; a rotating knife slices the ice cylinder into pieces as it slides from the evaporator.
There are numerous variants of these two main processes; ribbon ice, for example, is peeled from the outside of a flexible refrigerated tube that has been immersed in water long enough for a skin of ice to form.
Small ice machines are made in a range of sizes capable of producing any quantity from just a few pounds of ice a day up to 20 tons or more. Large plants are made by installing multiple units. Power requirements for the manufacture of flake ice are not much greater than those needed for block ice.
There has for long been argument about whether ice made at one port is better than another, whether natural ice is better than artificial ice, or stored ice is better than newly made ice, and so on.
The differences in properties of ices of different origin are so small that they will be of no significance to those using ice for cooling fish. The cooling capacity of ice is always the same; one pound weight of ice at 32°F requires 144 Btu of heat to change it all into water at the same temperature (1 Btu is the amount of heat required to raise the temperature of 1 pound of water by 1 degree Fahrenheit). The heat required to turn the ice into water at the same temperature is called latent or hidden heat, and the latent heat of fusion of ice is therefore 144 Btu/lb. No matter whether the ice has been made from hard tap water or soft rainwater, whether the ice is fresh or three months old, this figure for all practical purposes will remain the same.
If some of the ice has already turned to water, much of its value will have been lost; a slushy mixture of ice and water should never be compared with an equal weight of ice alone. Particles of ice made from hard water tend to stick together more during melting than pieces made from soft water, but their cooling properties are the same.
One pound of ice theoretically will cool a little more than 7 lb of cod from 55°F to 32°F. In practice a great deal of the cooling capacity of ice is absorbed in removing heat from the surroundings, so that even on trawlers fishing in Arctic waters 1 lb of ice is used to cool and keep cool only 2 lb of fish.
Ice in small pieces made by different methods will have different densities; a ton of crushed block ice may occupy only 56 cubic feet, a ton of tube ice 66 cubic feet, and a ton of flake ice 75 cubic feet. It is essential, therefore, when comparing different ices, to compare equal weights; what may appear to be the same amount of ice may be an equal volume, but one cubic foot of flake ice has far less cooling capacity than, say, a cubic foot of crushed block ice.
Ice is often made at a temperature somewhat below its melting point, but unless there is a very great lowering of its temperature, the increase in cooling capacity is unimportant. For example, ice cooled to 16 degrees F below melting point still has only 5% more cooling power, and since most ices are not manufactured at temperatures as low as this, the gain in cooling capacity can usually be ignored.
The size of particle, within limits, in any one type of ice makes little difference to either its meltage rate or the rapidity with which it cools fish; block ice finely crushed runs away no more swiftly than the same ice coarsely crushed. Ices whose pieces are of different shapes, however, do have somewhat different characteristics.
First of all, it is important to remember that direct comparison of costs of manufacture is impossible unless a complete calculation is made for each method using correct costs for the locality being considered. Cost of electricity, labour and water can vary enormously throughout the world, and it would be ridiculous to compare the price of ice from, say, a large, long-established block ice plant in Britain, having fairly cheap power supply, ample cheap, cold water but fairly high labour costs, with that from a very small flake ice plant in the Pacific, using expensive power and water but fairly cheap labour.
Where ice is wanted in hundreds of tons a day, then it is still difficult to find a cheaper method than the traditional brine-cooled block ice system; costs of thawing, storage, crushing and conveying must be included, however, for fair comparison with production of small ice.
Storage space requirements for uncrushed block ice are less than for any other form of ice, including blocks of ice made by the rapid ice system. Block ice can be made exclusively in off-peak hours, confining the use of peak power to the auxiliary harvesting load; full advantage can then be taken of cheap electricity, which in Britain may be half the ordinary price. Electricity in large plants can represent 40% of the total cost of production. Flake ice plants are often designed to work continuously for twenty-four hours, and cannot thus make full use of cheap power.
Large pieces of crushed block ice, when compared with Hake ice, make poor contact with the fish they are meant to cool, and may melt less rapidly; this is not necessarily a disadvantage for three reasons. First, some of the cooling is effected by cold melt-water running over the surface of the fish, rather than by direct contact between fish and ice; therefore there may not be such a marked difference in cooling effect as one would expect between flake ice and crushed block ice. Secondly, crushed block ice usually contains a proportion of fines, that is small pieces. Thirdly, where there is only a limited space available for ice, for example in a box containing a given weight of already chilled fish being transported inland, much of the meltage of ice in transit is caused by the warmth of the surrounding air. It may then be better to have in that space an ice that will combat the incoming heat for as long as possible; a greater weight of crushed block ice than of flake ice can be accommodated in the same space, and because the rate of meltage is less the already cooled fish will stay cool longer.
Crushed block ice, especially when the pieces are fairly large and coarse, can mark and bruise the fish more than smooth, flat pieces of small ice; block ice can of course be crushed to any required size of particle.
Block ice made by the rapid ice process requires less space for manufacture but needs slightly more storage space. Manpower requirements are about the same, but the cost of production will be higher because such a plant normally operates at a lower temperature. Crushed ice from blocks made by this process is for all practical purposes the same as other crushed ices.
The manufacture and use of small ice has a number of advantages. The plant takes up relatively little space, can be fully automatic and needs little maintenance. Much smaller sizes of units are available than for block ice manufacture, and therefore are of particular value to the small user remote from large ice factories. Manufacture begins very soon after the machine is started; there is no need to wait several hours before harvesting can begin. No crushing is required, although some tube ice machines producing pieces intermediate in size between flake ice and crushed block ice can be supplied with crushing facilities. The ice flows easily and is more easily stored, handled and transported; it lends itself well to mechanical, pneumatic or gravity distribution systems. The rate of meltage, however, may be higher than for crushed block ice during transfer from silo to chute, and steeper gradients are usually needed for chutes.
When made by a method that does not involve defrosting, the ice is dry and usually cooled well below its melting point, and is therefore less likely to congeal in storage. The flat shape of flake ice can make transfer of heat from fish to ice more rapid, although as pointed out earlier this does not necessarily mean faster cooling of the fish, since not all of the cooling is by contact between ice and fish; much depends on the manner in which the ice is used and upon surrounding conditions. The flakes are small and smooth and therefore do little damage to the fish. The ice can be made on board ship if required, and many machines can use either fresh or salt water.
Disadvantages are that flake ice takes up more room than crushed block ice or tube ice and therefore makes comparison with cooling capacity of other ices difficult, since this depends on use of equal weights of ice, not equal volumes. Flake ice sometimes bridges over the product being cooled, leaving an air space between the ice and the top of the fish, thereby reducing the flow of heat from fish to ice. Higher energy consumption is required for manufacture compared with block ice, since evaporation temperatures are generally lower, but when cost of crushing etc. is included for block ice, the costs of production are not vastly different.
It is difficult to compare one kind of small ice with another, since so many shapes and sizes are produced even of one general type. However, representative samples of the two main types, tube ice and flake ice, can be considered.
Typical pieces of tube ice are a little more than
1 inches in length and of
about the same outside diameter, with a hole through the middle about
inches in length and of
about the same outside diameter, with a hole through the middle about
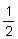 inch in diameter. Some of
the pieces are whole, some broken, usually longitudinally. The ice is often
moist on discharge since a hot gas defrost system is used to release the
ice.
inch in diameter. Some of
the pieces are whole, some broken, usually longitudinally. The ice is often
moist on discharge since a hot gas defrost system is used to release the
ice.
Representative pieces of flake ice can be from 1/16 inch to
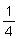 inch in thickness,
averaging about
inch in thickness,
averaging about 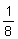 inch,
slightly curved in shape, and about an inch square. Usually they are fairly dry,
since they are normally removed from the drum mechanically and are sometimes
subcooled before leaving the machine. For comparison, crushed block ice in
Britain consists mainly of irregular lumps ranging from
inch,
slightly curved in shape, and about an inch square. Usually they are fairly dry,
since they are normally removed from the drum mechanically and are sometimes
subcooled before leaving the machine. For comparison, crushed block ice in
Britain consists mainly of irregular lumps ranging from
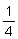 inch to 2 inches in
diameter.
inch to 2 inches in
diameter.
With the above sizes of particle, flake ice has over four times the surface area of tube ice, weight for weight. Flake ice should therefore melt more rapidly than tube ice and so cool the fish more rapidly, but this depends very much upon the circumstances in which the ice is used.
A greater weight of tube ice than of flake ice, with consequently greater cooling capacity, can be accommodated in a box of fish having limited space for ice, although it will probably melt more slowly than flake ice under similar conditions.
One therefore has to decide what duty is wanted from the ice. Flake ice takes up more space than tube ice, it melts quickly and thus should cool the product more quickly, but requires a larger volume for the same cooling capacity and to ensure that cooling is continued for the required length of time.
Flake ice should therefore be most useful where a warm product needs to be cooled rapidly, for example fish from the sea being stowed in a trawler’s fishroom; tube ice, on the other hand, will provide more cooling capacity in a small space than flake ice and will continue to cool slowly for a long time in a warm atmosphere; it should thus be more useful for keeping fish cool in transit, for example chilled fish in uninsulated containers consigned inland.
The larger the pieces of ice, the greater the possibility of damaging fish; tube ice is therefore more likely to make indentations than flake ice. Smooth pieces of tube ice should not mark fish so severely as jagged crushed ice.
Theoretically losses through meltage of flake ice in storage should be more severe than with tube ice. In practice this seems not to be so, since the surface of the heap soon forms a thin skin when melt water trickles down, thereby effectively retarding the melting of the remainder.
Tube ice is often wet on manufacture, and therefore storage should not be refrigerated, since the mass may congeal and the free-running properties be lost. Flake ice, too, can sometimes be wet through faulty manufacture. Caking of either type can occur at the sides or bottom of a heap if the container or room is not well insulated.
Sea water ice has been made and used many times for storage of fish, but the advantages gained have not always been clearly defined. It is doubtful whether any improvement in the quality of the fish has been directly attributable to the use of sea water ice.
Sea water ice must be made by a rapid process, otherwise ice will be formed first from fresh water, leaving a concentrated salt solution to be frozen last. Sea water ice, therefore, is often not homogeneous when made, and during storage brine again leaches out, leaving behind freshwater ice. For this reason, sea water ice has no fixed equilibrium temperature; some of the fish stored in such ice may become partially frozen or become too salty, while other fish will to all intents and purposes be in ordinary ice.
The difficulty of maintaining accurate control of temperature is the most serious disadvantage of sea water ice; the ice is no longer its own thermostat.
In use, flake ice has some advantage over crushed block ice in that the pieces are smaller, cause less indentation and melt more rapidly. Tube ice has properties somewhere between the two. It is doubtful whether the differences are sufficiently great to outweigh other considerations such as size of plant required, and the cost and speed of production.
For very large plants, it is likely that block ice manufacture may be still the most economical, but for moderate quantities in remote localities, the small-ice plant may very well be the answer.
Whatever type of ice is manufactured, you can never use too
much of it to keep fish fresh.