The L. monocytogens hazard identification, hazard
characterization and exposure assessment technical documents presented to the
consultation were discussed in detail by working groups. The full documents are
available on request from FAO or WHO and can be found at the following internet
addresses:
http://www.fao.org/WAICENT/FAOINFO/ECONOMIC/ESN/pagerisk/riskpage.htm
and
http://www.who.int/fsf/mbriskassess/index.htm
The executive summaries of these documents were updated during the consultation to take into account some of the questions and comments on the papers resulting from these discussions and are presented below. These are followed by a summary of the discussions of additional points that were not directly incorporated into the executive summaries of the discussion papers.
Introduction
RTE foods, per definition, are not cooked or submitted to other listericidal treatments immediately prior to consumption. Consequently, occurrence and possible growth of L. monocytogenes in these products can lead to human exposure. The overall objective of the hazard characterization and exposure assessment for L. monocytogenes in different RTE foods is to obtain estimates of the health risk for groups of consumers or the population as a whole. These risk estimates can then be used for identification strategies and actions that decrease the level of that exposure risk. Exposure assessment and hazard characterization of L. monocytogenes in different RTE food relay on the virulence characteristics and microbial ecology in particular products. The documents discussed in the consultation and the summaries-discussions presented below includes several examples of how hazard characterization and exposure assessment of L. monocytogenes in RTE foods can be carried out.
6.1.A Executive summary
Introduction
L. monocytogenes is widely distributed in the environment and has been isolated from a variety of sources including soil, vegetation, silage, faecal material, sewage and water. There is evidence to suggest that it is a transitory resident of the intestinal tract in humans, with 2 to 10 % of the general population being carriers of the organism without any apparent adverse consequences. The bacterium can grow at refrigerator temperatures and is resistant to various environmental conditions, allowing it to survive longer under adverse conditions than most other non-spore forming bacteria. Most cases of human listeriosis are sporadic and the source and route of infection is usually unknown, however, contaminated food is considered to be the principal route of transmission. Foods most often associated with human listeriosis are ready-to-eat products that support growth of L. monocytogenes, have long refrigerated shelf lives, and are consumed without further listericidal treatments. Invasive listeriosis (i.e., severe L. monocytogenes infections) is a relatively rare but often severe disease with incidence rates typically of about 4 to 8 cases per 1,000,000 individuals and fatality rates of 20 to 30 % among hospitalised patients.
L. monocytogenes causes illness by penetrating the lining of the gastrointestinal tract and then infecting normally sterile sites within the body. The likelihood that L. monocytogenes will invade the intestinal tissue depends upon a number of factors, including the number of organisms consumed, host susceptibility, and virulence of the specific bacterial isolate ingested. All strains of L. monocytogenes appear to be pathogenic but their virulence, as defined in animal studies, varies substantially. Listeriosis is an opportunistic infection that most often affects those with severe underlying disease (e.g. immuno-suppressive therapy, AIDS, and chronic conditions such as cirrhosis that impair the immune system), pregnant women, unborn or newly delivered infants and the elderly. The bacterium most often affects the pregnant uterus, the central nervous system or the bloodstream, and manifestations of listeriosis include but are not limited to bacteremia, meningitis, encephalitis, endocarditis, meningoencephalitis, miscarriage, neonatal disease, premature birth, prodromal illness in pregnant women, septicemia, and stillbirth. Incubation periods prior to individuals becoming symptomatic can be from a few days up to three months.
L. monocytogenes can also cause mild febrile gastroenteritis in otherwise healthy individuals. The public health significance of this type of listeriosis is much lower than that of invasive listeriosis.
Objectives
The scope and objectives of the present work were to quantitatively evaluate the nature of the adverse health effects associated with L. monocytogenes in ready-to-eat foods, and to assess the relationship between the magnitude of foodborne exposure (the dose) and the frequency of these health effects (the response).
Approach
The approach taken by the expert drafting group was to review and to summarize the literature relevant to hazard characterization for this pathogen, and the available dose-response models. In the absence of human feeding studies and surrogate pathogens, a number of dose-response models based on epidemiological data, animal studies, expert elicitation or combinations of these are compared and evaluated. The dose-response relationships model the probability of different biological end-points such as infection, morbidity, or mortality, as a function of the ingested dose of L. monocytogenes.
Key findings
The issue of what functional form of the dose-response relationship best describes the reality of the interaction between L. monocytogenes and humans is not resolved. However, the highly variable response to exposure to a foodborne pathogen of a human population, indicates that the likelihood that any individual will become ill due to an exposure to a foodborne pathogen is dependent on the integration of host, pathogen, and food matrix effects. Several empirical relationships encompassing a variety of assumptions have been applied to modelling L. monocytogenes dose-response relations. These models may fit the data equally well but give widely differing predictions in the dose region corresponding to levels of L. monocytogenes commonly found in food. The influence of host factors has been addressed by developing relationships specifically for susceptible or non-susceptible individuals. The potential effects of the food matrix on the dose-response relation were not considered as a variable within any of the models due to insufficient data.
Available models, which to a varying degree and sophistication have been evaluated against human epidemiological data, include (categorised by the end-point being modelled): 1) Infection (Weibull-Gamma model, Exponential model, Beta-Poisson model); 2) Morbidity (Exponential model, USFDA/USDA model); 3) Mortality (USFDA/USDA model, Exponential model). All the models have assumed that, in theory, a single bacterial cell has the potential of causing disease. In experimental models this probability is expressed by the "r value" (see Table 6.1). Each of the dose-response models reviewed has specific characteristics and limitations (Figure 6.1 and Table 6.1). Figure 6.1 is included for illustrative purposes and caution should be used in interpreting these curves since they are based on different endpoints, types of data etc., and in general, the predictions based on the models show a high degree of uncertainty and variation.
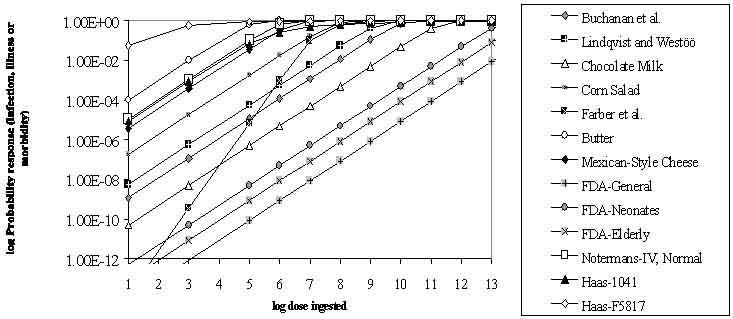
Figure 6.1. A comparison of available dose-response models for describing infection, morbidity or mortality.
NOTE: The points on the curves are only for legend purposes and do not represent data points. This figure is included for illustrative purposes and caution should be used in interpreting these curves since they are based on different endpoints, types of data etc., and in general, the predictions based on the models show a high degree of uncertainty and variation
Table 6.1. Summary of the selected dose-response models available for Listeria monocytogenes that were reviewed in the current document. The models are summarised in the order they are shown in the legend of Figure 6.1.
|
Model/Study |
Biological End Point |
Model/ Parameters |
Comments |
|
Buchanan et al. (1997) |
Morbidity (serious listeriosis) |
1Exponential |
Based on an estimate of immunocompromised individuals. It is purposefully conservative and assumed that all cases were caused by a single food category. Predicted Morbidity50 = 5.9 × 109 CFU. r-value is approximately equal to the probability that a single cell of L. monocytogenes could cause serious illness. |
|
Lindqvist and Westoo (2000) |
Morbidity (serious listeriosis) |
1Exponential |
Based on an estimate of immunocompromised individuals. It is purposefully conservative and assumed that all cases were caused by a single food category. Predicted Morbidity50 = 1.2 × 109 CFU. r-value is approximately equal to the probability that a single cell of L. monocytogenes could cause serious illness. |
|
Chocolate milk, Current Study |
Febrile gastroenteritis |
1Exponential |
Based on an outbreak associated with consumption of chocolate milk. The population was limited to immunocompetent individuals who suffered gastroenteritis symptoms only. |
|
Corn salad, Current Study |
Febrile gastroenteritis |
1Exponential |
Based on an outbreak associated with consumption of corn salad. The population was limited to immunocompetent individuals who suffered gastroenteritis symptoms only. The dose-response curve may be highly conservative due to highly limited dose data. |
|
Farber et al. (1996) |
Serious infection in humans. Based on expert elicitation |
2Weibull-Gamma, a = 0.25 |
The dose estimated for 50% of the population to become infected: High Risk: 480,000 CFU Low Risk: 48,000,000 CFU The model is of limited usefulness due to over prediction of the number of serious illnesses and a general lack of transparency regarding how the various assumptions were reached. |
|
Butter, Current study and FDA (2000) |
Morbidity (Serious listeriosis) |
1Exponential |
Based on an outbreak in Finland caused by butter. The effected population was a group of highly immunocompromised individuals in a hospital setting. Predicted Morbidity50 = 6.8 × 104 CFU |
|
Mexican-style cheese, Current study and FDA (2000) |
Morbidity |
1Exponential |
Based on an outbreak in pregnant women in the United States caused by Mexican-style cheese. Predicted Morbidity50 = 1.9 × 106 CFU. |
|
FDA-General, FDA (2000) |
Mortality |
3Original model based on weighted, multiple mathematical
models. The current study used an exponential model in conjunction with
predictions for the 1012 dose to represent |
Model includes individuals between the ages of 30 days and 60 years. It includes consideration of distributions for strain virulence. It is based on mouse lethality data “anchored” so that the model provides prediction consistent with incidence of lethal L. monocytogenes infections reported in FoodNet (The US Foodborne Diseases Active Surveillance Network). The number of cases of serious listeriosis was estimated by multiplying predicted fatalities by a factor of 5. The LD50 associated with this r-value should be considered notional and interpreted as indicating that a significant portion of the population is not susceptible. |
|
FDA-Neonates, FDA (2000) |
Mortality |
3Original model based on weighted, multiple mathematical
models. The current study used an exponential model in conjunction with
predictions for the 1012 dose to represent |
The model includes foetuses and neonates less than 30 days of age. It assumed that exposure is in utero. It includes consideration of distributions for strain virulence. It is based on mouse lethality data and “anchored” so that the model provides a prediction consistent with incidence of lethal L. monocytogenes infections reported in CDC FoodNet. The LD50 associated with this r-value should be considered notional and interpreted as indicating that a significant portion of the population is not susceptible. |
|
FDA-Elderly, FDA (2000) |
Mortality |
3Original model based on weighted, multiple mathematical
models. Current study used exponential model in conjunction with predictions
for the 1012 dose to represent |
The model includes individuals over 60 years of age and consideration of distributions for strain virulence. Based on mouse lethality data and “anchored” so that the model provides prediction consistent with incidence of lethal L. monocytogenes infections reported in FoodNet. The number of cases of serious listeriosis is estimated by multiplying predicted fatalities by a factor of 5. The LD50 associated with this r-value should be considered notional and interpreted as indicating that a significant portion of the population is not susceptible. |
|
Notermans-IV, normal, Notermans et al., (1998) |
Mortality in mice
|
1Exponential Model |
It is based on mice injected IV with L. monocytogenes. Mice were previously exposed to L. monocytogenes. Mice that were not previously exposed were more susceptible to L. monocytogenes. The use of mortality in mice without correction for the apparent decreased susceptibility of humans for L. monocytogenes led to a substantial overestimation of mortality in humans |
|
Haas et al. (1999) |
Infection in mice |
4Beta-Poisson and Exponential (no fit) Strain 1041 |
Using infection in mice without correction for the apparent decreased susceptibility of humans for L. monocytogenes led to a substantial overestimation of the incidence of infection in humans. The selection of the end point of infection of normally sterile sites in mice is difficult to correlate with human disease. |
* r = probability of a single cell causing infection
1 Exponential model: single parameter. Microorganisms are assumed to occur randomly and independent
2 Weibull-Gamma model: Three parameters. Based on Weibull model, host/pathogen interaction follows a distribution modified by two factors.
3 US FDA/USDA model: Surrogate experimental animal is used to establish the shape of the dose-response curve. US epidemiological data used to set constraint limits (anchor the results)
4 Beta-Poisson model: Two parameters. Heterogeneity of host/pathogen interaction
At this stage it is not possible to endorse a single dose-response model. In part, this reflects the fact that the models are based on different biological end points and use different types of data (e.g. annual disease statistics, animal models, outbreak investigations)(Table 6.1). The use of several dose-response model relationships is the recommended approach to deal with the uncertainty related to our current gaps in knowledge of dose-response relationships. Presently, there are only limited criteria on which to base the selection of a dose-response model and better ways to evaluate the models are needed. However, the choice of which models to use will depend on factors such as the purpose of the risk assessment and the level of resources and sophistication available to the risk assessors. This requires that the basis for the various dose-response relations and their impact on the overall risk assessment be adequately communicated to the risk managers requesting the assessment.
The absence of human data, the incomplete epidemiological information, the difficulties in extrapolating from animal data to humans, and a lack of mechanistic models are all limiting factors identified here that contribute to the uncertainty in the description of the dose-response relationship. The approach taken in the USFDA/USDA model is noteworthy since it addresses several of these limitations but it will need further evaluation and it would be interesting to evaluate that model with additional and independent data that has not been used to calibrate the model.
Gaps in the data
The limitations of the dose-response models reviewed, reflects the need for further data and scientific understanding of the pathogen’s mechanisms of pathogenicity and the host and food matrix effects that influence the potential to cause disease. Priority knowledge gaps that were identified in this evaluation include:
Impact of food type (food matrix) on the ability of L. monocytogenes to cause disease.
Identification of key virulence factor(s) within L. monocytogenes isolates that lead to the apparent diversity in the ability of strains of this pathogen to cause disease.
Determination of the distribution of virulence potentials among L. monocytogenes isolated from foods.
Enhanced epidemiological data related to outbreaks and sporadic cases of listeriosis, particularly data needed to calculate attack rates, to determine the dose consumed by individuals, and to assess the health and immune status of both symptomatic and asymptomatic individuals.
Better estimates of the actual proportion of the population at increased risk of invasive listeriosis.
Conclusions
While there are limitations associated with each of the dose-response models for L. monocytogenes evaluated in this report, it can be concluded that there are several models that could be useful in developing risk assessments for this microorganism. However, at the current time it would be advisable to consider the use of multiple dose response models when estimating risks. Higher consideration should be given by users of the models to those models that provide a more accurate picture of the dose-response relations by capturing the full interaction of host, pathogen, and food matrix effects.
Use of any of the models should be consistent with the fact that serious invasive listeriosis is a rare foodborne disease that largely, but not totally, affects specific high risk populations. However, even in these groups the likelihood of disease appears low. Thus, it is expected that dose-response models based on animal models that were selected for their susceptibility to L. monocytogenes would be of limited usefulness for predicting human response unless the dose-response relations for the animal model can be appropriately correlated to the disease response in humans. Without an appropriate basis in human disease, such models may not yield estimates of risk that are accurate and useful. Using animal data it appears that modelling lethality or severe invasive listeriosis is more effective in relation to human disease than modelling infection. At the current time, the public health significance of febrile gastroenteritis is largely unknown. The circumstances leading to these symptoms among the normal population appears sufficiently different so that the usefulness of using this as a biological end point for developing dose-response relations is limited.
It appears that dose-response relationships developed using epidemiological and annual health statistics from one country may be useful in predicting the dose-response relations in populations of other countries with a similar level of development. The same models may be applicable to other countries as well, but the influence of differences in demographics and the size of sub-populations at increased risk should be considered.
Recommendations
Consider the use of multiple dose-response models when estimating risk.
Develop criteria to form the basis for selecting dose-response models and tools to compare them.
Evaluate the dose-response models by testing them against independent data.
Avoid the use of febrile gastroenteritis as a biological end point for modelling.
Evaluate the effect of the type of food (food matrix) on the ability of L. monocytogenes to cause disease.
Identify key virulence factor(s) within L. monocytogenes isolates that lead to the apparent diversity in the ability of strains of this pathogen to cause disease.
Determine the distribution of virulence potentials among L. monocytogenes isolated from foods.
Obtain epidemiological data needed to calculate attack rates, determine the exposure dose, and assess the health and immune status of both symptomatic and asymptomatic individuals.
Develop estimates of the high risk population.
6.1.B Summary of discussions on hazard identification and hazard characterization
Limitations of dose-response models for hazard characterization of L. monocytogenes
There is epidemiological data indicating that low doses of L. monocytogenes can cause listeriosis. Conversely, quantitative exposure assessments indicate that all consumers are exposed to very high doses of L. monocytogenes in RTE foods many times per year. This suggests a missing element in our understanding of foodborne listeriosis due to RTE foods that is possibly related to variability in virulence between strains of this microorganism. For example, the establishment of "epidemic" strains in processing environments is recognized as an element common to several outbreaks of listeriosis.
Virulence of L. monocytogenes
All strains of L. monocytogenes are currently considered virulent and no acceptable biomarker has been developed to detect virulence of strains and host factors that may relate to increased susceptibility.
Application of dose-response models to different countries
Dose-response models may be generic. However, models would probably gain in international applicability through use of input data from different regions of the world. Validation of models by using such data has been encouraged.
6.2.A Executive summary
Introduction
The scope and objective of the present work were to quantitatively assess human exposure to L. monocytogenes in ready-to-eat (RTE) foods. The exposure assessment estimates the number of meals containing the pathogen and the number of organisms consumed. An exposure assessment can then be combined with a hazard characterisation, so that the magnitude and severity of risks to human health can be estimated.
Objectives
The aim of this report on exposure assessment of L. monocytogenes in RTE foods is to:
Provide an overview of issues that should be considered.
Describe and evaluate methods that can be used.
Collate and present relevant data and information.
Demonstrate the application of exposure assessment to specific risk management questions for use in both industrialised and developing countries.
Approach
The report considers issues related to assessment of exposure including an extensive review of general principles and modelling approaches, and provides a glossary of technical terms. Discussion of the merits of different approaches and their relevance to different risk questions is also presented.
Eleven examples of exposure assessments from existing qualitative/descriptive and quantitative risk assessments or related documents are reviewed and assessed using criteria based on the 1999 Codex principles and guidelines for the conduct of microbiological risk assessment. These show different approaches and ideas for modelling exposure to L. monocytogenes in specific RTE foods or in different countries or regions.
In addition seven new exposure assessments were initiated. The selection of these examples was based on various criteria, including:
different food commodities
lightly processed and highly processed RTE foods
a history of listeriosis associated with the food
potential for growth or inactivation during long-term storage
potential affect of temperature abuse
the effect of an inactivation step, e.g., pasteurization
potential for post-processing contamination
high consumption rates
use in international trade
The following RTE foods were modelled from retail to the point of consumption.
raw and unpasteurized milk
ice cream
soft mould-ripened cheese
The following RTE foods were modelled from production to the point of consumption:
minimally processed vegetables
smoked salmon
semi-fermented meats
In addition, there is a specific example comparing the effect of zero tolerance and a tolerance of 100 CFU/g at the point of consumption.
The aim of these examples is to illustrate the effect on exposure of:
processing
low contamination levels in products that do not permit growth of L. monocytogenes
long-term storage on increase or decrease of L. monocytogenes concentration
consumption frequency and meal (serving) size.
Gaps in the data that currently prevent completion of exposure assessments are identified and recommendations given on improving exposure assessments and their use for RTE foods. To supplement the report, a substantial bibliographic list of sources of relevant information and data is appended, but references specific to the different sections are also included in the report.
Key Findings
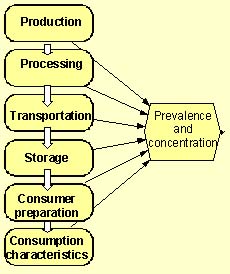
Figure 6.2: Product flow diagram indicating the tracking of the changes in prevalence and concentration of L. monocytogenes in a food.
The published and unpublished assessments that were reviewed included assessments of L. monocytogenes in bovine milk; seafoods; smoked salmon and trout; soft cheese made from raw milk; shredded cabbage; processed meats; and 21 RTE food groupings. These assessments were prepared in Australia, Canada, France, Sweden and the U.S. In addition, the report of an FAO expert consultation on the trade impact of Listeria in fish products was reviewed.
All assessments had clear goals, but these goals were quite different resulting in different approaches and levels of sophistication being adopted. All commented on the lack of data available and the consequential need to make a number of assumptions. The assumptions were incorporated into a modelling approach that was well developed in some examples, but less so in others, representing an evolution of sophistication over the six year period (1994 - 2000) since the first assessment of exposure to L. monocytogenes in RTE foods was published. No assessment critically considered the effect of the assumptions inherent in the model.
While all of the approaches that are recommended in the current report were used in one or other of the exposure assessments reviewed, none fully encompasses a “farm-to-fork” approach using a fully stochastic model. The most extensive study assessed exposure from many types of food grouped into 21 categories with L. monocytogenes growth modelled only from retail to consumption.
For the novel example exposure assessments a generic model structure was developed and is shown in Figure 6.2. It illustrates the need to track changes in the prevalence and concentration of L. monocytogenes in RTE foods as they move through the food system to the point of consumption. Figure 6.3 illustrates the interaction of factors that influence the level of exposure and the need to differentiate exposure to different population subgroups. In a completed exposure assessment the typical result is the simulated number of L. monocytogenes in a contaminated serving. For instance, in the model for exposure from soft cheese, the predicted distribution of L. monocytogenes concentration in servings is shown in Figure 6.4. The exposure assessment further predicts that for consumers at normal risk, from 4 to 22 servings of soft cheese are consumed per year, and that for consumers at high risk, from 3 to 17 servings of soft cheese are consumed per year. Of those soft cheese servings, 4 % (median) are predicted to be contaminated (Figure 6.4).
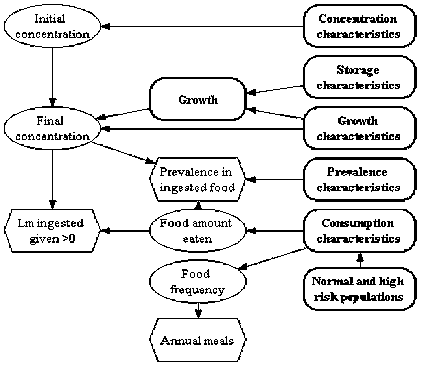
Figure 6.3. Influence diagram detailing factors that affect human exposure to L. monocytogenes in RTE food.
The starting point in the exposure assessment depends on the question that the risk manager seeks advice on. For example, in the case of RTE foods that do not receive a listericidal step, it may be necessary for the assessment to include potential sources of contamination in the harvest or growing area. Alternatively, for those products that do undergo a listericidal step but may become contaminated or re-contaminated, only the steps subsequent to the listericidal step may need to be modelled.
Food consumption characteristics of sub-groups of the population that are particularly susceptible to listeriosis need to be determined, but this proved difficult from available survey data.
An assessor often needs to be able to determine the prevalence and concentration at one point in the food chain from an earlier point in the chain because specific data relevant to the point of consumption is unavailable. This is also important in order to be able to assess the effectiveness of proposed intervention steps. To overcome lack of data, or to perform a process risk assessment, mathematical modelling will normally have to be used and the appropriate assumptions made. Growth, survival and death models for L. monocytogenes are now available, including models that reasonably predict growth rate in pure cultures and challenge tests. However, there is evidence that models may be less accurate for foods naturally contaminated with L. monocytogenes. Exposure assessments must explicitly recognize the limitations of the current generation of predictive microbiology models so that the risk assessment process is transparent.
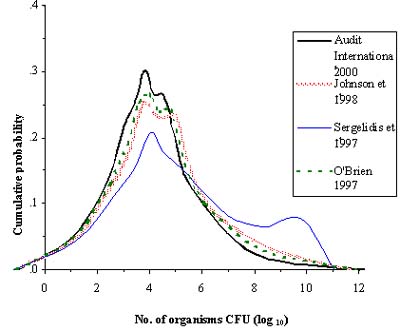
Figure 6.4: Simulated distribution of number of L. monocytogenes organisms in those 4% (median) of servings that are predicted to be contaminated. The 4 lines represent the outcome based on 4 different studies of home refrigerator storage conditions.
While the stochastic modelling approaches were found to be preferable, there are potential disadvantages. For example, as the complexity of the model increases, the bounds of uncertainty/variability become wider and may become so broad as to convey little useful information to the risk manager.
Useful insights can be obtained by using a risk assessment approach. However, the full range and quality of data required to complete risk assessments is not currently available. Specific gaps that were identified in the data are listed separately below.
Gaps in the data
L. monocytogenes incidence/prevalence in potential environmental sources, including i) agricultural environments, e.g. ground and well water; cultivated soil used for different crops or uncultivated soil for grazing pasture at different times of the year, silage, fresh and composted manure, farm equipment and farm workers; ii) aquatic environments, marine and freshwater where fish or shellfish are harvested including the effects of sewage or agricultural runoffs into water, fishing equipment, and commercial and recreational fishers.
L. monocytogenes incidence/prevalence and concentration in production including i) primary production: animals, fish, and crops; ii) secondary production, e.g. initial preparation, cleaned carcasses, gutted and stored fish, shucked shellfish, washed produce.
Product formulation information, e.g. pH, water activity and humectants, preservatives (e.g. nitrite and organic acids, lactic acid bacteria) to enable best estimates of microbial growth, survival or death.
Data for evaluating the validity of predictive models for L. monocytogenes in specific products, recognising the effect of prior history of the culture and potential differences between naturally contaminated products and those deliberately inoculated in challenge tests.
Identification of virulence markers for L. monocytogenes is needed so that exposure to those strains can be assessed specifically.
Identification of sources and levels of contamination and recontamination, both at the point of processing and at retail are needed, with information on frequency, and microbial load transferred.
Impact of microbiota including spoilage organisms on the growth and survival of L. monocytogenes in RTE foods or their ingredients, and on the shelf life of products.
Prevalence and concentration of L. monocytogenes on finished packages of RTE foods.
Retail and consumer handling practices, in particular, storage time and temperature and including more accurate measurements of home storage conditions including refrigeration temperatures by country or region.
Specific RTE product consumption data for meal servings and frequency by specific populations of individuals, including in developing countries, and particularly for those who are immunocompromised or otherwise susceptible.
Epidemiological data that distinguish serious or life threatening (usually systemic) vs. mild disease.
Conclusions
The report has demonstrated that it is feasible to develop exposure assessments useful to those managing food safety risks but that there are significant gaps in the data required to complete assessments for L. moncytogenes in RTE foods. For example, in the short term it will be necessary to characterize consumption frequency and meal size, and to obtain information on home storage times and temperatures, and handling and preparation practices.
Despite these limitations, immediate benefits are attainable using model exposure assessments by combining them with hazard characterizations to complete the risk characterization. As long as there are insufficient data, exposure assessment will remain reliant on the use of models of food production and distribution systems, of microbial ecology in foods, and on optimising the use of existing data. Specifically, to facilitate the development of exposure assessment by both experienced and inexperienced assessors, it is recommended that guidelines be developed and made widely accessible for the pooling of data from difference sources and of uncertain compatibility and quality. Also guidelines on the appropriate use and development of models, including testing of model validity, assessment of the effect of assumptions, and communication of the reliability (e.g. confidence intervals) of model results should be developed.
To maximise the use of models, they should be developed and described in a transparent manner so that they can be adapted and modified to the changing needs of the risk managers or adopted and adapted by other risk managers.
In the long term, the report identified the need for the establishment of an international data repository for exposure assessment data, including data from the food industry. The need to establish food-borne disease surveillance systems was also recognized.
Recommendations
Exposure assessments require data on prevalence (frequency of contamination and eating) and dose (contamination level and meal sizes for all ages including children and the elderly). To address some of the critical gaps in the data it is recommended that:
Member Countries be encouraged to identify and report on local practices of storage and handling of food in the home by consumers and in food-service establishments, including storage temperatures and times.
Coordination be sought with nutrition/consumption studies to develop knowledge of consumption data relevant to foodborne microbiological risks.
A central, international, data repository be established so that, once collated, data can be accessed and accumulated for use in future exposure assessments.
Systems of data input are developed whereby industry can contribute their data to the exposure assessment processes without fear of prejudicial use of their data including punitive action by government or release to competitive industries.
Active surveillance programs are established to develop information against which exposure assessments can be validated.
To advance the development of rigorous and reliable exposure assessments it is recommended that:
A process is established to indicate the types of exposure assessments that can be used by experienced assessors and those who are new to the process, as well as an approach to modify the model assessments to answer the types of questions that risk managers ask.
All assumptions used in the development of exposure assessment models should be stated explicitly, and where there are assumptions, the validity of those assumptions is tested and confidence in derived estimates of exposure be specified or characterised. The reliability (e.g. confidence intervals) of model results should be communicated.
Mathematical modelling guidelines be established for use in microbial food safety exposure assessment. An international workshop, such as that conducted for hazard characterization, may be an appropriate mechanism. This would include guidelines for predictive microbiology aspects of exposure assessment, pooling of data from difference sources and of uncertain compatibility and quality and data editing.
6.2 B Summary of discussions on Exposure Assessment
Comprehensiveness and costs of exposure assessments.
Development of an exposure assessment can be costly, particularly when the generation of new data is required, for example, for occurrence of growth of L. monocytogenes. The comprehensiveness and thereby the costs of an exposure assessment must reflect the importance of the problem under study.
Variability in the prevalence of L. monocytogenes in foods from similar processing plants/production lines.
There is evidence that at least for some RTE foods from similar processing plants/production lines the prevalence of L. monocytogenes in the products can vary from non-detectable to 100%. If quantified, this variability in prevalence of L. monocytogenes can be taken into account in exposure assessments. However, the factors resulting in this variability often remain unknown and these need to be identified to evaluate the potential and effect of lowering the prevalence of L. monocytogenes.
Collection of data on foodborne listeriosis
Collection of data from outbreaks is one possible way to increase knowledge about exposure to doses of L. monocytogenes from RTE foods. This type of data collection could be improved where there are guidelines provided by governments for outbreak investigations. However, collection of exposure data from outbreaks is difficult due to the long incubation period of listeriosis and the unavailability of the exposure food source at the time of illness. Co-ordination between food and health authorities to provide data relevant to risk assessment has rarely been effective. It is also recognized that large outbreaks of food-borne listeriosis are the exception. It appears that there are many sporadic cases of listeriosis that are not investigated or their food association is not determined.
Socio-economic factors
The relationship between the exposure to the microorganism and factors related to socio-economic status, food preparation and storage practices, food consumption patterns, frequency of consumption and other related aspects specific to RTE foods needs further research.
Gathering of information:
There is a need for collection of data related to all aspects of exposure assessment and hazard characterization. Some of the data needed is likely to be available, for example, within private industries and national or regional food inspection services. It is suggested that future efforts for data collection should also be targeted directly to these potential providers of specific information.
There is a general lack of quantitative data for use in exposure assessment, for example, data for levels of contamination as well as prevalence, growth or inactivation kinetics in RTE foods.
There is a need for information on consumption frequency and meal size, as well as data on home storage times and temperatures, handling and preparation practices.
There is also need for information on L. monocytogenes disease incidence, predisposing conditions, clinical manifestations (invasive vs. gastrointestinal) and probable exposure sources, giving consideration to regional differences.
It is further suggested that the potential for setting up an international data repository should be investigated.
Research:
Research is needed on virulence factors to better understand the diversity of L. monocytogenes virulence and to identify markers to differentiate between strains.
Increased understanding about the ecology of L. monocytogenes is needed to enable identification of routes of contamination and thereby reduce prevalence and growth in RTE foods.
Development of sensitive methods for enumeration of L. monocytogenes is needed to determine and quantify presence and growth of low levels (< 100/g) of the organism in RTE foods and environmental samples.
Collaboration is needed to explore new approaches to develop dose-response models and stimulate efforts to test the credibility of existing methods with independent data.
Criteria to form the basis for selecting dose-response models and tools to compare them need to be developed.
Potential intervention strategies to reduce the prevalence and concentration of L. monocytogenes in RTE foods should be explored through the stochastic modelling of exposure routes.
Technical support for developing countries
Sustainable support for training in the form of targeted, dedicated courses is required to assist the transfer of technology for microbiological risk assessment to developing countries.