Control measures to ensure safety and hygienic processing of food have traditionally included the use of criteria. Two types of criteria will be discussed in this Chapter:
Performance- and process criteria
Traditionally, control of microorganisms in food was demonstrated by microbiological testing of samples at various stages of production and the final product. Results were compared with criteria developed to give some degree of assurance that the food was safe and of good quality. It is now fully recognized that this type of activity can never give an absolute assurance of product quality and safety. A much higher degree of assurance can be provided by a preventative approach based on the application of the Hazard Analysis Critical Control Point (HACCP) principles at all steps in the food supply and processing system.
Nevertheless it is recognised that MC are widely used in the food industry and by government authorities. For this reason, the basic requirements of MC will be discussed below. As outlined by the Codex Alimentarius Commission (CAC, 2001) and in published opinion papers and recommendations by scientific bodies such as EU Scientific Committee for Food (EU, 1997) and the Institute of Food Science and Technology (Stannard, 1997). A part of this section (13.1) has already been published in Huss, 2001.
Three types of MC are generally recognized according to their use:
specifications
These terms have been defined and redefined a number of times, but it is generally recognised that the term "standard" is a MC contained in a law or regulation with mandatory compliance. In case of non-compliance some (specified) action is required by the regulatory agency. A microbiological "guideline" is a MC applied at any stage in food processing and aids in identifying situations requiring actions for food safety or quality reasons. Results obtained from testing assist in trend analysis and situations (products, processes) not complying with guidelines should result in investigative action to identify and rectify the cause. A "specification" is a MC used for contractual purposes by food business as part of their own safety management system and should not be confused with legal requirements. Codex Alimentarius and EU now operate with only one definition:
|
Microbiological Criteria Microbiological criteria for food defines the acceptability of a product or a food lot based on the absence or presence or number of microorganisms, including parasites and/or quantity of their toxins / metabolites per unit of mass, volume, area or lot (CAC,1997; EC, 1997) |
It is recommended in the documents (CAC, 2001; EC, 1997) that the components of MC for foods should consist of:
a statement of the microorganisms of concern and/or their toxins/metabolites and the reason for that concern
the analytical method for their detection
a sampling plan and the size of the analytical units
the microbiological limits (ML) considered appropriate for the food at the specified points(s) of the food chain
the number and size of analytical units that should be tested and conform to these limits
the food to which and where in the food chain MC applies
actions to be taken when the criterion is not met, (non-compliance).
The analysis of foods for compliance with mandatory microbiological criteria must be undertaken in an official laboratory in compliance with the Official Control of Foodstuffs Directive (EC, 1989).
Both the Codex- and the EU document specify that in situations of non-compliance with a mandatory MC some regulatory control actions are required such as sorting, reprocessing, rejection or destruction of product or further investigations into the situation. The decision on control action depends on an assessment of the possible risk to the consumer, the point in the food chain and the type of product. This means that non-compliance is not automatically followed by destruction of products. The decision on possible action depends on a carefully scientific evaluation of the whole situation and the stated microbiological limits in the MC are in reality guidelines to assist the authorities in choosing the correct control action.
In the EU directives applicable to fish (see Table 13.2) it is specified that if the products do not comply with the mandatory criteria set out in the Directives, the products cannot be placed on the market. This applies both to the criteria for pathogens and for organisms that are "indicators of poor hygiene" (Staphylococcus aureus, thermotolerant coli (44°C) and E. coli).
In contrast, it is stated that the "guidelines" used for indicator organisms (Standard Plate counts) are meant to help manufacturers decide whether their plants are operating satisfactorily and to assist them in implementing the production monitoring procedures.
Thus the EU Directives for fish have:
mandatory criteria for specified pathogens (Salmonella) and for "unspecified pathogens"
criteria for specific organisms as indication of poor hygiene and for the checking of GMP and HACCP (also mandatory criteria)
and use of other parameters ("coliforms" and "plate count") in guidelines to be used solely by the manufacturers.
MC should only be applied to products or processes when no other means of securing safety and shelf life are available and when the use of a MC enhances food safety. Thus, there must be scientific evidence that a MC is effective, practical and meaningful in terms of consumer protection.
MC may be useful in the following situations:
to indicate the microbiological status of raw materials, ingredients and final products of unknown origin (e.g. at port-of-entry)
as validation and verification of HACCP-based control systems, Good Manufacturing Practices (GMP) and Good Hygienic Practices (GHP)
to assess whether the prevalence of a pathogen in specific foods is increasing/decreasing relative to a target level (e.g. a FSO)
for contractual purposes by food business.
MC cannot stand in isolation, but should only be established and used within the framework of a general risk management programme. They should be based on scientific analysis and advice together with an assessment of the risk appropriate to the foodstuff and its use. Furthermore they should be developed in a transparent fashion and meet the requirements of fair trade.
When establishing MC, consideration must be given to the following (CAC, 2001):
evidence of actual or potential hazards to health
possibility of controlling the hazard by other means (e.g. at a CCP in a HACCP-programme or by use of process criteria)
likelihood of improved safety to consumers by applying a MC
microbiological status of the raw material. A number of potential pathogenic organisms are present as part of the normal microflora on the raw material and are therefore likely to be present on raw, non-heat treated final products. This is a potential hazard which need to be controlled by controlling the growth conditions for the pathogen (e.g. by salt, temperature control etc.)
effect of processing on the microbiological status of the food
likelihood and consequences of microbial contamination and/or growth during subsequent handling, storage and use
category of consumers concerned
intended use of the food. It must be included into the evaluation of a possible need for a MC if the risk will be eliminated by normal preparation before consumption
cost/benefit ratio associated with application of a criterion. It must be taken into consideration, if the microbiological examination can be carried out for a reasonable price
when establishing MC they must be based on a thoroughly well-documented examination of the particular product produced when GMP and GHP have been applied. The MC should be technically attainable and realistic in terms of achievability.
Based on these principles a number of situations can immediately be excluded as suitable for application of mandatory MC for pathogens:
ready-to-eat products where a Critical Control Point or a process criteria can be identified for growth/elimination of relevant pathogens.
According to the principal documents on MC (CAC, 2001; EC, 1997) the choice of a sampling plan should take into account:
statistical probability of detecting unacceptable food lots.
A sampling plan should include the sampling procedure and the decision criteria to be applied to a food lot based on the examination of a prescribed number of sample units by defined methods. A sampling plan should further be administratively and economically feasible. Statistically based, 2- or 3 class sampling plans are defined by ICMSF (1986).
A 2-class plan is used essentially for pathogens and/or where a presence/absence test is to be performed, while a 3-class plan is mainly used for hygiene indicators.
Microorganisms are not distributed homogeneously in fish and fish products and pathogens, if present, are usually at low levels. For these reasons, no practical sampling plan can ensure complete absence of a target microorganism, nor can it ensure that the concentration of a microorganism measured may be exceeded in a part of the fish product that was not sampled.
A 2-class sampling plan will mainly detect gross defects. Table 13.1 shows the calculated acceptance probabilities for 2-class sampling plans with different numbers of samples and percentages of defective lots. Application of a sampling plan with five samples of which none should contain pathogens (n = 5, c = 0) would lead to acceptance of a lot that contains 10% defective samples with a probability of 59.1%. When it comes to food safety, food processors would aim at much lower rate of defective samples in a lot.
Table 13.1 Effect of lot quality (% defective in a lot) on the probability of acceptance (%) for different 2-class sampling plans (EC, 1998).
|
% defective samples in lot |
probability of acceptance (%) given sampling plans with a total of n samples and allowance of "c" defect samples |
|||
|
n = 1; c = 0 |
n = 5; c = 0 |
n = 10; c = 0 |
n = 60; c = 0 |
|
|
1 |
99.0 |
95.1 |
90.4 |
54.7 |
|
2 |
98.0 |
90.4 |
81.7 |
30 |
|
5 |
95.0 |
77.4 |
59.9 |
4.6 |
|
10 |
90.0 |
59.1 |
34.9 |
0.18 |
|
20 |
80.0 |
32.8 |
10.7 |
0.00015 |
The most stringent sampling plan proposed by ICMSF (1986) is the sampling plan for Salmonella in baby food. In this plan 60 samples are analysed (n = 60) and none are allowed to contain Salmonella (c = 0) Even in this case there is a 30% chance of accepting products with 2% of sample units contaminated with Salmonella.
If the level of contamination in a lot is 0.5% it can be estimated that examination of 600 samples would be necessary for a 95% probability of detecting the contaminated lot. This probability would decrease to 45%, if the level of contamination is 0.1%. It can be concluded that if the level of contamination is not at least in the order of 5% or more, there is very little chance of detecting contaminated lots, and sampling and testing would therefore not improve the safety or decrease the risk. Sampling and microbiological testing is in this situation not suitable means for defining the acceptability of food lots and a MC is meaningless.
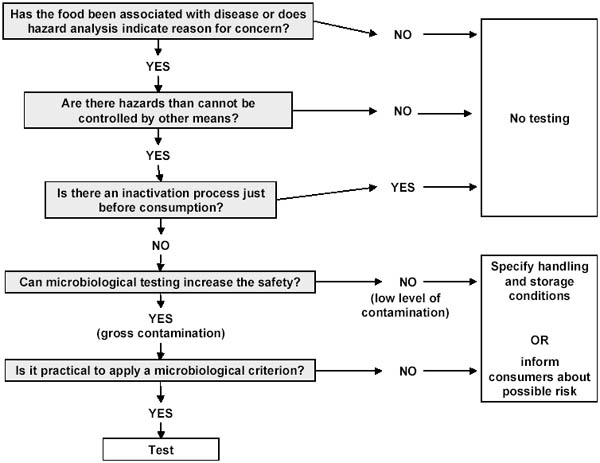
When evaluating the need for MC, the principle discussed above can be applied in a decision tree as shown in Figure 13.1.
Food safety and hygienic practices throughout the EU is controlled by a multitude of "Vertical Directives" dealing with specific products of animal origin (meat, milk, eggs, fish) or the so-called "Horizontal Directives" covering all foodstuffs entering the market. Directives that include MC for fish are:
Commission Decision on the microbiological criteria applicable to the production of cooked crustaceans and molluscan shellfish (93/51/EEC) (EC, 1993)
In addition a number of Directives have provision for MC to be added in the future (e.g. Council Directive 93/43/EEC on the hygiene of foodstuff).
The ML listed in the Directives above are shown in Table 13.2.
Table 13.2 Microbiological limits for fish and fish products laid down in EU Directives (EC, 1991a,b, 1993).
|
Products |
Microorganisms |
Microbiological standard |
Status/action |
|
Live bivalve molluscs |
Salmonella spp. |
absent in 25 g |
w |
|
" |
Escherichia coli |
< 230/100g |
w |
|
" |
Faecal coliforms |
< 300/100g |
w |
|
" |
Paralytic shellfish poison (PSP) |
(80mg/100g |
w |
|
" |
Diarrhetic shellfish poison (DSP) |
Negative in bioassay |
a |
|
Cooked crustaceans and molluscan shellfish |
Salmonella spp. |
Absent in 25g, n=5, c=0 |
w, n, r |
|
" |
Other pathogens and toxins thereof |
Not present in quantities such as to affect health |
w, n, r |
|
Whole products |
Mesophilic aerobic bacteria (30°C) |
m=10 000, M=100 000, n=5, c=2 |
r |
|
Shelled or shucked products |
Staphylococcus aureus |
m=100, M=1000, n=5, c=2 |
w, n, r |
|
" |
Escherichia coli (on solid medium) |
m=10, M=100, n=5, c=1 |
n, r |
|
" |
Thermotolerant coliforms (44°C on solid medium) |
m=10, M=100, n=5, c=2 |
n, r |
|
Shelled or shucked products except crabmeat |
Mesophilic aerobic bacteria (30°C) |
m=50 000, M=500 000, n=5, c=2 |
r |
|
Crabmeat |
Mesophilic aerobic bacteria (30°C) |
m=100 000, M=1 000 000, n=5, c=2 |
r |
w = withhold from market
n = notify the competent authorities of findings and action taken
r = review the methods and checking at CCPs.
The criteria listed in the EU Directives were developed 5-10 years ago, and there is a wide diversity and complexity in some of the MC selected. None of the MC is based on current Codex Alimentarius principles as outlined in this paper and many of the MC applied does not appear to be meaningful in terms of consumer health protection (e.g. aerobic plate counts, coliform counts in certain foods). The terminology used is not harmonised as MC is named as: MC, obligatory criteria, analytical criteria, guidelines, or standard without any clear definition of the meaning of these designations. Thus in the MC for cooked shellfish and molluscs the microbiological limit for aerobic mesophilic bacteria is called a "standard", while it is specified in the heading of the table to be a "guideline". None of the current MC is based on a formal risk assessment and sampling plans and detection methods to be used are not prescribed either.
The number of non-harmonised microbiological criteria in EU member states varies considerably. Thus France have more that 80 MC for foods while in Germany no microbiological criteria exist in the German Federal Legislation except those laid down by EC Directives. Countries which have non-harmonised, national microbiological criteria for fish and fish products are France, Norway, Spain, Denmark and Belgium (EC, 1998).
Some of the microbiological limits specified in the national non-harmonised MC mentioned above are given in Table 13.3. Other figures are from industry specification. However, it is claimed by Stannard (1997) that figures in Table 13.3 are considered to be practical, realistic and relevant.
Table 13.3 Microbiological limits for fish and fish products considered to be practical and relevant (extracted from Stannard, 1997).
|
Product |
Organism/Toxin |
GMP1 |
Maximum |
|
Raw fish/shellfish to be cooked |
Bacterial pathogens |
Criteria for absence not applicable |
|
| |
< 50 ppm |
50 ppm |
|
|
Non-heated, ready-to-eat products |
Salmonella spp. |
ND in 25 g |
ND in 25 g |
|
Cooked, ready-to-eat products |
Salmonella spp. |
ND in 25 g |
ND in 25 g |
1. see text
It must be emphasised that the figures in Table 13.3 are microbiological limits which may be included in MC. GMP-values are those expected immediately following production of food under good manufacturing conditions. Maximum values are those regarded as the maximum acceptable at any point in the shelf life of a product.
In the table it is pointed out that criteria requiring the absence of pathogens in raw foods are generally not practical. Absence of pathogens in raw food which are eaten raw is desirable but can never be guaranteed. In contrast, it is stated that pathogens (Salmonella, V. parahaemolyticus, L. monocytogenes) should be non-detectable in 25 g immediately following production of ready-to-eat products. This is clearly unrealistic for V. parahaemolyticus and L. monocytogenes in non-heated ready-to-eat products. The figures quoted under maximum values are more realistic and could be used as guidelines for microbiological testing also during and immediately after processing.
However, in many countries 102/g of Listeria monocytogenes is regarded as maximum.
S. aureus is a toxin-producing organism and growth in the fish product is required before there is any risk to the consumer. Since S. aureus competes very poorly with a large associate microflora, it is unlikely to grow in non-heated ready-to-eat products like cold smoked salmon. Thus the authors cannot agree that a MC for this organism in this type of products is relevant. In contrast, cooked peeled shrimp, which may have been recontaminated with S. aureus after cooking can be of risk and a MC for S. aureus may be very useful in this situation.
Many of the microbiological examinations of foods both by industry and regulatory agencies are meaningless and a waste of time and resources. Indiscriminate application of microbiological testing and criteria should be avoided. As the existence of a MC always requires some degree of microbiological testing, it needs very careful consideration, before any MC is established.
Testing foods for pathogens is not very effective as a tool to protect health of the consumer. Safety is obtained by the application of GMP, GHP and HACCP as safety management tools throughout the food chain. Microbiological analysis and MC can be used to support and to verify the effective application of these management tools. Any MC introduced for this purpose should not be used as rejection criteria but as guidelines taking into consideration all other factors of importance. In most cases the corrective action will be a re-evaluation of the processing- and HACCP procedures.
The real problem is how to control the food in international trade. At the port-of-entry the regulatory agency may not always know whether the incoming food was produced under hygienic conditions and application of the HACCP-principles. In this situation some MC will be needed, but then they should be established according to the principles described in the Codex documents. However, a much better and more modern approach would be to let control of foodstuffs in international trade be based on signed agreements between internationally recognised and competent authorities or business partners approved by such authorities (e.g. Memoranda of understanding, purchasing agreements). Alternatively, food may be passed without testing, but with some restrictions on storage condition (e.g. keep frozen until use), limitation on shelf life after thawing or some degree of warning to the consumer (see Figure 13.1).
Figure 13.1 Establishment of MC for pathogens (Modified after ICMSF, 2002).
|
Performance Criterion A performance criterion is the required outcome of one or more control measures at a step or combinations of steps which will assume the safety of a food (van Schothorst, 1998) |
Examples of performance criteria are:
the requirement of a 12 D-reduction in the number of Clostridium botulinum spores in sterilized canned food
the prevention of any growth of a given pathogen
a 6 D-reduction in number of Listeria monocytogenes
destruction of non spore forming pathogens that are known to occur in raw milk.
Process criteria are applied during the production of food, with the aim of building into the manufacturing process effective measures to control the risk of identified microbiological hazards.
|
Process Criteria Process criteria are the control parameters (e.g. time, temperature, pH, aw) at a step or combinations of steps, that can be applied to achieve a performance criterion (van Schothorst, 1998) |
Process criteria will commonly appear as critical limits for CCPs in HACCP plans. Some examples are shown in Table 13.4.
Table 13.4 Performance and process criteria in food processing.
|
Performance criteria |
Process criteria |
|
12 D Kill of C. botulinum |
2.4 - 3 min. At 121°c |
|
6 D Kill of non proteolytic C. botulinum |
90°C for 10 min |
|
6 D Kill of Listeria monocytogenes |
70°C for 2 min |
|
Kill of non spore formers in raw milk |
71,7°C for 15 sec. (pasteurisation) |
|
Destruction of pathogenic virus in shellfish |
90°C for 90 sec. (Lees 1995) |
|
No growth of C. botulinum |
pH < 4.6 or Water Phase Salt (WPS) > 10% |
|
No growth of C. botulinum |
WPS > 3.5%, Storage temperature <10°c |
In an early and extremely important report (NRC, 1985) it was pointed out very clearly, that microbiological testing has severe limitations as a control option. The report also came out with a strong recommendation of applying the HACCP system in all segments of the food industry.
With the increased use of the Hazard Analysis Critical Control Point (HACCP) system in the management of food quality and safety one may ask, if microbiological testing and -criteria are still necessary as the HACCP system aims at controlling hazards during processing. A number of microbiological criteria (MC) are still required by both national and international legislation, but there is a considerable debate whether MC are needed or necessary in all instance to increase food safety.
Already in 1970, Sir Grahame Wilson when summarizing a meeting on the use of microbiological, criteria, stated: "Bacteriologists are better employed in devising means to prevent or overcome contamination than in examining more and more samples". Processing concerns the whole volume of food; samples' only a minute fraction of it. Thirty years later two other distinguished and eminent microbiologists further pointed out that "It is an historical fact that the major advances in public health have been made by applying interventions, such as the use of milk pasteurisation or water chlorination to control specific microbiological hazards, i.e., application of performance and process criterion. We are not aware where significant food borne hazards to health have been reduced through the application of a microbiological criterion of a foodstuff as the primary means of control" (Baird Parker and Tompkin, 2000).
Thus it can be concluded, that while process criteria can be extremely effective in control of microbiological hazards and should be build into the HACCP system, microbiological testing and the use of microbiological criteria should never be carried out at the expense of a HACCP based system of control.
References
Baird -Parker, T.C. and R. Bruce Tompkin 2000. Risk and Microbiological criteria. In Lund, B., T.C. Baird-Parker and G.W. Gould (eds) The Microbiological Safety and Quality of Foods Vol II Aspen Publishers, Inc. Gaithensbury, Maryland, USA. pp.1852-1885.
CAC (Codex Alimentarius Commission) 1997. Principles for the Establishment and Application of Microbiological Criteria for Foods. Alinorm 97/13A, Supplement to Volume 1B, Appendix III CAC/GL, 21. Food and Agriculture Organization / World Health Organization, Rome, Italy.
EC (European Commission)1989. Council Directive 89/397/EEC of 14 June 1989 on the official control of foodstuffs. Official Journal of the European Communities L 186, 30/06/1989
EC (European Commission) 1991a. Council Directive 91/492/EEC of 15 July 1991 laying down the health conditions for the production and the placing on the market of live bivalve molluscs Official Journal of the European Communitites L 268, 24/09/1991 pp. 0001 - 0014
EC (European Commission) 1991b. Council Directive 91/493/EEC of 22 July 1991 laying down the health conditions for the production and the placing on the market of fishery products Official Journal of the European Communities L 268, 24/09/1991 pp. 0015 - 0034
EC (European Commission) 1993. Commission Decision 93/51/EEC of 15 December 1992 on the microbiological criteria applicable to the production of cooked crustaceans and molluscan shellfish. Official Journal of the European Communities L 013, 21/01/1993 p. 0011 - 0013
EC (European Commission) 1997. Report of the Scientific Committee for Food (39th series). Luxembourg: Office for Official Publications of the European Communies.
EC (European Commission) 1998. Report on tasks for scientific cooperation. Report of experts participating in Task 2.1. Luxembourg: Office for Official Publications of the European Communities.
ICMSF (International Commission on Microbiological Specifications for Food) 1986. Microorganisms in Food 2. Sampling for microbiological analysis: Principles and specific applications. 2nd ed. Blackwell Scientific Publications, UK.
ICMSF (International Commission on Microbiological Specifications for Food) 2002. Microorganisms in Foods 7. Microbiological testing in food safety management. Aspen Publishers.
Huss, H.H. 2001. Use and misuse of microbiological criteria for seafood. In Gudjonsson and O. Niclasen (eds) Proceedings of the 30th WEFTA Plenary Meeting. June 2000. Thorshavn, the Faroe Islands. Annales Societatis Scientiarrum Færoensis Supplementum XXV²²². pp.63-73.
Lees, D. 1995. Control measures in seafood. In Workshop on Foodborne Vital Infections Advisory Committee on the Microbiological Safety of Food, pp. HMSO, London, UK.
NRC (US - National Research Council) 1985. An evalution of the Role of Microbiological Criteria for Foods and Food Ingredients. National Academy Press. Washington DC, USA.
Stannard, C. 1997. Development and use of microbiological criteria for foods. Food Science and Technology Today 11, 137-177.
van Schothorst, M. 1998. Principles for the establishment of microbiological food safety objectives and related control measures. Food Control 9, 379-384.