Dr W. A. McIlmoyle
Animal Nutrition and
Agricultural Consultants
Northern Ireland, UK
INTRODUCTION
In the light of recent food scares, which have resulted from potentially harmful substances entering the human food chain, the issue of food safety has become particularly important for European consumers. Problems associated with food safety have resulted in intense media attention, particularly where the problems have resulted in the death of consumers, with attention being focused on every aspect of the food chain, including complete traceability within the chain.
Shortly after his appointment, the European Union Commissioner for Health and Consumers Protection, David Byrne, indicated that “Food safety is my No 1 priority, and I am setting about ensuring that Europe has the highest standards for our consumers”.
In order to achieve this objective, the European Commission published a ‘White Paper on Food Safety’ on the 12th January 2000, which set out a co-ordinated approach to food safety across the EU, and included the establishment of a new European Food Safety Authority (EFSA), with specific responsibility to ‘ensure a high level of human health and consumer protection’. The White Paper also clearly identified ‘the whole of the food chain, including animal feed production’, and attributed the ‘primary responsibility for safe food production to industry, producers and suppliers’.
The establishment of the European Food Safety Authority followed a trend already begun in some European Union Member States. In 1998, the United Kingdom Government, in an attempt to place the food chain under tighter scrutiny, set up the Food Standards Agency (FSA), with specific Terms of Reference to monitor the quality and safety of all food from ‘farm to fork’.
Animal feed
During 2001, some 124 million tonnes of animal feed was manufactured and marketed throughout the European Union. Feed offered to animals has long been recognised as a potential source of hazards in processed animal food products. Consequently, the animal feed industry, not just in Europe but worldwide, has come under intense scrutiny, as both governments and retailers of food strive to ensure that consumers are adequately protected.
Food scares
At the end of 1988, Mrs Edwina Currie, then a Junior Minister within the United Kingdom Government, condemned the United Kingdom egg industry for producing too many eggs that were positive for Salmonella. In the wake of media interest, egg sales plummeted and consequently, sales volumes of layers feed fell dramatically. This was indeed a watershed that changed the future of the animal feed industry.
Prior to 1988, the industry was quite happy to get on with the business that it knew best, namely the production of animal feed that met the requirements of its farmer customers, with particular emphasis being placed on unit cost and animal performance.
Since 1988, food scares have continued to receive major media attention. In November 1996, E.coli O157 caused an outbreak of food poisoning in Scotland, which resulted in the deaths of 18 adults. During 1998, the European Union feed industry encountered severe problems resulting from dioxin contamination of Brazilian citrus pulp. While this affected raw material markets and the animal feed industry, thankfully, there was no disruption to the food chain.
This was in stark contrast to the dioxin problems that hit the Belgian feed and food industry during 1999. Fat, destined for the feed industry, became contaminated with mineral oil, leading to a major food scare due to the presence of dioxin in animal feed and the possibility of dioxin contamination in processed animal products. One estimate put the cost of the problem to the whole Belgian feed/food industry at approximately GBP£1.5 billion (GBP£=US$1.52 at 5 July 2002), as a result of feed and processed animal products being withdrawn from the market for destruction. However, this estimate does not take account of the untold damage caused to consumer confidence across the whole processed animal product market, not only in Belgium but also throughout the European Union.
Bovine spongiform encephalopathy (BSE) in the cattle population across Europe, and it’s possible links with new Variant Creutzfeldt-Jakob disease (vCJD) in humans, has also taken its toll on consumer confidence in beef, while more recent speculation over scrapie in sheep has had implications for sheep meat sales. While not posing any direct threat to the consumer, the outbreak of Foot and Mouth disease in the United Kingdom and some other Member States of the European Union, also had severe repercussions for consumer confidence in food.
These problems have highlighted consumer concerns over food safety and the impact on consumer confidence has been dramatic.
In an attempt to restore consumer confidence, the animal feed industry in the European Union has introduced codes of good manufacturing practice (GMP). The implementation of codes, which are externally audited, and invariably incorporate a Hazard Analysis of Critical Control Points (HACCP) plan, have helped to provide transparency within the industry and, hence, restore some of the lost consumer confidence.
Other aspects, such as ‘open declarations’, which list all the raw materials used to manufacture the feed, have helped in this transparency, by providing more information to both animal feed customers and consumers alike.
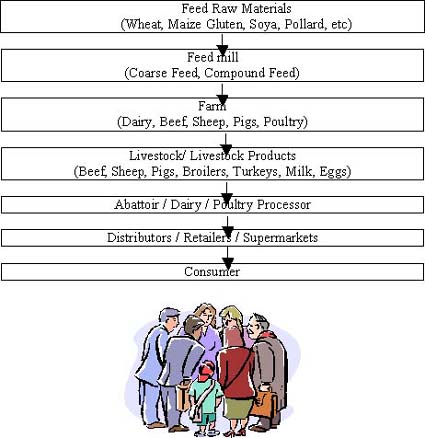
This paper is not about identifying who began to take the whole issue of ‘safe feed - safe food’ seriously. Suffice it to say that the feed industry worldwide now recognises that safe feed is part and parcel of the food chain (see Appendix 1) and, as such, must be open to scrutiny by supermarkets and consumer organisations if it is to survive. After all, without consumers, there would be no animal feed industry.
CODES OF PRACTICE
It is perhaps appropriate to point out the relative differences between a Code of Practice and legislation. The adoption of a Code of Practice is purely voluntary, with the ultimate decision to implement resting with the feed compounder. However, while the decision may be up to the compounder, the United Kingdom Government has made it clear that in view of the implications for consumer safety, any lack of action by the compound feed industry to police itself would result in legislation being drawn up to ensure that what should be done is in fact carried out. This realisation has spurred the industry to draw up it’s own Code of Practice to reflect the importance of animal feed in the food chain.
Within the United Kingdom, the animal feed industry is represented by the United Kingdom Agricultural Supply Trade Association (UKASTA). Association members, of which there are approximately 350, with an estimated annual turnover of £5 billion, include agricultural merchants, manufacturers of animal feed and road hauliers. The feed compounding membership manufacture approximately 90 percent of animal feed marketed in the United Kingdom.
In view of this overwhelming interest in the United Kingdom animal feed sector, the decline in consumer confidence in processed animal food products was of major concern to UKASTA. Accordingly, UKASTA set about developing a ‘Code of Practice for the Manufacture of Safe Animal Feedstuffs’, which has now become known as the UKASTA Feed Assurance Scheme (UFAS). The first UFAS Code was published in September 1998 and since then, there have been several revisions, with the latest version ‘Edition 2’ being published in November 2000.
The European Union, meanwhile, was following it’s own timetable. Council Directive 95/69/EEC, which later became known as the ‘Establishments & Intermediaries Directive’ was issued in December 1995. However, legislation introduced within each of the Member States under this Directive was not implemented until 1998/99.
In preparing the UFAS Code, UKASTA was able to incorporate all the necessary legislative aspects that required implementation under Directive 95/69/EEC. ‘The Feedingstuffs (Establishments & Intermediaries) Regulations, 1999’ embodies all aspects within the Council Directive and all United Kingdom feed compounders must now comply with these regulations. However, compounders able to comply with the standards within UFAS have no problem meeting the standards under the Regulations, since standards within UFAS more than satisfy the requirements of the regulations.
This is in contrast to the situation within some other Member States, where compounders have chosen to meet the requirements set by legislation, rather than attempt to comply with a voluntary Code of Practice, which incorporates standards that tend to be higher than those set by legislation.
Throughout this paper, implementation of the UFAS Code in feed mills will relate specifically to the United Kingdom. It is, however, recognised that other countries have also implemented codes of GMP to fulfil a similar objective (see Appendix 2).
Publication of the Pennington Report (April 1997), on the circumstances leading to the 1996 outbreak of food poisoning resulting from E. Coli O157 in Scotland, highlighted the need for the implementation of Hazard Analysis Critical Control Points. Included as one of the recommendations in the report was: “HACCP should be adopted by all food businesses to ensure food safety”
While Pennington targeted his comments directly at premises which prepared both raw and cooked meat for sale, the recommendation was nevertheless applied to “all food premises”, taking the wider definition of ‘all food premises’ to include feed mills manufacturing compound animal feed as part and parcel of the food chain. Careful consideration was given to ensure that UFAS incorporated the requirements of both Council Directive 95/69/EEC and HACCP into all aspects of feed manufacture.
UKASTA Feed Assurance Scheme
By implementing the UKASTA Feed Assurance Scheme, feed compounders can provide their customers, working at the production end of the food chain, and consumers alike, assured feed safety. Safety measures within UFAS have been designed to combat problems that may compromise feed safety.
During the implementation of UFAS, it was quickly realised that a feed mill is not an island. While management and staff may strive to minimise or even eliminate problems that may compromise feed safety during the manufacturing process, this alone will not be sufficient to ensure that all feed is safe.
Raw material sourcing
It must be recognised that a wide range of raw materials are utilised by modern feed mills in the manufacture of animal feed. While cereals and oil seed products make up a large proportion of these raw materials, a wide range of by-products (co-products) from the human food industry are utilized as raw materials in the feed industry. Storage times and conditions can influence quality parameters of raw materials, which, in turn, can affect feed safety.
It is important, therefore, if feed quality and safety is to be assured, that only high quality raw materials must be sourced. Raw material quality must feature high on any HACCP plan implemented by a feed mill. Sourcing raw materials exclusively from stores that have implemented a HACCP plan, and have been externally audited and ‘approved’, is a useful starting point, if raw material problems that can impact on feed safety are to be avoided.
Equally, constant monitoring and evaluation of all raw materials must be carried out to ensure that documented standards are maintained.
Transportation
Transportation, of both raw materials and finished feed products, can introduce hazards that may compromise feed safety. Good, well managed stores for raw materials will not prevent the introduction of hazards if vehicles used for their transportation are not clean or have previously been used to transport hazardous materials that may contaminate the load.
Clearly, this applies not only to vehicular transport, but also to railcars, ships, barges etc., and there have been several instances in recent years of ship’s cargoes being contaminated with heavy metals etc., with the contamination going undetected until animal health has been affected and it is too late. Raw material importers and brokers must also carry out their own HACCP, to ensure that raw materials arriving at the port are in good condition, have not been repeatedly treated with pesticides (to the extent that there are excessive pesticide residues) and have not been stored in unsatisfactory warehouses which have bird/vermin/roof leaks, etc.
Normally, in the United Kingdom, feed compounders only have control of the final leg of the transport chain, with raw materials entering the mill after being transported by truck from the port or store. Where raw materials are transported to the feed mill by road, only hauliers ‘approved’ by the mill should be used. This avoids the risk of feed safety being compromised by unscrupulous hauliers who may be tempted to utilize the same vehicles to transport raw materials and other products, such as broiler litter, domestic waste, glass, etc.
Code of Practice for Road Haulage. In an attempt to control what can and can not be transported in vehicles used in the feed industry, UKASTA have drawn up a Code of Practice for Road Haulage of combinable crops, animal feed materials and as-grown seeds. Road hauliers and vehicles carrying feedingstuffs in bulk at any stage must conform to the Code, including the requirements of the Haulage Exclusion List (Appendix 3) and the Haulage Contaminant Sensitive List (Appendix 4).
Hazards introduced via the raw material route, as a result of either poor storage or transportation, may not be eliminated by further processing through the feed mill. Consequently, storage and transportation must be tightly controlled, as these can be identified as possible Critical Control Points (CCP’s) in an HACCP plan for a feed mill.
Cross contamination
A contentious issue for feed mills over the years has been the avoidance of cross contamination between batches of medicated and non-medicated feed. In general, medication in feed must be included at pre-determined levels, as specified by either the manufacturer or the veterinary surgeon, or both.
Non-medicated feed, manufactured immediately following a batch of medicated feed, is likely to become contaminated with low and variable dosage levels of the medication included in the preceding batch. It is now accepted that cross contamination of non-medicated feed, which is frequently destined for non-target species, is unacceptable. Precautions must be implemented to minimise the risk of cross contamination, particularly where the medication has only been licensed for the target species.
Opportunity also exists for feed to become cross contaminated after it has left the mill on its way to the farm. Cross contamination can occur during transportation, as indicated below:
Over filling of compartments on the vehicle can lead to spillage between compartments during off-loading.
Ideally, only deliveries of non-medicated feed should be carried on the same vehicle. If this is not feasible, medicated feed should always be loaded at the front of the vehicle, so that non-medicated feed (loaded in compartments at the rear of the vehicle), is off-loaded first. This enables the vehicle to be cleaned and checked, prior to being reloaded.
UFAS CODE - THE BASIS FOR ‘GOOD MANUFACTURING PRACTICE’
The background
The UFAS Code was launched in September 1998. Once implemented, a mill must submit to an external audit to ensure that the Code is properly implemented and maintained. Only after audit will feed mills that reach the standards required by UFAS be listed as “approved” in the register.
After allowing a lead-in period of approximately 9 months to enable feed mills time to comply, the register went public in May 1999. By then, 53 mills had been fully “approved”, a further 11 had been audited and awarded “provisional approval”, while a further 72 mills had applied for first audit. According to the latest copy of the register, published by UKASTA on the 8th March 2002, there were 234 mills on the register, with 199 having been fully “approved”, while a further 35 are listed as having ‘applied for first audit’. Data published by UKASTA indicate that feed production from ‘approved’ mills now represents approximately 95 percent of commercially manufactured feed available throughout the United Kingdom.
UFAS guidelines
As required by Council Directive 95/69/EU, the UFAS Code provides a set of standards, based on Hazard Analysis Critical Control Points for the production of safe animal feed. This includes sourcing quality raw materials, which have been stored in audited storage facilities, transport of those raw materials to the feed mill for the manufacture of animal feed, and includes the transport of that feed from the mill to the farm.
The guidelines, incorporated within UFAS, embody the principles of good manufacturing practice as listed below:
design and maintenance of plant;
source and quality of feed materials;
manufacturing, including HACCP principles applied to operation of the plant, scheduling and packaging;
storage of both raw materials and finished products;
loading, transport and delivery;
quality control;
complaints;
product recall;
personnel and training;
documentation and traceability.
Special provisions are also included for the manufacture of safe additive premixes (vitamin/mineral premixes) and authorized intermediate products (high protein concentrates which are further mixed with cereals).
HACCP
HACCP was developed during the early sixties by Pillsbury Co., the United States Army Laboratories and NASA, to assist in the development of food for the American space programme. Food accompanying astronauts had to be 100 percent free of all pathogens and toxins, and it quickly became apparent that testing the finished product was incapable of achieving the necessary 100 percent safety target.
Hence, HACCP was conceived as a system that takes an in-depth examination of a product and all the components and manufacturing stages that go into producing that product. The process is scrutinised in a logical manner to determine ‘what can go wrong in the total system?’.
From its beginnings during the 1960’s, HACCP has come a long way. Various expert groups and committees have recommended the use of HACCP to demonstrate “due diligence”. In 1993, the Codex Alimentarius Commission published their guidelines on the application of HACCP, and this was followed in 1995/96 by recommendations from the World Health Organisation (WHO) which further encouraged the use of HACCP.
Also in 1995, in the United Kingdom, the Ministry of Agriculture Fisheries & Food (MAFF) as it was then, published a Code of Practice for the Control of Salmonella in Animal Feed. The use of HACCP was recommended to identify critical points in the manufacturing process. During 1997, Professor Pennington in his Report on the E. coli O157 outbreak in Scotland (referred to earlier) also recommended HACCP.
The HACCP principles are attached at Appendix 5, together with a list of the 14 stages involved in implementing an HACCP study attached at Appendix 6.
Given the concerns of consumers over safe food, UKASTA introduced the Code of Practice for the Manufacture of Safe Compound Animal Feeds in 1998. Within the Code, Section 1.3 deals specifically with the implementation of HACCP, as follows: “The whole process must be examined in detail to identify potential hazards with particular attention to those which may affect human or animal health, by carrying out an HACCP study”.
However, the fact that an HACCP plan is in place does not remove the responsibility of adequate quality control throughout the production process and there is a separate section on quality control which states that: “there must be a comprehensive system so designed, documented and controlled and so furnished with personnel equipment and other resources as to ensure that feedingstuffs will be consistently of a quality appropriate to their intended use.”
Benefits of HACCP
While some feed compounders have resisted the introduction of yet more administration and red tape into the industry, others quickly realised that the introduction of a Code that relied heavily on the implementation of a sound HACCP plan had benefits, as follows:-
HACCP embraces all aspects of product safety from crop production, raw material selection and storage, raw material haulage, manufacture through to finished product storage and transport to farm, etc.
Emphasis was shifted from testing occasional samples of finished product to a policy of preventative quality assurance.
Attention was focused on critical areas within the production chain to identify all possible hazards.
Any reduction in product losses or time spent dealing with re-works, would represent a reduction in overhead costs.
HACCP represents an accepted system of quality assurance, which is internationally recognised.
HACCP has become a useful ally in supporting a defence of “due diligence”.
The implementation of an HACCP plan is complimentary to ISO9002.
HACCP and the ISO9002 Quality Management System can be developed independently. While both are concerned with the prevention and detection of safety problems, in the case of ISO9002, the scope is widened to include all quality control measures, over and above those relating solely to feed safety. Feed mills already operating ISO9002, can incorporate an HACCP plan into their ISO9002 system.
Scope of HACCP
HACCP had been applied to a wide range of operations, ranging from small, local feed compounders with low daily outputs (blending a range of raw materials but with no pelleting facilities), to large manufacturers with computer controlled, sophisticated plant, capable of thousands of tonnes per day. Its use helps to ensure feed safety at all stages of the production chain. HACCP may be applied equally to both new and existing products, and its scope may be broadened to include the effectiveness of its support operations, such as cleaning and maintenance of the plant, as well as flushing operations, etc.
Product safety
Properly implemented and monitored, HACCP is applicable to issues of product safety that can be categorised under the following hazards:
biological
chemical
physical
Any one, or a combination of these hazards, can affect product safety, but more recently HACCP has also been used to identify control measures, that are more frequently associated with other aspects of product quality. These include issues such as ensuring that the specification satisfies the requirements of the feedingstuffs regulations or, alternatively, taking account of physical quality (pellet size, pellet hardness and proportion of meal relative to pellets, etc.).
HACCP implementation
In order to implement an HACCP plan, management must provide for the necessary team members to have sufficient time available to enable them to contribute to a series of meetings. The team members must have a thorough understanding of all the processes involved in the manufacture of feed through their particular plant. The time commitment and the number and duration of the meetings involved will depend on the complexity of the process and the number and types of hazards to be identified, and it is good policy that during the introductory phase, the Terms of Reference for the plan should be kept simple, dealing with a limited number of hazards that are likely to impact directly on product safety.
Auditing
A vital aspect of the UFAS Code is the fact that it’s implementation must be externally audited up to EN 45011 standards by a professional auditor who is totally familiar with feed manufacturing and the requirements of UFAS. The audit, can normally be completed in one day but may extend into a second, depending on the complexity of the operation. It is designed to check that the documentation encompasses all the requirements of UFAS, and that procedures are being implemented meticulously.
PROTEINS AND PROTEIN BY-PRODUCTS
Processed animal proteins and protein by-products have long been associated with an increased risk of microbiological hazards, the most common being Salmonella. Other animal derived organisms, such as Campylobacter and E. coli O157, have also been associated with increased incidence of food-borne hazards for consumers, but in the case of animal feedstuffs, Salmonella contamination remains the main cause for concern. During the 1980s and 1990s, the unprecedented rise of food-borne salmonellosis spurred the animal feed industry into closer examination of control measures designed to reduce the incidence of Salmonella in feed. The fact that Salmonella still features as a major hazard within the feed industry is confirmed by data published by the Department for Environment Food & Rural Affairs (DEFRA) on the incidence of Salmonella in animal feedstuffs and raw materials as shown in Table 1, covering the period January - December 2001.
While Table 1 shows a range of positives for Salmonella, ranging from 2.4 percent in processed animal protein raw materials for use in animal feedstuffs, down to 0.38 percent in heat treated, extruded pig feed, it is perhaps reassuring, that there were only 12 isolations of Salmonella enteritidis (Se) and S. typhimurium (St) from all feedstuffs and feed raw materials monitored by DEFRA during the 12 month period (see Table 2). This represents a decrease of 37 percent in the number of samples that tested positive for Se and St compared with the previous year, but it could be argued that there should be zero incidence of Salmonella contamination. Table 2 also confirms that the overall trend for the number of positives over the past 8 years is downward, from a high of 44 positive samples in 1994, to a low of 12 over the year January - December 2001.
The source of samples that tested positive for Salmonella enteritidis and typhimurium from products monitored by DEFRA, during the period January to December 2001, indicated that 2 samples of compound poultry feed and 1 sample of fishmeal tested positive for Salmonella enteritidis. In the case of Salmonella typhimurium, 3 samples of wheat tested positive together with one sample of pig feed. The remaining 5 samples were listed as ‘environmental’ (1), ‘other’ (1) and ‘unspecified’ (3). The fact that Salmonella typhimurium was isolated from 3 samples of wheat is very concerning, and highlights the need for constant vigilance, since all raw materials and not just processed animal products, should be regarded as potentially serious hazards.
TABLE 1
Incidence of Salmonella across a
range of animal feedstuffs and raw materials, tested by DEFRA, January -
December 2001
|
Product |
No. of tests |
No. of tests positive |
Percent positive |
|
Processed animal protein at a Great Britain protein processing premises. |
5,866 |
128 |
2.2 |
|
Great Britain and imported processed animal protein arriving for feedingstuffs use. |
1,350 |
33 |
2.4 |
|
Great Britain crushing premises - oil extracted seed meals (rape, sunflower, linseed, soya, palm) |
14,482 |
323 |
2.2 |
|
Non-oilseed meal vegetable products |
14,370 |
227 |
1.6 |
|
Pig and poultry meals |
5,274 |
58 |
1.10 |
|
Poultry extrusions |
6,320 |
27 |
0.43 |
|
Pig extrusions |
2,124 |
8 |
0.38 |
|
Ruminant concentrates |
2,655 |
24 |
0.90 |
|
Protein concentrates |
805 |
12 |
1.49 |
|
Minerals/other |
1,837 |
18 |
0.98 |
TABLE 2
Isolations of S. enteritidis & S.
typhimurium from all feedingstuffs and feed ingredients monitored under
DEFRA Codes of Practice
|
Type of material |
1994 |
1995 |
1996 |
1997 |
1998 |
1999 |
2000 |
2001 |
||||||||
|
Se |
St |
Se |
St |
Se |
St |
Se |
St |
Se |
St |
Se |
St |
Se |
St |
Se |
St |
|
|
Finished feeds |
4 |
25 |
2 |
20 |
0 |
18 |
2 |
7 |
0 |
8 |
0 |
7 |
0 |
9 |
2 |
4 |
|
Animal Protein |
0 |
4 |
0 |
1 |
0 |
10 |
0 |
2 |
0 |
0 |
0 |
1 |
0 |
2 |
1 |
0 |
|
Vegetable Material |
1 |
6 |
4 |
10 |
5 |
6 |
0 |
9 |
0 |
9 |
1 |
9 |
1 |
3 |
0 |
3 |
|
Minerals |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Miscellaneous |
0 |
4 |
1 |
5 |
1 |
2 |
1 |
6 |
2 |
3 |
1 |
1 |
1 |
3 |
0 |
2 |
|
TOTALS |
5 |
39 |
7 |
36 |
6 |
36 |
3 |
24 |
2 |
20 |
2 |
18 |
2 |
17 |
3 |
9 |
Se - S. enteritidis, St - S. typhimurium
From data presented in Table 3, feed mills sourcing imported, processed animal protein must be particularly vigilant due to the increased risk from imported compared with home processed product. For example, approximately 0.2 percent of samples out of a total of approximately 400 taken from processed animal protein and fishmeal produced in the United Kingdom, tested positive (see Figure 1), whereas over 15 percent of imported processed animal protein and fishmeal, taken from approximately 150 samples, were positive (see Figure 2).
The epidemiology of Salmonella infection in animals is extremely complex, involving two-way transmission between man, animals, the environment, animal feedstuffs, rodents, birds and flies.
Home Produced Animal Protein (2001 January - December)
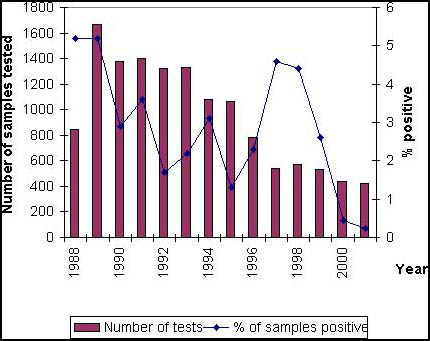
Figure 1. Home produced processed animal protein and fishmeal. Official (DEFRA) testing results: (January to December 2001) * Rendering and fishmeal premises must register under the Animal By-Products Order 1999. This Order requires monitoring of finished products for Salmonella (termed “Private” testing). Additional monitoring is carried out by Ministry Staff. This figure reports the results of the official testing
TABLE 3
Incidence of Salmonella positives
isolated from samples of a range of products (DEFRA, 2002)
|
Product |
Percentage testing positive |
|||||||
|
1994 |
1995 |
1996 |
1997 |
1998 |
1999 |
2000 |
2001 |
|
|
Processed animal protein at a Great Britain protein processing premises |
2.2 |
1.9 |
3.2 |
3.1 |
1.7 |
2.7 |
2.1 |
2.2 |
|
Great Britain and imported processed animal protein arriving for feedingstuffs use |
4.1 |
4.4 |
4.3 |
4.9 |
4.4 |
2.3 |
2.9 |
2.4 |
|
Oilseed meals and products for feedingstuffs use* |
4.9 |
3.3 |
4.6 |
3.5 |
1.7 |
3.3 |
1.9 |
2.2** |
|
Non oilseed meal vegetable products |
2 |
1.5 |
1.7 |
1.8 |
1.3 |
0.8 |
1.0 |
1.6 |
( ) Number of samples tested; * Linseed, rape, soya, and sunflower meals at a United Kingdom crushing premises, including all other oilseed meals and products arriving for feedingstuff use; **United Kingdom crushing premises only - oil extracted seed meals (rape, sunflower, linseed, soya and palm)
Imported animal Protein
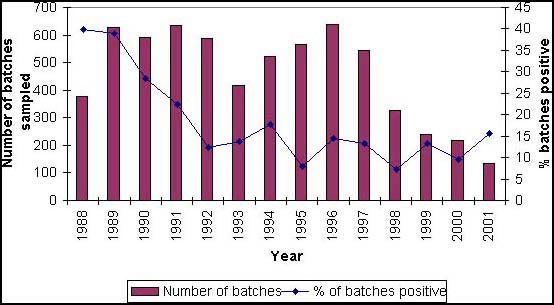
Percentage Salmonella contamination in samples tested under the Importation of Processed Animal Protein Order 1981 (as amended).
Figure 2 Imported processed animal protein and fishmeal. Official (DEFRA/MAFF) testing results. (January to December 2001)* Samples of imported processed animal protein are taken by Ministry staff and tested for Salmonella under The Importation of Processed Animal Protein Order 1981 (as amended). This figure records the results of that testing (termed “Official” testing) in terms of the number of contaminated consignments.
Vermin
A survey carried out in 1995 by Davies and Wray (Davies and Wray, 1995) reported that 35 percent of mice carried Salmonella enteritidis in the liver, 46 percent carried it in the intestine and 10 percent had it in their droppings; as a result, mice are probably recognised as being more of a threat than rats. Birds, particularly pigeons, seagulls and sparrows (Wray and Davies, 1996), are also a threat in and around feed mills. Uncovered bulk delivery vehicles, open raw material intakes, unsealed raw material and finished product silos are all at risk of Salmonella contamination from bird droppings and steps must be taken to prevent direct contamination. GMPs must, therefore, include adequate control of all vermin, including mice, rats and birds.
Similarly, drivers must avoid walking on loads of feed or raw materials. This prevents contamination from dirty yards which are popular with birds. In this respect, all spillages should be cleared immediately to minimise the amount of feed available to birds and vermin.
Heat treatment
Feed mill technology for heat treating feed has improved dramatically in recent years, with feed pasteurisation now being possible. However, under practical field conditions, complete control or “near zero tolerance” of Salmonella and other pathogenic bacteria cannot rely completely on heat treatment.
Salmonella are readily killed by heat, e.g. 71.7oC for 15 seconds, and by acid, e.g. less than pH 4. A combination of both heat and acidity can provide excellent control of Salmonella, and hence help to minimise the likelihood of animal feed becoming a vector in the transmission of Salmonellosis.
Maintaining the status of near-sterile feed right through to the stage that feed is consumed by animals, is extremely difficult. Bacterial multiplication downstream from the site of heat treatment can lead to significant bacterial counts in feed by the time it reaches the farm. This has meant that chemical control methods, using organic acids, organic acid salts, etc., have been introduced to complement heat treatment and help prevent recontamination, while in other cases, chemical treatment has completely replaced heat treatment.
Sampling and identification
Salmonella can be difficult to isolate in both feed and raw materials, due to the uneven distribution of the bacteria and associated sampling errors. Since Salmonella and E.Coli bacteria both belong to the family Enterobacteriaceae, both bacteria are often found together. Consequently, counts of Enterobacteriaceae in samples of raw material or finished feed can provide a useful assessment of the overall microbiological quality. Samples containing high Enterobacteriaceae counts should always be suspected as being positive for the presence of Salmonella, and accordingly, suitable precautions or controls should be put in place.
CONCLUSION
Compound animal feed forms a significant link in the chain of production of food products from animal origin (meat, milk and eggs). The production of safe animal feed is a question of good management practices at every stage of the process - from sourcing high quality raw materials, their storage and transportation, feed production, finished feed storage, through to delivery of feed to farm. It is imperative that each stage of the process is rigorously assessed for hazards or risks that could compromise either animal health, human health or both.
European Union legislation now requires that these objectives are achieved through the application of an HACCP plan covering every stage of the process. HACCP may be developed within an ISO9002 or equivalent quality management system and it is widely recognised that within such a system, HACCP will be stronger and more robust, since its effectiveness relies on proper implementation and ongoing maintenance.
REFERENCES
Davies, R. H. & Wray, C. 1995. Mice as carriers of Salmonella enteritidis on persistently infected poultry units. Veterinary Record, September 30, p. 337-341.
Wray, C. & Davies, R. H. 1996. A veterinary view of Salmonella in farm animals. PHLS Microbiology Digest 13(1): 44-48.
ANNEX 1
The Food Chain
ANNEX II
National Codes of Practice developed by FEFAC Members Associations
Codigo de boas practicas para a fabrica de premisturas e de alimentos para animais (IACA - Portugal).
GMP-regeling diervoedersector (Productschap Diervoeder - The Netherlands). Product Board Animal Feed (PDV) have implemented a ‘GMP - Regulation Animal Feed Sector’ together with a ‘Code for the Quality Control of Feed Materials for Animal Feed’.
Code GMP général pour le secteur de l’alimentation animale (BEMEFA/APFACA -Belgium).
Codice di buone pratiche per la produzione e la commercializzazione di alimenti composti per animali da reddito (ASSALZOO - Italy).
Code de bonnes pratiques pour la fabrication d’aliments médicamenteux - Guide de mise a niveau pour l’agrément des établissements fabricants des aliments pour animaux (SNIA - France).
Leitfaden fur eine Gute Herstellungspraxis von Futtermitteln (DVT - Germany). ‘Quality and Safety Charter’ - A Good Manufacturing Practice Code, based on HACCP, designed to provide ‘Quality and Security for food from producer to consumer’.
UKASTA Feed Assurance Scheme (UFAS) - Code of Practice for the Manufacture of Safe Compound Animal Feedingstuffs (UKASTA - UK).
Code of practice and general operating standard for poultry feed processing (DAKOFO - Denmark).
Leitfaden fur eine “Gute Herstellungspraxis von Futtermitteln”, GHF (VSF - Switzerland).
ANNEX III
Haulage Exclusion List
(Extract from the UKASTA Code of Practice for Road Haulage of Combinable Crops, Animal Feed Materials and As-Grown Seeds)
The following materials must not, since 1 July 1998, 1 July 2000 or 1 July 2001, as appropriate, have been carried in vehicles or trailers used for the transportation of goods covered by this Code of Practice. Hauliers must be prepared to give an undertaking to this effect if required:
Toxic & Corrosive Materials and any Packaging used for these Materials
Radio-active Materials
Livestock including Poultry, also including their carcasses
Animal & Poultry Wastes, including Manures/Litter
Mammalian Protein, e.g. Meat & Bone Meal, Meat Meal, Cull Cake and Other Mammalian Based Products. (Milk & Milk Products, Gelatin, Amino Acids, Dicalcium Phosphate, Dried Plasma and any other Blood Products are permitted to be carried)
Specified Risk Material/Cull Tallow
Mineral Clays which have been used for Detoxification purposes
Cereal & Other Seed Treated with Toxic Dressing (excluding Bagged or Packaged Seed)
Glass (including cullet)
Hides treated with Tanning Substances and its Waste
Scrap Metal, including Fragmented Metal and “Frag Rubber”
Sludge from Sewage Plants Treating Waste Waters
Solid Urban Waste, such as Household Waste
All wastes obtained from the various phases of the urban, domestic and industrial waste water treatment process, irrespective of any further processing of these wastes and also irrespective of the origin of the waste waters
Untreated Waste from Eating Places, except Food Stuffs of Vegetable Origin considered unsuitable for Human Consumption for Reasons of Freshness.
The following materials must not, since 1st July 2000, have been carried in vehicles used for the transportation of goods covered by this Code of Practice:
Bituminous products and other products not responsive to normal detergent cleaning
Any materials (e.g. timber) which have been treated with protection products
The following materials must not, since 1st July 2001, have been carried in vehicles used for the transportation of goods covered by this Code of Practice:
Processed Animal Protein, e.g. Bone Meal, Blood Meal, Dried Plasma and other Blood products, Hydrolysed Protein, Hoof Meal, Horn Meal, Poultry Offal Meal, Feather Meal, Dry Greaves, Dicalcium Phosphate and Gelatin.
ANNEX IV
Haulage Contaminant Sensitive List
Where vehicles are presented for the carriage of goods, their load carrying areas must, at all times, be kept in a clean, dry and fit state to avoid harm to the goods being carried. Vehicles for carrying liquids should be in a condition fit for the purpose. It must be remembered that the Food Safety Act requires that any surface which comes into contact with food must be clean.
Pressure Cleaning/Sanitising
Lorries must be pressure cleaned with a 1 percent hot (70-80°C) solution of any combined detergent/sanitiser after they are used for carrying the goods listed below. The vehicle sheet must also be pressure cleaned in this way. The vehicle and sheet must be drained and dry before re-use for other loads. Proof will be required to be given that appropriate cleaning operations have been undertaken and they must be recorded on the consignment note of a subsequent load.
Tallows (other than Specified Risk Material/Cull Tallow)
Strong smelling materials, excluding fish meals
Dicalcium Phosphate and Hydrolysed Protein from approved premises (other than Processed Animal Protein - see Appendix 3)
Any product known to be Salmonella positive
Packaging and parts of packaging from products used in agriculture or the food industry
Silage
Washing/Brushing/Vacuuming
Proof will be required to be given that appropriate cleaning operations have been undertaken, when the following materials have been carried prior to the carriage of goods covered by this Code. In most cases where the material is dry, thorough brushing or vacuuming will be sufficient. However, if the material is caked or damp, washing will be necessary.
Aggregates
Coal/Fly Ash/Coal By-Products
Fertilizer
Medicated Feed Products
Root Crops
Salt
Untreated Wood, Sawdust or other Materials derived from Wood
Moist Co-Products
Vehicles used exclusively for the delivery of one moist co-product, must be cleaned and sanitized with a food grade sanitizer once a week. The cleaning should include the vehicle body, trailer (if appropriate) and sheet inside and out. Vehicles must also be cleaned and sanitized between each load if different co-products are to be transported. Vehicles which have carried products included in the Haulage Contaminant Sensitive List must be cleaned, as detailed under (a) above.
Infested Products
Vehicles which have carried infested products must be thoroughly steam cleaned. The vehicle sheet must also be steam cleaned in this way. The vehicle’s load carrying area and sheet must be drained and dry before re-use for other loads. Proof will be required to be given that appropriate cleaning operations have been undertaken, and they must be recorded on the consignment note of a subsequent load. The use of smoke bombs is not likely to be effective and is not recommended.
Nuts, Nut Products and Sesame Seed
(NB. Attitudes towards and acceptance of nuts and nut products (including food waste containing nuts) and sesame seed, differ between end user companies. Hauliers must check individual companies’ policies, which are influenced by the allergic reaction to these products suffered by some people, resulting in severe anaphylactic shock).
Fish Meal
If a vehicle is used for the transport of Fish Meal and is subsequently used for the transport of other products, it must be thoroughly cleaned and sanitized, in accordance with (a) above, and inspected before and after the transport of the Fish Meal.
ANNEX V
HACCP Principles
Principle 1
Conduct a hazard analysis - Prepare a flow chart of the steps in the process - identify and list the potential hazards - specify the control measures to eliminate or control those hazards.
Principle 2
Determine the critical control points (CCPs) - Use a decision tree to identify the points at which control can eliminate or reduce the hazard.
Principle 3
Establish critical limits - These must be met to ensure that each CCP is under control. Target levels and tolerances must be met to ensure that the CCP is under control.
Principle 4
Establish a system to monitor control of the CCP - Regular scheduled testing or observations.
Principle 5
Establish the corrective action to be taken when monitoring indicates that a particular CCP is not under control (or is moving out of control).
Principle 6
Establish procedures for verification to confirm that HACCP is working effectively - may include appropriate supplementary tests, together with a review.
Principle 7
Establish documentation concerning all procedures and records appropriate to these principles and their application.
ANNEX VI
Stages in an HACCP study
|
PART 1 |
0 |
Management Commitment |
|
1 |
Scope (Terms of Reference) |
|
|
2 |
Select Team Members |
|
|
3 |
Write Product Description |
|
|
4 |
Describe Intended Use |
|
|
5 |
Draw up Mill Flow Chart |
|
|
PART 2 |
6 |
Confirm Flow Chart on site |
|
7 |
Identify all likely Hazards |
|
|
8 |
Decide on CCPs |
|
|
9 |
Set Targets and Limits for CCPs |
|
|
10 |
Set up Monitoring Procedures |
|
|
11 |
Set up Corrective Actions |
|
|
PART 3 |
12 |
Verification (Audits etc) |
|
13 |
Records |
|
|
14 |
Review the Study |