与会者名单
Australia
Dr Dennis Bittisnich
Manager
Food Regulation Section
Australian Government Department of Agriculture, Fisheries and Forestry
GPO Box 858
Canberra ACT 2601
Tel: 61.2.6272.3053
Email: [email protected]
Ms Melanie Fisher
General Manager
Food Standards
Food Standards Australia New Zealand
PO Box 7186
Canberra BC ACT 2610
Tel: 61.2.6271.2246
Fax: 61.2.6271.2204
Email: [email protected]
Ms Hikmat Hayder
Senior Scientist
Food Standards Australia New Zealand
PO Box 7186
Canberra BC ACT 2610
Tel: 61.2.6271.2645
Fax: 61.2.6271.2278
Email:[email protected]
Bangladesh
Mr Muhammad Habibur Rahman
Joint Chief
Ministry of Agriculture
Bangladesh Secretariat
Bhaban No. 04 Room 514
Dhaka
Tel: 880.2.7168161
Fax: 880.2.7167040
Email: [email protected]
Mr Md Ruhul Amin Talukder
Additional Director
Food Planning and Monitoring Unit
Ministry of Food
16, Abdul Gani Road
Dhaka-100
Bangladesh
Tel: 880-2955 6032
Fax: 880-2955 4159
Email:
[email protected]@hreworks.com
Mr Kazi Selim Anwar
Medical Officer/Bacteriologist
Institute of Public Health
Mohakhali
1212-Dhaka
Tel: 88.02.882.7434
Fax: 88/02.989.8853
Email: [email protected]
Bhutan
Mr Sonam Lhendrup
Legal Officer
Policy and Planning Division
Ministry of Agriculture
Thimpu
Tel: 323.745
Fax: 323.748
Email: [email protected]
Brunei Darusalaam
Mrs Nur Nisrinah Haji Awang Yusof
Scientific Officer
Department of Scientific Services
Ministry of Health
Jalan Menteri Besar
Berakas BB 3910 Negara
Tel: 673.2.3822424 Ext. 7744/7745
Fax: 673.2.381946
Email: [email protected]
Ms Hajah Rahimah Haji Ibrahim
Acting Assistant Fisheries Officer
Fisheries Department
MIPR
Brunei Darussalam
Email: [email protected]
Mrs Hajah Dahliana Haji Aliakbar
Senior Livestock Husbandry Officer
Department of Agriculture
Ministry of Industry and Primary Resources
BB 3510 Brunei Darussalam
Email:
[email protected]
Mrs Pengirah Hajah Masliati PSJ Pengiram
Haji Abd Momin
Agriculture Officer
Department of Agriculture
Ministry of Industry and Primary Resources
BB 3510 Brunei Darussalam
Email: [email protected]
Cambodia
Ms Pau Ann Sivutha
Chief
Food Safety Office
Department of Drugs and Food
Ministry of Health
Phnom Penh
Tel: 855.238.80248
Fax: 855.238.80247
Email: [email protected]
Mr. Thearith Lim
Assistant Quality Control Service
Deputy Chief of Sub technical Group of
National Codex Committee
No. 5060/144 Street
Phnom Penh
Tel: 855.2342.6166
Fax: 855.2342.6166
Email: [email protected]
China
Mr Yanqiu Zhang
Deputy Director General
Department of Market and Economy Information
Ministry of Agriculture
11 Nongzhanguan Nanli
Beijing 100026
Tel: 8610.6419.3179
Fax: 8610.6410.3154
Email: [email protected]
Mr Wen Shaohui
No. 59 Xueyuan Nanlu
Haidian district
Beijing 100081
Tel: 8610.6219.1437
Fax: 8610.6219.1434
Email: [email protected]
Ms Weiqin Wang
Deputy Director of Division
Department of International Cooperation
Ministry of Agriculture
11 Nongzhanguan Nanli
Beijing 100026
Tel: 8610.6419.2429
Email: [email protected]
Mr Fan Xuehui
Department of Food Safety Coordination
State Food and Drug Administration
A38 Beilishilu
Beijing 100810
Tel: 8610.6831.3344-0511
Fax: 8610.6831.3344-0511
Email: [email protected]
Mrs Liu Xiumei
National Institution of Nutrition and Food Safety
Beijing
Tel: 8610.8313.2928
Email: [email protected]
Mr Zhihua Ye
Director General
Science and Technology Management Department
Chinese Academy of Agricultural Sciences
12 Southern Street of Zhong-Guan-Cun
Beijing 100081
Tel: 8610.6891.9419
Fax: 8610.6897.5099
Email: [email protected]
Ms Lingping Zhang
Deputy Director
Division of Food and Cosmetic Administration
Department of Health Enforcement and
Supervision
Ministry of Health
Beijing
Tel: 8610.6879.2403
Fax: 8610.6879.2408
Email: [email protected]
Commonwealth of Northern Marianas Island
Mr John Tagabuel
Environmental Health Officer
Ministry of Health
PO Box 500409
Saipan MP 500409
Northern Marianas Island
Tel: 670.664.4870
Fax: 670.236.8700
Email: [email protected]
Democratic People’s Republic of Korea
Mr Kim Song Guk
Senior Officer
Academy of Health and Food Science
Pyongyang
Mr Ku Un Han
Researcher
Academy of Health and Food Science
Hygiene Inspection Bureau
Ministry of Public Health
Pyongyang
Fiji
Mr William G.L. Aalbersberg
Professor of Natural Products Chemistry and
Director, Institute of Applied Science
University of the South Pacific
Suva
Tel: 679.3312952
Fax: 679.3300373
Email: [email protected]
Mr Anthony Ian Chamberlain
Seafood Lecturer
HACCP Trainer, Food Safety Auditor
The University of the South Pacific
P.O. Box 1168 Suva
Tel: 679.321.2949
Fax: 679.330.1490
Mr Waisele Delai
Senior Health Inspector
Food Quality Control
Ministry of Health
PO Box 2223
Government Buildings
Suva
Tel: 679.330.6177
Fax: 679.330.6163
Email: [email protected]
India
Mr K.M. Appaiah
Director
Central Food Laboratory
CFTRI Mysore
Email: [email protected]
Mr S.R.Gupta
Joint Drugs Controller (India)
Room No. 351
A Wing
Directorate General of Health Services
Ministry of Health and Family Welfare
Nirman Bhawan
New Delhi 110011
Tel: 91.11.2301.7268
Fax: 91.11.2301.2290
Email: [email protected]
Mr Vijay Kumar
Adviser
Export Inspection Council of India
Ministry of Commerce and Industry
3rd Floor, NDYMCA
Cultural Centre Building
1 Jai Singh Road
New Delhi 1100001
Tel: 91.11.2374.8022
Fax: 91.11.2374.8186
Email: [email protected]
Indonesia
Prof Dr Dedi Fardiaz
Deputy III, The National Agency for Drug
and Food Control
Jl. Percetakan Negara No. 23
Jakarta
Tel: 62.21.425.3857
Fax: 62.21.425.3857
Email: [email protected]
Dr Shobar Wiganda
Director for Food Surveillance Centre
Food Security Agency
Ministry of Agriculture
Pusat Kewaspdaan Pangan
Badan Bimas Ketahanan Pangan
Departemen Pertanian
Gedung E Lantai 2
Jl. Harsono Rm 03
Jakarta
Tel: 62.21.781.6652
Fax: 62.21.780.6708
Email: [email protected]
Ms Agus Triyani
Bureau of International Cooperation
Ministry of Agriculture of Indonesia
A Building 6th Floor
Jalan Harsono RM No. 3
Jakarta Selatar
Tel: 62.21.7804350
Fax: 62.21.7804176
Email: [email protected]
Ms Saptarini Nuswamarhaeni
Bureau of International Cooperation
Ministry of Agriculture of Indonesia
A Building 6th Floor
Jalan Harsono RM No. 3
Jakarta Selatar
Tel: 62.21.708.30588
Fax: 62.21.7804.176
Email: [email protected]
Ms Tennike Erman
First Secretary
Embassy of Indonesia
Jalan Tun Izazak No. 233
Kuala Lumpur
Malaysia
Tel: 603.2141.0435
Fax: 603.2141.7298
Email: [email protected]
Japan
Mr Takehiko Suzuki
Deputy Director
Policy Planning and Communication Division
Department of Food Safety
Pharmaceutical and Food Safety Bureau
Ministry of Health, Labour and Welfare
1-2-2 Kasumigaseki
Chiyoda-ku
Tokyo 100-8916
Tel: 81.3.3595.2326
Fax: 81.3.3503.7695
Email: [email protected]
Mr Makoto Hirose
Deputy Director
Policy Planning and Communication Division
Department of Food Safety
Pharmaceutical and Food Safety Bureau
Ministry of Health, Labour and Welfare
1-2-2 Kasumigaseki
Chiyoda-ku
Tokyo 100-8916
Tel: 81.3.3595.2326
Fax: 81.3.3503.7695
Email: [email protected]
Mr Tadahiro Nagata
Director
Food Safety and Quality Division
National Food Research Institute
2-1-12 Kannon-dai
Tsukuba
Ibaraki 305-8642
Tel: 81.298.38.8008
Fax: 81.298.38.7996
Email: [email protected]
Mr Harumi Saka
Deputy Director
International Affairs Office
Food Safety and Consumer Affairs Division
Food Safety and Consumer Affairs Bureau
Ministry of Agriculture, Forestry and Fisheries
1-2-1 Kasumigaseki
Chiyoda-ku
Tokyo 100-8950
Tel: 81.3.5512.2291
Fax: 81.3.3597.0329
E-mail: [email protected]
Mr Ikuo Tsukamato
Deputy Director
Inspection Division
Department of Food Safety
Ministry of Health, Labour and Welfare
Food Quality Control Division,
Ministry of Health Malaysia
Level 2, Block A,
Jalau Cenderasari 50590
Kuala Lumpur
Malaysia
Tel: 603.2694.6601 Ext. 284
Fax: 603.2694.6517
Email: [email protected]
Kiribati
Mr Taeuea Tianuare
Chief Health Inspector
Ministry of Health and Medical Services
P.O. Box 268
Nawerewere
Tarawa
Tel: 686.28100
Fax: 686.28152
Email: [email protected]
Korea, Republic of
Mr DongHa Lee
Deputy Director
Food Standards Division
Korea Food and Drug Administration
5 Nokbun-Dong
Eunpyung-ku
Seoul 122-704
Tel: 2.380.1665
Fax: 2.382.4892
E-mail: [email protected]
Mr Jong Seok Park
Researcher
Food Microbiology Division
Korea Food and Drug Administration
Korea Food and Drug Administration
5 Nokbun-Dong
Eunpyung-ku
Seoul 122-704
Tel: 2.380.1682
Fax: 2.382.4892
Email: [email protected]
Mr Kim Pyung Tae
Director, Food Surveillance Division
Korea Food and Drug Administration
5 Nokbun-Dong
Eunpyung-ku
Seoul 122-704
Tel: 2.380.1633
Fax: 2.352.9445
Email: [email protected]
Laos
Mrs Sivilay Naphayvong
Chief
Food Control Division
Food and Drug Department
Ministry of Health
Vientiane
Tel: 856.21.214014
Fax: 856.21.214015
Email: [email protected]
Malaysia
Dato' Dr Shafie Ooyub
Deputy Director General
Public Health Department
Ministry of Health Malaysia
Health Offices Complex
Level 2, Block A, Jalan Cenderasari
50590 Kuala Lumpur, Malaysia
Tel: 603.2694.6382
Fax: 603.2694.6390
Email: [email protected]
Dr Abd Rahim bin Hj Mohamad
Director
Food Quality Control Division
Public Health Department
Ministry of Health Malaysia
Health Offices Complex
Level 3, Block B, Jalan Cenderasari
50590 Kuala Lumpur
Tel: 603.2694.6512
Fax: 603.2694.6517
Email: [email protected]
Dr Hasan Abd Rahman
Director State Health Department
Pejabat Kesihatan Negeri Johor
Tingkat 4, Blok B
Wisma Persekutuan
Jalan Air Molek
80590 Johor Bharu
Tel: 607.2245.188
Fax: 607.2232.603
Email: [email protected]
Ms Noraini Dato’ Mohd Othman
Deputy Director (Codex)
Food Quality Control Division
Ministry of Health Malaysia
Health Offices Complex
Level 3, Block B, Jalan Cenderasari
50590 Kuala Lumpur
Tel: 603.2694.6601 ext 230
Fax: 603.2694.6517
Email: [email protected]
[email protected]
Ms Shamsinar Abdul Talib
Principal Assistant Director
Food Quality Control Division
Public Health Department
Ministry of Health Malaysia
Level 3, Block B, Jalan Cenderasari
50590 Kuala Lumpur, Malaysia
Tel: 603.2694 6601 ext 230
Fax: 603.2694 6517
Email: [email protected]
Mr Raj R. D'Nathan
Deputy Under Secretary
Ministry of Agriculture
19th Floor, The Mall
100 Putra Place, Jalan Putra
50350 Kuala Lumpur, Malaysia
Tel: 603.4045.3050
Fax: 603.4045.8900
Email: [email protected]
Mr Amarjit Singh Sarjit Singh
Assistant Secretary
Multilateral, Economic and Environment Division
Ministry of Foreign Affairs
No 1, Jalan Wisma Putra, Presint 2
62602 Putrajaya, Malaysia
Tel: 603.8887.4441
Fax: 603.8889.2788
Email: [email protected]
Maldives
Mr Adam Manik
Senior Project Officer
Ministry of Fisheries, Agriculture and Marine Resources
Malé
Ms Fathimath Safoora
Laboratory Technologist
Public Health Laboratory
Malé
Micronesia, Federated States of
Mr Moses E. Pretrick
Environmental Health Coordinator
FSM National Government
Department of Health, Education and
Social Affairs
Division of Health Services
Environmental and Community Health
Section
P.O. Box PS-70
Palikir, Pohnpei FM 96941
Tel: 691.320.8300/1280/1909
Fax: 691.320.8460/5263
Email: [email protected]
Mongolia
Mrs Tumur-Ochir Enkhjargal
Officer of the Department of Strategic Planning and Policy
Ministry of Agriculture
Government Building #9
Enkhtaivan Avenue 16A
Ulaanbaatar 210349
Tel: 976.1.351.261908
Fax: 976.11.452967
Email: [email protected]
Myanmar
Ms Yi Yi Htwe
Deputy Director (Food)
Food and Drug Administration
35 Minchaung Street
P.O. Box 11191
Yangon
Tel: 95.01.381902
Fax: 95.01.379004
Email: [email protected]
Nauru
Mr Vincent Melvin Scotty
Health Inspector
Ministry of Health
Yaren
Tel: 674.444.3702
Fax: 674.444.3106
Email: [email protected]
Nepal
Mr Poorna Prasad Manandhar
Secretary
Ministry of Agriculture and Cooperatives
Singh Durbar
Tel: 977.1.422.5108
Fax: 977.1.422.5825
Email: [email protected]
[email protected]
New Zealand
Mr Neil A. McLeod
Programme Manager (Market Access - Asia-Pacific)
New Zealand Food Safety Authority
PO Box 2835
Wellington
Tel: 64.4.463.2613
Fax: 64.4.463.2643
Email: [email protected]
Mr Peter D. Gollan
Programme Manager –South and South-east Asia - South & Central America
New Zealand Food Safety Authority
PO Box 2835
Wellington
Tel: 64.4.463.2614
Fax: 64.4.463.2643
Email: [email protected]
Pakistan
Mr Mubarik Ahmed
Project Director
Grain Testing Laboratory
Ministry of Agriculture, Food and Livestock
Karachi University Campus
Pakistan Agricultural Research Council
Karachi
Tel: 21.924.3188
Fax: 51.482.1474
Email: [email protected]
Palau
Mrs Joanne Maireng Sengebau-Kingzio
Chief
Division of Environmental Health
Ministry of Health
P.O. Box 6027
Kororg
Palau 96940
Tel: 680.488.6073
Fax: 680.488.6194
Email: [email protected]
or [email protected]
Papua New Guinea
Mrs Rose Pepa Kavanamur
Technical Advisor – Food Safety
and Quarantine
Department of Health
P.O. Box 807
Waigani
Nationbal Capital District
Papua New Guinea
Tel: 675.3013705
Fax: 675.3013604
Email: [email protected]
Philippines
Mr Gilberto F. Layese
OIC – Director
Bureau of Agriculture Fisheries Product
Standards
Department of Agriculture
BPI Compound
Visayas Avenue
Diliman
Quezon City
Tel: 632.920.6131/33
Fax: 632.920.6134
Email: [email protected]
Mr Clarito Barron
Assistant Director
Bureau of Plant Industry
San Andres
Malate
Manila
Tel: 632.525. 3470
Fax: 632.523.0765
Email: [email protected]
Dr Minda S. Manantan
Deputy Executive Director
National Meat Inspection Commission
Visayas Avenue
Diliman
Quezon City
Tel: 632.924.3119
Fax: 632.924.7973
E-mail: [email protected]
Ms Norma Z. Granada
Chief
Product Quality Control and Research Division
Philippine Coconut Authority
Elliptical Road
Diliman
Quezon City
Tel: 632.920.0417
Fax: 632.928.1063
Email: [email protected]
Dr Sonia yo Leon
President
Foundation for the Advancement of Food Science
and Technology
99 Mother Ignacia Ave.
Quezon City
Tel: 632.372.6688
Fax: 632.372.7350
Email: [email protected]
[email protected]
Samoa
Dr Nuualofa Potoi
Assistant Chief Executive Officer
Public Health Services
Ministry of Health
Private Mail Bag
Apia
Tel: 685.21212 Ext. 376
Fax: 685.21106
Email:
[email protected]
Mr Pimalolo Maiava
Registrar of Pesticides
Quarantine Division
Ministry of Agriculture, Forestries, Fisheries and Meteorology
Apia
Singapore
Mr Tan June Keong Owen
Surveillance Executive
Ministry of Health
College of Medicine Building
16 College Road
Singapore 169854
Tel: 63252013
Fax: 62241677
Email: [email protected]
Solomon Islands
Mr Robinson Stanley Fugui
Director of Environmental Health
Ministry of Health and Medical Services
P.O. Box 349 Honiara
Tel: 677.25513
Fax: 677.25513
Email: [email protected]
Sri Lanka
Dr C.K. Shanmugarajah
Director (Environmental and Occupational Health)
Ministry of Health Care, Nutrition and UVA
Wellassa and Development
Suwasiripaya
385 Deans Road
Colombo 10
Fax: 9411.2672694
Mr Sinnathamby Nagiah
Assistant Director
Food Control Administration
Food Control Administration Unit
Ministry of Health Care, Nutrition and Uva Wellassa Development
385 Deans Road
Colombo 10
Tel/Fax: 94.112672004
Email: [email protected]
Thailand
Mr Supachai Kunaratanapruk
Secretary General
Food and Drug Administration
Ministry of Public Health
Tiwanont Road
Nonthaburi 11000
Tel: 66.2591.8441
Fax: 66.2591.8636
Email: [email protected]
Mr Kraingsak Dangprom
Veterinary Officer
Bureau of Livestock Standard and Certification
Department of Livestock Development
Ministry of Agriculture & Cooperative
69/1 Pyathai Road
Bangkok 10400
Tel: 662.281.6569
Fax: 662.280.3899
Email: [email protected]
Ms Duangtip Hongsamoot
Food and Drug Administration
Ministry of Public Health
Tiwanont Road
Nonthaburi 11000
Tel: 662.590.7318
Fax: 662.590.7359/591.8477
Email: [email protected]
Ms Yupa Laojindapun
Standards Officer
National Codex Contact Point
National Bureau of Agricultural Commodity and Food Standards
Ministry of Agriculture and Cooperative
Bangkok 10200
Tel: 662.281.6569
Fax: 662.280.3899
Email: [email protected]
Mrs Kanokphan Srimanobhas
Senior Food Technologist
Department of Fisheries
Kaset - Klang, Chatuchak
Bangkok 10900
Tel: 662.558.6138
Fax: 662.558.0138
Email: [email protected]
Ms Warunee Sensupa
Food Specialist
Food and Drug Administration
Thailand Ministry of Public Health
Tiwanont Road, Nonthaburi 11000
Tel: 662.590.7023
Fax: 662.591.8476
Email: [email protected]
Mr Pisan Pongsapitch
Standards Officer
National Codex Contact Point
National Bureau of Agriculture and Food Standards
Ministry of Agriculture Commodity and Food Standards
Bangkok 10200
Tel: 662.280.3887
Fax: 662.280.3899
Email: [email protected]
[email protected]
Ms Tipicha Posayanonda
Pharmacist
Food and Drug Administration
Thailand Ministry of Public Health
Tiwanont Road, Nonthaburi 11000
Tel: 662.590.7322
Fax: 662.591.8460
Email: [email protected]
Ms Akanid Wapeewuttikorn
Pharmacist
Food and Drug Administration
Ministry of Public Health
Tiwananont Road
Nonthaburi 11000
Tel: 662.5907001
Fax: 662.591.8636
Email: akanid@fda,moph.go.th
Tonga
Mr Viliami Manu
Chief of Food Division
Ministry of Agriculture, Forestry and Food
P.O. Box 14
Nuku’ Alofa
Tonga
Tel: 676.25.355
Fax: 676.23.093
Email: [email protected]
Vanuatu
Mr George Taleo
Acting Director
Public Health Services
Ministry of Health
Private Mail Bag 009, Port Vila
Republic of Vanuatu
Tel: 678.22512
Fax: 678.25438
Email: [email protected]
Vietnam
Mr Chu Quoc Lap
Deputy Director
Food Administration
Ministry of Health
138A Giang Vo
Hanoi
Tel: 84.04.8464490
Fax: 84.04.8463739
Email: [email protected]
INTERNATIONAL
GOVERNMENTAL AND NON-
GOVERNMENTAL
ORGANIZATIONS
CODEX SECRETARIAT
Mr Yoshide Endo
Food Standards Officer
Codex Alimentarius Commission
FAO
Viale delle Terme di Caracalla
00100 Rome
Italy
Tel: 39.06.57054796
Fax: 39.06.57054593
E-mail: [email protected]
Consumers International (CI)
Ms Kavitha Anand
Assistant Coordinator
Citizen, Consumer and Civic Action Group (CAG)
8 Fourth Street
Venkateswara Nagar Adyar
Chennai 600 020
India
Tel: 91.44.24460387/24914358
Fax: 91.44.24914358
Email: [email protected]
Ms Alice de Cruz
Programme Officer
Consumers International Asia Pacific Office
5-1 Wisma WIM
7 Jalan Abang Haji Openg
TTDI Kuala Lumpur
Malaysia
Tel: 603.7726.1599
Fax: 603.7726.8599
Email: [email protected]
Mr Ngo Thi Phi Yen
Education and Communication Department
Ho Chi Minh City
Nutrition Center
178 Le Van Sy Street, Ward 10
Phu Nhuan District
HCM City
Viet Nam
Tel: 84.8.8423160
Fax: 84.8.8448405
Email: [email protected]
Crop Life International
Mr Nik Yahaya Abdul Razak
Regulatory Leader ASEAN
Crop Life Asia
28th Floor, Rasa Tower
555 Phaholyyothin Road
Chatuchak, Bangkok 10900
Tel: 603.7969.4309
Fax: 603.7955.6292
Email: [email protected]
Food and Agriculture Organization
of the United Nations (FAO)
Mr Hartwig de Haen
Assistant Director-General
Economic and Social Department
FAO
Viale delle Terme di Caracalla
00100 Rome
Italy
Tel: 39.06.57053566
E-mail: [email protected]
Mr Carlos Eddi
Senior Officer
Animal Health Service
FAO
Viale delle Terme di Caracalla
00100 Rome
Italy
Tel: 39.06.57054159
E-mail: [email protected]
Ms Daniela Battaglia
Animal Production Officer
Agriculture Department
FAO
Viale delle Terme di Caracalla
00100 Rome
Italy
Tel: 39.06.57056773
Fax: 39.06.57055749
E-mail: [email protected]
Mr Biplab Kanti Nandi
Senior Food and Nutrition Officer
FAO Regional Office for Asia and the Pacific
39 Pra Atit Road, Maliwan Mansion
Bangkok 10200, Thailand
Tel: 662.6974.2143
Fax: 662.6974.445
Email: [email protected]
Mr Diderik de Vleeshauwer
FAO Information Officer
FAO Regional Office for Asia and the Pacific
39 Pra Atit Road, Maliwan Mansion
Bangkok 10200, Thailand
Tel?: 662.697.4126
Fax: 662.697.4445
Email: [email protected]
Mr Dirk H. Schulz
Food and Nutrition Officer
Subregional Office for the Pacific Islands
Private Mail Bag Apia
Samoa
Tel: 685.221.27
Fax: 685.221.26
E-mail: [email protected]
Industry Council for Development (ICD)
Dr Rina Agustina-Ahmad
ICD/SEAMEO Associate
ICD/SEAMEO Cooperative Program
SEAMEO TROPMED Regional Center for Community Nutrition
SEAMEO Building, Salemba 4
University of Indonesia
Jakarta 10430
Indonesia
Tel: 62.21.390.9205
Fax: 62.21.391.3933
Email: [email protected]
Ms Georgina Cairns
Executive Director
Asian Food Information Centre
P.O. Box 140 Phrakanong Post Office
Bangkok 10110
Thailand
Email: [email protected]
International Life Sciences Institute (ILSI)
Ms Pauline Chan
Senior Program Manager
ILSI Southeast Asia
1 Newton Road
Golhil Plaza
Podium Block #03-45
Singapore 308899
Tel: 65.6352.5220
Fax: 65.6352.5536
Email: [email protected]
Ms Veronita Rusli
Scientific Program Executive
ILSI Southeast Asia
1 Newton Road
Golhil Plaza
Podium Block #03-45
Singapore 308899
Tel: 65.6352.5220
Fax: 65.6352.5536
Email:
[email protected]
Secretariat of the Pacific Community
Ms Wendy Snowdon
Nutrition Education & Training Officer
Secretariat of the Pacific Community
(SPC), BP D5
Noumea Cedex 98848,
New Caledonia
Tel: 687.2600 00
Fax: 687.2638 18
Email: [email protected]
United Nations Industrial Development Organization (UNIDO)
Mr Karl Schebesta
Industrial Development Officer
Agro Iundustries and Sectoral Support Branch
UNIDO
Vienna International Centre
P.O. Box 300
A-1400 Vienna
Austria
Tel: 431.26026.3490
Fax: 431.21346.3490
Email: [email protected]
World Health Organization (WHO)
Dr Han Tieru
WHO Representative in Malaysia, Brunei Darussalam and Singapore
PO Box 12550
50782 Kuala Lumpur
Malaysia
Tel: 603.2093.9908
Fax: 603.2093.7446
E-mail: [email protected]
Mr Anthony Roy Hazzard
Technical Officer Food Safety
Regional Office for the Western Pacific
P.O. Box 2932
Manila 1000
Philippines
Tel: 632.528.9872
Email: [email protected]
Mr Md. Shamsul Huda
Environmental Health Adviser
Country Office
World Health Organization
UN House
Pulchowk
Kathmandu
Tel: 977.1.552.3993
Fax: 977.1.552.7756
Email: [email protected]
Mr Alexander Hildebrand
Regional Advisor – Food Safety
World Health Organization
South-east Asia Office
Ring Road
New Delhi 110002
India
Ms Cristiana Salvi
Technical Officer for Communication
and Advocacy
WHO Regional Office for Europe
European Centre for Environment and Health - Rome Office
Via Francesco Crispi, 10
00187 Rome
Italy
Tel: 39.06.4877543
Fax: 39.06.4877599
Email: [email protected]
World Organization for Animal Health (OIE)
Dr Yoshiyuki Oketani
Deputy Regional Representative
OIE Regional Representation for Asia and the Pacific
East 311, Shin Aoyama Building
1-1-1 Minami Aoyama, Minato-ku
Tokyo 107-0062
Japan
Tel: 81.3.5433.0520
Fax: 81.3.5411.0526
Email: [email protected]
MALAYSIAN OBSERVERS
Mr Jamal Khair Hashim
Principal Assistant Director
Food Quality Control Division
Ministry of Health Malaysia
Health Offices Complex
Level 3, Block B, Jalan Cenderasari
50590 Kuala Lumpur.
Tel: 603-2694 6601 ext 278
Fax: 603-2694 6517
Email: [email protected]
Ms Nik Shabnam Nik Mohd Salleh
Principal Assistant Director
Food Quality Control Division
Ministry of Health Malaysia
Health Offices Complex
Level 3, Block B, Jalan Cenderasari
50590 Kuala Lumpur.
Tel: 603-2694 6601 ext 203
Fax: 603-2694 6517
Email: [email protected]
Mr Mohd Salim Dulatti
Principal Assistant Director
Food Quality Control Division
Ministry of Health Malaysia
Health Offices Complex
Level 3, Block B, Jalan Cenderasari
50590 Kuala Lumpur.
Tel: 603-2694 6601 ext 130
Fax: 603-2694 6517
Email: [email protected]
Mr Chin Cheow Keat
Principal Assistant Director
Food Quality Control Division
Ministry of Health Malaysia
Health Offices Complex
Level 3, Block B, Jalan Cenderasari
50590 Kuala Lumpur.
Tel: 603-2694 6601 ext 289
Fax: 603-2694 6517
Email: [email protected]
Ms Zaleenah Zainuddin
Principal Assistant Director
Food Quality Control Division
Ministry of Health Malaysia
Health Offices Complex
Level 3, Block B, Jalan Cenderasari
50590 Kuala Lumpur.
Tel: 603-2694 6601 ext 250
Fax: 603-2694 6517
Email: [email protected]
Dr A’aisah Senin
Principal Assistant Director
Food Quality Control Division
Ministry of Health Malaysia
Health Offices Complex
Level 3, Block B, Jalan Cenderasari
50590 Kuala Lumpur.
Tel: 603-2694 6601 ext 132
Fax: 603-2694 6517
Email: [email protected]
Dr Azriman Rosman
Principal Assistant Director
Food Quality Control Division
Ministry of Health Malaysia
Health Offices Complex
Level 3, Block B, Jalan Cenderasari
50590 Kuala Lumpur.
Tel: 603-2694 6601 ext 286
Fax: 603-2694 6517
Email: [email protected]
Ms Norrani Eksan
Principal Assistant Director
Food Quality Control Division
Ministry of Health Malaysia
Health Offices Complex
Level 3, Block B, Jalan Cenderasari
50590 Kuala Lumpur.
Tel: 603-2694 6601 ext 256
Fax: 603-2694 6517
Email: [email protected]
Ms Sharizat Ahmad
Principal Assistant Director
Food Quality Control Division
Ministry of Health Malaysia
Health Offices Complex
Level 3, Block B, Jalan Cenderasari
50590 Kuala Lumpur.
Tel: 603-2694 6601 ext 251
Fax: 603-2694 6517
Email: [email protected]
Dr Haliza Abd Manaf
Assistant Director
Food Quality Control Division
Ministry of Health Malaysia
Health Offices Complex
Level 3, Block B, Jalan Cenderasari
50590 Kuala Lumpur.
Tel: 603-2694 6601 ext 270
Fax: 603-2694 6517
Email: [email protected]
Dr Lokman Rejali
Assistant Director
Food Quality Control Division
Ministry of Health Malaysia
Health Offices Complex
Level 3, Block B, Jalan Cenderasari
50590 Kuala Lumpur.
Tel: 603-2694 6601 ext 228
Fax: 603-2694 6517
Email: [email protected]
Ms Nor Kamilah Mohamad Alwi
Assistant Director
Food Quality Control Division
Ministry of Health Malaysia
Health Offices Complex
Level 3, Block B, Jalan Cenderasari
50590 Kuala Lumpur.
Tel: 603-2694 6601 ext 254
Fax: 603-2694 6517
Email: [email protected]
Ms Norzitah Abu Khair
Assistant Director
Food Quality Control Division
Ministry of Health Malaysia
Health Offices Complex
Level 3, Block B, Jalan Cenderasari
50590 Kuala Lumpur.
Tel: 603-2694 6601 ext 279
Fax: 603-2694 6517
Email: [email protected]
Ms Laila Rabaah Ahmad Suhaimi
Assistant Director
Food Quality Control Division
Ministry of Health Malaysia
Health Offices Complex
Level 3, Block B, Jalan Cenderasari
50590 Kuala Lumpur.
Tel: 603-2694 6601 ext 227
Fax: 603-2694 6517
Email: [email protected]
Ms Fatimah Sulong
Assistant Director
Food Quality Control Division
Ministry of Health Malaysia
Health Offices Complex
Level 3, Block B, Jalan Cenderasari
50590 Kuala Lumpur.
Tel: 603-2694 6601 ext 252
Fax: 603-2694 6517
Email: [email protected]
Ms Nor Aini Muhd Supian
Assistant Director
Food Quality Control Division
Ministry of Health Malaysia
Health Offices Complex
Level 3, Block B, Jalan Cenderasari
50590 Kuala Lumpur.
Tel: 603-2694 6601 ext 131
Fax: 603-2694 6517
Email: [email protected]
Ms Roziaton Abdul Wahab
Assistant Director
Food Quality Control Division
Ministry of Health Malaysia
Health Offices Complex
Level 3, Block B, Jalan Cenderasari
50590 Kuala Lumpur.
Tel: 603-2694 6601
Fax: 603-2694 6517
Email: [email protected]
Mr Shanmugam s/o Supramaniam
Assistant Director
Food Quality Control Division
Ministry of Health Malaysia
Health Offices Complex
Level 3, Block B, Jalan Cenderasari
50590 Kuala Lumpur.
Tel: 603-2694 6601
Fax: 603-2694 6517
Email: [email protected]
Ms Ruhana Abdul Latif
Assistant Director
Food Quality Control Division
Ministry of Health Malaysia
Health Offices Complex
Level 3, Block B, Jalan Cenderasari
50590 Kuala Lumpur.
Tel: 603-2694 6601 ext 253
Fax: 603-2694 6517
Email: [email protected]
Dr Nirmala Amplavanar
Principal Assistant Director
Disease Control Division
Ministry of Health Malaysia
Health Offices Complex
Level 2, Block A, Jalan Cenderasari
50590 Kuala Lumpur.
Tel: 603-2694 6601
Fax: 603-2694 6404
Dr Mariam Mohamad
Assistant Director
Disease Control Division
Ministry of Health Malaysia
Health Offices Complex
Level 2, Block A, Jalan Cenderasari
50590 Kuala Lumpur.
Tel: 603-2694 6601
Fax: 603-2694 6404
Dr Rohani Jahis
Assistant Director
Disease Control Division
Ministry of Health Malaysia
Health Offices Complex
Level 2, Block A, Jalan Cenderasari
50590 Kuala Lumpur.
Tel: 603-2694 6601
Fax: 603-2694 6404
Dr Norlen Mohamed
Assistant Director
Disease Control Division
Ministry of Health Malaysia
Health Offices Complex
Level 2, Block A, Jalan Cenderasari
50590 Kuala Lumpur.
Tel: 603-2694 6601
Fax: 603-2694 6404
Mr Ishak Jaidin
Senior Assistant Principal Environmental Health Officer
Disease Control Division
Ministry of Health Malaysia
Health Offices Complex
Level 2, Block A, Jalan Cenderasari
50590 Kuala Lumpur.
Tel: 603-2694 6601
Fax: 603-2694 6404
Dr Yusingh Muhamad
Epidemiology Officer
State Health Dept of Negeri Sembilan
Jalan Lee Sam, Seremban
Fax: +606-7675 506
Dr Khairiah Ibrahim
Epidemiology Officer
State Health Dept of W. Persekutuan
Health Offices Complex
Level 2, Block A, Jalan Cenderasari
50590 Kuala Lumpur.
Tel: 603-2694 6601
Mrs Nurul Izzah Ahmad
Researcher
Environmental Health Research Center
Institute for Medical Research
Jalan Pahang
50588 Kuala Lumpur
Tel: 603-4040 2469
Fax: 603-4040 2431
Email: [email protected]
Mr Ibrahim Baba
Chief Assistant Principal Environmental Health Officer
Ministry of Health
Health Offices Complex
Level 1, Block B, Jalan Cenderasari
50590 Kuala Lumpur.
Tel: 603-2694 6601
Fax: 603-2694 6602
Email: [email protected]
Dr Arumugam Lingam Selladurai
Director
National Public Health Laboratory
Lot 1853, Kampung Melayu
47000 Sungai Buloh, Selangor
Tel: 603-6156 5109 ext 1000
Fax: 603-6157 1036
Dr Palaniyappan Etty Goundar
Deputy Director
State Health Deparment of Perak
Jalan Panglima Bukit Gantang Wahab
30000 Ipoh, Perak
Tel: 605-2540 284
Fax: 605-2427 564
Email: [email protected]
Dr Anwa Sulaiman
Deputy Director
State Health Dept of Terengganu
Level 5, Wisma Persekutuan
Jalan Sultan Ismail
20920 Kuala Terengganu
Tel: 609-6222 866
Fax: 609-6245 829
Email: [email protected]
Dr Ismail Abu Taat
Deputy Director
State Health Department of Kedah
Jalan Perak off Jalan Putera
05150 Alor Setar, Kedah
Tel: 604-7335 533
Fax: 604-7306 421
Email: [email protected]
Dr Lila P Mohamed Meeran
Deputy Director
State Health Department of Kelantan
Level 5, Wisma Persekutuan
Jalan Bayam
15590 Kota Bharu, Kelantan
Tel: 609-7483 288
Fax: 609-7472 818
Email: [email protected]
Mr Kamarudzaman Ahmad
Food Technologist
Makmal Kawalan Mutu Makanan
Laka Temin, Bukit Kayu Hitam
Kedah Darul Aman
Tel: 604-9222 873
Fax: 604-9222 873
Email: [email protected]
Ms Sanimah Abd Rahman
Food Technologist
State Health Department of Melaka
Level 6, Wisma Persekutuan
Jalan Hang Tuah
75300 Melaka
Tel: +606-2828 344
Fax: +606-2839 233
Mr Mohamad Jefry Crossley
Food Technologist
State Health Dept of Negeri Sembilan
Jalan Lee Sam, Seremban
Tel: +019-3022 120
Fax: +606-7675 506
Email: [email protected]
Mr Ahmad Nadzri Sulaiman
Food Technologist
State Health Department of Kelantan
Level 5, Wisma Persekutuan
Jalan Bayam
15590 Kota Bharu, Kelantan
Tel: 609-7483 288
Fax: 609-7472 818
Email: [email protected]
Mr Fadzil Othman
Food Technologist
National Public Health Laboratory
Lot 1853, Kampung Melayu
47000 Sungai Buloh, Selangor
Tel: 603-6156 5109
Fax: 603-6140 2249
Email: [email protected]
Mr Abdullah Ma'amor
Assistant Secretary
Ministry of Foreign Affairs
No 1, Jalan Wisma Putra, Presint 2
62602 Putrajaya
Tel: 603-8887 4441
Fax: 603-8889 2788
Email: [email protected]
Ms Fauziah Arshad
Senior Research Officer
Malaysia Palm Oil Board (MPOB)
No 6, Persiaran Industri
Bandar Baru Bangi
43000 Kajang, Selangor
Tel: 603-8925 9432
Fax: 603-8920 1918
Email: [email protected]
Mr Mohd Fairus Mohd Hidzir
Research Officer
Malaysia Palm Oil Board (MPOB)
No 6, Persiaran Industri
Bandar Baru Bangi
43000 Kajang, Selangor
Tel: 603-8925 9432
Fax: 603-8920 1918
Email: [email protected]
Mr Jumali Suratman
Senior Research Officer
Cocoa Board Malaysia
Lot 3, Jalan P/9B, Seksyen 13
43650 Bandar Baru Bangi, Selangor
Tel: 603-8926 7800
Fax: 603-8925 5386
Email: [email protected]
Ms Chin Hui Han
Research Officer
Cocoa Board Malaysia
Lot 3, Jalan P/9B, Seksyen 13
43650 Bandar Baru Bangi, Selangor
Tel: 603-8926 7800
Fax: 603-8925 5386
Email: [email protected]
Mr Zehnder Jarroop
Science Officer
Pepper Marketing Board
P.O. Box 1653
93916 Kuching, Sarawak
Tel: 6082-331811
Fax: 6082-336877
Mr Hussain Moh
Principal Assistant Director
Ministry of International Trade and Industry (MITI)
Level 8, Block 10, Jalan Duta
50622 Kuala Lumpur
Tel: 603-6203 4690
Fax: 603-6203 1303
Email: [email protected]
Ms Aedreena Reeza Alwi
Assistant Secretary
Ministry of Agriculture and Aero-Based Industries
International Section, Wisma Tani
Jalan Sultan Salahuddin
50624 Kuala Lumpur
Tel: 603-2617 5069
Fax: 603-2694 6500
Email: [email protected]
Ms Faridah Aini Muhammad
Assistant Director
Department of Agriculture
Level 5-7, Wisma Tani
Jalan Sultan Salahuddin
50624 Kuala Lumpur
Tel: 603-2617 5422
Fax: 603-2694 7151
Email: [email protected]
Ms Norma Othman
Assistant Director
Department of Agriculture
Level 5-7, Wisma Tani
Jalan Sultan Salahuddin
50624 Kuala Lumpur
Tel: 603-2617 5449
Fax: 603-2691 8563
Email: [email protected]
Mr Arpan Shah Satu
Director, Agribusiness and Industry
Farmers' Organization Authority
Block C North, Damansara Town Centre, Damansara Heights
P.O. Box 11005
50990 Kuala Lumpur
Tel: 603-2096 2113
Fax: 603-2095 4239
Email: [email protected]
Ms Norma Mohd Salleh
Director
Lembaga Pemasaran Pertanian Persekutuan (FAMA)
Bangunan FAMAPOINT
Lot 17304 Jalan Persiaran Satu
Bandar Baru Selayang
68100 Batu Caves, Selangor
Tel: 603-6138 1304
Fax: 603-6120 4685
Email: [email protected]
Ms Thalathiah Haji Saidin
Director
Fish Quarantine Management and Quality
Assurance
Department of Fisheries Malaysia
Wisma Tani
Jalan Sultan Salahuddin
50624 Kuala Lumpur
Tel: 603-2617 5616
Fax: 603-2698 0227
Email: [email protected]
Ms Badariah Mohd Ali
Head
Fish Quality Control Section
Department of Fisheries
Wisma Tani
Jalan Sultan Salahuddin
50624 Kuala Lumpur
Tel: 603-2617 5623
Fax: 603-2698 0227
Email: [email protected]
Ms Shamsiah Muhammad
Director
Pesticide Control Division
Department of Agriculture
Jalan Gallagher
50480 Kuala Lumpur
Tel: 603-2697 7220/1
Fax: 603-2697 7225
Email: [email protected]
Mr Yeoh Ngoh Sum
Assistant Director
Pesticide Control Division
Department of Agriculture
Jalan Gallagher
50480 Kuala Lumpur
Tel: 603-2697 7240
Fax: 603-2697 7225
Email: [email protected]
Dr Noor Zaleha Awang Saleh
Science Officer
Department of Chemistry
Jalan Sultan
46661 Petaling Jaya, Selangor
Tel: 603-7985 3146
Fax: 603-7955 6764
Email: [email protected]
Ms Maimunah Yeop Kamaruddin
Science Officer
Department of Chemistry
Jalan Sultan
46661 Petaling Jaya, Selangor
Tel: 603-7985 3033/22
Fax: 603-7955 6764
Email: [email protected]
Mr Hussal Mizzar Hussain
Assistant Director
Department of Standards Malaysia
Level 1 & 2, Block C4, Parcel C
62662 Putrajaya
Tel: 603-8885 8182
Fax: 603-8889 4200
Email: [email protected]
Mr Abd Rahim Dal
Jabatan Kerajaan Tempatan
Blok K, Aras 4
Pusat Bandar Damansara
50644 Kuala Lumpur
Tel: 603-2099 2447
Fax: 603-2094 8996
Email: [email protected]
Dr Noraini Mohd Khalid
Deputy Director
Malaysian Agriculture Research and Development Institute (MARDI)
P.O. Box 12301
50774 Kuala Lumpur
Tel: 603-8943 7722
Fax: 603-8942 2906
Email: [email protected]
Prof. Mayda Dr Nik Ismail B. Nik Daud
President
Malaysian Institute of Food Technology (MIFT)
D/A Unit Penyelidikan Makanan
Universiti Kebangsaan Malaysia
43600 UKM Bangi
Selangor
Mr Mohd Khairuddin Md Tahir
Chief Executive Officer
International Tropical Fruits Network
P.O. Box 334, UPM Post Office
43400 Serdang, Selangor
Tel: 603-8941 6589/90
Fax: 603-8941 6591
Email: [email protected]
Ms Noraza Aziz
Manager
SMIDEC
701 D, Level 7, Tower D, Uptown 5
No 5, Jalan SS 1/39
Damansara Uptown
47400 Petaling Jaya, Selangor
Tel: 603-7628 7400
Fax: 603-7660 1919
Email: [email protected]
Mr Reza Kamal Ramli
SMIDEC
701 D, Level 7, Tower D, Uptown 5
No 5, Jalan SS 1/39
Damansara Uptown
47400 Petaling Jaya, Selangor
Tel: 603-7628 7400
Fax: 603-7660 1919
Email: [email protected]
Dr Muhamad Lebai Juri
Operation Manager
SINAGAMA
Mint Tech Park
Malaysian Institute for Nuclear
Technology Research
43000 Bangi, Selangor
Tel: 603-8920 2097
Fax: 603-8920 1099
Mr Che Hassan Pahmi Che Mamat
Director
Islamic Applied & Food Research Div
Jabatan Kemajuan Islam Malaysia
Aras 1, Blok D7, Pusat Pentadbiran Kerajaan Persekutuan
62519 Putrajaya
Tel: 603-8888 4135 / 8886 4043
Fax: 603-8889 4951
Email: [email protected]
Dr Puziah Hashim
Senior Manager
SIRIM QAS International
No 1, Persiaran Dato' Menteri
40911 Shah Alam, Selangor
Tel: 603-5544 6655/52
Fax: 603-5544 6688
Email: [email protected]
Ms Seri Azalina Mohd Ghazali
Senior Executive
National Standard Development Section
SIRIM Berhad
No 1, Persiaran Dato' Menteri
40911 Shah Alam, Selangor
Tel: 603-5544 6914
Fax: 603-5510.6389
Email: [email protected]
Ms Zainorni Mohd Janis
Senior Executive
National Standard Development Section
SIRIM Berhad
No 1, Persiaran Dato' Menteri
40911 Shah Alam, Selangor
Tel: 603-5544 6001
Fax: 603-5510 6389
Email: [email protected]
Prof Dr Aminah Abdullah
Deputy Dean
Faculty of Science and Technology
Universiti Kebangsaan Malaysia
43600 Bangi, Selangor
Tel: 603-8921 5420
Fax: 603-8925 6086
Email: [email protected]
Assoc Prof Dr Mohd Khan Ayob
Lecturer (Food Science Programme)
Faculty of Science and Technology
Universiti Kebangsaan Malaysia
43600 Bangi, Selangor
Tel: 603-8921 5992
Fax: 603-8921 3232
Email: [email protected]
Assoc Prof Dr Rokiah Mohd Yusof
Lecturer
Faculty of Medicine & Health Sciences
Universiti Putra Malaysia
43300 Serdang, Selangor
Tel: 603-8946 8494
Fax: 603-8942 6769
Email: [email protected]
Prof Dr Thong Kwai Lin
Lecturer (Microbiology)
Faculty of Science
Universiti Malaya
50603 Kuala Lumpur
Tel: 603-7967 4437
Fax: 603-7967 5908
Email: [email protected]
Assoc Prof Datin Dr Toh Poh See
Lecturer (Food Safety / Public Health)
Faculty of Hotel and Tourism Mgt /
Faculty of Applied Science
Universiti Technologi MARA
40450 Shah Alam, Selangor
Tel: 603-5543 5663
Fax: 603-2096 2626
Email: [email protected]
Dr Siti Noorbaiyah Abd Malek
Lecturer
Faculty of Applied Sciences Technologi
Universiti MARA
40450 Shah Alam, Selangor
Tel: 603-5544 4611
Fax: 603-5544 4562
Email: [email protected]
Mr Azahar Amir
Assistant Principal Environmental Health Officer
Health Department
Dewan Bandaraya Kuala Lumpur
KM 4, Jalan Cheras
56100 Kuala Lumpur
Tel: 603-9284 5166 ext 210
Fax: 603-9200 6441
Email: [email protected]
Mr Norhisham Mohammad
Senior Assistant Principal Environmental Health Officer
Health Department
Majlis Bandaraya Shah Alam
Level 4, Wisma MBSA
Persiaran Perbandaran
40000 Shah Alam, Selangor
Tel: 603-5510 5133 ext 270
Fax: 603-5512 7014
Email: [email protected]
Dr Tee E Siong
President
Nutrition Society of Malaysia (NSM)
c/o 46, Jalan SS 22/32
47400 Petaling Jaya, Selangor
Tel: 603-7728 7287
Fax: 603-7728 7426
Email: [email protected]
Dr Amin Ismail
Council Member
Nutrition Society of Malaysia (NSM)
c/o Faculty of Medicine and Health Sciences
Universiti Putra Malaysia
43400 Serdang, Selangor
Tel: 603-8946 8557
Fax: 603-8942 6769
Email: [email protected]
Mr Danny Wee Kong Heng
Malaysia Association of Environmental Health (MAEH)
c/o Public Health Institute
Jalan Bangsar
50590 Kuala Lumpur
Tel: 603-2284 8160
Fax: 603-2284 5644
Mr Koris Atan
Vice President
Federation of Malaysian Consumers Association (FOMCA) Penang
28, Lintang Pekaka 4, Sungai Dua
11700 Penang
Tel: 604-6575 406
Fax: 604-6590 752
Email: [email protected]
Ms Indrani Thuraisingham
Legal Advisor
Federation of Malaysian Consumers Association (FOMCA)
No 24, Jalan SS 1/22A
47300 Petaling Jaya, Selangor
Tel: 603-7877 4741
Fax: 603-7873 0636
Email: [email protected]
Ms Zalifah Mohd. Kasim
Lecturer
Food Science Program
School of Chemical Sciences and Food Technology
Faculty of Science and Technology
Universiti Kebangaan Malaysia
43600 UKM Selangor
Tel: 603.8921.5991
Fax: 603.8921.3232
Email: iffa.pkrisc.cc.ukm.my
Dr Norrakiah Abdullah Sani
Lecturer
Food Science Program
School of Chemical Sciences and Food Technology
Faculty of Science and Technology
Universiti Kebangaan Malaysia
43600 UKM Selangor
Tel: 603.8921.4053
Fax: 603.8921.3232
Email: [email protected]
Assoc. Prof Dr. Ayub Yabno
Lecturer
Food Science Program
School of Chemical Sciences and Food Technology
Faculty of Science and Technology
Universiti Kebangaan Malaysia
43600 UKM Selangor
Tel: 603.8921.5963
Fax: 603.8921.3232
Email: [email protected]
Dr Sharifah Nor Ahmad
Assistant Director
Food Quality Control Division
Ministry of Health
Health Offices Complex
Level 3, Block B, Jalan Cenderasari
50590 Kuala Lumpur
Tel: 603-2694 6601
Fax: 603-2694 6517
Email: [email protected]
Dr Devan Kurup
Deputy Director
Disease Control Division
Ministry of Health Malaysia
Health Offices Complex
Level 2, Block A, Jalan Cenderasari
50590 Kuala Lumpur
Tel: 603-2694 6601
Fax: +2694 6404
Mr Abdullah Hisham Ahmat Yaya
Pharmacist
National Pharmaceutical Control Bureau
Jalan Universiti, P.O. Box 319
46730 Petaling Jaya, Selangor
Tel: 603-7957 3611
Fax: 603-7956 7075
Email: [email protected]
Ms Siti Aida Abdullah
Principal Assistant Director
Pharmaceutical Services Division
Jalan Universiti, P.O. Box 319
46730 Petaling Jaya, Selangor
Tel: 603-7968 2200
Fax: 603-7968 2251
Email: [email protected]
Ms Zawiah Ahmad
Research Officer
Institute for Medical Research
Jalan Pahang
50588 Kuala Lumpur
Tel: 603-4040 2388
Fax: 603-2694 3575
Email: [email protected]
Dr Mohd Yusof Haji Ibrahim
Deputy Director
State Health Department of Sabah
1st Floor, Rumah Persekutuan, PS 11290
88814 Kota Kinabalu, Sabah
Tel: 6088-256749
Fax: 6088-217740
Email: [email protected]
Dr Zainal Che Mee
Health Officer
Pejabat Kesihatan Pintu Masuk
Blok C, Kompleks LKIM
06050 Bukit Kayu Hitam, Kedah
Tel: 604-9221 157
Fax: 604-9221 491
Email: [email protected]
Ms Wee Bee Wah
Food Technologist
State Health Department of Selangor
Level 10 & 11, Wisma Masalam
Jalan Tengku Ampuan Zabedah C 9/C
40100 Shah Alam, Selangor
Tel: 603-5518 6023
Fax: 603-5512 6695
Mr David Lau Chien Loung
Food Technologist
State Health Department of Sarawak
Tel: +6082-417995
Fax: +6082-258849
Email: [email protected]
Ms Norlida Md Darus
Food Technologist
State Health Department of Sabah
1st Floor, Rumah Persekutuan, PS 11290
88814 Kota Kinabalu, Sabah
Tel: 6088-217408/412
Fax: 6088-217740
Email: [email protected]
Mr Shamsuddin Ahmad
Food Technologist
State Health Department of Sabah
1st Floor, Rumah Persekutuan, PS 11290
88814 Kota Kinabalu, Sabah
Tel: 6088-217408/412
Fax: 6088-217740
Email: [email protected]
Ms Sitti Haji Aralas
Food Technologist
State Health Department of Terengganu
Tingkat 5, Wisma Persekutuan
Jalan Sultan Ismail
20200 Kuala Terengganu, Terengganu
Tel: 609-6222 866
Fax: 609-6235 001
Email: [email protected]
Ms Zalilah Nasir
Food Technologist
State Health Department of Johor
4th Floor, Block B, Wisma Persekutuan
Jalan Air Molek
80590 Johor Bharu, Johor
Tel: +607-2245 188
Fax: +607-2277 577
Email: [email protected]
Mr Chandran Thangayah
Food Technologist
State Health Department of Selangor
Level 10 & 11, Wisma Masalam
Jalan Tengku Ampuan Zabedah C 9/C
40100 Shah Alam, Selangor
Tel: 603-5518 6001
Fax: 603-5512 6695
Email: [email protected]
Mr Hermy Mohd Yeet
Food Technologist
Public Health Institute, Bangsar
Tel: 603-2297 9491
Fax: 603-2282 3114
Email: [email protected]
Ms Karitha Anand
Researcher
Citizen Consumer and Civic Action Group (CAG)
No. 8 4th Street
VenkaTESWANA Nagar
Adyar Chennai – 600020
Tel: 91.44.24460387
Fax: 91.44.24914358
Email: [email protected]
Mr Azhar Ahmad
Food Technologist
State Health Department of Perlis
Tel: 012-4709 424
Fax: 604-9760 764
Email: [email protected]
Mr Abdul Ghani Abu Samah
Food Technologist
Level 1 & 2, Bangunan Hospital Lama
41000 Klang, Selangor
Tel: 603-3371 8822
Fax: 603-3373 7154
Email: [email protected]
Ms Afiedah Munir
Food Technologist
Food Quality Control Laboratory
State Health Department of Sabah
1st Floor, Rumah Persekutuan, PS 11290
88814 Kota Kinabalu, Sabah
Tel: 6088-761007
Fax: 6088-762473
Email: [email protected]
Ms Juliana Jaafar
Food Technologist
Public Health Laboratory Ipoh
Jalan Jelapang
30020 Ipoh, Perak
Tel: 605-5287 829
Fax: 605-5287 836
Mr Wan Azuan Wan Omar
Environmental Officer
Department of Environmental
Level 3-7, Block C4
62662 Putrajaya
Tel: 603-8885 8200 ext 8447
Fax: 603-8889 1975
Email: [email protected]
Dr Muhmad Kamarulzaman Muhmad Sarif
Director
Veterinary Public Health Division
Department of Veterinary Services
8th Floor, Wisma Chase Perdana
Bukit Damansara, Off Jalan Semantan
50630 Kuala Lumpur
Tel: 603-2094 0367
Fax: 603-2093 5804
Email: [email protected]
Dr Maznah Ahmad
Director
Veterinary Public Health Laboratory
Department of Veterinary Services
Jalan Nilai Banting
Bandar Baru Salak Tinggi
43900 Sepang, Selangor
Tel: 603-8706 8681/6762
Fax: 603-8706 8675
Email: [email protected]
Dr Moktir Singh Gardir Singh
Veterinary Officer
Department of Veterinary Services
8th Floor, Wisma Chase Perdana
Bukit Damansara, Off Jalan Semantan
50630 Kuala Lumpur
Tel: 603-2094 0077
Fax: 603-2093 5804
Email: [email protected]
Dr Abidin Hamid
Deputy Director
Biotechnology Center
MARDI, GPO Box 12301
50774 Kuala Lumpur
Tel: 603-8943 7743
Fax: 603-8942 2906
Email: [email protected]
Ms Normah Omar
Deputy Director
Food Technology Research Institute
MARDI, GPO Box 12301
50774 Kuala Lumpur
Tel: 603-8943 7160
Fax: 603-8942 2906
Email: [email protected]
Ms Faridah Mohd Som
Research Officer
Food Technology Research Institute
MARDI, GPO Box 12301
50774 Kuala Lumpur
Tel: 603-8943 7737
Fax: 603-8942 2906
Email: [email protected]
Ms Hasimah Hafiz Ahmad
Research Officer
Food Technology Research Institute
MARDI, GPO Box 12301
50774 Kuala Lumpur
Tel: 603-8943 7502
Fax: 603-8942 2906
Email: [email protected]
Mr Madzlan Kasran
Research Officer
Food Technology Research Institute
MARDI, GPO Box 12301
50774 Kuala Lumpur
Tel: 603-8943 7504
Fax: 603-8942 2906
Email: [email protected]
Mr Abu Hanipah Abdul Rahman
Senior Assistant Director
Jabatan Kastam
Wisma Kastam, Jalan Paroi-Senawang
Karung Berkunci No 16
70450 Seremban
Tel: +606-6793 700
Fax: +606-6793 666
Prof Dr Gulam Rusul Rahmat Ali
Deputy Dean
Sekolah Pengajian Siswazah
Zon 4, Off Jalan Stadium
43400 UPM Serdang, Selangor
Tel: 603-8946 4204/5/6
Fax: 603-8946 4233
Email: [email protected]
Assoc Prof Dr Zaiton Hassan
Lecturer
Faculty of Science and Biotechnology
Universiti Putra Malaysia
43400 Serdang, Selangor
Tel: +019-255 4029
Email: [email protected]
Ms Nor Ilyani Mohamed Nazar
Pharmacist
Pusat Racun Negara
Universiti Sains Malaysia
11800 Minden, Penang
Tel: 604-657 0099
Fax: 604-656 8417
Email: [email protected]
Mr Azman Husain
Lecturer
Universiti Teknologi MARA
Jalan Othman
46000 Petaling Jaya, Selangor
Tel: 603-7965 2011
Fax: 603-7695 2012
Dr Clemente Michael Wong Vui Ling
Biotechnology Research Institute
Universiti Malaysia Sabah
Locked Bag 2073
88999 Kota Kinabalu, Sabah
Tel: +012-233 5056
Fax: 6088-320993
Email: [email protected]
FAO/WHO Secretariat
Mr Ezzeddine Boutrif
Senior Officer
Food Quality and Standard Service
Food and Nutrition Division
FAO
Viale delle Terme di Caracalla
00100 Rome
Italy
Tel: 39.06.57056156
Fax: 39.06.57054593
Email: Ezzeddine.Boutrif @fao.org
Mr Peter Karim BenEmbarek
Food Safety Department
WHO
20 Avenue Appia
CH-1211 Geneva 27
Switzerland
Tel: 41.22.791.4204
Fax: 41.22.791.4807
Email: [email protected]
Ms Londa Vander Wal
Associate Professional Officer
Food Quality and Standard Service
Food and Nutrition Division
FAO
Viale delle Terme di Caracalla
00100 Rome, Italy
Tel: 39.06.57055636
Fax: 39.06.57054593
Email: [email protected]
Ms Jenny Murcott
Food Safety Department
WHO
20 Avenue Appia
CH-1211 Geneva 27
Switzerland
Tel: 41.22.791.3557
Fax: 41.22.791.4807
Email: [email protected]
粮农组织/世界卫生组织亚洲及太平洋区域食品安全会议
第一次筹备会议
2003年4月29日星期三14:00时
罗马粮农组织总部马来西亚厅(B-227)
引 言
粮农组织/世界卫生组织亚洲及太平洋区域食品安全会议(下称“区域会议”)第一次筹备会议于2003年4月29日在粮农组织总部举行。除哈萨克斯坦、缅甸、新西兰和斯里兰卡以外,亚洲及太平洋区域所有被邀请的国家(在罗马派驻代表处的国家)出席了会议(见附件1的与会者名单)。粮农组织经济及社会部助理总干事德汉(Hartwig
de Haen)先生代表粮农组织和世界卫生组织欢迎各位代表与会,并强调了交流共同关注的问题和协同努力对改进亚洲及太平洋各国食品安全的重要性。
德汉先生忆及2002年1月举行的第一届全球食品安全管理人员论坛、2002年2月举行的欧洲食品安全会议以及2004年即将举行的第二次全球论坛。他还指出了粮农组织理事会第一二三届会议关于其他区域食品安全会议的建议、亚洲食典协调委员会第十三届会议的建议以及马来西亚表示有兴趣作为区域食品安全会议的东道国。根据这些背景情况,粮农组织召开本次会议,争取该区域各国确认有兴趣召开这届区域会议,并就区域会议的内容和组织事项提供指导,讨论为这项活动筹集资金。世界卫生组织也将于2003年5月份或6月初在日内瓦与常驻世界卫生组织使团召开一次类似的会议。
确认有兴趣召开这届区域会议
代表们表示普遍支持召开区域会议的提议,并欢迎有机会讨论这一事项,对马来西亚政府表示愿意作为拟议召开的区域会议的东道国表示赞赏。本次会议就控制区域会议的费用向秘书处提供了一些想法,并为降低总开支提供实物和预算捐助(见本报告议题8和附件4经过修正的预算)。代表们表示希望创造性地解决区域会议的其余资金需要。到2003年6月底食品法典委员会会议召开时将通知各国秘书处是否为区域会议筹足资金。
讨论工作文件
1) 会议目的
本次会议强调了以下活动的重要性,即区域会议考虑到粮农组织理事会的指导,并得出各国政府可利用的实际结果和开展能力建设行动,但未必提出政策建议,因为这将重复其他论坛如食品法典委员会的目的。
对区域会议目的的修改如附件2所示。
2) 草拟的会议议程和时间表
会议讨论了拟议的主题并提出了修正和改进的建议。修改后的主题列在附件2中。会议认为应当连同第一次筹备会议报告向本区域所有政府分发这些经过修改的主题以征求意见。主题名单一旦确定,将与区域各国协商以便自愿编写有关文件。此外,秘书处将寻找编写其中某些主题文件的顾问。
本次会议讨论了区域会议的形式。得出的一致意见是由各国政府/顾问就四项主题包括各项主题中列出的各点组织四场全会发言。列入名单但全体会议未加讨论的任何主题应通过参考文件,包括任何有关的会前出版物加以讨论。文件可由一个以上的国家联合编写,或由非政府组织撰稿。
本区域各国可编写会议厅文件,论述与会议主题有关的国家形势。这些文件可在会前提供给与会者。各国也可组织会外活动或设立宣传画亭,介绍为解决具体的食品安全问题而执行的计划和行动事例。如果愿意,在为期三天的会议之前或之后,可在未得到粮农组织/世界卫生组织秘书处支持的情况组织非正式会议。
会议表示普遍支持召开两个工作组会议的建议。这两个工作组可各自涉及两个问题,每届会议讨论一个问题。因此,全会所有四项主要主题的实际行动要点可在工作组中提出。最好每个国家派两名代表参加会议,从而使所有国家都能够对工作组的讨论作出贡献。
在讨论工作组主题的挑选问题时,会议认为这些主题应使各国有机会就改善食品安会提出国家和区域两级的具体行动建议。会议认为“C)能力建设活动的优先次序和协调”和“D)信息交流、教育和宣传”这些主题提供这种机会。
会议认为消费者权利、增强消费者意识和生产者义务等问题需要包括在这些主题中加以处理。
3) 与会者
出席区域会议的代表应既代表国家食品安全系统的技术方面又代表其政策方面。代表的资望应足以影响决策,但又仍然接近食品安全措施的实际应用。
会议同意本区域以外的国家派代表以观察员身份列席区域会议。仅允许得到粮农组织承认的(国际)非政府组织作为观察员列席会议,而国家非政府组织可作为其国家代表团的一部分参加会议。应邀请东盟和其他有关政府间区域组织作为观察员参加会议。
建议每个国家限派5名代表和每个观察员国家/组织限派2名观察员。出席区域会议的正式与会者总数不应超过200名。对马来西亚来说,除了5名官方代表之外,将允许大约100-150人作为观察员列席会议。
4) 会议的语言
会议审议了仅使用英语组织区域会议来削减费用及后勤等事宜。一些代表强调使本区域所有国家能够充分参与的重要性。中国代表指出他无法在本次会议上就区域会议的语言问题发表任何官方意见,他将与其政府联络以期得到有关这一事项(无中文)的指示。
5) 文 献
澳大利亚愿意对有关以下主题的文件编写和发言者作贡献:A) 食品安全法规-科学和以风险为基础的协调方法和B) 风险分析在食物控制中的应用-挑战和利益或E)
食源疾病的检测和监视系统。日本也表示愿意对会议筹备活动作知识性贡献。秘书处将与本区域所有国家联络以争取它们在文献方面作出贡献。
6) 会议的长短、日期和地点
本次会议商定区域会议将在马来西亚举行。东道国的具体责任需要在将由秘书处编写的“责任备忘录”中明确界定。
马来西亚建议于2004年5月25日-27日在吉隆坡召开为期三天的会议,或者于2004年5月24日-27日召开为期四天的会议。如果使用两种语言组织会议,因而需要翻译报告草案,则可能需要第四天。
7) 筹备活动的时间表
见附件3。
8) 所需支持
附件4提供了经过修订的概算,它考虑到与会者提出的限制会议开支的各项建议。秘书处将与潜在的捐助者联络争取他们支持为会议提供资金。
会议强调,除了直接财政支持之外,各国将提供(i)
临时借调人员以协助区域会议的筹备,(ii) 通过编文件提供支持,或(iii) 为低收入国家代表的旅行提供直接支持。
马来西亚表示愿意为区域会议文件的印刷提供经费(估计费用为500美元)和会议厅、设备、咖啡、服务员、接待和本地交通(不包括住宿)等地方费用,估计为65000-70000美元。马来西亚同意努力为与会者安排费用较低的住宿。马来西亚还表示愿意在会议之前和会议期间提供一名联络官员,会议筹备期间该官员最好在马来西亚而不是在罗马。筹备会议与会者表示赞赏马来西亚的这些表示。
正如报告前面所指出的那样,其他国家也为会议文件编写提供实物支持。
会议建议中等收入国家可支持由其挑选的或通过秘书处安排的至少一个低收入国家的一名或二名代表的旅行。
其他事项
会议承认国家一级事先筹备以提高各国参与会议的质量的重要性。为了促进这项活动,建议向本区域粮农组织驻国家代表处/世界卫生组织代表处发送一份通函,争取他们积极协助筹备过程(即讨论会/会议)。这应当由从事国家一级食品安全活动的所有政府机构和非政府组织参加。为使代表做好充分准备和确定各国可能编写的会议厅文件,这些讨论会/会议最好在区域会议前约六个月时举行,即2003年10月-11月举行。粮农组织/世界卫生组织将不大可能为此类讨论会/会议提供资金;然而,可鼓励在本区域旅行的粮农组织/世界卫生组织的官员出席这些活动。
会议指出,食品法典委员会下届会议(2003年6月30日-7月7日,罗马)和第二届全球论坛的筹备会议(2003年7月8日,罗马),将为组织也在出席这些会议的常驻代表和国家食品安全官员召开第二次筹备会议提供良好的机会。
本次会议于2003年3月29日16:40时结束。
粮农组织/世卫组织亚太区域食品安全会议
第二次准备会议报告
2003年7月3日,星期四14:30
粮农组织罗马总部,马来西亚厅(B-227)
引 言
粮农组织/世卫组织亚太区域食品安全会议的第二次准备会议于2003年7月3日在粮农组织总部举行。代表亚太区域14个国家的26名代表参加了这次会议(见附件1出席会议人员名单)。粮农组织经济与社会部助理总干事哈特威格·德汉先生代表粮农组织和世卫组织欢迎各位代表出席会议。
德汉先生回顾了粮农组织第123和124届理事会关于召开区域食品安全会议的建议,以及马来西亚表示愿意作为大会东道主的意愿。第一次准备会议的主要成果强调如下:总体支持召开大会的建议;赞赏马来西亚政府愿意作为大会东道主的提议;有关大会经费安排的意见;为减少整个大会费用的有关实物和预算支持承诺。会议还注意到粮农组织第124届理事会关于大会应当使用的区域性官方语言的建议(中文和英文)。
大会主题
会议强调了举行区域食品安全会议的重要意义,即加强区域内各国之间有关实质性食品安全问题的经验交流和信息交换,推动制订有关克服区域内各国在完善各自食品安全体系过程中所面临的实际困难和问题的具体行动计划。一些代表指出,应当把那些低成本、易操作的切实行动置于高度优先的会议主题来进行讨论,而不仅仅限于对理论问题的探讨,主题应包括:“C)能力建设活动的重点与协调”;“D)信息交换、教育与情况通报”以及“E)食源性疾病的监督与监测体系”。会议注意到,在准备每份大会文件时,应考虑大会主题所产生的结果。为了控制大会的成本,提高区域主人翁意识,会议同意由区域内有关国家,而不是外聘专家,准备大会文件。会议鼓励区域内各国向大会文件处(CRDs)提出与大会主题相关的、各自国家感兴趣的具体问题(例如:伊朗代表团提出,关于杀虫剂施用设备对粮食作物安全的影响)。会议鼓励各国在准备文件方面做出表率,或对任何尚无牵头国的主题主动做出贡献。
会议同意,把消费者权益、增强消费者意识和生产者责任等问题列入大会的相关主题进行详细讨论。
A) 食品安全立法—利用基于科学和风险的方法实现协调一致
澳大利亚同意准备这份文件并将在大会上就该议题带头发言。该文件将着重介绍执行和强化食品安全立法方面的一些实际经验,从而为其他国家在制定各自的食品安全法律方面提供帮助。这份报文件强调协调国家立法的重要意义,而这种国家立法的协调可能会最终导致区域内各国立法的一致性和趋同性,这些是对整个区域都十分重要的问题。此文件还应当包括有关消费者权益和参与等内容。文件中包含的有关基于风险的食品安全立法内容,将对有关食品管理中应用风险分析的其他文件进行补充而非重复。
这份文件可对该区域其他国家的食品安全立法状况作一个简短的评述,但不应是对所有国家的食品法律进行综合评述。应鼓励各国通过向大会文件处(CRDs)提交有关文件,以交流在此方面的经验。
这份文件将简要地论及食品安全立法对经济的影响,其详细情况将在其他的背景文件中详尽论述。最近几年,经合发组织(OECD)已经就这个题目准备了大量的报告,有些报告可以被用作此目的。
会议建议,把兼备食品/药品作用的产品所需立法情况列入此主题。秘书处通知会议,此问题有待食品法典委员会(CAC)第26届会议的批准,粮农组织/世卫组织计划拟就“功能性食品”召开专家咨询会议。这个含有“功能性食品”立法内容的咨询报告,可被用作大会的背景文件。
B) 食品管理中风险性分析的应用 – 挑战与利益
澳大利亚同意准备这个文件并将在大会上就该议题带头发言。由于对风险性分析的需求众所周知,所以这份文件应仅强调在欠发达国家中克服采用风险性分析问题的具体实际行动。
C) 能力建设活动的重点与协调
在秘书处的支持下,印度暂时同意负责撰写此文件。这个文件应该简要涉及在食品管理体系中各个不同方面的益处和缺陷,以及各国能够自我评估其能力建设需求的方式方法。粮农组织已经公布了一份关于评价国家能力建设需求的调查报告,具体可见CAC/26INF/5文件的附件。这份报告可被作为准备本次大会主题文件的一个支持性材料。根据亚洲地区食品法典协调委员会第13届会议的建议,粮农组织和世卫组织正在考虑就能力建设召开一个技术咨询会议。咨询会议的成果也可成为对这份文件的一个贡献。
一些国家还提到了能力建设中的有关具体问题,例如,对新鲜农产品的检查/认证问题,它们希望在这个文件中也涉及此类问题。
D) 信息交流、教育和情况通报
会议认为,文件应当论及有关国家内部和国家之间的信息交流情况,建议文件列举出有关国家的一些成功实例。这个文件还可提供在有关中小型食品生产企业和街道食品部门中成功推行教育计划的实际信息。会议还建议,作为这次大会行动计划的一部分,该地区各国可同意建立一个向其他国家通报有关食源性疾病发生信息的快速预警系统。
尽管注意到日本还没有就准备文件表明态度态,但秘书处将直接与日本联系,讨论由日本负责撰写这份文件的可能性。
E) 食源性疾病的监督与监测体系
会议同意,世卫组织西太平洋和东南亚地区办公室在该区域各国的帮助下,联合起草这份文件。文件将重点提出建立一个区域食源性疾病报告和统计信息中心的可行性。作为大会行动计划的一个内容,各国代表可承诺报告各自国家的疾病情况,保证提供食源性疾病发生的真实情况和次数以及采取的控制措施。如果这项行动计划要获得成功,各国与会代表需承诺为报告的努力做出贡献。公共卫生与监督部门的参与被认为是这个主题的重要组成部分。
大会的主题和具体要点已经修改,详见附件2。
大会的时间表和召开形式
会议同意,大会将由五个国家的政府按五个主题分别进行全会发言,每个主题下列出了讨论要点。会议确认并支持成立两个工作组,每个组都具有跨学科特征。在讨论为工作组选配主题的过程中,会议同意这些主题应能为各国完善各自乃至整个区域的食品安全体系而拟订切实行动的建议提供机遇。会议认为以下主题可提供有关的发展机遇,如:“C)能力建设活动的重点与协调”和“D)信息交流、教育和情况通报”。然而,只有根据全会的讨论情况,才能够最后确定有关工作组会议的讨论主题。
确定大会的日期为2004年5月24日至27日。马来西亚已经决定为与会代表第三天活动提供安排,但不包括起草报告的人员。
附件3是修改后的大会时间表。
预算事务
一些国家指出,为低收入国家的代表出席大会提供旅行费用还存在着后勤困难。
尽管文件打印费用的预算估计为500美元,但马来西亚同意为打印费用最高提供5000美元。秘书处将直接与马来西亚就当地费用问题做进一步讨论。
大会的成本将相对较低,特别是因为各国将负担各自文件的准备费用。粮农组织/世卫组织将通过正常计划预算经费提供直接工作人员的费用,但会议的其他费用将由预算外资源解决。大会秘书处将于9月确认能否获得足够的财务支持以确保举行这次大会。由于到目前为止还没有任何直接财政支持的提议,所以会议鼓励各国分享其对捐赠人的意见。
其他事务
会议认为,为使各国较快地收到大会文件,配有电子邮件的各常驻机构更倾向使用电子邮件系统而并非邮寄方式接收文件。
粮农组织一旦完成“责任备忘录”的定稿,将立即发致马来西亚,并且将表明大会的召开有待获得足够的资金。责任备忘录将包括被邀请参加大会的国家名单,它将作为会议的一个信息性文件,具体见附件4。
尽管对大会仍需要额外的财务和实物支持,但会议确认了赞成召开大会的意愿以及政治上的支持态度。
会议于2003年7月3日16时40分结束。
粮农组织/世界卫生组织亚洲及太平洋区域食品安全会议
Royal Adelph酒店,马来西亚芙蓉,2004年5月24-27日
暂定议程
议 题 主题事项 参考文件
1.会议开幕
2.选举主席团成员
3.通过暂定议程和时间表 CAP 04/1
4.主旨讲话 CRD
5.食品安全立法—利用基于科学和风险的方法实现协调一致 CAP 04/2
6.对食品管理应用风险分析—挑战与利益 CAP 04/3
7.能力建设活动的重点与协调 CAP 04/4
8.信息交流、教育与传播 CAP 04/5
9.食源性疾病的监测及监督系统 CAP 04/6
10.其他事项
11.通过报告
马来西亚食品质量管理司司长Abd.
Rahim bin Hj Mohamad博士
代表马来西亚卫生部副部长(公共卫生)
Y. Bhg. Dato’ Dr Shafie B. Ooyub博士所致的
开 幕 词
尊敬的代表们,女士们、先生们:
首先,我想代表马来西亚政府和马来西亚卫生部向各位尊敬的代表致以热烈的欢迎。马来西亚对成为本次区域会议的东道国感到十分荣幸,这次区域会议是粮农组织和世卫组织为满足成员国政策指导和能力建设需要而正在举行的一系列会议之一。这次会议符合2002年1月28日至30日在摩洛哥举行的“第一次粮农组织/世卫组织全球食品安全管理人员论坛”和2002年9月17日至20日在吉隆坡举行的亚洲食典协调委员会第十三届会议提出的各项建议。
女士们、先生们,
我们今天聚集在一起是因为我们有着一个共同的目标,即改进食品安全和保护公共健康。无论我们的思想或方法多么不同,我们都在努力实现这一共同目标。在这一方面,我可以有把握地说,我们都是这项奋斗中的盟友,我们的敌人是微生物和其他食物危害。
女士们、先生们,
我想我们今天在座的大多数人都认识到“安全”并不意味着“无风险”。想查明一种食品各种可能的不利影响简直就是不可行的。分析安全往往意味着明智地权衡利益与风险。为此,我们必须查明和评估食品风险,努力消除或尽量减少这些风险,并向所有相关者通报风险情况。以下谚语/俗话恰当地总结了这一情况:“样样东西都有毒;没有一样不是毒物。适当的剂量区分毒物和药物”。
还必须强调,在确保安全方面,不采取适当行动或出现任何差错可能付出惨痛的代价,正如最近二恶英造成食物污染和疯牛病事件所表明的那样。每一种食源性疾病爆发可能不仅仅是对生产与疾病爆发有关的产品的公司带来经济灾难,而且可能是整个行业的经济灾难,影响到整个国家。这是因为大部分公众无法区分批号或甚至区分商标。公众可能大笔一挥,将整个产业而不仅仅是某种商标或某一批号列入黑名单。
女士们、先生们,
我们大家都意识到,食品安全是需要作出全球反应的一种全球问题。我们国内所面临的许多食品安全问题也正是全世界共有的问题。疾病和病原体并无国界。因此,区域和国际合作对提出有效的解决办法至关重要。所以,除了我们努力在国内食品链中形成伙伴关系之外,同样重要的是开展各种有效的区域和国际合作,交流信息,相互学习经验,共同互利。
然而,跟上这种发展和采用或改进新的方法,以便防止和应对新的危害,需要所有利益相关者,尤其是没有充足的资源或能力但又依靠食品出口作为一种外汇来源的发展中国家作出巨大的政治承诺和努力。
女士们、先生们,
在这一方面,本次区域会议为我们讨论共同感兴趣的食品安全问题,促进信息交流,学习各自的经验,以便进一步改进食品安全提供了一个适当的论坛和机会。
同样重要的是指出这次会议为食品安全领域进一步的合作活动提供了动力,在为能力建设和科学合作建立联盟方面尤其如此。我确信,这种联盟将有助于集合资源和专业力量,比单独行动时取得更快和更大的成果。同时还将可能在国内、区域和国际各级动员专业力量。
在结束讲话时,我想感谢在座的各位对食品安全这一重要领域的关注,并期望在今后几天中与各位就本次会议的所有主题进行积极开放的讨论。最后但并非最不重要的是,我还想借此机会祝愿所有代表在马来西亚逗留期间愉快。
联合国粮食及农业组织经济及社会部助理总干事
Hartwig de Haen先生所致的
开 幕 词
诸位阁下、各位贵宾、尊敬的代表们、女士们、先生们:
我荣幸地代表联合国粮食及农业组织欢迎各位出席粮农组织/世卫组织亚洲及太平洋区域食品安全会议。这次会议是粮农组织和世卫组织正在组织的一系列区域会议之一,它提供一个论坛,使本区域的食品安全官员能够走到一起,分享如何才能改进食品安全的信息和经验。
本次会议是根据2002年1月份在摩洛哥马拉喀什组织的粮农组织/世卫组织第一次全球食品安全管理人员论坛的建议,并为筹备2004年10月12日至14日将在泰国曼谷举行的第二次全球论坛而组织。
我想强调作为本次会议取得成功的根本的一些概念,第一是食品安全至关重要;第二是改进食品安全面临挑战;以及第三是粮农组织和其他伙伴机构正在为促进粮食安全采取实际行动。
食品安全的重要性
·确保安全和健康的食品是粮食安全的一项重要先决条件。改善所有国家无论是发达国家还是发展中国家的人的生活至关重要。
·粮农组织于1996年召开的世界粮食首脑会议认识到,获得安全食品本身是粮食安全的一项要素。获得足够的安全、富营养食品供应,是人人应有的权利,而不是成为富人的一种奢侈。
·食源性疾病的每次爆发所产生的代价包括一系列直接和间接成本。在发达国家中,估计的平均代价为每年每人100美元,而在发展中国家这一代价可能更大。食源性疾病造成的死亡人数令人震惊:仅仅污染食物和不清洁的饮水所引起的痢疾每年就造成180万人死亡。
·旨在改善食品安全的做法也减少食品损失,从而增加食品可供量。各国可采用一系列先进技术和实际控制措施来改善食品安全,从而延长其可食用期。
·本区域各国充分意识到食品安全对出口和进口的重要性。例如,在运往欧盟的产品中检测到氯霉素残留造成最近欧盟禁止进口海鲜和禽类产品,使亚洲某个国家失去了3.35亿美元的出口机会。
改进食品安全所面临的挑战
改进食品安全尽管有这些众所周知的重要理由、不断增加的全球知识和可用于改善食品安全的先进手段和技术,但仍然面临许多挑战。请允许我仅仅提及以下五项挑战:
1) 标准的实施往往使食品生产者和加工者的成本上升,可能迫使一些供应商破产。
2)
努力实现完全安全食品即“零风险”食品的利益,应与所涉及的往往很高的成本造成竞争能力的丧失实现平衡。
3)
除非在区域合作网络中实现一体化,否则单个国家往往没有能力应对紧急情况中的食品安全问题。
4)
消费者普遍缺乏食品安全意识,各国需要通过适当的沟通和教育政策加以解决。
5)
许多国家实施食品安全措施和监测食源性疾病的能力不足。
前两天媒体头条新闻报道了两个事件,最及时地肯定了采取一致行动的重要性。其中一个事件是加利福尼亚检测到生杏仁受到沙门氏菌污染,致使从市场召回大量生杏仁,包括已经出口到亚洲地区的杏仁。另一个事件是肯尼亚25-40人死亡。虽然尚未肯定,但媒体报告怀疑这些死亡因食用受到黄曲霉毒素严重污染的玉米所致。
这次会议仅仅是粮农组织在全世界和尤其是在亚洲及太平洋地区努力改进食品安全的一个事例,而本地区有充分的理由特别处理食品安全风险问题。请允许我仅仅提及其中三项理由:
1) 迅速城市化
随着生活在(大)城市中的人口数量不断增加,人们食用更多的加工食品、销售链和货架寿命延长,街头食品增加等等。所有这些都产生食品安全含义。
2) 贸 易
本地区各国作为出口国和进口国都积极参与贸易活动。因此必须遵守贸易伙伴的食品质量和安全标准。
3) 畜牧革命
通过畜牧革命我们指的是畜产品消费的迅速发展,在收入迅速增加的本地区尤其如此。结果,畜牧生产不断发展,而动物疾病的风险也不断增加。人口与动物尤其是禽类密切接触,产生了额外的健康风险。
粮农组织和伙伴促进食品安全的实际行动
粮农组织参与了全世界促进食品安全的许多实际行动。其中包括:
1)
粮农组织和世卫组织向食品法典委员会成员提供有关食品安全风险的科学咨询。
2)
就公开问题组织专家磋商会(往往与世卫组织一起)。最近的事例包括微生物风险评估、食品中的丙烯酰胺和转基因食品的安全评估。
3)
粮农组织、世卫组织、世界动物卫生组织、世贸组织以及世界银行最近设立了标准及贸易发展基金,协调这些组织在食品安全、植物和动物卫生领域的能力建设活动。最近已经批准利用这项基金为粮农组织/世卫组织的一个项目提供资金,帮助亚洲及太平洋地区低收入国家在风险分析框架内制定食品标准,并将在今后几个月中执行这一项目。
4)
手册和准则。例如,2003年发表了粮农组织/世卫组织加强国家食品管理系统准则。正在编写一本风险分析手册和成套食典培训材料。
5)
粮农组织和世卫组织设立了一项参与食典活动信托基金,以增加发展中国家和转型国家参与食品法典委员会重要工作的活动。
6)
能力和技术援助。事例包括过去三年中在亚洲及太平洋地区就不同的食品安全方面召开的八次区域和分区域研讨会以及国家技术援助项目。
7)
食源性疾病监视。我们将在本次会议上讨论本地区一项新的举措,并希望商定具体步骤。
8)
确保获得信息。我高兴的宣布,粮农组织将在本次会议期间启动国际食品安全、动物和植物卫生门户网站。这一门户网站将向国家政府和贸易伙伴提供获取官方相关信息的手段。
9)
世界卫生大会刚刚通过了一项全球膳食、体力活动和健康战略,以便通过促进健康膳食和生活方式,消除非传染性疾病带来的不断增加的负担。粮农组织决心与世卫组织和成员国合作实施对食品安全具有间接重要性的这项全球战略。
结束语
亚洲及太平洋国家在改进其人口的粮食安全和加强其粮食和农业产品竞争能力方面取得了显著的进展。这些成就需要得到加强和扩大。各国还必须增加食品安全投资,不仅使本地区进一步改善其贸易机遇,同时保护其国内消费者的健康。
必须加强各级的区域合作和信息交流机会。粮农组织和世卫组织联合召开亚洲及太平洋区域食品安全会议就是为了实现这些目标。
我想事先感谢各位将在今后四天中努力处理各位将面临的许多重要问题。我对各位的审议活动表示最良好的祝愿,并期盼这些审议活动的结果。
世界卫生组织驻文莱、马来西亚和新加坡代表
Han Tieru博士所致的
开 幕 词
诸位阁下、各位贵宾、尊敬的代表们、女士们、先生们:
我仅代表世界卫生组织总干事Jong-wook
Lee博士欢迎各位参加粮农组织/世卫组织本次亚洲及太平洋区域食品安全会议的开幕式,并与来自联合国粮食及农业组织(粮农组织)的同事一起感谢马来西亚政府对我们的款待。
食品安全在世界各地受到不断关注,亚洲及太平洋地区最近的事件向我们表明了本地区也绝对没有摆脱这些关注的影响。
在世界各地,无疑在本地区也是如此,每年有许多人,包括许多儿童因食物感染而死亡。过去,这些问题往往仅仅因新闻报道惊人的疾病爆发时才引起公众的注意。一定程度上仍然如此,但我们需要确保人人认识到,在关于疾病爆发的新闻报道背后,还有许许多多零星的病例和小规模爆发,而这些构成了与食品有关的真正疾病负担。其中许多情况没有进入报告系统,肯定也不会成为头条新闻。
在本地区,世卫组织对死亡原因的一般报告系统表明,每年有70多万人死于因微生物造成的食源和水源性痢疾。除了痢疾之外,不安全的食品也造成其他一些种类的严重疾病,包括通过我们的农业生产系统在食物中自然出现或添加的某些化学物质造成人体虚弱的长期影响。
此外,即使本地区最近一些公共卫生紧急情况,如禽流感、Nipah病毒和非典等显然不是食源性疾病,但也在一定程度上都与生产食物的方式或处理食用动物的方式有关。
最后,食品污染也对贸易和国民经济产生影响,因为贸易壁垒和与食品安全有关的禁令给出口国带来了重大的经济损失。
因此,有许多充分的理由来研究我们通过食品生产系统,尤其是我们的食品安全系统来保护公共健康的方式。本次会议是在重要的时刻举行的,希望我们能够产生影响,在国家重点活动背景中推动这一重要事项。
作为今后工作的一部分,我们需要确保公众能够获得对其食品问题的解答。食品总有一定程度的风险,这一信息确定了我们需要处理的问题的规模,但我们也需要能够提供当局正在采取何种行动和公众本身能够开展哪些活动来降低受到伤害的可能性的情况。
我们需要确保公众认识到,如果得了食源性疾病(我相信我们大多数人都经历过),假如比较幸运,这一情况可能仅仅造成几天的不适。然而,这并非是唯一可能的结果:也可能最终无法上班和进一步失去能力,甚至在最严重的情况下导致死亡。
食源性疾病的结果也对卫生系统产生了压力,降低了经济生产率。美国最近的估计表明,食源性疾病使该社会承担的年度卫生费用超过60亿美元。这些数字并不包括生产和贸易系统中进一步承受的经济损失。
世卫组织是世界上专门从事人体健康活动的政府间机构。它帮助各国政府、民间社会、消费者团体、私营实体、媒体和其他利益相关者获取有关食物可能造成伤害及如何才能尽量减少伤害的方式的最佳证据。其中包括确保通过监视系统和风险分析适当了解政策响应,帮助规划适当组合的宣传运动、立法和改变食品行业中的安全文化和系统。
世卫组织通过帮助整合不同的疾病监视系统产生作用。我们将在这次会议上讨论建立和加强本地区监视网络的方法,我们希望也将能够在这些领域向前迈进。
世卫组织确保食品安全的职责,要求采取以证据为基础的新的预防战略,以便降低食品造成危害的风险。这些战略可在“从农场到餐桌”的整个食物生产链中加以实施。在世界其他地区,食物链越来越长,而在本地区许多地方,从生产到消费的距离非常近:在我们的审议活动中,我们应努力确保未来的食品安全举措考虑到我们各种不同的粮食生产系统。
本次区域食品安全会议得到世卫组织和粮农组织共同努力支持。为什么呢?因为我们需要从多学科角度研究这些问题!过去许多问题的根源在于我们未能使负责食品生产链不同环节的所有伙伴和政府部门共同努力。我们希望粮农组织/世卫组织的联系也将鼓舞农业、卫生和其他部门在国家和国际一级的合作。
各位代表,扩大我们在食品安全方面的集体努力,着手收集证据,在国家一级进行监视,作出有效的回应,并确保这一事项列入政治议程的重要位置的时机已经来临。我们需要分享经验,无论是好的经验还是坏的经验,从而使今后的食品安全系统得到改进和避免以往的错误。
我祝愿各位会议圆满成功,并期待各位在公共健康这一关键领域的审议结果。
谢谢诸位。
马来西亚卫生部副部长
Datuk Abdul Latiff Ahmad阁下所致的
开 幕 词
尊敬的代表们、女士们、先生们:
首先,请允许我向各位表示热烈欢迎,欢迎来到马来西亚。我愿代表马来西亚政府和马来西亚卫生部向粮农组织/世卫组织表示我诚挚的感谢,使马来西亚能够荣幸地成为审议确保食品安全这一极其重要事项的本次区域会议的东道国。同时高兴地注意到众多代表和国家参加了这次会议,表明许多国家包括粮农组织和世卫组织对食品安全的高度重视。
女士们、先生们,
当今食品安全方面的挑战复杂而具有多面性。政府和公众对食品安全的重视已经提高,受到最近与食品安全危机有关的事件如二恶英污染和疯牛病等的推动。食品贸易全球化、经济相互依赖程度提高以及东西方文化交流,使口味和喜爱逐渐同化。这导致种类广泛的食品进入市场。这些食品使用高科技设施生产、经过长距离运输,以新的方法包装和储存,并经过更多的步骤处理,使食品链中污染的机会增加。在这种动态变化过程中,马来西亚也不例外。诸位中有机会参观我们的一些超级市场的代表将注意到能够获得世界各地生产的食品,在这种情形下,任何单一来源的污染都可能传播到全国众多社区,甚至传播到世界各地,其后果或影响大幅度增加。
虽然主要由于世卫组织和粮农组织提供必要的领导和指导,过去几十年世界各地食品安全方面的进展巨大,但我仍然认为我们能够也必须做得更好。许多工作仍然有待进行。随着食品安全的挑战继续不断变化,除了改变我们的食品安全系统以满足更好地保护公共健康的这些不断变化的需要,我们别无选择。我们必须确保食品安全系统能够通过最有效的手段,应对和预防食源性疾病和食品危害。
我们必须尽我们所能,作为一个国家单独或通过区域和国际合作发展有效的解决办法。因此,除了我们努力在国内食品链中发展伙伴关系之外,同样重要的是开展各种区域和国际合作,交流信息,学习相互的经验,共同互利。我相信这对帮助各国不断加强和定期调整其食品安全计划,紧跟知识和技术的变化,适应与区域和国际贸易食品的安全和质量有关的新义务和权利尤其重要。
女士们、先生们,
我确信,在处理食品安全问题包括减轻食源性疾病负担方面,我们拥有知识和经验,应当分享和推广,使其他国家受益,甚至适用时在全球范围内加以应用。
因此,我高兴地注意到本次会议的主题是“采取实际行动,促进食品安全”。它充分强调需要在加强食品安全方面采取切合实际的、能够作为最佳前进方法加以实施的行动。这些行动必须考虑到从最近的食品安全危机和禽流感及口蹄疫等疾病爆发中吸取的经验教训。这一危机暴露了我们的食品安全系统的一些弱点,在各国内部或各国之间都是如此,帮助我们决定应在哪些方面加强我们的预防机制。换句话说,如果在如何避免未来的危机方面,我们能够善于学习,越战越强,则食品安全危机可能并非都是消极的。
因此,这样一次会议提供了一个极好的平台/机会。我还高兴地看到本次会议的安排包括对以下领域的最新认识:
·首先,基于风险的方法,包括应用风险分析,这为国家和国际两级的管理措施奠定了以科学为依据的新的预防性基础;
·其次,食源性疾病监测和监视系统,需要这一系统以提供有关食源性疾病的可靠数据,并将其与食品污染相联系,以便采取以证据为基础的干预行动,降低疾病发生率;
·第三,确定能力建设活动的重点并加以协调,这对加强食品安全尤其是在发展中国家的公共卫生职能方面的作用至关重要。
·最后,信息交流、教育和沟通,这些构成了提高认识、制定战略和建立改进食品安全所必需的专门力量的基础。
最后,我希望各位代表能够相互交流,使本次会议取得最佳成效。我还将鼓励各位花一些时间欣赏和游览我们因丰富的文化、多民族构成和烹调而闻名的美丽的国家,留下在马来西亚度过的快乐记忆。
我十分荣幸地以此宣布本次“粮农组织/世卫组织亚洲及太平洋区域食品安全会议—采取实际行动,促进食品安全”正式开幕。我谨祝愿各位的审议活动圆满成功,并期待各位就我们如何能够集体改善亚洲及太平洋区域食品安全的方式方法提出的结论和建议。
“采取实际行动,促进食品安全”
泰国食品和药品管理局秘书长
Supachai Kunaratanapruk博士的
主 旨 演 讲
尊敬的各位代表,女士们、先生们:
我谨感谢组织委员会邀请我参加在全世界食品安全挑战处于历史性时刻组织的这次重要会议。
无疑,食品安全是每个国家的主要关注。人们日益无法孤立地考虑食品安全问题。食品安全已成为发展议程一个不可分割的部分。人们应认识到食品安全、卫生和经济繁荣之间的相互联系。
禽流感和疯牛病的爆发仅仅是证明食品安全与经济之间的关系的几个事例。自2003年年底以来,为了防止禽流感病毒的传播,已经至少屠宰了1亿只鸡。在禽流感之前,世界上爆发了疯牛病,经济损失足以影响爆发这些疾病的国家的国内生产总值的增长率。这种疾病爆发总能引起人们大量关注并获得巨额预算加以管理。
另一方面,对某些食品安全问题监视不足。受粮食短缺影响的人口数以百万计,而受微生物和化学食品污染影响的人有几十亿,对健康产生已知和未知的长期影响。对此经济影响无法加以准确估算,但只要想一下癌症病人人数可能不断增加和随之造成生产性生命的丧失。转基因食物、防腐剂、着色剂、调味剂以及现代食品加工工艺应是我们值得骄傲的食品行业的发明创造。然而,它们是否肯定安全?人们是否应对此始终保持警惕?我相信本次会议有助于指明未来的方向。
食品安全问题如何和能否促进健康和经济发展?我是一个乐观主义者;因此,我相信这些问题并没有超出我们集体的能力范围。这次会议无疑是一个重要的步骤。然而,从现实出发,食品安全领域存在使情况复杂化并需要加以消除的利害冲突。
食品不仅仅为人们提供食物,而且也是一个巨大的产业。各国在食品生产和贸易方面具有优势和弱势。因此,这一状况不利于促进各国之间的协调。
协调一致是使各国共同努力的一种新颖方法,希望能够确定全球食品安全、食品消费和公平食品贸易方法的标准。然而,良好的意愿结果是成为对那些处于不利境地的国家的技术贸易壁垒。亚洲地区各国面临对呋喃类药和氯霉素强制实施的零容忍度。与此同时,即使仅发生一例牛海绵状脑病的出口国的牛肉产品被普遍禁止进入我们的国家。我们是否真正拥有足够的科学证据表明这是保护我们消费者的健康所必需的措施?这些情况不利于交流信息和透明度要求,而信息交流和透明度正是各国之间开展良好合作和相互谅解的根本。
实施基于风险的管理方法是否将或能否更加具有成效和诚信?这一概念不仅仅注重确定危害,而且包括对这些危害的暴露、其可容忍性及消除。一个良好的事例是黄曲霉素。虽然已知黄曲霉素为致癌物质,但鉴于无法从食物链中完全消除黄曲霉素这一事实,食典允许每公斤含有15微克。我肯定鉴于我们掌握的先进研究信息,有可能量化与食物有关的风险,然后作出决定。不必要的食品安全限制肯定将妨碍食品出口国乃至全世界的经济发展,结果是增加这一相互依存的世界上的贫困。
除了实施基于风险的方法之外,我将促请与会国家在各自可能已经建立的地方警报系统之外,分享或发展一个“国家间警报系统”。这种系统将成为及早预防危机的一个有益平台。禽流感的爆发就是表明良好的警报和监视系统的重要性的一个非常好的事例。然而,只有充分建立了信任和公平之后这才能成为现实。
一个根本的事实是,发达国家仍然普遍存在食物和水传播的疾病,而在发展中国家情况更糟。贫困者为最易受害群体。结果,食品安全不应仅仅针对出口。每一个公民都有权获得和消费安全食品。因此,他们应能获得安全食品。危害分析关键控制点的理念在工厂规模的生产中得以成功实施,但应同样实施(或平行实施)“良好卫生规范”。在泰国,村庄一级的食品生产占总产量的至少30%。食品管理机构有义务向公众保证小规模生产与大规模生产同样安全。
改进现代技术使其适应地方生产正是发展中国家所缺乏的。在生产领域促进以证据为基础的研究和方法,应当成为大会将讨论的能力建设活动的一个主要问题。当泰国政府应用“双轨”政策—即在促进利用本地固有技能的中小企业的同时鼓励外来投资时,我们面临种种困难。如果不对高成本技术进行大量投资,村级食品生产者就无法满足良好加工规范或危害分析关键控制点系统要求。如果不存在这种差距,我们泰国的地方食品生产者就能对我们的经济作出更大的贡献。然而,从这一经验来看,我们认识到最适宜的技术未必是最昂贵的技术。因此,小型加工业也能以承受得起的代价生产安全食品。
食品从根本上来说是一种农产品。因此,改进农业标准是食品安全取得成功的关键。所谓的“从农场到刀叉”或“良好农业规范”的理念,假如不属于“高技术农作方法”,就应当广泛而大力实施。
有效的消费者食品安全教育是我想强调的另一项重要活动。不应仅仅从供应方面来考虑食品安全问题,还应同时把改进需求方面作为目标。各国与所有相关组织进行合作的主管当局有责任改进消费者的食品安全知识。
为了向消费者提供如何为自己选购安全食品并加强有关食品消费的消费者意识提供咨询,应通过不同的媒体如电视、广播、活页宣传品、小册子和报纸等向公众提供教育。各级教育课程中也应当包含有关食品安全的课程。此外,食品制备过程中的卫生方法也应当以消费者为重点,并需要改变喜欢生食或微煮食物的那些人的不良消费行为。
总的来说,各个食品加工步骤涉及的所有人员的能力建设在实现食品安全方面发挥重要作用。令人遗憾的是,我们在官员、生产者、批发商、零售商和消费者各级使理论、态度和方法相联系方面存在差距。这一问题值得考虑,使我们能够在行动和未来的合作中取得进步。
主管食品安全的机构负责挑选有关食品的最佳政策:即立法、控制战略、执法机制、私营与公共部门合作、研究、教育和公共关系。食品安全政策不应成为“仅仅涉及食品”的边缘化问题。食品安全对健康和经济产生的影响应得到承认。
“人人获得安全食品”是世界发展的组成部分。如果能够在诚挚的氛围中以整体方法作出协调一致的努力,则各国现有的资源就
可能绰绰有余。我想感谢联合国粮食及农业组织和世界卫生组织为完成其使命而作出的不懈努力。
尊敬的来宾、女士们、先生们,我希望这次会议成为一个良好的开端。我促请所有与会者共同努力,使亚洲及太平洋区域各国实现更高的食品安全水平。
谢谢各位。
新西兰食品安全管理局计划经理Neil
Macleod先生的
主 旨 演 讲
新西兰建立了一个管理食品安全的新机构,并制定了对食品生产、加工和出口实行风险管理的新立法。食品安全管理局于2002年7月1日开始工作,集中了原先由农业和林业部管理局(后称农林部食品质量保证管理局)行使的管理职能和卫生部公共卫生领域的食品方面。
本文力图介绍新西兰为管理本次会议与会者详细论述的一些问题和挑战而提出和实施的一些解决办法。这样做并不是为其他人限定这些要素作为解决办法,而是提出本区域成员国之一的理由和取得的结果。
新西兰视角
人们要求新西兰从“发达”国家的角度来处理这些问题。这可能并不完全合适。新西兰如同参加本次会议的许多国家一样,十分依赖以陆地为基础的生产和捕鱼。新西兰人口少,主要产业属于初级生产。因此它与本地区其他农业或捕捞经济有着许多共同的关注。
讨论食品安全时新西兰管理人员常说的是:
·必须仅仅利用适当的条例(而不是应用无充分正当理由的措施)来管理风险;
·区分管理金字塔即管理人员、审核人员和生产者的作用;
·从被管理者—食品行业回收管理费用;
·以绩效或结果为基础的措施;
·与外国市场的联系。
法规的明确目的是确保公众对安全食品供应的信任,每个活动者的明确责任以及(在食品法规中少见的)确保适当进入外国市场的必要性,这反映了新西兰生产的大量食品出口这一事实。新西兰食品安全管理局万维网站(www.nzfsa.govt.nz)上详细规定了管理关系以及作为标准制定者的政府、一个单独的审核层面和发挥确保食品安全主要作用的群体即食品行业本身的作用。
外部因素
作为一个食品贸易国,新西兰关心了解贸易伙伴的食品安全措施的设计目的:这些措施有无正当理由和应用时是否公正?它们能否实现预定目的:即保护消费者健康?鉴于新西兰出口其生产的如此多的食品,新西兰管理人员和产业似乎有一种双重义务。国内消费者必须能够获取安全食品,而海外消费者也能通过任何谣传的问题,关闭新西兰向该市场或甚至更广阔范围的食品出口。新西兰的出口食品标准高,因为食品行业已成为全国一个非常严肃的行业。
风险管理:立法
1999年11月1日起生效的畜产品法提供了新西兰如何实施风险管理的一个实际事例。其目标非常明确:管理动物材料和动物产品对人体和动物健康的风险并促进进入市场。其范围包括所有动物材料(人除外)但将于2004年年底包括奶和奶制品。新西兰立法中涉及其他食品项目的其余部分也有类似的计划,但其中一些计划仍属自愿适用。
风险管理系统包括公司一级实施的强制性风险管理计划、为使一系列活动保持一致性而执行的管理控制计划(如国家化学残留物或微生物调查计划)、认证所采用的官方保证规定以及对食品行业所有参与者明确规定的职责,包括对本身可能并非食品生产者的出口方的登记注册,以及对可能在法律范围以外经营者的严重处罚。风险管理计划是食品行业建立的记录系统,对其进行独立评价,如果可以接受,由经新西兰食品安全管理局登记注册。它必须表明如何查明所有已知的生物、化学和物理危害并管理有关风险。它还延伸到众所周知的危害分析和关键控制点原则范围以外。这可能包括有益健康问题,其中食品必须满足消费者的希望或其标签说明真实情况。可取的结果是产品适合其预定用途—鉴于动物可能提供的产品范围广,产品可能并非始终供人消费。
新西兰的食品安全管理问题
在评价风险管理计划时,应当指出,这些计划仅仅从2000年11月1日才开始执行。虽然所有新的活动都必须在《1999年法》的范围内进行,但原有的一些企业仍然应用过渡性安排。迹象表明,一些比较传统的经营活动在使用新规定方面没有多大动力;有些企业选择由中央政府规定其活动的老系统。规模较大的经营者实现了过渡,但还有规模较小的经营者遇到了与风险管理计划形式所涉及的文献程度问题。对它们来说,可能有必要更多地使用模块来帮助指导它们遵守该法律的行动。无疑,希望创新的经营者发现,能自由发展对其本身的经营或产品生产线特定的风险管理方式,充分利用《畜产品法》所提供的灵活性。新西兰食品安全管理局在一定程度上仍然受到以下关切的支配,即如果对经营者管得过多,它们可能失去主动思考其企业正在努力实现何种食品安全目标的动力,结果使其遵守法律的可能性下降。
鉴于如此多的新西兰食品进入贸易,与海外对应部门的关系中出现问题就十分显然。新西兰食品安全管理局虽然欢迎海外对应部门来访,了解新西兰食品安全管理局实施的食品安全系统真正发挥预期的功能,但对海外机构似乎认为需要参观新西兰的工厂并批准这些工厂适合出口能有任何实用意义都不太肯定。新西兰食品安全管理局认为这是新西兰的有关任务,认为海外人员来访一天获得的“简单印象”根本无法成为评价连续食品安全管理的最佳基础。另一个值得关注的问题是假冒的新西兰证书或产品标签的欺骗行为,为利于打击这一行为而采取的措施之一是目前澳大利亚也积极参与的确定出口食品安全电子认证的一个系统。
与其他国家一样,有时新西兰食品安全管理局与特定贸易伙伴国内交涉的机构数量大,妨碍了问题的顺利解决。但新西兰也是由生物安保当局来处理动物和植物卫生问题,新西兰食品安全管理局处理进口食品的人体健康成分。海外对应部门应用卫生和植物检疫措施方面的一致性也并非始终显而易见。有时并没有明确提及国际标准(或偏离这些标准的理由),甚至没有提及原先有关类似进口建议的决定。由此可能得出的结论是由于农民或地方生产者等正在受到保护,而不是出于食品安全措施的预期重点即人体健康。
新西兰食品安全管理局根据食品贸易经验提出,在通常需要应用风险分析原则时,可能存在海外管理人员可应用的一些简单工具。可以进行定性评价。例如,出口国是否已经向该市场出售类似产品而无食品安全问题?是否向拥有适当的严格进口管理制度的其他“基准”市场(如欧盟或美国)出口?正在出口的产品是否来自例如一种不同的动物品种但在出口国又受到同一管理系统的监督?
一些贸易伙伴主张它们必须对所有出口方平等应用同一措施,但这忽视了世贸组织卫生和植物检疫协定条款所承认的情况,即两个国家可认定采取的措施等效并又未必落实另一方法规的每一项细节,但仍然获得可取的食品安全结果。同样可以要求这些“特殊”处理必须有互惠性。通常并非如此。在许多情形中,食品贸易将是单向的,平衡只能通过贸易本身这一事实才能实现:即一个伙伴想出售,而另一个伙伴想购买。
在所有这些管理关系中,无论选择何种关系,除非以诚信为基础,即产业为其最佳利益生产安全食品、出口当局适当信任及其履约保证可信,否则食品安全得不到任何保证。没有这种诚信和信任,任何食品安全系统都无法存在或延续。就新西兰的情况而言,新西兰食品安全管理局为(食典委定义的)主管当局。该管理局相信其提供的保证是值得信赖的,并希望贸易伙伴同样予以接受。如果新西兰的食品有问题,新西兰食品安全管理局将希望了解这些情况—请各位告知。
新西兰应用的食品管理模式
议讨论文件清单
文件编号 标 题
CAP 04/2 食品安全法规;利用基于科学和风险的方法实现协调一致
CAP 04/3 对食品管理应用风险分析-挑战与利益
CAP 04/4 能力建设活动的重点与协调
CAP 04/5 信息交流、教育与传播
CAP 04/6 食源性疾病的监测及监督系统
议题5 CAP 04/2
粮农组织/世卫组织亚洲及太平洋区域食品安全会议
2004年5月24-27日,马来西亚芙蓉
食品安全法规;利用基于科学和风险的方法实现协调一致
(澳大利亚撰文)
1.概 要
实现国家和国际食品安全法规的协调一致面临着诸多的挑战和机遇。本文讨论基于风险的重要原则和方法,例如风险分析、“从农场到餐桌”方法以及“危害分析关键控制点”过程控制体系的利用,为了促进食品安全法规的协调一致和透明度,目前正在越来越多地制定原则和方法。风险管理对于一些国家尤为困难,本文也讨论确保所有的利益相关者均能参与法规制定过程的重要性。在实施食品安全法规过程中应将重点置于公众健康,与此同时还应通过对法规影响的评估充分考虑其社会经济的影响,本文还讨论这种方法的重要性。最后探讨在国际上实现食品安全法规协调一致所面临的诸多挑战和机遇,尤其是国际贸易和贸易协议对其影响以及食品法典委员会(食典委)标准的应用。本文还简要阐述国际上的联合问题,例如区域联盟、信息交流网络和技术合作,这种联合已被作为帮助实现国际食品法规协调一致并加强全球公众健康和贸易的方法。为了实施一致的和基于风险的国家及国际食品安全法规的战略,本文概述所建议的一些方法。
2.实现国家食品安全法规协调一致所面临的挑战和机遇
按照惯例,许多个国家的食品法总是包括了不安全食品的定义和杜绝此类食品在市场上出现并处罚市场外责任方的强制措施等规定。但是,食品法通常不会对食品控制机构的职责和食品安全的预防问题做出明确的规定。其结果是促使许多国家在指导和鼓励采用预防性和总体措施,以减少食源性疾病的风险之外,还建立了有效的食品监管体系,并将重点置于法规的强制实施。
虽然许多国家已经制定了明确的食品安全目标并意识到食品监管体系必须建立在系统和公正的食品法规原则之上,但许多国家还在致力于探索这些方法的价值。因此,这些国家的食品安全法规依旧是大量零散规定拼凑起来的混合体,这样的法规与以科学为基础的食品安全目标是不相符的。这些监管体系往往不能有效地保护公众健康,并可能危及国内食品市场和贸易能力。
近年来,有许多因素推动对食品安全控制和监管体系采取主动措施,这些因素包括:
·科学上对食品安全风险的了解更加清楚,并具备了评估这些风险对公众健康影响的手段(包括不断出现的食品病原体);
·新的食品生产技术和食品安全控制/管理体系;
·日益增多的食品贸易及在贸易协议规定的义务;
·消费者/市场对食品安全日趋关注;
·由食典委制定的国际食品标准。
为了帮助成员国强化和更好地协调食品监管体系,国际机构(例如联合国粮食及农业组织和世界卫生组织[1])面对着这些因素的挑战(在竞争的时代),采取积极的行动,制定了公正的、基于风险的决策标准,以便在该领域建立透明并具主动性的食品安全监管体系。这些行动计划的关键在于制定和应用风险分析原则。
2.1 风险分析
食品法规的客观合理性在国际上日趋为人们所接受,反映了公众健康和安全的需要应与社会经济诸多因素保持一致[2]。为了实现这种协调一致,许多国家和国际组织越来越多地采用风险分析体系来阐述风险的特征(从食品安全和经济影响的角度),确定适宜的风险管理方案并在此过程中与所有的利益相关者开展信息交流。
风险分析包括三个相互关联的部分:风险评估、风险管理和风险交流。每一部分的内容将在会议主题B(风险分析在食品控制中的应用:挑战和益处)进行讨论,其中包括国际机构应如何制定风险分析的框架,以及由澳大利亚-新西兰食品标准局开展的特定案例研究。
许多国家在制定食品安全法规过程中已经将风险分析综合成为一种系统的方法,食典委也将其确定为诸多标准的基础。食典委是粮农组织和世卫组织的联合机构,负责制定国际食品标准(例如标准、准则和操作规范)以提高全球公众健康,并帮助实现标准的协调一致,以促进国际贸易。食典委已经认识到采用基于风险原则的重要性,将“风险分析工作原则”引入食典委程序手册中[3],并由食典委的各规范委员会遵循执行。
风险分析的基本原则和益处已得到人们广泛承认,在国际上的应用也日益增多,但是不同的国家在应用过程中采用了不同的方式,并在国家法规框架内规范其应用的范围。
引起争论的关键问题包括,应用方式(其富集科学的不确定因素,即科学证据不足)、等效标准(不同的机构或不同国家在同一产品上采用了风险分析却得到不同的风险管理方法)以及在制定食品安全法规时应当考虑的“其他合理”因素的方式及范围。这些问题是目前国际上颇有争议的主题。
为了帮助本区域各国政府按照食品法典和国际贸易协议制定有效而透明的风险分析框架,粮农组织和世卫组织在国际生命科学学会(ILSI)及促进发展工业理事会(ICD)的通力合作下,正在制定大量的方法,其中包括风险分析手册[4],并组织风险分析的区域研讨会。
·由食典委制定并应用的风险分析原则,应成为所有国家在食品法规上的决策基础。各国政府在建立各自的食品控制和监管体系时,应充分利用粮农组织和世卫组织的风险分析方法。
2.2 风险评估
风险评估是风险分析的首要步骤,是以下述四个内容为基础,即(i)危害确定,(ii)危害定性,(iii)影响评估,以及(iv)风险定性(见主题B的文件)。
虽然不是所有的国家均拥有足够的科学资源、能力或资料来开展风险评估,但可能也没必要为此让所有的国家均拥有综合的体系。目前在国家、区域和国际各个层面上,用于风险评估的资源日趋增多,各国政府在制定国内法规时应利用这些评估结果。例如,由粮农组织/世卫组织的联合专家委员会(如食品添加剂和污染物联合专家委员会、农药残留联合专家委员会、微生物风险评估联合专家会议)开展的风险评估,均是国际上通用的。
最近粮农组织对许多发展中国家食源性疾病的资料进行了检查,并评价利用这些资料是否有助于风险评估及确定风险管理的方案(包括评估这些决定在经济上影响的方法)[5]。在会议议题E-食源性疾病监测及监督体系中,将对相关问题进行讨论。
·各个国家要尽可能地采取切实可行的方法,提高利用和评价国际风险评估结果的能力,特别是那些由食典委及其下属或相关的机构开展的评估结果。此外,为了促进所制定的国内食品安全法规不仅在国内(例如,与当地的饮食方式和饮食条件)而且与国际均能保持协调一致,还应采用具有相似环境和社会经济状况的其他国家资料。
2.3 风险管理和法规效果评估
风险管理是与风险评估截然不同的一个步骤,其中包括和所有的利益相关者进行磋商,权衡已确定的法规方案。该步骤包括考虑风险评估的结果和其他的相关因素,例如成本效益问题,包括对产业和执行机构的影响以及潜在的法规干预措施(例如操作规范和强制性标准)。
总而言之,应通过透明的国家食品政策来确定风险管理,包括与利益相关者开展磋商的正式程序。确定可接受的风险水平从本质上讲是一种政治决策过程,要全面地考虑公众健康、社会经济及环境的各方面因素。然而,在做出这些决定的过程中,各国政府需要考虑预期的保护水平是否属于履行国际协议义务范围内的必要限制,而未构成贸易限制性措施。许多国家采用了公开透明的法规效果评估(RIA),从而实现风险管理中的更好平衡。法规效果评估是对政府决策所产生的潜在影响进行系统和持续地检查和讨论的方法之一,也是向决策者通报其决策结果的方法之一。食品法规效果评估旨在确定和评估不同法规方案,以便实现公众健康和社会经济利益的平衡。
在确定公开透明的食品安全风险管理和法规效果评估框架方面,具有大量的国家法规模式。例如,澳大利亚-新西兰食品标准局在制定食品标准过程中,将风险管理的公开性和澳大利亚政府委员会制定的法规效果评估要求相结合(见澳大利亚-新西兰食品标准局的“应用和建议指南”[6])。经济合作与发展组织(经合发组织)在《经合发组织理事会关于改进政府法规质量的建议》[7]中也已确定了法规效果评估的指南。
1999年由当时的澳大利亚-新西兰食品局(现为澳大利亚-新西兰食品标准局)在澳大利亚实施的引进强制性食品安全计划,是对食品法规进行法规效果评估的范例之一。在澳大利亚(还有其他许多国家),依照联邦的规定对所有的拟议法规均必须进行法规效果评估。澳大利亚已经通过了四个食品安全标准:其中三个为强制性标准,它们分别阐述了食品安全、可接受的食品安全操作以及食品加工场所与设备的规定。第四个为自愿性标准,建议食品企业制定明确的食品安全计划,并确定食品加工操作中的有害物(注:这一自愿性标准在澳大利亚的一些州作为强制性标准予以实施)。
成本效益研究预示,食品企业实施这些安全标准的运行费用每个企业大约为1?000澳元,比实施原有法规的费用低35%,原有的法规是若干法规的集合体。间接的益处包括新标准允许企业在食品安全控制体系和食品生产的创新方面具有较大的灵活性。政府为了执行新标准而实施的行政管理和审核制度的费用估计会增加近50%(大约为4
800万澳元),地方政府承担了其中的绝大部分费用。本研究结果推断,食源性疾病的发生率下降20%将促成该标准的通过,因为在生产力和保健费用上相当于节省了5亿澳元。
在这一基础上,澳大利亚政府决定将食品安全标准作为一项切实可行的的方法,以达到极为显著的节约目标并惠及整个澳大利亚社会,政府还批准了绝大多数的标准作为食品标准规范。标准的效果将以十年为限进行评估。应注意的是,由于实施食品安全法规的不同政策所致,这些食品安全标准并未包括新西兰,预计现行的国家食品安全措施在新西兰将具有同等效力。
*
政府应充分利用由国际机构(如经合发组织)制定的风险管理和法规效果评估的模式,并建立共享这些信息的机制,以确保决策的透明度并实现食品法规方法的协调一致。
2.4 风险交流和法规透明度
有关在食品上存在或可能存在的有害物所带来的风险问题,有效的信息及意见交流是风险分析基本和必不可少的内容,无论其是否涉及即将出现的食品安全危机或者将成为食品法规的一项内容。
风险交流使利益相关者了解风险评估每一阶段的进程。这有助于确保所有的利益相关者能够清楚地理解风险分析的逻辑性、结果、重要性以及局限性。从许多利益相关者还可获得有关评估的信息。例如,产业界的利益相关者可能拥有对风险评估极为重要的、尚未发表的数据,这些数据可能会影响到风险评估的方案及决策。在切实可行的合理范围内,利益相关各方应参与管理方案的确定、选择方案标准的制定,并为战略的实施和评估做出贡献。
风险交流可以由国际、国家或地方一级的官方发起,也可以由其他方发起,例如,产业界、商业界、消费者以及其他有关各方。
当风险管理的最终决策已经形成时,应将该决策的依据准确无误地告知所有的有关方,这一点至关重要。有效的风险交流应具有建立和维护信任及信心的目标。这将有助于所有的有关方在所拟议的风险管理方案上达成高度的一致意见并予以支持。
粮农组织和世卫组织联合专家会议的报告-《风险交流在食品标准和安全事宜上的应用》[8]详细地阐述了风险交流的原则和准则,各国政府应采用这些原则和准则,以巩固食品法规工作的透明度和广泛性的基础。
·各国政府应更加努力地开展风险交流,以传播食品安全的重要信息,并促使利益相关者广泛地参与国家的风险评估和管理进程(这些努力也同样有助于确立国家在食典委的地位)。
·粮农组织/世卫组织联合专家会议有关风险交流的指导方针及建议应广泛应用于各国政府的风险分析框架内,而且还应和风险分析的能力建设相结合。
3.食品安全法规采用的“从农场到餐桌”以及基于操作的方法-危害分析及关键控制点的作用
近年来,食品安全法规的范围已经扩大到整个食品供应链――从农产品生产直至消费过程。这种从“从农场到餐桌”的方法是为了在食品供应链中确定可能危及食品安全的多种环节,并确立旨在保障食品安全所需要的协调一致的干预措施。这种保障食品安全的方法源自于用“预防”风险取代在最终产品中测定风险的愿望,该方法尤其重视农场的管理和生产操作过程。
许多国家已经采取对食品法规进行逐步评估的措施,从制定详细的规格或产品标准逐步转向基于结果的行业操作标准。基于操作的标准是确定拟要实现何种食品安全目标,而不是规定如何实现这种目标,此类标准在一些国家日趋普遍,在食品安全和监管体系的核心和本质上均包括一些具有共性的内容。
操作标准的实施取决于食品生产者对加工过程的控制情况,是通过各种证明文件来证实是如何实现食品安全的目标的。因此,操作标准使得产商对安全食品的生产负有更大的责任,同时也使加工技术的创新更具灵活性。但是,对于高风险食品的生产和加工,仍然需要采用严格的专门法规。
操作标准使生产者能够主动地采用自愿的食品安全控制方法,包括良好卫生操作规范,以及加工控制体系如危害分析及关键控制点。基于危害分析及关键控制点的食品安全标准,在规定的风险分析框架中完全符合以科学为基础的食品安全评估及管理原则,而且可以适用于整个食品链的各个环节。
基于操作过程的标准和危害分析及关键控制点系统在许多国家中正越来越多地应用于高风险食品的生产。例如,澳大利亚食品标准要求发酵肉末制品的生产必须采用危害分析及关键控制点系统。新西兰乳制品法规要求在奶及奶制品的生产过程中必须应用危害分析及关键控制点系统。澳大利亚在未来几年中,将逐步把采用危害分析及关键控制点原理的强制性食品安全计划引入其他的高风险食品企业中。欧盟将采用在食品生产中实施危害分析及关键控制点的同类规定,而且美国也将对海产品、肉类、禽类及果汁颁布类似的规定。
由于中小规模的食品企业的能力和知识有限,引进以危害分析及关键控制点为基础的系统可能较为困难,但借助于食品工业内的协作以及国家和国际的培训及法规机构的介入,可以实现该系统的最佳实施(参见以下所述)。
在商业和法规上引进危害分析及关键控制点的另一结果是,希望在发达国家市场开展贸易的发展中国家日益需要采用危害分析及关键控制点系统。例如,马来西亚卫生部实施了一项危害分析及关键控制点的自愿认证计划,以应对欧盟对渔产品加工厂实施危
害分析及关键控制点的要求。截止至2001年年底,有40多个食品加工厂在此计划中进行了认证。因此,在其他以出口为主的部门中,食品卫生法规的优先重点将是实施基于法典的危害分析及关键控制点系统。
危害分析及关键控制点是以科学为基础的产业和法规的食品安全体系,其日趋广泛的应用也促使食典委通过并完善危害分析及关键控制点的准则[9]。食典委正在将危害分析及关键控制点纳入卫生操作规范之中,这些卫生操作规范是由食品卫生规范委员会负责制定的。因此,危害分析及关键控制点系统日益成为国际食品安全标准和贸易的基准点,并将成为所有国家食品安全法规的支柱。
为了帮助采纳危害分析及关键控制点的过程控制并将其纳入食品生产之中,各种国际机构均制定了方法和计划。这包括粮农组织/世卫组织的“危害分析及关键控制点:实用教师手册[10]”。粮农组织还实施了以不同国家的食品加工企业为对象的培训项目[11],这些企业需要提高其技术能力,以改善其产品的质量并满足出口的要求。联合国工业发展组织(工发组织)也在许多发展中国家努力开展了基于危害分析及关键控制点的能力建设工作[12]。
·在制定和审议食品安全法规措施过程中,各国政府应考虑为食品安全管理采取“从农场到餐桌”以及预防性方法,并将基于操作的食品安全标准纳入其中。应考虑将基于危害分析及关键控制点的食品安全控制系统作为所有高风险食品的强制性措施。
·各国政府应充分利用危害分析及关键控制点准则,以及粮农组织、联合国工发组织、世界卫生组织和其他国际组织在能力建设上所做的努力。
4.国际食品安全法规的协调一致
近几十年来,由于许多国家日益增加的人口的需求和不断扩大的收入增长以及出口机遇的增加,散装粮食产品和加工食品的国际贸易已经显著扩大。
全球食品贸易的扩大均聚焦在不同国家所采用的食品安全控制体系(无论是私营的还是公共的)和法规方法的差异之上。不同的国家在食品安全上的不同重点及努力均在国际论坛上突出地表现出来。此外,这些差异均涉及到国际争端,从而阻碍了国际食品贸易。即使在食品安全标准上已建立了基于风险的科学框架的国家之间,在食品安全法规上也具有差异。
国家之间存在的食品法规上的差异具有诸多的缘故,其中包括:
·在食品供应中,食品安全风险具有内在的和独特的差异(包括在生产操作、植物和家畜病原体和/或气候条件上的差异);
·评估和管理食源性风险的原则和协议不同;
·制定和实施法规计划的能力不同,以及控制食品安全风险和确保法规强制实施的基础设施不同;
·国家的文化偏爱和在处理食品安全风险中可接受的保护水平(例如欧盟在某些情况下允许采用未经高温消毒的牛奶,而澳大利亚则严禁采用和出售此类牛奶);
·国内监管体系包括了新旧不同的政策和标准,它们在不同产品或不同国家往往不一致;
·食品安全法规被用作贸易技术壁垒的手段,用以保护国内产业抵御外国的竞争。
4.1 食品安全法规的统一
为了促进贸易,不论是通过双边还是多边的协议,国家之间可以而且也已经进行了食品安全法规的合作。双边协议通常是贸易合作伙伴之间为了促进贸易而签订的。例如澳大利亚和新西兰于1996年决定开展合作共同将许多食品标准协调一致,以减少法规上的贸易壁垒和产业成本。1998年美国和加拿大签署了一份协议,在此协议下许多部门的农产品贸易得到了加强,包括某些食品安全标准的协调一致。
最为全面的多边协议是世贸组织的卫生和植物检疫措施(SPS)协议,其旨在将那些不一致的和偶尔不协调的食品、卫生及环境法规措施对贸易的影响减至最小程度。诸如世界动物卫生组织(OIE)和国际植物保护公约(IPPC)等其他国际组织制定的标准也分别致力于动植物领域法规的协调一致。虽然世贸组织的技术性贸易壁垒协定(TBT)并非针对食品安全法规,但也要求各国采用的针对传统质量因素、欺诈性行为、包装、标识等技术性法规对进口产品的限制不应大于其对国内产品的限制。技术性贸易壁垒协定还鼓励采用国际标准。
卫生和植物检疫措施协议确定了世贸组织成员国为保护人类、动植物生命和健康而采取措施的权利。该协议适用于所有的相关法律、法令、规则;检测、检验、验证和批准程序以及直接涉及食品安全的包装和标识要求。要求成员国只能采用基于科学原理的措施-主要是风险评估,并只能在必要的范围内实施,该范围是根据他们所确定的适宜保护水平界定,这些措施的实施不得构成
对国际贸易的变相限制。
在卫生和植物检疫措施协议框架下,食品卫生法规的合理性可以不同的方式实现。主要包括:
·采用国际标准,或
·通过对食品安全法规措施进行基于科学的风险分析。
卫生和植物检疫措施协议认为食典标准(包括与食品添加剂、兽药和农药残留、污染物、分析和取样方法有关的标准以及卫生操作规范和准则)与卫生和植物检疫措施协议的规定是相一致的。因此,食典标准可以用作国家卫生检疫措施比较的基准。虽然它并未强制性要求成员国采用食典标准,但是根据卫生和植物检疫措施协议,促使国家食品标准与食典委所制定的标准保持一致是符合成员国的最大利益的。
如同会议主题B以及以上所述,根据卫生和植物检疫措施协议,也可通过法规措施的风险分析来确定食品法规措施的合理性。
·为了提高公众健康并促进贸易,各国政府应建立各种机制,以促进食品安全法规与他们所签署的世贸组织各种协议保持一致。这包括采用食品安全标准和食典委制定的准则,或使二者统一。
4.2 食典标准可用作法规统一的基准
食典委制定的食品安全标准为食品安全的协调一致奠定了基础,因为这些标准反映了合理的、符合现代要求的、国际上认可的科学以及日趋采用的风险分析。
这些食典标准还反映了国际上对制定食品安全标准所采用方法的一致意见,这种方法是建立在科学数据和其他技术信息的基础之上(例如,如何考虑国家之间的饮食方式差异)。此外,食典标准是采用协商一致的方法而制定的,而且几乎每一标准均是采用协商一致的方式通过的。
虽然还无法获得全面的数据,但众所周知,食典标准已经为许多国家广泛地采用并以此作为其食品标准的基础。例如,在2001年食品法典非洲协调委员会指出,西非国家经济共同体自由贸易区的许多成员国决定采用食典标准,以使区域食品标准保持协调一致。
随着食典标准越来越多地被引用或采用,应保持它们在技术上的合理性并采用基于风险的一致原则阐明当代食品安全的风险,这一点至关重要。食典标准还必须赢得产业、消费者及贸易伙伴的信赖,并适用于发达国家和发展中国家。
·各国政府应认识到积极参与法典委工作的重要性。
4.3 等效性[13]
在通常的情况下,进口和出口国家采用不同的食品检验和验证系统。采用不同系统的原因包括特定食品安全危害发生情况不同,各国所选择的食品安全风险管理系统不同,以及食品控制系统的发展历史不同。
在这种情况下,为了在保护公众健康的同时促进贸易,进口国和出口国可彼此合作,探讨出口国卫生检疫措施如何有效地符合进口国适宜的卫生检疫保护要求。采用等效原则对进口国和出口国双方均有益。在保护消费者健康的同时,允许出口国依据自己的情况采用最为方便的措施来达到进口国适宜的保护水平,这将有助于促进贸易并最大限度地减少政府、产业、生产者和消费者因法规而带来的费用。进口国在对出口国所采用措施的等效性做出判断之后,也能减少检验措施的实施次数和范围。
食典委还为各国政府拟签订的双边或多边等效性协议制定了操作准则[14]。
·各国政府应与贸易伙伴开展合作,采用食典委的操作规范评估并协调法规方法,以促进安全食品的贸易。
4.4 技术援助和《卫生和植物检疫措施协议》
卫生和植物检疫措施协议鼓励向成员国特别是向发展中国家提供技术援助,这些援助的提供或是通过双边协议或是通过国际组织(例如,粮农组织/世卫组织资助发展中国家参与食典委活动的信托基金,为加强发展中国家出席和参与食典标准制定工作而提供资助)。卫生和植物检疫措施协议中涉及能力建设的这些条款,在提高发展中国家的法规能力上的作用有限,这是由于经费有限或发展中国家对这些权利的缺乏重视所致。如果要真正地解决发展中国家在食品安全法规上所面对的挑战,需要发达成员国、相关的国际组织、国际银行以及非政府组织/产业伙伴之间开展协调并投入大量的资金。
标准与贸易发展基金(STDF)就是这种伙伴关系的范例之一,旨在加强捐助方在帮助发展中国家制定标准方面上的协调,这些标准与食品安全、卫生和植物检疫措施协议以及动植物卫生等方面有关。标准与贸易发展基金是世界银行、粮农组织、世界动物卫生组织、世界卫生组织及世界贸易组织的联合计划,其运行规则为:(a)为发展中国家制定和完善标准能力建设的试点项目提供小额资助;(b)帮助政府和私营部门符合国际标准的要求,例如在世贸组织协议中所涉及的标准;(c)在提供标准方面的技术援助上,加强机构间的协调和捐助方之间的合作。标准与贸易发展基金还在食品安全、动植物健康领域方面实施了一项能力建设数据库的项目,该项目是由参与该基金的所有机构联合实施,有助于加强在能力建设方面的协调。
卫生和植物检疫措施协议在促进国际贸易和食品安全上成功与否,取决于所有国家能够在多大程度上建立全面而有效的食品控制体系。随着食品法典和国内法规标准之间的互相影响,很可能会出现食品安全标准日趋一致,尤其是那些用于制定标准和确保这些标准有效实施的系统的标准。
·为了促进食品安全法规的协调一致,无论是捐赠国还是受援国政府均应认识到食品法规能力建设工作的重要性,其中包括由相关的国际机构和双边渠道提供的援助。
5.实现食品安全法规协调一致的区域战略
许多国家特别是最不发达的国家,既无能力也无资源来应对这些挑战或利用因引进基于风险的食品安全法规而带来的机遇。在这些国家加强和建立食品安全监管体系,将提高粮食安全和公众健康,从而增加国际贸易的机会。
解决能力建设的问题应建立在区域基础之上。一旦确定了某一区域内各国的共同需求,该区域的各国政府应开展合作,交流信息并共享技术援助的资源,以满足其需求。
会议主题C-能力建设活动的优先重点及协调对这些问题进行了更加全面的探讨。
为了强化国家食品安全监管体系,粮农组织、世卫组织和食典委[15]已经将能力建设确定为食品安全的优先重点。这些问题也是粮农组织/世卫组织于2002年召开的“全球食品安全管理人员论坛[16]”的重要主题。第二次全球论坛将于2004年10月12-14日在泰国曼谷举行,其主题是“建立有效的食品安全系统”。将要讨论的两个领域为:
·加强官方食品质量控制服务;以及
·食源性疾病监测与食品安全快速预警系统(会议主题E-食源性疾病的监测和监督系统,将对此问题进行更加全面的探讨)。
国际组织(例如粮农组织和世卫组织)所处的地位,决定了他们应在建立联盟、制定信息交流框架以及协调与食品安全有关的能力建设方面发挥领导作用。这种协调将避免工作重复和重叠,促使对特定国家的技术援助能遵循适宜的顺序,有助于从有关国家吸取教训并强化所有权。同时也为公开和定期地回顾和评估能力建设计划提供一个体系,以确保这些计划的成功实施和可持续性。
建立联盟的首要步骤之一,是在区域政府机构之间建立畅通无阻的综合信息交流机制,以便定期交流有关食品安全活动的信息。在会议主题D-信息交流、教育和沟通中,将对这些问题进行更加全面的讨论。
为了促进有关食品法规的信息交流,粮农组织与卫生和植物检疫措施协议的标准制定机构以及世贸组织一道,建立了“食品安全、动植物健康国际门户网站[17]”,为获得国际标准、布告及相关文本和国家法规及规定提供了访问站点。
区域信息交流网络的例子包括东南亚国家联盟(东盟)的东盟粮食安全网络,东盟国家通过这一网络做出了承诺,将在食品安全方面进行更好的协调。在世卫组织粮食安全战略中有关东南亚区域的一项重要条款,也包括了该区域各国政府提出的要求,旨在
确保能定期地修订和评估食品安全法规,尤其要根据食典委的标准进行修订和评估。
实现国家和国际食品法规协调一致的另一个重要方面,就是要通过对国家需求的评估,评估单一食品控制体系的能力和限约因素(亦见会议讨论主题C)。
编写国别概况有助于促进需求评估的过程,其中包括对现行法规和规则、食品检验活动、实验室能力、公众健康问题、出口重点渠道等情况的回顾。国别概况旨在全面地评价能力建设和技术合作的需求,包括近期和长期的技术援助和人力资源及机构能力建设需求。
需求评估的结果将提供有用的信息,以便为国家内部行动和外部援助设计一个协调一致的总体方案,从而满足各个国家的具体需求。所提供的援助应由国际机构和有关各方进行协调,其中应考虑这些机构相应的职责、资源及专业技术的情况。
目前正在进行区域需求的评估,例如,通过世卫组织西太平洋区域办事处进行的评估,该办事处正在执行一项战略[18],通过实施世卫组织太平洋食品安全计划以加强太平洋岛屿各国之间有关食品安全的信息共享。其中包括有关食品进口和食品法规的国别信息联机数据库。
澳大利亚-新西兰食品标准局始终积极地和世卫组织西太平洋区域办事处及其他国际机构保持密切联系,共同帮助东南亚国家,包括越南、印度尼西亚以及南太平洋国家对其食品法规结构进行回顾,从而促进基于风险的、透明的食品安全法规的引入,这些法规均符合国际贸易的要求(参见主题B的文件)。
·粮农组织和世卫组织在食品安全控制系统的全球能力建设上继续发挥领导和协调作用,并与世贸组织、世界银行等其他国际组织、非政府组织以及成员国合作,建立联盟,建立信息交流的框架并实施与食品安全有关的技术援助项目。
·该区域各国政府和协会应继续开展合作,一起致力于解决食品安全控制的普遍问题,从而在公众健康和贸易机会上共同获益。
议题6 CAP 04/3
粮农组织/世卫组织亚洲及太平洋区域食品安全会议
2004年5月24-27日,马来西亚芙蓉
对食品管理应用风险分析-挑战与利益
(由澳大利亚-新西兰食品标准局撰文,澳大利亚堪培拉)
1.引 言
顾名思义,澳大利亚-新西兰食品标准局(澳新食品标准局)为澳大利亚和新西兰制定食品标准。然而,在本文中,澳大利亚-新西兰食品标准局的作用仅限定于澳大利亚本土。作为澳大利亚食品监管体系的组成部分,澳新食品标准局采用风险分析作为其决策过程的基础。澳新食品标准局按科学的方法已为食品标准制定设立了严格而透明的程序,并很高兴地和与会的同仁共享此经验。
风险分析是用于估计人类健康和安全的风险,确定和实施适当的风险控制措施,并将风险情况及所采取的措施与利益相关者进行交流。为了有效地实施风险分析,还必须通过基础设施支持与风险分析有关的各种活动,这些基础设施包括法规、测试实验室、强制实施体系以及权限和明确责任体系之间的协调。有关澳新食品标准局的详细信息可从澳新食品标准局的网站上获取[19]。
本文将阐述了风险分析的总体框架以及澳大利亚为维持和加强食品供应安全而应用风险分析的经验。
2.风险分析
2.1 风险分析:总体框架
为了帮助在决策中采用基于风险和证据的方法,已在食品和人类健康及安全的监管体系中采用了风险分析,因而,目前国际上对风险分析的框架已达成广泛的共识[20]。该框架具有三个重要组成部分:风险评估、风险管理和风险交流。在此框架内制定食品标准,首先要考虑的是其对人类健康和安全的作用。然而,在制定风险管理方案时,通常要考虑很多方面的问题,例如:经济影响和实施的可行性,考虑这些问题是为了选择最适宜的方案。
风险评估:风险评估旨在确定与食品相关的风险程度,并回答以下三个问题:会出什么问题(情况)?这个问题可能会如何出现
(可能性)?如果出现了,会产生什么结果(严重性)?风险评估的过程可以清楚地分为四个步骤――危害确定、危害定性、影响评估以及风险定性。从这些步骤获得的综合信息并根据有害事件发生的可能性以及后果的严重性,可以用来估计健康和安全的风险情况。
风险管理:该步骤的目的是为了确定是否需要和需要何种食品监管措施方可将风险降低至社会可以接受的水平。在处理健康和安全风险时,应制定风险管理方案并对其有效性进行评估,与此同时应考虑每一方案对于有关的利益相关者的影响,例如初级生产者、食品制作商、零售商、消费者以及政府等。
风险交流:风险交流包括社区磋商,是风险分析过程的基本内容。在风险分析过程的不同阶段开展风险交流活动很有帮助,可促使利益相关者积极参与。有效的风险交流将有益于所有的参与者,可确保利益相关者充分了解严格而透明的风险分析过程,并促进社区高度地信赖监管体系。
以下是风险分析框架的图解:
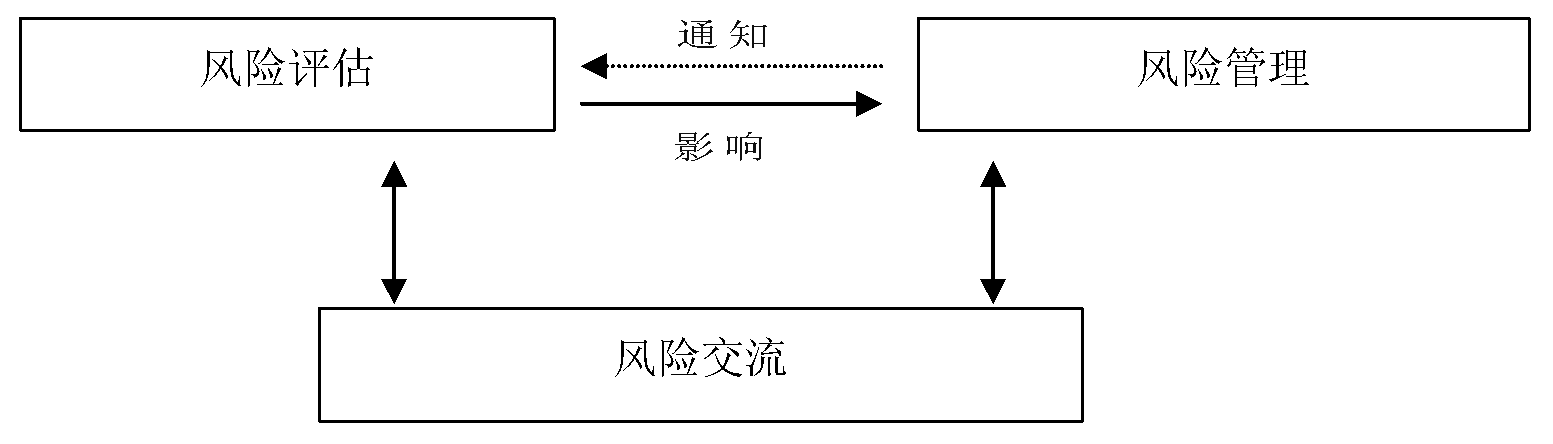
图1. 风险分析框架由风险评估、风险管理及风险交流三个重要部分构成。
2.2 国际环境
将风险分析应用于食品法规的制定,是确保一个国家在世贸组织贸易协议下履行其权力和义务的关键要素之一。就本文而言,世贸组织诸多协议中与食品法规关系最为密切的是“实施卫生和植物检疫措施协议[21]”(卫生和植物检疫协议)。卫生和植物检疫协议要求成员国采用的监管措施必须以科学原理为基础,在具有充足的科学证据下方可实施。要求成员国所采取的措施应建立在对人类生命和健康的风险进行评估的基础上。国家一级进行的风险评估应考虑采用由相应国际组织制定的风险评估方法并与实际情况适宜。就食品安全而言,相应的国际标准制定机构是“食品法典委员会”。食典委及其各种规范委员会和其他组织通常称之为“食典委”。
食典标准是评价国家食品措施和法规的基准。只有在具有科学的正当理由,或者确定了一个较高的适宜保护水平(ALOP)的情况下,各成员国方可采用具有比食典标准更高保护水平的措施。在确定适宜保护水平过程中,各国必须考虑将不利于贸易的影响减
至最小之目标。
加强卫生和植物检疫协议的实施具有许多非常重要的原则,其中包括:
·在成员国之间尽可能地使食品安全措施协调一致,从而避免不必要的贸易障碍;
·虽然出口国所采取的措施不同于进口国,但经证实可达到同等的安全保护水平时,应接受来自出口国的“等效性”措施,
·透明度-提供食品安全和贸易措施变化的信息或公告。
2.3 风险分析在食典委内的应用
风险分析为评估已有的与食源性危害或潜在危害有关的信息提供了框架,并有助于确定治理这些危害的最佳途径,从而减少疾病的风险或其他不利于健康的影响。风险分析还考虑了与受影响各方及利益相关者开展信息交流的必要性,例如和潜在的消费者、其他风险评估者、公众健康专家以及政府其他机构的交流。换言之,实施风险分析必需:
·评估食源性风险;
·确定并实施针对这些风险的最有效管理方法;
·采用有针对性和及时的方法与有关各方进行信息交流。
食典委已经界定了风险分析的三个组成部分,在2003年年中,食典委将风险分析纳入食典委程序手册中,从而最终确定并通过了其风险分析的工作原则[22]。这些原则为在风险分析的基础上制定食典标准和相关文本提供了方法和准则。目前,食典委正在致力于编撰有关风险分析的指导文件,以供成员国使用(食品安全风险分析工作原则的拟议草案),食典委总原则规范委员在2004年5月的会议上已对该文件进行了讨论。
工作原则规定,食典委在应用风险分析时应当:
·持之以恒;
·公开、透明和文件记载;
·按照“关于科学在食典决策过程的作用以及考虑其他因素的限定范围的原则声明”和“关于食品安全风险评估作用的原则声明
4”进行实施;
·根据新产生的数据,对其进行必要的评估和审议。
为此还制定了许多原则,这些原则规定了风险分析的构成应围绕着其三个部分进行,风险评估和风险管理的作用要分清,以避免作用的混淆和利益的冲突并确保风险评估在科学上的真实性。
最后,由于在风险评估和风险管理上可能存在诸多的不确定因素,所选定的任一风险管理方案均应考虑其真实程度以及危害的特性。
2.4 风险分析在国家一级的应用
澳大利亚已经将风险分析的原则及方法纳入到食品监管体系中。风险分析不仅应用于食品标准的制定,也用于应对因危害发生或控制系统失效而导致的粮食紧急情况。
在澳大利亚,澳新食品标准局在食品安全问题上发挥风险评估者的作用。在国家部一级的指导下并依照部的基本职责,也起到风险管理者的作用,特别是在食品安全紧急情况下,例如以下所列举的实例。最后作为食品的监管部门,澳新食品标准局承担了尽管不是全部但却是绝大部分的风险交流工作。依照问题,卫生和农业部门的同仁们在该领域可能也发挥其作用。
还应认识到,风险分析的深度和广度是取决于拟处理问题的紧急程度和复杂性。在正常情况下,有可能安排充足的时间全面地实施风险分析的所有步骤。但是在紧急情况下,则必须在很短的时间内作出决策。在这种情况下,应尽可能地维持风险分析的框架,以确保决策能够建立在合理的基础之上。在紧急情况下可采用临时的风险分析措施,但在合理的期限内随着新信息的获得,必须对其进行修订。
在澳大利亚本土上,风险分析适用于涉及食品的所有危害及对人类健康的影响。这些危害及影响包括:化学危害物、传染病原(例如微生物危害物和朊病毒)、营养素(无论是缺乏营养还是营养过剩)以及整个食品(例如转基因食品、辐照食品和其他新食品)。
对于不同类型危害所采用的风险评估方法不尽相同,其取决于现有可用的方法和信息。例如,完全定量的方法通常是用于化学危害物,而定性或半定性的方法是用于解决微生物危害。风险管理措施可采取食品标准的形式,包括禁令、限制、污染物的最大限量和/或标识要求。其他风险管理措施还包括对处于风险的群体提出建议、与产业联合采取监管措施(例如准则和操作规范)以及对整个社会的一般性建议。
在过去几年中,澳大利亚采取了重要的措施,以提高风险交流活动的有效性。多年来,在食品监管措施上,澳大利亚始终将其决策所依据的理由公布于众,现在还可获得电子版的文件。此外,澳大利亚还编撰有关监管措施的资料,旨在让资料通俗易懂并在社区、产业及专业层面上的各项活动中积极地开展广泛的交流。
2.5 挑战和益处
风险分析在国家一级的应用具有诸多的挑战和益处。其中列举部分如下:
2.5.1 挑 战:
·国家一级可利用的数据。澳大利亚在收集和分析食品消费和食品污染物数据的系统上的投入相当大。但是,在专门针对澳大利亚食品供应和社区的风险分析上,有时仍然难以获得足够和高质量的数量信息。这一问题与基础设施情况紧密相关,例如试验室能力。在国家基础设施之外,还有很多数据资源应当收集,包括国际专业机构(例如,食品添加剂联合专家委员会、农药残留联合专家会议和微生物风险评估专家联合会议),由其他国家开展的评估以及世卫组织开发的区域膳食。这些资源均可为本区域各国所用。
·训练有素职员的来源情况-具有全面的科学和专业技能的资历,是有效应用食品安全方面的风险分析所必需的。所需要的科学技能包括微生物学、毒理学、食品技术、营养学、免疫学和分子遗传学。其他专业技能,例如法律、经济和通讯等也是需要的。目前,澳新食品标准局在科学的风险评估和风险管理方面为本区域的政府官员提供了培训项目,以便他们能应用风险分析技术。
·将复杂的概念尤其是科技概念更为广泛传播到社会上是很困难的,这制约了有效的风险交流。目前,澳新食品标准局正在开发风险交流的培训方法,这些方法可为本区域的政府采用。
2.5.2 益 处
·能更好地确定和瞄准公众健康问题,从而最终改善食品安全的管理。
·通过集中解决食品安全风险最高的问题,实现更有效地利用资源。
·贸易机遇-通过客观地证实无危害的事实或有效地控制危害并生产安全食品,风险分析可为进入其他国家市场的谈判奠定坚实的基础。
·可以更加有效地与社会进行风险交流,从而改善食品生产、制作和贸易的操作规范。
3.实际应用/案例研究
以下讨论四个案例研究的结果,根据澳大利亚的经验,举例阐明风险分析原则在食品安全问题上的应用。
3.1 案例研究1:酱油和蚝油中的氯丙醇
3.1.1 问 题
近年来,在科学上日益重视称之为氯丙醇一类的化学物质。氯丙醇主要有3-氯-1,2-丙二醇(3-MCP)和1,3-二氯-2-丙醇(1,3-DCP)。这两种化合物均可在许多食品中发现。尽管几年前就知道氯丙醇存在于由水解植物蛋白制成的酱油和蚝油中,但英国2001年公布的对酱油和蚝油的调查结果表明,在某些产品中3-氯-1,2-丙二醇的含量很高。
后来,澳新食品标准局开始了自己的食品安全风险评估,对澳大利亚零售市场现有的酱油和蚝油进行了研究调查,以确定澳大利
亚食品供应中氯丙醇的风险水平。
3.1.2 风险分析
在国家风险评估中的膳食暴露评估部分,起先采用了来自英国调查样本的污染物发生率和含量水平以及澳大利亚的食品消费数据。但是,随着澳大利亚试验数据的增多,在膳食暴露评估中就使用了这些数据。澳大利亚风险评估的危害确定和定性内容很大程度上依赖于食品添加剂联合专家委员会[23]2001年第57届会议的工作。
国家风险评估结果断定,酱油和蚝油中的氯丙醇含量较高会对消费者产生不能接受的食品安全风险。在三个方面做出了风险管理决定:
1.
许多酱油和蚝油产品的3-氯-1,2-丙二醇含量已高达不可接受的水平,其零售商和制作商负责召回这些产品。
2.
按40%干物质含量计算,酱油和蚝油中3-氯-1,2-丙二醇的最大限量为0.2毫克/公斤,1,3-二氯-2-丙醇为0.005毫克/公斤,这些限量被纳入《食品标准法典》。这些标准不仅保证了污染物处于安全水平,而且其中也采用了“低到可实现的合理水平”原则,企业建议的含量通过良好生产操作规范是可以实现的。1,3-二氯-2-丙醇0.005毫克/公斤的最大限量是该物质的报告限量,采用了可接受的分析方法。
3. 采用酱油和蚝油产品的进口测试旨在防止进口产品具有不可接受的氯丙醇含量。
在处理这一问题的整个过程中,和有关机构及相应产业组织进行了密切的风险交流。一旦确定某些产品对食品安全构成不可接受的风险,就通过因特网上的公告和报纸宣传向消费者发出警报,并召回受到污染的各个产品。随后,将澳大利亚的整个风险评估结果(食品中的氯丙醇――公众健康风险的分析,技术系列报告15)在澳新食品标准局的网站上予以公布[24]。
3.1.3 问 题
食品中氯丙醇的形成至今尚不清楚,澳大利亚政府机构继续与相关的产业部门保持联系,以便测定食品中氯丙醇的来源,同时还密切关注着国际上的文献报道。在澳大利亚食品供应链上起初几乎没有与氯丙醇有关的数据,只有在具备了测试能力之后方能收集到这些数据。在此过渡期间,澳大利亚只能利用国际专业组织和其他监管者的工作结果,以加快风险管理的决策。
3.2 案例研究2:对虾中的硝基呋喃残留物
3.2.1 问 题
在一些国家,硝基呋喃类化合物是用于人类和动物上的广谱性合成抗菌剂。根据科技文献的报道,硝基呋喃类具有四种化合物,即呋喃唑酮、呋喃它酮、呋喃妥因和呋喃西林。
1993年食品添加剂联合专家委员会撤消了这些硝基呋喃类化合物的卫生标准(即每日允许摄入量),这是因为毒物学数据库的不完全性以及在动物研究上出现令人担忧的致癌性。因此,包括澳大利亚在内的许多国家随后纷纷限制或禁止在食用动物上使用硝基呋喃类化合物,后来在食品中不允许含有能检测到的残留物。
2003年10月,有资料表明在一些进口的对虾中发现了微量的呋喃唑酮代谢物,3-氨基-恶唑烷酮。在检测到残留物的产品中,含量极小仅为十亿分至几(微克/千克)。但是,在《食品标准法典》没有专门规定的最大残留限量的情况下,这些残留物是不允许存在的。
3.2.2 风险分析
鉴于这些检测的结果,澳新食品标准局开展了风险评估,以确定对虾中所检测到的残留物含量对消费者构成的食品安全风险的程度。开展风险评估是为了使执法机构知悉是否要采取任何风险管理行动,以保护消费者的健康,例如对对虾进行检测和(或)召回某批含有残留物的对虾。风险评估中的膳食暴露评估部分,采用了在一家企业发现的残留物含量,危害鉴定和定性是根据食品添加剂联合专家委员会论文集所概述的资料进行重新评估的结果。
风险评估表明,由对虾中这些微量残留物而导致的风险极小,因此可以安全的食用这些对虾。澳大利亚没有必要考虑召回那些已经进入市场的的对虾。然而,假如这些残留物不符合《食品标准法典》的规定,则建议执法机构检测进口对虾的硝基呋喃类化合物含量。采用的检测比例相对较低,这与消费者食品安全的风险程度是相称的。
3.2.3 问 题
在澳大利亚,进口对虾硝基呋喃残留物的问题已经引起新闻媒体的极大关注,澳新食品标准局和其他政府机构业已强调这些对虾可以安全食用。但是,毒理学资料不足和缺乏每日允许摄入量的规定,对风险评估构成了特殊的挑战。在其他国家已经检测到这些残留物,但澳大利亚在建立检测技术之前,却不能证实它们的存在。这也使得与社区开展风险程度的交流更加困难,尤其是在出现危言耸听的媒体报道情况下。
3.3
案例研究3:甲壳动物熟肉中的单核细胞增多性李斯特杆菌
3.3.1 问 题
在某些种类的食品中,单核细胞增多性李斯特杆菌对公众健康和安全构成了潜在威胁,特别是易受害的子群体,例如孕妇、免疫力缺乏者以及老年人。虽然由食品中的单核细胞增多性李斯特杆菌导致全身感染的发病率较低,但其后果极为严重,甚至死亡。澳新食品标准局于2002年开展了一项微生物学风险评估,以估计食用受李斯特杆菌污染的甲壳动物熟肉对澳大利亚和新西兰消费者所构成的风险。
3.3.2 风险分析
风险评估采用的数据是来自澳新食品标准局于2002年对甲壳动物熟肉中李斯特杆菌的调查结果[25]以及有关李斯特杆菌病发病率的公众健康资料。开展该项调查的原因是,澳大利亚缺乏有关食品中李斯特杆菌污染程度的全国性资料。采用几率模型作为风险评估组成部分,该模型估计,如果在国内贮藏期间李斯特杆菌没有出现生长,澳大利亚因食用受污染的甲壳动物肉类而致病的几率为每1600年出现一例。按最严重情况的模型,即假设熟虾在出售后置于家中冰箱贮藏最长达三天(导致李斯特杆菌滋长),预计澳大利亚因甲壳动物熟肉而导致李斯特杆菌病的几率是每2.5年出现一例。
风险分析的结论是,因此类食品而患李斯特杆菌病的风险很低。该结论是基于:
·几率模型的研究结果;
·甲壳动物熟肉受李斯特杆菌的污染率很低(约3%)而且含量也很低(小于每克50个菌落数[26]);
·制作过程包括了灭菌步骤(蒸煮);
·产品的货架期很短,因此限制了食品中病原菌的生长机会,食品中的病原菌是加工后污染所致。
根据风险评估的结果,甲壳动物熟肉中的李斯特杆菌对整个群体构成的风险很低,因此不要设置微生物限值。然而,应当认识到,对于某些子群体而言,风险可能会较高,因此需要备用的风险管理措施。
3.3.3 问 题
针对易受害群体的另外建议(采用忠告宣传页)可以视为是最为适宜的风险管理战略。澳新食品管理局以前曾经编写过这样的建议材料,目前正在修订针对处于风险的人们的宣传页。
有关微生物风险管理的全部材料可从澳新食品标准局的网站上获取[27]。
3.4 案例研究4:生物技术食品的风险分析
3.4.1 问 题
首先,如何证实转基因作物和其他转基因生物的食品是和传统的食品一样安全,其次,如何通过适当的标识确保消费者知悉他们所购买的食品情况。
3.4.2 风险分析
在澳大利亚采用基因技术生产的食品须经科学的安全性评估之后,方可作为食品供应。强制性的销售前批准程序适用于所有的转基因食物,无论是种植于国内,还是在其他国家。其他国家也有类似的转基因食品评估程序。例如加拿大、美国、英国和欧洲其他国家。此外,澳新食品标准局转基因食品的安全性评估程序与食品法典委员会制定的方法相一致。
澳新食品标准局开展的转基因食品安全性评估,是依据以下五项重要原则:
1.
安全性评估是采用基于风险的科学方法:用于安全性评估的科学数据从各种不同的来源获得(主要是来自申请者,但也来自于科学文献、一般的技术资料、中立的科学家、其他监管机构和国际组织以及整个社会),并采用国际上公认的基于风险的科学方法对这些数据进行评估。
2.
安全性评估是建立在逐案评估的基础之上:转基因食品的安全性评估不能作为单一类别进行,因为安全性问题取决于食品的类型和遗传修饰的性质。对于被用作食品或用在食品制作中的转基因品种,安全性评估是针对该品种的若干部分或组成成分进行的。例如,以不同方法进行遗传修饰的大豆制作成的食品必须分别予以评估。
3.
新的遗传材料、新的蛋白质和转基因食品的其他特性均应予以考虑:澳新食品标准局认为,转基因食品中的新成分(新的遗传材料/DNA,和由该DNA编码产生的新蛋白质)应予以逐一地全面考虑。食品的其他特性,例如营养水平和天然存在的过敏原、毒素和抗营养素,也应予以详细的考虑,因为这些特性可能会受到遗传修饰作用的直接影响。
4.
应考虑转基因的预期和非预期结果:引入新基因的预期结果(直接结果)是产生新蛋白质,从而表现出预期的性状。作为安全性评估过程的组成部分,应全面地评估转基因的直接后果,例如任何新蛋白的潜在毒性/过敏性,抗生素水平抗性基因出现转移的可能性,或任何期望的营养成分改变结果。成分改变等非预期结果(间接结果),如脂肪酸模型的显著变化,产生新毒素或过敏原的可能性,维生素和矿物质的显著改变等,也需要予以详细的考虑。这些结果因转基因性质的不同而不同。
5.
必要时,可以与传统生产的食品进行比较:新产品可与传统生产的食品进行比较。假如传统方式生产的食品长期以来均可安全食用,转基因食品就可以与其进行比较。比较法常用于确定转基因食品的过敏原、毒素、营养素或抗营养素的水平是否不同于传统食品中天然的含量水平。转基因食品和传统食品之间的任何显著差异均要对其不利于健康的潜在作用进行评估。值得注意的是,虽然转基因食品的成分可能会不同于传统食品,但是这并非意味着该食品就是不安全或营养不充分。每一种转基因食品需要逐一地进行评估。
3.4.3 问 题
澳大利亚为规范转基因食品而制定的风险分析框架,是当今世界上最健全、磋商性的、透明的但也有争议的框架之一。澳新食品标准局正在利用已取得的颇有价值的经验,完善转基因植物食品的监管体系,帮助制定转基因食品的未来的法规准则,这些食品是指来自转基因微生物、动物和鱼类的食品以及采用新的生产技术或含有新成分的食品。
4a. 澳大利亚已经采取的实际行动
每一国家都要采用自己的并符合特定情况的方式,来应对食品法规的挑战。澳大利亚在国际和区域如何面对这些挑战的部分实例列举如下:
在国际上对风险分析活动的促进作用
澳新食品标准局在食品法规和采用风险分析解决食品安全问题方面具有专业技术优势,借助于这一优势,它为促进区域和国际在食品安全问题特别是风险分析上的对话做出了真正的贡献。通过食典委的会议、专家小组、以及世界卫生组织的国际磋商会,澳新食品标准局为食品安全问题上的国际对话做出了贡献。例如,澳新食品标准局积极促进粮农组织/世卫组织食品添加剂联合专家委员会(JECFA)、粮农组织/世卫组织农药残留联合专家会议(JMPR)以及粮农组织/世卫组织微生物风险评估专家联合会议的工作,并为粮农组织/世卫组织的专家磋商会议提供专家咨询意见和资料。
风险分析上的能力建设
澳新食品标准局还与相邻区域的一些国家一道参与了技术援助计划。技术援助通常是借助于合作项目予以提供,并得到一些机构的支持,例如:澳大利亚国际开发署、亚太经合组织、粮农组织、世卫组织、经济合作与发展组织以及新西兰外交及贸易部。这些援助计划力图:
·通过提高发展中国家食品监管体系的一致性和公开性,从而增加安全食品的贸易;
·制定有助于亚太区域食品标准协调一致的方法;
·提高发展中国家的科技能力,尤其是提高他们参与食典委工作的能力;
·建立和加强发展中国家的科技可靠性;
·帮助建立亚太经合组织/东盟国家之间的战略伙伴关系,尤其是在食典委事务上。
国际上的能力建设形式不尽相同,其包括如下:
·食品标准的信息交流、标准制定、调查的设计和实施以及风险评估方法;
·研究方面的合作和信息交流以及科学风险评估;
·交流有关国际食品安全性工作和食品标准机构的观点,并确定共同的食品安全性前景;
·专家和管理人员的访问。
近年来实施的各种类型计划的实例如下:
| 活 动 | 国 家 | 合作机构 |
| 食品召回计划 | 斐济 | 世卫组织 |
| 与农业有关的转基因生物的安全性和风险评估的培训研讨会 | 东盟国家 | 东盟/国际生命科学学会 |
| 食品召回计划 | 瓦努阿图 | 世卫组织 |
| 食品标识和成分标准的用户指南 | 印度尼西亚 | 澳大利亚国际发展署 |
| 风险评估培训(2个计划:化学和微生物学风险评估) | 12个区域经济 | 亚太经合组织/澳大利亚国际发展署 |
| 制定食品安全性培训计划 | 越南 | 世卫组织 |
| 食品法规的制定 | 越南 | 世卫组织 |
| 转基因食品的安全性评估 | 区域经济 | 亚太经合组织 |
最近六个月以来,澳新食品标准局对其在该地区能力建设方面的贡献进行了较为全面的评估。调整了其贡献内容,目前提供了一项全面的培训计划。详细情况可见澳新食品标准局的网站。
建立战略联盟
为了广泛地提高食品安全性,澳新食品标准局一直致力于与区域及国际组织建立联系和联盟。这样的联盟强化了澳新食品标准局获得外部资金的能力,从而能够提供技术合作项目。已经与以下机构建立了联盟:
(a) 世界卫生组织(WHO)西太平洋区域办事处
和世卫组织西太平洋区域办事处一道开展了非常重要的活动。澳新食品标准局的主要重点已置于越南的培训活动上。除了其对此特殊活动的兴趣之外,世卫组织西太平洋区域办事处已表示,希望探讨将澳新食品标准组织制定的有关风险评估和标准制定的培训计划推广至亚太区域的其他国家,尤其是南太平洋地区。
(b) 国际生命科学学会(ILSI)
澳新食品标准局和国际生命科学学会已经就开展联合工作问题进行了讨论,主要是在食品安全、转基因食品,以及食品/药品兼用产品的领域。澳新食品标准局和(东南亚)国际生命科学学会正在制定此类合作的议定书/协议。
(c) 双边联系
在双边的基础上,澳新食品标准局还向个别国家提供有关风险分析的专业建议和指导。最近的一项计划涉及2003年为文莱政府进行食品安全体系的战略评估。
案例研究:开展风险评估的培训以支持食品安全措施
澳新食品标准局利用亚太经合组织和澳大利亚国际发展署的资助,实施了两项旨在提高发展中成员国开展风险评估的能力的培训计划。
该培训计划是由两个为期两周的培训班组成。第一个培训班(河内,2002年7月)着重于食品的污染物、天然毒素和农药残留物。第二个培训班是检查微生物风险评估,于2003年10月在胡志明市举办。
参与培训人员来自本区域的许多国家,包括文莱达鲁萨兰国、中国、中国台北、香港、印度尼西亚、老挝人民民主共和国、马来西亚、巴布亚新几内亚、菲律宾、韩国、新加坡、泰国、南朝鲜和越南。这些培训人员获得了完整的培训教材;在强化实践的项目中,他们学习了如何开展风险评估并了解风险评估者的作用和责任。
最近结束的计划涉及在转基因食品安全性评估方面的培训。在亚太经合组织的资助下,澳新食品标准局举办了为期一周的有关转基因食品安全性评估的培训班。该培训班倾重于国际上用于转基因食品安全性评估的各种方法,包括食典和粮农组织/世卫组织制定的检验方法和准则,国际批准体系的概况以及目前采用方法的总体情况。
4b. 结 论
首先,各国在进行食品安全风险管理过程中应采用风险分析原则。这不仅是世贸组织(尤其是卫生及植物检疫协议)之要求,而且是开展有效的风险确定和实施适宜风险管理战略的有效手段。
澳新食品标准局认为,国家应明确规定食品监管机构在管理食品安全问题上的各种职责,拥有这种职责的食品监管体系是最有效的体系。作为一个拥有比多数国家更加复杂的食品监管体系的国家,是十分清楚这一重要性的。在出现食品安全紧急状况的时候才决定由谁来负责某一特定问题,并非明智之举。
出现食品安全紧急状况是检验一个食品监管体系的最佳时期。食品安全紧急状况通常具有数据不足、公众关注和没有时间开展全面的风险评估等特点。在这种状况下,国家应实施临时的风险评估,在获得更多的科学数据之后必须对此评估进行修订。在21世纪中,确保一个食品安全体系有效运行所需要的科技和管理技能并不容易获得。发达国家有义务实现其专业技术的共享,不仅要实现信息共享,还要向发展中国家提供技术援助(尤其是培训)。
作为信息共享的组成部分,各国应互相交流有关食品安全危害的风险评估文件,因为促进对食品危害的了解能够实现协调一致并达成共识。澳新食品标准局发现,将技术报告和风险评估结果置于其网站上,是促进这种交流的最好机制。
最后,如果不能有效地与利益相关者尤其是消费者开展交流,即使有最好的风险评估技术和最佳的风险管理战略也无济于事。上一世纪九十年代的显著特征就是,许多国家对其食品监管部门在保护本国公众健康和食品安全上的能力失去了信任,一个重要的原因就是风险交流不够。公众信任的程度是和监管措施的公开与透明程度紧密相关。随着新的食品安全风险的上升,食品监管部门必须确保有关这些风险的信息能够准确无误并持续不断地传播至产业和消费者之中,反之,监管部门应认真听取这些产业和消费者的反馈意见,食品安全人人有责,必须使每一个人意识到应为更加安全的未来发挥自己的作用。
议 题7 CAP 04/4
粮农组织/世卫组织亚洲及太平洋区域食品安全会议
2004年5月24-27日,马来西亚芙蓉
能力建设活动的重点与协调
(印度撰文)
1.引 言
随着全球化、乌拉圭回合多边贸易谈判的进程,以及世界贸易组织随后的卫生和植物检疫措施协议(SPS)和贸易技术壁垒协议(TBT)的实施,食品质量和安全问题已成了粮食安全、公众健康保护和粮食贸易方面的重要问题。大家充分认识到,要提高食品质量和安全,就要在这方面进行能力建设,这不仅在发展中国家要这样做,许多发达国家也应这样做,其目的就是与世界贸易组织保持一致。
卫生和植物检疫措施协议第9条和贸易技术壁垒协议第12条强调,必须向发展中成员国提供‘技术援助’,特别是要符合发达的进口国家对卫生和植物检疫措施的要求,以促进发展中成员国的市场准入。协议进一步规定,技术援助可包括基础设施建设、研究活动及加工技术。这不仅为发展中的成员国提供了技术知识和培训机会,也为其出口市场所需的卫生和植物检疫保护的适当标准提供了必须的硬件。
因此,国际上食品安全能力建设的活动日益增多。联合国粮农组织和世界卫生组织前不久对这些活动提供了支持,从以下网址可以获得食品法典委员会第26届会议的参考文件(http://www.fao.org/DOCREP/MEETING/006/y9805e.htm)。
本文阐述了各国在能力建设方面确定特别急迫和重要需求及其优先重点、协调能力建设活动,以及能力建设活动长期可持续性之必要性。
2.确定国家一级特别急迫和重要的能力建设需求
能力建设过程必须从实事求是的评估开始。通过评估,国家(包括所有的利益相关者)将确定该国特别急迫和重要的需求。需求评估过程需要评估现有的结构,包括法规、相关机构、现有能力,以及优先领域。援助需求可广泛包括人力资源和机构能力建设的各个方面。粮农组织正在编写评估食品安全能力建设需求的指南,该指南包括评价食品控制系统不同组成部分的工具,以帮助各国确定某个地区特定国家的需求内容。
2002-2003年,粮农组织食品质量和标准处编制了食品安全能力建设方面的问卷,开展问卷调查并将其汇编成册。根据非洲、亚洲、拉丁美洲、西南太平洋和近东至少48个国家的反馈结果,确定了需要加强的领域。这些问卷的报告可在下列网址获得:ftp://ftp.fao.org/es/esn/food/CB_questionaire.doc。
2003年,粮农组织和世界卫生组织联合出版了《保障食品安全和质量 –
强化国家食品控制体系指南》,旨在帮助各国主管部门,特别是发展中国家,改进其食品控制系统。该指南概述了各种食品控制系统的优劣情况,并力求对强化食品控制系统的战略提出建议,以保护公共健康,防止欺诈和欺骗,避免食品掺假和促进贸易。除国家主管部门外,该指南还有益于对其他各利益相关方提供帮助,包括消费者组织、产业和贸易组织、农民团体及影响国家食品安全和质量政策的其他任何团体。
3.能力建设活动的协调
尽管各个国家均开展了不同形式的能力建设和技术援助活动,强化了食品安全和质量控制的特定方面,但这些活动往往未能协调
一致或纳入食品安全和质量总体战略或发展计划之中。因此,许多活动显得效率低下或无法达到最佳或可持续结果。所以,无论是在特定的国家、区域还是在世界范围,均必须加强与食品安全能力建设有关的各机构之间的合作与协调。
3.1 区域方法
许多能力建设活动都不能充分满足区域需求或解决共同关心的问题。只有确定了区域合作领域,才能开展适当的援助,从而加强整个地区的能力。此外,应确定各种国家体系的优势,并制定一个向该地区其他国家提供技术援助的系统。某些业已确定的领域包括检查、检验和出具证书,包括出口证书。
3.1.1 共同参与国际标准的制定
随着食典标准日趋为各国所接受,成员国对食品法典委员会的各种活动的兴趣日益增加。卫生和植物检疫措施协议也明确强调,各国应参与制定国际标准工作。
由于费用和能力方面的局限,个别国家难以参加国际标准制定组织的工作。欲了解一个发展中国家的观点可能需要付出极大的努力。旨在增加区域凝聚力的区域能力建设,将有助于该地区的所有国家在食典制定过程充分发表各自的意见。必须帮助这些国家向食典委系统提供相关数据,并全面参与标准制定过程,包括出席食典委会议的自然人数。
3.1.2 安全管理体系方法
目前,正在将基于危害分析及关键控制点的方法纳入食典委正在制定的新卫生规范以及许多国家(包括澳大利亚、加拿大、美国、欧盟成员国以及其他国家)的法规要求之中,尤其是针对风险比较大的产品,如海产品、肉、禽和奶产品。
为了在地区一级建立产业和管理机构,可在轮流的基础上制定涉及下列内容的区域计划:
i)
研究发达国家正在实施的危害分析及关键控制点系统;
ii) 开发适用于各种重要行业的危害分析及关键控制点模型,以促进产业发展;
iii) 为产业和主管当局提供危害分析关键控制点的全面培训,包括审计。
3.1.3
建立食品安全和质量的人力资源开发区域中心
在开发和提高人力资源技能以及实施符合国际要求的食品质量和安全计划过程中,培训已被公认是重要活动内容之一。应努力建立食品安全及质量人力资源开发区域中心,以满足地区一级在食品检验、实验室分析、危害分析关键控制点的应用等领域的培训需求。可从潜在的捐赠方寻求所需的资金。这样就可以满足该地区所有国家的需求。
3.1.4 等效性
在卫生和植物检疫措施协议中已经确定了等效性的概念,在国际上也得到食典委员会的不断支持,其目的就是为了更加有效地使用共有资源,避免重复检验和检测,并且确保有效地满足卫生和安全要求。这些行动也是促进贸易的重要手段,即通过承认出口国的标准和证书体系,提供与进口国一致的预防健康风险的标准和证明体系,同时可减少拒收率,减少出口产品在海外市场的检验。
此类等效协议通常是在个别进口国和出口国之间签署。然而,如果在地区一级制定这类协议-通过区域协议的形式,确定该地区所有国家特定的卫生和植物检疫措施具有等效性,这不仅有益于该地区内的贸易,也有利于在区域基础与第三方国家谈判等效协议。这类协议还有助于减少各成员国的财务负担。
3.1.5 基于风险的方法与地区一级的协调
食品中的危害物给消费者带来的风险在国际上已公认是一个重要问题。卫生和植物检疫措施协议第5条规定,卫生和植物检疫措施应以风险评估为基础,同时应考虑开展与有关实际风险相适应的评估,如果出口国提出要求,应公布此类评估的详细情况。在制定和通过标准时,食典委也提倡所有食典规范委员会采用风险分析方法。在确定标准供国内使用前,尤其是在这些标准因当地产品或因当地或区域条件而与国际标准有差异时,必须不时地开展风险评估。建议在各国利用各自力量联合开展风险评估研究的基础上,制定区域一级的标准。同时还要在区域范围内使这些标准协调一致。
3.1.6 建立实验室联网
实验室里的仪器和食品分析方法日趋复杂,给许多发展中国家造成障碍。所以,加强实验室里的设备、人力和基础设施的建设一直是个重要领域。在区域范围内,建立实验室联网系统十分有益,便于一个国家的设备能为其他国家所使用,这样的话,没有必要重复购置这些设备,同时又可用于该地区的所有国家。使这些实验室符合国际上的通用标准是实现上述目标的先决条件。
还应组织实施实验室工作人员的联合培训计划。建立一个特定的实验室体系,负责制定和修改标准方法并共享这些方法,这也许很有帮助。在区域范围内,还可以组织实验室间的标定试验。这样也可促进供决策用的分析试验数据的共享,并促进标准的制定。
3.1.7 证 明
不同地区出具证明的方法,如出口证明、危害分析关键控制点、国际标准化组织9000,等等,应在该区域内得到认可,而不管其国内的运行情况。一个在某个领域拥有先进经验的国家可以帮助在此领域没有现行系统的一些国家,直至它们能够建立自己的系统。
3.1.8 安全与质量区域委员会
建议本区域建立一个区域委员会,以负责处理世界贸易组织范围内所有食品安全和质量问题,该委员会应由该地区所有国家的代表组成,每个季度召开一次会议,讨论上述领域的合作与协调问题,确定援助需求等等。委员会主席可轮流担任,会议也可在各国轮流召开。
3.2 国际水平
食品安全能力建设活动必须在国际一级协调一致,这些活动包括如下:
·安排区域或全球食品安全活动,讨论和交流全球关注的食品安全问题;
·组织召开应用危害分析关键控制点方法预防和控制污染物、毒素等研讨会;
·组织和实施各国均可参加的食品安全项目。这样可以共同提高各国贸易密集型产品的质量。这些项目也可以包括动植物病害跨境移动及其预防;
·实施一项国际生物技术和食品安全与质量的综合计划,以加强人力资源建设以及卫生与植物检疫措施的实施。
4.能力建设活动成果的长期可持续性
为了使技术合作活动具有长期有效和可持续性,除专业能力建设外,应包括所有利益相关者。特殊重点应置于比较敏感的关键人物之上,如政策制定者,还要着重于制定与食品安全有关的公共教育计划,如将质量和安全方面的内容纳入教育课程中。必须通过实施国际系统的标准加强食品控制系统,这些标准包括食品法典、ISO
17020、ISO 17025、ISO/IEC准则62和65,此外还应寻找信赖,确保国际标准的持续实施。
5.结 论
能力建设必须符合发展中国家的需求、重点和条件。虽然已经开展了一些与能力建设有关的活动,但所作的努力都是零星的,不足以达到乘数效应。大部分能力建设的活动都集中在培训和研讨会方面,而基础设施的开发如实验室的设备一直有限。从国家、区域及国际的长远观点,能力建设必须采取综合方式进行。
议 题8 CAP 04/5
粮农组织/世卫组织亚洲及太平洋区域食品安全会议
2004年5月24-27日,马来西亚芙蓉
信息交流、教育与传播
(日本政府撰文)
1.引 言
当今全球社会的发展,要求食品链上的所有利益相关者,包括政府机构、研究和学术单位、粮食生产和加工部门以及消费者,必须加强信息交流、教育和风险交流。增加这方面的透明度可以促进食品安全并提高消费者的信心。一个国家在这些方面吸取的重大教训应与其他国家共享,这有助于在提高食品安全方面加强区域合作。
2.风险交流-日本的若干事例
最近,日本经历了诸多事件,导致消费者对食品安全性失去了信心。在这一系列事件中,包括2000年6月一家经危害分析及关键控制点[28]认证过的工厂所生产的奶制品导致了大范围食品中毒事件,以及2001年9月发现了第一例染有牛海绵状脑病(即疯牛病)的牛。2002年4月,日本政府建立了“食品安全管理大臣委员会”,由该委员会负责考虑并提出新的管理组织必须具备的职能,以确保食品安全。大臣委员会由公共管理省、家政省、邮电省、厚生劳动省、农林水产省的大臣们以及内阁官房长官组成。大臣委员会根据顾问委员会提交的有关疯牛病问题调查报告及从其他渠道获取的信息,决定:(1)为了体现保护消费者健康这一首要重点,政府应当开展风险分析,以解决食品安全问题,并成立食品安全委员会这样一个新机构,负责风险评估;(2)认真制定一部新的综合法律《食品安全基本法》(暂定名),旨在保护消费者并确保食品安全。因此,于2003年5月制定了《食品安全基本法》,2003年7月成立了内阁办公室的附属机构-食品安全委员会。该委员会独立于厚生劳动省及农林水产省的风险管理机构。
日本政府做出的上述决定和行动表明,为了平息消费者对日本疯牛病的关注,不仅要成功地进行风险交流,还要对政府体系进行重大改革。这些改革包括引进风险管理措施,制定综合法律,以及建立风险评估机构,日本政府希望这些改革能恢复消费者的信心。
2.1 实施风险分析前开展风险交流的若干事例
2.1.1 奶制品引起的食品中毒事件
这第一个案例就是因公司管理不当所致,该事件在柳叶刀医学杂志上也作了报道(第356卷,第573页;2000年8月12日)。
因食用奶制品(包括雪牌公司大阪厂生产的“低脂肪奶”)而引起的食品中毒,致使14,780人受害(受害者人数是指2000年6月27日第一份报告以来出现症状的人数),造成了空前的大规模食品中毒。通过调查清楚地表明,导致大规模中毒的原因是多方面的,所有这些均极大地降低了消费者对制造商的信任。这些原因包括:拖延向当局报告中毒事件,造成受害人数增加;尽管厚生劳动省根据《食品卫生法》第13条对加工厂进行了危害分析及关键控制点认证,但卫生措施不当;危害分析及关键控制点计划实施不力;在多次新闻发布会上提前公布了监管当局和雪牌公司有关中毒事件调查的中间报告内容;持续销售可能含有葡萄球菌毒素的其他产品;生产记录保存不全及其他一些原因。
在该案例中,发现该公司存在的问题主要包括:缺乏信息公布机制、食品安全控制不力、缺少危机感,以及与消费者的交流不当等。
2.1.2 日本染有疯牛病的牛
该案例包括一些典型问题。因安全控制措施不当和政府危机通讯不畅,使这些问题进一步恶化。
2001年9月10日,农林水产省正式宣布,运往千叶县屠宰场的一头奶牛不能站立,被怀疑是感染了疯牛病。这一公布使整个国家陷入恐慌状态。
出现第一例疯牛病后,日本政府立即向公众做出解释,称日本的疯牛病病例数很低。但是,消费者错误地感觉到,疯牛病的威胁远远大于政府声明所述,并开始对政府失去信任和产生不满。
为了保证上市牛肉的安全性,厚生劳动省及农林水产省采取了一系列措施,其中包括:
·停止销售出现疑似病牛的农场所生产的牛肉(9月11日);
·全国中止运输30个月龄或超过30个月龄的任何牛(9月19日);
·清除和焚化来自12个月龄或超过12个月龄牛体的特定危险物质(9月27日);
·清除特定危险物质,并对所有年龄段的所有牛进行筛查试验(10月18日),以及其他措施。
由于政府实施了这些确保牛肉安全性的措施,并将这些措施公布于众,消费者的关注逐步下降。
2.2. 实施风险分析后的若干事例
自2003年7月以来,日本政府在风险分析框架内一直积极地开展风险交流。例如,在2003年7月至2004年3月间,召开了34次利益相关者(如管理人员、消费者、生产者和食品安全专家)会议,交换日本各方面的意见。这些会议旨在倾听利益相关者的意见,并使这些意见在风险管理措施中得以体现。现将在这种新框架下所获得的经验详述如下:
2.2.1 加拿大和美国染有疯牛病的牛
2003年5月,加拿大发现了一头染有疯牛病的牛;2003年9月美国也发现了一头染有疯牛病的牛。立即中止从这些国家进口牛肉;处置被特定危险物质污染的牛肉;迅速公布加拿大和美国鉴定染有疯牛病牛的情况;日本政府采取的所有这些有效控制措施,未使日本消费者的购买和饮食行为发生大的变化。
2.2.2 日本禽流感情况
在禽流感事件中,尽管获得禽流感爆发的消息后就禁止从受感染地区进口家禽,但遗憾的是在日本还是发现了一些病例。不过,这些病例是由迁飞鸟引起的,与国际食品贸易无关。
2004年1月16日报告了山口县首次爆发情况,2004年2月16日报告了在大分县爆发的另一例情况,这些都没有对消费者的购买或饮食行为造成显著变化。
但是,2月26日首次报告京都发现禽流感后,导致鸡和蛋的销售急剧下降。2月26日前,尽管出现大量的鸡死亡而且在最后6天每天的死亡数都在增加,但京都府动物卫生部门没有接到任何报告。这些迹象本应引起农场对禽流感或其他不适情况的察觉。尽管鸡的死亡数量之大,但在2月25和26日,农场仍将鸡运往两个鸡肉加工公司,一个在兵库县,另一个在爱知县。虽然努力召回了产自该农场的所有产品,但还是有些鸡肉进入了食品链。由于消费者认为农场的行为极不道德,各县政府之间的联系不够,以及兵库县政府的召回情况报告被篡改,从而消费者失去了信心。
山口县爆发禽流感后,县政府立即通过因特网和其他方法发布信息,促进公众清楚地了解该情况。在京都爆发禽流感后,由于国民关注急剧上升,3月9日日本政府开始发起一场运动,向日本人民提供有关禽流感的准确信息。此外,3月22日,除与当地利益相关者召开了一次交换意见会议外,还在京都召开了一次由执行官员和禽流感专家参加的会议。从而加深了相互之间的理解。
一家名为“OISIX”的公司(该公司向其邮寄杂志的读者销售蔬菜和其他食品)于2004年3月7-8日做了一次因特网调查。结果证明,尽管有84%回答者知道目前尚无流行病学信息可以证明该病可通过污染的鸡和蛋传播,但是仍有44%的回答者拒绝购买。这说明尽管人们知道某些食品是安全的,但并不能保证人们会去购买这些食品。这些结果说明了风险交流的难度。
有关禽流感问题,尽管农场在该问题上处理不当显然是增加公众关注的重要因素,但据估计消费者的关注程度与新闻界对禽流感的报道力度是成比例的。
3.加强食品安全相关信息的收集和交流活动
收集信息必须准确,并要在研究单位、政府机构、粮食生产与食品加工工业及消费者之间进行有效交流,以便增加透明度,努力促进食品安全。
由于疯牛病事件所致,日本强化了处理食品安全问题的系统,尤其是信息收集和交流活动。
3.1 日本国内的信息收集和交流
根据日本食品卫生法第58款规定,食品中毒控制措施的内容之一就是信息收集体系。诊断出食源性疾病的任何医生必须通过该地区的卫生中心向政府报告病例情况。
实践证明,该体系在大规模食物中毒事件中发挥了有效的作用,如雪牌事件。医院和其他医疗机构十分关注患有呕吐、腹泻和其他具有食物中毒症状的病人,因此能够确定食物中毒所出现的迹象。在雪牌案例中,此类医院和其他医疗机构在发现病人的当日分别向各自卫生中心报告了食源性疾病的发生情况。市里的官员接到卫生中心的情况报告后,于次日对该工厂进行了紧急调查,并将结果报告给厚生劳动省。
通过食物中毒报告收集到的信息还被用于以下方面:确定特定食品与病原结合体所增加的风险,撰写风险概况材料,以及制定风险管理的决策,以控制食品中的特定危害。
3.2 收集其他国家的信息并开展交流
日本在出现第一个疯牛病病例后,才意识到对国外食品安全信息的收集和分析做得不够,需要予以加强。继这些事件之后,日本加强了及时并有效地从国际组织及海外各国收集信息的机制。
对日本而言,能够尽快地从其他国家获得食品安全的相关信息极为重要。因为在日本,进口食品约占整个供应食品的60%。此外,危害物日趋在全球范围传播的概念尚未被日本广泛接受,因为日本在地理上被大海所隔离,所以近几十年来,不可能有外来危害物入侵。
其他疾病如非典(SARS)的防治突出了信息收集和及时交换信息的重要性。例如,由于收集了大量信息并采取一些应对措施,日本才成功地预防了非典的发生。
4.食品安全方面的教育
采用各种不同的教育方法,对各阶层所有利益相关者开展食品安全方面的教育至关重要。通过有效的教育和交流,可以培养各个国家的食品安全文化。该地区的许多国家已在这方面开展了一些活动。多年来,在食品卫生和食品安全其他领域,日本已经建立了对小规模食品生产者/加工者及食品销售商开展教育的系统,为改善日本的食品卫生条件做出了贡献。
日本的食品卫生法制定于1947年。按食品卫生法的规定,食品制造商、生产者、加工者和销售商必须遵守这一法律。1948年,日本成立了由食品制造商、生产者、加工者和销售商组成的食品卫生协会。该协会的宗旨就是提高其成员对食品卫生的了解,自觉实施食品控制体系,从而预防食源性疾病、食物中毒和其他与食品和饮料有关的任何问题。
根据食品卫生法,日本的食品卫生检验由隶属于各地方政府卫生中心的食品卫生检查员负责进行,他们还负责提供指导。在地方政府和国家政府的卫生中心配合下,上述食品卫生协会也在该领域开展了工作。
每当修改与食品安全有关的法律或法规时,国家政府和卫生中心总要召开各种会议,向食品制造商、生产者、加工者和销售商解释修改情况。食品卫生协会在这一方面开展的活动包括:将每年的8月确定为全国“食品卫生月”;为食品制造商、生产者、加工者、销售商和消费者举办情况通报会以及培训班;提供食品安全方面的食品卫生教员指南;以及为新开餐馆提供咨询意见。在食品安全领域,这些活动提供的指导和建议比政府开展的活动更具有针对性,而且更多集中于小规模的食品制造商、生产者、加工者和销售商。
近年来,尽管食品制造商、生产者、加工者和销售商所承担的食品安全相关责任日趋加大,但除了政府目前更加重视消费者的教育外,与食品安全有关的教育体系变化甚小。
5.结 论
尽管日本政府自2003年7月以来一直在积极介入风险交流活动,但日本民众似乎尚未完全了解食品安全方面的风险分析概念。因此,日本政府今后应努力倡导风险分析概念和不断加强风险交流。一般来讲,日本人崇尚和睦,在其他多数人面前发表自己的意见或争论并不是日本的传统。在国际公认的风险分析框架内,如何使风险交流更加实用和更加有效是日本政府的职责,在此过程中应充分考虑日本人民的传统、文化和特点。
收集日本国内与食品安全有关的信息并与其他国家开展交流十分重要。由于全球的食品贸易日益增加,应密切注视出口国的食品安全问题。要收集其他所有国家的信息并进行认真分析,以便在科学信息的基础上做出更加合理的风险管理决策。
6.建 议
在开展风险交流时,尽快提供准确信息至关重要。由于在食品安全问题上零风险是不可能实现的,为了确定和实施风险管理方案,在可操作范围内尽量减少风险,在所有利益相关者之间(尤其是可能受到不利影响的利益相关者之间)开展风险交流极为重要,这有利于在实施风险管理战略上达成一致意见。
随着粮食生产和贸易快速全球化,国际上出现微生物和化学污染食品的潜在可能性日趋增大。定期交流有关食品安全信息,在出现食品安全紧急情况下迅速获得信息,对于应对上述情况十分重要。为了及时而适当地开展食品安全风险管理,必须建立一个有助于促进食品安全信息交流并加强国内外食品安全部门之间合作的机制。鉴此,日本拟致力于开发一个新的国际食品安全部门网络(INFOSAN),世界卫生组织食品安全司最近已经提交了这一方案。
为了减轻食源性疾病的负担,必须向食品链上的大众(包括小规模企业和街头销售商)提供有关食品安全的教育和信息,并确保所宣传的食品安全信息协调一致。在这一方面,粮农组织和世界卫生组织应向全球提供科学合理的食品安全信息。
议 题9 CAP 04/6
粮农组织/世卫组织亚洲及太平洋区域食品安全会议
2004年5月24-27日,马来西亚芙蓉
食源性疾病的监测及监督系统
(由马来西亚政府撰文)
1.引 言
事实上,食源性疾病通常属传染性疾病或中毒性疾病,主要是因摄取食物使病原物进入体内所致。食源性疾病严重影响了人类的健康和经济的发展,日趋成了公共卫生问题。在发展中国家和发达国家,食源性疾病的爆发引起了媒体的重视,唤起了消费者的关注。
尽管许多人因食用不安全食品而致病和死亡,但全球食源性疾病的发生率却难以估计。在工业化国家,每年有30%的人受食源性疾病为害,而在发展中国家,该问题可能更加普遍。据世界卫生组织报道,2002年有210万人死于腹泻,其中绝大多数是欠发达国家的儿童。例如在美国,估计每年发生的食源性疾病达7
600万例,导致325,000人住院和5
000人死亡;而医疗费用和生产力损失估计达350亿美元(1997年)。秘鲁1991年爆发的霍乱,导致当年鱼类和渔产品的出口损失达5亿美元;近来食源性病原菌大肠杆菌(Escherichia
coli O157:H7)正在逐渐蔓延,可导致血性腹泻和肾衰竭,1996年,日本大肠杆菌的爆发导致6 300多所学校儿童受到感染,2人死亡。
在马来西亚,应报告的食源性疾病即霍乱、伤寒病、食品中毒、甲肝和痢疾的发生率少于十万分之五,事实上属于零星发病,爆发仅局限于特定的地区。
由于爆发范围广泛、新的食源性病原物的不断出现,以及抗菌药物耐药性的发展,食源性疾病发病率上升已经对我们的食品安全议程构成了威胁。导致上述现象的原因可归咎于食品供应的全球化、食品生产和加工技术的发展、农业和畜牧业生产方式的改变、人口结构的演变以及生活方式的变化。
由于资料不全和缺乏可信赖的数据,食源性疾病负担的实际大小尚不得而知,这限制了我们对该疾病在公共卫生重要性的了解,也妨碍了我们努力争取有效控制食源性疾病所需要的资源和支持。
在预防和控制食源性疾病及降低减少风险战略中的不确定性因素过程中,加强对人类食源性疾病的监控以及对污染物的监测十分有助于确定和评估其优先重点。2000年召开的第53届世界卫生大会通过了一项决议,明确了食品安全在公共卫生上的重要作用,并呼吁制定旨在减轻食源性疾病负担的全球战略。世界卫生大会53.15号决议鼓励成员国“建立和维持旨在监控食源性疾病适宜的国家和区域机制”。2002年世界卫生组织公布了一份文件,即“全球食品安全战略:增进健康需要更加安全的食品”,以此来应对这一问题。加强食源性疾病监控的总体目标是为了向成员国提供必要的数据,通过改进各国的食品安全体系来减少食源性疾病的负担。
为了开展食源性疾病和食品污染物的调查研究,需要创新的战略和方法。制定减少与食品有关的风险的战略需要了解各个层面的食源性疾病现状,这一战略还必须建立在有关食源性有害物和食源性疾病发生率的最佳科学证据上。
根据世界贸易组织(WTO)实施卫生和植物检疫措施协议,世贸组织成员国应当确保其卫生或植物检疫措施是建立在科学的风险评估基础上,充分考虑了相关国际组织开发的风险评估技术。在这一点上,食品法典是卫生和植物检疫措施协议下食品安全的国际参考标准,食品法典委员会通过了“在食品法典的框架下应用风险分析的工作准则”,与此同时食典委还制定供各国政府采用的风险分析工作准则。风险分析包括风险评估、风险管理和风险交流。在开展风险评估乃至最后形成风险管理方案和实施风险交流的过程中,监控数据具有极为重要的作用。
2.食源性疾病监控
食源性疾病监控有助于评估食源性疾病的负担、确定公共卫生的重点领域、制定政策和评价计划结果,还有助于预防、确定和控制该疾病的爆发以及促进有关研究的进程。该监控还可发觉不断出现的食品安全问题。
所有国家均拥有各自不同的公共卫生体系,使各监控体系存在极大差异,并在食源性疾病的领域上具有不同的重点。2002年,世卫组织就特定地方食源性疾病监控方法问题举行了磋商,磋商会审议了现有的食源性疾病监控体系,并根据其信息产出能力,将这些体系分为四大类别(参见附录1)。其中包括从不具有正规监控的体系到对整个食品链实施全面监控的体系。一个国家可能归属于一种类别,但可能拥有其他多个类别的监控内容。食源性疾病的监控还可能属于国家需上报的传染性疾病体系的组成部分。然而,由于目前尚无十分明确的“最佳监控方法”,世卫组织磋商会提出了加强食源性监控体系的五条行动建议(参见附件2)。
马来西亚现行的特别重要食源性疾病强制性报告措施对监控十分有益,但是不足以应对不断出现的新食源性疾病的情况。目前收集的监控数据主要是通过以医生为主的监控和对爆发事件的调查,因为目前对实验室的报告尚无强制性要求。通过这一体系,所收到的报告主要来自政府卫生机构,包括卫生中心、门诊部和医院以及私人医院和一般的医务工作者。此清单上所列的食源性疾
病包括霍乱、伤寒及副伤寒、病毒性甲型肝炎、食物中毒和痢疾。
因此,尽早确定未知的病原物并予以报告的系统方法十分必要。马来西亚卫生部已经编制了一份有关传染性疾病报告和实验室检测系统方法手册,弥补了现有其他特定疾病报告方法的不足,而且有助于对不断出现的新疾病或重新出现的疾病做出快速反应。此类报告是针对综合病症而言,而不是针对特定疾病,而且与食源性疾病有关的报告只有“国家急性肠胃炎监控报告”。卫生部还针对特定的传染性疾病实施了基于实验室的监控,其中包括因沙门氏菌、志贺氏菌、伤寒沙门氏菌和弧菌诱致的食源性疾病。
在马来西亚的公共卫生和大学的研究实验室,已经具备了脉冲场凝胶电泳DNA指纹分析和电泳影像分析设备。然而,脉冲场凝胶电泳所带来的一些问题及挑战包括:电泳步骤、反应试剂、化学药品、电泳条件的标准化和费用问题,以及缺乏训练有素的操作人员。建立马来西亚食源性致病菌DNA检验的国家电子网络(PulseNet),需要对试验人员进行采用标准步骤的培训,以便使DNA指纹分析结果能够在实验室之间和国家之间进行比较,这样,在食源性疾病爆发的情况下就能快速鉴定其病原物。食源性致病菌DNA检验的国家电子网络并非仅仅针对脉冲场凝胶电泳,而是实验室、监控部门和流行病学部门的各类人员用于快速确定即将爆发的食源性疾病的通讯网络。
食品中毒事件可归咎于食品加工者对食品的处理不当和不卫生的操作。马来西亚卫生部致力于在食品卫生和食品加工者应使用的卫生设备方面实施基本培训计划。目前,食品卫生操作规范和地方当局的法规对食品加工场所提出了一般和特定的卫生要求。拟议的食品卫生条例要求所有食品加工者必须接受经卫生部认定的机构的培训,该条例现在正处于在公报上公布阶段。卫生部还积极促进食品安全保障体系的应用,例如危害分析及关键控制点、良好操作规范和食品产业的良好卫生操作规范。食品卫生措施的执行将极大地减少食品的污染,这些措施包括:从农场到餐桌的危害分析及关键控制点、以及采用国家食品安全标准对食品或农场操作场所进行认证,在食品安全加工方面对雇员开展持续性培训等。
马来西亚的兽医服务部针对家畜产品上的食源性病原物即沙门氏菌、大肠杆菌O157、弯曲杆菌、耶尔森氏菌和耐万古霉素肠球菌(VRE),实施了国家监控计划。兽医服务部的流行病和兽医处已经制定了家畜/动物若干疾病的监视、控制、监测和根除计划/方案,这些疾病包括沙门氏菌、禽流感、耐万古霉素肠球菌、布鲁氏菌、肺结核、强尼氏病、尼帕病毒和牛海绵状脑病(疯牛病)。
除了实施疾病控制和根除以及无疫病区的计划或方案外,还实施了家畜农场认定计划。该计划包括基础设施协议、生物安全协议、同群畜禽健康计划、良好畜牧生产操作规范、兽药使用控制、产品标识和追溯系统及质量控制系统。该计划的实施将确保安全食品的供应。
食源性疾病监控数据来源包括疾病报告、实验室报告、环境指数(食品公司检验的原始数据;农业、兽医和食品分析)、爆发调查报告、科学研究、发病率报告、病例调查、观察岗报告、调查报告、人口普查和媒体报道。由若干机构收集的有关治病因子、疾病特征、运输车辆的大量信息可以成功地用于减少食源性疾病的发生范围。
马来西亚就此题目于2003年7月7-9日组织召开研讨会,着重讨论了建立区域实验室网络事宜。会上一致同意下列战略:
·弄清每一成员国现有实验室的基础设施、技术专长和测试能力;
·建立一个实现信息收集、整理、分发和定期更新的机制;
·确定每一国家的实验室联系点;
·和国际机构建立联系
在菲律宾,一个旨在完善基于实验室的食源性病原物监控体系项目已经开始和现有的若干监控计划联合实施。这些系统相互之间各自独立,至今为止也未能实现数据的系统集成。该项目旨在将这些系统结合起来成为一整体,从而形成一个食源性疾病的监控体系,该体系将包括抗菌药物耐药性数据。近来为微生物学家提供了有关沙门氏菌血清分类和抗血清方面的培训,以便使他们可以进行这些试验,并将所有的分离物送到热带医学研究所。
目前越南正在进行一项研究,以加强开展食源性疾病监控和确定食源性疾病负担的能力建设。在疾病控制和预防中心(CDC)和世卫组织西太平洋区域办事处的指导下,越南卫生部负责实施这一研究项目。该项目包括以下四方面的研究:
(1)
积极监控-在四个设有观察点的医院中进行监控,以确定肠道感染病的发病率,包括已确定的致病菌;
(2) 病例对照研究-通过对已确定致病菌的病例进行访谈,确定肠道病的风险因子,每一病例均按年龄和性别设置两个相应的对照;
(3) 实验室调查-对126个医院实验室进行信件调查,以确定实验室的能力;
(4) 人口调查-对3 000人进行访问调查以确定访谈前四个星期中的肠道疾病发生率。
在斐济卫生部的协助下,斐济卫生学校、世卫组织西太平洋区域办事处和疾病控制和预防中心最近制定了一项非伤寒沙门氏菌监控和实验室支持的国家合作计划。该计划旨在为涉及公共卫生的所有人员提供有关的技术和程序信息,这些信息是对带有非伤寒沙门氏菌患者进行监控的相关信息,包括为确定感染源和相关风险因子必须实施的特定步骤。
美国、英国、澳大利亚和荷兰已经建立了国家食源性疾病主动监控体系。美国食源性疾病监控网络(US FoodNet)就是疾病控制和预防中心(CDC)、美国农业部(USDA)和美国食品及药物管理局(FDA)之间的一个协作项目,该网络建于1996年。网络具有9个观察点,对许多食源性病原物进行了主动监测,还负责计算疾病负担、通过大量的病例对照研究来确定零星病例的传染源,而且还负责评估控制措施对这些传染病的控制效果。澳大利亚于2000年建立了OzFoodNet网络,这是国家和各州及属地卫生当局共同协作的一个项目,旨在更好地了解各社区食源性疾病的病因和发生情况,并为政策制定提供证据基础。
近年来,美国借助于PulseNet国家网络显著地提高了发现疾病大规模爆发的监控能力,该网络是食源性病原物分子分型国家网络。PulseNet网络使不同实验室能够将各自的试验结果在线进行相互比较或者和全国数据库资料进行比较。当对一个菌簇进行标记时,详细的流行病学调查通常就可以确定其来源。随着在其他国家的推广应用,该网络已经成为“国际化”网络,即加拿大PulseNet、欧洲PulseNet、新西兰PulseNet,以及最近建立的亚州太平洋PulseNet。
继2003年4月29日召开的东盟领导人关于非典型肺炎特别会议和2003年6月10-11日召开的东盟+3卫生部长关于非典型肺炎会议之后,东盟国家一级都在致力于加强本地区传染病监控的合作和网络建设。为了加强区域合作,现已确定了三个卫生项目。拟定泰国负责协调加强疾病监控体系的项目,印度尼西亚负责加强东盟疾病监控网络建设,马来西亚负责协调加强实验室能力建设以及东盟+3各国之间传染性疾病监控的质量保障。
在国际一级,为了加强在全世界范围进行沙门氏菌的分离、鉴定、血清分类以及抗菌药物耐药性测试,世界卫生组织于2000年开始建立全球沙门氏菌监控网络。该网络由人类健康、兽医和与食品有关的学科等领域的机构和个人(流行病学家和微生物学家)组成。其活动包括微生物学家的区域培训、外部质量保障和基准测试、中等范围的电子讨论小组以及基于网络的数据库,其中含有实验室在沙门氏菌血清分类上结果的年度概要。
世卫组织的全球沙门氏菌监控网络还在中国举办了微生物学家的培训班,并将在2004年为流行病学家举办第三次培训班。还计划制定一项疾病负担研究项目,类似于在越南开展的研究。该研究应和世卫组织和世界银行的其他行动协调一致。
在信息和通讯技术时代,信息交流变得更加容易也更加迅速。信息和通讯技术的应用将加强监控体系,使其更加高效也更具效果。网络建设、网络的网络化、在线报告以及电子讨论等,在此就不一一列举,均为各个机构提供了诸多手段,利用这些技术造益于不同层面的食源性疾病监控体系。
高效而富有成果的食源性疾病控制体系将有助于确保所消费食品的质量和安全。为了加强对食源性疾病的检测和反应,应当采取全球性方法,以便在疾病爆发或危机方面发挥预警系统的作用,这些爆发和危机可能会出现在任一层次,即国家、区域和全球一级。因此该体系应是全面的,还应和整个饲料-食品链上的所有监测数据结合起来。这势必产生一个强大的监控体系,并有利于确定适宜的优先重点和开展公共卫生干预活动。目前,各个层次和多个学科的一些机构和利益相关者已经参与到食源性疾病监控体系中,其中包括卫生部、兽医服务部门、农业和渔业、食品企业、大学、实验室。虽然这些机构中大多数是独立开展工作,但这些机构开展的相关活动均在努力开展合作,使它们协调一致,从而形成一个统一的监控体系。
3.今后的行动步骤
为了加强和提高现有食源性疾病监控体系,世界卫生组织应发挥领导作用,在未来的方向和任务上为成员国提供指导。所制定的目标及战略应能被所有的成员国所接受,从而促进成员国实施这些战略,并履行其义务。众所周知,由于所带来的相互作用的复杂性,成员国的政治承诺至关重要,可确保所实施的卫生计划的成功和可持续性。
为了加强我们现有的食源性疾病监控体系,在从事食源性疾病监控和食品安全工作的所有部门之间开展协调一致的行动至关重要。采取公认的和标准化的监控方法并利用流行病学上可靠和适宜的技术,是确保系统发挥作用所必须的。利用信息和通讯技术开展高效的相互交流,将进一步加强和维持这一成功的监控体系。协调中心的选择应当根据流行病学水平和实验室设施及技术专长而定。该中心应负责举办培训班,并开展流行病学和实验室方法及监测方面的研究。
创新战略和方法是开展食源性疾病和食品污染物调查所必需的。开展食品病原物和人类疾病之间联系的研究,将有助于量化食源性疾病的风险。从事食源性疾病监控和食品安全工作的所有部门开展合作十分重要,可使风险分析产生内容丰富的结果。此外,应充分利用区域和国际上基于实验室的、拥有连网设施的监控体系,这样可以提供食源性疾病监控信息。这些信息是利用现代风险分析框架对不断出现的新病原物进行监控的结果。
在较不发达国家和发展中国家,食源性疾病监控体系可能不存在或未成体系,应考虑在这些国家建立或强化这些监控体系的资源。人力资源、技术专长、实验室和科技基础设施以及系统的成本效益等问题均需加以适当的重视。
在食源性疾病监控过程中,这些国家的能力建设内容必须加以考虑,这不仅要在国家一级上考虑还要扩展到区域一级和全球水平上。为了改善基础设施和提高技术专长,必须考虑能力建设所需要的足够资源,特别是不发达和发展中国家。应当努力地促进与食源性疾病监控有关的知识、技能和技术专长的共享。在衡量食源性疾病实际负担过程中,应克服所存在的资金不足的困难,以
便建立相应的观察点。
最后但确是很重要的一点,在《国际卫生条例》的修订过程中应将食源性疾病问题纳入其中。修改和加强此类的法规也需要不同的政府机构之间的合作和协调一致,这些机构包括农业。渔业、兽医服务和卫生等部门。
4.结 论
食源性疾病是十分重要的公共卫生问题,因为它不仅影响到人类的健康,而且对经济和贸易问题产生极大的影响。对人口增长、生活方式、国际食品贸易、粮食生产和加工、农业和畜牧业生产方式以及抗菌药物耐药性等产生影响的全球变化,构成了食源性疾病不断上升的威胁。由于绝大多数的食源性疾病并未报道,该问题的严重程度尚不得而知。加上缺乏食源性疾病负担的可靠数据,也严重妨碍了对其在公共卫生上重要程度的了解,尽管最近公开的食源性疾病的爆发已经引起消费者和政策制订者对食品安全问题的重视,
在评估食源性疾病的负担以及快速发现并应对这些疾病的爆发方面,食源性疾病的监控能够为我们提供很有价值的信息。为了使其更加有效,食源性疾病的监控必须和从农场到餐桌的整个食品链上的食品监测数据相结合,这有助于将食品中的病原物和人类疾病联系起来。必须强调的是,国家、区域和全球各级相关机构强有力的领导作用加上政治承诺,以及开展合作和协调一致的活动至关重要,有助于加强和提高现有的和正在建立的监控系统,同时降低食源性疾病的风险程度。
5.建 议
本区域各国应:
1.
加强各自的食源性疾病监控能力,以便通过国家、区域和国际监控网络促进快速确定不断出现的食源性疾病,正如最近爆发的非典型肺炎和禽流感情况。
2. 加强食源性疾病的监控并由相关机构收集食品污染物数据,可以对食源性疾病的负担进行适当的文件记载和评价。
3. 建立监控食源性疾病观察站,以便获得食源性疾病方面的数据、衡量食源性疾病负担,以及适当地确定在预防和控制食源性疾病方面众所关注的优先领域。
4. 研究各国不同食源性疾病监控体系,努力使这些体系在地区和国际上协调一致。
5. 加强监控不仅要监控人患疾病,还要应用系统微生物风险评估技术监控整个食物生产链中的病原物,从而提高确定食物中的病原物与人患疾病之间关系的能力。
6. 加强基于实验室的重要食源性疾病的国家监控能力和基础设施建设,同时还要加强在出现耐抗菌剂食源性病原物紧急情况下,进行检测、监控及应对的国家能力建设。
7. 制定培训和能力建设计划,用足够的资源改进基础设施和开发技术专长。
6.参考文献:
Australian Government
Department of Health and Ageing. 2003. OzFoodNet: A health network to enhance
the surveillance of food-borne diseases in Australia (available at
www.ozfoodnet.org.au/index.htm).
US CDC. 2001. Updated
Guidelines for Evaluating Public Health Surveillance Systems. Recommendations
from the Guidelines Working Group. Morbidity and Mortality Weekly Report,
50(RR13): 1-3
WHO. 2001. Global
surveillance of food-borne disease: Developing a strategy and its interaction to
risk analysis. Report of a WHO Consultation, Geneva, Switzerland, 26–29 Nov
2001. WHO/CDS/CSR/EPH/2002.21. Geneva.
WHO. 2002. Emerging Food-borne Diseases. WHO Fact Sheet 124. Geneva
WHO. 2002. Global
Strategy for Food Safety: Safer food for better health. Food Safety Issues
Series. Geneva
WHO. 2002. Methods for food-borne disease surveillance in selected sites. Report
of a WHO consultation, Leipzig, Germany, 18–21 March 2002.
WHO/CDS/CSR/EPH/2002.22. Geneva.
WHO. 2003. The present
state of food-borne disease in OECD countries. By J. Rocourt, G. Moy, K. Vierk &
J. Schlundt. Geneva
WHO. 2004. Food-borne
Disease (available at
www.who.int/foodsafety/food-borne_disease/en/).
WHO. 2004. Global
Salmonella Surveillance Site (available at
www.who.int/salmsurv/en/)
附件 1
CATEGORIES OF
FOODBORNE DISEASE SURVEILLANCE
(WHO/CDS/CSR/EPH/2002.22 : Methods for Food borne disease surveillance
in selected sites: Report of a WHO consultation)
Category 1 No formal
surveillance
Description of system
This situation typically exists in countries with political instability, recent
history of war, or extreme poverty. The public health system is very low
priority or non-existent. Some elements of surveillance may be undertaken by
outside agencies.
Data elements
None.
Information expected
Large or unusual outbreaks may be detected and investigated by an outside agency
(e.g., non-governmental organizations).
Category 2 Syndromic
surveillance
Description of system
Syndromic surveillance is the collection, analysis and interpretation of
syndromic data (e.g., diarrhea or food poisoning) from at least selected sites.
The surveillance system should use standard case definitions for classifying
syndromes. Data should be routinely reported, collated at a central level and
promptly disseminated to the public health community. These systems may function
with or without laboratory capacity (ministry of health or hospital) but there
is no formal laboratory-based surveillance system.
Data elements
Case counts (e.g., see WHO cholera guidelines).
Information expected
Trends over time, seasonal variation.
Define at-risk and high-risk populations.
Recognition of point source outbreaks at the local level.
Recognition of large or unusual outbreaks at the national level.
Category 3
Laboratory-based surveillance
Description of system
Laboratory-based surveillance is the collection, analysis and interpretation of
laboratory data from at least selected sites. The surveillance system should use
standard case definitions for classifying diseases. Laboratories should use
standardized methods for pathogen identification with recognized international
quality assurance systems. Data should be routinely reported, collated at a
central level and promptly disseminated to the public health community.
Laboratory-based surveillance provides higher quality data that syndromic
surveillance; countries should strive to develop this type of surveillance
system.
Data elements
Etiologic identification
Etiologic agent-specific case counts
Pathogen characterization (e.g., serotyping, antibiogram, etc.)
Information expected
Etiologic agent-specific trends over time, seasonal variation
Define at-risk and high-risk populations
Recognition of point source at the local and diffuse outbreaks at the national
level
Category 4 Integrated
food-chain surveillance
Description of system
Integrated food-chain surveillance (IFCS) is the collection, analysis, and
interpretation of data from animals, food, and humans. The surveillance system
should use standard case definitions for classifying diseases. Data should be
routinely reported, collated at a central level and promptly disseminated to the
public health community. IFCS allows the attribution of burden of illness to
specific food categories through the use of detailed information from monitoring
food and animals.
Data elements
Etiologic identification
Etiologic agent-specific case counts in the population
Etiologic agent-specific prevalence in animals and foods
Pathogen characterization (e.g., serotyping, antibiogram, etc.)
Community-level case counts
Information expected
Etiologic agent-specific trends over time, seasonal variation
Reliable incidence rates
Define at-risk and high-risk populations
Recognition of point source at the local and diffuse outbreaks at the national
level
Ability to use food and/or animal data to generate hypotheses for human disease
outbreaks
Comprehensive estimates of burden of food borne disease
Ability to assess the effectiveness of food safety policy interventions
Ability to attribute burden of food borne disease by food category
Ability to detect and control hazards in food
Ability to recognize emerging pathogens in animal
附件 2
FINAL GENERAL
RECOMMENDATIONS
(WHO/CDS/CSR/EPH/2002.22 : Methods for Food borne disease surveillance
in selected sites: Report of a WHO consultation)
·WHO should encourage
development of an inventory of existing studies on burden of food borne disease
and a comparison of their results;
·WHO should encourage Member States to conduct studies to determine the burden
of food borne disease and provide technical support to these countries;
·WHO should select countries using the criteria identified in this report and
identify resources to support burden of illness studies;
·WHO should seek resources to enhance laboratory-based surveillance and outbreak
detection and response for food borne disease;
·Member States should seek to improve their existing food borne disease
surveillance system.
会议厅文件清单
|
Country/Organization |
Conference Room
Document Reference |
| Keynote speeches | Annexes 8and 9(CRD 1) |
|
Programme for Consumers International side event |
CRD 2 |
| Programme for ILSI side event | CRD 3 |
| Information document for Portal launch | CRD 4 |
| Malaysia - 1 | CRD 5 |
| Malaysia - 2 | CRD 6 |
| Malaysia - 3 | CRD 7 |
| Malaysia - 4 | CRD 8 |
| Thailand - 1 | CRD 9 |
| Thailand - 2 | CRD 10 |
| Korea | CRD 11 |
| Philippines -1 | CRD 12 |
| Philippines -2 | CRD 13 |
| ILSI Informative document | CRD 14 |
| Bangladesh | CRD 15 |
| Japan | CRD 16 |
| ASEAN | CRD 17 |
| PDR Laos | CRD 18 |
| Cambodia | CRD 19 |
| ICD | CRD 20 |
| Tropical Fruit Network | CRD 21 |
| New Zealand | CRD 22 |
| China - 1 | CRD 23* |
| Indonesia | CRD 24 |
| Pakistan | CRD 25* |
| Sri Lanka | CRD 26 |
| Myanmar | CRD 27 |
| Singapore | CRD 28 |
| China – 2 | CRD 29 |
All Conference Room
Documents except those marked with an asterisk (*) are available electronically
from the Conference website: www.foodsafetyforum.org/asian.
Conference Room Document 2
English only

Safety of Street Vended Foods: A Side Event Organized by
Consumers International Asia Pacific
at the FAO/WHO Regional Conference on Food Safety for Asia and the Pacific
Royal Adelphi Hotel, Seremban, Negeri Sembilan, Malaysia
9:00 AM – 12:30 PM, 26 May 2004
PROGRAMME
9:00 – 9:15 Opening Remarks by Dr. Sothi Rachagan, Regional Director, Consumers
International Asia Pacific, Kuala Lumpur, Malaysia
9:15 – 9:45 Safety of Street Vended Foods in India by Ms. Kavitha Anand,
Researcher, Citizen, Consumer and Civic Action Group, Chennai, India
9:45 – 10:15 Safety of Street Vended Foods in the Philippines by Dr. Sonia de
Leon, President, Foundation for the Advancement of Food Science and Technology,
Quezon City, Philippines
10:15 – 10:45 BREAK
10:45 – 11:15 Safety of Street Vended Foods in Vietnam by Dr. Ngo Thi Phi Yen,
Medical Doctor, Ho Chi Minh City Nutrition Center
11:15 – 11:45 FAO/WHO Activities in Street Food Vending
11:45 – 12:30 Q&A
Conference Room Document 3
English only
FAO/WHO Regional Conference on Food Safety for Asia and Pacific
Seremban, Malaysia, 24-27 May 2004
Tentative Seminar Programme
Enterobacter sakazakii in Powdered Infant Formula:
Current Updates and Issues
(A side event (co-organized by ILSI Southeast Asia Region)
at the FAO/WHO Regional Conference on Food Safety for Asia and the Pacific)
9.00 hours, 26 May 2004
|
Plenary Session |
|
|
9.00 – 9.15 am |
ILSI SEA Region |
|
9.15 – 9.45 am |
Introduction to Enterobacter
sakazakii |
|
9.45 – 10.15 am |
Current knowledge and Issues on
Enterobacter sakazakii Contamination in Infant Foods |
|
10.15 – 10.45 am |
Break |
|
10.45 – 11.15 am |
Minimizing Enterobacter sakazakii
Contamination in?the Manufacture of Infant Formula – a Perspective from
Industry |
|
11.15 – 11.45 am |
Preventing Cross-contamination of
Enterobacter sakazakii during Infant Formula Preparation |
|
|
Present Work and Future Research
Needs on Enterobacter sakazakii |
|
12.15 – 1.15 pm |
Discussion |
|
1.15 pm |
Adjournment |
Conference Room Document 4
English only
FAO/WHO Regional Conference on Food Safety for Asia and the Pacific
Seremban, Malaysia, 24-27 May 2004
THE INTERNATIONAL PORTAL FOR FOOD SAFETY,
ANIMAL AND PLANT HEALTH
(Information paper- May 2004)
THE NEED FOR THE PORTAL
1. The changing patterns of food and agricultural production, greater attention
to the environmental impact of agriculture, emergence of new technologies and
potential contaminants, increase in international trade, and a heightened
consumer awareness of food safety and animal health issues have all combined to
increase interest in global sanitary and phytosanitary measures.
2. This interest comes from international agencies; national trade, agriculture,
food safety, environment and consumer protection services; international trading
partners; and private sector organizations. Each requires reliable information
on standards, regulations, scientific evaluations, and other supporting
information whether to make decisions on trade-related issues or to prepare
relevant regulations or measures.
3. Despite the broad level of interest, obtaining accurate and current
information can be difficult. There are two common problems: 1) information may
be difficult to locate, and 2) it may not always be clear which source
represents the current official position on a given subject. A user may need to
search the websites of a number of different international standard setting
bodies to retrieve all the relevant information on a particular commodity or on
a specific topic. The same scenario is repeated at the national level where
standards, regulations, and related information may be spread across the
websites of several agencies. Further, some valuable information may not
currently be electronically available to the general public.
THE PURPOSE OF THE PORTAL
4. Based on the above considerations, FAO is leading an interagency initiative,
involving the relevant international agencies, standard setting bodies and
national authorities to develop and maintain an internet-based portal - the
International Portal on Food Safety, Animal and Plant Health - which enables an
authoritative search for current standards, regulations and other relevant
official materials from a single search tool.
5. Some important user groups are expected to include:
·those involved in promotion and enforcement of safe production and trade
(relevant ministries, the commercial sector and development agencies)
·international trade regulators (customs and SPS-related authorities)
·WTO SPS inquiry points
·(policy) researchers
6. The types of questions to be addressed by the portal include the following:
·International standards – what standards exist for a particular commodity or
process?
·Scientific evaluations – what supporting scientific evaluations underpin the
international standards?
·Import regulations– what are the technical regulations for import (of a
particular commodity) into a given country or trade zone?
·Recent regulations - what new regulations have been implemented or are planned
which impact food safety, animal and plant health?
·Trade disputes, concerns and notifications – which commodities or processes
have led to international concerns being raised, between which trading partners
and for what reasons?
·Risk analysis – what official risk assessments have been published on a
specific subject/about a particular commodity?
·Pest/disease reports – what pests/diseases have been officially reported
recently for a given country or trade zone?
·Contact points – who are the official contact points in a given country for
animal health, biosafety, food safety, plant health and WTO inquiries?
·Inspection/quarantine service - how is the (country’s) inspection/quarantine
service organized?
DESIGN CONSIDERATIONS
Ownership
7. To date, systems created to collect and disseminate sanitary and
phytosanitary information have had only limited success. Those interpreting
official sources usually fail to satisfy as users need to revert to the original
source to be confident that essential components have not been lost. Systems for
countries and organisations to publish materials have been undermined by users
failing to supply (or provide updates for) the requested data, often because
material is already published elsewhere. With this in mind, the project aims to
avoid duplication of effort and minimise the cost of participation by linking to
existing sites wherever possible. At the same time, the portal offers
publication facilities for any countries without pre-existing websites to allow
them to post information that would not otherwise be available electronically.
Maintenance
8. One way of ensuring cost-effective maintenance of the information is to match
the descriptions of content (keywords, document types, etc) used on official
partner source sites to those in the portal. Data is then viewable through the
portal based on how it is described by these sources. The quality of the
information in the portal is therefore critically dependent on the quality of
these descriptions, and on the mapping of these descriptions to the portal’s own
set of keywords and information types. A long-term goal of the portal would be
the adoption of standard descriptions over time, by participating source sites.
Language
9. The portal makes documents available in whatever language it was supplied.
The aim is to ensure a multilingual capacity by using a controlled vocabulary
for the keywords describing the documents, translated into English, French and
Spanish. In addition, the full text search feature of the portal will retrieve
documents containing the requested search expression, independent of language
considerations.
Accessibility
10. The portal has been designed to be as accessible as possible over slow
internet connections. Although the portal is essentially a database- driven
website, technology has been applied to ensure that commonly-retrieved pages are
‘cached’ to ensure that they are presented as if they were produced from a
conventional static website
CURRENT STATUS OF THE PROJECT AND TIMETABLE
The project is being implemented in the following stages:
Develop a prototype and test with users
11. The portal currently (May 2004) contains content drawn from the three SPS-recognized
standard setting bodies, as well as from CBD, FAO (including FAO’s internal
legislation and regulatory database (FAOLEX)), WHO, and WTO, in addition to
demonstration ‘nodes’ of nearly 400 items from the US, a further 2,000 from the
EU, and smaller data sets from selected developing countries. There are
currently over 15,000 items referenced by the portal, each described using a set
of keywords supported by a powerful free text search facility.
12. The portal has undergone testing within FAO and with partners from the
content providers. Further work is planned over the coming months with external
users from the user groups identified above.
Extension of international and national coverage
13. In addition, plans are in hand to include further EU and US information, as
well as sample data sets from larger developing countries. Coverage will also be
extended over the coming months by the progressive inclusion of supporting
material to the official standards (for instance, more detailed information from
the Joint FAO/WHO Expert Consultation on Food Additives and Contaminants (JECFA)
and the Joint FAO/WHO Meeting on Pesticide Residues (JMPR)).
Launch Version 1.0 of the portal
14. The portal will formally be launched on 25 May,2004, on the occasion of the
FAO/WHO Regional Conference on Food Safety for Asia and the Pacific (Seremban,
Malaysia, 24-27 May 2004). It is now freely accessible from the FAO Biosecurity
PAIA webpage (www.fao.org/biosecurity) or directly from www.ipfsaph.org.
Interested users are invited to utilise the portal, share the link with other
users, and provide feedback to the FAO project team on the portal content and
usability.
Provide capacity building assistance
15. A programme of capacity building integrated into existing capacity building
efforts by the various agencies is planned to follow this launch, with further
initiatives to be well coordinated between agencies. This will include:
·building awareness and
understanding of the benefits of information exchange to support safe trade;
·development of national strategies for the exchange of official information;
·assistance in the development of computing facilities and information
management capacity to support information exchange;
·human resource development and training sessions for national authorities on
the use of the portal and data input and editing functionality; and
·tailored training sessions to targeted audiences of end users on the use of the
portal.
16. Through this capacity building programme, it is envisaged that further
arrangements can be made with other countries for automatic and/or manual
loading of their national information.
17. For further information on the portal please contact:
Mike Robson: IPFSAPH Project Manager
at [email protected]
Niek van der Graaff: Chairman FAO Biosecurity Inter-Departmental Working Group
at: [email protected]
Ezzeddine Boutrif: Secretary FAO Biosecurity Inter-Departmental Working Group
at [email protected]
Agenda Item 5 Conference Room Document 5
English only
FAO/WHO Regional Conference on Food Safety for Asia and the Pacific
Seremban, Malaysia, 24-27 May 2004
FOOD SAFETY LEGISLATION IN MALAYSIA
(Malaysia)
Abstract
Food safety legislation should be developed and updated taking into
consideration specific needs of consumers and food producers, development in
technology, emerging hazards, changing consumer demands and new requirements for
trade, harmonization with international and regional standards, obligations
under the World Trade Organization (WTO) agreements, as well as social,
religious and cultural habits. The implementation of food safety legislation
throughout the food chain is essential in establishing an effective food safety
system.
The Food Act 1983 and the Food Regulations 1985 are the Malaysian food
legislations that form the backbone of the food safety programme. Other
legislations that have an impact on food safety are the Pesticide Act 1974, the
Fisheries Act 1983, the Veterinary Surgeon Act 1974 and the Animal Ordinance
1953, all under the Ministry of Agriculture and Agro-based Industry, the Trade
Description Act under the Ministry of Domestic Trade and Consumer Affairs, etc.
The continuous revision and updating of the Food Regulations 1985 is conducted
by the Technical Advisory Committee on the Food Regulations 1985 chaired by the
Director of the Food Quality Control Division, Ministry of Health. It is an
inter-agency committee consisting of relevant government agencies involved in
food safety from farm-to-table, the food industry, professional bodies and the
consumers. Proposals for new legislations as well as revision and updating of
legislations are elaborated in a 14-step procedure, which closely follow the
Codex step procedure in the elaboration of Codex standards. Proposed new
legislations, revised and updated legislations are posted on the Food Quality
Control Division Website for comments. Notification to the WTO is undertaken to
fulfill obligations under the WTO. Efforts in harmonization of legislation with
international standards are actively undertaken using Codex standards as a
benchmark. As such, a risk-based approach has been taken into consideration by
the risk assessment conducted by Joint Expert Committee on Food Additives (JECFA)
(for chemical additives) and Joint Expert Meeting on Microbiological Risk
Assessment (JEMRA) (for microbiological parameters).
Harmonization of the national legislation with Codex standards which is an
enormous task as well as the generation of scientific data are challenges in
standard setting work.
Introduction
Food safety should be addressed throughout the food chain from farm to table.
The establishment and updating of food safety legislation throughout the food
chain is essential in establishing an effective food safety system. Food safety
legislation should be developed and updated taking into consideration specific
needs of consumers and food producers, development in technology, emerging
hazards, changing consumer demands and new requirements for trade, harmonization
with international and regional standards, obligations under the World Trade
Organization (WTO) agreements, as well as social, religious and cultural habits.
Background
Malaysia has realized that food safety can no longer be considered solely as a
domestic entity nor can it be the responsibility of a single agency. Even though
the mandate for food safety rests with the Ministry of Health, other government
agencies are also responsible for food safety in the country. The Vision of the
Food Quality Control Division (FQCD) of the Ministry of Health to uphold the
nation’s integrity by ensuring safe food through shared responsibility and
accountability on the basis of tripartite management is testimony that food
safety is not only the responsibility of the government but also the industries
and consumers.
The Food Act 1983 and the Food Regulations 1985 are the Malaysian food
legislations that form the backbone of the food safety programme. These
legislations replace the Sale of Food and Drug Ordinance and Regulations 1952.
The objective of the Food Act 1983 and the Food Regulations 1985 is to ensure
that the public is protected from health hazards and fraud in the preparation,
sale and use of foods and for matters connected therewith. It is enforced by the
Ministry of Health and the Local Authorities. The legislation, applicable to all
foods sold in the country either locally produced or imported, covers a broad
spectrum from compositional standards to food additives, nutrient supplements,
contaminants, packages and containers, food labeling, procedure for taking
samples, food irradiation, provision for food not specified in the regulations
and penalty.
Since food safety is addressed throughout the food chain, legislations
pertaining to food safety under the jurisdiction of other agencies are also
enforced by the relevant agencies. At the primary production level, the
Pesticide Act 1974, the Fisheries Act 1983, the Veterinary Surgeon Act 1974 and
the Animal Ordinance 1953, all under the Ministry of Agriculture and Agro Based
Industry are implemented. At the processing and retail levels, apart from the
Food Act 1983 and the Food Regulations 1985, other legislations that were
mentioned earlier are also applicable to a certain extent. The Trade Description
Act under the Ministry of Domestic Trade and Consumer Affairs also play an
important role in terms of protecting consumers from misleading and false
labelling of food product.
Present activities
Updating and harmonization of food safety legislation
In order to ensure that the food safety legislations are in tandem with the
development in food technology as well as to keep abreast with the changing
consumer demands, it has to be continuously revised and updated.
The continuous revision and updating of the Food Regulations 1985 is conducted
by the Technical Advisory Committee on the Food Regulations 1985 chaired by the
Director of the FQCD, Ministry of Health. It is an inter-agency committee
consisting of relevant government agencies involved in food safety from
farm-to-table, the food industry, professional bodies and the consumers. Request
for updating the food safety legislations is mainly made by the food industries
(especially on the use of new ingredients and additives) and consumers (who
demanded to be informed of new technology and new processes so as to be able to
make an informed choice of the food they buy).
To assist the Committee in ensuring that specific areas of concern in food
safety is addressed, various working groups comprising experts in the
specialized areas are established to undertake work, namely in the following
areas:
·Food Additives and Contaminants
·Food Labelling
·Food Commodity Standards
·Nutrition and Claims
·Microbiology
·Pesticide Residue
·Drug residue
·Fats and Oils
Currently new Acts and Regulations being drafted to enhance the implementation
of the food safety programme include:
·Food Hygiene Regulations
·Food Irradiation Regulations
·Food Import Regulations
·Food Analyst Act
·Animal Feed Act
Proposals for new legislations as well as revision and updating of legislations
are elaborated in a 14-step procedure, which closely follow the Codex step
procedure in the elaboration of Codex standards. Proposed new legislations,
revised and updated legislations are posted on the FQCD Website for comments.
Notification to the WTO is undertaken so as to ensure transparency and
fulfillment of obligations under the WTO.
Efforts in harmonization of legislation with international standards are
actively undertaken. Codex Alimentarius is the benchmark for food safety
legislation, whilst standards under the International Plant Protection
Convention (IPPC) and the International Organization on Epizootics (OIE) are
benchmarks for plant and animal health, respectively. Efforts to harmonize the
classification of food categorization and food additives and food undertaken
with the assistance of a local university. This is a follow-up of the
recommendation of the ASEAN Food Safety Standards Harmonization Project
undertaken with the International Life Sciences Institute (ILSI) South-East
Asia.
At the ASEAN level, harmonization of maximum residue levels (MRLs) with Codex
for pesticide residue is actively undertaken, wherever appropriate. However,
where Codex standards are absent especially for products that are peculiar to
the region, efforts to harmonize using regional standards are carried out.
Science and risk-based approach
In general, the continuous process of revision and updating of legislations
incorporates a science and risk-based approach to determine acceptable level of
control involving the entire food supply chain from primary production (use of
pesticides and veterinary drugs, contamination from the environment) to food
processing (use of additives and ingredients). Using the Codex Alimentarius as a
reference in the promulgation of legislation, a risk-based approach has been
taken into consideration by the risk assessment conducted by Joint Expert
Committee on Food Additives (JECFA) (for chemical additives) and Joint Expert
Meeting on Microbiological Risk Assessment (JEMRA) (for microbiological
parameters).
The Ministry of Health has proposed a regulation for genetically modified food (GMF).
The regulation utilizes a risk-based approach in the approval of GMF. In the
proposed regulation, risk assessment will be conducted by GMAC under the
Ministry of Natural Resources and Environment before it is allowed to be
marketed in the country. The Biosafety Bill, which is being formulated, is the
overall legislation pertaining to biosafety in Malaysia. Streamlining the
approval process for risk assessment under the Biosafety Bill and the proposed
regulations on GMF is a challenge to both ministries.
Currently, the risk-based approach is also used in the updating of ingredients
to be added to food. Application for the addition of new ingredients must be
justified so as not to cause any risk to consumers especially the vulnerable
groups.
Consumer’s rights
The food safety legislation has always given due recognition to consumer rights
e.g. the new regulations for nutrition labelling and claims ensure that
consumers are getting the right information and able to make a wise choice when
buying food. The new regulations not only benefit the industries in making
nutrition labels and claims but also ensure that the consumers are not misled by
the attractive claims on the food labels. The consumers too, should play their
role by reading the labels before making the right choice in buying food
especially in ensuring that they are eating correctly based on the Food Pyramid.
Besides this regulation, the proposed regulation for GMF also incorporates
provision on the labelling of GMF to provide consumer choice. The Consumer
Protection Act enforced the Ministry of Domestic Trade and Consumer Affairs,
although not specific to food safety do offer consumer protection.
Economic impact of food safety measures
The implementation of food safety legislation throughout the food chain is
essential in establishing an effective food safety system. Such legislation
should be updated to cater for the rapid development in food technology, the
internationalization of food tastes and also emerging and re-emerging food borne
diseases. Failure to adhere to such needs could lead to contaminated food and
occurrence of food borne disease as a consequence, loss of trade and
productivity.
The implementation of the Food Quality Control Programme has decreased health
care costs, improved worker productivity due to decrease disease burden. It has
also increased the availability of palatable and wholesome food through the
reduction of substandard food into the country. The implementation of the
programme has also increased consumer confidence in the food supply with the
enforcement of food standards and food labelling regulations. Provisions of
standards in the regulations have increased trade opportunities and export
premiums.
Recommendations/Lessons learnt
Harmonization of the national legislation with Codex standards is an enormous
task especially as it involves human and financial resources, and expertise. It
is essential that personnel undertaking such work have a good understanding of
the current national legislations as well as Codex Standards. In Malaysia, the
establishment of committees and sub-committees that specifically undertake this
work has benefited the country in ensuring that food safety legislations are
harmonized to the extent possible.
In the promulgation of food safety legislation, availability of scientific data
is very important, as the scientific and risk-based approach is the basic
requirement for standard setting work. In Malaysia, scientific data is gathered
through surveys and research conducted in collaboration and cooperation with
research institutes and institutes of higher learning. This smart partnership
has benefited Malaysia in terms of the optimization of limited resources,
expertise and funds. However, this effort could still be enhanced.
The implementation and enforcement of food safety legislation remains the
sovereign right of a country. However, the SPS Agreement under the WTO requires
that food safety legislations enforced in a country do not discriminate between
domestically produced foods and imported foods. Food safety legislations
enforced in Malaysia are applicable to domestic as well as imported foods.
Agenda Item 6
Conference Room Document 6
English only
RISK ANALYSIS IN MALAYSIA
(Malaysia)
Abstract
Malaysia acknowledges the importance of Risk Analysis to be the basis for all
food safety management actions, development of food safety standards and
managing risks associated with food hazards. Risk Analysis will complement the
numerous existing food safety initiatives that are currently being implemented.
To realize this aspiration, Malaysia has established a National Committee on
Risk Analysis under the Food Quality Control Division (FQCD), Ministry of Health
in 2002. The main objectives of this committee are to look into the development
and application of Risk Analysis in the country and also commission national
risk assessment projects.
The FQCD have been promoting the usage of Risk Analysis as a decision making
tool in food safety activities through a series of training, workshops, meetings
and collaboration with Japan International Cooperation Agency (JICA). Malaysia
has also actively participated in risk assessment training and meeting at the
ASEAN level and Codex work on GMOs.
Getting good information and sound scientific data will be one of the real
challenges in using risk analysis as a tool in food safety. Malaysia appreciates
the contribution of international expert bodies and feels that this effort
should continue to provide information and data needed for risk assessment
especially on dose-response data and animal feeding studies.
Training of risk managers and risk assessor will also pose a real challenge to
Malaysia. There is a need to strengthen expertise in risk analysis framework
especially in computer modelling and predictive microbiology and analytical
capabilities.
Recognizing that there is a certain level of expertise in research institutions
and universities in risk assessment, smart partnership should be developed with
such parties so as to optimally utilize the limited resources.
Background Information
Malaysia acknowledges the call for Risk Analysis to be the basis for all food
safety management actions by the international community. Malaysia also
recognizes Risk Analysis as the fundamental methodology underlying the
development of food safety standards at both international and national levels.
It is the methodological basis for assessing and managing risks associated with
food hazards.
The Ministry of Health has been implementing an active food surveillance
programme which includes routine compliance sampling, routine food premises
inspection and food import inspection activity. The routine compliance sampling
involves an annual sampling target of 40,000 samples from all over the country
and analyzed for various parameter specified under the National Work Plan.
As a preventive approach, the Ministry of Health has also implemented a
voluntary Hazard Analysis and Critical Control Point (HACCP) certification
scheme. The Malaysian Certification Scheme for HACCP requires the food
industries to implement the HACCP system that meets the criteria for
certification. The certification process includes adequacy, compliance and any
follow-up audits by appointed certified auditors. The Ministry of Health will
verify the maintenance of the certified HACCP system through surveillance audit.
To date, more than 75 food industries have been certified and most of the
products are exported to the EU, Japan and the USA.
The Ministry of Health also conducts a food monitoring activity on specific food
contaminants and additives. This is to provide accurate data and information on
levels and extent of food contamination for microbiological, chemical, chemical
migration and food additives. This information is needed for the formulation of
an effective preventive action and guidance for updating regulations on specific
problems to ensure food safety.
The Ministry of Health has also prepared a Draft Regulation on Genetically
Modified Foods (GMFs) which requires that all GMFs to be imported, prepared for
sale or sold in the country be approved by the Director General of Health before
allowed to be marketed. The draft regulations also require GMFs to be labelled
in accordance with the provisions stipulated.
Malaysia, being party to the Cartagena Protocol on Biosafety, has to conform to
obligations under that protocol. As such, the Ministry of Natural Resources and
Environment has drafted a Biosafety Bill which coordinates the application,
approval and notification of Living Modified Organisms (LMOs) and products
thereof in the country.
Currently, the National Guidelines for the Release of Genetically Modified
Organisms (GMOs) into the Environment, a document which provide a national
framework for addressing biosafety issues, with regard to regulation, assessment
and management of risks associated with the use and release of GMOs into the
environment, is the reference for the conduct of risk assessment on GMOs. This
was formulated by the former Ministry of Science, Technology and Environment.
Risk Analysis in Malaysia
Malaysia has established a National Committee on Risk Analysis under the Food
Quality Control Division (FQCD), Ministry of Health in 2002. The main objectives
of this Committee are to look into the development and application of Risk
Analysis in the country and also commission national risk assessment projects.
The members include all government agencies and Non Government Organizations
(NGOs). The first national risk assessment project commissioned by this
committee was on V. parahaemolyticus in frozen prawns and is currently in
progress.
One of the first awareness programme in the ASEAN region was initiated in 1999
by the International Life Sciences Institute (ILSI) and the Food and Agriculture
Organization (FAO), when a two and a half day Risk Assessment Workshop was
conducted in Kuala Lumpur, Malaysia. The participants include government
agencies responsible for food safety, industries and NGOs.
Subsequently, the FQCD has been promoting the usage of Risk Analysis as a
decision making tool in food safety activities in Malaysia. A series of
workshops and meetings have been conducted on risk management, microbiological
and chemical risk assessment among the food safety officers all over the country
and relevant government agencies.
A number of personnel have been trained in microbiological, chemical and
genetically modified food risk assessment conducted by International
Organizations.
In collaboration with Japan International Cooperation Agency (JICA) under the
Japan Technical Cooperation Programme, the FQCD has conducted workshops on
microbiological risk modelling and a risk assessment project on V.
parahaemolyticus in frozen prawns is currently in progress.
Risk Analysis principles is presently being used as a tool in the development of
food standards and in taking enforcement action (risk management options) to
ensure food safety.
The National Guidelines for the Release of GMOs into the Environment outline the
general principles governing standards of practice for the introduction of GMOs
and products containing or consisting of GMOs to the environment. These
Guidelines provide procedures for risk assessment on the release of GMOs into
the environment.
The risk assessment of GMOs is conducted by the Genetic Modification Advisory
Committee (GMAC) under Ministry of Natural Resources and Environment. This
committee comprises members from relevant agencies and research institutions and
will convene when there are applications for the release of GMOs. This is the
central scientific committee that deals with issues on the application and
approval of GMOs.
With the eventual gazettement of the Draft Regulation on GMFs under the Ministry
of Health, GMAC will be the committee that conducts the risk assessment / safety
assessment on GMFs. The draft regulation will complement the Biosafety Bill in
terms of the application and approval procedures.
At the ASEAN level, three (3) workshops had been conducted to train participants
on the risk assessment of GMOs, particularly on GMOs of plant origin.
Malaysia also participated in the Codex work on GMOs, namely the labelling
requirements for GMFs under the Codex Committee on Food Labeling and the
Principles for the Risk Analysis of Foods Derived from Modern Biotechnology and
Guidelines for the Conduct of Foods Safety Assessment of Foods Derived from
Recombinant-DNA Plants and Recombinant-DNA Microorganisms.
The challenges
a) Data and Information
Getting good information and sound scientific data is one of the real challenges
in using risk analysis as a tool in food safety.
The Food Consumption Pattern is very crucial when conducting exposure
assessment. In Malaysia, the first national survey on food consumption pattern
of adult diet will only be completed by the end of 2004. The food consumption
pattern for specific vulnerable groups such as the elderly, children and etc.,
has yet to be undertaken and will done in the near future.
Malaysia appreciates the contribution of international expert bodies and feels
that this effort should continue to provide information and data needed for risk
assessment especially on dose-response data and animal feeding studies.
b) Risk Management
Changing the existing approach in the management of food safety issues to the
application of risk management framework will also pose a real challenge to
Malaysia. We need to continuously train the food safety officers. At present
Malaysia is in the process of developing a simple guideline for risk managers on
the application of Risk Management Framework in making risk management decision.
c) Risk Assessment
Training of risk assessors will also be the greatest challenge to Malaysia.
There is a need to strengthen expertise in computer modelling and predictive
microbiology as well as expertise in analytical and Risk Assessment / Safety
Assessment of GMFs. With the implementation of the regulations on GMFs, training
for the purpose of enforcement should be strengthened.
In terms of analytical capabilities, the National Public Health Laboratory,
under the Ministry of Health, laboratories in the Chemistry Department, under
the Ministry of Science and Innovation, and laboratories in Institute of Higher
Learning are capable of detecting GMOs in primary products. Analytical
capability in the detection of GMOs in composite food products remains a
challenge.
d) Risk Communication
The FQCD is planning to increase human resources in risk communication at all
levels, comprising professionals capable of translating risk assessment results
and risk management decision to all stakeholders and the public.
Summary/Lessons Learned
To fully implement Risk Analysis, Malaysia is continuously enhancing its
expertise and directing all efforts to ensure Risk Analysis will be used as a
basis for the development of food safety standards and making decision on food
safety risk management options. An important aspect in the implementation of
risk analysis is the availability of funds. Funding could be sourced from
government, international organizations and agencies, as well as donor
organizations.
To ensure continuity and development of expertise in chemical and
microbiological risk assessment, a core group of personnel who are involved
(food standard officials) or interested in risk assessment activity should be
trained. Continuous training of this core team will further enhance effective
utilization of risk assessment.
Recognizing that there is a certain level of expertise in research institutions
and universities in risk assessment, smart partnership should be developed with
such parties so as to optimally utilize the limited resources.
Risk managers should be adequately trained on the application of risk management
framework in food safety. To promote the application of risk management options
to risk managers, a good promotion and education programme needs to be
developed.
Agenda Item 7 Conference Room Document 7
English only
FAO/WHO Regional Conference on Food Safety for Asia and the Pacific
Seremban, Malaysia, 24-27 May 2004
FOOD SAFETY CONTROL SYSTEM IN MALAYSIA
(Malaysia)
Abstract
Food safety is increasingly becoming a global challenge both by virtue of its
public health impact as well as its economic and political implications.
Malaysia has established the National Food Safety and Nutrition Council in 2001,
a multi- sectoral forum to set clear policies and strategies for the continuous
improvement of the food safety programme. This Council is chaired by the
Honorable Minister of Health and formulates policy on food safety and nutrition,
which will be integrated, with other national policies to address, amongst
others, health, economic and trade issues. The National Food Safety Policy was
formulated in 2002 and it aims at providing direction to all stakeholders in
establishing and implementing food safety measures, through collaborative
efforts to safeguard human health. To effectively implement the National Food
Safety Policy in a more coordinated and integrated manner, a National Plan of
Action on Food Safety had also been formulated in 2002. The action plan clearly
defines the role of each stakeholder and its successful implementation will
depend on the support and commitment of all the relevant government agencies and
stakeholders.
Malaysia has taken measures to strengthen the following activities: Formulation
and Review of Food Legislations; Certification; Enforcement; Laboratory
Services; Research and Monitoring and Participation in International and
Regional Fora.
Malaysia is currently collaborating with the Japan International Cooperation
Agency (JICA) specifically in the areas of data management, electronic
networking in import control, laboratory capability and technical exchange
programmes.
At the ASEAN level, Malaysia has offered expertise to other ASEAN countries,
example, Viet Nam in the promulgation of food legislations and development of
HACCP certification and in enforcement and prosecution activities as well as the
development of HACCP certification in Brunei Darussalam. Malaysia has also
conducted consultations on food safety and Codex under WHO to the Pacific
islands namely Solomon Islands, Kiribati and Samoa, as well as on Codex under
FAO to Iran. Malaysia is willing to offer expertise in the following areas:
promulgation of food legislations, laboratory capability, implementation of good
practices on farm, food processing, animal husbandry and aquaculture,
establishment of certification schemes, on-line control of food imports, crisis
management in the prevention and control of Nipah virus, food safety
consultancy, development of Codex Contact Point and the setting up of National
Codex Committee.
Introduction
Food safety should be addressed throughout the food chain from farm to table,
that is from the stage of production, processing, storage and distribution. This
requires close collaboration and cooperation among all stakeholders along the
food chain, clearly defined jurisdiction and responsibilities, mechanism of
cooperation and means of dealing with existing and emerging food safety
challenges. Resources such as manpower and finance should be allocated and
utilized in a coordinated manner to achieve optimal results.
Background
Food safety is increasingly becoming a global challenge both by virtue of its
public health impact as well as its economic and political implications.
Malaysia has realized that food safety can no longer be considered solely as a
domestic entity nor can it be the responsibility of a single agency. The
Ministry of Health has established the National Food Safety and Nutrition
Council in 2001, a multi- sectoral forum to set clear policies and strategies
for the continuous improvement of the food safety programme. It involves
relevant government agencies, industry and consumer representatives as well as
other stakeholders from farm to table. The Honorable Minister of Health chairs
the council and within the offices of this council, policies on food safety and
nutrition will be integrated with other national policies to address, amongst
others, health, economic and trade issues. This would enable a more
comprehensive approach to food safety on the basis of smart partnership and
shared responsibility and encourage industry’s commitment and consumer
involvement in implementing the strategies for food safety.
The Ministry of Health formulated a National Food Safety Policy in 2002 which
aims at providing direction to all stakeholders in establishing and implementing
food safety measures, through collaborative efforts to safeguard human health.
The Policy addresses food safety throughout the food chain and requires multi
agency and multi disciplinary collaboration and cooperation involving relevant
government agencies, food industries, consumers, the scientific community and
others. Essential elements include food safety infrastructure, food safety
legislation, inspection and enforcement services, food laboratory, ICT,
scientific information gathering and analysis, product tracing, management of
food safety crisis, management systems for food safety assurance, education on
food safety, safety of imported and exported foods, novel foods and technologies
and participation in international food safety fora.
To effectively implement the National Food Safety Policy in a more coordinated
and integrated manner, a National Plan of Action on Food Safety had also been
formulated in 2002. The action plan clearly defines the role of each stakeholder
and the action to be taken. It is the concerted effort by various government
agencies and the non-governmental organizations (NGOs) and its successful
implementation will depend on the support and commitment of all the relevant
government agencies and stakeholders. The approach undertaken is farm to table.
Capacity building activities on food safety in Malaysia
In view of increased need for food safety in international trade, globalization
and to meet with obligations under the World Trade Organizations (WTO)
agreements, in addition to the traditional programme, new initiatives are
undertaken to meet the challenging demands towards ensuring food safety whilst
minimizing barriers to trade. The Sanitary and Phytosanitary Measures (SPS) and
Technical Barrier to Trade (TBT) Agreements under the WTO encourage the
harmonization of food standards. For food safety purposes, the SPS Agreement
recognizes Codex standards, guidelines and recommendations as the benchmark for
national measures and regulations.
Programme strategies that have been strengthened include formulating laws that
meet with international requirements, emphasis on import and export control and
upgrading analytical capabilities. These are achieved through activities such as
formulation and review of legislations and standards, strengthening enforcement,
promoting certification, improving data management, and increased participation
in international activities related to food safety. Other new initiatives
include outsourcing of laboratory capabilities, enhancement of consumer
empowerment through increased informative labeling and consumer education,
promotion of self-regulation towards industry accountability, and incorporating
Information and Communication Technology (ICT) in food safety. In order to
improve transparency as well as to meet current demands, dialogue sessions are
being held regularly with industry and consumer representatives.
1. Formulation and Review of Food Legislations
Malaysia is constantly revising the food laws, regulations and standards so as
to be in line with current needs as well as international requirements. The Food
Regulations 1985 is amended from time to time under the Technical Advisory
Drafting Committee of the Food Regulations 1985 supported by technical sub
committees and expert task forces. Reference is made to the Codex standards and
guidelines where available.
2. Certification
Malaysia has initiated and implemented the Malaysian Certification Scheme (MCS)
for Hazard Analysis and Critical Control Points (HACCP) since 1997 and has
formulated four (4) guidelines to facilitate successful implementation of the
system by the food industries. The Ministry of Health practices third party
certification and is involved in the surveillance auditing of food factories.
The European Union (EU) has recognized government authorities as the competent
authority for certification of seafood exports to the EU. HACCP certification is
now extended to other food industries with auditing being done by third parties.
More than 75 food industries have been certified under the MCS for HACCP by the
Ministry of Health. The Veterinary Services Department of the Ministry of
Agriculture and Agro-based Industry also certifies various Quality Assurance
Schemes and Good Animal Husbandry Practice (GAHP) at the farm level. The
Department of Agriculture and the Department of Fisheries under the Ministry of
Agriculture and Agro-based Industry have also implemented farm accreditation
schemes to ensure Good Agriculture Practice (GAP) and Good Aquaculture Practice
(GAqP), respectively.
Malaysia has also formulated the Guidelines on Good Hygiene Practices for Small
and Medium Scale Food Industries towards HACCP. These guidelines provide a guide
on basic hygienic and sanitation requirements and to fulfill prerequisite
requirements for the implementation of HACCP to small and medium scale
industries.
Various government agencies play an active role in promoting Good Manufacturing
Practice (GMP), Good Hygiene Practice (GHP), the application of HACCP and Halal
Certification.
3. Enforcement
Enforcement activities such as sampling, premise inspection, import and export
control have been intensified. This is achieved in part by improving
infrastructure with regards to data collection and monitoring as well as
equipment. The Food Safety Information System of Malaysia (FoSIM) has been
established to link FQCD, 34 entry points, 11 food laboratories and the Customs
Department in order to better manage safety of imported food. FoSIM was launched
in 2003.
4. Laboratory Services
Laboratory capacity building has always been given top priority, as they are one
of the most crucial infrastructures needed for an effective and efficient food
safety system. Various approaches are being undertaken by the Ministry of Health
to ensure that laboratories are able to meet the increasing demand and
complexities of food analysis including new requirements imposed by the Food
Regulations 1985.
·Continuously upgrade the existing laboratories in the Ministry of Health
(instrumentation, quality system and skills).
·Optimizing existing government laboratories’ facilities including those from
other ministries such as the Ministry of Agriculture and Agro-based Industry and
the Ministry of Science, Technology and Innovation.
·Buying of services from Universities to conduct analysis on parameters
currently not carried out by Ministry of Health laboratories.
·Engaging expert consultants such as that from the Japan International
Cooperation Agency (JICA).
·Collaboration with Institutes of Higher Learning on food safety including
research and surveys.
·Recognition of Private Laboratories accredited for ISO/IEC 17025 by the
Department of Standards Malaysia.
5. Research and Monitoring
In view of the need for a science based approach to food safety, the research
activities have now focused on data collection for purposes of risk assessment.
These activities are to monitor incidence of food-borne diseases, including food
poisoning; to monitor environmental problems related to food; and to monitor
food for microbiological and chemical contamination. The baseline information on
the status of specific contaminant levels in relevant foodstuffs will also help
prioritize issues of concern.
6. Participation in International and Regional Fora
In meeting the global challenges, Malaysia is playing a bigger role in
activities related to food safety at international and regional levels including
Codex, ASEAN, APEC, ASEM, WTO (SPS and TBT) and others.
The National Codex Contact Point is located in the Ministry of Health and serves
as the National Contact Point for international food safety issues. Malaysia
currently has a National Codex Committee, 21 Codex Sub-Committees, 3 Codex Task
Forces and 7 Codex Working Groups. Malaysia had so far played an active role in
Codex. Malaysia will be hosting the 33rd Session of the Codex Committee on Food
Labelling from 9 to 13 May 2005.
At the ASEAN level, Malaysia has been appointed as the coordinator of the
programme area on food safety under the ASEAN Expert Group on Food Safety (AEGFS).
Malaysia chaired and hosted the First Meeting of the AEGFS in 2001 and chaired
the Second Meeting of the AEGFS in 2003. The AEGFS discusses issues pertaining
to food safety from farm to table. The Draft ASEAN Food Safety Improvement Plan
(AFSIP) drawn up by the AEGFS has been endorsed by the Senior Officials Meeting
on Health. Malaysia also hosted the First Meeting of the ASEAN Task Force on
Codex (ATFC) in 2001. The ATFC was formed to formulate ASEAN common positions on
issues of concern to ASEAN Member countries.
Networking with other regional and national activities
Networking at the regional and national levels can be achieved through the
establishment of focal points or the utilization of existing focal points. The
creation of a website dedicated to food safety is ideal but could involve
significant implications in terms of financial, human resources as well as
infrastructure at the regional and national levels.
The introduction of the new International Portal on Food Safety, Animal and
Plant Health by the FAO, provides a single access point to the wide range of
official materials relating to SPS measures in food and agriculture. This portal
will enhance transparency in SPS measures and facilitate trade among trading
partners. The utilization of this portal will assist in facilitating networking
at the international and regional levels.
Technical assistance among countries within the region
Malaysia is currently collaborating with JICA specifically in the areas of data
management, electronic networking in import control, laboratory capability and
technical exchange programmes.
At the ASEAN level, Malaysia has offered expertise to other ASEAN countries.
Malaysia has assisted Viet Nam in the promulgation of food legislations and
development of HACCP certification and trained food control officials in
enforcement and prosecution activities as well as the development of HACCP
certification in Brunei Darussalam. Other technical assistance includes
attachment of food control officials from various ASEAN countries at the
Ministry of Health.
Malaysia has also offered consultations on food safety and Codex under WHO to
the Pacific islands namely Solomon Islands, Kiribati and Samoa, as well as on
Codex under FAO to Iran.
Malaysia is willing to offer expertise in the following areas: promulgation of
food legislations, laboratory capability, implementation of good practices on
farm, food processing, animal husbandry and aquaculture, establishment of
certification schemes, on-line control of food imports, crisis management in the
prevention and control of Nipah virus, food safety consultancy, development of
Codex Contact Point and the setting up of National Codex Committee.
Agenda Item 8
Conference Room Document 8
English only
FAO/WHO Regional Conference on Food Safety for Asia and the Pacific
Seremban, Malaysia, 24-27 May 2004
INFORMATION EXCHANGE, EDUCATION AND COMMUNICATION
THE MALAYSIAN PERSPECTIVE
(Malaysia)
Abstract
Information exchange, education and communication through cooperation between
Food Quality Control Division, Department of Public Health, the Ministry of
Health as the lead agency for food safety and with the Department of
Agriculture, the Department of Fisheries and the Department of Veterinary
Services, Ministry of Agriculture and Agro-based Industry and relevant other
Ministries, agencies and organizations is essential for the nation. Policies and
programmes on food safety are science-based, to the extent possible.
The National Food Safety Policy endorsed by the National Food Safety and
Nutrition Council 2001 has identified several key elements including education
on food safety and scientific information gathering and analysis which has an
impact particularly contributing towards strengthening the nations’ food safety
programme and a society of well-informed consumers.
Current initiatives in information exchange and communication in food safety is
the Food Safety Information System of Malaysia (FoSIM) that utilizes ICT, to
improve food safety control measures on imported foods. Another initiative such
as the Crisis Alert Team for both periods of crisis and pre-crisis is seen
vital. Communication of information related to agriculture, fisheries and
veterinary services, trade, education and those related to food safety is also
available through the web-based portals of the relevant Ministries and public
and private agencies and also through traditional media of information
dissemination of brochures, pamphlets and posters.
Food safety education in Malaysia continuously receives good response through
the demand for the Food Handlers Training Programme by food handlers and food
industry operators. The demand for recognition under the MCS HACCP certification
by the Ministry of Health and accreditation of the Veterinary Health Mark Logo
by the Department of Veterinary Services, Ministry of Agriculture and Agro-based
Industries is also good.
The future challenge at the national level is the implementation and
practicalities of all relevant stakeholders to the National Food Safety Policy.
At the regional level, Malaysia plays an active role in the ASEAN Expert Group
on Food Safety and other ASEAN bodies related to food safety. At the
international level, Malaysia plays an active role in Codex and other meeting
related to food safety.
Introduction
Food safety in Malaysia is under the purview of the Food Quality Control
Division (FQCD), Department of Public Health, Ministry of Health. Since food
safety should be addressed from farm to table, other agencies such as the
Department of Agriculture, the Department of Fisheries and the Department of
Veterinary Services, Ministry of Agriculture and Agro-based Industry are also
responsible in ensuring safe food for consumer protection. Increased cooperation
with other relevant Ministries, agencies and organizations linked to public
health and food safety is essential to meet the nation’s requirements and needs.
Significant progress in this arena has been achieved in the last decade by
strengthening the education of the workforce through training and utilization,
as well as upgrading information communication technology (ICT). One of the
essential elements to Malaysia’s food safety activities is ensuring policies and
programmes, including legal requirements, are based on science, to the extent
possible.
Throughout the process of developing scientifically-based policies, the relevant
Ministries have aggressively sought input from the scientific community and
others. The Ministries conduct inter-agency technical meetings based on
scientific information that allows the agency to share information with, and
gather input from stakeholders from both public and private sectors on food
safety topics. Our policies and their effectiveness affect all our partners
along the food safety chain.
However, establishing science-based programmes and policies is only the first
step. In order for those policies to work, training provisions are recognized
for a scientifically and technically trained workforce that can operate in
modern food safety systems as well as existing small industries. One step
Malaysia is taking in that direction is to increase our cadre of scientifically
trained personnel in the field of implementing and auditing food safety systems
such as the Hazard Analysis and Critical Control Point (HACCP) in food
industries, as well as implementing and auditing quality systems i.e. ISO/IEC
17025 in the food laboratories. Furthering this effort through an extensive
training and education programme is one of our future initiatives and identified
under the National Food Safety Policy.
Background
The National Food Safety Policy
The National Food Safety Policy endorsed by the National Food Safety and
Nutrition Council in 2001, has identified several key elements including
education on food safety and scientific information gathering and analysis.
Scientific information gathering and analysis recognizes the need for
identification and evaluation of potential hazards in food and animal feed. One
of the many methods and indicators to identify food safety problems include data
derived from monitoring carried out along the food chain, disease surveillance,
research and epidemiological investigation. The data is imperative for correct
analysis to facilitate study of the evolution of known hazards and the
identification of new ones. All these would form the basis for control measures
taken to ensure food safety based on scientific principles, recognizing the
limits of science.
Malaysia recognizes reliable scientific information on food safety as one of the
main pillars to ensure food safety. There is great need for strengthening the
strategic plan to collect and analyze scientific information on food safety
throughout the food chain. Therefore, adoption of appropriate information
technology to facilitate accurate, relevant and timely information flow is one
of the identified priorities. The strategic plan for scientific information
gathering and analysis include monitoring and surveillance, research and
scientific co-operation and networking. The monitoring and surveillance
programme is needed to generate accurate data on the levels and trends in food
contamination, which can be used as the basis for the promulgation of
regulations and preventive measures. It also helps to strengthen Malaysia’s
position in the international market by ensuring the safety of imported and
exported food.
Issues and problems related to food safety is becoming increasingly complex and
challenging, where science-based information particularly through research or
studies will continue to be an important component for supporting the
development of food safety policies. At the international level, the
internalization of science particularly in the development of food standards
based on accepted scientific basis has a tremendous imposition in Malaysia’s
food trade in the international market. One of the first steps in building
scientific alliances is the establishment of clear lines of communication
between interested agencies in scientific cooperation and technical assistance.
This communication could be in the form of regular meetings of relevant
agencies, an inventory of scientific cooperation or technical assistance needed
or provided for, a list of experts and reference centers in various subject
areas, compilation of reference materials and other means. The establishment and
maintenance of a database would help to reduce overlap and duplication in
scientific cooperation. In addition, it is also important that needs assessment
be carried out to identify specific needs and to design and prioritize a
coherent and integrated approach. The above approach are expected to facilitate
the creation of a network of centers of excellence, enabling Malaysia to draw
from the leading edge scientific expertise in all relevant disciplines at the
national and international level.
Another element of the National Food Safety Policy is education on food safety
that recognizes education programmes on food safety as a critical part of the
strategy to prevent or reduce the incidence of food-borne diseases and
furthering co-operation between the government and non-governmental organisation
including the consumer’s association as very important in ensuring the success
of these programmes.
Through collaborations, there is substantial amplification of key health
messages that can enhance the effectiveness of the campaigns for all involved.
More importantly, the consumer is the one who benefits the most from the
extensive reach of essential and timely messages. The co-operation in education
emphasises several components such as information sharing, consistent messages
from agencies to the consumers, research-based education approaches based on
public perception and behaviour, targeting high-risk consumers or those more
prone to food-borne illnesses, co-operation with the media in ensuring accurate
information and facilitating conveyance of education messages, education and
training to all relevant parties and introducing education for the young for a
lasting impact.
Taking into consideration the above components, it is essential that educational
programmes are supported by comprehensive food safety awareness campaign for
consumers with tailored information, communication channel and approach to suit
different audience especially high-risk consumers through broadcast media, in
multilingual to ensure widest coverage and including, effective food safety
educational materials to cater to target groups of different cultures and
languages and different ways of eating and preparing foods.
Current Initiatives
Information Exchange and Communication
The Food Safety Information System of Malaysia (FoSIM)
The Food Safety Information System of Malaysia (FoSIM) launched in 2003 utilizes
ICT, to improve food safety control measures on imported foods and enhance the
safety of food for the consumers. This project involves, amongst others, the
installation of the necessary network cables and hardware and the development of
the system application based on web technology. The system when fully
implemented will link all 34 entry points in the country with the 14 food
quality control laboratories, 13 state health departments and the FQCD, Ministry
of Health. This system has an interface with the Customs Information System to
allow communication between the two systems.
Among the important elements in this system is firstly, the future need for
prior notification using the system by the importer which preferably should be
24 hours before a food consignment is brought into the country through any entry
point, usually by sea or air (this precludes the importation of foods which
could not be determined earlier than 24 hours, such as fishes and small
quantities of food brought in by land); secondly, the registration of all
importers for access to the system; thirdly, the need for food sampling by the
health inspectors followed by the analytical results authorized by food analysts
using the system and lastly, the on-line availability and the accessibility of
all information in real-time pertaining to the importation of food, enabling
immediate decisions regarding enforcement activities on any particular
consignment of food.
Crisis Alert Team (CAT)
The establishment of a dynamic crisis alert team (CAT) at the FQCD, Ministry of
Health has proven to be a success in particular when Malaysia was faced by the
contamination of dioxin in the food chain in 1999. Effective communication and
networking between the various Malaysian government departments and the affected
stakeholders including the industries, importers and foreign government agencies
via foreign embassies and High Commissions based in Kuala Lumpur enabled
information exchange on imported food and ingredient to be scrutinized before
approval.
Another concept of the CAT in practice, outside the limits of a crisis, is the
establishment of a team of officers on a weekly rotational basis to monitor and
scrutinize via the mass media and ICT, latest developments in the food safety
arena globally and locally. The existence and functioning of such a team which
operates 7 days a week enables the FQCD to function as in a pre-crisis period
better equipped to face the challenges of a crisis.
Web-Based Information
Malaysia believes that communication is essential to safer foods, better health
and greater security. Therefore, the Ministry of Health has made a milestone
progress in establishing communication through the world-wide-web enabling
consumers, including industries and governmental agencies to contact the
Ministry on food safety aspects and to enable them to view and comment on the
latest issues including food alerts, proposed legislations, forthcoming
conferences, provide on-line registration for HACCP certification, access the
FoSIM system for on-line registered parties and obtain much needed information
on food safety. The Ministry of Agriculture and Agro-based Industry and other
governmental departments also provides web-based gateway to information related
to agriculture, fisheries and veterinary services, trade, education and others.
The websites of several agencies relevant to food safety in Malaysia are
available in Appendix 1.
Dissemination of Information
Dissemination of information on food safety is carried out via brochures, radio
talks and commercials including talks and necessary training to educate
personnel involved in enforcement activities. Contentious issues with the
industries and consumer bodies are discussed and resolved through separate
dialogues involving the relevant ministries and chaired by the respective
Ministers. Communication is seen vital to link the industries with government
requirements.
Food Safety Education
The Food Handlers Training
The Ministry of Health launched the Food Handler’s Training Programme in 1996
and accredited private institutions to conduct a one-day Food Handlers Training
Programme on basic aspects of food hygiene and handling. 39 accredited
institutes are actively conducting food handlers training as of April 2004 and a
total of 150,000 food handlers had undergone training. The Ministry of Health
conducts the Food Handlers Training Programme with a comprehensive Training the
Trainers Module since 1998. Identified Lead Trainers consists of officers from
the Ministry of Health. The Ministry also promotes food handlers training at the
state level to ensure basic education and knowledge before licensing of food
premises. Inter-governmental and inter-agency support and communication is
essential towards achieving a trained workforce in the food service industry.
HACCP in Industries
The Ministry of Health also promotes food safety through the certification of
the Malaysian Certification Scheme for Hazard Analysis and Critical Control
Point system (MCS HACCP) that describes procedures which apply to food premises
in gaining HACCP certification. The certification process includes adequacy,
compliance and any follow-up audits by appointed certified auditors. The
Ministry will verify the maintenance of the certified HACCP system through
surveillance audit. As of April 2004, more than 75 industries were certified
under the MCS HACCP.
The Veterinary Health Mark Logo is a mark of quality and safety given to plants
processing livestock products, awarded under the Veterinary Inspection and
Accreditation Program of the Department of Veterinary Services (DVS), Ministry
of Agriculture and Agro-based Industry. It also signifies the complete
compliance by the plants to the minimum standards of hygiene and sanitation,
quality assurance and food safety set by DVS, verified through the process of
plant inspection, examination and auditing (adequacy, compliance, follow-up,
surveillance and review audits) of the food safety quality system such as HACCP
and Good Manufacturing Practices. As of December 2003, a total of 58
establishments are participating in this programme, whereas 33 establishments
are being provided advisory services. Veterinary inspectors are involved in the
veterinary inspection at the export-oriented poultry processing plant. The
Veterinary Health Mark Logo scheme is recognized by relevant competent
authorities and this enables the establishments to export their products.
Future challenges
National Level
The endorsement of the National Food Safety Policy by all relevant agencies and
organizations has paved the way for a new era of strengthening existing
commitment and creating new found areas in food safety for the nation. The
implementation and practicalities involved to abide the policy by all relevant
stakeholders in the next decade is hoped to achieve safe and quality food and
build a nation based on a society of smart consumers.
Regional Level
The 3rd Meeting of the ASEAN Expert Group on Food Safety (AEGFS), held in
Jakarta, Indonesia in February 2004 discussed the draft ASEAN Food Safety
Improvement Plan (AFSIP) that encompasses proposed regional activities at the
ASEAN level including activities on information sharing, education and training,
and research and development. Emphasis was given to exchange of information on
food safety including during crisis between ASEAN Member Countries through
existing national web-sites, through AEGFS focal points, and other means.
Malaysia proposed the need to develop common format for compilation of data
amongst ASEAN Member Countries in the form of research data, directory of
experts, list of accredited laboratories, food business directory and training
programs and courses. In the area of education and training, Malaysia proposed
the establishment of training centers and joint training programmes taking into
account the existing models and institutions, strengthening mechanism for
informing ASEAN Member Countries of training opportunities and expertise in
other ASEAN Member Countries, to develop an ASEAN module for training of
trainers and food handlers and the development of training modules on food
safety for healthcare workers with the assistance of the World Health
Organization (WHO) for ASEAN Member Countries’ needs. Malaysia also proposed the
need to encourage publication and dissemination of research findings through ICT
and journals. The decisions agreed at the meeting need to be followed through by
all ASEAN Member Countries to strengthen food safety in the ASEAN region. The
Meeting agreed on information sharing as one of the three priorities. The draft
AFSIP has been endorsed by the Preparatory Senior Officials Meeting for the 7th
ASEAN Health Ministers Meeting (AHMM), Penang, Malaysia, 19-20 April 2004.
International Level
Malaysia recognizes the need to exchange views at meetings and therefore, is
committed to actively participate at the international fora in food safety and
food trade. Malaysia has and will continue to participate at food
standard-setting meetings such as the Codex Alimentarius Commission and their
various committees and working group to exchange views and data to facilitate
trade and also to enable better understanding and harmonization of food laws.
Issues discussed in other fora, such as the Committees for Sanitary and
Phytosanitary Measures (SPS) and Technical Barrier to Trade (TBT) under the
World Trade Organization are also followed closely, as Malaysia’s role as a
trading partner with developed countries warrants the need for fair trade
practices.
Agenda Item 7
Conference Room Document 9
English only
FAO/WHO Regional Conference on Food Safety for Asia and the Pacific
Seremban, Malaysia, 24-27 May 2004
Regional Coordination in Strengthening Countries’ Participation and
Implementation of International Food Safety Standards
National Bureau of Agricultural Commodity and Food Standards,
Ministry of Agriculture and Cooperatives, Thailand
1) Introduction
Food safety issues have become a main issue for international food trade since
the establishment of the World Trade Organization (WTO) in 1995. The WTO’s
Agreement on the Application of Sanitary and Phytosanitary (SPS) Measures has
completely changed the situation of international food trade and resulted in the
high importance of international food standards. Under the SPS Agreement,
members have the right to take sanitary and phytosanitary measures necessary for
the protection of human, animal or plant life or health. The Agreement also
accepts sanitary or phytosanitary measures which conform to international
standards, guidelines or recommendations to be necessary to protect human,
animal or plant life or health. In the scope of food safety, the Agreement
recognizes the standards, guidelines and recommendations established by the
Codex Alimentarius Commission (known as Codex) as international standards.
Furthermore, the Agreement emphasizes that members shall play a full part,
within the limits of their resources, in the relevant international
organizations and their subsidiary bodies, in particular the Codex Alimentarius
Commission and other organizations to promote within these organizations the
development and periodic review of standards, guidelines and recommendations
with respect to all aspects of sanitary and phytosanitary measures.
Even though every country recognizes and accepts the high values of
participation in the international food standard organizations e.g. Codex, few
developing countries can play their full part effectively in this system because
of the limitation of resources and capabilities.
2) Problems of developing countries on participation and implementation of Codex
standards
Codex is the Joint Food Standards programme established almost 40 years ago by
the Food and Agriculture Organization of the United Nations (FAO) and the World
Health Organization (WHO). Membership is currently 170. Participation of members
in the Codex standard setting process is varied among members. Information was
collected to analyze the participation of Codex’s members in four Codex
Committee concerning food safety in the year 2003. These Codex Committees are:
·Codex Committee on Pesticide Residues (CCPR)
·Codex Committee on Residues of Veterinary Drugs in Foods (CCRVDF)
·Codex Committee on Food Additives and Contaminants (CCFAC)
·Codex Committee on Food Hygiene (CCFH)
The analysis of the information provides interesting results as follows:
·Only 67 out of 170 member countries participated in at least one meeting of the
above Codex Committees.
·Ratio of participations in the meetings between developed countries and
developing countries is approximately 55:45.
·Ratio of participations in drafting groups/working groups preparing working
documents under the above ·ntioned Codex Committee between developed countries
and developing countries is approximately 80:20.
·Ratio of pesticide residue data submitted for evaluation for establishing
pesticide residue standards in the year 2003 from developed countries compared
to developing countries is approximately 90:10.
This information shows that several member countries did not attend the meetings
of the above mentioned Codex Committees. This may be the result of their limited
resources and their expectations from attending the meetings. For those
countries who attended the meetings, their expected benefits may be high enough
for them to invested. From the above information, the level of participation
between developed countries and developing countries are much different.
Abilities of developing countries, by numbers of countries attending the
meetings, is very different to developed countries. However, the abilities of
developing countries measured by the level of participation in drafting
standards/working documents or submitting scientific data are far less than
developed countries. As it is a fact that Codex standards are principally based
on scientific data and information, it can be assumed that Codex standards are
mostly directed by developed countries unless developing countries improve their
ability and participation in the Codex process.
3) Improving developing countries’ participation and implementation of
Codexstandards
The 26th Session of the Codex Alimentarius Commission (CAC) welcomed the
establishment of FAO/WHO Trust Fund for Participation of Developing Countries in
Codex Standard Setting Procedures. The Trust Fund, which started in 2004, aimed
to increase the participation of developing countries in Codex meetings. The
Trust Fund, while solving the problem of limited budget of developing countries
attending Codex meetings, cannot significantly improve their ability and
participation in the Codex standards setting process. Other solutions such as
capacity building within each developing country or regional coordination have
to be introduced and implemented.
3.1 Regional coordination: ASEAN experience
The Association of South-east Asian Nations (ASEAN) is a regional body that
brings together and provides coordination among the 10 members of ASEAN. The
ASEAN vision 2020 envisaged “ a stable, prosperous and highly competitive ASEAN
economic region in which there is a free flow of goods, services and
instruments, a free flow of capital, equitable economic developments and reduced
poverty and socio-economic disparities”.
Goals of ASEAN countries are to produce and supply foods that are safe and meet
requirements of importing countries as well as international standards.
Strengthening Member Countries’ participation and implementation of
international food safety standards, especially Codex standards setting process,
is also the main goal of the region. However, due to the different stages of
development of food safety and quality standards in Member Countries, the
regional approach has been concentrated on harmonization and networking of food
safety and food standards among member countries. Some of the many working
groups/task forces have been established for this purpose.
3.1.1 ASEAN cooperation under ASEAN Ministers on Agriculture and Forestry
a) ASEAN Task Force on Codex (ATFC)
The ASEAN Task Force on Codex has been established to serve as a forum for
Member Countries to discuss Codex issues of common interest and to possibly
identify common positions on Codex issues of importance to Member Countries. It
is also a forum to harmonize standards and regulations in ASEAN by using Codex
standards as references. Four meetings of the Task Force have taken place in
Kuala Lumpur, Bali, Bangkok and Cebu. Many joint ASEAN positions on Codex issues
were discussed and agreed. Activities on the strengthening and improving
participation of ASEAN countries in Codex, for which a member country was
nominated to be a focal point for each Codex Committee, have commenced.
b) Expert Working Group on the Harmonization of Maximum Residue Limits (MRLs) of
Pesticide among ASEAN Member Countries
The main objectives of the programme of this Expert Working Group are for the
harmonization to protect consumer’s health, the harmonization of standards on
agricultural and food commodities among ASEAN Member Countries and to provide
Member Countries with a means for coordination and information sharing to reach
international standards.
Eight meetings of the yearly Expert Working Groups meeting have taken place. The
total number of harmonized MRLs of pesticides which have been endorsed by the
ASEAN Ministers on Agriculture and Forestry (AMAF) is 369, involving a total of
28 pesticides. The number of draft harmonized MRLs in the process of
consideration is 258. Even though most of the harmonized MRLs are based on Codex
MRLs, more focus is on the harmonization of MRLs for minor crops important to
the region for which no Codex MRLs are available. Issues on regional
collaboration in the generation of residue data for harmonization and the
principles and criteria on harmonization of MRLs are being considered in the
Expert Working Group.
c) ASEAN Food Safety Network
The 25th meeting of the ASEAN Ministers on Agriculture and Forestry which was
held on 21 August 2003 in Malaysia expressed full support for Thailand’s
initiative on the establishment of the ASEAN Food Safety Network. The Meeting
noted that the proposed establishment of the Network would provide cohesive
direction for the ASEAN working groups to help resolve recurring problems of
non-tariff barriers encountered in the trade of food and agricultural products
for ASEAN regarding food safety. Thailand was assigned as the coordinator of the
ASEAN Food Safety Network.
An electronic coordination has been set up with the aim of coordinating,
networking, information sharing and providing early warning among various
national authorities and also working groups/task forces in ASEAN. This is also
a forum for on-line bilateral or multilateral discussions.
3.1.2 ASEAN cooperation under ASEAN Health Minister
ASEAN Expert Group on Food Safety (AEGFS)
The ASEAN Expert Working Group on Food Safety under the ASEAN Cooperation on
Health Development is developing an ASEAN Food Safety Improvement Plan, which
forms part of a comprehensive programme of action to address the impact of
globalization and trade liberation in the health sector.
3.1.3 Inter-regional cooperation
ASEAN has made major strides in building cooperative ties with states in the
Asia-Pacific region and shall continue to accord them a high priority.
Cooperation with East Asian countries has accelerated with the holding of an
annual dialogue among the leaders of ASEAN China, Japan and the Republic of
Korea which is called ASEAN+3. In 1999, the leaders of ASEAN+3 countries issued
a Joint Statement on East Asia Cooperation outlining the areas of cooperation
among them. On 8-9 March 2004, a Symposium of ASEAN+3 on Food Safety and its
Related Techniques was held in Chiang Mai, Thailand with funding support from
the Association for International Cooperation of Agriculture and Forestry (AICAF)
of Japan. The Symposium was placed under the ASEAN+3 cooperation framework and
served as a platform for the exchange of information and experience on a chosen
theme. Food safety was chosen to be a particular topic for this year’s
symposium. One of the conclusions arising from the discussion during the
Symposium was that the presence of developed countries in the Symposium, such as
Japan and the Republic of Korea, as well as those more advanced Members of
ASEAN, are seen as a great opportunity for the less advanced ASEAN Member
Countries to tap the information and experiences in an effort to further improve
and strengthen their respective national food safety policies and
implementation.
3.2 Capacity building of individual country
To support the successfulness of regional coordination, each member country has
to prepare itself to a level that can support the regional activities.
Individual countries also need to be ready for the implementation of
international and regional food safety standards. Some actions are recommended
for individual countries for example:
·Governments should take the necessary steps through national food safety
policies, systems and programmes to ensure that food safety considerations form
an integral part of their system. The implementation of such controls throughout
production, handling, processing and marketing leads to improved food safety and
increased competitiveness. These national programmes need to be reviewed to
determine level of compliance with international and regional standards. Through
national food control systems, governments should provide policies and
supporting infrastructures to reach the level of international and regional
standards.
·Capacity building to participate in international arena allows developing
countries to engage in food safety standards establishment. Developing country
government officials need to be able to more effectively use existing trade
rules and agreements and to argue for changes in them in a more powerful manner.
In order to do this, they should have the capacity to participate effectively in
the international standard-setting organizations. Furthermore, they must have
the capacity of negotiation not only in international standard setting process
but also for market access.
·Investments in infrastructure, technology and research are necessary as part of
the overall food safety system development. Investments to improve food safety
infrastructure that support better hygiene at the primary step of the food
supply chain is needed. Advances in the technology of food analysis for
microbes, additives, pesticide and veterinary drug residues, and other foreign
matter permit faster, cheaper detection of potential safety problems in foods.
4) Conclusions and recommendations
International food safety standards become highly important for the global trade
of food commodities. Many countries, both developed and developing, have
recognized the need to actively participate in the international food standards
organization, particularly the Codex Alimentarius Commission. However, most
developing countries are not able to participate effectively in Codex process
because of the limitation of budget, expertise and knowledge. One of the ideas
for this problem is regional coordination among developing countries.
The experiences of ASEAN on food safety and food standards coordination can be
seen as a case study of regional coordination. Some of the main programmes
include coordination on Codex issues, harmonization of regional standards,
coordination and networking on food safety within the region. The processes of
coordination are on-going and need further improvement especially in the area of
scientific knowledge and expertise that need to be strengthened. The work
programme on data generation/sharing to support standards establishment is in
the beginning stages and needs significant supports internally and externally.
It is recommended that programmes on regional coordination be continued and
improved to strengthen developing countries’ participation and implementation of
international food safety standards. The existing programmes may be expanded
either by improving capabilities/efficiencies or by increasing members joining
the programme. Technical assistance or support from international organizations
e.g. FAO, WHO or from developed countries can improve scientific capabilities of
the programme and also of each countries in the region.
Agenda Item 9
Conference Room Document 10
English only
FAO/WHO Regional Conference on Food Safety for Asia and the Pacific
Seremban, Malaysia, 24-27 May 2004
Country Report
Foodborne Diseases: Situation of Diarrheal Diseases in Thailand
(Food Control Division, Food and Drug Administration, Thailand)
Microbial agents and
chemical contaminants, including other hazards that may make food injurious to
health of the consumers, are among the public concerns and are involved with the
global increase in the incidence of food borne diseases.
Food borne diseases do not only affect people’s health and well-being, but they
also have economic impacts on individuals and countries. In many developing
countries, food borne disease outbreaks from bacteria, such as Escherichia coli
and Salmonella spp., impose a substantial burden on health care systems and can
markedly reduce the economic productivity of the countries.
In addition to food borne diseases from microorganisms, food containing toxic
chemical substances, either those occurring naturally (e.g. tetrodotoxin in
puffer fish), or those resulting from contamination with chemicals (e.g. toxic
metals) can also lead to diseases.
Situation and Problems
Diarrheal diseases([29]) have been a major public health problem in Thailand for
many years(1) (see Figure 1). Food is considered as a main route of transmission
of microorganisms causing diarrheal diseases. There are approximately a million
cases of acute diarrhea reported each year, and the reported cases of food
poisoning are more than 120,000 per year(2).
Bureau of Epidemiology reported that, although there was still a high number of
acute diarrheal cases, the diarrheal disease incidence started to decrease in
2003. There were 956,313 cases of acute diarrhea (146 deaths); 23,113 cases of
dysentery (3 deaths); 126,185 cases of food poisoning (11 deaths); and 9,633
cases of enteric fever (3 deaths)(1). (Data from 1 January to 31 December 2003
as presented in Table 1)
The most recent report from Bureau of Epidemiology indicates that there are
146,325 cases of acute diarrhea; 2,421 cases of dysentery; 17,128 cases of food
poisoning; and 797 cases of enteric fever (4 deaths)(2). (Data from 1 January to
29 March 2004)
The investigation of these reported cases show that consumption of microbial
contaminated drinking water and food is the major cause of the diseases in
Thailand. Diarrheal diseases are usually found among those living in poor
environmental sanitation and those with poor personal hygiene. The disease
incidence in children under five years of age is also reported high.
Inappropriate consumption behaviours among people in some areas, who always
consume raw or undercooked food, are one of the major causes of diarrheal
diseases in Thailand(2).
Causes of Diarrheal Diseases
In Thailand, bacteria mostly isolated from diarrheal cases are Shigella spp.,
Salmonella spp., and Escherichia coli. Factors leading to the incidence of
diarrheal diseases in the country are described in the following(2,3).
1. Contaminated food and drinking water
Contaminated food and drinking water is the major cause of diarrheal diseases.
Food and drinking water contamination can happen at all stages of the food chain
(described as ‘from-farm-to-table’). It needs to be ensured that contamination
of food and drinking water with pathogens leading to diarrheal diseases is
minimized in every process involved with the food practice (e.g. food
production, food transportation, and food preparation). Source of drinking water
and water used in the cooking process, clean cooking utilities, and sanitation
in food preparing areas are very important factors need to be focused.
2. Poor personal hygiene
Poor personal hygiene can lead to food contamination and diarrheal diseases. It
is not only those who do the cooking or food preparing processes, but it is
‘everybody’ who needs to have a good personal hygiene. Washing hands before
cooking/consuming food or preparing milk for children, and washing hands every
time after using restrooms are a basic personal hygiene which needs to be
emphasized to everybody.
3. Poor consumption behaviours
In some regions of Thailand, such as the north-eastern region, people like to
consume raw or undercooked meat, which is a cause of diarrheal diseases and
other diseases, such as worm diseases, among people in the region. Public
education in the region, focusing on consumption of clean and cooked food, is
still needed. Also, in a general practice, it is necessary to advise everybody
to drink clean or boiled water, consume clean or newly cooked food, reheat the
leftovers every time before consumption, and not consume raw food (especially
raw meat).
Hence, to minimize the food contamination, hygienic practice in food preparation
processes is very important. In addition, behaviours of those preferring to
consume raw or undercooked food need to be changed.
Figure 1 Morbidity rate of diarrheal diseases in Thailand, 1992-2003.
Source: Bureau of
Epidemiology, 2003
Table 1 Case report of diarrheal diseases in Thailand, 2003.
| Diarrheal Diseases | Reported Cases (persons) | Deaths (persons) | Morbidity Rate (per 100,000 population) |
Mortality Rate (per 100,000 population) |
| Acute diarrhea | 956,313 | 146 | 541.26 | 0.05 |
| Dysentery | 23,113 | 3 | 541.26 | 0 |
| Food poisoning | 126,185 | 11 | 67.79 | 0 |
| Enteric fever | 9,633 | 3 | 3.75 | 0 |
Source: Bureau of
Epidemiology, 2003
Food Safety
Implementation in Thailand
According to the food safety policy pursued by the Thai Government: “Safe and
Clean Food for All in 2004”, the Ministry of Public Health is authorized to be
responsible for the Food Safety Programme. This programme has been strictly
implemented, aiming at keeping the standard and quality of all foods produced
and consumed in Thailand high and able to meet the international food standard,
which could consequently lead the country to become the kitchen of the world.
- Programme for Prevention and Control of Diarrheal Diseases in Thailand:
An effective epidemiological system focusing on active surveillance on food
borne diseases in the high-risk areas has been implemented. Thailand has a
special team composed of epidemiologists, sanitation personnel, health education
personnel, and personnel from Department of Disease Control, working
cooperatively in both district and provincial levels to effectively prevent and
control the diseases. This team will give a prompt response to investigate the
source of diarrheal disease cases and/or outbreaks, and try to control the
outbreaks within 10 days(3).
‘Programme for Prevention and Control of Diarrheal Diseases’ is aimed for the
reduction of morbidity and mortality rate of the diseases, especially the
incident rate of diarrheal diseases in children under five years of age. Oral
rehydration therapy (ORT), which can prevent the electrolyte loss, shock, and
subsequent death, is aimed to be used in more than 95 percent of diarrheal cases
in the country. This programme has been effectively implemented together with
other programmes, such as sanitation, environmental health, mother and child
health care, nutrition, and communicable diseases control programmes, in order
to reduce the chance of disease transmission, particularly among young children.
The following operation strategies have been used in the Programme for
Prevention and Control of Diarrheal Diseases in Thailand(3):
Strategy 1: Diarrheal disease prevention
The diarrheal disease prevention programme is focused in the high-risk areas.
Public education on diarrheal disease prevention is provided by health care
personnel and other authorities involved, emphasizing on hygienic-sanitary
cooking utilities, sanitation in food preparing areas, personal hygiene, and
appropriate consumption behaviours.
Strategy 2: Investigation, monitoring, and reporting of diarrheal cases
In case of an outbreak or incidence, sources of diarrheal diseases would be
immediately investigated and eradicated. Monitoring of the outbreak or incidence
in the reported area would be conducted for 10 consecutive days after the cases
reported.
Routine reporting data, data collected in households, and data from health
facility surveys are used to monitor and evaluate the programme.
The community can also participate in the programme by working with health care
personnel in planning and implementing on diarrheal disease monitoring,
reporting, and controlling activities.
Strategy 3: Treatment
It is essential to encourage the health care personnel to use ORT, which is a
proper treatment for diarrheal diseases, as the first line treatment for
diarrhea instead of jumping for antidiarrheal drugs and antibiotics. It is
necessary to ensure that all public health facilities and pharmacies have a
sufficient supply of oral rehydration salts (ORS). In Thailand, the training
programme on ORT provided for health care personnel emphasizes on the importance
of an increase in fluid intake, the continued feeding and breast feeding during
diarrhea, intravenous therapy for patients with severe dehydration, the use of
ORS in the treatment or prevention of dehydration, and the dangers of misused
antidiarrheal drugs and antibiotics.
Home management of diarrhea using the recommended home fluids is also
emphasized. Caretakers are trained for a better care providing for diarrheal
patients at home, especially child patients.
- Five strategies implemented for food safety in Thailand
Additionally to the regular programme for prevention and control of diarrheal
diseases in the country, following five strategies have been implemented in
Thailand in order to accomplish the goals of the food safety policy(4):
Strategy 1: Development of laws and regulations to comply with international
standards
Laws and regulations related to food control have been revised and amended for
complying with the international food regulations. It must be ensured that the
enforcement of laws and regulations in Thailand provides an equity in consumer
protection on all food products (including all foods produced, imported, and
exported).
Strategy 2: Strengthening of food safety monitoring system
The Ministry of Public Health, Thailand, has implemented measures for a strict
and regular surveillance, and monitoring on chemical and microbial contaminants,
including toxins in food. All food production sites, distribution sites, and
restaurants are regularly inspected on its good manufacturing practice (GMP) or
Hazard Analysis And Critical Control Point (HACCP), hygiene, and food sanitary
system.
Strategy 3: Development of consumer power
The consumer power is developed by providing public education through the media
(e.g., television, radio, leaflets, pamphlets, newspapers, etc.) in order to
make the consumers know how to select and buy safe food for themselves and to
increase a consumer awareness on food consumption. In addition, organizations in
central and local areas are pushed to set up responsible groups (including
responsible groups at schools) for the increase in consumer protection on food.
Strategy 4: Development of responsible personnel and working processes
Training and education programmes related to food safety have been provided for
food safety personnel by responsible organizations. Also, the information system
linkage and the cooperation between related organizations have been focused in
order to develop and strengthen the working capability, and to ensure that
working processes are going in the same direction.
Strategy 5: Development of laboratory capability
The Thai Government has developed the capability of analysis by supporting on
necessary equipment and facilities in laboratories and food testing mobile
units.
Summary
Among food borne
diseases, diarrheal diseases in those living in poor environmental sanitation
and those with poor personal hygiene have been a major public health problem in
Thailand for many years. Major causes of the diseases include contaminated food
and drinking water, poor personal hygiene, and poor consumption behaviours.
Thailand has implemented a programme for prevention and control of diarrheal
diseases in the country, focusing on prevention, investigation, monitoring,
reporting, and treatment of the diarrheal cases. According to the programme, it
is reported a decrease in the diarrheal disease incidence in 2003.
In addition to diarrheal diseases control programme, the prevention of food
borne diseases generated from contamination with other microbiological agents
(e.g. worm diseases and hepatitis-A), toxins, and chemical agents (e.g.
pesticides and toxic metals) is also a strategy included in the ‘Food Safety
Programme’ in Thailand. This programme is emphasized and implemented by the
Ministry of Public Health cooperated with other related organizations, aiming at
making all foods produced and consumed in Thailand safe and able to meet the
international food standard, which could consequently lead the country to become
the kitchen of the world.
References
1. Bureau of Epidemiology. Situation of diarrheal diseases. Bangkok, Department
of Disease Control, Ministry of Public Health, 2003.
2. Bureau of Epidemiology. Situation of diarrheal diseases. Bangkok, Department
of Disease Control, Ministry of Public Health, 2004.
3. Bureau of Epidemiology. Control of diarrheal diseases (CDD) programme in
Thailand. Bangkok, Department of Disease Control, Ministry of Public Health,
2001.
4. Thai Food and Drug Administration. Food Safety Programme: A Report from Food
and Drug Administration, Thailand. Bangkok, Thai Food and Drug Administration,
Ministry of Public Health, 2004.
Agenda Item 9
Conference Room Document 11
English only
FAO/WHO Regional Conference on Food Safety for Asia and the Pacific
Seremban, Malaysia, 24-27 May 2004
PREVENTION AND MANAGEMENT SYSTEM FOR FOOD POISONING IN KOREA
(Republic of Korea)
I. Introduction of food
safety systems in Korea
In order to contribute to the improvement of national health by preventing
sanitary dangers and harm caused by food and improving the quality of food
nutrition, the Food Sanitation Act was established in 1962. There have been many
amendments to reflect mainly to ensure food safety. During the period, the
public interests shifted from the quantity to the quality of the foods. To
fulfil the public demands and secure the national food safety, a specialized new
government agency, the Korea Food and Drug Administration (KFDA) was established
in 1998 with 6 regional branches and a subsidiary institute, National Institute
of Toxicological Research. The KFDA is in charge of regulating food, drugs,
cosmetics, and medical devices. In food sector, the responsibilities of KFDA
are, among others, food safety policy, promulgation of standards and
regulations, risk assessment, surveillance, analytical method research and
audits. The essential principles of making decision on food policy in Korea are
as follows:
- Food regulations (including standards and specifications) based on sound
scientific evidence,
- Reflection of consumer’s demands for the higher level of food safety,
- Transparent procedures in developing the regulations,
- International harmonization on food safety control,
- Encouragement of food industry development
- National institutional structure
In Korea, the Food Sanitation Act and Processing of Livestock Products Act are
the two major acts to control food safety. However, the food safety control
systems are scattered with several agencies so that sound food safety control
might not be maintained efficiently. The responsibilities and relations for
these agents are shown in Figure 1. Therefore, integration of those government
bodies is undertaking to construct effective and intensive food safety control
system, In near future, we will have sound and better control system then ever
for food safety assurance.
II. Discussion for food poisoning management in Korea
The term “food poisoning” is using in Korea rather than “foodborne disease.” In
the Food Sanitation Act, the food poisoning is defined as infectious or toxic
diseases caused by microorganisms or toxic agents that enter the body through
the ingestion of food. Also, any doctor who has made a diagnosis of patient
poisoned or suspected to be poisoned by foods or has made an autopsy on his
remains, shall report it without delay to the head of the competent public
health center, the Mayor/Province governor and the Minister of Health and
Welfare/Commissioner of the KFDA step by step. The Food Surveillance Division
and Food Microbiology Division take charge of food poisoning control in the KFDA.
Although food poisoning is described in the Food Sanitation Act as the
definition of WHO, it is interpreted in a narrow sense in Korea. In other words,
we are not accepting the concept of foodborne disease yet. In fact, diseases
through the ingestion of food are controlled by the Food Sanitation Act and the
Infectious Diseases Prevention Act. Contagiousness is the main difference
between two acts. For example, such as salmonellosis and listeriosis are
controlled by the Food Sanitation Act, but hepatitis, shigellosis,
Enterohaemorrhagic E. coli are managed by the Infectious Diseases Prevention
Act. The KFDA is a main body to control and prevent national food poisoning in
Korea and the Korea Center for Disease Control and Prevention (KCDC) is an
agency to control contagious diseases. The KCDC is a new organization belonging
to the Ministry of Health and Welfare that has launched in this year by
expansion and remodel of former Korea National Institute of Health.
For epidemiologic investigation of food poisoning outbreaks, KFDA is operating a
Central Food Poisoning Headquarter which is mad up of experts from KFDA, KCDC
and MOE (Ministry of Education and Human Resources Development). The
responsibilities of it are, among others, establishment of national food
poisoning control plan, administration of statistics, development of food
poisoning manual, support of Local Food Poisoning Investigation Teams, and
education and public relations. There also are Local Food Poisoning
Investigation Teams composed of personnel from KFDA, public health center and
local governments. Once food poisoning outbreaks occur, the Local Food Poisoning
Investigation Teams conduct the actual epidemiologic investigation and report
the results to Central Food Poisoning Headquarter.
In these days, food poisoning outbreaks showed different patterns in compared to
the past in Korea. First, inversely proportional to the improvement of food
hygiene and food safety level, the numbers of outbreaks and patients have
increased continuously (Table 1). This is estimated due to the increment and
activation of food poisoning reports according to increased concern of doctors
and consumers about food poisoning. Secondly, Korea has distinctive four seasons
so that food poisoning has typically happened in summer (May/September).
Recently, however, it tends to occur steadily irrelevant to seasons or
temperatures. It is assumed on account of global warming and constant
temperature maintaining inside buildings by social development. Thirdly,
outbreaks occurring in group and school meals have increased tremendously which
resulted in the increase of patients. School meal program has been obligated in
full-scale in Korea from 1998 and large scaled food poisoning outbreaks have
happened frequently since then. Development of the food-service or restaurant
industry is also a cause. Control and improvement of hygienic condition for
group meal is the most important field in the prevention of food poisoning in
Korea at present. Lastly, major microorganisms causing food poisoning are
changing (Table 2). Salmonella spp, Staphylococcus aureus, Vibrio
parahaemolyticus are the three major food poisoning bacteria in Korea for a long
time. Meanwhile, in addition to these bacteria, virus (Norovirus) is emerging as
an important agent recently. Also, failure rates of identifying causative agents
from outbreaks or epidemiologic investigations keeps increasing and it makes
KFDA difficult to carry out its food poisoning control or preventative plan
efficiently.
Fig 1. Food poisoning outbreaks and patients in Korea
| Year | 1996 | 1998 | 2000 | 2002 | 2003 |
| Outbreak | 81 | 119 | 104 | 78 | 135 |
| Patients | 2,797 | 4,577 | 7,269 | 2,980 | 7,909 |
Fig 2. Major causative agents of food poisoning outbreaks in Korea (2003)
| Salmonella | S. aureus | V. parahaemolyticus | Norovirus | Unknown | |
| No | 416 | 808 | 732 | 1,422 | 2,180 |
| Rate | 5.3% | 10.2% | 9.3% | 18.2% | 27.6% |
III. Conclusion
KFDA devotes its all strength to prevent and control food poisoning in Korea.
Major efforts, among others, are:
- Development of HACCP model for group and school meal
- Intensive surveillance or food hygiene inspection to large scaled restaurants,
food suppliers, group meal places and frequently consumed food products of
concern
- Attempt to conversion of food poisoning definition to foodborne disease
concept
- Upgrade and improve food poisoning report, statistics and epidemiologic
investigation system
- Reinforcement of education and public relations
- Operation of hot-line for food poisoning report and consultation
- Strengthening of penalty or fine for regulation violators in Food Sanitation
Act
In the globalization era, food poisoning is no longer a sole issue in a country
or region. To improve the public health and secure the food safety in global
aspect, every country should try its efforts to control its own food safety.
Exchange and share of food poisoning statistics, epidemiologic investigation
results and food safety information are very important factors for this purpose.
Agenda Item 5 Conference Room Document 12
English only
FAO/WHO Regional Conference on Food Safety for Asia and Pacific
Seremban, Malaysia, 24-27 May 2004
Regulatory Procedure for Health Risk Assessment of GM Foods
(Philippines)
Food safety, as defined by FAO (2003) involves all hazards, whether chronic or
acute, that may make food injurious to the health of the consumer. IT is NOT
NEGOTIABLE. However, the US Congress has recognized that “zero” risk and
“absolute safety” are impossible to achieve in a food system. Foods can easily
be rendered unsafe by abuse in storage, preparation, or consumption; food
allergies, food intolerances, food insensitivities, etc.
The Substantial Equivalence Concept
The substantial equivalence concept provides useful framework for safety
assessment. In this analysis, each product is compared with its conventional
counterpart and similarities and differences are then made the focus of the
evaluation. The safety assessment of genetically modified foods requires an
integrated and stepwise, case-by-case approach, which can be aided by a
structured series of questions.A comparative approach focusing on the
determination of similarities and differences between the genetically modified
food and its conventional counterpart aids in the identification of potential
safety and nutritional issues and is considered the most appropriate strategy
for the safety and nutritional assessment of genetically modified foods.
Presently, no alternative strategies exist that would provide a better assurance
of safety for genetically modified foods than the appropriate use of the concept
of substantial equivalence. This concept was developed as a practical approach
to the safety of genetically modified foods. It is a key step in the safety
assessment process although it is not a safety assessment in itself; one must
remember that it does not characterize a hazard, but it is used to structure the
safety assessment of a genetically modified food relative to a conventional
counterpart.
The concept of substantial equivalence involves methods to detect and evaluate
the impact of unintended effects, such as the acquisition of new traits or loss
of existing traits. Such is the case of genetically modified foods with
intentional nutritional effect that may provide improved products for developed
and developing countries. The change in nutrient levels in a particular crop
plant may impact on overall dietary intake. In such cases, it is important to
determine alterations in nutrient content and bioavailability, and their
stability with time, processing and storage, as well as to monitor changes in
dietary patterns as a result of the introduction of the genetically modified
food and evaluate its potential effect on nutritional and health status of
consumers. However, an assessment of the impact on the nutritional status of
consumers is important for all significant dietary changes and not specific to
the introduction of genetically modified foods.
Risk Assessment Process
The strategy of the risk assessment process that is applied to
biotechnology-derived crops is based directly on the changes that have been made
in the plant. The hazard identification process reveals that there are three
principal issues that merit further risk assessment:
·The safety of the inserted DNA
·The safety of the newly introduced component
·The safety of the balance of the whole food
In the food safety evaluation of GM foods specific questions that must be
answered should be:
·Is the transferred DNA safe to consume?
·If an antibiotic resistance marker is used, is it safe?
·Are the newly produced proteins safe to consume?
·Have potential allergens been introduced into the food?
·Are the composition and nutritional value changed?
·Are there changes in the content of important substances?
·In what forms will the food or food products isolated from it be consumed?
·Do the newly introduced substances survive processing, shipment, storage, and
preparation?
·What is the expected human dietary exposure?
Discussions on the safety aspect of Genetically Modified foods have covered a
broad range of aspects. According to WHO (2002), there are three main issues
debated:
2.1 Allergenicity. As a precautionary principle, the transfer of genes from
commonly allergenic foods is discouraged unless it can be demonstrated that the
protein product of the transferred gene is not allergenic.
2.2 Gene transfer. Gene transfer from GM foods to cells of the body or to
bacteria in the gastrointestinal tract would cause concern if the transferred
genetic material adversely affects human health. This would be particularly
relevant if antibiotic resistance genes, used in creating GM foods, were to be
transferred. Important considerations for the assessment of the consequences of
the transfer and expression of this gene in transformed cells would be the
clinical and veterinary importance of the antibiotic in question, the levels of
natural resistance and the availability of effective alternative therapies.
Although the probability of transfer is low, the use of technology without
antibiotic resistance genes has been encouraged by a recent FAO/WHO expert
panel.
2.3 Outcrossing. The movement of genes from GM plants into conventional crops or
related species in the wild (referred to as “outcrossing”) may have an indirect
effect on food safety and food security.
3. Response of the Philippines regarding the safety of biotech foods
In 2001 President Gloria Macapagal Arroyo declared a national biotech policy,
that is “…promote the safe and responsible use of modern biotechnology and its
products as one of the means to achieve food security, equal access to health
services, sustainable and safe environment and industry development.” With the
above policy statement on modern biotechnology, coupled with the objective of
the Department of Agriculture to accelerate agricultural development, enhance
production, and diversify products for food security and global competitiveness,
the need for a legal and strong framework on the importation and use of GMOs was
emphasized.
3.1 Establishment of Administrative Order 8 and GMO Approval process
Finally, in 2002, the Department of Agriculture issued Administrative Order No.
8, which clearly addresses the risks of GM plant and plant products to human and
animal health and the environment.
Under AO 8, no person shall be allowed to import or release into the environment
any regulated article without a satisfactory risk assessment. The assessment of
GM crops shall be:
·Science-based – identification and evaluation of risk based on scientific
studies
·Transparent – basis for decision is open for public scrutiny
·Case by case – different GMOs pose different types and levels of risk and
should be assessed accordingly
·By transformation event – unit of analysis in evaluating GMOs
The Bureau of Plant Industry is the central agency overseeing the approval
process of importation, release and propagation of GM crops. As shown in Figure
2, the applicant submits his application for the approval of a regulated article
to the BPI. After processing and evaluating the documents submitted to them, the
BPI farms out the papers to the different concerned agencies involved in the
risk assessment process. Each of the independent reviewers conducts their
assessment. Inquiries and questions on the submitted scientific documents are
channeled back to the applicant for clarification. Upon the conduct of the risk
assessment, the reviewers shall then submit a report to the BPI, together with
their decision whether they find the regulated article substantially equivalent
to its conventional counterpart. The BPI thus collates the evaluations and the
appropriate decision is given by the BPI. The functions of the following
independent reviewers:
·Scientific and Technical Review Panel (STRP) – assess scientific quality of
reports; assess feed safety and environmental safety of Bt Corn
·DA regulatory Agencies:
o Bureau of Agriculture and Fisheries Product Standards– assess food safety
o Fertilizer and Pesticide Authority – safety of pest-protected plants
o Bureau of Animal Industry – assess feed safety
o Bureau of Plant Industry - environmental safety
3.2 Food Safety Assessment – Bureau of Agriculture and Fisheries Product
Standards
In line with its commitment to provide safe foods and under the provisions of AO
8, the Bureau of Agriculture and Fisheries product standards has come up with a
specific framework in the Risk assessment of Foods derived from Modern
Biotechnology. The process carried out was based on:
·Sound scientific evidence
·Pursued in accordance with recognized risk assessment guidelines
·Identify and characterize risks associated with the regulated article for
direct use as food
·Evaluation shall be focused on the possible adverse effect of GM foods which
exclude risk assessment attributed to discrete chemical entities such as
additives, residues, chemical or microbiological contaminants
·Evaluation shall be carried out in a transparent manner
·Evaluation shall take into account the history of safe uses as food source from
the importing country
·Evaluation of GM food shall consider the safety of its conventional counterpart
The food safety assessment of BAFPS focused on:
- Horizontal Gene transfer
- Safe use of Antibiotic Resistance Marker gene
- Nutritional composition
- Possible allergenicity
- Unintended effects
The agricultural industry relies on science and makes choices based on the
consultation of the experts – the scientist and regulators to either: APPROVE or
CHALLENGE each new product of biotechnology. A legal system, that is, AO 8, is
crucial in ensuring food safety and positive environmental results. In the
absence of knowledge and unavailability of information, the consuming public
relies on trusted sources to validate what is “SAFE”.
4. Conclusion and Recommendations
Many future products developed through biotechnology will be intentionally
designed not to be equivalent in composition or nutritional content with their
conventional counterparts; products that are intended to directly provide
consumer benefits through enhanced nutrition and health, address food security
and/or nutritional adequacy in developing countries, and improve animal feed.
New crop foods or food ingredients should be assessed on a case-by-case basis.
Extending the paradigm used for assessing food safety should include foods that
are designed to be not compositionally equivalent to their conventional
counterparts. In addition, estimated dietary intake and effect on total nutrient
intake are important additions to the safety assessment paradigm for
non-equivalent foods.
Periodic review of legal instruments and subsequent identification of gaps,
emerging needs and issues in the regulation of biotech products need to be done.
Current regulatory framework does not address all problematic organisms and
their products. The guidelines set by the National Committee on Biosafety of the
Philippines (NCBP) guidelines only regulates research and development work on
GMOs and Potentially Harmful Exotic Species. Similarly, DA AO 8 regulates only
GM plants and selected plant products. Current problems on unregulated entry of
some food ingredients derived from GMOs (i.e. unregulated entry of GM microbes,
safety assessment of bioremediation/biocontrol agents are not required), as well
as future entry of transgenic fish and farm animals need to be addressed.
Agenda Item 9 Conference Room Document 13
English only
FAO/WHO Regional Conference on Food Safety for Asia and Pacific
Seremban, Malaysia, 24-27 May 2004
AFLATOXIN CONTAMINATION IN FOODS AND FEEDS IN THE PHILIPPINES
(Philippines)
INTRODUCTION
Mycotoxins are toxic secondary metabolites, representing a wide diversity of
chemical species, which are produced by certain toxigenic molds during their
development on foods and feeds. The molds which appear to be the main producers
of mycotoxins belong to the Aspergillus, Fusarium and Penicillium genera.
Mycotoxin contamination, particularly aflatoxin, is commonly found in locally
produced agricultural crops such as corn, peanuts, coconuts and cassava and
their food and feed products.
The consumption of a contaminated feed by a domestic animal results in the
contamination of foods such as meat, milk and other dairy products. These toxins
elicit both acute and chronic toxicities. Some mycotoxins are acutely toxic to
the liver, while others damage the kidney, the central nervous system or the
circulatory system.
OCCURRENCE
The aflatoxins are produced by two molds, Aspergillus flavus and A. parasiticus.
Its specific forms are designated as B1, B2 , G1 , G2 , M1 & , M2 . Aflatoxin B1
is the most potent naturally occurring carcinogen known. Aflatoxin M1 is a toxic
metabolite, derived from Aflatoxin B1 which occurs in milk when aflatoxin-contaminated
feed is consumed by dairy cattle. B designations of Aflatoxin B1 & B2 resulted
from the manifestation of blue fluorescence under UV light and the G
designations refer to the yellow-green fluorescence of the aflatoxin group.
In the Philippines, the growth of the aflatoxin-producing fungi is favored by
climatic conditions, i.e., high temperature coupled with high relative humidity
(80-90% wet season and 50-70% dry season). Aflatoxin contamination in
agricultural crops, food and feed commodities is a serious problem in the
country.
Coconut products and by-products are considered to be the Philippines’ most
important agricultural commodity in the international and local market. Almost
every available region especially in the rural areas engages in coconut
production. Coconut farmers depend on them for their livelihood.
The white meat from the coconut is dried directly or indirectly by various
methods like sun-drying, tapahan or kukum method. High moisture copra can easily
be contaminated with aflatoxin.
CARCINOGENICITY
Aflatoxin are known to be human carcinogens based on sufficient evidence of
carcinogenocity in humans (IARC 1987, 1993). Aflatoxicoses is a poisoning
resulting from ingestion of aflatoxin-contaminated food in feed. A case-control
study in the Philippines, where mean aflatoxin contamination levels in dietary
items were established and individual levels of aflatoxin consumption were
determined retrospectively, demonstrated an increased, dose-related risk of
developing hepatocellular cancer in persons with higher ingestion of aflatoxin.
Aflatoxins, especially B1, have been tested extensively for genetoxicity. It
induces DNA damage, gene mutation, chromosomal anomalies and cell transformation
in mammalian cells in vitro. Aflatoxins are genetoxic carcinogens. For this type
of carcinogen, it is generally felt that there is no threshold dose below which
no tumor formation would occur. In other words, only a zero level of exposure
will result in no risk.
Aflatoxin M1 is a metabolic hydroxylation product of B1. Aflatoxin M1 produced
DNA damage in rodent cells in vitro and gene mutation in bacteria. With respect
to the carcinogenicity of aflatoxin M1, International Agency for Research on
Cancer (IARC, 1993) concluded that there is inadequate evidence in humans but
sufficient evidence in experimental animals (liver tumor). The overall
evaluation (IARC) was “Aflatoxin M1 is possible carcinogenic to humans.
EVIDENCE FROM ANIMAL EXPERIMENTS
Mixtures of aflatoxins and aflatoxin B1 were tested extensively for its
carcinogenicity in experimental animals like mice, rats, hamsters, fishes, ducks
and monkeys. Oral administration of mixtures of aflatoxins and aflatoxin B1
caused hepatocellular and or cholangiocellular liver tumors, including
carcinomas in all species tested except mice. In mice, aflatoxin administered
increase the incidence of lung adenomas.
In 1979, a study on the toxic effects of aflatoxin to rats were tested (Norted).
Finely ground corn mixed with aflatoxin were given to rats by stomach tube. The
rats received high toxic doses (10 or 20 mg aflatoxin/kg body weight). Signs of
typical aflatoxicoses were observed.
A feeding trial was done by Marin, et al (2002) to determine the effect of
aflatoxin-contaminated diets on growth and hematological and immunological
parameters. Low doses of aflatoxin (140 and 280 ppb) were included in a
corn-soybean diet of weanling piglets for a period of four (4) weeks. The result
showed that there was depressed growth as well as alteration on the humoral and
cellular immunity aspects in pigs.
A study of the carcinogenicity of dietary aflatoxin M1 in male Fischer rats
compared to aflatoxin B1 was done by Cullen, et al. Aflatoxin M1 and aflatoxin
B1 (0.0 to 50 ug/kg) were fed to male Fischer rats starting at seven (7) weeks
to 21 months of age. Hepatocellular carcinomas were detected in 2 of 37 rats and
neoplastic nodules were found in six of 37 rats fed with 50 ug/kg afla M1
between 19 and 24 months. Nineteen(19) of 20 rats fed a diet containing 50ug/kg
of afla B1 developed hepatocellular carcinomas by 19 months of age. The results
indicated that aflatoxin M1 is an hepatic carcinogen although it is considerably
less potent that aflatoxin B1.
Research was done evaluating the excretion of aflatoxin B1 on eggs of laying
Japanese quails (Oliveira, et al). The quails were fed with prepared rations
containing 0, 25, 50 or 100 ug Aflatoxin B1 /kg feed for 90 days. The results
showed that the average egg production was not affected. However, the egg weight
was significantly lowered. Residues of aflatoxin B1 were detected in eggs at
levels that ranged from 0.01 to 0.08 ug/kg .
In swine, the effects of feeding aflatoxin-contaminated corn depend on the age
of the pig and concentration of the toxin in the feed. Low levels (20 to 200ppb)
can affect the feed intake of the pig and suppression of the immune system. High
levels (1000 to 5000 ppb) result in death.
There is sufficient evidence in humans for the carcinogenicity of naturally
occurring mixtures of aflatoxins and aflatoxin B1. However, there is inadequate
evidence of the carcinogenicity of aflatoxin M1 in humans but may possibly be
carcinogenic to humans.
Regulatory Actions of Countries
The United States Food and Drug Administration (USFDA) enforces the following
action levels for aflatoxins present in human food, animal feed and animal feed
ingredients.
Aflatoxin level Commodities and species
(in parts per billion)
10 All products, except milk, designated for humans
0.5 Milk
20 Corn for immature animals and dairy cattle
100 Corn for breeding beef cattle, swine and mature poultry
200 Corn for finishing swine
300 Corn for finishing beef cattle
300 Cottonseed meal (as a feed ingredient)
20 All feedstuff other than corn
In India, tolerance limit of 30 ppb aflatoxin in all foods has been fixed.
Brazil adopted the maximum allowed levels of 20 ppb for corn.
The European Union adopted the following levels of aflatoxin in feeding stuffs
Maximum content (ppm) Commodities and Species
relative to a feeding stuff with
a moisture content of 12%
0.05 Feed materials with the exception of
0.02 - groundnut copra, palm-kernel, cottonseed, babassu, maize and products
derived from the processing thereof
Regulatory Action of the Philippines in Food and Copra Meal on Aflatoxin
The incidence of aflatoxin in coconut and its by-products was first presented as
a problem when the USFDA discovered and isolated aflatoxin from copra and copra
meal sometime in 1970. In effect, the USFDA set a tolerance limit of 20 parts
per billion (ppb) of aflatoxin for coconut products beyond which the use of any
contaminated commodity for edible use was banned.
Due to the stringent regulations on aflatoxin level imposed by the European
market, the Philippine Coconut Authority set up an aflatoxin laboratory for the
purpose of monitoring aflatoxin in copra and its by-products. In 1990-1992, the
RP-UK Aflatoxin Project in Copra was conducted in coordination with the PCA and
Natural Resources Institute (NRI) of the United Kingdom.
Other projects/programs/measures implemented by PCA with regards to the copra
quality improvement are:
a) NAFC fund Copra Quality Improvement Project (CQIP) in selected Mindanao
Provinces (October 1989- January 1992)
b) DBM funded nationwide CQIP (January 1990-January 1992)
c) Issuance of AO # 01 Series of 1991
d) Massive Information Campaign (August 1991 – June 1992)
e) Philippine-German Coconut Project (June 1992-December 2000)
Despite the program/measures/projects to resolve the possible threat of losing
the European market for copra meal, the copra quality and aflatoxin level of
Philippine copra and copra meal have yet to be improved and corrected. It
strongly appears that the copra quality problem in the Philippines cannot be
solved by technical solutions alone. The best dryers and the best copra drying
technologies would not be able to solve the copra quality problem in this
country unless the following barriers are removed/corrected;
a) Lack of Quality-Based Pricing system in the Copra Marketing Chain
b) Overcapacity in the Oil Milling Sector
c) Wrong Copra Storage Practice
d) Trading of “green copra”
Recommendations
a) Establishment of an honest to goodness and implementable quality based
pricing system for copra by oil millers
b) Training program, seminars, workshops for farmers traders and consumers
c) Monitoring and surveillance studies be continued in all aflatoxin-susceptible
agricultural crops and their food and feed products and the data obtained will
served as basis for setting the aflatoxin regulatory limits in the country.
d) Massive or intensive campaign on the effects of mycotoxins, particularly
aflatoxins in radios, televisions to educate the people.
e) BFAD should continue testing market products to ensure the safety of the
foods for human consumption as well as to safeguard the health of the consumers.
REFERENCES:
1. Arim, Rosario H. Mycotoxin Contamination of Foods and Feeds in the
Philippines, FNRI, Taguig, Metro Manila, Philippines
2. Coker, R.D., Jones,B.D., Nagler, M.J., et al. Mycotoxin Training Manual.
Tropical Development Research Institute, October 1984.
3. Cullen, Joan M., et al. Carcinogenicity of Dietary Aflatoxin M1 in Male
Fischer Rats Compared to Aflatoxin B1.
4. Directive 2002/32/EEC of the European Parliament and of the Council of 7 May
2002 on undesirable substances in animal feed. Official Journal of the European
Communities. OJL 140/10.30.5.2002.
5. IARC. International Agency for Research on Cancer. IARC Monographs on the
Evaluation of Carcinogenic Risks to Humans. Overall Evaluations of
Carcinogenicity. Supplement 7. Lyon, France: IARC, 1987.
6. IARC. International Agency for Research on Caner. IARC Monographs on the
Evaluation of Carcinogenic Risks to Humans. Some Naturally Occurring Substances:
Food Items and Constituents, Heterocyclic Aromatic Amines, and Mycotoxins. Vol.
56. Lyon. France: IARC, 1993.
7. Marin, D.E., Taranu, I., Bunaciu, R.P. Pascale, D.S. 2002. Changes in
performance, blood parameters, humoral and cellular immune responses in weanling
piglets exposed to low doses of aflatoxin. Journ. Animal Sci. 80(5):1250-1257.
8. Norted, W.P., 1979. Effect of ammoniation on the toxicity of corn
artificially contaminated with aflatoxin B1, Trop. Sci. 25:139-154.
9. Oliveira, C.A., Rosmaninho, J.F. Castro, A.L. Undated. Aflatoxin residues in
eggs of laying Japanese quail after long=term administration of rations
containing low levels of aflatoxin B1.Food Additives & Contaminants
10. Oliveira, C.A., Kobashigawa, E., Reis, T., Mestieri, L. 2000. Aflatoxin B1
residues in eggs of laying hens fed a diet containing different levels of the
mycotoxin. Food Additives and Contaminants 17(6):459-462.
11. RP-UK Reduction in Aflatoxin Contamination of Copra in the Philippines. Jan.
1990 to June 1992. Vols. 1-3, NRI.
13. Safety Evaluation of Certain Mycotoxins in Food. WHO Food Additives
Series:47, 2001.
14. United States Food and Drugs Administration. FDA Regulatory Guidance for
Toxins and Contaminants.
Conference Room Document 14
English only
FAO/WHO Regional Conference on Food Safety for Asia and Pacific
Seremban, Malaysia, 24-27 May 2004
Background information for the seminar and workshop
Enterobacter sakazakii in Powdered Infant Formula:
Current Updates and Issues
(A side event (co-organized by ILSI Southeast Asia Region) at the
FAO/WHO Regional Conference on Food Safety for Asia and the Pacific)
9.00–13.15 hours, 26 May 2004
Several outbreaks of Enterobacter sakazakii infections among infants, primarily
neonates fed milk-based, powdered infant formula have highlighted the risk
associated with this microorganism and the importance of scientific knowledge,
updates and information sharing to address this risk. The Joint FAO/WHO Workshop
on Enterobacter sakazakii and other Microorganisms in Powdered Infant Formula
held in Geneva, Switzerland in February 2004 recognized the need for increased
understanding and awareness of the species and its life-threatening
consequences.
While E. sakazakii can cause disease in all age groups, infants – in particular,
low birth weight, premature, and immuno-compromised infants – are those at
higher risk of infection. As developing countries may have a higher proportion
of such infants, this issue may impact a greater number of individuals. Powdered
infant formula has been identified as the main source or vehicle of such E.
sakazakii–related infections and mortality in many of the reported cases,
although implicated products were generally in conformance with the
microbiological requirements of the current Codex Code of Hygienic Practices for
Foods for Infants and Children.
It is important to note that contamination from the environment could also occur
during reconstitution of powdered infant formula. Good hygienic practices during
the reconstitution of powdered infant formula, rapid cooling of the prepared
formula and minimizing the time-lapse between preparations and feeding times are
critical to reduce E. sakazakii contamination. As caregivers play the pivotal
role in both preparation and possible contamination routes, continuous education
and awareness of hygienic practices are called upon to minimize potential
contamination and growth during preparation and prior to consumption.
ILSI Southeast Asia Region, working in conjunction with FAO/WHO at the
conference, aims to increase the awareness of E. sakazakii by facilitating the
exchange of science-based information in the capacity building efforts related
to Microbial Risk Assessment in the region. In addition, current studies,
identification of data and future research gaps will be discussed for input and
future action.
Agenda Items 5-9 Conference Room Document 15
English only
FAO/WHO Regional Conference on Food Safety for Asia and Pacific
Seremban, Malaysia, 24-27 May 2004
Bangladesh Country Paper
1.0 Introduction
1.1 Background Information
This paper is an excerpt of the report made by the National Taskforce on Food
Safety formed by the government following the request and suggestions of FAO
Country Representative in Dhaka.
This paper attempts to describe status of food safety (standards, control system
and mechanism) in Bangladesh to match the themes of the FAO/WHO Conference;
identify issues and constraints, suggest policy options to over come those, and
possible stands of Bangladesh to the regional action plan to be framed.
1.2 Food Security Situation: Bangladesh Context
□ Bangladesh has made substantial progress in increasing food grain production
over the last two decades. The production of food grains in 2002-03 was 26.70
million metric tons, which is expected to reach 28.60 million tons in the year
2003-04. This has led to improve overall food security situation, where per
capita availability of food grains (rice+wheat) for FY03 was estimated to be 202
kgs/capita/year.
□ Poverty head count ratio remains at the level of 44.3% ( 5.5 million people
lying under food-based absolute poverty line). The hard core poverty head count
ratio, though, declined over the years still counts more than 24.5 million
people. Both rural and urban Poor have low incomes and thus low purchasing
powers, which increase the chances of consuming food of poorer quality that may
well be also unsafe.
□ Nutrition and food utilization are increasingly recognized as key components
of food security in Bangladesh- having one of the highest rates of malnutrition
in the world. Economic analyses indicated that without improvements in the
nutritional status of the population, 22.9 Billion US$ in productivity will be
lost to the country between 2000 & 2010 (UNICEF & ADB, 1980). (HFA in
Bangladesh: Lessons Learnt, Henry B Parry, 2000).
1.3 Importance of Food Safety
□ Food safety and sanitation are considered to be a key issue to ensure overall
food security in Bangladesh.
□ Food is the major source of human exposure to pathogenic agents, both chemical
and biological (viruses, parasites, bacteria), from which no individual is
spared. The importance of food safety stems from: (1) food being the primary
mode of transmission of infectious disease; (2) the intricate linkage with
development- governs individual and community health, national productivity, and
promotes export potential & thus earn foreign exchange; (3) emerged as prominent
sources of conflict in international agricultural trade.
□ Biotechnology has raised some food safety concerns as new scientific methods
to assess the safety of food derived from biotechnology have yet to be developed
and agreed upon internationally.
□ In Bangladesh >90 % tube wells of 61 districts (out of 64) are contaminated
with arsenic.
□ Urban population are gradually shifting from cereal-based diets and would
likely generate a demand for fish, livestock, horticultural, forest produce as
well as processed items, in turn necessitating safety load of associated
transport, storage and marketing infrastructure.
2.0 National Position on the objectives and revised draft agenda themes
Bangladesh pays due importance on the objectives and themes of the conference
having huge outcome on the national and regional food safety. Though there seems
to have some over laps in themes of the conference. Below are some descriptions
about national position related to these themes.
2.1 Food safety legislation-science and risk-based approaches to harmonization
2.1.1 Potential risks in food
□ A wide range of food borne diseases (endemic----hyperendemic---epidemic---pandemic)
is encountered in Bangladesh.
□ Naturally occurring toxins, such as mycotoxins, marine biotoxins, cyanogenic
glycosides and toxins occurring in poisonous mushrooms, periodically cause
severe intoxication.
□ Bovine Spongiform Encephalopathy (BSE, or "mad cow disease"), are suspected to
cause new variant Creutzfeldt-Jakob Disease in humans. Recently occurring “bird
flue” disease in poultry caused by avian viruses is also a threat.
□ Persistent organic pollutants (POPs): Dioxins and Polychlorinated Biphenyls
(PCBs) exist in the environment and in the human body, which are carcinogenic.
□ Metals, such as lead and mercury, cause neurological damage in infants and
children. Exposure to cadmium can also cause kidney damage, usually seen in the
elderly.
2.1.2 Food Safety load: Major areas
·Primary Production:
o Yearly food grain (rice+wheat) production is about 26.69 million metric ton (mmt)
from 117 million farm holdings; Maize 0.2 mmt; Vegetables about 1.52 mmt; Fruit
1.49 mmt; Oil seed 0.36 mmt; Spices 0.37 mmt;
o 95 % of cereal seed requirement is met through farmers to farmers exchange and
the rest by public sector corporation;
o Chemical Fertilizer consumption-3.2 million metric ton; Pesticides
consumption- 16.5 thousand metric ton;
o Cattle & buffalos-223 million; goat & sheep-146 million; fowls & ducks-1266
million (Agri census-1996)
·Post harvest processing: Rice mills-10000; Flour mills-650; about 630
registered food processing industries in Bangladesh (Formuzul, 1999); fish
processing- 129 plants, no of depots-2300, ice plant-653.
·Transport, handling and storage: 193 cold storages; 636 LSDs, 13 CSDs and 5
Silos (1.0 million ton per year);
·Import and export:
|
Item |
Import (000 metric ton)
|
Export (000 metric ton)
|
| Rice | 1500 | 2.5 |
| Wheat | 1600 | - |
| Maize | 800 | - |
| Pulses | 140 | - |
| Edible oil | 2600 | - |
| Vegetables | 16 | 10 |
| Fruits | 109 | 2.5 |
| Fish | N/A | 47 |
Item Import
(000 metric ton) Export
(000 metric ton) Rice 1500 2.5 Wheat 1600 - Maize 800 - Pulses 140 - Edible oil
2600 - Vegetables 16 10 Fruits 109 2.5 Fish N/A 47* Domestic Market and
consumption: Population is nearly 137 million; No. of household – 26 million;
About 13135 markets in the country (DAM, 2000)
2.1.3 Bangladesh Food Safety Laws and Regulations
Pure Food Ordinance, 1959 and Pure Food Rules, 1967; The Animals Slaughter
(Restriction) and Meat Control (Amendment) Ordinance – 1983; BSTI ordinance 1985
(has been amended as BSTI (amendment) Act 2003); Destructive Insects and Pests
Rules (Plant Quarantine) 1966, amended up to 1989; Agricultural Produce Market
Act 1964 (revised in 1985); Fish Protection & Conservation Act, 1950(latest
amendment in 1995); Marine Fisheries Ordinance 1983 and Rules 1983; Fish & Fish
Products (Inspection & Quality Control Ordinance, 1983; Fish & Fish Products
(Inspection & Quality Control) Rules’1997; The Essential Commodity Act 1957, 58,
64; The Food or Special Courts Act 1956; The Food Grain Supply (Prevention of
Prejudicial Activity) Ordinance 1956; The pesticides Ordinance-1971 & The
pesticides Rules-1985.
2.1.4 Policy Linkages
Food Safety in all stages of the food chain, that is, from farm to table has
been focused with due importance in all the relevant policies of GOB. The
policies are- Bangladesh Environment Policy; Bangladesh Food and Nutrition
Policy 1997 and NPAN 1997; Bangladesh Food Policy 1988; Comprehensive Food
Security Policy 2001 and New National Food Policy 2001(draft); National
Agriculture policy; Bangladesh Health Policy; The Exim Policy etc.
Though Food Safety has been focused in all relevant policies of GOB, the basic
food laws, Pure Food Ordinance 1959 & the Pure Food Rules 1967, did not
accommodate Codex standards, guidelines & practices including HACCP (Hazard
Analysis and Critical Control Point). However, Fish & Fish Products (Inspection
& Quality Control Ordinance 1983 and Fish & Fish Products (Inspection & Quality
Control Rules 1997) have received due importance of HACCP principles. The
Bangladesh Standard and Testing Institution (BSTI) also adopted HACCP as
Bangladesh standard.
2.1.5 Food Standards:
□ 107 food items are covered under Pure Food Rules 1967.
□ 190 food standards by BSTI of which 52 should have compulsory certification
marks.
□ 28 Codex standards adopted as Bangladesh standards.
2.1.6 Major Stakeholder Ministries and Departments for Food Control in
Bangladesh
|
Sl. No
|
Ministry |
Department/Organization |
Major Activities |
| 1. |
Ministry of Agriculture |
Plant Protection Wing, DAE |
· Phyto Sanitarycertificate for Import/Exported plants/plant products · Pesticide Use Control · Fertilizer Use Control |
| 2. | Ministry of Food | Directorate General of Food (DGF) |
· Quality Control of PFDS, Stock, Procured Food grains/Food Stuff, Imported food etc. · Food Control in the Market (not doing at present) |
| 3. | Ministry of Health & Family Welfare | Directorate General of Health; District & Upazila Health Administration and Institute of Public Health. |
· Food Quality and Sanitation Control in Upazila/District level · esting |
| 4. | Ministry of LGRD | City Corporation & Pourashava Health Units | Have Sanitary Inspector, Labs and Public Analyst for food quality control in their command areas. |
| 5. | Ministry of Fisheries & Livestock |
A) Department of Fisheries (FIQC Wing) B) Department of Livestock |
· Fish Quality Control & Certification for export · Same for the domestic market |
| 6. |
Ministry of Industries |
BSTI |
· Frame Standards of Food Products · Testing & Certification Marks and Surveillance.
|
| 7. |
Ministry of Science, Information and Communication Technology |
BAEC |
Testing of Food Items; Research and Development |
|
IFST, BCSIR |
Testing of Food Items; Research and Development |
||
| 8. |
Ministry of Education |
DG, Primary, DG, Secondary, Text Book Board, Universities
|
Food safety, Nutrition & Environmental issues in the text book of all level of education |
| 9. |
Ministry of Information |
PIB BTV Radio Bangladesh
|
Broad cast issues for awareness building |
| 10. |
Ministry of Home |
Bangladesh Police |
Assist the Inspection Agencies |
| 11. |
Ministry of Law, Justice& Parliamentary Affairs |
Formulation, Vetting, Parliamentary Approval etc. |
So, food control in Bangladesh is a multi-sectoral responsibility.
2.1.7 Conformity Assessment Infrastructure:
There are about 25 (excluding the branches) food laboratories under various
government, autonomous and international organizations in Bangladesh. However
very few are operating down to the regional and district level. It was observed
that only a few of the laboratories are well equipped and well maintained. They
have shortages of maintenance budget, inadequate technological resources (equipment+manpower)
and, above all, lack of coordination in procedures/methods of testing.
International accreditation is also missing except a few.
2.1.8 Coordinating Mechanism:
·Policy Structure: Cabinet is the only universal coordinating and controlling
infrastructure. No separate coordinating mechanism exists in respect of food
safety in the policy structure.
·Food control (Management and Inspection): No single organization exists in
Bangladesh to oversee/coordinate food control activities.
·Mandatory Minimum Standard Formulation: There is no structure of Food Safety
Advisory Committee or Minimum Standard Fixing Committee.
·Auxiliary Standard making: Standard Wing of BSTI formulated about 365 food &
agricultural product standards and services among those only 190 are Food
Standards. BSTI has right to adopt International Standards (ISO, IEC, Codex etc
) as Bangladesh Standards. Till now 150 International Standards have been
adopted as Bangladesh Standards. Standards Wing of BSTI is being assisted by
6(six) Divisional Committee and 70 Sectional/Technical Committees. 17-sectional
committees under Agricultural & Food Divisional Committee are working for Food
Standards. The members of the committee include representatives from stakeholder
Ministries and departments, universities/research organizations, CAB, Business
and trade associations/chambers etc.
·Lab Activities and Research: Coordinating mechanism among the laboratories
should be strengthened in terms of research and routine test methods.
·Accreditation Body- A draft act has been prepared and sent to different
ministries for comments.
2.1.9 Empowering countries to enforce food legislation
Each and every food safety related laws and rules in Bangladesh have empowered
respective authorities to enforce the provisions regarding inspect, sample, test
& sue, if applicable, against the producers/marketing agents.
2.2 Application of risk analysis in food control- challenges and benefits
2.2.1 Trade impact due to new stringent standards
Asaduzzaman and Hossain (2003) shows that the Bangladesh face welfare loss of
US$ 16.3 million. She losses mainly on two counts- due to terms of trade effect
as well as endowment effect. Endowment effect is the loss of income of the
unskilled (production) labourer. In terms of changes in output, GDP or export or
import trade, none appear to be substantial. While the results are preliminary,
there are two explanations for the apparent small adverse effects. The first is
that except for shrimps, the rest of the food trade does not account for more
than 2% of total trade and hence the changes become almost imperceptible to
them. Secondly, and probably more importantly, there is little change in shrimp
related magnitude because, the changes (investment) in the shrimp sector had by
and large taken place and adjustments made against the new standards to affect
the sector now. It is needed to analyze industry by industry situation.
2.2.2 Impact of EU ban on Bangladesh shrimp in 1997
·Simulation exercises based on with or without ban scenarios: US$65.1 million
as the cost of the EU for Bangladesh.
·Investment to ensure HACCP compliance: US$18 million (facilities and
equipment, training staff and workers for achieving acceptable SPS and technical
standards; annual cost of US$ 2.4 million for maintaining HACCP programme.
2.2.3 Bird flu impact
·In Bangladesh, the quantitative impact was not yet available but the poultry
industry have suffered a huge set back as the domestic poultry market collapsed
for months and import of poultry was banned with a bid to restrict influx of the
pathogenic avian influenza.
2.2.4 Food Borne Disease Load: Some evidence for Bangladesh
·'Diarrhea diseases' is one of the major public health problems in Bangladesh
around 70% of which are food and water borne. UNICEF (2000) study revealed that
prevalence of diarrhoeal diseases among under five children is 16.7%. A Report
of the Directorate General of Health Services (DGHS,2001) showed that the
diarrhoeal diseases is the most prevailing one among all age groups including
5.9 % deaths (1997).
·Diarrhoeal diseases in Bangladesh still causes 5.7 million disabilities
adjusted life years 61% of total DALYs (Disability Adjusted Life Years).
·The Government epidemic surveillance system reported a total of 16,57,381
cases and 2,064 deaths from acute diarrhea in 1998 (ICDDR,B Annual Report, 1998)
excluding post flood (1998) diarrhea (14,86,197 cases and 1,836 deaths).
·Hygiene related diseases in Bangladesh costs US $ 80 million each yr for
treatment alone (BBS, 1998)
Salmonella infections (typhoid):
It is highly endemic in Bangladesh and is an important cause high morbidity and
economic loss, which are known to cause a wide spectrum of disease syndromes in
man and animals (Bowmer, 1968) like gastroenteritis, enteric fever, bacteremia,
focal abscess or as an asymptomatic infection i.e. carrier state (Rubin et al.,
1977):
Ashdown et al., 1990). However, data on salmonellae in Bangladesh remain
scanty (Blaser et al,1982) & limited to few clinical reports (Stoll et al.,1983;
Roy et al.,1985;Butler et al., 1991) & Salmonella Meningitis (Hook, 1991).
Cholera:
·In Bangladesh, cholera outbreaks occur regularly twice a year, both before and
after monsoon. (ICDDR, B Annual Report, 1998)
·Case-control studies have shown that, in Bangladesh, the rate of contamination
of household water with V. cholerae 01 is significantly higher in water used for
cooking than in water used for drinking.
·However, only 0.13% of the food samples cultured were contaminated with V. cholerae 01. indicating the risk of food-borne transmission of cholera during
the non-epidemic season.
·Nevertheless, V cholerae 01 has been isolated from aquatic flora and fauna in
this region (Islam MS, Miah MA, Hasan MK, Sack RB), including blue- green algae
(reservoir of V. cholerare 01. (Islam MS, Miah MA, et al)
·Transmission of cholera through contaminated foods served by street vendors
and restaurants should be considered: in Dhaka, there were two outbreaks of
cholera in 1974 and 1975.
Post Flood health consequences in Bangladesh:
In 1998, the devastating flood affected 52 districts causing shock to 30.52
million people and killed 918 persons. The water was contaminated with faucal
coliform (94% of WASA and 86% of surface H2o, and none from TW water, as
expected. (Yesmin J & D Banu. PHAB journal 1999, VI (172):1-5).
2.3 Food Safety Monitoring Results: Though not representative still raise
concern
Organization Monitoring results 2002-03 Bangladesh Standard and Testing
Institute (BSTI) Number of Surveillance team/mobile court-250; Number of sample
collected from open market for testing-226; Number of show cause notice
issued to manufacturer of sub-standard products-117; Number of cancellation of license-45; Number of legal
actions-35; Ministry of Food: DGF Central laboratory
Rice Sample-242 of which 206 were found out of specification;
Wheat Sample-291of which 73 were found out of specification;
Oil Sample- 6; out of specification- none; MOHFW: Institute of Public Health
·Around 3000-6000 food samples are usually tested per annum in IPH. Test results
showed about 50 percent of the samples as adulterated.
·Samples of Butter oil, Banspati Dalda, and Condensed milk were seen 100
percent adulterated.
·Percentage of contaminated water samples is decreasing.
·Food hygiene of street vended food and personnel hygiene of the vendors have
been of great concern. Ministry of Agriculture: DAE Plant Protection Wing
·2731
imported vegetables and fruit sample tested; all satisfactory
·1500 exported vegetables and fruit sample tested; all satisfactory
·imported (2928.00 MT) vegetable seed sample tested
·58979 Cereal sample tested; all satisfactory MOFL: Deptt. of Fisheries
Total lot exported- 3940; Rejected lot in country- 49; Rejected lot outside the
country-8; Causes- Salmonella, v-cholera, e-coli, filth, antibiotic; Action
taken as per FIQC/97 MOLGRD: Public Health Laboratory of Dhaka City Corporation
Food samples are-Cereals and Pulses, Fruits, Vegetables and their products, Fats
and oils, Spices & condiments, Sweetmeats etc.;
Physical and routine chemical analysis and some biological tests are carried
out; 960 samples tested, 713 found adulterated 2.4 Information exchange,
education and communication
Two projects are running currently, one under the Ministry of Food (under FAO
technical assistance) and another under the Ministry of Health and Family
Welfare (under WHO technical assistance), and are working for capacity
strengthening, training, research and awareness building in the area of food
safety. Following the completion of HACCP implementation project in 97-98 in the
Dept. of Fisheries, a total of 2000 personnel engaged in fish
processing
business were trained on various components of HACCP. Ministry of Agriculture
have their own training and research activities and awareness building program.
2.5 Case studies: Impact of Awareness Building and Training; Impact of IPM
activities in Boro paddy and vegetables during 2002-03
Boro 2002-03 (winter): Applications of pesticides were reduced by 88% on an
average while yields increased by 11% in the sample farms.
Vegetables 2002-03 (winter): Applications of pesticides were reduced by 82.0% on
an average while yields increased by 6.0% in the sample farms.
2.5 Current Status of Utilization of Biotechnology in Agriculture in Bangladesh
Bangladesh is a signatory to the convention on Biological diversity and the
Cartagena Protocol on Bio safety. While acknowledging the benefits of
Biotechnology in increasing food production, management of pest and
diseases,
improved diagnostics, high nutritional quality etc, she is also concerned with
the associated risk if any and developing risk management system. Use of
biotechnology is at infancy stage in Bangladesh. Nearly 150 agri-scientists are
doing some biotech research and development works. In fisheries and livestock
sector the progress is also insignificant.
Funding in different organization of Bangladesh involved in Biotech Research
during the last 10-yrs shows that total funding was Tk 60.777 crore of which GOB
funding was Tk 39.720 crore (Lump grant and revenue expenditure Tk 39.09 crore;
Regular research funds Tk 63.0 lakh). Funding by foreign agencies was Tk 17.057
crore, while investment by private Biotech labs was Tk 04.00 crore.
Enabling Regulatory Measures:
- Bio-safety Guidelines (2002) are being revised and National Institute of
Bio-tech established and formation of NCBB, IBC, BSO, FBC are in progress;
- A national steering committee headed by Principal Secretary to the Prime
Minister has been working;
- Draft Bio-safety Act & Draft Plant Variety Protection Act are under
consideration;
- The Seed ordinance/Act, 1997; The Seed Policy, 1993 & Rules, 1998 are in
place.
- National Biosafety Monitoring and Control Authority has been proposed.
- Bio-tech policy is required.
- A TAPP is under consideration to start a project on the whole issue.
2.6 Food borne disease monitoring and surveillance systems
Food borne diseases (FBD) plays important role in country’s public health, the
GOB has its own monitoring and surveillance system in its paradigm of
epidemiological back up mechanism (good reporting systems/accurate
information/robust data base/quick-response oriented rapid action plan).
Moreover it has a ready-in-hand Epidemiological Control Preparedness Team to
combat such disease outbreaks.
The cause specific types of FBD that are generally encountered by the public
health authorities, are: i) Sudden disease outbreaks (diarrhoea, vomiting,
intoxications, etc.) are reported following large parties (after mass
consumption of same food); ii) Gradual increasing trend in primary & secondary
attacks rates following the consumption of a particular food / food habit /
practice; iii) Chronic outbreaks due to environmental pollution like heavy
metals that may contaminate our food, viz., recent catastrophic contamination of
ground water with high levels of trivalent Arsenic; iv) Seasonal outbreaks of ‘
Cholera’ or ‘Dysentery’, related to seasonal changes.
To address the above mentioned vital epidemiological components towards taking
accurate & in time measures the GOB has developed the following mechanisms,
based on the following steps:
Factors leading to the Prevalence of food borne diseases are rapid
industrialization; Urbanization; Changing lifestyle; Changing Population;
Polluted environment; Poverty; Lack of food preparation facilities, etc.
3.0 Efforts towards establishing food safety by the GOB
- Formulating/updating food safety laws/policies related to food safety,
standard and certification system. The Pure Food Ordinance, 1959 is in the
process of amendment.
- Building Policy linkages
- Public-Private Sector Interaction & Human resource / lab infrastructure
development
- Adoption of International standards: 28 Codex (including Guideline for the
application of HACCP, Code of practice General Principle of Food Hygiene etc.
- Phytosanitary measures adopted by DOF-SoQ etc.
- Membership in Trade Arrangements-WTO SPS and TBT
- Strengthening the information, surveillance and alert service at DLS.
- Draft Consumer Protection Act; Draft Feed Act; Animal Quarantine Act and
Animal Disease Control Act are also awaiting approval;
- Working with SAARC for harmonization of standards on food products.
3.1 Efforts by NGOs:
A wide range of activities on food safety awareness are being undertaken by a
series of NGOs as follows-
i) Consumers Association of Bangladesh (CAB)
ii) Bangladesh Paribesh Andolon (BAPA)
iii) DOSHER Bangladesh , etc.
4.0 Activities in Regional/International Arena
·SAARC initiatives
SAARC member states has recently identified food safety as a topic of priority
concern to ensure that both consumers and smallholder farmers in SAARC countries
duly benefit from food trade and also to prevent farmers from marginalization
due to food safety concerns in the process of globalization. SAARC Food Security
Reserve Board (SFSRB), at its 9th Meeting held in Islamabad, Pakistan in
December 2002, urged the member states to consider harmonizing food laws,
regulations, standards, quality control system, and control mechanism to
facilitate maintenance of food safety for enhancing food trade. SAARC (under
RIPA) also organized a workshop in Kathmandu, Nepal which adopted some
recommendations likely to be communicated to the respective government.
·FAO-ILSI initiatives: 4-workshops/seminars have already held.
·WHO 10-Points Regional Strategy for Food Safety in the South East Asia Region
5.0 Issues and Constraints for Bangladesh
·Food control activities are implemented unorganized form, including scanty
information on food contamination.
·Food laws & regulations do not embody recent development/ recommendations by
Codex, SPS & TBT Agreements
·Weak coordination among activities like plant quarantine, food control,
standards, enforcement and labs
·Weak Consumer/public awareness programs
·GM food, one of the main issues of concern for human health
·Compliance cost.
·Financial resource constraints.
·Dynamic Factors Influencing Food Safety Policies;
·Food Safety Concerns in Technology Improvement Research: Production technology
research; Post harvest Technology Research; Policy Research and Food Safety
·Absence of proper enforcement
·Knowledge of standards, laws/regulations are too low among the producers and
consumers.
·Safe limits of arsenic in food yet to be ascertained.
6.0 Conclusion and Recommendations
6.1 Recommendations for Improving Bangladesh Food Safety System
Food Safety Policy
·A comprehensive Food Safety Policy should be formulated having an appropriate
institutional framework to operationalize it.
Laws, Regulations, Standards
·The Food Ordinance/Rules and Regulations and other relevant Acts should be
updated from time to time in view of the changing requirements.
·Enactment of Consumer Protection Act, Feed Act etc. should be made as early as
possible.
·Harmonization of provisions/standards in various laws/rules are also
necessary.
·At the retail level, work to ensure the adoption of science-based standards
and to foster HACCP-type preventive approaches-largely through the Food Code
process.
·Guidelines should be issued on Good Agricultural Practices and Good
Manufacturing Practices (GMPs) for all foods including fruits and vegetables.
·CAC standards do not fully take care of a number of foods manufactured/grown
in the country for their quality and safety standards. In such cases, the
internationally accepted food certification system should rely on the National
standard for marketing.
·Food Standards in PFR and those set by BSTI should have similarities in
respect of parameter definition/limits/testing methods etc.
·A comprehensive labelling law with appropriate labelling provisions for local
and imported packaged food inconformity with CODEX should be formulated and
properly implemented.
Technical Assistance (TA) needs:
Training for the national regulation agencies concerning the preparation of
technical regulations (TBT principles); for implementing certification,
accreditation and reinforcement; evaluating the impact of the
standards/procedures/guidelines; Seminar/workshop for creating awareness among
private and public sectors; Preparation of a SPS and TBT accomplishment
guidelines.
Infrastructure of Food Control
·Include measures to modernize food inspection, manufacturing procedures,
research on food borne disease outbreaks;
·Establishment of bodies for accreditation, regulation and certification;
·Development of consultants ;
·Feasibility and methods for post-marketing monitoring of GM food products
·Institutional changes- complement HACCP, ISO management System etc.
·An apex body for policy formulation and development task of Food Quality and
Safety Programs (FQSP) should be formed including all stakeholders;
·Institutional mechanism such Food Safety Council, Food Safety Technical Comm.
Monitoring Committee etc.
·A National Food Control Agency should be established.
Training and Human resource development
Should be targeted for i) food inspectors, ii) food scientists and analysts,
iii) policy makers, iv) microbiologists, v) public health physicians, vi) food
technologists, vii) serial librarians and documentation officials, viii) food
law experts etc.
Conformity Assessment Infrastructure
·Adequate testing facilities including microbiological and safety parameters
analysis should be developed from farm to production to assist the compliance of HACCP, quality certification system and for continual improvement of a produce.
·Food safety and other certification system should be reviewed through unified
efforts of industries, farmers, regulators, scientists, academicians & consumers
to develop a state-of-the-art, food safety quality system.
·Cleaning, grading, testing, standardization, packing, storage, labeling and
marketing based on well documented ·inciples of good practice, HACCP,
scientific storage should be encouraged at farmers’ level so as to promote
direct integration of food processing units with producers.
·Capacity strengthening is required for the laboratories of different
institutions.
·Strong co-ordination among the conformity assessment infrastructure.
·There is a need to establish/review safe limits for food additives and
contaminants, study data on these mycotoxins, level of risk and recommendation
of Codex. Laboratories should follow simple method for detection and
quantification of toxins in mycotoxin prone food items.
·An electronic certification (E-cert) system may be developed.
·It is very important that the sampling procedure is standardized and it is
transparent.
·The procedures for inspection and drawing samples should be laid down in
accordance with the standards prescribed and should be in tune with the
international practice.
Food Safety Database
·Adequate data should be generated for pesticide residues, toxic metals in
different food crops for use in risk assessment work, for ensuring consumer’s
protection and for harmonization of standards with that of safety standards
under Codex.
·The food safety information database should be expanded to provide more
complete information on the incidence of food borne disease by pathogen and by
food.
·Surveillance program to collect more precise information about the incidence
of food borne illness, especially illness caused by chemical and microbiological
poisoning including Salmonella and E. coli O157:H7.
·Growing a networking system on nationwide food borne diseases and its risk
assessments.
·Share information/data for mutual usage & wider utilization (particularly to
reduce risks).
Production and Market Places Development
·Encouragement to organic production of farmed and processed farm products
should be on the priority food list, which is an alternative to safe food,
produced through conventional techniques.
·Integrated quality system from farm to market should be developed to ensure
that there is no deterioration in the quality of the organic food and it is
properly transported, stored and marketed. Depending upon food crops, concept of
ISO 9000 and HACCP should be developed and practiced at farm level. Integrated
quality marketing approach should be adopted at farm, from pre-harvest to post
harvest including scientific storage, grading, standardization, certification,
labeling, traceability, transportation and marketing.
·Technology, innovation and enterprise development should be the keys in
attaining food safety.
·Development of scientific storage facilities in food chain would facilitate in
retaining the quality of the produce.
Coordination among GOB organizations and GO-NGO activities
·Domestic harmonization of activities, procedures, method of testing etc. among
the GOB agencies are very much required in the first place.
·A national commitment and the collaboration of all ministries concerned with
health, agriculture, finance, commerce, food, industry, municipality and
concerned NGOs are to be ensured.
Law Enforcement
·The laws in place should be implemented with full force and hurdles in
implementing the existing laws against adulteration to be eliminated.
Facing Arsenic
·Scientists, researchers, consumers, NGOs, donors should come forward with
integrated efforts to formulate a strategic plan to solve the problem.
·Awareness should be built up among the consumers to select and purchase the
safe food from open market.
Awareness building
·Education, awareness and training through manuals, material, and practical
demonstration as a priority to regulatory measures should be given to farmers,
food processors, govt. regulators, policy makers, vendors and other persons
involved in the system for compliance.
· Adequate knowledge and guidance should be available to farmers for strict
application of good agricultural and marketing practices for their food crops &
programs to educate consumers about food safety should be launched.
Research and Study
·Food Safety Policy research should be launched in the fields of production,
processing, marketing and consumption.
·Study of collective impact of unsafe food intake should be carried out for
which a technical assistance might be sought.
6.2 Some recommendations for Regional Action Plan for food safety
·Review of laws/infrastructure/coordinating mechanism and provide technical
assistance to update those towards regional harmonization.
·Review of standards and certification systems in purview of international
requirements.
·Review of research and study programmes and help conducting research and study
projects.
·Technical assistance in 10 years’ training/awareness building programmes.
·Assist in developing risk analysis infrastructure and making risk limits for
adulterants/contaminants.
·Provide support for publishing a regional food safety bulletin containing news
and views on food safety data/events/information/development.
·Assist in establishing food safety cell or commission or council at the SAARC
secretariat.
·All of the SAARC countries should prioritize their efforts in establishing and
evaluating priorities in food borne disease prevention & control.
·Establish a Regional epidemiological network among the SAARC countries on all
possible ways to combat FB-disease outbreaks, particularly the possible risks of
being contaminated with a used range of FB-diseases in all countries of SAARC.
·Chalk out long term & sustainable resources, means & ways to fight back FB-illnesses
from respective countries.
References:
1. Government of Bangladesh: Various documents.
2. MOA (2004): Report of the National Task Force on Food Safety Bangladesh for
FAO-WHO Regional Conference on Food Safety for Asia and the Pacific Seremban,
Malaysia, 24-27 May 2004.
3. MOF (2004): Report of the Working groups for Harmonization of food safety
laws, regulations, control system, control mechanism and standards for
facilitating food trade among the SAARC countries.
4. Rouf, Abdur (2004): Enhancing Certification System for Better Marketing,
Country paper prepared for APO Seminar to be held in Tokyo, Japan, January 2004
5. Zahangir, Alam Md. (2002): Sanitary and Phytosanitary Measures, Bangladesh
Country Paper presented in APO Seminar, Tokyo, Japan.
Agenda Item 7 Conference Room Document 16
English only
FAO/WHO Regional Conference on Food Safety for Asia and Pacific
Seremban, Malaysia, 24-27 May 2004
The progress, experience and lessons learned in the bilateral technical
cooperation
in the food safety fields through “JICA project for
Strengthening Food Safety Programme in Malaysia”
(Japan)
1. Introduction
The need for safe food is fundamental throughout the world and is a major
concern globally. However, with the expansion and globalization of food trade as
well as advancement and development in farming practices and food processing,
ensuring food safety has become more complicated. In these circumstances,
capacity building in food safety is crucial to keep up with new issues on food
safety due to advances in science and technology.
The Food Safety Department of the Ministry of Health, Labour and Welfare has
fully supported capacity building in food safety fields through the Japan
International Cooperation Agency (JICA)[30]technical cooperation. JICA has been
engaged in two “Technical Cooperation projects”[31]in support of capacity building
in the food safety area through the sharing of knowledge and experience. One is
in Thailand (April 1994-April 2000) and the other is in Malaysia (June 2001-May
2005), which is still on-going. “Technical Cooperation Project” activity was
basically implemented in an integrated manner through the dispatch of Japanese
long- and short-term experts for hands-on trainings, counter-part training in
Japan and the provision of necessary equipment. In each project, long- and
short-term experts stayed at a relevant food safety authorities and
laboratories.
The approach applied for capacity building, the current progress made and
lessons learned through Technical Cooperation Project “The Project for
Strengthening Food Safety Programme in Malaysia” (hereinafter referred to as
“the project”) are introduced in this paper.
2. Overviews of the project
2.1 Background
In order for Malaysia to progress towards the status of a developed country,
which is scheduled for 2020, food safety is a vital and indispensable component,
as it contributes not only to health and productivity but also provides an
effective platform for development. Realizing this Malaysia has undertaken
various initiatives to strengthen its food safety programme. One of the main
initiatives recently undertaken to strengthen food safety in Malaysia is the
joint technical cooperation project between JICA and Malaysia for 3 years (2001
June to 2004 May).
2.2 Outline of the Project
Before the commencement of the Project, a Japanese long-term expert was
dispatched to look into the existing food safety management system in Malaysia.
Based on this information and with the help of JICA consultation teams, a
Project Design Matrix, which outlined the project’s purpose, its outcomes,
activities and input to be carried out, was developed. The project purpose is to
increase the availability of safe food for Malaysian consumers and its outputs
are as follows:
o Food hygiene management is strengthened;
o Means to prevent food in the market which is not in compliance with the Food
Act and Regulations, are strengthened;
o Means of providing information on food safety for consumers is improved.
To achieve purpose and to gain output, the project carried out a series of
activities such as strengthening food safety administration, capacity building
for food analysis, strengthening food inspection and technical guidance, and
development and promotion of food safety information for consumers and food
handlers.
2.3 Approach for capacity building in food analysis
Capacity building in food analysis is a prerequisite not only for identification
of hazards in food for strategic planning purposes but also to assess and manage
these risks. Upgrading analytical capability will facilitate the generation of
reliable and accurate data for assessing risk management options and making risk
management decisions related to policy and regulatory enhancement. However, food
analysis technology, which requires precise and sophisticated instruments, has
been advancing rapidly and analysts need to keep up with such developments.
During the initial phase of the Project, the following limitations were
identified;
o Insufficient human resources;
o Inadequate analytical skills;
o Inadequate precision instruments and their operation/maintenance.
Taking the identified limitations into consideration, a consensus was reached to
adopt the following approach for capacity building in food analysis:
o Identification of area of concern according to the priority of food analysis;
o Appointment of a core laboratory and personnel as counterparts in each field;
o Counterparts were sent to Japan and trained by appropriate laboratories based
on their knowledge and experiences;
o Provision of instruments, if necessary;
o Send short-term experts from the same laboratory which trained the
counterparts in Japan to Malaysia to conduct hands-on training and to help
establish analytical methods and procedures which are documented as Standard
Operating Procedures (SOPs);
o Counterparts plan and implement “echo-training” sessions, where counterparts
act as trainers to train other analysts.
2.4 Current progress and outcomes
Since the project started almost 3 years ago, JICA has dispatched three
long-term experts and 24 short-term experts, accepted 17 counterparts from
Malaysia to Japan for training, and provided state-of-the-art machinery. As a
result of collaborative efforts, progress has been made in capacity building in
food analysis, food import control network systems and monitoring programmes.
2.4.1 Analytical capability
With respect to capacity building for food analysis, the project focused on the
transfer of analytical skills in the areas of pathogenic bacteria, pesticide
residue, veterinary drug residue, mycotoxins, nutrients, genetically modified
food and food packages. As a result of the combined hands-on training on food
analysis, both in Japan and Malaysia, analytical capability at the identified
core laboratory was strengthened and analytical methods were developed and
documented as Standards Operating Procedures (SOPs).
Table 1: Numbers of analytical parameters before and after the project
Before Project After Project Microbiology 12 17 Pesticide 17 56 Veterinary drugs
2 18 Mycotoxins 1 3 GMO 0 5 Nutrition 0 6 Food package 0 102.4.2 Import Food
Inspection
During the process of developing an import food control network system, JICA
experts were dispatched to transfer their knowledge based on the Japanese
experience. The interactions between JICA experts and their Malaysian
counterparts enhanced motivations among the Malaysian counterparts, which could be one of the keys to its
success. The development of the import food control
network system, called "Food Safety Information Management System in Malaysia (FoSIM)",
will facilitate the application of an integrated and scientific based import
food control system.
2.4.3 Monitoring
With the enhancement of laboratory capability, additional baseline data are
currently being collected through the initiation of monitoring programmes such
as school hostel’s kitchen survey, pesticide residue and veterinary drug
residues monitoring, and microbiological risk assessment of Vibrio
parahaemolyticus. These data will facilitate the identification of problematic
areas and contribute further to the strengthening of the food safety programme.
For example, based on the assessment of the bacterial survey of school hostel
kitchens, critical points, which needed to be controlled, were identified. Based
on these critical points identified, food hygiene guidelines for management and
food handlers at school hostel kitchens were developed.
In addition, 15 educational materials such as a poster “Bacteria causing Food
Poisoning” were developed and distributed.
3. Conclusion
JICA’s final evaluation based on the five criteria of relevance, effectiveness,
efficiency, impact and sustainability of this Project was evaluated as "high".
This evaluation concluded that the Projects purpose has almost been achieved
except for a quality assurance programme in food analysis and a strengthening of
monitoring programme, and this success is largely due to the high level
commitment and ownership by the Malaysian side. This commitment and ownership is
key for the success and further improvement of food safety programmes in a
sustainable manner.
3.1 Lessons learned
The commitment and ownership by the recipient side is crucial to ensuring the
sustainability of the Project. This was very obvious during this Project as can
be seen by the Malaysia commitment as follows:
o Initiative in establishing the“National Food Safety and Nutrition Council” in
2003 which is chaired by the Minister of Health and consists of all relevant
Ministries, NGOs and private sectors to address food safety issues from farm to
table.
o Increase in the number of human resources is crucial for analytical capability
and capacity.
Japanese experts who trained Malaysian counterparts in Japan were sent to
Malaysia to carry out hands-on training sessions for the same Malaysian
counterparts. This approach was very effective both for the Japanese experts and
Malaysian counterparts as this made it easy to share the experiences and
knowledge, which facilitates technical cooperation smoothly.
Echo training means counterparts who have received technical transfer from
Japanese experts, act as trainers to train other food analysts in Malaysia.
Through echo trainings, the counterparts can also learn by teaching as well as
the trainees can learn. The above two approaches are effective for human
resource development.
To ensure the technical transfer of food analysis using precision instruments
such as GC-MS in sustainable manner, a more integrated instruments' maintenance
mechanism including communication with supplier/manufacture support for trouble
shooting and supplier/manufacture regular prevention checks should be taken into
consideration in advance.
Agenda Item 7 Conference Room Document 17
English only
FAO/WHO Regional Conference on Food Safety for Asia and the Pacific
Seremban, Malaysia, 24-27 May 2004
WORK OF ASEAN EXPERT GROUP ON FOOD SAFETY (AEGFS)
(ASEAN)
BACKGROUND
Food safety is an essential public health issue. It is a major concern for
consumers, industry and government. As such, the need to address it throughout
the food supply chain is compelling. The importance of food safety has increased
significantly in recent years following a series of global events associated
with incidences of contamination and outbreaks like contamination of Escherichia
coli O157:H7, dioxin, MCPD, the mad cow disease and the foot and mouth disease.
In relation to that, the advancement in the field of food science and technology
has stimulated the growth of the food industry. However, it has also contributed
as a source of health hazard. Changes in consumers’ taste and preferences that
result in the influx of a wide variety of foods impose on the government’s
limited resources to ensure food safety. Obligations under the WTO compounded
this situation and the establishment of an ASEAN Economic Community by 2020,
will further liberalize food trade. All these have immense impositions on the
national governments to ensure food safety.
INTRODUCTION
ASEAN Seminar and Workshop on “ASEAN Risk Assessment and Harmonization”
organized by ILSI Southeast Asia, ILSI Risk Science Institute and the Food and
Agriculture Organization (FAO) Kuala Lumpur, Malaysia from 3-5 November 1999
noted differences in stages of development of food quality and safety standards,
harmonization efforts as well as policies and programmes affecting food safety.
It was recommended the establishment of an ASEAN framework as a platform to
promote and facilitate harmonization of food standards
The ASEAN Senior Officials Meeting for the 5th ASEAN Health Ministers Meeting on
Healthy ASEAN 2020 held in Yogyakarta, Indonesia from 24-27 April 2000 agreed on
the need to formulate an “ASEAN Food Safety Policy and an ASEAN Framework on
Food Safety” as one of the “ASEAN Regional Action Plans on the Impacts of
Globalization and Liberalization of Trade and Services in the Health Sector”.
Malaysia tabled a concept paper on ASEAN Food Safety Policy and ASEAN Framework
for Food Safety at the 18th Meeting of the ASEAN Sub-Committee on Health &
Nutrition (ASCHN) in Ha Noi, Vietnam, 24-26 October 2000. The meeting endorsed
the concept paper and agreed that Malaysia be the coordinator for the programme
on food safety. The meeting also agreed on the establishment of an ASEAN Expert
Group on Food Safety (AEGFS) to be initially chaired by Malaysia, and for
Malaysia to convene the first meeting of the AEGFS.
1. The 1st Meeting of the AEGFS, Kuala Lumpur, Malaysia, 3-4 September 2001
The 1st Meeting of the AEGFS chaired by Malaysia was held in Kuala Lumpur, 3-4
September 2001. The Meeting was of the view that the mechanism of collaboration
should be improved to minimize overlapping and duplication of work on food
safety among the ASEAN bodies and particularly the working and expert groups and
task forces under the Senior Officials Meeting on Health Development (SOMHD),
Senior Officials Meeting – ASEAN Ministers on Agriculture and Forestry (SOM-AMAF),
the Committee on Science and technology (COST),the ASEAN Working Group on
Technical Cooperation on Pharmaceuticals (AWGTCP) and the ASEAN Consultative
Committee on Standards and Quality (ACCSQ). To overcome this problem the Meeting
agreed:-
·Malaysia as the Coordinator of the programme area on food safety, in
collaboration with the ASEAN Secretariat, and with assistance from the WHO and
FAO, will prepare a concept paper on the proposed umbrella body using a multi-sectoral
approach taking into consideration the following:
a) Consolidation of roles and functions of all relevant ASEAN bodies dealing
with food safety into the proposed body;
b) Mechanism for better collaboration in food safety among the ASEAN bodies,
including the possibility of organizing a joint meeting among the Chairpersons
of the various ASEAN bodies dealing with food safety.
·The Meeting agreed in principal on the Revised Concept Paper “Proposed ASEAN
Food Safety Policy and ASEAN Framework on Food Safety” prepared by Malaysia,
which had taken into consideration Thailand’s written comments.
The Meeting agreed that Malaysia in collaboration with the ASEAN Secretariat and
with assistance from the WHO to engage a consultant and conduct a workshop to
further develop the concept paper in the four major areas identified as: 1)
capacity building, 2)harmonization of regulatory standards, 3)research and
development and, 4)networking.
2. The ASEAN Workshop on Food Safety Policy, Kuala Lumpur, Malaysia,
28 October– 1 November 2002
An ASEAN Workshop on Food Safety was organized by the Ministry of Health
Malaysia in collaboration with the WHO The Workshop recognized that food safety
is an important public health issue, and to enable the ASEAN Vision 2020 to be
realized and to be consistent with the spirit of the Hanoi Plan of Action
developed a Draft ASEAN Food Safety Improvement Plan (AFSIP) to be considered by
the AEGFS and endorsed by the SOMHD.
The Workshop made several recommendations, including the following:
··In the spirit of the Hanoi Plan of Action and consistent with the ASEAN
Vision 2020, it should be recognized that food safety is an important public
health issue, and for the vision to be realized, commitment at the highest level
will be required.
·The AEGFS should consider the proposed AFSIP and recommend its endorsement by
SOMHD.
·A proposal should be made to SOMHD to consider nominating a lead country or
countries to provide secretariat and coordination of the plan.
··The AEGFS should have the continuing role of managing the AFSIP and as the
coordinating body on food safety within ASEAN.
··t would not be appropriate at the present to create a single umbrella body
for food safety within ASEAN.
··The chairs of the relevant bodies within the ASEAN system that are concerned
with food safety issues should meet for purposes of better coordination, and
that the ASEAN Secretariat should be involved in this meeting.
··The Terms of Reference and Rules of Procedures of all relevant committees,
working groups and task forces should be reviewed by the appropriate bodies to
address duplication and gaps.
··The relevant bodies (i.e. bodies dealing with food safety issues within the
ASEAN system other than those reporting to SOMHD) should be invited to consider
incorporating the activities for which they had responsibility within the
proposed AFSIP Plan.
3. The 2nd Second Meeting of the AEGFS, Siem Reap, Cambodia, 1-2 April 2003
The 2nd Meeting of the AEGFS was held in Siem Reap, Cambodia from 1-2 April 2003
and chaired by Malaysia. This meeting endorsed the recommendation of the
Workshop on Food Safety Policy, Kuala Lumpur, Malaysia, 28 October – 1 November
2002 as above.
The Meeting had an extensive discussion on the Draft AFSIP and agreed on the
following:
·The Plan should address as a first priority national or country programmes on
food safety in terms of capacity-building taking into account the expertise
which is offered by Member Countries in various areas; the Plan may also
identify priorities at the national level.
·The Plan should also include joint regional activities developed using data
and information from Member Countries taking into account criteria for
prioritization adopted by the 1st SOMHD held in Ha Noi, Viet Nam, 2001.
·Identify priority activities that are regional, concrete and practical for
immediate implementation, including projects that could be implemented through
existing mechanisms as non-project activities, without the need for project
development such as information sharing
·Expedite the implementation of projects on the basis of cost-sharing with the
assistance of international organizations;
·Format of the AFSIP should be simplified, consisting of programme areas of
cooperation, existing activities, proposed activities and time-frame;
The Meeting agreed on the following priority areas under the Draft AFSIP:
1. Center of Excellence for food inspection
2. Consumer participation in food safety; and
3. Information sharing on food safety through existing national website or other
means of communication.
The Meeting noted views and concern expressed by some Member Countries in
implementing activities under the AFSIP due to limited resources and that the
AEGFS should consider consolidating meetings relating to food safety.
The Meeting requested Member Countries to consider volunteering to be a country
coordinator for each of the areas under the Draft AFSIP and inform Malaysia as
the Chair of the AEGFS on the following:
o Legislation
o Laboratories
o Monitoring and surveillance
o Implementation of food safety systems
o Food inspection and certification
o Education and training
o Information sharing on food safety
o Research and Development
o International participation
o Consumer participation in food safety
The 2nd SOMHD held 3-4 April 2003 following this Meeting agreed that the AEGFS
would provide overall oversight, facilitation and coordination of the AFSIP
activities while actual management of specific projects/activities under the
AFSIP should be done by the countries carrying out the project, and the country
coordinator of the AFSIP programme areas.
4. The 3rd Meeting of the AEGFS, Jakarta, Indonesia, 24-26 February 2004
The 3rd Meeting of the AEGFS was held in Jakarta from 24-26 February 2004. The
meeting noted the need for greater coordination among the various ASEAN bodies
working on food safety related issues and agreed that the ASEAN Secretariat
prepare a paper identifying the opportunities and strategies for improving
coordination for consideration at the 4th AEGFS. The Meeting requested the ASEAN
Secretariat to continue information dissemination to AEGFS Focal Points on
relevant projects and activities of other ASEAN bodies.
The Meeting endorsed the revised documents on the Draft ASEAN Food Safety
Improvement Plan, The Matrix of Proposed Regional Activities and Terms of
Reference of the Programme Coordinator and the Lead Country/Center of
Excellence. The Meeting reconfirmed the three priority areas as food inspection
and certification with Malaysia as coordinator, information sharing and consumer
participation in food safety. To expedite implementation of AFSIP, the Meeting
agreed that:
·Malaysia as coordinator of priority area on food inspection and certification
and will start some activities, on a cost-sharing basis, before the 4th AEGFS
·Requested other ASEAN Member Countries to confirm at the 4th AEGFS their
interest in coordinating programme areas, which will involve among other tasks,
taking leadership to develop regional activities and to open up relevant
on-going or planned national activities for participation by other Member
Countries on the basis of cost-sharing
The Meeting agreed that Malaysia as overall coordinator of AFSIP would
coordinate the preparation of longer term Phase II Action Plan by doing the
following:
·Approach WHO/FAO, with assistance from the ASEAN Secretariat, to obtain the
services of experts/consultants to develop a longer term action plan of food
safety which also includes a comprehensive needs assessment (including
identification of centers of excellence , gaps, opportunities and risks) and
·Draft a Terms of reference for the project on preparing the longer term plan
of action, for consideration by ASEAN Member Countries and the ASEAN Secretariat
before the 4th AEGFS.
The Meeting noted that the matrix on the Proposed Center of Excellence on Food
Safety is comprehensive in scope and provides an overall summary of work in food
safety in ASEAN as well as would be useful as a survey
instrument in obtaining
information on the level of expertise and resources available in the Member
Countries. In the matrix on the Proposed Center of Excellence on Food Inspection
and Certification, the Meeting agreed that Malaysia would use the matrix to
develop regional activities and identify lead countries/center of excellence and
expertise and other ASEAN bodies whilst a more comprehensive survey for the
other programmes be undertaken when the preparation of the longer term AFSIP
commences or when other lead countries are identified.
The Preparatory Senior Officials Meeting for the 7th ASEAN Health Ministers
meeting (AHMM), Penang, Malaysia, 19-20 April 2004 adopted the AEGFS report and
the AFSIP with minor amendments as proposed by Philippines and Thailand.
Agenda Items 5-9 Conference Room Document 18
English only
FAO/WHO Regional Conference on Food Safety for Asia and the Pacific
Seremban, Malaysia, 24-27 May 2004
Country Overview on food safety
(People’s Democratic Republic of Laos)
In the Lao People’s Democratic Republic, health is a major component of the
government’s approach to achieve economic growth and sustainability of its
social progress. However, the country’s state of health services at this time is
severely challenged with the burden of diseases as reflected in high infant and
maternal mortality rates, as well as high prevalence of diarrhea diseases,
particularly in many areas where potable water supply and environmental
sanitation conditions remain a problem.
In the year 2000, 12,440 cases of severe diarrhea were reported and 1,449 cases
for dysentery, food poisoning, typhoid fever and hepatitis A. Helminthes
infection is also a major health problem such as Opisthorchis, which is
transmitted via consumption of contaminated raw fish.
The cost of food borne disease and food contamination to the Lao PDR community
are great which affect personal suffering, loss of family income, community
health costs and industrial productivity.
A. Food safety legislation
The Food and Drug Administration Commission (FDA) was established in 1991. It
comprises member representatives from 7 ministries and a permanent bureau has
been located in FDD, MOH which carries out all activities of the commission.
This commission is chaired by Minister of Ministry of Health and is the body
that ensures each of the key ministries are part of decision making processes
related to food safety regulation and enforcement in Lao PDR.
Since 1995, Lao PDR has been a member of the Codex Alimentarius Commission and
the function of Codex Contact Point is carried out by the Director General of
Food and Drug Department which is responsible for all activities of Codex.
Besides, the National Codex Committee was established and approved by the
chairman of FDA Commission. This technical committee consists of members from
related ministries such as Organization of Science Technology and Environment,
Min. of Trade, Min. of Agriculture and Forestry, Min. of Industry and
Handicraft, Min. of Health (Food and Drug Depart., Food and Drug Quality Control
Center, Hygiene and Prevention Depart.). This committee is responsible for the
implications of various standardization of food and food control issues of the
international agencies and the region.
Food law has just been adopted by the National Assembly from this month and will
be disseminated to the population of the whole country. Veterinary law has also
been drafted and is under consideration. The
Food Safety Policy and Food Safety Programme has been developed by getting
commitment of all key ministries to address food safety along the length of the
food chain. The vision is setting as “Health for all through national economic
development and safe food production and trade in safe food” and the goals are
to reduce morbidity and mortality due to food borne illness and to promote safe
food production and international and domestic trade in safe food.
Food safety has been controlled by using some regulations that have been
established and developed throughout the whole food chain by different sectors
such as:
- Agriculture sector which is responsible for the primary production, has
formulated guidance related to SPS measures and has developed decree, some
regulations on Livestock and Pesticide Management.
- Health, Industry, Commerce sectors which are responsible for the processing,
importation, exportation and distribution, have developed regulation on
Importation–Exportation of food, regulations on processing of food safety,
regulation of bottled drinking water and other regulations related to food
additive, inspection, hygiene and some codes of practice..
Therefore various food control efforts from farm to table are undertaken and
cooperate with MAF, MI, MOH, MC and network of each area in provincial and
district level. Besides promoting food production with contribute to food
exportation. The private sector is involved in controlling food by following the
regulations, rules and codes of practice related to food safety.
The control of domestic foods products is a multidisciplinary activity which
requires the involvement and cooperation of all concerned. Food quality is the
responsibility of the Food and Drug Department of the Ministry of Health.
Therefore, the issue of release certificates of quality and analysis for
domestic foods product is related to some sectors such as Department of
Industry, Department of Agriculture, Department of Livestock and Fishery and
Laboratories.
For food importation, mostly all sanitary and phytosanitary certificates from
exporter countries have been adopted by complying with the standards and Codex
Alimentarius. Also, the manufacturer analysis is often accepted because of the
limited capacity to analyze food in the country.
For food exportation, the organization for delivering certificates of food
analysis and quality assurance certificate are the responsibility of the Food
and Drug Department and Food and Drug Quality Control Center, Ministry of
Health. And the releasing sanitary and phytosanitary of agricultural and animal
production are responsible by Agriculture Department and Livestock and Fisheries
Department, Ministry of Agriculture. Food quality has to analyze, comply with
the requirements of the importation countries.
Food safety has been controlled by using some legislative documents from related
ministries. Some existing regulations need review such as:
- Revise the regulation on Import-Export food, safe food processing, bottled
drinking water
- Revise the regulation and technical norms on livestock and fisheries;
regulation on plant quarantine in line with international plant protection.
- Some necessary regulations are needed to develop such as regulations on food
additives, food hygiene, market hygiene, livestock farming system and
management, fish culture and fish production management, feed management.
There were some necessary standards addressing a limited range of foods and are
being reviewed in line with Codex wherever appropriate to food law. Several
standards of zoonosis and contaminant residues of meat, milk, egg, fish and
their products, fish and feed are formulated and also standards of producing in
agriculture (Pre-Post harvest, Agro processing), technical guidelines on related
crop production, fertilizers and pesticide use need to developed to improve a
control measure and inspect in plant growing.
B. Application of risk analysis in food control
Quality assurance in food analysis is an essential component of food control
that is an important tool for ensuring food quality and safety. In Lao PDR,
there are two main laboratories located in Vientiane which are responsible for
analyzing food samples collected by food inspectors.
- Food and Drug Quality Center, Ministry of Health has carried out a physical,
chemical and microbiological analysis of food samples. This is the main
laboratory for issuing certificates of analysis for food processors and conduct
quality assessment for the FDD.
- Laboratory of Livestock and Fishery Department have carried out analyses of
animal parasites and diseases and certify the sanitary condition of meat
products.
Besides, the food analysis capabilities of staff are limited and the modern and
appropriate equipment is still inadequate. In addition, chemicals, reagents, and
standard methods of analysis are unavailable. These affect the quality assurance
system. Therefore risk analysis has never been applied in food control.
C. Prioritization and coordination of capacity building activities
In 1993-1994, FAO provided technical assistance to assist in capacity building
related to food quality and safety measures such as establishing food
legislation, food inspection and quality assurance training, food import and
export inspection and certification system, food analysis, and laboratory
management and quality assurance system. Since then, food control measures have
been implemented.
Since 1994, WHO has provided a technical consultant to organize food safety
courses and a workshop on Good and Manufacturing Practice and on Application of
the HACCP system in controlling the safety of food industry for inspectors and
producers. In addition, a training course on food inspection on Sanitation
Standard Operation Procedures (SSOP) was recently conducted for inspectors in
the whole country.
FDD require assistance from International agency to strengthen capacity to
participate in multi-sectoral approach to food safety and food law
dissemination, to prepare food hygiene regulations, draft necessary regulations
and codes of practice, to enable participation in Codex process and to develop
and disseminate food safety guidance documents for industry sectors. Besides,
the study on contaminants of food and drinking water (supported by WHO) has been
conducted in order to initiate data collection related to contamination in food
and in drinking water and also to strengthen analytical capacity.
Specific urgent and important capacity building needs
- Revise and formulate food regulations in line with food laws and international
regulations.
- Strengthen capacity of food inspection by providing training course on food
inspection in each sector from primary production to distribution, formulate
inspection forms and provide equipment and facilities for inspection.
- Strengthen capacity of food analysis to analyze for priority hazards in food
products, analyze on zoonosis disease, chemical, antibiotic, hormone residue in
meat, egg, on pesticide in vegetable and fruit.
- Strengthen the capacities of manpower to implement the application of GMP,
SSOP, HACCP in food premises and GAP in farm production by providing adequate
and efficacy training to food producer and inspectors.
D. Information exchange, education and communication
The Food and Drug Administration Commission has regularly taken the lead for
communicating food safety regulations to stakeholders to exchange information
and opinions concerning risk. However, the participation of concerned agencies
is still limited.
Food producers do not understand the relationship between food sanitation
measures and the quality of the final product. Therefore producers need to train
on safety food practices in production, processing and distribution. Also,
groups of agriculture producers and farms need to be established and regulations
related to crop production such as seed/planting materials, fertilizer,
pesticide and rearing pathogen and natural enemy for controlling pests need to
be published and informed.
Furthermore, consumers generally do not appreciate the health implications of
food safety and personal hygiene because of insufficient information on the
rights with regard to safe food. Therefore, the consumer association is required
to establish. Available information on food safety needs reviewing such as
advertising, educational pamphlets, brochures, radio spots and the development
of IEC material and mass media are required for rural population.
E. Food-borne disease monitoring and surveillance system
Many pathogens are being spread to and by an increasing variety of foods. Health
statistics indicate that food borne disease is the major cause of morbidity.
Diarrhea diseases, frequently associated with food borne agents, are one of the
leading causes of mortality, particularly among children.
Regarding the report of the Hygiene and Prevention Department, outbreak of
cholera/severe diarrhea in 1998, 1999, and 2000 was reported for illness in the
number of 6110 cases, 10120 cases and 12440 cases respectively and was reported
for death in the number of 109 cases, 453 cases and 520 cases respectively. In
2000 dysentery disease was reported for 803 cases and 4 deaths. In addition, the
record of food and beverage analysis at Food and Drug Quality Control Center
that E.Coli, Salmonella and Vibrio Cholera were found in various foods. Also,
many studies on food contaminants were conducted and revealed various
contaminants especially on microbiological contaminants.
The investigation team has been established in case of emergency to support and
conduct immediate action to solve problems, advise farmers/producers, give
public information in order to prevent the extension of food borne diseases.
Weakness of food control situation
- Insufficient trained manpower on enforcement of legislation to develop food
control capacity;
- Lack of food scientists, researchers, technologists on performance
surveillance and monitoring of food contaminants to control the incidence of
pathogens in the food supply and also for getting the information and data of
food contaminants and food borne diseases;
- Inadequate information and education on food safety for food producers to
perform effectively guidelines and practices of food handlers;
- The capacity of analysis are very limited and lack fresh reagents and modern
laboratory equipment to control food quality and conduct a risk assessment;
- Poor dissemination on food safety for consumers that contribute to the
unawareness of consumers to purchase safe and quality food;
- Limited in participating in the work of Codex especially limited capacity to
enforce Codex standards because of analytical limitation, and human resource and
others limitations;
- Future Plans for Strengthening food safety: Disseminate food law and revise
existing regulation and standards;
- Develop Plan of Action of food safety programme;
- Improve the capacity of food inspection from production to consumers;
- Strengthen the analysis capacity of necessary hazards by training analysts and
provide laboratory equipment;
- Strengthen the capabilities of manpower and producers to implement effectively
the application of GMP, GHP, SSOP & HACCP in food premises (SME) and GAP in farm
production for producers and inspectors.
Agenda Items 5-9
Conference Room Document 19
English only
FAO/WHO Regional Conference on Food Safety for Asia and the Pacific
Seremban, Malaysia, 24-27 May 2004
Cambodia Country Report on Food Safety
1. Country Overview
Cambodia is an agricultural country located in South East Asia with a total
landmass of 181,035 square kilometers. It has a tropical climate with two
distinct monsoon seasons (dry and rain seasons). The latest population census in
1998 recorded a total of 11.5 million people with an annual growth rate of 2.5
%. In Cambodia 84% of the population lives in rural areas. In 2002 the GDP was
US$360. However, thirty six percent (36%) of the total population live below the
poverty line, but in rural areas the percentage of the population that live
below the poverty line rises to 79%.
Agriculture, mainly rice production, accounts for 40% of the GDP employing more
than 70% of the workforce. The growth rate in agricultural employment is,
however, slowing down. National disasters (annual flooding and drought) result
in year-to-year fluctuations in the agricultural sector
Agro-based food production is one of the main characteristics of the Cambodia
food production. The middle and small industrial sector play a crucial role in
the nation's economic framework. Besides raw agricultural products (rice, maize,
soy bean, green bean, fresh meat, fish, sea products, fruits, fresh water),
milk, flour, soy sauce, fruit juices, beverages, bottled drinking water and etc.
are locally processed. Food safety remains a major public health concern.
Food-borne disease outbreaks are common and caused by mishandling of perishable
food and unhygienic preparation, especially ready to eat food (in restaurants,
street food, and hawkers).
2. Morbidity and Mortality
Life expectancy at birth among females is 58.3 and male 54.5. Cambodia
Demographic and Health Survey (CDHS) 2000 revealed high level of mortality among
children and women: infant mortality rate 95 deaths per 1,000 live births, under
five mortality rate 124 deaths per 1,000 live births, and maternal mortality
rate 437 deaths per 100,000 live births. These mortality rates are highest in
the region. Diarrheoal diseases, acute respiratory infection and vaccine
preventable diseases cause about half of all deaths in the under-five age group.
Health Statistics:
1) In Patients for Cholera, Diarrhea, and Dysentery (source: MoH report)
| Diarrhea syndromes | 1997 | 1998 | 1999 | 200 | 2001 | 2001 |
| Cholera | 146 | 212 | - | 244 | - | - |
| Diarrhea | 5,293 | 6,243 | 8,701 | 8,709 | 10,847 | 10,178 |
| Dysentery | 2,078 | 2,071 | 2,266 | 1,976 | 3,470 | 2,632 |
| Total (%) | 7,517(5.0) | 8,526(5.4) | 10,967(5.60) | 10,929(3.8) | 14,317(5.1) | 12,810(5.1) |
| Total in patients reported | 150,433 | 156,945 | 195,790 | 291,248 | 283,140 | 250,301 |
2) Cases and Fatality Rate of Diarrhea, and Dysentery among In Patients
| Fatalityrate(%) | 1997 | 1998 | 1999 | 2000 | 2001 | 2002 |
| Diarrhea | 35(0.66) | 41(0.66) | 103(1.18) | 70(0.80) | 54(0.5) | 54(0.5) |
| Dysentery | 14(0.67) | 12(0.58) | 7(0.31) | 13(0.66) | 63(1.8) | 63(2.4) |
In 2002, fatality rates by dysentery were increased 3 times
higher comparing with cases fatality rates in 1997.
3) Out Patients for Diarrhea
| Cases | 1997 | 1998 | 1999 | 2000 | 2001 | 2002 |
| Diarrhea | 382,604 (11.4%) | 354,743 (11.4%) | 266,410 (9.2%) | 301,224 (8.7%) | 304,834 (7.5%) | 333,612 (7.3%) |
| Total out patients | 3,347,367 | 3,108,329 | 2,909,986 | 3,477,485 | 4,078,688 | 4,595,939 |
4) Food Safety Outbreak in Cambodia 1996 – 2004
| Year | Outbreak/Problem | Effect |
| 1996 | Drinking rice wine that mixed with some pesticide (to make it strong) | 40 deaths |
| 1998 | Drinking rice wine that mixed with Methanol (to make it strong) in rice wine | 30 deaths |
| 2000 | Eating puffer fish that had poison | 7 deaths |
| 2001 | School children eating contaminated rice | 212 children/hospital |
| 2002 | Eating meat from a dead marine turtle | 3 deaths/100 affected |
| 2003 | Drinking contaminated well-water | 1 death/112 affected |
| 2004 | Bird flu in farms near Phnom Penh and other two provinces | 3 outbreaks in provinces |
3. Food Safety Policy and Legislation
In 1993 a new system of developing legislation was introduced in Cambodia.
Subsequently, there has been only one framework law that has been enacted and is
related to products such as food. The Law on The Management of Quality and
Safety of Products and Services (N0 0600/001) was approved on June 21, 2000. The
law addresses "Production and Commercialization”; consumers’ rights and economic
operators’ obligations; labelling; commercial fraud repression; action against
products or services, which are likely to induce grave or imminent dangers;
Inspection procedures for quality and safety of products, goods and services;
and offences.
Since the Law has been approved, several actions have been taken to disseminate
information about the law by using media, meetings at schools, and consumer
education. In addition, the law has been applied in legal actions against any
violators of the law's provisions. In addition, a number of sub-decrees have
been endorsed since 1997. These include the following:
a) Sub- Decree N0 54 (22/09/97) – Sub-Decree on the Organization and Functioning
of the Ministry of Commerce. Under this sub- decree the Ministry of Commerce
assigns duty to the Department of Cambodia Import and Export Inspection and
Fraud Repression (CAMCONTROL ) as follows:
- Inspecting and repressing goods with fraudulent quality while being in
circulation at markets.
- Analyzing the quality of both foodstuff and consumer goods.
- Inspecting and certifying conformity with the national standards on the
quality, safety, and labelling of food and consumer goods (except medical
products, medical equipment and cosmetics).
- Inspecting both imported and exported products.
b) Sub-Decree N0 64 (29/ 07/ 2001) relating to the designation and management of
border control at the border gates, airport and the seaports of the Kingdom of
Cambodia.
c) Sub-Decree N0 69 (October 20, 2003, Ministry of Planning) concerning the
Management of Exploitation of Iodized Salt in the Kingdom of Cambodia. The
purpose of this sub-decree is to eradicate iodine deficiency disorder by
supplying properly iodized salt. The responsible agency in charge of quality and
safety control is the quality control agency of the Ministry of Commerce, in
cooperation with relevant ministries.
d) Sub-Decree No 67 (October 22, 1997) Identified that the Ministry of Health (MoH)
is responsible for controlling the safety and the management of food. As a
result, the MoH added ‘Food and Cosmetics’ to its existing Department of Drugs
and Medical Devices. At present, the Department of Drugs and Food is responsible
for food safety for the MoH. This sub-decree No 67 clearly and unambiguously
defines the duty of the Ministry of Health to control the safety (and
wholesomeness) of the food supply.
e) Sub-Decree No. 12 (February 2002) established the Department of Industrial
Standards of Cambodia which is in charge of national standards development in
order to improve the quality of local products, including food.
4. Food Safety Administration
The system of food control in Cambodia is complex. To enhance coordination of
the inspection of quality safety of products and services, an inter-ministries
committee was established under sub-decree No 5: Establishment of an
Inter-Ministerial Committee Coordinating Inspection of Quality and Safety of
Products and Services. Another step to enhance coordination was the formation of
the Cambodia national Codex committee (CNCC) in 2001. It is the same committee
as the Inter-ministerial Committee Coordinating Inspection of Quality and Safety
of Products and Services. Eight Members have been appointed to the CNCC. The
CNCC is to consider matters related to policy on safety and quality of products
and services, consumer protection and fair trade and to ensure coordinated
action by relevant ministries. The Codex Contact Point is located within
Camcontrol (Cambodia Import Export Inspection and Fraud Repression Department)
of the Ministry of Commerce.
Significant control is exercised by the Ministry of Commerce and the Ministry of
Industry, Mines and Energy (MIME). Camcontrol's officers inspect imported foods
at the point of entry as well as foods for export. The inspectors also perform
market monitoring throughout the country. The major focus of Camcontrol's
activities is on preventing the distribution of unsafe, poor quality,
adulterated, misbranded or contaminated products, including food.
The MIME is responsible for quality control in manufacturing industries. Under
the sub-decree No. 4 (February 1992) on the Management and Quality Control of
Industrial Products of Factories and Handicrafts and its related law, every
three months, inspectors sample processed foods and undertake microbiological
and/or chemical analyses in their laboratories on a diversity of products e.g.
bottled water, rice wine, fish sauce and vinegar. Before issuing production
licenses, the results of analytical testing should be satisfactory. Where there
are no Cambodian food standards against which to interpret the results, Codex
standards are applied. A limited number of national standards have been approved
by the Industrial Standards Technical Committee.
The Ministry of Agriculture, Forestry and Fisheries has a key role in managing
the safety and quality of agricultural products as they enter the food chain.
Concerns exist that some meat entering the Phnom Penh market has not been
inspected and may have been processed at illegal slaughtering operations. There
are also concerns about movement of animals through Cambodian border
checkpoints. The Ministry has nine checkpoints to control this issue.
The Ministry of Health has responsibility for all matters of public health,
including assuring the safety and wholesomeness of food offered for sale in
Cambodia. The Department of Drugs and Food has been requested to provide the
Ministry with guidance in the creation of a fully integrated food control
structure, involving all stakeholders in the food supply and food control chain.
Implementation of the objectives of this program will help achieve the Ministry
of Health's goals of safe food for all Cambodians. Ministry of Health is the
focal point of the Asian Expert on Food Safety.
5. Food borne Disease Surveillance and Contaminant Monitoring
There are several sections with a laboratory in each of the key ministries
including the National Institute of Public Health and the Department of Drugs
and Food in MOH; fisheries, agronomy and animal health in MAFF; and laboratories
in MIME, MOC and the Ministry of the Environment. In addition there are private
laboratories such as the Pasteur Institute. There is however no coordinated
programme of food surveillance and little analytical data regarding
microbiological or chemical contamination of food.
Ministry of Health, Department of Drugs and Food, conducts monitoring of
microbial and chemical hazards in foods. In relation to chemical hazards,
analyses are limited but the Department is capable of the basic assessment of
food colours. Ministry of Commerce, Camcontrol, also conducts assessment of
microbial and chemical hazards in foods. Ministry of Industry, Mine, and Energy
also conducts assessment of microbial hazards in foods. Ministry of Agriculture,
Forestry, and Fisheries also conducts assessment of microbial hazards in foods.
The Department of Agronomy and Agricultural Land Improvement laboratories
conducts assessment of pesticide formulation and is planning to develop
pesticide residue capability. Animal health authorities have recently set up a
veterinary public health laboratory and are receiving support from Japan and
Norway for training in analytical procedures of relevance. Upcoming training
will focus on veterinary drug residues in imported meats and milk and in meat
slaughtered in slaughterhouses in Cambodia. The Pasteur Institute can also
determine antibiotic residues and evaluate the antimicrobial resistances of
isolated cultures.
Cambodia does not have an active programme of food borne disease surveillance
and outbreak response is often limited by the lack of available expertise.
6. Food Safety Education and Training
Information, education, training and advice to producers, industry, consumers
and regulators are quite variable across the different sectors of the food
chain.
Cambodian legislation does not stipulate training requirements for food
inspectors. Inspectors do not have degree level qualifications and have minimal
training in risk-based approaches to food safety. However, key Camcontrol
inspectors have received some training on quality control of food with the
assistance of FAO. The purpose of this training was to ensure Camcontrol could
effectively implement its responsibilities in relation to inspection of imported
food.
The Ministry of Health plays a leading role in relation to food safety education
for consumers. Within the Ministry of Health is the National Centre for Health
Promotion. It is actively involved in health education and food safety for the
public and for school children. Even though active the National Centre for
Health Promotion has developed and uses only limited information, education and
communication materials.
Agriculture authorities have conducted an extensive integrated pest management (IPM)
programme in 15 provinces between 1998 and 2004. This programme (supported by
DANIDA and WB) has trained 80,000 (less than 1-2% of the total farming
population) farmers in IPM rice farmer communities. Village animal health
workers conduct training as part of their regular duties.
The Ministry of Commerce has conducted several seminars and workshops at a
national level on topics related to food safety including on how Codex works. In
addition, the Ministry has a staff exchange program with other Asian countries.
However, because of the limitation of such efforts most farmers, fishermen, food
processors, food handlers and consumers are still in need of education and
training in relation to food safety. In order for food safety education and
training to have a significant impact on the safety of food there is a great
need for any programme to both be better focused and better resourced.
7. The Future for Food Control in Cambodia
Still food control activities are weak and many examples of failure to control
unsafe, fraudulent and improperly labeled food can be observed. Consequently
Cambodia’s food control authorities really need to ask how safe is the food in
Cambodia, what the burden of food borne disease is both in terms of health and
the economy of Cambodia, whether they want to better protect the population and
facilitate economic development through enhanced trade in safe food and what
they can do to improve on what is currently being done. This report looks at
current concerns in Cambodia’s food control system and identifies corrective
actions to be taken.
Globalisation opens a window of opportunity that should allow Cambodia to learn
from the mistakes of the developed countries that have spent significant time
and resources trying to get their own food control strategies right. The country
is at a point in the path to safer food where authorities can choose to either
better coordinate food control activities using a risk-based farm-to-table
approach or to make the mistakes already identified in developed countries.
These mistakes have included:
·Establishing multiple and overlapping laws, regulations and standards;
·Disagreements regarding food control management responsibilities;
·A lack of training, transparency and ethics in enforcement;
·Poorly focused enforcement activities;
·Expensive sampling and analysis of food for little risk management effect; and
·Ineffective communication, training and education.
Accession to the World Trade Organization and the signing of key agreements
could very well provide the same momentum for a view of what is being done and
what to do better as it has in other countries of the region, such as China and
Viet Nam. Grasping this opportunity could provide an important catalyst for not
only improving health but also improving the economy of the community.
In looking at how to implement an effective food control strategy focusing on
risk reduction a number of guiding principles need to be applied. The principles
include:
· Priority to the health of the consumer,
·Developing science-based food control strategies,
·Establishing initiatives, along the full farm-to table continuum, that target
both risk and economic development;
·Recognizing that food control is a shared responsibility between all
stakeholders
·Food control is best achieved by addressing all key building blocks of a
national food control system.
Cambodia needs to grasp these guiding principles and employ the building blocks
as laid out by FAO and WHO from farm-to-table in a sector-wide approach to
strengthen its food safety efforts, to better protect the health of consumers
and to enable it to find an appropriate niche in the international trading
market in food and food products.
A five-year action plan has been proposed by a Joint FAO/WHO mission to develop
the food control system in Cambodia. The action plan emphasizes the importance
of a coordinated and risk-based farm-to table approach. It provides for
strengthening all key building blocks of a food control system including:
1. Food law and regulations;
2. Food control management;
3. Inspection services;
4. Laboratory services, monitoring and surveillance; and
5. Information, education, communication and training.
Agenda Item 8 Conference Room Document 20
English only
FAO/WHO Regional Conference on Food Safety for Asia and the Pacific
Seremban, Malaysia, 24-27 May 2004
Communicating Food Safety to Consumers
(Industry Council for Development & Asian Food Information Centre)
Introduction: The Role of Communications
‘Education is the basis of effective and long lasting improvements in food
safety’ WHO 1984
The importance of food safety education in prevention of food-borne illness is
universally recognized. There is general consensus that a knowledge of food
safety provides the basis for the development of intervention strategies and
initiatives aimed at preventing food-borne illness, at all stages of the food
chain - from production of raw commodities, through processing and distribution,
right through to the point of consumption.
Of course, content and delivery modes for food safety education must be adapted
to fit the very different characteristics of multiple stakeholders involved in
this supply chain. This paper is intended to outline the rationale and
objectives, which provided direction for content and delivery modes selected for
a specific food safety communications and education initiative which targeted
consumers. It is hoped that this sharing of experience will provide some useful
insight for others with a responsibility for consumer food safety education and
communications.
Background Information
Industry Council for Development (ICD) is a private non-profit corporation
granted NGO status with WHO and liaison status with FAO. Since 1992, ICD in
association with a number of other institutions and organizations has delivered
regional cascade training programme in food safety for nutritionists and other
health professionals.
The Asian Food Information Centre (AFIC) is a not-for-profit organization whose
remit is to provide science-based, but easily understandable information on
nutrition and food safety topics to non-specialists. Specifically, AFIC works to
support the most important information-sources for some of Asia’s 3 billion
resident population, namely mass media and health practitioners and educators.
In 2002, AFIC and ICD embarked on a collaborative project to develop a resource
which would emphasize prevention of food borne illness, by providing factual
information on prevention measures in laypersons language. The project also
benefited from the technical expertise and input of the Regional Asia Pacific
Office, Nutrition Division of the UN FAO, and SEAMEO TropMed Nutrition
Institute.
The resource was intended not only to provide guidance to consumers on what
measures they could take to safeguard their own health, but also to provide
guidance on what consumers could, and indeed should expect from the multiple
elements of the food supply chain.
Rationale for Resource – Identification of an Information Gap
Consumers can play a very positive role in efforts to reduce cases of food-borne
illness, not only by observing good hygiene practices in their own food
handling, but also by expressing demand for improved food safety measures.
Strong consumer demand (i.e. ‘pull’ forces) for effective food safety measures,
provides a highly complementary force to the ‘push’ forces provided by national
and international agencies, the food industry, health professionals and others
who are working towards improved food safety standards and practices.
A number of surveys, including AFIC’s own consumer perception surveys, find that
mass media is the most common source of information on nutrition and food safety
for lay persons. For example, a survey of consumer perceptions on food safety in
2002, found mass media sources cited as preferred information source almost 50%
more frequently than all other sources (such as government departments, medical
professionals and agencies) combined.
However, content analysis of consumer information on food safety and prevention
of food borne illness available through mass media, indicates very limited
consumer education is being delivered through this channel: .
For example, qualitative analysis of media tracked in 2003 found 11% of total
media coverage focused on food-borne illness, and approximately 5% on chemical
and physical hazards. Less than 10% of these news reports include science-based
information on prevention, such as advice on the importance of hand washing,
avoiding cross-contamination between cooked and raw foods, correct temperature
control, appropriate response on recognition of food products do not meet
acceptable standards (eg what to do with products sold after sell-by-date, what
sell-by-date means) etc..
In contrast, qualitative analysis of nutrition topics reveals a very different
approach. For example, approximately 9% of total food and health media coverage
in Asia region tracked by AFIC was devoted to the topic of overweight/obesity,
and approximately 7% of total regional food and health coverage addressed the
role of diet and lifestyle in the onset and progress of non-communicable chronic
degenerative disease, such as heart disease, Type 2 diabetes, and hypertension.
More than 50% of the reports and features tracked include advice on
prevention/reducing risk exposure.
Similarly, a very large proportion of new reports on food safety do not include
any reference to actual prevalence or nature of food-borne illness cases but
approximately 20% nutrition-themed issues, provide quite detailed information on
prevalence and epidemiological trends.
Finally, reporting of strategic actions at national, regional or international
level on efforts to reduce food safety risks are noticeably less common, than
reports on public health proposals and measures to address some of the most
important nutrition and health issues in the Asia region, such as heart disease,
high blood pressure, overweight and micronutrient deficiency.
The implicit message that consumers may take from this state of affairs is that
food safety is in crisis, and furthermore, mass media provides little evidence
that substantial measures to address challenges in food safety are underway.
In the light of the focus by mass media on FBI crises, combined with the very
high prevalence of FBI (WHO estimate 1.8-3.1 billion cases of FBI will occur in
2004), it is perhaps not surprising that consumer confidence in safety and
quality if the food supply is falling. For example, an Ipsos-Reid reported in
Nature and Health magazine April/May 2002 found that the majority of adults in a
survey of 18,650 in 34 countries felt that their food was less safe than 10
years ago.
Therefore a resource, intended to fill an information gap on best practice
preventive measures, which might in turn contribute to the push-pull dynamic,
driving progress in food safety forwards, was developed.
Objectives of ‘Preventing Food-Borne Illness from Farm to Table: Highlights of
Best Practise – A Pro-Active Risk Communications Resource
The Highlights of Best Practice resource was developed as a series of short
briefing documents each addressing different stages in the farm to table supply
chain. Each section has been prepared so that it may be used alone, or in
conjunction with other sections of the document. Further additions to the basic
package or compendium are planned. For example, explaining the role of Codex,
HACCP and other measures and agencies which have responsibility for food safety.
These sections are also intended to complement communications on emerging food
safety crisis issues. Food safety crisis issues such as mass food poisoning
incidents, or detection of substances potentially harmful to human health,
nearly always evolve very rapidly, and those responsible for their
communications are required to respond under severe time pressures, incomplete
technical information and safety assessments, and in the face of public response
which is driven by many factors, some of which are unrelated to the magnitude of
risk. This phenomenon, has been described by many risk communication specialists
as the ‘risk information vacuum’ The risk information vacuum is characterised by
an absence of adequate information addressing a particular food safety concern,
leading to a collective public perception, shaped by a combination of technical
information which is poorly understood, and anecdotal comment and interpretation
influenced by extrinsic factors, ultimately resulting in misunderstanding and in
many instances, magnification of perceived risk.
The Highlights of Best Practice in Prevention of Food Safety aims to pre-empt
this scenario by providing non-technical information about preventive measures
that are intended to reduce food safety risk or exposure to risk and
counteracting some of the characteristics common in risk focused journalism. The
Best Practice resource therefore, emphasizes:
> Reassurance, rather than alarm
> Consensus rather than extreme opinion
> Fact, rather than opinion wherever possible
> Quantifiable assessment of hazard, rather than personal evaluation.
> Actionable advice and tips on prevention, rather than non-specific alarm
commentary.
Conclusion
The key message of this resource is that consumers can and should take an active
role in preventing food-borne illness, by staying informed, and actively seeking
and rewarding the best practices and standards, which are recognized to
eliminate or significantly reduce food safety risk.
Future evaluation of this resource will test response to this message.
Agenda Items 5-9 Conference Room Document 21
English only
FAO/WHO Regional Conference on Food Safety for Asia and the Pacific
Seremban, Malaysia, 24-27 May 2004
TROPICAL FRUIT: FOOD SAFETY, NUTRITION & HEALTH*
(Tropical Fruit Network)
The Asia-Oceania region is the largest producer of tropical fruits accounting
for about 66% of world output (FAO 2002). However, less then 5% of the
production is exported. Nevertheless, the sector provides substantial foreign
exchange earning with export value of fresh tropical fruits amounting to US
1.3billion while processed fruits, USD640 million (2002). The global growth
rates in production and export are also encouraging, estimated at 3.8% and 3.1%
respectively per annum.
Tropical fruits are packed with valuable nutrients: vitamins, micronutrients, phytochemicals and fibres. However, the per capita consumption of tropical
fruits is relatively low in the region, about one-third the recommended rate of
150kg/capita/year. At the same time, according to WHO, incidences of Non
Communicable Diseases (NCD), such as cardiovascular disease, cancer, diabetes
are on the increase. The region has also a fair share of malnutrition in vitamin
A and protein especially among the young which may affect the productivity of
many countries. Tropical fruits have the potential of addressing these health
issues because of their inherent nutritional values.
An integrated campaign involving the public and private sectors as well as
civil societies need to be intensified to increase consumption of tropical
fruits. In addition, more R&D needs to be undertaken to identify fruit species
with the desired nutrient contents especially, phytochemicals, and
epidemiological studies on the impact of fruit intake on disease prevention and
reduction.
On the supply side, tropical fruit requires Good Agricultural Practice (GAP), HACCP and other best practice protocols, including organic farming to ensure
safe and nutritious food from farm to table.
___________
·Presented by Khairuddin Md. Tahir, Chief Executive Officer, International
Tropical Fruits Network (TFNet).
Agenda Item 5 Conference Room Document 22
English only
FAO/WHO Regional Conference on Food Safety for Asia and the Pacific
Seremban, Malaysia, 24-27 May 2004
A Performance – Based Approach to Food Safety Regulatory Inspections
(New Zealand)
Introduction
The cost of regulatory compliance inspections is of concern to those involved
the food industry. As food safety knowledge increases and new processes are
introduced, inspections are required to be more intensive and inspectors are
required to have more technical knowledge. This results in increased costs to
processors, exporters, importers and regulators.
This paper proposes a method of using inspection resources in an efficient
manner by applying a performance-based approach to food regulatory inspections.
This approach can be used for a number of regulatory inspection activities.
The Performance – Based System
Performance – based inspection systems are those where the frequency and
intensity of inspections is based on the:
·Food safety risk involved with the product (e.g. fresh meat for human
consumption compared with processed pet food), and the
·The performance of those producing the product. Good performance is rewarded
by fewer inspections and therefore less cost, and poor performance receives
intensified inspections at greater cost. We all want to be able to confidently
manage risks and reduce costs of regulation, while ensuring consumers are
provided with the safest food possible.
Key Elements
Performance based systems must have:
·Clearly defined audit/inspection procedures and frequencies
·Clearly defined pass/fail criteria
·Meaningful and useful performance ratings.
The programme must be robust so that any actions taken based on the results of
the programme can be justified nationally and internationally. It must also
provide meaningful data on the performance of those that are audited or
inspected.
Food Safety Regulatory Applications for Performance – Based Systems
Performance – based systems can be applied to:
·Processing verification / inspections
·Import Inspection and testing
·Approval of exporter’s establishments
·Acceptance of new species/products from exporting countries
·Enforcement of new regulations on exporting countries (by providing evidence
of performance which can allow exemptions to be made for a country or
countries).
Example
Performance-based Verification (PBV) of Animal Product Processing in New Zealand
The New Zealand Food Safety Authority (NZFSA) PBV programme is an example of
a performance-based system in action. This programme was introduced in 1999
after consultation with the animal product industry. It took approximately two
years for all parties to become familiar with it, and has been subject to much
discussion. It has been revised and is working efficiently although there is
still some debate on inspection frequencies.
The programme covers the verification by NZFSA inspectors (both veterinarians
and other inspectors) of processors’ Risk Management Programmes (registered by
NZFSA) for processing animal products for human or animal consumption. The
verification also includes processors compliance with overseas market access
requirements. It is important to note that every verification audit visit is
paid for by the processor being audited.
The PBV programme is currently split into a series of verification steps
which have an associated audit frequency. Each Risk Management Programme is
allocated a verification audit step. The steps are:
Step 6 – audit every 6 months
Step 5 – audit every 3 months
Step 4 – audit every 2 months
Step 3 – audit every 6 weeks
Step 2 – audit every month
Step 1 - audit every two weeks
After 3 consecutive acceptable audit outcomes, the reward for performance is
that the processor moves up a step, for example, from Step 3 to Step 4, and
therefore is audited less frequently. If a processor fails an audit e.g. on Step
3, they move to Step 2. This increases costs and subjects the processor to more
intense audit.
There may be sanctions applied for failure at any step. However, when a
processor reaches Step 1, then the processor must provide NZFSA with a
management plan to address the fundamental failure of the processor to process
in accordance with the approved Risk Management Programme.
The development of the programme included decisions on:
·A starting audit frequency for processors – depending on risk of product
processed and compliance history.
·The number of steps and verification frequencies for each step
·The pass/fail criteria for audits
·The criteria for moving from step to step
·The actions/sanctions for reaching the minimum step.
When the programme was introduced all processors began at a frequency
consistent with their previous compliance rating. Producers of animal products
such as meat and bone meal started at a higher step, i.e. fewer audits, than
slaughter and boning operations. Some considerations/decisions were:
·That the highest audit frequency was practical – decided as one audit per 2
weeks.
·That the lowest frequency still provided adequate compliance assessment.
·The pass criteria was ‘processing was in substantial compliance with
requirements’.
In New Zealand, the ability to reduce compliance costs is a very strong
motivator, and the PBV system has contributed to the ongoing performance
improvement of New Zealand animal product processing. Also there is increased
financial and market pressure on poorer performers to improve performance, as
markets take note of the PBV step individual processors are on.
Some processors have made savings of up to 50% in compliance costs. These are
those that have taken an especially active role in managing their own
compliance, rather than relying on the regulator (NZFSA) to identify compliance
deficiencies.
So the advantages of a performance-based verification system for food processing
are:
·Reduced compliance costs for good performers. Good performers actively manage
their own compliance, with the result being fewer non-compliances at regulatory
inspection. This is important, as it is this that improves the safety and
quality of food.
·Poor performers can be targeted and there is factual evidence of this and so
any sanctions can be justified.
·Improved performance by processors will ultimately improve food safety and
quality for both domestic consumers and consumers in export countries.
Other Possible Applications of the Performance-based Approach
Import Inspection and Testing
Initial inspection frequencies can be based on previous country import
inspection history. Benefits are:
·The regulatory focus is on countries of highest food safety risk, which will
receive increased inspections on product from those countries
·Importer costs are reduced if importing from good performing countries,
because of fewer regulatory inspections
·Importers move towards importing from good performing countries because
inspection costs are less
·Fewer import inspections means the flow of product from good performing
countries can be quicker through ports, and therefore reach markets in a fresher
state.
·Importing countries can ultimately apply very low frequency inspections or no
inspection, if the confidence in a product from a country, or all product from
that country, is of good quality and considered safe (this can be managed by
customer response).
·Importing countries ultimately provide safer food to their consumers using
this approach.
Approval of Establishments in Exporting Countries
When an importing country approves approving establishments in exporting
countries, consideration should be given to the performance of the competent
authority of the exporting country.
A rating for exporting countries applied by importing countries can be
applied retrospectively applied based on previous country inspection records.
Any performance-based system should also include import compliance history when
deciding starting frequencies. A performance-based system used for this purpose
could be linked to the results of a performance-based system used for import
inspection and testing.
This system can provide evidence, i.e. a good rating, for the importing
country to be able to approve establishments and new processes for export to
their country, without the need for an inspection visit specifically for that
purpose. Alternatively, it can provide evidence that an inspection visit is
needed.
Inspection visits of importing countries will always occur. However there is
a cost saving to both countries if these can be limited and if they are not
required for every new establishment or every new species/product. Any country
inspection should always audit a representative sample of establishments, and
use this as a basis of acceptance of all similar establishments within the
country. The inspection should also assess the effectiveness of the regulatory
system. Is it adequate?
Advantages of using a performance based system for this purpose:
·The inspection resources of an importing country can be best directed towards
those countries of higher import risk.
·It allows an importing country to make quick, justifiable decisions on
approvals.
·Performance –based ratings can be used to decide whether exporting countries
should be required to apply new regulations introduced by an importing country.
Regulations that are designed to prevent inferior quality food from entering a
country are often based on the controls required to prevent import of inferior
product from the poorest performing countries.
Sometimes proposed regulations do not allow discretion for exempting good
performing countries from the requirements of the regulations. Once these
regulations become law, it can be difficult or impossible for an importing
country to exempt a country from them, or to accept an equivalent system. The
use of performance – based ratings provide performance data that can be useful
in deciding on country exemptions from regulations or requirements.
Summary/Conclusion
The application of a performance-based approach to food safety regulatory
inspections can:
·Focus resources on high risk poor performers
·Detect more non-compliant product
·Reduce compliance costs
·Reward good exporters/countries
·Reduce importers inspection costs
·Increase imports from compliant countries
·Improve product flow through ports
·Provide more confidence in quality of domestic and imported food and therefore
provide safer food for consumers.
Agenda Item 8 Conference Room Document 24
English only
FAO/WHO Regional Conference on Food Safety for Asia and the Pacific
Seremban, Malaysia, 24-27 May 2004
INTEGRATED FOOD SAFETY SYSTEM IN INDONESIA
(Republic of Indonesia)
1. INTRODUCTION
Since 1998, a team of Indonesian and Australian specialists has been funded
by the Indonesian Government, the Australian Agency for International
Development (AusAID) and the Australian Government Analytical Laboratories (AGAL)
to improve food safety in Indonesia and through this improve public health and
increase trade. The team has developed and introduced a national Integrated Food
Safety System (IFSS). The IFSS is based on the WHO document “Guidelines for
Strengthening National Food Safety Programmes”. By identifying key stakeholders
and the responsibilities of the National Agency for Drug and Food Control (NADFC),
Republic of Indonesia it was possible to map the Indonesian system against the
WHO model. Three functional stakeholder groups were identified and the team
realized that the WHO model could be refined to better fit with Indonesia’s
existing system and at the same time reflect risk analysis principles. The
resultant National Integrated Food Safety System model implements risk analysis
principles on a national level through three networks: (1) Food Intelligence
Network (Risk assessment), (2) Food Control Network (Risk management), and (3)
Food Promotion Network (Risk communication). The National Integrated Food Safety
System was officially launched by Coordinator Minister for People’s Welfares at
the NADFC in Jakarta on May 13, 2004.
2. THE NATIONAL IFSS AND ITS NETWORKS
The National Integrated Food Safety System consists of three networks as
follows:
·Food Intelligence Network - Risk assessment. This brings together agencies
involved with foodborne disease monitoring, food surveillance and food
assessment including government, industry, academia and consumers.
·Food Control Network - Risk management. This brings together agencies involved
with administration of food law, the inspectorate and analysis of food.
·Food Promotion Network - Risk communication. This brings together government,
industry and consumers to communicate on food safety issues.
The networks enable improved communication between stakeholders, provide for
greater knowledge sharing and build food safety capital at local, regional and
national levels. To prevent each network from working in isolation, projects
were required to synergise and focus food safety activities. Diagram below shows
how the three networks link each other under the National IFFS.

The National Integrated Food Safety System
As shown in the diagram above, three sub-programs were developed to put
policy into practice at national, provincial and local levels. These programs
are:
·Food Watch - a national food monitoring program that:
o uses analytical results to identify food safety problems,
o works with key stakeholders to find practical solutions and
o produces user-friendly reports/information for industry to improve practices
·Food Stars - a voluntary award program that introduces three levels of food
safety training across all industry sectors from paddock to plate. The levels
are as follows:
o one star - basic hygiene training,
o two star - good manufacturing practice/ good agricultural practice etc
depending on the industry sector
o three star - implementation of a food safety program based on hazard analysis
critical control point principles (HACCP)
·Rapid Response - a future program to enable effective communication between
agencies during times of crisis and also includes strategies for food recalls
etc.
The revised model provided the basis for the IFSS program logo (See Logo below).
The IFSS is now gaining momentum as more government agencies are becoming
involved. The team has developed the first versions of the Food Watch and Food
Stars programs and are now trialling them.
Food Watch
A food surveillance system relies on the quality of information in it. If
laboratory results are suspect there can be no confidence in a food monitoring
program. Over the years thousands of food laboratory technicians (from
government and private laboratories) have been trained as part of this project.
A food reference laboratory infrastructure is currently being established to
support the accredited laboratories and to provide more confidence and rigor to
laboratory results.
The team has also worked hard to make the most of available information. For
example, by collating results of routine food testing it has been possible to
produce two draft Food Watch reports in Indonesia. The Food Watch reports looked
at hazardous chemicals in foods and also drinking water. The aim of the Food
Watch reports is to turn laboratory results into improved food safety practices.
The reports present results, identify where the major problems are and
provide solutions to improve compliance with standards. The Food Watch reports
can be used by food inspectors and sanitation officers to help raise awareness
and provide education to break the cycle of ignorance. The reports do more than
say "don't do this" they explain how to do things safely in a practical way.
The first draft Food Watch report explained the problems with using illegal
hazardous chemicals in food. For example,
·borax is sometimes used by street food vendors as a preservative or to improve
the texture of certain foods especially bakso (a boiled meatball).
·formalin (formaldehyde in water) is sometimes added to wet noodle or tofu as
preservative.
·rhodamine B – a prohibited red colouring, is sometimes used to colour certain
drinks.
The report explained the dangers of using these chemicals and explained which
additives could legally be used with pictures to show how to measure them
accurately e.g. 1g/kg = one teaspoon per 5kg.
The second report looked at drinking water. There are two grades of water
available for sale. Clean water is used for cleaning and drinking water is used
for drinking or cooking. Many businesses treat their water on site, that’s why
it is important to include water in the food safety program. The drinking water
report showed that all the commercially bottled water was safe to drink. In
Indonesia shop-front ‘drinking water depots’ are popular. People bring their 19L
water bottles (on motor bikes) to be refilled at the depots. Some of the samples
tested contained small numbers of E. coli bacteria so a simple food safety chart
was developed (with photographs) to help businesses identify hazards, assess
risks and make changes. A business can use the information from the Food Watch
report to construct a basic food safety program.
Food Stars
The Food Stars program is all about introducing HACCP-based food safety
programs from farm to table throughout Indonesia. Government departments are
supporting the three-stage Food Stars approach, which has been developed by a
team from the Australian Government Analytical Laboratories, National Agency for
Drug and Food Control, Ministry of Industry and Trade, Ministry of Agriculture
and the Institut Pertanian Bogor (Bogor Agriculture University). The Australian
team members provided a catalyst to bring everyone together. They used their
experience to facilitate workshops, helping the team to work through problems
and find practical solutions.
HACCP cannot be introduced in one step; therefore, the voluntary “Star Award
System” takes three food safety steps to reach HACCP with achievements being
acknowledged on the way.
·The One Star Award provides basic food safety training to everyone in the
business. (This involves one day training for proprietors, who go back to their
business and overview in-house training of all staff. This is assessed by a
trained food safety officer.)
·The Two Star Award is aligned with Codex principles of food hygiene –
developing good food safety practices ie procedures and worksheets to support
cleaning, sanitation, pest control, hygiene, deliveries, water quality, food
storage etc (This involves one day training for 1-2 from business, documents and
practices audited by trained food safety officer from provincial government)
·The Three Star Award is HACCP! (This requires attending a five day accredited
course, implementing a program in the business and having it audited by a
suitably trained food safety auditor.) After achieving the Three Star Award a
business will be well-placed to implement an internationally accredited quality
system if it chooses to.
In the last year, officers from the National Agency for Drug and Food Control
have visited every province providing professional development training for over
1200 food inspectors which included socialising the IFSS and introducing the
Food Watch and Food Stars Programs. The Food Stars Award has been piloted in
four provinces and materials finalised for the One Star and Two Star levels for
the food manufacturing sector. Materials have also been drafted for the Food
Service sector.
Where next for the IFSS?
The Integrated Food Safety System is not a quick fix. It is all about
providing a safe food supply by making the most of what is available. By
communicating with each other, sharing information and working towards the
common goal everyone is working together to improve food safety in Indonesia.
Future initiatives will include:
·Producing materials to train food safety auditors in the practical aspects of
auditing small to medium sized food businesses. Many food safety officers know
the theory, but have very little practical experience.
·Developing Food Stars materials suitable for the Agriculture sector
·Developing the Rapid Response team communication strategy to synergise
resources across governments during national emergencies
·Co-ordinating national food monitoring surveys, targeting areas of concern
e.g. pesticide residues in food so that information from Food Watch reports may
be developed for industry to improve practices
·Strengthening the laboratory network to support the Food Watch program.
The Integrated Food Safety System provides the national framework for food
safety – it transcends government departments, academia, industry and consumers
and enables them to work together to maximise resources and improve food safety
Indonesia.
Agenda Item 5 Conference Room Document 26
English only
FAO/WHO Regional Conference on Food Safety for Asia and the Pacific
Seremban, Malaysia 24 – 27 May 2004
Food Safety Legislation – Science and Risk Based Approaches
to Harmonization
(Sri Lanka)
1. INTRODUCTION
All countries should have an effective Food Control Administration System to
ensure that the food reaching the consumers is safe from farm to the fork. Food
legislation is one of the important tools to ensure that not only the food is
safe, but also honestly presented. If an organized food control system is not
effectively supported by a regulatory system, deceptive practices in the food
trade are bound to take place.
Considering the implications arising out of WTO negotiations and the prescribed
SPS measures under the Agreement, all member countries are expected to harmonize
their legislation taking into account of the Codex guidelines and model
legislation as reference. This would also facilitate international trade in
food. Provision is also made to deviate from such guidelines and models with
justification, depending on the national requirements.
In the light of the above background Sri Lanka found it necessary to review the
current Food Act No. 26 of 1980 and its subsequent amendment and several
regulations published under the Food Act and also to draft a number of new
regulations relating to food safety areas that have not been covered earlier.
Sri Lanka is also is actively collaborating with the FAO in the current process
of harmonization of Food Regulations in the SAARC Region.
2. HARMONIZATION
Harmonizations of Food Regulations in the SAARC countries were initiated in 1998
by the FAO in collaboration with ILSI-India and assisted the countries of the
region in their effort modernize the food control systems and activities. A
series of seminars were conducted in which members of the Food Control
Administration systems in the SAARC countries participated and exchanged useful
information.
Simultaneously Sri Lanka initiated action to revise / review the existing Food
Regulations in keeping with the guidelines of the Codex Alimentarius Commission
and other texts to ensure that a set of science-based, risk-based regulations
are in place to strengthen the country’s regulatory system with the assistance
of a national consultant and prepared a complete set of draft regulations under
the following titles:
1. Food (Hygiene) Regulations;
2. Food (Additives) Regulations;
3. Food (Standards – Milk & Milk Products) Regulations;
4. Food (Registration of Premises) Regulations;
5. Food (Registration of Bottled Water Processing Premises) Regulations;
6. Food (Standards – Herbs & Spices) Regulations;
7. Food (Powers & Duties of Authorized Officers) Regulations;
8. Food (Labelling & Advertising) Regulations;
9. Food (Packaging Materials & Articles) Regulations;
10. Food (Irradiation) Regulations;
11. Food (Iodization of Salt) Regulations;
12. Food (Cereals, Pulses & Legumes) Regulations;
13. Food (Sugars & Sugar Products) Regulations;
14. Food (Sweeteners) Regulations;
15. Food (Fish & Fish Products) Regulations;
16. Food (Meat & Meat Products) Regulations;
17. Food (Fruits & Vegetables) Regulations;
18. Food (Fats & Oils) Regulations;
19. Food (Preparation & Distribution of Cooked Food) Regulations;
20. Food (Control of Import of Food & Sale) Regulations;
2.2 SCIENCE-BASED, RISK-BASED APPROACH
It has been emphasized that a risk-based approach should include Risk Analysis,
Risk Characterization, Risk communication and Risk Management. Even though
capacity building within the national food control system should be enhanced to
cope up with the prescribed trends, attention was paid to enshrine the
principles within the Food Laws. Action is increasingly being taken to publish
all existing food laws and finalized draft laws in the web (http://www.health.gov.lk/).
Seminars and workshops are being held to all the stake-holders – regulatory
officials, consumers, food industries, food trade and other interested groups.
Feed-backs from the participants are discussed at the Food Advisory Committee
level before being published in the form of official documents. This process not
only ensures transparency, but also provides valuable information leading to
several amendments.
One example is the food hygiene regulations relating to food handling/catering
establishments in the country. During a discussion on the draft Food Hygiene
Regulations it transpired that catering establishments – particularly the medium
and small type restaurants are established on ad-hoc basis and attempts are
subsequently to regularize. Most of these establishments did not conform to the
required hygienic standards under the regulations and it becomes virtually
impossible for the regulators to bring them into conformity. The regulation
itself did not have any pre-establishment requirements. As a result of this feed
back, the Food (Hygiene) Regulations are being re-drafted to ensure that all new
establishments obtained prior approval / registration and they should satisfy
conditions stipulated in the regulations before commencement.
2.3 TRANSPARENCY
Transparency is the is the key objective of the risk analysis approach and its
importance cannot be over-emphasized as reflected in the Codex Statement of
Principles relating to Food Safety Risk Assessment, the Codex Committee on Food
Hygiene (CCFH).
Applying these principles it is envisaged to appoint a permanent committee for
risk assessment of imported food as well as food in the local market and to
prescribe measures for sampling, analysis, characterization and communication.
The risk management part would be undertaken by the Food Control Administration
Unit under the guidance of the Chief Food Authority. All identified risks would
be communicated to all stake-holders, nationally and internationally through
electronic and printed media and also through publication in the Health Ministry
Website: http://www.health.gov.lk/ A quarterly “Food Safety News” bulletin is
being published from the Food Control Administration Unit with the WHO
assistance.
3. ISSUES AND CHALLENGES
There is an acute shortage of properly trained personnel – particularly Food
Inspectors, Analytical Chemists and Food Microbiologists. Technical Assistance
and Capacity Building pledged by the FAO and WTO have not
materialized so far.
Centers of Excellence for further training of these officials have not yet been
identified. There is a considerable delay is publishing food regulations on
account of the need for publishing them in three languages and non-availability
of competent translators in the country. There is also a need for more
provincial food laboratories. There is no programme of regular pesticide
residues monitoring in the country. This is one area where capacity building and
Technical Assistance is required.
4. CONCLUSION
In Sri Lanka there is a Food Control System under the Ministry of Healthcare,
Nutrition and Uva Wellassa Development. Director General of Health Services is
the Chief Food Authority and also the Chairman of the Food Advisory Committee (FAC)
established in terms of the Food Act No. 26 of 1980. The FAC is comprised of 19
members. They represent various stake-holders in food safety from Government
Departments / Ministries as well as trade and consumers. There is also a Food
Advisory Technical Sub Committee that deliberates on issues referred to it on a
regular basis. The main function of the FAC is to advise the Minister in charge
of the subject of health on food safety policy matters. The Food Control
Administration Unit is in charge of the general administration of regulatory and
training activities of the country. There are over 1700 Public Health
Inspectors, 256 Medical Officers of Health, 44 Food & Drugs Inspectors who are
Authorized Officers under the Food Act implementing the provisions of the Food
Act and Regulations published under the Act. A food-borne diseases surveillance
mechanism is also in place.
There are five approved food laboratories for chemical analysis and one
microbiological laboratory in the country. Action has been initiated to
establish a pesticide residue surveillance system.
Publication of regulations is under way and science-based, risk-based system is
enshrined in the revised regulations. A codex contact point and an SPS enquiry
point had been established in the Food Control Administration Unit. The
anticipated Technical Assistance/ Capacity Building have not materialized so
far.
Agenda Item 9 Conference Room Document 28
English only
FAO/WHO Regional Conference on Food Safety for Asia and the Pacific
Seremban, Malaysia, 24-27 May 2004
EXAMPLES OF SINGAPORE’S INFECTIOUS DISEASE SURVEILLANCE
AND INFORMATION SHARING PLATFORMS
(Singapore)
FOOD SAFETY OVERVIEW
Food safety and the control of food-borne diseases are integral components of
public health. Despite heavy reliance on food imports, Singaporeans today enjoy
a food supply that stands as one of the safest in the world. This safeguarding
of public health against outbreaks of food-borne disease is the responsibility
of the Ministry of Health working in concert with two agencies, the Agri-Food
and Veterinary Authority (AVA) under the Minister for National Development and
the National Environment Agency (NEA) under the Ministry of the Environment.
FOOD SAFETY POLICY AND LEGISLATION
The food safety and hygiene legislation includes the Sale of Food Act, Food
Regulations, the Environmental Public Health Act, the Environmental Public
Health (Food Hygiene) Regulations; the Animals and Birds Act; the
Slaughter-houses and Meat Processing Factories Act; the Control of Plants Act;
the Wholesome Meat and Fish Act; the Fisheries Act; the Endangered Species
(Import and Export) Act; the Feeding Stuffs Act; and the Agri-Food and
Veterinary Authority Act. These laws provide for high standards of food safety
and hygiene, and are administered through the AVA and NEA.
FOOD SAFETY ADMINISTRATION
AVA is responsible for ensuring a resilient supply of safe food. It regulates
the safety of food from production, import to just prior to retail. The agency
also sets and enforces food safety standards and oversees food labelling. It
adopts an integrated food safety management system to ensure that imported and
locally produced foods are safe for human consumption. Specific programmes exist
for the inspection of imported food, imported livestock, local poultry and pig
slaughter-houses, local food processing establishments, cold stores as well as
local farms.
Import control in Singapore is an important dimension of the food safety system
as more than 90% of the nation’s food is imported. Sources supplying to
Singapore must meet the import requirements for food safety. AVA accredits
overseas establishments that export meat, meat products, livestock and eggs to
Singapore to ensure that requirements for biosecurity, freedom from diseases and
food safety standards are met. It also has in place inspection and sampling
programmes for food imported into Singapore.
AVA licenses local poultry and pig slaughter-houses, food processing
establishments, cold-stores and farms and undertakes regular inspection of these
premises to ensure the safety of the food produced and manufactured. It is also
responsible for the issue of export health certificates for local food
processing establishments to export their products.
NEA’s primary role is to ensure that all food sold at food retail outlets are
prepared hygienically and are safe for consumption. It takes a pro-active
approach in ensuring good food hygiene at the retail food outlets, and has
implemented several programmes to achieve this over the years. To ensure that
food handlers appreciate and practice good food and personal hygiene, any person
wishing to work in the retail food trade as a food handler is required to pass
the Basic Food Hygiene Course and also be inoculated against typhoid and be
screened for TB if they are over 45 years of age. In order to ensure compliance
of good food and personal hygiene practices, good housekeeping and cleanliness
of food premises, a Points Demerit System (PDS) was put in place whereby demerit
points are given to licensees for offences committed under the regulations. The
food retail outlets will be liable for suspension for up to two weeks if the
maximum points allowed are exceeded.
The Food Grading Scheme was introduced in 1997 for these outlets to be appraised
in a more structured way and to motivate the licensees to improve on their
hygiene practices. NEA officers conduct routine checks on the food outlets, the
frequency of which depends on the grading, with premises having poorer grading
being checked more often. Since the scheme started, the number of food retail
outlets with better grades had increased steadily. A programme has also started
recently to encourage school canteens and food courts to achieve “A” grade
status by training their food vendors to maintain an in-house food hygiene
monitoring system.
To further raise the standards of hygiene and sanitation of restaurants and food
caterers, the Food Hygiene Officer (FHO) Scheme was introduced in 2001 where
suitable personnel such as sous chef or chief cook etc are trained as FHOs so
that they have the knowledge and competence to audit the hygiene of food
operations in their own establishments daily. This self-regulation by the food
operators is complementary to the routine audits conducted by NEA officers.
FOOD SAFETY TRAINING AND EDUCATION
Singapore recognises that total food safety management is a continuum from farm
to fork requiring a tri-partite strategy, i.e. the combined effort of the
government, food industry and consumers.
The food industry is responsible for the safety of food it offers to the
consumer. AVA collaborates both with the local food industry to encourage the
adoption of HACCP systems and local farms to adopt Good Agricultural Practices.
The agency has initiated a Food Safety Partnership Scheme in 2003 to give
recognition to local food industry players with commendable food safety
assurance and food safety consumer education efforts in Singapore.
Consumers are responsible for carrying out precautionary measures to reduce the
risk of food-borne illnesses. AVA started its Food Safety Awareness Programme in
2002 to educate consumers on food safety. The programme targets consumers
through road shows and the distribution of food safety information through
printed collaterals, advertorials and via the AVA website.
At the food retail end, NEA works intimately with its partners such as the
hotel, restaurant and coffee shop associations to upgrade the hygiene and
sanitation of food establishments. This is done through regular dialogues,
seminars and participation in Committee workgroups.
FOOD-BORNE DISEASE SURVEILLANCE AND CONTAMINANT MONITORING
Singapore has a well-established epidemiological surveillance system for
food-borne diseases. Hospitals and general practitioners encountering patients
with cholera, enteric fevers and viral hepatitis infections are required legally
to notify the Ministry of Health. The Ministry is also administratively notified
of cases of salmonellosis, shigellosis, campylobacteriosis, listeriosis and
Vibrio parahaemolyticus infections. Diarrhoeal disease surveillance is carried
out based on weekly returns from the polyclinics. In addition, an enteric fever
carrier registry is maintained, documenting all carriers since 1974.
The food contaminant monitoring system is backed by the testing capabilities of
AVA’s Veterinary Public Health Laboratory (VPHL). The VPHL offers a
comprehensive range of analytical services covering a wide spectrum of chemical
and microbiological hazards. Its capabilities include tests for disease, food
poisoning and spoilage organisms, harmful chemicals and toxins. VPHL is also the
national reference laboratory for pesticide residues. The laboratory uses
internationally recognized procedures and standards, as well as state-of-the-art
technology to provide multidisciplinary laboratory testing services. Test
capabilities are continuously developed to keep pace with the challenges of, and
to maintain vigilance against, newly-emerging chemical and microbiological
hazards that may be present in food. The laboratory practices a rigorous quality
assurance programme and participates actively in inter-laboratory proficiency
testing schemes. It is accredited under ISO17025.
Agenda Item 9 Conference Room Document 29
English only
FAO/WHO Regional Conference on Food Safety for Asia and Pacific
Seremban, Malaysia, 24-27 May 2004
The National Surveillance System for Foodborne Disease in China
(People’s Republic of China)
First of all, we would like to thank FAO/WHO supporting the Regional Conference
on Food Safety for Asia and Pacific, and have the agenda on foodborne disease
monitoring and surveillance systems. Thanks Malaysia for preparing the document
and made the good suggestions.
Now, we appreciate to share some information with you, about the national
foodborne diseases surveillance system in China
·The report system
There two report systems for foodborne diseases separately in China.
1. Infectious diseases: Aaccording to “The Peoples Republic of China Law for the
Infectious Diseases Prevention and Cure”, ccholera, virus hepatitis, bacterial
and ameba diarrhea, typhoid and paratyphoid, and other infectious diarrhea, must
be reported.
4. Food poisoning events: According to “ The Peoples Republic of China Law for
Food Hygiene” and “The Management Means for the Food Poisoning”, the important
food poisoning events, including the more than 30 cases, or more than 1 death in
an outbreak, or happened in the school, must be directly reported to the
Ministry of Health. The general outbreaks could be reported to the local health
agency.
In 2003, the reported events are significant more than those during the past
years. Other than the events fact, it is important that The State Department
issued “The Byelaw for the Meet an Emergency Outburst Public Health Happening”
during the SARS prevalence. The situation of fail to report food poisoning is
getting better, and the media made the strong support.
·National Foodborne Monitoring and Surveillance System
In 2001, the National Foodborne Monitoring and Surveillance System have
established. There are more than 50 surveillance points among 13 provinces in
China, which covered about 643 millions population, as 50.8% of total.
According to the EU model WHO recommended, the data of acute foodborne diseases
were collected from 1992 to 2001. It was found that the microbial foodborne
disease was at the first position, and then followed by chemical foodborne
disease. There were 31.1% of food poisoning caused by Vibrio parahemelitics and
17.9% of caused by Salmonella spp. The most dangerous foods are meat and seafood
products.
·Microbial Risk Assessment
- The rapid examination methods (PCR) and the trace to the source techniques for
Salmonella, E.coli, Vibrio parahemelitics and Listeria were established in our
laboratory.
- The quantitative detecting inspection for Salmonella in poultry and eggs,
Vibrio parahemelitics in oyster were carrying out.
- The consumption assessment model of shelled eggs in Chinese population, the
transmit assessment model of foodborne pathogen from fly to human, exposure
assessment model for raw oyster in the littoral areas, were established at the
research stage.
- After the 36th CCFH, the studies on the Enterbacter sakazakii in the infant
formulas are going on in China.
·Comments to FAO/WHO
1. The prevention and control of foodborne disease is the main objective to
protect consumers, which is more scientific and difficult than the food
inspection and control. It is necessary to have another consultation meeting for
foodborne disease control, and invite some experts not only food safety area,
but also the epidemiologist, clinical medicine, veterinary, educationist,
economist and sociologist.
2. We support FAO and WHO will try to establish the globe foodborne disease
surveillance network, and to share the information. Meanwhile, the food
terrorist and biosafety are the focus problems worldwide recent years. How to
resolve this antinomy? We suggest FAO/WHO to consider and have a consultation
meeting, and to ensure the nations safety.
关于婴幼儿奶粉配方中的坂歧肠杆菌的研讨会
内容概要
2004年5月26日,马来西亚芙蓉
2004年5月26日,在马来西亚芙蓉组织了关于婴幼儿奶粉配方中的坂歧肠杆菌(Enterobacter sakazakii)的研讨会,作为粮农组织/世卫组织亚洲及太平洋区域食品安全会议的一项卫星活动。2004年2月份在瑞士日内瓦举行粮农组织/世卫组织关于婴幼儿奶粉配方中的坂歧肠杆菌和其他微生物的联合研讨会之后,本次会议具有充分的重要性。研讨会认识到必须加深对坂歧肠杆菌及其后果的认识和意识。
关于婴幼儿奶粉配方中的坂歧肠杆菌的研讨会的总目标是通过促进交流以科学为依据的信息,提高对坂歧肠杆菌的认识,增强与微生物风险评估有关的能力建设活动,分享有关当前研究的信息,确定未来研究的数据。
来自业界、学术界和国际组织的科学家应邀在研讨会上讲话。来自亚太区域管理机构和卫生部门的总共100名与会者出席了半天的活动。讨论的主题包括关于婴幼儿食品受坂歧肠杆菌污染的现有知识和问题,尽量减少在制造婴幼儿配方食品时受坂歧肠杆菌污染,防止婴幼儿配方食品制备过程中受到坂歧肠杆菌的交叉污染以及关于坂歧肠杆菌的当前工作及未来的研究需要。
坂歧肠杆菌情况介绍
新西兰纽西兰乳品公司的Sally Hasell博士首先在研讨会上发言,总体介绍了这种微生物的特征。人们已知,坂歧肠杆菌是一种机会致病菌,可能造成所有年龄组的疾病。在健康成人和儿童中,感染情况不常见,但低出生体重、早产和免疫受损婴幼儿比较容易受到与坂歧肠杆菌有关的感染。
坂歧肠杆菌普遍见于环境和食品中,食物中发现该细菌时,土壤、水和蔬菜很可能是其主要来源。坂歧肠杆菌与食物中往往出现的其他密切相关的细菌难以区分。目前,还没有专门认证的检测坂歧肠杆菌的方法。最常用的方法是国际标准化组织和联邦药管局记录的那些方法。
该细菌能在6-47oC之间生长。一项新的研究表明高于70oC时该细菌迅速灭活。一项研究还表明该细菌能够在干燥环境中很好地存活。
最近有风险婴幼儿中爆发严重的疾病如脑膜炎,并与婴幼儿配方食品的消费有联系,尽管这些产品通常符合当前的食典委婴幼儿食品卫生行为规范的微生物要求。
关于婴幼儿食品受坂歧肠杆菌污染的现有知识和问题
婴幼儿奶粉配方受到坂歧肠杆菌和沙门氏菌污染与婴幼儿爆发严重疾病有关,其中包括严重的发育后遗症和死亡。来自世界卫生组织(瑞士)的Peter Ben Embarek博士指出,新生儿,尤其是早产儿、低出生体重婴儿和免疫受损婴儿受坂歧肠杆菌感染的风险最大。
当前的食典标准是许多年前确立的,需要根据新的发展变化和知识进行审查。建议对当前食典准则的修改包括使用肠细菌(Enterobacteriaciae)取代大肠菌(coliforms)作为指标,增加对肠细菌的限制,并对不同年龄组采用不同的水平。
婴幼儿奶粉配方中的低度坂歧肠杆菌污染被认为是一种严重的风险因素。然而,根据初步风险评估,在制备点包括一个致病菌控制步骤和缩短保持和喂养时间将有效降低风险。
鼓励食品行业采取有关步骤,尽量减少坂歧肠杆菌在加工环境及婴幼儿奶粉配方中的含量和传播。还建议业界为高风险婴幼儿提供更多的经过商业消毒的替代性配方产品。
尽量减少婴幼儿配方食品加工过程中受坂歧肠杆菌的污染—业界的看法
瑞士雀巢公司的Anthony Huggett博士指出,业界消除婴幼儿配方食品中沙门氏菌的经验为管理坂歧肠杆菌提供了见解。虽然目前不可能完全从加工环境中消除肠细菌,但可能大大降低肠细菌包括坂歧肠杆菌在婴幼儿配方食品中的含量。
鉴于婴幼儿配方食品的加工包括潮湿和干燥步骤,指出在热处理之后添加成分是可能的污染源。重要的是这些成分在添加到产品之前必须消毒。加工厂的干洗也表明在减少坂歧肠杆菌和其他细菌在最终产品中的存在有着积极的结果。
只有通过采取一系列综合补充预防措施,包括限制坂歧肠杆菌在婴幼儿配方食品中的存在和确保使用产品的方法防止在婴幼儿喂食之前坂歧肠杆菌在配方产品中的出现或生长,才能有效地减轻坂歧肠杆菌产生的风险。这可能通过提供关于婴幼儿配方食品的使用和制备的明确说明和提供教育照料者的手段来实现。
防止婴幼儿配方食品准备过程中坂歧肠杆菌的交叉污染
2001年4月,美国田纳西州新生儿加护病房一名早产婴儿死亡与坂歧肠杆菌有关。这导致疾病控制与预防中心进行调查,结果是对医院在婴幼儿配方食品的准备、储藏和喂食方面进行了政策改革。
泰国Mead Johnson营养品公司的Michael
Stein先生指出,到目前为止,没有专门提供的关于婴幼儿配方食品喂养方法的准则。疾病控制与预防中心的建议是根据营养需要挑选配方产品,而且如有可能使用取代婴幼儿粉末产品的替代品。还应由受过训练的人员使用无菌技术在指定的配制室配制婴幼儿配方奶粉制品。应遵循制造商的说明,产品应立即冷冻或如果在配制后24小时内没有食用应废弃。最后,连续肠内营养的喂食或“滞留时间”应不超过4小时。
还应向保健提供者提供参考文件以加强其意识。
关于坂歧肠杆菌当前的工作和未来的研究需要
印度尼西亚Bogor农业大学的Sri Estuningsih博士介绍了以下情况,即目前人们很少了解坂歧肠杆菌在婴幼儿配方奶粉制品中的生物学、病理毒性因素、致病性以及流行性。也没有关于坂歧肠杆菌在发展中国家现有的婴幼儿配方奶粉制品中的普遍性的可靠数据。
重要的是通过适当的报告和调查感染来源和载体,确定坂歧肠杆菌在发展中国家婴幼儿食品中的普遍性。与此同时,应对坂歧肠杆菌的致病性和毒性因素进行研究,增加对这些细菌、尤其是婴幼儿配方食品中的这些细菌的了解。发展具体而迅速的微生物学和分子学检测方法以及对各种方法的认证研究,应考虑到发展中国家的现状。
研讨会最后举行了一次讨论会议,与会者交流了各国在坂歧肠杆菌数据收集和检测方面的技术制约因素和资源限制。不妨探讨在学术界和业界科学家之间进行合作,对商业化婴幼儿食品的微生物污染进行调查和核查。关于儿童医院的卫生方法和对坂歧肠杆菌的影响的研究也可能成为考虑的主题。对母亲和与婴幼儿直接接触的其他照料者进行有关婴幼儿配方食品的配制、使用和处理的公众教育,以及对减少与坂歧肠杆菌有关的感染等也都具有重要性。
消费者国际亚太办事处在粮农组织/世卫组织亚洲及太平洋区域
食品安全会议上组织的
关于街头销售食品的安全的会外活动
2004年5月26日09:00-12:00时,马来西亚芙蓉
为了支持这次会议确定亚太区域促进食品安全的实际行动和提出能力建设建议的目的,消费者国际亚太办事处组织了关于街头销售食品的安全的一次会外活动。来自亚洲及太平洋区域国家的大约100名政府官员出席了这次半天的会议。
消费者国际亚太办事处(马来西亚)区域主任Sothi Rachagan博士在其开幕词中简要介绍了消费者国际的食品计划工作,尤其是关于街头销售食品安全的项目。Sothi博士强调了街头食品对城市地区尤其是对城市贫民的粮食安全和就业的根本性,会上还提到了街头食品在保存和促进地方和传统食品方面的作用。他还概述了与街头销售食品带来的健康风险有关的消费者关注。
公民、消费者和公民行动组研究员Kavitha Anand女士扼要介绍了该行动组未来的干预行动计划。计划包括游说市议员将街头食品销售纳入地方城市规划和建立模范销售设施并培训食品处理人员。
食品科学技术促进基金(菲律宾)总裁Sonia de
Leon博士介绍了地方组织参加街头食品部门的工作。他列举的事例包括:食品安全培训研究所向食品处理人员和从业人员提供培训和干预行动计划;Wintex技术公司提供食品安全方面的创新和实用模型;食品培训所提供有关食品安全、质量和加工的咨询和课程。
代表越南胡志明市营养中心的一位医生Ngo Thi Phi
Yen博士介绍了改善胡志明市街头食品销售的食品安全状况的一项拟议计划。该计划的第一步涉及选择一个地点,为其设计一个街头食品销售模型。第二步是注重加强消费者、销售者和管理人员(政府)的食品安全知识,建立一个合理的管理系统。第三步是对该模型进行评价,以确定是否应当在其他地区推广。
联合国粮食及农业组织(粮农组织)的准专业官员Londa VanderWal女士概述了粮农组织/世卫组织今后为改进街头食品安全拟采取的举措。其中包括与市政当局合作,改善街头食品制备和销售条件;加强食品质量管理能力,改进销售者所使用的生食和加工食品的全面质量;培训食品处理人员和教育消费者。
如欲了解更多的情况,请通过[email protected]电子邮件联络或访问http://www. consumersinternational.org/ roap网站。
[1] 见粮农组织/世卫组织联合出版物《保障食品的安全和质量:强化国家食品控制体系指南》,网址为:http://www.fao.org/DOCREP/006/Y8705E/Y8705E00.HTM
[2] 《食品安全法规:当代问题综述》,S. Henson 和 J. Caswell,食品政策 24:第589-603页,1999年。
[3] 网址:www.codexalimentarius.net/procedural_manual.stm
[4] http://www.fao.org/es/ESN/food/capacity_tools_ramanual_en.stm
[5] 《发展中国家食品安全的经济学》,S. Henson,粮农组织,欧洲环境署工作文件03-19,2003年
[6] http://www.foodstandards.gov.au/_srcfiles/revised_Guide_Props_Appls_July02.pdf
[7] http://193.51.65.78/puma/regref/pubs/rco95/foreword.htm
[8] http://www.fao.org/DOCREP/005/X1271E/X1271E00.htm#TOC
[9] 国际推荐卫生规范-食品卫生总则,食典委,1999年。
[10] http://www.who.int/foodsafety/publications/fs_management/haccp_teachers/en/
[11] http://www.fao.org/es/ESN/food/capacity_projects_africa_en.stm
[12] http://www.enyox.com/dev/unido-tcb/index.php?cccpage=projects_list&set_z_sidenav=0
[13] 与食品检验和验证系统有关的卫生检疫措施等效性判断准则,ALINORM 03/30A,附件II,2003年7月由食品法典委员第26届会议通过。
[14] 制定有关食品进出口检验和验证系统的等效性协议的准则(CAC/GL 34-1999)
[15] http://www.fao.org/DOCREP/MEETING/006/y9805e.htm
[16] http://www.foodsafetyforum.org/
[17] 食品安全、动植物健康国际门户网站概念文件,(INF/6, 食品法典委员会第26届会议,2003年7月): http://www.fao.org/docrep/pdf/meeting/006/y9489e.pdf
[18] http://fsi.wpro.who.int/index.asp
[19] http://www.foodstandards.gov.au/
[20] 粮农组织/世卫组织联合专家会议有关风险分析在食品标准问题上应用的报告,1995年3月13-17日;粮农组织/世卫组织联合专家会议有关风险管理和食品安全的报告,1997年1月27-31日,粮农组织/世卫组织联合专家会议有关风险交流在食品标准及安全问题上应用的报告,1998年2月2-6日。
[21] http://www.wto.org/english/tratop_e/sps_e/spsagr_e.htm
[22] http://www.codexalimentarius.net/procedural_manual.stm
[23] 粮农组织/世卫组织食品添加剂联合专家委员会
[24] http://www.foodstandards.gov.au/media releasespublications/technicalreportseries/ technicalreportserie2094.cfm
[25] 澳新食品标准局熟虾中李斯特杆菌的调查;2002年
[26] 所采用的调查方法的检测最大限值
[27] http://www.foodstandards.gov.au/_srcfiles/P239listeriaFAR.pdf
[28] 危害分析及关键控制点
[29] Thailand defines diarrheal diseases as a group of diseases including acute diarrhea, dysentery, food poisoning, and enteric fever.
[30] JICA has supported socio-economic and human-resource development in order to facilitate the autonomous, sustainable development of developing countries as one of Japan’s Official Development Assistance (ODA) implementing bodies. JICA is mainly responsible for implementing technical cooperation for developing countries.
[31] “Technical Cooperation Project” involves providing support for the training of personnel required to achieve social and economic progress in developing countries and for the development and diffusion of technology and skill. Elements such as the dispatch of experts, acceptance of training participants and provision of equipment are organically linked so as to realize a form of technical cooperation in which every aspect (from the formulation of plans to their execution and evaluation) falls within a fully integrated whole.