Selecting the proper site for a bivalve hatchery is the most important consideration when deciding to build a hatchery, yet it is a factor that has often been overlooked when some hatcheries were built. Several factors may have contributed to the inappropriate location of a facility including the lack of one or more of the components of the essential infrastructure, e.g. land availability at reasonable cost, the local availability of electricity and freshwater, a qualified labour force, or good communications. A further consideration has often been that an individual or company may have wished to build a hatchery at a site adjacent to an existing bivalve growout operation. In such cases the hatchery became an add-on feature to an existing operation. Yet, another factor is that an individual or company may own or have rights to a particular location and it proves to be the only place where a hatchery could conveniently be built. While it is true that it may be impossible to build a hatchery at an ideal location, nevertheless certain criteria must be met or a hatchery will likely be doomed to failure.
The first consideration is to determine if government regulations permit construction of a bivalve hatchery at the desired site. This can be done quickly by making enquiries of local, state, provincial or federal authorities. If regulations do not permit construction of a hatchery at the desired site one must decide if it is preferable to find another location where construction is permitted, or attempt to change existing government regulations to allow construction at the desired site.
It is likely that a number of permits and licences will be required to ensure compliance with local building codes and national and local environmental regulations before any construction is allowed. This can be a lengthy, costly and time consuming process and may require an assessment of the potential impact of the hatchery on the local environment before permission is either granted or not granted to begin the construction phase.
Before committing to what is considered to be a suitable location for a hatchery it is of paramount importance to ensure that good quality seawater exists year-round at the prospective site. This point cannot be overemphasized. If a good seawater source is not available, it will be difficult, if not impossible, to develop an efficient and profitable hatchery operation. For this reason every effort should be made to obtain as much information as possible about the quality of the seawater throughout the year at a potential site - or sites. Information is required not only for surface waters but also for the entire water column, since thermoclines may develop or upwelling may occur periodically. If previous oceanographic surveys have been undertaken in the area, copies of the data should be examined. If such surveys have not been undertaken, one should be prepared to undertake a detailed sampling of the waters at the proposed site for at least a year.
Environmental parameters of seawater that need to be examined will depend in part on geographic location and the intended species for culture. Bivalve larvae as well as juveniles and adults have strict physiological requirements, such as water temperature, salinity and oxygen levels and these must be maintained in a hatchery operation. Water temperatures are higher in the tropics than in temperate regions and indigenous bivalves are well adapted to tolerate these conditions. But in a hatchery situation temperatures must not be allowed to drop too low or larval and juvenile survival and growth will be adversely affected. In temperate areas water temperatures must not be allowed to exceed upper or lower lethal levels to larvae and juveniles. Salinity can vary widely and tolerance to these fluctuations differs among bivalve species. Some require high oceanic levels of salinity while euryhaline (estuarine and brackish water) species exhibit much wider tolerance. Periods of heavy rainfall may not only cause periods of low salinity, but heavy associated runoff can increase quantities of silt and other materials which may lead to problems in a hatchery. Dense concentrations (blooms) of some marine algal and bacteria species may release toxic substances that may cause reductions in both the survival and growth of bivalve larvae or juveniles, or mass mortalities in extreme cases. As much data as possible on these parameters should be collected prior to deciding on the adequacy of a site for a bivalve hatchery. Remedial measures to improve inadequate quality seawater can be extremely costly and may adversely effect the profitability of a venture.
Locations possibly influenced by effluents discharged from industrial plants should be avoided. The lethal and sublethal effects of many industrial pollutants are not completely understood, nor are the additive effects they may exert when several industries are discharging a range of potentially toxic wastes in nearby waters. Effects of such effluents can be extremely damaging to bivalve larvae. For example, an anti-fouling ingredient added to marine paints, tributyltin (TBT), has been found to be highly lethal to bivalve larvae even at concentrations of a few parts per billion. Drawing a seawater supply from the vicinity of marinas and commercial docks needs to be avoided. If feasible it is advisable to undertake bioassay studies using bivalve embryos to help determine the quality of the water at the potential hatchery site. The presence of deleterious materials may be transitory or seasonal in nature, so sampling for bioassays should be carried out over a period of at least a year and be done preferably on a weekly basis.
Agricultural - forestry included - and domestic sources of pollution should also be avoided. It has recently been shown that runoff from some cultivated lands can carry concentrations of pesticides at levels deleterious to the growth and survival of bivalve larvae. Domestic pollution may not only contain pollutants that are toxic to bivalve larvae but the high organic content can cause depletion of oxygen levels and increased levels of bacteria that could also lead to reduced growth and mortalities of larvae.
Another consideration when deciding upon the location of a bivalve hatchery is whether "civilization" will soon encroach on the site. Urbanization with its ancillary problems is one of the main concerns in bivalve culture. If the site will soon be encompassed by urbanization then every effort must be made to ensure that sources of potential pollution will be kept to a minimum. This will require working closely with planners and developers.
The hatchery should be located close to the ocean so that the distance required to pump water is kept to a minimum. This negates the necessity of having to maintain great lengths of pipe. It should also be located as close to sea level as possible to avoid problems of pumping water any great vertical distance. If fluctuations in surface seawater temperature and salinity occur regularly, the intakes for the pipes will need to be located at depth (up to 20 m below the surface) to maintain more constant water temperature and salinity. Depending on the nature of the geological strata, it may be possible to drill wells close to the shore to access seawater aquifers. A water source of this nature will be at a more constant temperature year-round and will already be pre-filtered by percolation through the strata. It may, however, require oxygenating before use. It is always wise to consult with a suitably qualified engineer when making decisions on the best methodology and technology to procure the water supply.
Sufficient area needs to be available at the site to accommodate the hatchery and ancillary buildings and also to allow for any future expansion. The need for adequate surveillance should also be considered.
Other considerations that need to be kept in mind for a site include an adequate supply of electrical power, a source of freshwater and a skilled labour force to operate the hatchery. Good communications should exist so that required materials and supplies can be acquired quickly and larvae and seed can be quickly shipped to their various destinations. The proximity of institutions such as universities, government laboratories and libraries should also be considered since such resources can be of great assistance in operations and in helping towards solutions to problems that may arise.
It is a worthwhile preliminary to prepare a check list of parameters that must be met, or at least reviewed, when considering a site for a bivalve hatchery and work through the list to ensure the site meets as many of the requirements as possible.
There is no rigid design for a bivalve hatchery. The layout of hatcheries varies from site to site, with species produced, geographic location, funds available, the target production species and personal preferences (Figure 3). Some hatcheries are small and supply seed for their own bivalve on-growing culture operations. Others are large and may only produce seed for sale, or they may produce seed for their own operations and also an excess to sell to other growers. Hatcheries may or may not include a nursery component and some may only produce mature larvae for shipment elsewhere while others may grow and supply seed varying in size from 1 to 12 mm shell length. Much depends upon the nature, requirements and the level of sophistication of the growout operations that collectively make the customer base.

Figure 3: A selection of photographs of hatcheries depicting the variability in size and sophistication of construction that exists around the world. Clockwise from top left: Tinamenor S.A. (Pesues, Spain), Turpiolito hatchery, (Gulf of Cariaco, Venezuela), Bermuda Biological Station’s scallop hatchery based on insulated cargo containers and the SMS oyster hatchery (Point Pleasant, Nova Scotia, Canada).
Many hatcheries were built with little advance planning or forethought for possible future development. A hatchery was built to produce a required quantity of seed and when the initial objective was achieved a decision was made to expand and add extra capacity. The resulting facility is often neither efficient nor worker friendly. Other hatcheries were built to produce seed of a single species but other species are produced now and the resulting hatchery is somewhat inefficient in its new role.
Considerable time will be saved and many frustrations avoided if a hatchery is carefully planned before construction begins. Several considerations must be remembered when designing a hatchery and two are of great importance. Firstly, the hatchery operation must be worker friendly and efficient to make the operation as profitable as possible, and secondly, the need for future expansion must be kept in mind.
There are two basic parts to a bivalve hatchery, the salt water system and the physical plant.
The need for a supply of high quality seawater was previously discussed. It is important to ensure that the seawater source and system to pump and treat it is located conveniently close to the hatchery and optimum use made of it to keep capital and operating costs to a minimum.
The hatchery should be located as close to sea level as possible to avoid lifting water. Intakes for the seawater should be as short as possible and conveniently located so they can be serviced and maintained with minimum effort. Intakes for the salt water should be located at depth to avoid fluctuations in temperature and salinity and also to reduce the number of organisms and amount of detritus that will enter the system. In temperate areas, intakes should be located below any thermocline that occurs in summer to reduce temperature variability. In areas where periods of heavy rain occur, the intakes should be deep enough to avoid sudden fluctuations in salinity and heavy siltation that may occur with the rains. Intakes at depth avoid major plankton blooms, some of which may be harmful to bivalve larvae and also greatly diminishes the number of fouling organisms entering the system. Fouling organisms can settle in pipes and greatly reduce water flow into the hatchery. Many of the above sources of variability can be avoided by accessing seawater from drilled wells. This possibility should be investigated before any other solution is considered.
Size of pumps and the diameter of the pipes required will depend on the scale of the operation and the volumes of seawater required to meet all aspects of production. Pumps are available through commercial outlets and the type and size of pump required can be determined after discussions with dealers. It is important to ensure that surfaces that come into contact with the seawater are non toxic. Most plastics, cast iron and certain grades of stainless steel are suitable. Pumps that contain mild steel or brass components should be avoided.
Seawater pumped directly from the ocean is first passed through sand filters that filter out most particulate material greater than 20-40 µm in size (Figure 4). A well maintained sand filter will remove the major portion of detritus and organisms from the water that may interfere with bivalve larvae. It also eliminates many of the fouling organisms that could settle and grow in pipes in the hatchery. They not only can cause problems with water flow but when they die they can produce anaerobic conditions that can be toxic to bivalve larvae. They may also harbour and eliminate bacteria that can be deleterious to larvae. Sand filters are commercially available and are the same or similar to those used to filter water in swimming pools. A series of two or more such filters are generally installed and they are regularly back-flushed to avoid clogging of the filter media. Other types of filters may be used depending on personal preference and cost considerations. Self-cleaning, rotating drum filters offer an alternative to remove larger particulate material and large surface area cartridge or bag filters are available and are extremely effective in removing smaller sized particulates.
Another method to obtain seawater for a hatchery is to pump it from seawater wells. This has become the preferred method for hatcheries to obtain their water supply in recent years. A well is dug or drilled close to the hatchery and is deep enough to provide a sufficient supply of seawater for the hatchery. Water from such wells is of high quality and generally has a constant temperature and salinity. It has already been filtered through sedimentary or porous rock, contains little detritus and few, if any, fouling organisms. Water abstracted in this way requires little if any further filtration. Constructing seawater wells can be expensive initially but the high capital cost is offset by reduced operating costs.
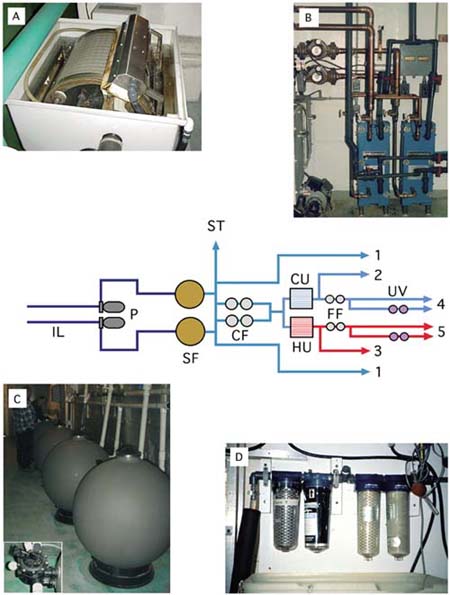
Figure 4: A diagram of the various stages of seawater treatment for hatchery usage from the intake pipes (IL) to the points at which water is used in the different aspects of the operation (1 to 5). Key: P - seawater pumps; SF - sand filters (photograph C) or alternatively self-cleaning drum filters (photograph A); ST - to storage tanks (if required); CF - cartridge filters of 20 µm and 10 µm; CU - seawater chilling unit (if required); HU - seawater heating unit (if required - photograph B); FF - final filtration (5 µm and 1 or 2 µm - photograph D); UV - ultra-violet light disinfecting units (if required).
A guide to typical usages (treatment levels vary from hatchery to hatchery):
1 - Unheated, sand-filtered water for broodstock and larger juveniles (if water requires to be heated, then 3).
2 - Chilled seawater filtered to 10 µm for spawning broodstock or for large-scale algal culture of hardy species. Chilled (or ambient temperature) seawater is often mixed with heated seawater to provide intermediate temperatures for a variety of purposes.
3 - Heated seawater filtered to 10 µm for conditioning and spawning broodstock and for growing larger spat. Some hatcheries have a separate heating system for either unfiltered or sand-filtered seawater for broodstock conditioning.
4 - Chilled water filtered to 1 µm and either UV-disinfected or not for algal culture.
5 - Heated water filtered to 1 µm and either UV-disinfected or not for larval culture.
After filtration, all or part of the seawater may be pumped to a storage tank that may be made of either concrete or fibreglass. Use of a storage tank may be a matter of preference and many hatcheries do not have them. They are useful when water can only be obtained at a particular time, e.g. at high tide. Sometimes this method is used in areas where electrical power is unreliable to ensure a supply of seawater is always available. Sufficient water is pumped into the storage tank so it can supply the hatchery until the tank can be refilled. The tank is located at height so that the effect of gravity maintains a sufficient water flow through the hatchery. In other hatcheries, the salt water system is a flow-through system and water is pumped continuously through the hatchery for use where it is needed and then is discharged to waste. Recently, many hatcheries have installed recirculating or partial recirculating systems to reduce operating costs. This is particularly true if seawater is in short supply or if it has been heated or chilled. Recirculated water may be passed over biologically activated filters to remove metabolic wastes of the animals and held before it is reused. If the water has been heated or chilled it may be passed through heat exchangers to partially heat or chill incoming water and thus reduce energy costs.
All piping must be non-toxic, usually PVC (polyvinylchloride) schedule 40 or 80, although ABS or polyethylene pipes and fittings are also sometimes used as alternatives. The diameter of the pipes depends on water demand. In most hatcheries the main distribution lines within the hatchery are 50 mm diameter or less although the main intake pipes may be up to 15 cm diameter. The piping should be well supported and high enough off the ground so that it is out of the way but readily accessible for cleaning. Valves and outlets should be conveniently located. If the water is sufficiently filtered there should be little need to clean the lines frequently. Cleaning may be required periodically, hence, it is important to have clean-out ports or screw unions located conveniently so that the lines can be easily cleaned in situ or quickly dismantled for more thorough cleaning.
In most hatcheries in temperate areas there needs to be the capability to heat and sometimes to chill part of the seawater supply. There are commercial units available for this purpose and discussions and calculations on required capacity with dealers will ensure that an adequate supply at the required temperatures is available. Again, it is essential to ensure that surfaces of such units coming in contact with the seawater are non-toxic to bivalve larvae. Most commercially available heat exchange units utilize titanium as the heat transfer surface and this material is preferred by most hatcheries.
Hatchery managers may wish to sterilize (or more correctly, disinfect) all or part of the seawater before use, particularly if disease problems arise. Seawater can be sterilized with either UV (ultra-violet) light or ozone. Commercial units are available and simple calculations will determine the size of unit that is required. Commercial units are normally rated for their performance in sterilizing freshwater. In seawater situations where organic loadings and turbidity caused by colloidal materials are frequently higher than for freshwater, it is recommended that such units are used at half (or less) of the recommended flow rate for satisfactory performance. If UV-light sterilization is used, the water must be filtered to about 1 µm prior to sterilization since UV-light is readily absorbed by particles in the water reducing the efficiency of the unit. Filtration can easily be incorporated into a UV unit and many available units have both filters and the UV lamps combined.
Government regulations may exist in some areas that control the discharge of effluent from a hatchery. Before constructing a hatchery, government regulations controlling discharge of effluents should be reviewed and if they exist they must be followed. Large floor drains sunk into the floors of wet areas are essential and should be located conveniently throughout the hatchery. Periodically large volumes of water must be discharged, e.g. when emptying tanks, and the drains must be able to handle such discharges.
Some hatcheries may wish to breed exotic species or strains or races of a species that do not occur locally. Depending on government regulations, this may entail installation of a quarantine facility to ensure that pests, parasites and diseases are not introduced with the exotic species or larvae accidentally escape into the natural environment. This will require a separate drainage system in the area of the hatchery designated for quarantine that empties into special holding tanks where the effluent can be sterilized with a strong hypochlorite solution. The sterilized water is then treated with thiosulphate to neutralize any residual chlorine before it is discharged back into the environment. Quarantine facilities may require a separate room to hold, condition and spawn adults. Drains from this room will also empty into the quarantine treatment tanks.
Careful thought should be given to hatchery design to permit convenient and efficient operations. The hatchery should be adaptable so that changes can be made readily without involving major rebuilding. In some hatcheries, tanks have been constructed of concrete and changes cannot be made easily. It is much better to have plastic or fibreglass tanks so they can be easily moved or changed if needed. Floors should be of concrete and have sufficient drains. All surfaces should be covered with a durable, mildew resistant finish to facilitate cleaning. Floor standing cabinets and storage units made from wood should be mounted on concrete plinths to prevent them being damaged by immersion in seawater. Where this is not possible, wood surfaces need to be painted with a good quality epoxy resin.
A hatchery has several areas that are all inter-related. For convenience they have been divided into algal culture, broodstock conditioning and spawning, larval rearing, juvenile culture and service areas (Figure 5).
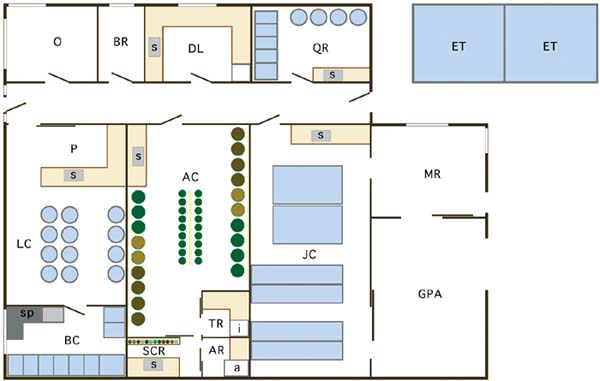
Figure 5: A generalized floor plan for a purpose-built bivalve hatchery (see the following text for explanation).
The success of a bivalve hatchery depends on the production of algae. Large quantities of high quality algae must be available when needed. It is a most important part of any hatchery and considerable thought should be given to providing a sufficient and efficient working area for this purpose (AC - Figure 5). Since algae are used in all phases of production, the facility should be located centrally and conveniently. Space required for algal culture depends partly on levels of production, methods of culture and whether algae will be raised entirely inside the hatchery with artificial illumination, or if it will be raised outside under natural light, or a combination of the two. A well ventilated greenhouse is required if algae is grown in natural light and this structure needs to be placed so as to obtain the maximum amount of sunlight. Shading may be need to protect younger, less dense cultures from strong sunlight.
A small room is required to maintain stock (also known as master) cultures of algae (TR). Dimensions vary but it can be as small as 2 x 3 m. The room should be insulated and the temperature kept cool. Shelving is needed with flourescent lights at the back to provide the light source. An air supply is also required. Test tubes with algal slants and small flasks with stock culture that are monospecific and axenic are kept in this room often in a refrigerated, illuminated incubator. Methods are described in a Part 3.
The next phase of culture uses stock cultures from the cool room and grows them in 4 l flasks and 20 l carboys against a bank of fluorescent lamps (SCR). This can be part of the main algal culture area or a small room off it. The space required depends on the number of species and amount of algae being produced. This area requires an air and carbon dioxide supply and needs to be kept at 15 to 18ºC. Another adjacent small room (AR) houses an autoclave (a), which is used to heat sterilize medium for the small cultures. Some hatcheries use alternative methods to prepare culture medium and these are described in Part 3.
The size of the main algal culture area depends on the number of species being cultured and the amount of algae required. This area can occupy a substantial part of the hatchery. If most of the algae is raised inside the hatchery by the batch culture method then there must be sufficient space for a series of tanks that can measure up to 3-4 m in diameter and 2 m in depth. If the bag or tall cylinder culture methods are used the amount of floor area required can be reduced. Ballasts for fluorescent lamps used to illuminate cultures need to be of the "cool running" type or isolated in a separate area from which the heat they generate can be dissipated. This area should ideally be maintained at 15 to 20ºC.
In many hatcheries, considerable portions of the algae, if not all, are raised in greenhouses. These can be stand-alone structures or attached to one side of the hatchery - preferably the south side in the Northern Hemisphere and northern side in the Southern Hemisphere, so as to receive as much sunlight as possible. The size of the greenhouse depends on the method of culture and quantities of algae that need to be produced.
Sufficient electrical power must be available for artificial lighting when natural sunlight is inadequate. Compressed air and carbon dioxide supplies are essential. There should be adequate ventilation or installed air-conditioning to maintain temperatures at or below 20°C on days when bright sunlight heats the facility. A generator will be required in areas where the electricity supply is unreliable and may be off for several hours or more at a time. Although survival of the algal cultures is not at risk in the absence of light for an hour or two, cultures need to be aerated. Diatoms will settle to the bottom of cultures without aeration and cultures may collapse.
Space is required to hold and condition broodstock (BC - Figure 5). The amount of space needed depends in part on the number of species being held and whether some or most of the conditioning will be undertaken in the open environment rather than in the hatchery. Heated or chilled seawater may be required for this aspect of operation at certain times of the year. The ability to isolate tanks so that photoperiod can be adjusted is desirable since it has been shown that varying periods of light and dark can affect gonadal maturation.
Space is required for spawning trays (sp) but this can be part of the larval rearing area since the space is not require continuously. Spawning trays or dishes can be stored when not in use. Methods for broodstock conditioning, spawning and fertilization are described in Part 4.
Another major part of the hatchery is occupied by the larval rearing facility (LC) and dimensions of this area depend on the scale of production. The space is occupied with tanks, the number needed depending on production levels and the techniques used to rear larvae. On the Pacific coast of North America the tendency has been to raise larvae at low densities of 2-3 per ml in large tanks that measure 3-4 m in diameter, 4-5 m in height and hold 40 000 to 50 000 l. In other hatcheries larvae are raised in smaller tanks of up to 5 000 l in volume at higher larval densities. A manager must decide on required production to meet market demand and the methodology that will be used to rear larvae when planning this part of the hatchery.
Larval rearing tanks are generally made of fibreglass or of a suitable plastic and should be thoroughly leached prior to use. Regardless of the size of tanks used, there should be large sunken floor drains to handle large volumes of water when the tanks are drained. A preparation area in the larvae culture room (P) is required for washing, grading, counting and measuring larvae and for accommodating the equipment used for these purposes. This area requires cupboards and shelves for the storage of equipment when not in use.
Once mature larvae have set (settled and begun metamorphosis) they are moved to tanks in the juvenile culture room (JC) for culture until they are of sufficient size to transfer to nursery systems, which may be part of the hatchery or at another location. This is generally when the juveniles (known as spat) exceed 2 mm shell length. The size and types of tanks in terms of volume and surface area used for this purpose vary according to species.
Mature larvae are set in the hatchery or in outside (sometimes remote) facilities. When this procedure occurs within the hatchery it is generally done in the larval culture area, frequently directly in the larval tanks. Space for additional tanks may be required specifically for this process. Spat (early juveniles) are subsequently transferred to tanks systems in a separate area specifically for juvenile culture (JC). The size and types of tanks in terms of volume and surface area used for this purpose vary according to species. They may be upwellers, downwellers or tray systems of varying configuration and the juveniles are grown in these until they exceed 2 mm shell length. To grow spat to a larger size within the hatchery on cultured food is uneconomic since food requirement increases exponentially with size. If the nursery system is located outside the hatchery, sufficient space must be allotted for this operation.
Methods for the culture larvae are described in Part 5 and for spat in Part 6.
Hatcheries dealing with broodstock from outside the immediate region or with exotic species may, as already mentioned, be required to quarantine stock and rear the progeny in isolation. Such hatcheries will include a quarantine room (QR) for this purpose, the effluent from which is discharged into treatment tanks (ET).
Other rooms include a dry laboratory (DL), office (O) and bathroom (BR). The dry laboratory is where algal transfers can be made (if no specific space is allocated elsewhere), chemicals weighed and mixed, microscopes kept for examining cultures, records maintained and for the storage of scientific equipment.
Static machinery such as the main pumps, sand filters and pre-filters (to remove particles down to 10 µm), seawater heating/chilling units, furnaces, the air ventilation system, air blowers/compressors, a standby generator for emergency power supply, together with electrical panels and control equipment, are housed in a soundproof machinery room (MR). Duplication of essential equipment is preferred in the event of electrical or mechanical failure. Compressed air is required in all phases of culture and carbon dioxide is required for algal culture. In many hatcheries the seawater intake pumps and sand filters are located in a separate pump house close to the point of intake and the final filtration of seawater may take place at the point of use rather than at a central, fine filtration unit.
Since storage is always an issue in a hatchery, it is useful to have a large general-purpose area (GPA) that can be used for storing materials and equipment, packing seed and as a workshop. Most of the working areas should be fitted with benches and sinks (s).
It is preferred that the various parts of the hatchery can be isolated in the event of a disease outbreak.
A bivalve hatchery is a business and like any other business it must be run efficiently and it must be economically viable. Government subsidies or grants may help offset costs particularly during initial stages of operation, but eventually the hatchery must stand on its own and be profitable. The economics of building and operating a bivalve hatchery will vary from business to business, from area to area and country to country but eventually all must turn a profit.
Hatcheries are expensive operations. Considerable capital is required to build a hatchery and finance operations. The owner must have sufficient working capital to carry on operations until income is generated. Before deciding to build a hatchery, one needs to carefully examine all facets of building and operating a hatchery and determine at what level a hatchery will be economically viable. Many costs need to be considered including purchase of the site, construction of the hatchery, installation of the seawater system, equipment needed for all phases of production, maintenance, supplies and utility overheads, loan repayments and the need for a trained staff.
Profitability can vary greatly with other factors including geographic area, the scale of the operation and whether it is part of a fully integrated bivalve culture operation.
In temperate areas a major operating cost is heating (and chilling) seawater, but this cost is generally avoided in tropical areas. This may influence location of a hatchery in temperate areas to sites where warm seawater exists at least for part of the year to help reduce heating costs.
Some hatcheries are small family operated ventures that only produce sufficient seed for their own culture needs. Such hatcheries are generally operated for only a few months a year, production is limited, and costs are much lower than for other larger hatcheries.
Large hatcheries may be part of a fully integrated bivalve culture operation or they may be in business only to supply seed. Where a hatchery is part of an integrated culture operation, the hatchery may be operated to simply break even and show no profit or may even operate at a small loss. Profits for the company are made in other phases of the culture operation. Where the hatchery exists only to produce seed to sell to other growers, a profit must be made solely on the hatchery operation. This emphasizes the fact that before building a hatchery one must make an accurate assessment of the market for whatever seed will be produced and not only the quantity of seed that can be sold but also the price people are willing to pay for seed.
Another consideration in operating a bivalve hatchery is that a critical level of production must be maintained to permit profitability. A hatchery cannot exist by simply producing a few thousand juveniles each year. The cost to do so is too high. In fact the costs associated with producing a few thousand juveniles are almost the same as producing several million - economies of scale apply. A manager must determine the critical level of production that needs to be attained to make the operation profitable and this again points to the necessity of knowing the extent and value of the market for the product.
Accurate records of costs, production and sales must be kept to assess whether the hatchery is being profitably run.
Anon. 1979. Feasibility study for a commercial oyster hatchery in Tasmania. Tas. Fish. Devel. Authority: 115 pp.
Breese, W.P. & Malouf, R.E. 1975. Hatchery manual for the Pacific oyster. Sea Grant Program Pub. No. ORESU-H-002. Oregon State Univ. Corvallis, Oregon, USA: 22 pp.
Castagna, M. & Kraeuter, J.N. 1981. Manual for growing the hard clam Mercenaria. VIMS Spec. Rep. In Applied Mar. Sci. and Ocean Eng. 249: 110 pp.
Curtin, K. 1983. Oyster hatchery pilot scheme; setting up, operation and future role of hatcheries. N.Z. MAF: 16 pp.
Dupuy, J.L., Windsor, N.T. & Sutton, C.E. 1977. Manual for design and operation of an oyster seed hatchery for the American oyster, Crassostrea virginica. Spec. Rep. Applied Mar. Sci. Ocean. Eng. 142. VIMS, Gloucester Point, Virginia.
Helm, M.M. 1994. Towards reliable bivalve seed supply in Nova Scotia. Bull. Aquacul. Assoc. Canada 94 (4): 9 - 14
Holliday, J.E. 1984. International developments in oyster hatchery technology. Misc. Bull. 1. Div. Fish, Dept. Agriculture. New South Wales, Australia: 101 pp.
Huguenin, J.E. & Colt, J. (eds.). 1989. Design and operating guide for aquaculture seawater systems. Dev. Aquaculture Fish. Sci. Elsvier. 20: 264 pp.
Hurley, G., Henderson, K., Percy, M. & Roscoe, D. 1987. Design of a small scale shellfish hatchery. Nova Scotia Dept. Fish. Halifax, NS, Canada: 45 pp.
Im, K.H. & Langmo, R.D. 1977. Hatchery produced Pacific oyster seed: economic feasibility on cultch in the Pacific Northwest. Sea Grant, Oregon State Univ. Corvallis, Oregon, USA. Pub. No. ORSESU-T-77-010: 80 pp.
Neima, P.G. & Kenchington, E. 1997. Report on commercial scallop hatchery design. Can. Tech. Rep. Fish. Aquat. Sci., 2176: 55 pp.
Robert, R. & Gerard, A. 1999. Bivalve hatchery technology: the current situation for the Pacific oyster, Crassostrea gigas, and the scallop Pecten maximus in France. Aquat. Living Resour. 12 (2): 121 - 130
Spencer, B.E., Helm, M.M. & Dare, P.J. 1977. Recommended quarantine measures for marine molluscs. MAFF Fish. Res. Tech. Rep., Lowestoft, No. 32: 7 pp.
Utting, S.D. & Helm, M.M. 1985. Improvement of seawater quality by physical and chemical pre-treatment in a bivalve hatchery. Aquaculture, 44: 133 - 144
Wickins, J.F. & Helm, M.M. 1981. Sea water treatment. p 63 - 128. In: Hawkins, A. D. (ed.) Aquarium Systems. Academic Press, London: 452 pp.