Some knowledge of bivalve biology is necessary to understand operations of a bivalve hatchery and to assist in solving problems that arise. It is not the intention here to give a detailed description of bivalve biology but to provide a brief resume of information pertinent to operations of a hatchery. There are several excellent texts on molluscan biology readily available and there are extensive reviews of groups and individual species of oysters, scallops, mussels and clams. The reader is directed to these publications at the end of this section for additional information.
Bivalves belong to the phylum Mollusca, a group that includes such diverse animals as chitons (chain shells), gastropods, tusk shells, cephalopods (squid and octopus) as well as clams, oysters, mussels and scallops. The phylum has six classes of which one is Lamellibranchia or Bivalvia. These animals are compressed laterally and the soft body parts are completely or partially enclosed by the shell, which is composed of two hinged valves. The gills or ctenidia of animals in this class are well developed organs, specialized for feeding as well as for respiration.
The most prominent feature of bivalves is the two valves of the shell that may or may not be equal and may or may not completely enclose the inner soft parts. They have a variety of shapes and colours depending on species. The valves are composed mostly of calcium carbonate and have three layers; the inner or nacreous layer, the middle or prismatic layer that forms most of the shell, and the outer layer or periostacum, a brown leathery layer which is often missing through abrasion or weathering in older animals.
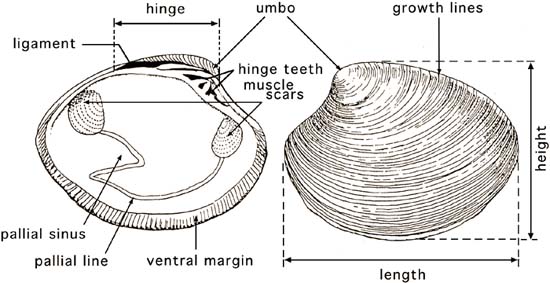
Figure 6: External and internal features of the shell valves of the hard shell clam, Mercenaria mercenaria. Modified from Cesari and Pellizzato, 1990.
Bivalves do not have obvious head or tail regions, but anatomical terms used to describe these areas in other animals are applied to them. The umbo or hinge area, where the valves are joined together, is the dorsal part of the animal (Figure 6). The region opposite is the ventral margin. In species with obvious siphons (clams), the foot is in the anterior-ventral position and the siphons are in the posterior area (Figure 7). In oysters the anterior area is at the hinge and in scallops it is where the mouth and rudimentary foot are located.
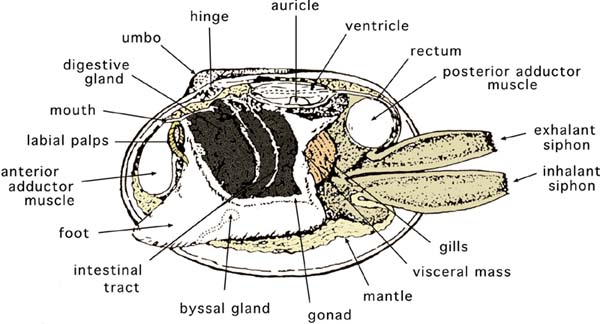
Figure 7: The internal, soft tissue anatomy of a clam of the genus Tapes. In this view, the uppermost gill lamellae have been removed to reveal the foot and other adjacent tissues. Modified from Cesari and Pellizzato, 1990.
Careful removal of one of the shell valves reveals the soft parts of the animals. The differences in general appearance of an oyster and scallop can be seen in Figure 8.
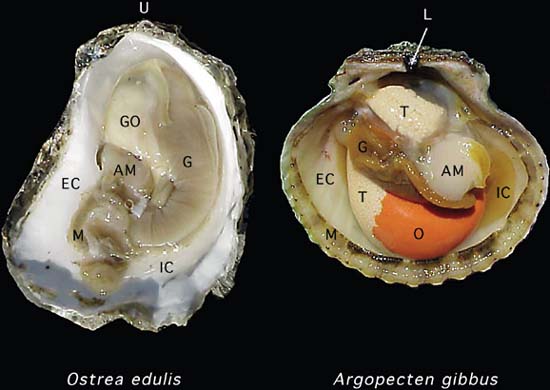
Figure 8: The soft tissue anatomy of the European flat oyster, Ostrea edulis, and the calico scallop, Argopecten gibbus, visible following removal of one of the shell valves. Key: AM - adductor muscle; G - gills; GO - gonad (differentiated as O - ovary and T - testis in the calico scallop); L - ligament; M - mantle and U - umbo. The inhalant and exhalant chambers of the mantle cavity are identified as IC and EC respectively.
Mantle
The soft parts are covered by the mantle, which is composed of two thin sheaths of tissue, thickened at the edges. The two halves of the mantle are attached to the shell from the hinge ventral to the pallial line but are free at their edges. The thickened edges may or may not be pigmented and have three folds. The mantle edge often has tentacles; in clams the tentacles are at the tips of the siphon. In species such as scallops the mantle edge not only has tentacles but also numerous light sensitive organs - eyes (Figure 9).
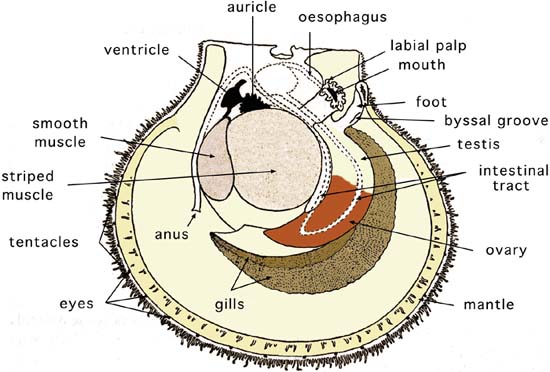
Figure 9: The internal, soft tissue anatomy of a hermaphroditic scallop.
The main function of the mantle is to secrete the shell but it also has other purposes. It has a sensory function and can initiate closure of the valves in response to unfavourable environmental conditions. It can control inflow of water into the body chamber and, in addition, it has a respiratory function. In species such as scallops, it controls water flow into and out of the body chamber and hence movement of the animal when it swims.
Adductor muscle(s)
Removal of the mantle shows the underlying soft body parts, a prominent feature of which are the adductor muscles in dimyarian species (clams and mussels) or the single muscle in monomyarian species (oysters and scallops). In clams and mussels the two adductor muscles are located near the anterior and posterior margins of the shell valves. The large, single muscle is centrally located in oysters and scallops. The muscle(s) close the valves and act in opposition to the ligament and resilium, which spring the valves open when the muscles relax. In monomyarian species the divisions of the adductor muscle are clearly seen. The large, anterior (striped) portion of the muscle is termed the "quick muscle" and contracts to close the valves shut; the smaller, smooth part, known as the "catch muscle," holds the valves in position when they have been closed or partially closed. Some species that live buried in the substrate (e.g. clams) require external pressure to help keep the valves closed since the muscles weaken and the valves open if clams are kept out of a substrate in a tank.
Gills
The prominent gills or ctenidia are a major characteristic of lamellibranchs. They are large leaf-like organs that are used partly for respiration and partly for filtering food from the water. Two pairs of gills are located on each side of the body. At the anterior end, two pairs of flaps, termed labial palps, surround the mouth and direct food into the mouth.
Foot
At the base of the visceral mass is the foot. In species such as clams it is a well developed organ that is used to burrow into the substrate and anchor the animal in position. In scallops and mussels it is much reduced and may have little function in adults but in the larval and juvenile stages it is important and is used for locomotion. In oysters it is vestigial. Mid-way along the foot is the opening from the byssal gland through which the animal secretes a thread-like, elastic substance called "byssus" by which it can attach itself to a substrate. This is important in species such as mussels and some scallops enabling the animal to anchor itself in position.
Digestive system
The large gills filter food from the water and direct it to the labial palps, which surround the mouth. Food is sorted and passed into the mouth. Bivalves have the ability to select food filtered from the water. Boluses of food, bound with mucous, that are passed to the mouth are sometimes rejected by the palps and discarded from the animal as what is termed "pseudofaeces". A short oesophagus leads from the mouth to the stomach, which is a hollow, chambered sac with several openings. The stomach is completely surrounded by the digestive diverticulum (gland), a dark mass of tissue that is frequently called the "liver". An opening from the stomach leads to the much-curled intestine that extends into the foot in clams and into the gonad in scallops, ending in the rectum and eventually the anus. Another opening from the stomach leads to a closed, sac-like tube containing the crystalline style. The style is a clear, gelatinous rod that can be up to 8 cm in length in some species. It is round at one end and pointed at the other. The round end impinges on the gastric shield in the stomach. It is believed it assists in mixing food in the stomach and releases enzymes that assist in digestion. The style is composed of layers of mucoproteins, which release digestive enzymes to convert starch into digestible sugars. If bivalves are held out of water for a few hours the crystalline style becomes much reduced and may disappear but it is reconstituted quickly when the animal is replaced in water.
Circulatory system
Bivalves have a simple circulatory system, which is rather difficult to trace. The heart lies in a transparent sac, the pericardium, close to the adductor muscle in monomyarian species. It consists of two irregular shaped auricles and a ventricle. Anterior and posterior aorta lead from the ventricle and carry blood to all parts of the body. The venous system is a vague series of thin-walled sinuses through which blood returns to the heart.
Nervous system
The nervous system is difficult to observe without special preparation. Essentially it consists of three pairs of ganglia with connectives (cerebral, pedal and visceral ganglia).
Urogenital system
Sexes of bivalves can be separate (dioecious) or hermaphroditic (monoecious). The gonad may be a conspicuous, well defined organ as in scallops or occupy a major portion of the visceral mass as in clams. The gonad is generally only evident during the breeding season in oysters when it may form up to 50% of the body volume. In some species such as scallops, the sexes can be readily distinguished by eye when the gonad is full since the male gonad is white in colour and the female is red, even in hermaphroditic species. Colour of the full gonad may distinguish the sexes in some species such as mussels. In other species, microscopic examination of the gonad is required to determine the sex of the animal. A small degree of hermaphrodism may occur in dioecious species.
Protandry and sex reversal may occur in bivalves. In some species there is a preponderance of males in smaller animals indicating that either males develop sexually before females or that some animals develop as males first and then change to females as they become larger. In some species, e.g. the European flat oyster, Ostrea edulis, the animal may spawn originally as a male in a season, refill the gonad with eggs and spawn a second time during the season as a female.
The renal system is difficult to observe in some bivalves but is evident in such species as scallops where the two kidneys are two small, brown, sac-like bodies that lie flattened against the anterior part of the adductor muscle. The kidneys empty through large slits into the mantle chamber. In scallops, eggs and sperm from the gonads are extruded through ducts into the lumen of the kidney and then into the mantle chamber.
In most bivalves sexual maturity is dependent on size rather than age and size at sexual maturity depends on species and geographic distribution. Production of eggs and sperm is termed gametogenesis and size of the bivalve along with temperature and quantity and quality of food are undoubtedly important in initiating this process. The gonad is composed of many-branched, ciliated ducts from which numerous sacs, termed follicles, open. Gametes arise by proliferation of germinal cells that line the follicle wall. The gonad undergoes continuous development until it becomes fully mature but this development has been divided into several stages for convenience, e.g. resting, developing, mature, partially spawned and spawned. When the gonads or gonadal tissue are fully mature they are very evident and form a significant portion of the soft parts of the animal. Gonaducts that will carry the gametes to the body chamber develop, enlarge and are readily observed in the gonad. At this time the animal is frequently referred to as being ripe.
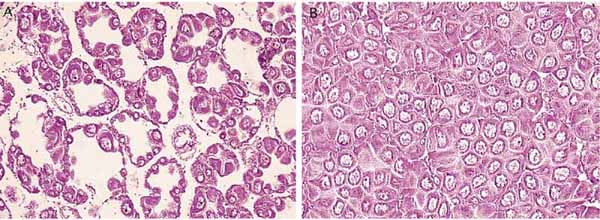
Figure 10: Photomicrographs of histological sections through the ovary of the scallop, Argopecten gibbus, during gametogenesis. To the left (A), developing eggs can be seen lining the walls of the numerous follicles. The right hand photograph (B) shows the follicles filled with mature eggs (courtesy: Cyr Couturier and Samia Sarkis).
Several methods have been used to determine if a bivalve is ripe and ready to spawn. The most accurate method is to make histological slides of the gonad (Figure 10) but this is costly, time consuming and the animal must be sacrificed. Making smears of the gonad or extracting small samples of the gonad from a few individuals of a stock and examining them microscopically is an alternative and the most frequently used technique. In scallops, the gonadal index (weight of the gonad divided by the weight of the soft parts, multiplied by 100) is sometimes used. A rigid routine is generally followed in hatcheries to condition adults for spawning and with practice, most hatchery managers quickly develop the ability to know if the animal is ripe and ready to spawn by examining the gonad macroscopically.
Bivalves that reach the size of sexual maturity and spawn for the first time are sometimes referred to as virgins. Although these animals attain sexual maturity, the number of gametes produced is limited and sometimes not all are viable. During subsequent spawning the number of gametes produced greatly increases.
The period of spawning in natural populations differs with species and geographic location. Spawning may be triggered by several environmental factors including temperature, chemical and physical stimuli, water currents or a combination of these and other factors. The presence of sperm in the water will frequently trigger spawning in other animals of the same species. Some bivalve species in tropical environments have mature gametes throughout the year and limited spawning may occur continuously during the year. In temperate areas, spawning is usually confined to a particular time of the year. Many bivalves undergo mass spawning and the period of spawning may be brief. Virtually the entire contents of the gonad are released over a short period during spawning activity. Other species of bivalves have a protracted spawning period and it may extend over a period of weeks. These species are sometimes referred to as dribble spawners. Limited spawning occurs over a protracted period with one or two major pulses during this time. In some species there may be more than one distinct spawning in a year. In hermaphroditic species, spawning is timed so that either the male or female part of the gonad spawns first. This minimizes the possibility of self-fertilization.
In most bivalve species of commercial interest, gametes are discharged into the open environment where fertilization occurs. Sperm is discharged in a thin, steady stream through the exhalent opening or exhalent siphon. Discharge of eggs is more intermittent and they are emitted in clouds from the exhalent opening or siphon. In species such as scallops or oysters, females frequently clap the valves to expel the eggs. This may be done to clear eggs lodged on the gills. After spawning the gonads of many species are emptied and it is impossible to macroscopically distinguish sex in individuals. The animal is then said to be in the resting stage. In dribble spawners, the gonad may never be completely emptied.
Some bivalves, e.g. the European flat oyster, are larviporous and the early stages of larval development occur in the inhalant chamber of the mantle cavity of the femalephase oyster. Eggs when spawned are passed through the gills and are retained in the mantle chamber. Sperm is taken in through the inhalant opening. The length of time larvae are held in the mantle chamber and subsequently the length of time larvae are free living in surface waters varies with species. In some genera, e.g. Tiostrea, larvae may only be part of the plankton for as little as one day.
Occasionally, particularly in temperate areas, spawning may not occur in some years. This can be a consequence of several factors but probably mostly is related to water temperatures, which remain too low to trigger spawning. When this occurs in oysters, the eggs and sperm may be reabsorbed into the gonadal tissue, broken down and stored as glycogen. In clams and scallops the gonad may remain in a ripe condition until the next year.
These topics are covered more fully in later sections, but a brief description is given here for continuity. Larval development is similar whether initial development occurs in the mantle chamber of the female or completely in the open environment.
Eggs undergo meiotic division at fertilization to reduce the number of chromosomes to a haploid number before the male and female pronuclei can fuse to form the zygote. Two polar bodies are released during meiotic division and when apparent, indicate successful ferilization. Cell division begins and within thirty minutes after fertilization the egg divides into the two-celled stage. The eggs are heavier than water and sink to the bottom of the tank where cell division continues.
The time taken for embryonic and larval development is species specific and temperature dependent (Figure 11). Within 24 hours the fertilized egg has passed through the multicelled blastula and gastrula stages and in 24 to 36 hours has developed into a motile trochophore. Trochophores are somewhat oval in shape, about 60-80.m in size and have a row of cilia around the middle with a long apical flagellum and these permit them to swim.
The early larval stage is referred to as the straight-hinge, "D" or Prodissoconch I stage. Shell length of the initial straight-hinge stage varies with species but it is generally 80-100.m (larger in larviparous oysters). The larva has two valves, a complete digestive system and an organ called the velum that is peculiar to bivalve larvae. The velum is circular in shape and can be protruded from between the valves. It is ciliated along its outer margin and this organ enables the larva to swim but only well enough to maintain itself in the water column. When the larva swims through the water column the velum collects phytoplankton upon which the larva feeds.
Larvae continue to swim, feed and grow and within a week the umbones, which are protuberances of the shell near the hinge, develop. As larvae continue to grow, the umbones become more prominent and the larvae are now in the umbone or Prodissoconch II stage. Prodissoconch II stage larvae have distinct shapes and with practice it is possible identify larvae of different bivalve species in the plankton. This has been used by biologists to forecast oyster sets in the natural environment for the industry. Duration of the larval stage varies with species and environmental factors such as temperature but it can be 18-30 days. Size at larval maturity also varies with species and can be 200-330.m.
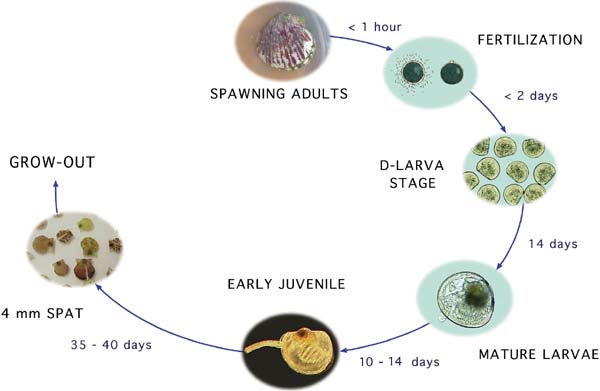
Figure 11: Representation of the developmental stages of the calico scallop, Argopecten gibbus, which take place within a hatchery. The duration of the period between the various stages is given in hours or days for this particular species and may differ for other species of bivalves.
When larvae approach maturity, a foot develops and gill rudiments become evident. Small dark circular dots, the eye-spots, develop near the centre of each valve of some species. Between periods of swimming activity, larvae settle and use the foot to crawl on a substrate. When a suitable substrate is found the larva is ready to metamorphose and begin its benthic existence. Mature oyster larvae secrete a small drop of cement from a gland in the foot, roll over and place the left valve in it. They remain attached in that location for the rest of their lives. In other species, the larva secretes byssus from the byssal gland in the foot and this serves as a temporary holdfast to attach to a substrate. The larva is now ready to metamorphose.
Metamorphosis is a critical time in the development of bivalves, during which the animal changes from a swimming, planktonic to a sedentary benthic existence. Considerable mortalities can occur at this time both in nature and in hatcheries. The subject is dealt with in detail later since it is an important aspect in the hatchery production of juvenile bivalves.
Bivalves are filter feeders and feed primarily on phytoplankton - microscopic plant life. In juveniles and adults, the ctenidia, or gills, are well developed and serve the dual purpose of feeding and respiration. The ctenidia are covered with cilia - tiny vibrating hairs - whose concerted and co-ordinated beat induces a current of water. When resting on or in a substrate, water is drawn into the animal through the inhalant opening or the inhalant siphon, through the gills and then is returned to the surrounding water through the exhalant opening or siphon. The gills collect plankton and bind it to mucous. Strands of food-laden mucous are passed anteriorly by means of ciliary action along special grooves on the gills to the labial palps whose role is to assist in directing food into the mouth. Bivalves can exercise some selection of their food and periodically the palps reject small masses of food, pseudofaeces, that are expelled from the mantle cavity, often by the vigorous "clapping" together of the shell valves.
What constitutes optimum foods for bivalves remains largely unknown however phytoplankton undoubtedly forms the major portion of the diet. Other sources of food may be important such as fine particles of non-living organic material (detritus) along with associated bacteria and also dissolved organic material.
Only general statements can be made about growth in juveniles and adults since it varies greatly between species, geographic distribution, i.e. climate, location in the subtidal or intertidal zones, as well as differences in individuals and in their genetic make up. Growth can also vary greatly from year to year and in temperate areas there are seasonal patterns in growth.
Growth can be measured in bivalves by different methods including increments in shell length or height, increases in total or soft body weight, or a combination of all of these factors. In tropical areas growth can vary with season, being faster during or after rainy periods when nutrients are washed into the ocean leading to increased production of phytoplankton. In temperate areas, growth is generally rapid during spring and summer when food is abundant and water temperatures are warmer. It virtually ceases in winter, resulting in annual checks in the shell. These winter checks can be used to age some bivalves. Some species are short lived but others may live to over 150 years.
In culture operations the important considerations in bivalve growth are length of time taken to grow to sexual maturity and to market size. The goal of bivalve culture is to grow bivalves to commercial size as quickly as possible to make the operation as economically attractive as possible.
Bivalves in the larval, juvenile and adult stages can die from a variety of causes, which can be environmental or biological in origin. The subject is much too large to consider in detail here but a brief synopsis is given to highlight a number of pertinent points, which could be important in hatchery operations.
The physical environment can cause severe mortalities to bivalves in all three stages. Too high temperatures or prolonged periods of cold temperatures can be lethal to bivalves as can be sudden swings in temperature. Severe extremes in salinities, particularly low salinities after periods of heavy rain or run off from melting snow, can also cause extensive mortalities. Heavy siltation can smother and kill juveniles and adults.
Pollution, particularly industrial pollution, can cause extensive mortalities in juvenile and adult bivalves. Both industrial and domestic pollution can be problems for hatchery operations and must be avoided. Domestic pollution can increase organic and bacterial loads in water as well as contributing a wide range of potentially toxic materials. Little is known of the combined effects of sub-lethal levels of the wide range of organic and organo-metallic compounds of man-made origin that may be present in such effluents.
Bivalves in the larval, juvenile and adult stages are preyed upon by a wide variety of animals that can cause severe mortalities. In the natural environment plankton feeders probably consume large quantities of larvae. In hatcheries, predation is largely a nonissue since the water used is filtered and any predators are removed.
Bivalves are hosts to parasites that can cause mortalities, particularly in the adult stage. Shell boring worms, Polydora sp., and sponges burrow into the shells and weaken them, thus causing mortalities.
Probably the major cause of mortalities in bivalves, particularly of larvae and juveniles in hatcheries, is disease. Considerable research effort has been expended in studying bivalve diseases and trying to develop methods to control them.
Diseases can be devastating to adult bivalves as witness the demise of some populations in the world. A few examples include,
Dermocystidium:
a fungal disease of bivalves caused by Perkinsus marinus;Delaware Bay Disease (MSX):
a disease caused by the haplosporidian protozoan, Haplosporidium (Minchinia) nelsoni;SSO (seaside organism disease):
a disease caused by the haplosporidian protozoan, Haplosporidium costale, (which together with H. nelsoni has decimated large populations of Virginia oysters on the Atlantic coast of the USA and now extends northwards into Atlantic Canada).Aber Disease:
A disease caused by the protozoan, Marteilia refringens;Bonamiasis (Haemocytic Disease):
A disease caused by the microcell parasite, Bonamia ostreae;
(Aber disease and Bonamiasis have resulted in the virtual demise of the European oyster in some parts of Europe).
Although considerable work has been carried out on these diseases, no practical methods have been developed to control them and restore oyster populations to previous levels. The severity of these diseases points to the care that must be taken when transporting adult bivalve stock into a hatchery.
In hatcheries it appears that diseases which do occur are caused by bacteria and not by protozoans. Bacteria are present to some degree in both algal and larval cultures. Indeed, bacteria may form an important part of the diet of larvae. However, periodically, large groups of larvae will die suddenly and an entire culture is lost. High bacterial counts are almost always associated with such large-scale mortalities. Bacteria may cause mortalities (pathogenic) or they may be simply present as opportunistic bacteria (saphrophytic), feeding on the dying larvae. Bacteria that cause diseases largely belong to the genus Vibrio sp. and every precaution must be taken to prevent them from causing epidemics in hatcheries. The best method to prevent such epidemics is to observe strict hygienic procedures and ensure that the larvae are well fed with high quality food. Larvae should be inspected regularly. If a disease occurs or is suspected, tanks and equipment should be sterilized with a bleach solution and rinsed well with freshwater. To protect larvae from further contamination, tanks should be refilled with UV-irradiated or ozone treated seawater. Use of antibiotics to control diseases is largely avoided in hatcheries. They are expensive and add to cost of operations and also there is the fear of a strain of bacteria developing that will be resistant to the antibiotics, which could lead to even more severe disease problems in the future.
Balouet, G., Poder, M. & Cahout, A. 1983. Haemocytic parasitosis: morphology and pathology of lesions in the French flat oyster, Ostrea edulis L. Aquaculture 34: 1 - 14
Bower, S.M. 1992. Diseases and parasites of mussels. In: The Mussel Mytilus: Ecology, Physiology, Genetics and Culture. E.G. Gosling (ed). Elsevier. Devel. Aquaculture Fish. Sci. 25: 465 - 510
Bower, S.M., McGladdery, S.E. & Price, I.M. 1994. Synopsis of infectious diseases and parasites of commercially exploited shellfish. Annual. Rev. of Fish. Diseases. Elsevier, 4: 1 - 199
Cesari, P. & Pellizzato, M. 1990. Biology of Tapes Philippinarum, p 21-46. In: Tapes Philippinarum: Biologia e Sperimentazione. Regione Veneto, Ente di Sviluppo Agricolo, Venice: 299 pp. (text in Italian and English)
Elston, R.A. 1990. Mollusc Diseases; Guide for the Shellfish Farmer. Washington Sea Grant. Univ. Washington, USA. SH179.S5E44: 73 pp.
Ford, S.E. & Tripp, M.R. 1996. Diseases and defense mechanisms. In: The Eastern Oyster, Crassostrea virginica. V.S. Kennedy, R.I.E. Newell and A.F. Eble (eds). Maryland Sea Grant, Univ. Maryland, College Park, Maryland, USA. ISBN-0-943-676-61-4: 423 - 441
Ford, S.E. 2001. Pests, parasites, diseases and defense mechanisms of the hard clam, Mercenaria mercenaria. In: Biology of the Hard Clam, J.N. Kraeuter and M. Castagna (eds). Elsevier. Devel. Aquaculture Fish. Sci. 31: 591 - 628
Getchell, R.G. 1991. Diseases and parasites of scallops. In: Scallops: Biology, Ecology and Aquaculture. S.E. Shumway (ed). Elsevier. Devel. Aquaculture Fish. Sci. 21: 471-494
Gosling, E. (ed). 1992. The Mussel, Mytilus: Ecology, Phytiology, Genetics and Culture. Elsevier. Devel. Aquaculture Fish. Sci. 25: 589 pp.
Gosling, E. 2002. Bivalve Molluscs, Biology, Ecology and Culture. Fishing News Books. Blackwell Publishing, UK: 443 pp.
Grizel, H., Miahle, E., Chagot, D., Buolo, V. & Bachere, E. 1988. Bonamiasis: a model study of disease in marine molluscs. In: Disease Processes in Marine Bivalve Molluscs W.S. Fisher (ed). Amer. Fish. Soc. Spec. Publ. 18. Bethesda Maryland: 1-4
Jorgensen, C.B. 1990. Bivalve Filter Feeding: Hydrodynamics, Bioenergetics, Physiology and Ecology. Olsen and Olsen, Fredensborg, Denmark: 140 pp.
Kennedy, V.X., Newell, R.I.E. & Eble, A.F. (eds). 1996. The eastern oyster Crassostrea virginica. Maryland Sea Grant, Univ. Maryland, College Park, Maryland, USA. ISBN-0-943-676-61-4: 734 pp.
Koringa, P. 1976. Farming the Flat Oyster of the Genus Ostrea. Elsevier. Devel. Aquaculture Fish. Sci. 3: 238 pp.
Kraeuter, J.N. & Castagna, M. (eds). 2001. Biology of the Hard Clam. Elsevier. Devel. Aquaculture Fish. Sci. 51: 751 pp.
Manzi, J.J. & Castagna, M. 1989. Clam Mariculture in North America. Elsevier. Devel. Aquaculture and Fish. Sci. 19: 461 pp.
Mason, J. 1983. Scallop and Queen Fisheries in the British Isles. Fishing News Books Ltd, Surrey, UK: 143 pp.
Morton, J.E. 1960. Molluscs: An Introduction to their Form and Function. Harper Textbooks, New York, USA: 232 pp.
Quayle, D.G. 1988b. Pacific oyster culture in British Columbia. Can. Bull. Fish. Aquatic Sci. 218: 241 pp.
Shumway, S.E. (ed). 1991. Scallops, biology, ecology and aquaculture. Elsevier. Devel. In Aquaculture Fish. Sci. 21: 1095 pp.
Yonge, C.M. & Thompson, T.E. 1976. Living Marine Molluscs. Will Collins, Sons and Co. Ltd, Glasgow: 288 pp.