by
S.M. BANERJEA
and
S.C. BANERJEE
Central Inland Fisheries Research Institute Sub-Station
Cuttack, India
Abstract
For increased production of plankton in fish ponds a series of experiments were conducted in cement cisterns and in ponds using trace elements like manganese, copper and iron along with phosphatic fertilizer. Two soil types were used in this investigation; one a slightly acidic soil, high in phosphorus but low in manganese and the other also with a slightly acidic reaction but low in phosphorus and relatively high in manganese. The results showed that both the soils responded fairly to treatment with manganese showing an increase of plankton weight by about 14 percent due to manganese alone. In the case of high phosphorus and low manganese soil the additional increase of plankton for a dose of 1 kg/ha of manganese was 16.4 percent, which compares well with an increase of 14.6 percent by 20 kg/ha of P2O5. In the other soil with low phosphorus and high manganese the additional increase in plankton weight due to manganese was 21 percent against 48 percent by phosphate alone, but when the effect of phosphate plus manganese was compared with that of phosphate minus manganese, it was observed that both the soil types exhibited about 14 percent increase in plankton weight due to manganese alone showing that for the range of water soluble manganese in soil from 0.25 to 2.8 ppm and soluble manganese in water from 0.25 to 2.8 ppm and soluble manganese in water from 0.001 to 0.02 ppm, the degree of response was rather independent of manganese status of soil and water. The effect of incorporation of two other trace elements, copper and iron, at the same dose did not show any marked increase in production of plankton.
FERTILISATION DES ETANGS DE PISCICULTURE AVEC DU MANGANESE A FAIBLE DOSE POUR ACCROITRE LA PRODUCTION DE PLANCTON
Résumé
Pour accroître la production de plancton dans les étangs de pisciculture une série d'expériences ont été faites dans des citernes cimentées et dans des étangs avec des oligoéléments tels que le manganèse, le cuivre et le fer associés à des engrais phosphatés. Les expériences ont été pratiquées sur deux types de sol: l'un légèrement acide, à teneur élevée en phosphore mais faible en manganèse, l'autre également légèrement acide mais ayant une faible teneur en phosphore et une teneur relativement forte en manganèse. Les résultats montrent que dans les deux cas le traitement au manganèse a donné des résultats positifs puisque l'adjonction de ce seul élément a provoqué une augmentation en poids du plancton d'environ 14 pour cent. Dans le cas du sol à haute teneur en phosphore et à faible teneur en manganèse, l'augmentation de plancton pour une dose de 1 kg/ha de manganèse était de 16,4 pour cent, ce qui se compare favorablement à l'augmentation de 14,6 pour cent obtenue avec 20 kg/ha de P2O5. Dans l'autre sol, celui à faible teneur en phosphore et à teneur élevée en manganèse, l'augmentation pondinale du plancton était de 21 pour cent avec le manganèse alors qu'il atteignait 48 pour cent avec le phosphate employé seul, mais en comparant les effets obtenus avec phosphate plus manganèse à ceux obtenus avec phosphate moins manganèse on a constaté, avec les deux types de sol, une augmentation d'environ 14 pour cent du poids du plancton attribuable au manganèse seul. Ainsi, lorsque la teneur du sol en manganèse soluble dans l'eau varie entre 0,25 et 2,8 ppm et que la teneur de l'eau en manganèse soluble se situe entre 0,001 à 0,02 ppm le degré de réaction est relativement indépendant de la quantité de manganèse contenue dans le sol et dans l'eau. L'incorporation de deux autres oligo-éléments, cuivre et fer, à des doses analogues, n'a pas eu d'effet sensible sur la production de plancton.
FERTILIZACION DE LOS ESTANQUES PISCICOLAS CON MANGANESO COMO OLIOGOELEMENTO PARA EL AUMENTO DE LA PRODUCCION DE PLANCTON
Extracto
Para elevar la producción de plancton en los estanques piscícolas se llevaron a cabo una serie de experimentos en cisternas de cemento y en estanques utilizando oligoelementos como el manganeso, el cobre y el hierro junto con fertilizantes fosfáticos. Se usaron dos tipos de suelos en esta investigación: uno ligeramente ácido con elevada cantidad de fósforo pero con poco manganeso, y el otro también con una reacción ligeramente ácida pero con poco fósforo y relativamente elevada proporción de manganeso. Los resultados demostraron que ambos suelos respondieron bastante bien al tratamiento con manganeso, mostrando un aumento del peso del plancton del 14 por ciento, aproximadamente, debido solamente al manganeso. En el caso del suelo con elevadas cantidades de fósforo y poco manganeso, el aumento adicional de plancton para una dosis de 1 kg/ha. de manganeso fué del 16,4 por ciento, lo cual se aproxima mucho al aumento de 14,6 por ciento por 20 kg/ha. de P2O5. En el otro suelo con poco fósforo y elevada proporción de manganeso, el aumento adicional del peso del plancton debido al manganeso fué del 21 por ciento frente al 48 por ciento con fósforo solamente, pero cuando el efecto del fosfato unido al manganeso, se comparó con el del fosfato sin manganeso, se observó que ambos tipos de suelo exhibían alrededor de un 14 por ciento de aumento en el peso del plancton debido al manganeso solamente, mostrando que para los márgenes de variación de manganeso hidrosoluble en el suelo de 0,25 a 2,8 ppm y de manganeso en el agua de 0,001 a 0,02 ppm, el grado de respuesta era bastante independiente de la cantidad de manganeso en el suelo y en el agua. El efecto de la incorporación de otros dos oligoelementos, cobre y hierro, a las mismas dosis, no mostró ningún aumento sensible de la producción de placnton.
Increased production of phyto-plankton, or more precisely speaking, acceleration of the rate of primary production in fish ponds is of vital importance in successful fish culture operations. Depending primarily on the fertility status of water and soil in a pond for their growth, planktonic algae form the first and important link in the food chain for fish. Natural and inherent fertility of ponds has often been found to be unsatisfactory due to deficiency in one or more of the nutrient elements in soil and water. Correction of this deficient condition by the application of fertilizers in proper form and in optimal quantities forms an important problem for investigation. Though a considerable amount of work has been done with fertilizer elements like nitrogen, phosphorus, potassium and calcium (Mortimer,1954) practically very little work has been done with micro-elements like manganese, copper, iron, boron, vanadium, molybdenum, etc. Two obvious reasons are responsible for it. Firstly, the importance of these elements in plant and animal nutrition has been recognized only in comparitively recent years; secondly the function of these elements in metabolic processes still remains obscure in many cases and so more concerted efforts have been directed towards elucidating the role of micro-nutrients in biochemical processes in plants and animals rather than conducting field experiments to study growth promoting properties.
Among the micro-elements probably manganese has been the subject of more extensive investigations. In the early part of this century evidence was presented by McHargue (1922) and others to show that manganese must be regarded as essential for plant growth. Since then so many investigators have recorded observations on manganese requirements of higher plants from different parts of the world. In India Patnaik (1955) from a series of sand culture studies showed that in the absence of manganese, growth of rice plants was poor, the plants were lighter green in colour and the leaves were spotted in appearance. When manganese was added at a concentration of 1 ppm the yield was nearly doubled, but a concentration over 5 ppm proved toxic, the plants becoming stunted and chlorotic. In more recent years the role of micro-nutrients in plant metabolism has been studied under laboratory conditions by many workers (Arnon, 1958; Pirson, 1958; Brown et al., 1958; Eyster et al., 1958) using planktonic algae of simple structure like Ankistrodesmus, Scenedesmus and Chlorella sp. It is now almost well established that manganese plays an important role both in photosynthesis and in heterotrophic growth of phyto-plankton. The requirement of manganese for entirely autotrophic physiological processes is comparatively much higher than that required for heterotrophic growth. It was shown that under laboratory conditions, growth of phyto-plankton (Scenedesmus) estimated by cell count, packed cell volume and dry weight, was arrested under manganese deficient condition. For aquatic micro-plants earlier work of Hopkins (1930a, b and c), Clark and Fly (1930), Clark (1933) and Saegar (1933) showed that under laboratory conditions addition of manganese in culture solution increased the growth of Chlorella, Lemna minor and five other species of Lemnaceae, to a marked extent.
In an attempt to explore the possibility of using the existing laboratory findings for increasing production of plankton in fish ponds a series of experiments were conducted in laboratory, in cement cisterns under semi-field conditions and in fish ponds under purely field conditions. The results of these experiments are presented and discussed in this paper.
In the case of land plants there are recognized deficiency symptoms specific for individual trace elements which can be of diagnostic help. Such deficiency symptoms under extremely low concentrations of the element under laboratory conditions have been recorded for planktonic algae, but under field conditions manganese concentration in water is not likely to show such extremely low values. So to know what actually constitutes the deficient condition for a particular trace element, we have to depend entirely on the concentration of that element in soil and water and study the response of the soil type to treatment with salts of that element using growth of plankton as an index of response.
In preliminary observations on the manganese status of soil and water of fish ponds in Orissa, the authors (Banerjee and Banerjea - unpublished) have shown that the manganese content of soil in various forms (water soluble, exchangeable, reducible, total) and the soluble manganese in water varies from pond to pond even within a radius of forty miles. Of the different forms of manganese, water soluble manganese in soil showed highly significant correlation with manganese in water. Among the fish ponds studied it was noticed that soluble manganese in water and soil was very poor in Jobra farm showing an average of 0.014 ppm and 0.420 ppm respectively, most of the ponds having a concentration of 0.005 ppm in water, while Chaudwar farm had relatively higher manganese concentration in water and soil with an average of 0.073 ppm in water and 2.520 ppm in soil. These two soils representing low and high manganese status of pond soils studied so far, were selected for the experimental study.
The experiments were conducted in three series. In the first series, the low manganese soil of the Jobra farm was studied in cement cisterns under semi-field conditions using manganese as a trace element. The second series of experiments were undertaken in small ponds in Chaudwar fish farm, having a relatively high concentration of manganese in water and in soil, using only manganese as a trace element. In the third series of experiments a combination of three trace elements, viz., manganese, copper and iron was used. In all the experiments a basal dose of superphosphate at the rate of 20 kg of P2O5 per hectare was added so that phosphorus deficiency in soil may not act as a limiting factor in plankton production.
This experiment was carried out in six cement cisterns of approximate dimensions, 1.0 m × 0.7 m × 1.0 m (length × breadth × depth), and about 0.5 m3 capacity. The cisterns were drained, cleared and allowed to dry. A bottom substratum of about 15 cm thickness of soil from a pond in the Jobra fish farm was provided in each cistern. The soil was collected in bulk, dried, powdered and spread uniformly on the bottom of the cisterns. They were then refilled with water from the parent pond. A week's time was allowed for the establishment of equilibrium conditions between soil and water. In order to ensure sufficient seed for plankton, each cistern was inoculated with a concentrated plankton sample of about 5 cc collected from the parent pond. A week later manuring was done. Two cisterns were kept as control without any treatment; two were treated with superphosphate alone at the rate of 100 Kg/ha, equivalent to 20 Kg P2O5 per hectare, while the remaining two were manured with superphosphate at the rate of 100 kg/ha + manganous sulphate (MnSO4,H2O) at the rate of 3 Kg/ha, equivalent to 1 Kg Mn per hectare, so that the effective concentration of added manganese on volume basis was 0.14 ppm. The fertilizers were mixed with a quantity of mud from the parent pond, formed into small pellets and distributed uniformly all over the water area. Soil samples were collected and analysed before treatment, three months after treatment and finally after six months when the experiment was concluded. Water samples for chemical analysis and plankton analysis were collected fortnightly. Plankton samples were collected by straining 10 litres of water from 20 different places through a bolting silk net, and this being only 2 percent of the total volume of water, the plankton concentration in the cistern was not affected appreciably. From each cistern 250 cc of water was collected for chemical analysis, the loss of water due to this and due to evaporation was made up by adding water from the pond strained through a bolting silk net. The plankton sample after being strained was concentrated to a volume of 20 cc and a uniform mixture was obtained by thorough shaking from which 1 cc was preserved in 4 percent formalin for estimating the percentage of zoo- and phyto-plankton and also to determine the predominant plankters. The remaining portion was centrifuged, the supernatant liquid decanted and the sample filtered through a quantitative filter paper (Whatman No.42). After washing the residue with distilled water, it was transferred to a tared platinum basin, dried in an electric oven at a constant temperature of 103°C and weighed to a constant weight. The monthly averages for water and plankton analysis are given in Tables I(a) and I(c), while Table I(b) presents the results of periodic examination of soil. The duration of the experiment was six months, as indicated earlier.
TABLE I(a)
Water quality of treated and untreated cisterns1
| Control (No treatment) | 20 Kg P2O5 per hectare | |||||||
| Month | pH | Alkalinity (CaCO3) | Phosphate (P2O5) | Manganese (Mn) | pH | Alkalinity (CaCO3) | Phosphate (P2O5) | Manganese (Mn) |
| December | 8.1 | 81 | 0.04 | 0.001 | 8.1 | 75 | 0.05 | 0.001 |
| January | 8.7 | 78 | 0.06 | 0.001 | 8.7 | 77 | 0.10 | 0.001 |
| February | 8.8 | 71 | 0.06 | 0.001 | 9.3 | 61 | 0.15 | 0.001 |
| March | 8.6 | 85 | 0.08 | 0.001 | 8.7 | 70 | 0.28 | 0.002 |
| April | 8.8 | 82 | 0.11 | 0.001 | 9.0 | 73 | 0.45 | 0.002 |
| May | 8.8 | 80 | 0.32 | 0.001 | 8.8 | 86 | 0.48 | 0.002 |
| Average | 8.6 | 80 | 0.11 | 0.001 | 8.8 | 74 | 0.25 | 0.0015 |
| 20 Kg P2O5 + 1Kg Mn per hectare | ||||||||
| Month | pH | Alkalinity (CaCO3) | Phosphate (P2O5) | Manganese (Mn) | ||||
| December | 8.2 | 76 | 0.04 | 0.001 | ||||
| January | 8.7 | 87 | 0.06 | 0.001 | ||||
| February | 8.9 | 81 | 0.08 | 0.002 | ||||
| March | 8.7 | 93 | 0.12 | 0.003 | ||||
| April | 8.6 | 103 | 0.40 | 0.003 | ||||
| May | 8.9 | 84 | 0.51 | 0.003 | ||||
| Average | 8.7 | 87 | 0.20 | 0.0022 | ||||
TABLE I (b)
Soil condition of treated and untreated cisterns 1
| Control (no treatment) | |||||||
| Month | pH | P2O5 | N | Mn | |||
| Total | Available | Total | Available | Total | Water Soluble | ||
| December | 6.3 | 1260 | 120 | 455 | 214 | 550 | 0.22 |
| March | 6.4 | 1280 | 130 | 440 | 230 | 490 | 0.24 |
| June | 6.3 | 1250 | 150 | 450 | 252 | 519 | 0.28 |
| Average | 6.3 | 1262 | 133 | 448 | 232 | 517 | 0.25 |
| 20 Kg P2O5 per hectare | |||||||
| pH | P2O5 | N | Mn | ||||
| Total | Available | Total | Available | Total | Water Soluble | ||
| December | 6.4 | 1240 | 120 | 450 | 218 | 570 | 0.24 |
| March | 6.6 | 1300 | 140 | 450 | 239 | 570 | 0.25 |
| June | 6.5 | 1260 | 150 | 465 | 262 | 580 | 0.28 |
| Average | 6.5 | 1267 | 137 | 455 | 240 | 573 | 0.26 |
| 20 Kg P2O5 + 1 Kg Mn per hectare | |||||||
| pH | P2O5 | N | Mn | ||||
| Total | Available | Total | Available | Total | Water Soluble | ||
| December | 6.4 | 1260 | 130 | 460 | 220 | 570 | 0.23 |
| March | 6.5 | 1260 | 140 | 445 | 240 | 580 | 0.26 |
| June | 6.3 | 1280 | 150 | 445 | 262 | 580 | 0.31 |
| Average | 6.4 | 1267 | 140 | 453 | 241 | 577 | 0.27 |
TABLE 1 (c)
Plankton concentrations in treated and untreated cisterns
| Control (No treatment) | |||||
| PLANKTON | |||||
| Month | Total weight γ/litre | % | Predominant phyto-plankters | % | Predominant zoo-plankters |
| December | 1875 | 32 | Cladophora, Scenedesmus | 68 | Diaptomus, Cladocerans |
| January | 1875 | 54 | Cosmarium, Amphora | 46 | Nauplii, Conochilus |
| February | 1825 | 36 | Volvox, Scenedesmus | 64 | Keratella, Cladocerans |
| March | 1925 | 74 | Microcystis, Closterium | 26 | Cyclops, Nauplii |
| April | 2000 | 88 | Volvox, Microcystis | 12 | Copepods, Rotifers |
| May | 2025 | 56 | Volvox, Anabaena | 44 | Copepods, Rotifers |
| Average | 1921 | ||||
| Increase over control (%) | - | ||||
20 Kg P2O5 per hectare | |||||
| PLANKTON | |||||
| Total weight γ/litre | % | Predominant phyto-plankters | % | Predominant zoo-plankters | |
| December | 1925 | 40 | Scenedesmus, Spirogyra | 60 | Copepods, Rotifers |
| January | 2175 | 62 | Oscillatoria, Pandorina | 38 | Diaptomus, Rotifers |
| February | 2040 | 26 | Volvox, Spirogyra | 74 | Copepods, Cladocerans |
| March | 2325 | 80 | Microcystis, Volvox | 20 | Diaptomus, Nauplii |
| April | 2525 | 92 | Microcystis, Oscillatoria | 8 | Brachionus, Keratella |
| May | 2225 | 62 | Volvox, Oscillatoria | 38 | Cyclops, Arcella |
| Average | 2203 | ||||
| Increase over control (%) | 14.6 | ||||
20 Kg P2O5 + 1 Kg Mn per hectare | |||||
| PLANKTON | |||||
| Total weight γ/litre | % | Predominant phyto-plankters | % | Predominant zoo-plankters | |
| December | 2050 | 36 | Spirogyra, Cladophora | 64 | Rotifers, Copepods |
| January | 2400 | 80 | Volvox, Spirogyra | 20 | Cladocerans, Cyclops |
| February | 2225 | 22 | Scenedesmus, Lyngbia | 78 | Brachionus, Diaptomus |
| March | 2600 | 82 | Microcystis, Oscillatoria | 18 | Cladocerans, Rotifers |
| April | 3000 | 95 | Microcystis, Volvox | 5 | Nauplii, Brachionus |
| May | 2825 | 72 | Microcystis, Anabaena | 28 | Cladocerans, Rotifers |
| Average | 2517 | ||||
| Increase over control (%) | 31.0 | ||||
| Increase over phosphate treatment(%) | 14.2 | ||||
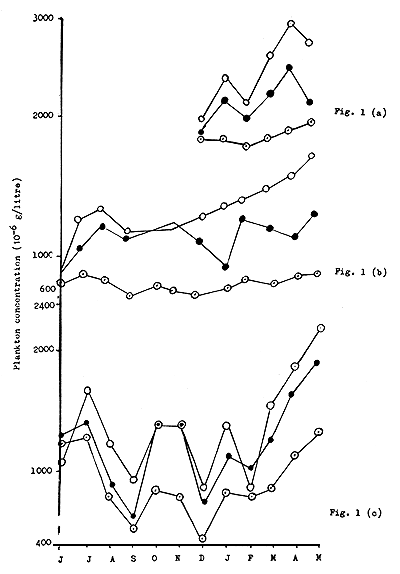
Fig. 1 Fluctuations in plankton concentrations for the control and treated series
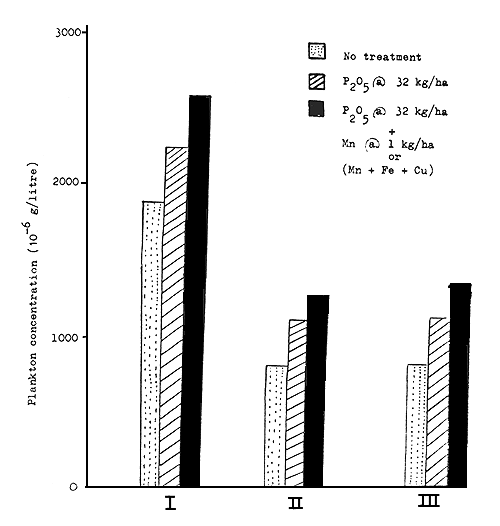
Fig. 2 Average plankton concentration for different treatments
The second series of experiments was conducted in small nursery ponds at Chaudwar fish farm, ranging in size from 0.048 to 0.076 hectare. The ponds though perennial in nature retained only about one meter of water during the summer months of May–June, when they were cleared of all the previous stock of fish by repeated nettings with a fine-meshed net. Submerged and floating vegetation, as well as marginal grasses were completely removed by manual labour. As the ponds had high bunds on all sides, very little water entered them from the catchment area. After making collections of soil, water and plankton in the month of June during pre-treatment condition, two of the ponds were treated with superphosphate alone at the rate of 20 Kg P2O5/ha and two were treated with superphosphate at the rate of 20 Kg P2O5/ha + manganous sulphate at the rate of 1 Kg mn/ha, during the last week of June. The remaining two ponds were kept as control without any treatment. Regular periodic observations on soil condition, water quality and plankton population were continued for one complete year. The data are presented in Tables II(a) to II(c).
In the third series of experiments all the procedures were the same as in the second series, excepting that three trace elements, viz., manganese, copper and iron were used in combination at the rate of 1 Kg mn/ha + 1 Kg cu/ha + Kg fe/ha instead of only 1 Kg mn/ha, added in the second series. Manganous sulphate, copper sulphate and ferrous sulphate were used for the respective elements. As all the ponds had nearly one meter of water during the period of treatment, the effective concentrations of added trace elements were 0.1 ppm on volume basis. The observations on plankton concentration, water quality and soil condition were continued for one year and the data are presented in Tables III(a) to III(c). Fluctuations in plankton concentrations for the control and treated series are given in Fig. 1, while the variation in average plankton concentrations for the control and treated series in different experiments are shown in Fig. 2.
That trace elements added to fish ponds along with fertilizers might possibly increase plankton production was probably first pointed out by Mehring (1918). While explaining the variable behaviour of potassium fertilizers, he suggested that presence of trace elements in kainite might be responsible for its beneficial effect. His remark, however, received only scant attention by workers on pond fertilization. Swingle (1947) during his work on pond fertilization at Alabama found that results are quite unpredictable. Sometimes fertilization was followed by delayed algal growth and attempts to hasten production of algae in such cases by the addition of minor elements such as zinc, manganese, boron, iron and copper were all without any effect. In the absence of any knowledge regarding the concentration of these elements in the soil and water phases, these findings did not lead to any definite conclusions. Harvey (1947) working with marine plankton found that as little as 0.0001 ppm of manganese increased the growth of some forms of planktonic algae. Experiments conducted by Henderson (1949) in concrete pools showed that manganese may be of some help in causing and maintaining water blooms.
In the present study the results of experiments in the first series carried out in cement cisterns under more controlled conditions showed that both treatment with superphosphate alone and with superphosphate + manganese sulphate result in definite increase in weight of plankton, but the increase in the latter case was higher than in the former (Table I(a) and Fig. 1(a)). Plankton concentrations in all the cisterns showed irregular fluctuations during the period of experiment, but the total weight of plankton produced by superphosphate + manganese sulphate treatment was always higher than that for the untreated or superphosphate treated sets. This experiment was initiated in the winter month of December and the increase of plankton concentration in the treated cisterns over untreated ones became more pronounced during the summer months of March-May. The average plankton concentrations for the three sets were 1921,2203 and 2517 /litre showing an increase of 14.6 percent and 31.0 percent respectively for superphosphate alone and superphosphate + manganese sulphate. Thus it is seen that the effect of adding 1 Kg/ha of Mn compares well with that of adding 20 Kg/ha of P2O5. It has, however, to be remembered that the soil under investigation was fairly rich in total and available phosphorus (Table I(b)) and was not expected to show a marked response to single phosphatic fertilizer.
TABLE II (a)
Water quality of treated and untreated ponds1
| Control (No treatment) | ||||
| Month | pH | Alkalinity (CaCO3) | Phosphate (P2O5) | Manganese (Mn) |
| June | 8.0 | 92 | 0.12 | 0.03 |
| July | 8.4 | 109 | 0.08 | 0.02 |
| August | 8.0 | 82 | 0.04 | 0.02 |
| September | 7.2 | 70 | 0.03 | 0.01 |
| October | 7.2 | 68 | 0.07 | 0.01 |
| November | 7.1 | 56 | 0.03 | 0.01 |
| December | 7.0 | 52 | 0.02 | 0.01 |
| January | 7.4 | 83 | 0.02 | 0.01 |
| February | 8.2 | 104 | 0.04 | 0.02 |
| March | 8.4 | 105 | 0.09 | 0.02 |
| April | 8.4 | 110 | 0.015 | 0.04 |
| May | 8.2 | 104 | 0.017 | 0.04 |
| Average | 7.8 | 86 | 0.07 | 0.02 |
| 20 Kg P2O5 per hectare | ||||
| pH | Alkalinity (CaCO3) | Phosphate (P2O5) | Manganese (Mn) | |
| June | 7.9 | 76 | 0.14 | 0.04 |
| July | 8.2 | 72 | 0.09 | 0.02 |
| August | 7.7 | 66 | 0.03 | 0.02 |
| September | 7.3 | 62 | 0.03 | 0.01 |
| October | 7.3 | 62 | 0.07 | 0.01 |
| November | 7.1 | 62 | 0.05 | 0.01 |
| December | 7.1 | 58 | 0.02 | 0.01 |
| January | 7.4 | 76 | 0.02 | 0.02 |
| February | 7.5 | 84 | 0.04 | 0.02 |
| March | 7.6 | 86 | 0.17 | 0.04 |
| April | 7.7 | 92 | 0.18 | 0.04 |
| May | 7.6 | 94 | 0.23 | 0.06 |
| Average | 7.5 | 74 | 0.09 | 0.02 |
| 20 Kg P2O5 + 1 Kg per hectare | ||||
| pH | Alkalinity (CaCO3) | Phosphate (P2O5) | Manganese (Mn) | |
| June | 8.0 | 80 | 0.13 | 0.03 |
| July | 8.5 | 84 | 0.09 | 0.05 |
| August | 7.7 | 68 | 0.10 | 0.03 |
| September | 7.4 | 73 | 0.02 | 0.02 |
| October | 7.5 | 75 | 0.02 | 0.02 |
| November | 7.4 | 74 | 0.04 | 0.01 |
| December | 7.4 | 76 | 0.02 | 0.01 |
| January | 7.8 | 82 | 0.02 | 0.01 |
| February | 7.9 | 82 | 0.03 | 0.03 |
| March | 7.9 | 84 | 0.15 | 0.04 |
| April | 8.0 | 84 | 0.22 | 0.04 |
| May | 8.1 | 86 | 0.13 | 0.04 |
| Average | 7.8 | 79 | 0.08 | 0.03 |
1 Expressed in mg/litre, except pH
TABLE II (b)
Soil condition of treated and untreated ponds1
| Control (No treatment) | |||||||
| Month | pH | P2O5 | N | Mn | |||
| Total | Available | Total | Available | Total | Water Soluble | ||
| June | 6.6 | 680 | 36 | 790 | 396 | 627 | 3.1 |
| July | 6.7 | 650 | 30 | 760 | 375 | 630 | 2.8 |
| December | 6.7 | 640 | 31 | 710 | 345 | 625 | 2.4 |
| June | 6.5 | 650 | 34 | 770 | 380 | 630 | 3.0 |
| Average | 6.6 | 655 | 33 | 757 | 371 | 628 | 2.8 |
| 20 Kg P2O5 per hectare | |||||||
| pH | P2O5 | N | Mn | ||||
| Total | Available | Total | Available | Total | Water Soluble | ||
| June | 6.6 | 680 | 32 | 780 | 372 | 645 | 3.6 |
| July | 6.7 | 660 | 40 | 748 | 352 | 640 | 3.2 |
| December | 6.9 | 640 | 32 | 700 | 330 | 630 | 2.8 |
| June | 6.6 | 660 | 36 | 770 | 360 | 625 | 3.8 |
| Average | 6.7 | 660 | 35 | 749 | 353 | 632 | 3.3 |
| 20 Kg P2O5 + 1 Kg Mn per hectare | |||||||
| pH | P2O5 | N | Mn | ||||
| Total | Available | Total | Available | Total | Water Soluble | ||
| June | 6.5 | 660 | 32 | 760 | 380 | 640 | 3.2 |
| July | 6.6 | 680 | 42 | 740 | 368 | 625 | 3.6 |
| December | 6.8 | 670 | 30 | 770 | 322 | 610 | 3.0 |
| June | 6.5 | 650 | 36 | 750 | 382 | 640 | 3.9 |
| Average | 6.6 | 665 | 35 | 755 | 363 | 629 | 3.4 |
TABLE II (c)
Plankton concentrations in treated and untreated ponds
| Control (No treatment) | |||||
| PLANKTON | |||||
| Month | Total weight γ/litre | % | Predominant phyto-plankters | % | Predominant zoo-plankters |
| June | 778 | 56 | Ulothrix, Spirogyra | 44 | Copepods, Rotifers |
| July | 822 | 50 | Oscillatoria, Spirogyra | 50 | Cladocerans, Rotifers |
| August | 778 | 28 | Anabaena, Amphora | 72 | Diaptomus, Cladocerans |
| September | 620 | 12 | Cladophora, Scenedesmus | 88 | Copepods, Cladocerans |
| October | 720 | 36 | Oscillatoria, Scenedesmus | 64 | Cladocerans, Diaptomus |
| November | 650 | 24 | Amphora, Closterium | 76 | Copepods, Rotifers |
| December | 600 | 20 | Cosmarium, Ulothrix | 80 | Cladocerans, Rotifers |
| January | 738 | 56 | Volvox, Scenedesmus | 44 | Brachionus, Copepods |
| February | 822 | 62 | Oscillatoria, Anabaena | 38 | Rotifers, Keratella |
| March | 744 | 70 | Volvox, Anabaena | 30 | Cladocerans, Nauplii |
| April | 860 | 60 | Microcystis, Oscillatoria | 40 | Brachionus, Copepods |
| May | 844 | 68 | Microcystis, Volvox | 32 | Difflugia, Copepods |
| Average | 748 | ||||
| Increase over control (%) | - | ||||
20 Kg P2O5 per hectare | |||||
| PLANKTON | |||||
| Month | Total weight γ/litre | % | Predominant phyto-plankters | % | Predominant zoo-plankters |
| June | 835 | 36 | Anabaena, Amphora | 64 | Diaptomus, Nauplii |
| July | 1010 | 72 | Oscillatoria, Microcystis | 28 | Cladocerans, Difflugia |
| August | 1140 | 66 | Volvox, Pandorina | 34 | Cladocerans, Conochillus |
| September | 1000 | 42 | Lyngbia, Oscillatoria | 58 | Rotifers, Brachionus |
| October | 1065 | 42 | Oscillatoria, Scenedesmus | 58 | Keratella, Nauplii |
| November | 1140 | 32 | Pandorina, Cenedesmus | 68 | Rotifers, Diaptomus |
| December | 1060 | 36 | Cosmarium, Anabaena | 64 | Keratella, Cladocerans |
| January | 930 | 10 | Pandorina, Closterium | 90 | Cladocerans, Copepods |
| February | 1268 | 72 | Volvox, Anabaena | 28 | Cyclops, Nauplii |
| March | 1246 | 76 | Volvox, Oscillatoria | 24 | Keratella, Brachionus |
| April | 1214 | 80 | Microcystis, Volvox | 20 | Diaptomus, Cladocerans |
| May | 1428 | 86 | Microcystis, Volvox | 14 | Copepods, Keratella |
| Average | 1111 | ||||
| Increase over control (%) | 48.0 | ||||
| 20 Kg P2O5 + 1 Kg Mn per hectare | |||||
| PLANKTON | |||||
| Month | Total weight γ/litre | % | Predominant phyto-plankters | % | Predominant zoo-plankters |
| June | 844 | 40 | pandorina, Cladophora | 60 | Difflugia, Copepods |
| July | 1220 | 62 | Scenedesmus, Mycrocystis | 38 | Rotifers, Copepods |
| August | 1240 | 60 | Microcystis, Volvox | 40 | Rotifers, Copepods |
| September | 1024 | 52 | Volvox, Cosmarium | 48 | Copepods, Cladocerans |
| October | 1086 | 40 | Cosmarium, Anabaena | 60 | Keratella, Cladocerans |
| November | 1114 | 36 | Oscillatoria, Closterium | 64 | Cladocerans, Rotifers |
| December | 1260 | 42 | Closterium, Scenedesmus | 58 | Keratella,Brachionus |
| January | 1296 | 56 | Oscillatoria, Scenedesmus | 44 | Diaptomus, Rotifers |
| February | 1374 | 68 | Anabaena, Volvox | 32 | Rotifers, Brachionus |
| March | 1496 | 70 | Volvox, Oscillatoria | 30 | Cladocerans, Copepods |
| April | 1624 | 86 | Microcystis, Oscillatoria | 14 | Brachionus, Nauplii |
| May | 1824 | 92 | Microcystis, Volvox | 8 | Diaptomus, Keratella |
| Average | 1267 | ||||
| Increase over control (%) | 69.0 | ||||
| Increase over phosphate treatment (%) | 14.4 | ||||
TABLE III (a)
Water quality of treated and untreated ponds1
| Control (No treatment) | ||||
| Month | pH | Alkalinity (CaCO3) | Phosphate (P2O5) | Manganese (Mn) |
| June | 8.0 | 85 | 0.09 | 0.018 |
| July | 7.8 | 87 | 0.045 | 0.027 |
| August | 7.1 | 76 | 0.036 | 0.012 |
| September | 7.1 | 68 | 0.016 | 0.008 |
| October | 7.0 | 54 | 0.020 | 0.003 |
| November | 7.3 | 58 | 0.024 | 0.002 |
| December | 7.4 | 70 | 0.022 | 0.002 |
| January | 7.5 | 80 | 0.037 | 0.001 |
| February | 7.5 | 80 | 0.050 | 0.001 |
| March | 7.6 | 94 | 0.070 | 0.010 |
| April | 7.8 | 98 | 0.060 | 0.055 |
| May | 8.0 | 96 | 0.065 | 0.049 |
| Average | 7.5 | 79 | 0.040 | 0.016 |
| 20 Kg P2O5 per hectare | ||||
| pH | Alkalinity (CaCO3) | Phosphate (P2O5) | Manganese (Mn) | |
| June | 8.2 | 107 | 0.080 | 0.018 |
| July | 8.0 | 102 | 0.270 | 0.022 |
| August | 7.1 | 83 | 0.120 | 0.011 |
| September | 7.1 | 76 | 0.068 | 0.008 |
| October | 7.1 | 65 | 0.045 | 0.005 |
| November | 7.6 | 70 | 0.025 | 0.002 |
| December | 7.6 | 75 | 0.020 | 0.004 |
| January | 7.5 | 75 | 0.025 | 0.003 |
| February | 7.5 | 72 | 0.040 | 0.001 |
| March | 7.9 | 110 | 0.090 | |
| April | 7.9 | 125 | 0.085 | 0.082 |
| May | 7.8 | 128 | 0.080 | 0.080 |
| Average | 7.6 | 91 | 0.079 | 0.022 |
| 1 Kg Mn 20 Kg P2O5 + 1 Kg Cu per hectare 1 Kg Fe | ||||
| pH | Alkalinity (CaCO3) | Phosphate (P2O5) | Manganese (Mn) | |
| June | 8.1 | 109 | 0.100 | 0.022 |
| July | 7.8 | 103 | 0.220 | 0.037 |
| August | 7.2 | 80 | 0.080 | 0.018 |
| September | 7.2 | 72 | 0.032 | 0.009 |
| October | 7.0 | 54 | 0.025 | 0.006 |
| November | 7.3 | 69 | 0.025 | 0.005 |
| December | 7.6 | 72 | 0.030 | 0.003 |
| January | 7.8 | 80 | 0.040 | 0.003 |
| February | 7.8 | 85 | 0.060 | 0.001 |
| March | 8.0 | 105 | 0.080 | 0.028 |
| April | 8.3 | 124 | 0.095 | 0.120 |
| May | 8.2 | 123 | 0.095 | 0.110 |
| Average | 7.7 | 90 | 0.076 | 0.030 |
TABLE III (b)
Soil condition in treated and untreated ponds1
| Control (No treatment) | |||||||
| Month | pH | N | P2O5 | Mn | |||
| Total | Available | Total | Available | Total | Water Soluble | ||
| June | 6.2 | 740 | 450 | 630 | 38 | 670 | 3.5 |
| July | 6.5 | 700 | 390 | 650 | 35 | 620 | 4.4 |
| December | 6.5 | 680 | 370 | 635 | 30 | 650 | 4.0 |
| May | 6.2 | 730 | 410 | 640 | 35 | 630 | 4.0 |
| Average | 6.4 | 713 | 405 | 639 | 35 | 642 | 4.2 |
| 20 Kg P2O5 per hectare | |||||||
| pH | N | P2O5 | Mn | ||||
| Total | Available | Total | Available | Total | Water Soluble | ||
| June | 6.2 | 810 | 450 | 680 | 35 | 630 | 3.6 |
| July | 6.6 | 830 | 370 | 680 | 45 | 660 | 4.6 |
| December | 6.6 | 780 | 320 | 670 | 30 | 680 | 4.4 |
| May | 6.3 | 810 | 390 | 670 | 38 | 630 | 5.3 |
| Average | 6.4 | 808 | 383 | 675 | 37 | 647 | 4.1 |
| 1 Kg Mn 20 Kg P2O5 + 1 Kg Cn per hectare 1 Kg Fe | |||||||
| pH | N | P2O5 | Mn | ||||
| Total | Available | Total | Available | Total | Water Soluble | ||
| June | 6.2 | 870 | 470 | 610 | 35 | 630 | 3.4 |
| July | 6.5 | 800 | 380 | 620 | 45 | 640 | 4.8 |
| December | 6.6 | 770 | 340 | 610 | 34 | 660 | 4.6 |
| May | 830 | 450 | 615 | 39 | 620 | 5.4 | |
| Average | 6.4 | 818 | 410 | 614 | 38 | 637 | 4.5 |
TABLE III (c)
Plankton concentrations in treated and untreated ponds
| Control (No treatment) | |||||
| PLANKTON | |||||
| Month | Total weight γ/litre | % | Predominant phyto-plankters | % | Predominant zoo-plankters |
| June | 1267 | 32 | Scenedesmus, Cladophora | 68 | Copepods, Nauplii |
| July | 1290 | 60 | Oscillatoria, Pandorina | 40 | Cladocerans, Keratella |
| August | 778 | 22 | Anabaena, Pandorina | 78 | Copepods, Cladocerans |
| September | 556 | 12 | Oscillatoria, Scenedesmus | 88 | Copepods, Cladocerans |
| October | 822 | 66 | Oscillatoria, Scenedesmus | 34 | Brachionus, Keratella |
| November | 778 | 70 | Volvox, Anabaena | 30 | Keratella, Nauplii |
| December | 444 | 50 | Dictyosphaerium, Volvox | 50 | Rotifers, Diaptomus |
| January | 822 | 58 | Volvox, Anabaena | 42 | Cladocerans, Keratella |
| February | 737 | 42 | Pandorina, Oscillatoria | 56 | Rotifers, Brachionus |
| March | 778 | 68 | Anabaena, Volvox | 32 | Cladocerans, Cyclops |
| April | 1000 | 78 | Volvox, Anabaena | 22 | Rotifers, Cyclops |
| May | 1290 | 82 | Microcystis, Anabaena | 18 | Brachionus, Difflugia |
| Average | 844 | ||||
| Increase over control (%) | - | ||||
| 20 Kg P2o5 per hectare | |||||
| PLANKTON | |||||
| Total weight γ/litre | % | Predominant phyto-plankters | % | Predominant zoo-plankters | |
| June | 1334 | 42 | Ulothrix, Anabaena | 58 | Copepods, Cladocerans |
| July | 1467 | 42 | Anabaena, Spirogyra | 58 | Rotifers, Copepods |
| August | 892 | 18 | Anabaena, Spirogyra | 82 | Rotifers, Cladocerans |
| September | 637 | 5 | Pandorina,Ulothrix | 95 | Copepods, Rotifers |
| October | 1334 | 78 | Volvox, Oscillatoria | 22 | Diaptomus, Conochilus |
| November | 1334 | 60 | Volvox, Oscillatoria | 40 | Rotifers, Brachionus |
| December | 637 | 42 | Cosmarium, Anabaena | 58 | Cyclops, Keratella |
| January | 1067 | 62 | Closterium, Cosmarium | 38 | Cyclops, Nauplii |
| February | 955 | 38 | Volvox, Oscillatoria | 62 | Diaptomus, Cladocerans |
| March | 1334 | 72 | Oscillatoria, Anabaena | 28 | Keratella, Brachionus |
| April | 1604 | 72 | Volvox, Anabaena | 28 | Cyclops, Nauplii |
| May | 1890 | 88 | Microcystis, Volvox | 12 | Keratella, Nauplii |
| Average | 1140 | ||||
| Increase over control (%) | 35.0 | ||||
| 1 Kg Mn | }per hectare | ||||
| 20 Kg P2O5 + 1 Kg Cn | |||||
| 1 Kg Fe | |||||
| PLANKTON | |||||
| Month | Total weight γ/litre | % | Predominant phyto-plankters | % | Predominant zoo-plankters |
| June | 1110 | 50 | Oscillatoria, Dictyosphaerium | 50 | Copepods, Nauplii |
| July | 1667 | 52 | Lyngbia, Oscillatoria | 48 | Diaptomus, Cladocerans |
| August | 1222 | 32 | Oscillatoria,Cosmarium | 68 | Copepods, Cladocerans |
| September | 892 | 10 | Pandorina, Lyngbia | 90 | Rotifers, Cladocerans |
| October | 1344 | 58 | Volvox, Pandorina | 42 | Rotifers, Nauplii |
| November | 1322 | 60 | Cladophora, Cosmarium | 40 | Diaptomus, Nauplii |
| December | 778 | 42 | Pandorina, Cladophora | 58 | Copepods, Cladocerans |
| January | 1322 | 68 | Oscillatoria, Anabaena | 32 | Keratella, Difflugia |
| February | 778 | 20 | Scenedesmus, Closterium | 80 | Rotifers, Copepods |
| March | 1512 | 86 | Volvox, Anabaena | 14 | Difflugia, Arcella |
| April | 1922 | 78 | Microcystis, Volvox | 22 | Cladocerans, Keratella |
| May | 2222 | 92 | Microcystis, Volvox | 8 | Rotifers, Cyclops |
| Average | 1367 | ||||
| Increase over control (%) | 62.0 | ||||
| Increase over phosphate treatment (%) | 19.9 | ||||
The second series of experiments was conducted under complete field conditions in ponds in a fish farm. Here also it was noticed that ponds treated with both superphosphate alone and with superphosphate + manganese sulphate showed higher plankton production than untreated ones, production in the latter case being more than in the former. Fluctuations in plankton concentration in ponds were also irregular under the field conditions; the plankton concentration curves, however, did not show any sharp rise and fall as in the case of cistern experiments (Fig.2). This experiment was continued for one complete year, June-May, so that the plankton concentration and water quality of treated and untreated ponds could be studied for all seasons. It will be seen from Fig.1(b) that in the month of July, one month after treatment, plankton concentrations in both superphosphate treated ponds and in ponds treated with superphosphate + manganese sulphate showed a marked increase over that in untreated ponds, the increase in ponds treated with superphosphate + manganese sulphate being much higher. The difference between the latter two sets was less pronounced in August and during September; October and November the plankton concentration curves overlapped each other, showing no significant difference between the two treatments. From December, however, this difference again became very pronounced and continued until the end of the experiment in May, with an exception in the month of February which is difficult to explain. It was noticed that during summer months of March to May the effect due to manganese as well as phosphate treatments became more marked. It is likely that added nutrients lost from the water phase and bound in the soil by physical or chemical processes, were released during summer months under higher temperature by the increased rate of reduction processes in the soil. During this period the proportion of phyto-plankton in all the ponds was higher ranging from 60 to 92 percent with green and blue green forms like Anabaena, Oscillatoria, Volvox and Microcystis predominating. The added manganese has possibly been utilized for the auto-trophic growth of phyto-plankton with an enhanced rate of carbohydrate synthesis during long periods of sunlight and high temperature, the prevailing initial concentration being sufficient for heterotrophic growth. Though thick dispersions of plankton were noticed at times in some ponds, in no case was there a heavy bloom as observed by Henderson (1949). The average plankton concentrations for the three sets: (1) control, (2) treated with superphosphate and (3) treated with superphosphate + manganese sulphate, were 784,1111 and 1267/ litre respectively, showing an increase of 48 percent and 69 percent respectively for the two treatments. Compared to low manganese Jobra soil here we find an additional increase of 21 percent due to manganese as against 48 percent due to phosphate. Chaudwar soil, however, being deficient in phosphorus showed a marked response (48 percent increase in plankton) to phosphatic fertilizers and comparatively less to manganese. Considering the effect of manganese alone, we find, however, an increase of 14.2 percent for manganese deficient Jobra soil and 14.4 percent for Chaudwar soil with relatively high manganese status. It may therefore be concluded that for pond soils having water soluble manganese ranging from 0.25 to 2.8 ppm and pond water having manganese concentration 0.001 to 0.02 ppm, the addition of this element is efficient and economical to increase the production of plankton in fish ponds. The degree of response to manganese treatment is independent of the manganese status of soil and water in this range.
In the third series of experiments conducted under field conditions with similar procedures as in the previous one, two other common micro-nutrient elements (iron and copper) were incorporated along with manganese. Both these elements are known to take part in metabolic reactions in plants and animals and their role as micro-nutrients for planktonic algae has been studied by many workers (Marsh et al., 1963; Hopkins, 1930a, b and c; 1933; Sommer, 1931). These two elements behave like manganese in their chemical reactions in aquatic environment. Iron is practically insoluble in ionic form at the pH values and oxygen concentrations generally encountered in fresh-water fish ponds. It remains partly as ferric hydroxide either in suspension or adsorbed on colloidal clay miscels and partly as a soluble or colloidal iron-organic complex which appears to be quite stable. The experimental evidence obtained by Harvey (1937) showed that both these forms are assimilable by diatoms and other phyto-plankton organisms. Rodhe (1948) has shown that only a small fraction (about 10 percent) of this iron is available to iron-starved cells of Scenedesmus sp. In the ponds under study the concentration of iron in water ranged from 0.4 to 0.9 ppm as compared to copper and manganese concentrations which ranged from 0.008 to 0.017 ppm and 0.01 to 0.04 ppm, respectively showing an Fe:Mn ratio of 40-23:1. The element copper, thoug a micro-nutrient, is used for control of algal blooms which sometimes become a nuisance in fish ponds. In alkaline waters only an insignificant fraction of added copper remains in ionic form because of its reaction with carbonate in water, producing basic copper carbonate (Banerjea & Mitra, 1954). A minor fraction may remain as colloidal hydroze. Certain species of Coelastrum and Navicula may be sensitive to copper ion in concentrations of the order of 0.03 ppm, while Anabaena, Aphanizomenon, Tabellaria and Synura can tolerate about 0.05 ppm (Hale, 1922). Pillai (1954) while working with blue-green algae found that an amount of copper inhibiting Phormidium tenue stimulated Spirulina and Oscillatoria sp. It is also often noticed that after an algal population has been destroyed by copper sulphate treatment a secondary maximum of total phyto-plankton of transient nature often develops, possibly due to the nutrient effect of copper or due to nutrients released from decomposed algae. The copper concentrations in the ponds under study range from 0.008 to 0.017 ppm an is thus fairly below the toxic concentration for plankton. The effective concentration of copper and iron on volume basis added in this experiment was 0.01 ppm. As observed in the previous experiment, one month after treatment in July, plankton concentration showed an increase both for phosphate treated ponds and ponds treated with phosphate + manganese sulphate, the increase in the latter case being more than that in the former. This continued up to September, but during October-November the two curves of plankton concentration overlapped, showing that the effect of trace elements is minimal in this period. Later on, however, in the plankton curve for ‘phosphate + trace element’ an exception was noted in February when plankton concentration in ‘phosphate + trace element’ treated ponds was markedly lower than that in the other. A somewhat similar variation was noticed in February in the previous experiment, though not to the same extent. During the summer months of March, April and May, the curves showed a trend of regular rise, the effect of phosphate as well as trace elements being more marked.
Comparing the average weight of plankton for the whole period for the three sets of experiments it will be seen that while phosphate alone raised the plankton concentration by 35 percent, there was an additional increase of 27 percent by the combined effect of the three trace elements. However, calculating the effect of trace elements (Mn + Cu + Fe) only an increase of 19.9 percent was recorded in the phosphate treated ponds as against 14.4 percent in ponds treated with manganese alone in the previous experiment, which means an increase of 5.5 percent only due to copper + iron. Thus the copper and iron status of soil and water does not appear to present a deficient condition to the extent of showing a marked response when treated with these elements.
It is interesting to note that during summer months of March, April and May in all the three series of experiments the plankton concentrations showed a trend of regular increase for treated and untreated ponds. At the same time the effect of trace elements and phosphate also become more pronounced as exhibited by difference in plankton weight. Increased proportion of phyto-plankton, particularly green and blue-green forms associated with longer period of sunlight, higher temperature and release of more nutrients under reduced condition in bottom soil and increased bacterial activity, may be responsible for the enhanced rate of auto-trophic synthesis of carbohydrate in summer. From the tables showing chemical quality of water it will be seen that total alkalinity and dissolved nutrients are generally in higher concentrations in summer than in any other season.
To explore the possibility of using trace elements like manganese, copper and iron for increased production of plankton in fish ponds series of experiments were conducted in cement cisterns under semi-field conditions and in ponds under purely field conditions. Two soil types were used in this investigation, one a slightly acidic soil high in phosphorus but low in manganese and the other also with a slightly acidic reaction but low in phosphorus and relatively high in manganese status. The former was treated with only manganese and the latter with manganese alone and in combination with copper and iron. The results showed that both the soils responded fairly to treatment with manganese, showing an increase of plankton weight by about 14 percent due to manganese alone. In the case of high phosphorus and low manganese soil the additional increase of plankton for a dose of 1 Kg/ha of Mn was 16.4 percent which compared well with an increase of 14.6 percent by 20 Kg/ha of P2O5, showing that for this type of soil, manganese can be used efficiently and economically as a fertilizer element. In the other soil with low phosphorus and high manganese, the additional increase in plankton weight due to manganese at the rate of 1 Kg/ha was 21 percent against 48 percent by phosphate alone at the rate of 20 Kg/ha of P2O5, but when the effect of phosphate + manganese was compared with that of phosphate-manganese it was observed that both the soil types exhibited about 14 percent increase in plankton weight due to manganese alone showing that for the range of water soluble manganese in soil from 0.25 to 2.8 ppm and soluble manganese in water from 0.001 to 0.02 ppm the degree of response was independent of manganese status of soil and water. The effect of incorporating two other trace elements, copper and iron at the same dose for the latter soil did not result in any marked increase in production of plankton.
The authors express their sincere thanks to Dr. B.S. Bhimachar, Director, Central Inland Fisheries Research Institute, Barrackpore and Dr. H. Chaudhuri, Officer-in-Charge, Central Inland Fisheries Research Sub-Station, Cuttack, for kindly going through this paper and suggesting valuable improvements. We also extend our thanks to Shri P.R. Sen of Cuttack Sub-Station for his help in the study of plankton in this investigation.
Arnon, D.I., 1958 The role of micronutrients in plant nutrition with special reference to photo-synthesis and nitrogen assimilation. Proc.Conf.Ohio.agric.Exp.Sta., (1957):1–33
Banerjea, S and E. Mitra, 1954 Preliminary observations on the use of copper sulphate to control submerged aquatic weeds in alkaline waters. Indian J.Fish., 1(1&2):204–16
Brown, T.E., H.C. Eyster and H.A. Tanner, 1958 Physiological effects of manganese deficiency Proc.Conf.Ohio.agric.Exp.Sta., (1957):135–56
Clark, N.A.,1933 Manganese and the growth of Lemna. Plant Physiol., 8:157–61
Clark, N.A. and C.L. Fly, 1930 The role of manganese in the nutrition of Lemna. Plant Physiol., 5:24–248
Eyster, H.C., T.E. Brown and H.A. Tanner, 1958 Mineral requirements of Chlorella pyrenoidosa under autotrophic and heterotrophic conditions. Proc.Conf.Ohio.agric.Exp. Sta., (1957):157–74
Hale, F.E., 1922 Tastes and odours in New York water supply. J.Amer.Water Wks.Ass., 9:829–37
Harvey, H.W., 1937 The supply of iron to diatoms. J.mar.biol.Ass.U.K., 22:205–19
Harvey, H.W., 1947 Manganese and the growth of phytoplankton. J.mar.biol.Ass.U.K., 25:562–79
Henderson, C., 1949 Manganese for increased production of water bloom algae in ponds. Progr.Fish.Cult., 11:157–9
Hopkins, E.F.,, 1930a Iron ion concentration in relation to growth and other biological processes. Bot.Gaz., 89:209–40
Hopkins, E.F., 1930b Manganese as an essential element for green algae. Amer.J.Bot., 17:1047
Hopkins, E.F., 1930c The necessity and function of manganese in the growth of Chlorella sp. Science, 72:609–10
Hopkins, E.F., 1933 The effect of small amounts of copper on the growth of Chlorella and Lemna. Amer.J.Bot., 29:681
Marsh, H.V., H.J. Evans and G. Matrone, 1963 Investigations on the role of iron in chlorophyll metabolism. Pl.Physiol., 38-632-64
Mc Hargue, J., 1922 The role of manganese in plants. J.Amer.chem.Soc., 44:1592–8
Mehring, H., 1918 Die Fisch Teichdüngung. Fish pond manuring. Dtsch.landw.Pr., 45:293
Mortimer, C.H. and C.F. Hickling, 1954 Fertilizers in fish ponds. Fish.Publ.,Lond., (5):155 p.
Patnaik, S., 1955 Role of micronutrients on the growth and metabolism of rice plants. Bull. nat.Inst.Sci.India, 8:65–8
Pillai, V.K., 1954 Some factors controlling algal production in soil water lagoons. Proc. Indo-Pacif.Fish.Coun., 5:78–81
Pirson, A., 1958 Manganese and its role in photosynthesis. Proc.Conf.Ohio.agric.Exp.Sta., (1957):81–98
Rodhe, W., 1948 Environmental requirements of fresh-water plankton algae. Symb.bot.upsaliens., 10:149
Saegar, A., 1933 Manganese and the growth of Lemnaceae. Amer.J.Bot., 20:234–45
Sommer, A.L., 1931 Copper as an essential element for plant growth. Pl.Physiol., 6:339–45
Swingle, H.S., 1947 Experiments on pond fertilization. Bull.Ala.agric.Exp.Sta., (264)