by
YOSHIHIRO SATOMI
Fresh-water Fisheries Research Laboratory
Ministry of Agriculture and Forestry
Tokyo, Japan
Abstract
The sources of carbon, especially from the standpoint of biological production, are discussed. There are certain cases where carbon sources become a limiting factor in the growth of phytoplankton. In such cases phytoplankton absorbs N and P excessively. The adequate composition of C-N-P of phytoplankton is approximately 50 : 7 : 1 by weight. Acetic acid is the only organic acid available for phytoplankton in the presence of CO2. Favourable results were obtained by the application of acetic acid with inorganic fertilizers. The author found that high alkalinity exerts a favourable influence on the production of zooplankton.
It is assumed that organic matters in bottom soil quickly decompose at higher C-N ratios, eventually to raise alkalinity and the productivity of water.
As is seen from the favourable effects upon zoo- and phyto-plankters and bacteria, alkalinity has physiological importance not only to primary products but also to all organisms in the food chain. It is closely connected with the turnover rate of materials in fish ponds.
IMPORTANCE PHYSIOLOGIQUE DES SOURCES DE CARBONE DANS LES ETANGS PISCICOLES FERTILISES
Résumé
L'auteur passe en revue les sources de carbone, notamment sous l'angle de la production biologique. Dans certains cas, les sources de carbone peuvent devenir un facteur limitatif de la croissance du phytoplancton et celui-ci alors absorbe des quantités excessives de N et P. La composition adéquate en C-N-P du phytoplancton est d'environ de 50 : 7 : 1, en poids. L'acide acétique est le seul acide organique que le phytoplancton puisse assimiler en présence de CO2; de bons résultats ont été obtenus en incorporant de l'acide acétique aux engrais inorganiques. L'auteur a constaté qu'une forte alcalinité exerce une influence favorable sur la production de zooplancton. Il est supposé que les matières organiques contenues dans le sol du fond de l'étang se décomposent rapidement lorsque le rapport C-N est élevé, ce qui a pour effet en définitive d'augmenter l'alcalinité et la productivité des eaux.
Comme le montre les effets favorables sur les zooplanctontes, les phytoplanctontes et les bactéries, l'alcalinité a une importance physiologique non seulement pour les produits primaires mais aussi pour tous les organismes de la chaîne alimentaire. Le taux d'utilisation des matériaux de l'étang est étroitement lié au degré d'alcalinité.
IMPORTANCIA FISIOLOGICA DE LAS FUENTES GENERADORAS DE CARBONO EN LOS ESTANQUES PISCICOLAS FERTILIZADOS
Extracto
Se examinan las fuentes generadoras de carbono, especialmente desde el punto de vista de la producción biológica. Existen ciertos casos en que las fuentes generadoras de carbono constituyen un factor limitante en el desarrollo de fitoplancton. En tales casos el fitoplancton absorve N y P con exceso. La composición adecuada de C-N-P en el fitoplancton es aproximadamente 50 : 7 : 1, en peso. El ácido acético es el único aćido orgánico disponible para el fitoplancton en presencia de CO2. Se obtuvieron resultados favorables mediante la aplicación de ácido acético con fertilizantes inorgánicos. El autor comprobó que la elevada alcalinidad ejerce una favorable influencia en la producción de zooplancton.
Se supone que las materias orgánicas del fondo del suelo se descomponen fácilmente cuando las proporciones de C-N son elevadas y, eventualmente, aumentan la alcalinidad y la productividad del agua.
Como puede verse de los favorables efectos en los consumidores de zooplancton y de fitoplancton y en las bacterias, la alcalinidad tiene importancia fisiológica no solamente para los productos primarios sino también para todos los organismos de la cadena alimentaria. Está estrechamente relacionada con el coeficiente de renovación de materiales en los estanques piscícolas.
The techniques of fertilized fish farming are covered by many detailed reports. This paper deals with the problems of the sources of carbon from physiological and biological production standpoint.
Inorganic carbon is available in inland waters in the form of CO2, (H2CO3), HCO3'and CO3'', in proportions determined by pH, water temperature and dissolved salt content. The approximate content of total carbon dioxide is estimated on the basis of pH, temperature and alkalinity.
The alkalinity of inland waters depends on their origin and the kinds of rocks they have encountered (Satomi, 1963), in particular whether they have passed through volcanic rock or limestone areas.
Although all organic matter contains carbon, sources which can be supplied as organic fertilizer, and are in a form readily available for living organisms, particularly phytoplankton, are rather limited.
Phytoplankton converts inorganic carbon into organic carbon by photosynthesis, utilizing in most cases CO2 and HCO3'. In addition, certain species make use of CO3".
The formula for such conversion is as follows:
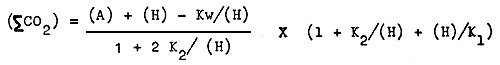
| where ∑CO2 | = total carbon dioxide |
| A | = alkalinity |
| (H) | = concentration of hydrogen ion |
| Kw | = ionization constant of water |
| K1 & K2 | = first and second dissociation constants respectively |
When total carbon dioxide is exhausted or ∑CO2 = 0
A = Kw/(H) - (H)
Then if water temperature is kept constant, (H) can be determined in theory by alkalinity.
For example, when ∑CO2 = 0 at 25°C, one gets the following relationships:
| Alkalinity | Theoretical pH-value |
| m.equiv./ℓ | |
| 0.5 | 10.7 |
| 1.0 | 11.1 |
| 2.0 | 12.0 |
One can confirm the above relationships by use of Scenedesmus cultured in a glass container. However, in the case of Chlorella, one finds that the pH-value does not rise beyond 9.6 – 9.8, with inorganic nitrogen compounds (N) and phosphates (P) still remaining. In other words, there still remain HCO3' and CO3" in the culture medium even when CO2 = 0 (Matsue & Hirano, 1955; Satomi, 1959a).
This seems to indicate that there are certain cases where carbon sources become a limiting factor for the growth of phytoplankton, even though succession of species depends on pH.
It is further observed that phytoplankton absorbs N and P excessively in a culture medium with insufficient supply of carbon sources. This was demonstrated by the author, who successfully induced phytoplankton to grow by addition of CO2, N, and P to the culture medium deficient in C, N and P respectively (Satomi, 1959b). Post-mortem examination further detected inorganic N and P accumulated in their bodies (Satomi, 1962a).
On the basis of the above evidence, the author concluded that N and P taken in excess have nothing to contribute to the production of phytoplankton. He further found that adequate composition of C-N-P of phytoplankton is approximately 50 : 7 : 1 by weight, which is almost identical with that of marine phytoplankton (Satomi, 1959a). It is pointed out that from the standpoint of fish farming the use of inorganic fertilizer tends to result in a deficiency of C, relative to N and P.
Alkalinity of land waters is generally low in Japan due to the volcanic rocks, its average for rivers being 0.51 milliequiv./ℓ (Kobayashi, 1960). The average for lakes is much the same (Satomi, 1962b). Furthermore, the diffusion of CO2 from the atmosphere into water is known to be very slow (Verduin, 1956 & 1960; Gessner, 1959).
Judging from the velocity constants for the hydration of CO2 and the dehydration of H2CO3, it is easily seen that the release of CO2 from the water is much faster than its diffusion into the water (Edsall & Wyman, 1957). In addition, mineralization of phytoplankton further reduces alkalinity (Satomi, 1962a). In fact, the pH-value was observed to rise up to the point where ∑CO2 = 0 in a two-ha pond fertilized only by inorganic fertilizers.
Then the question comes to the sources of organic carbon. The author examined the effect of organic acids from plankton bodies, or produced in the process of their decomposition on phytoplankton (Satomi, 1964). It was demcnstrated by this study that acetic acid is the only organic acid available for phytoplankton in the presence of CO2. It is true, as shown by many reports, that a number of organic acids are utilized by phytoplankton, but this needs to be re-examined from the viewpoint of whether they can be fully utilized in the presence of CO2 in order to prevent alkalinity decreasing and pH value increasing.
The author obtained the following favourable results by application of acetic acid with inorganic fertilizers (N and P).
| Number of fish per 3.3m2 | Silver carp | 4 | 1 |
| Kawachi-buna | 1 | 4 | |
| (crucian carp) | |||
| Common carp | 1 | 1 | |
| Lot with CH3COOH added | Silver carp | 2.23 ton/ha | 1.28 ton/ha |
| Kawachi-buna | 0.40 | 1.02 | |
| Common carp | 0.32 | 0.33 | |
| Lot without CH3COOH added | Silver carp | 1.52 | 1.04 |
| Kawachi-buna | 0.30 | 0.72 | |
| Common carp | 0.35 | 0.17 |
Note(1) Total amount of inorganic fertilizer applied: 4.4 mg-P/l, 25.5 mg-N/l, 60 mg-C/l
(2) Period of experiment: 20 March 1962 – 1 November 1962
Since the availability of various carbon sources is important for the production of phytoplankton, which is the primary product of a fertilized fish farm, it is easily seen that it will necessarily affect the production of phytoplankton-feeding zooplankton (e.g. Hazelwood and Parker, 1961; Borecky, 1956).
Fertilization by inorganic fertilizers results in the rise in pH value, adversely affecting the production of Daphnia.
Since there has been no direct evidence to demonstrate the positive relationship between alkalinity and zooplankton. Suginome and Satomi (1966) studied this with a sufficient supply of phytoplankton at constant temperature. From this experiment it was found that high alkalinity exerts a favourable influence on the growth, reproduction and longevity of zooplankton. It was also found that acetic acid, good for the growth of phytoplankton, also accelerates the growth of zooplankton.
High alkalinity also has a favourable effect on aquatic insects, which may be the consequence of a similar action (e.g. Armitage, 1958).
As is well known in soil science, bacteria are generally activated at high carbon - nitrogen ratios, to quicken decomposition of organic carbon, which eventually works to reduce this ratio.
The author and Oya (1964) observed the relationship between carbon - nitrogen ratios in the bottom soil and the fish production in a pond fertilized with organic fertilizer (chicken faeces) (Fig 1). It was found that higher alkalinity, if less than 2 milliequiv./ℓ, gives higher fish production. On the basis of these facts, it is assumed that organic matter in bottom soil decomposes faster at higher carbon - nitrogen ratios, eventually to raise the alkalinity and the productivity of water.
Accumulation of cellulose deteriorates the quality of bottom soil (Schäperclaus, 1961) and it is known that decomposition of cellulose depends upon alkalinity and calcium content (Brand, Klust and Mann, 1956). It is thus obvious that fertilization with organic fertilizer should be done with due consideration for the carbon - nitrogen ratio.
Since Ohle (1938), there have been many reports published in which the alkalinity is used an an index of fish production. However, the use of this index is based, in most cases, on observation and is not substantiated by experimental evidence.
As is seen from the favourable effects on zoo- and phytoplankton and bacteria, the alkalinity has physiological importance not only for the primary products but also for other organisms at higher levels in the food chains. It is closely connected with the velocity of turnover of materials in fish ponds. Its physiological significance for aquatic insects is not yet known.
A linear relationship between alkalinity and fish production is recognized in the United States (Carlander, 1955), while in Japan a linear relationship is observed between alkalinity and the logarithm of fish production (Satomi, 1962c). This discrepancy is assumed to be due to the difference in the fish utilized; carnivorous fish in the former, and omnivorous and plankton-feeding fish in the latter case.
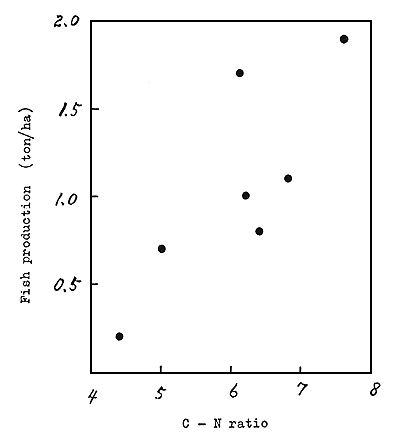
Fig. 1 Production of Kawachi crucian carp at different carbon - nitrogen ratios in the bottom soil in ponds fertilized with organic fertilizer (chicken faeces)
Lastly, it may be pointed out that inorganic carbon is directly assimilated into body-composing organic matter (Sorvacher and Belokopytova, 1960). However, the physiological significance of this phenomenon, and whether there is any organic carbon which is directly assimilated into body-composing organic matter, is not known. The ability of fish to convert inorganic carbon into the organic form (as with inorganic Ca, P and S) should be given due consideration in future in fertilized fish farming, as well as in the study of production biology.
Armitage, K.B., 1958 Ecology of the riffle insects of the Firehole River, Wyoming. Ecology, 39:571–80
Borecky, G.W., 1956 Population density of the limnetic Cladocera of Pymatuning reservoir. Ecology, 37:719–27
Carlander, K.D., 1955 The standing crop of fish in lakes. J.Fish.Res.Bd Can., 12:543–70
Edsall, J.T. and J. Wyman, 1957 Biophysical chemistry. Vol. l. New York, Academic Press, 699 p.
Gessner, F., 1959 Hydrobotanik. Bd 2. VEB. Deutsch.Vertag der Wissensch., Berlin, 701 p.
Hazelwood, D.H. and R.A. Parker, 1961 Population dynamics of some fresh-water zooplankton. Ecology, 42:266–74
Kobayashi, J., 1960 A chemical study of the average quality and characteristics of river waters of Japan. Ber.Ohara Inst.landw.Biol., 11:313–58
Matsue, Y. and R. Hirano, 1955 On the relation between the carbon dioxide supply and the phytoplankton growth. Jap.J.Limnol., 17:133–40
Ohle, W., 1938 Teichwirtschaftliche Kalkkontrolle und die PH-SBV-Tasche. Z.Fisch., 36:185–91
Satomi, Y., 1959a Beziehungen zwischen dem geeigneten Stickstoff-Phosphor Verhaeltnis und Alkalinitaet für Phytoplanktonwuchs. Bull.Freshw.Fish.Res.Lab., Tokyo, 8:21–39
Satomi, Y., 1959b Beiträge zur Kenntnis der Teichdüngungen durch Stickstoff und Phosphordüngermittel bei höherer Planktonsdichte, insbesondere in Bezug auf Kohlensäuregehalt des Wassers. Bull.Freshw.Fish.Res.Lab., Tokyo, 9:1–10
Satomi, Y., 1962a Untersuchungen über die Remineralisation des Phosphors und Stickstoffs im Phytoplankton, insbesondere in Bezug suf Alkalinität der Medien und andere Milieufaktoren. Bull.Freshw.Fish.Res.Lab., Tokyo, 11:1–9
Satomi, Y., 1962b Untersuchungen über die Alkalinität des Süsswassers 2. Über den untersuchten Werte und seinen Schwankungen in japanischen hauptsächlichen Seen und Flüssen. Bull.Freshw.Fish.Res.Lab., Tokyo, 12:51–63
Satomi, Y., 1962c Uber die Bedeutung der Alkalinität als Indikator für BinnenfischereiProduktion. Bull.Freshw.Fish.Res.Lab., Tokyo, 12:65–74
Satomi, Y., 1963 Untersuchungen über die Alkalinität des Süsswassers 4. Herausgelöste Menge beim Kontakt mit den verschiedenen Gesteinen. Bull.Freshw.Fish.Res.Lab., Tokyo, 13:1–6
Satomi, Y., 1964 Über die Verwertung organischer Säuren durch Phytoplankton und dabei begleitende Veränderung der Alkalinität. Bull.Freshw.Fish.Res.Lab., Tokyo, 13:77–92
Satomi, Y. and S. Oya, 1964 April 1964 Annual meeting of the Japanese Society of Scientific Fisheries
Schäperclaus, W., 1961 Lehrbuch der Teichwirtschaft. Berlin, Paul Parey, 582 p.
Sorvachev, K.F. i O.V. Belokopytova, 1960 Polgloshenie rybami neogranicheskogo ugleroda iz okruzhaiushchei sredy i uchastie vobmene veshchestv. Biokhimiia, 25:459–64
Suginome, M. and Y. Satomi, 1966 Über den Einwirkungen der pH-Werte und Alkalinität auf Wuchs und Erzeugungsfähigkeit von Daphnia carinata King, aus Dauereier geschlüpfter Generation. Bull.Freshw.Fish.Res.Lab., Tokyo, 15:113–22
Verduin, J., 1956 Energy fixation and utilization by natural communities in western Lake Erie. Ecology, 37:40–50
Verduin, J., 1960 Phytoplankton communities of western Lake Erie and the CO2 and O2 changes associated with them. Limnol.Oceanogr., 5:372–80
Vel'tishcheva, I.F., 1961 Proniknovenie ugleroda (C14) karbonata iz vody i raspredelenie ego v tele ryby. Trudy vsesoiuz.nauch.-issled.Inst.morsk.ryb.Khoz., 44:23–6
von Brandt, A., G. Klust and H. Mann, 1956 Der Zelluloseabbau in Fischteichen. Arch.Fisch. Wiss., 7(1):31–40