by
P. VEDAVYASA RAO
Central Marine Fisheries Research Institute
Mandapam Camp, India
Abstract
Some aspects of the maturation and spawning of the commercially important prawns of the southwest coast of India, Metapenaeus dobsoni, M. affinis, Penaeus indicus and Parapenaeopsis stylifera have been studied.
In all the species the mature ovary consists of anterior, middle and posterior lobes. Five maturity stages: immature, early maturing, late maturing, mature and spent-recovering stages are distinguished and the maturation process in the female of each species is described. The minimum size at first maturity was estimated to be 64.1 mm for M. dobsoni, 88.6 mm for M. affinis, 130.2 mm for P. indicus and 63.2 mm for P. stylifera. M. dobsoni and P. stylifera breed throughout the year, with peaks in June, November-December and April, while in P. indicus and M. affinis the breeding period extends over the period from October to March or April. The spawning of individual prawns of each species is restricted to short and definite periods, as shown by ova diameter studies, and individuals are estimated to spawn five times during their lifetime. Highest spawning activity was observed when the water temperature was relatively high. The relationship between length of prawn and fecundity was found to be logarithmic in all the species.
MATURATION ET REPRODUCTION DES PENAEIDAE DE LA COTE SUD-OUEST DE L'INDE
Résumé
La communication étudie certains aspects de la maturation et de la ponte des crevettes d'importance commerciale de la côte sud-ouest de l'Inde : Metapenaeus dobsoni, M. affinis, Penaeus indicus et Parapenaeopsis stylifera.
Dans toutes les espèces étudiées, l'ovaire mûr comprend un lobe antérieur, un lobe moyen et un lobe postérieur. On distingue cinq stades de maturité : immature, début de l'évolution, fin de l'évolution, fin de l'évolution, mature et post-ponte (récupération), et l'auteur décrit le processus de maturation chez la femelle de chaque espèce. Estimation des tailles minimales à la premiére maturation : 64,1 mm pour M. dobsoni, 88,6 mm pour M. affinis, 130,2 mm pour P. indicus et 63,2 mm pour P. stylifera. M. dobsoni et P. stylifera se reproduisent toute l'année, avec des pointes en juin, novembre-décembre et avril, tandis que chez P. indicus et M. affinis, la période de reproduction s'étend d'octobre à mars ou avril. La ponte des individus de chaque espèce est limitée à des périodes brèves et bien définies, comme l'a révélé l'étude du diamètre des oeufs, et l'on estime que les individus pondent 5 fois durant leur vie. On a observé le maximum d'activité reproductrice lorsque la température du substrat était relativement élevée. Pour toutes les espèces, le rapport entre la longueur de la crevette et la fécondité s'exprime sous forme logarithmique linéaire.
MADURACION Y DESOVE DE LOS PENEIDOS DE LA COSTA SUDOCCIDENTAL DE LA INDIA
Extracto
Se han estudiado algunos aspectos de la maduración y desove de los camarones de importancia comercial de la costa sudoccidental de la India, a saber: Metapenaeus dobsoni, M. affinis, Penaeus indicus y Parapenaeopsis stylifera.
En todas las especies, el ovario maduro consta de lóbulos anterior, medio y posterior. Se distinguen cinco fases de madurez: inmaduros, en principios de maduración, maduración avanzada, maduros, frezados en recuperación, y se describe el proceso de maduración en la hembra de cada especie. La talla mínima en la primera madurez se ha calculado en 64,1 mm para M. dobsoni; 88.6 mm para M. affinis, 130,2 mm para P. indicus y 63,2 mm para P. stylifera. M. dobsoni y P. stylifera crían durante todo el año con máximos en junio, noviembre-diciembre y abril; P. indicus y M. affinis lo hacen durante un largo período que se extiende de octubre a marzo o abril. El desove de los camarones individuales de cada especie se limita a períodos breves y definidos, como han relevado los estudios del diámetro de los huevos, y se calcula que desovan cinco veces durante su siclo vital. La mayor actividad de desove se ha observado cuando la temperatura del sustrato es relativamente alta. Se ha descubierto que la relación existente entre la longitud y la fecundidad del camarón adopta una forma logarítmica lineal en todas las especies.
Studies on the breeding habits of some Indian penaeid prawns have been carried out by Menon (1952; 1953), Shaikmahmud and Tembe (1960; 1961), Rajyalakshmi (1961), George (1962) and Subrahmanyam (1963). Most of these investigations were restricted to the determination of the time of spawning, and the conclusions arrived at were based entirely on the data on incidence of mature prawns in the catches obtained in different months. No detailed study on the process of maturation, spawning periodicities and the factors influencing successful spawning, have been so far attempted. The present investigation pertains to the study of different aspects of spawning of four commercially important species of prawn in the Cochin area: Metapenaeus dobsoni (Miers), M. affinis (H. Milne Edwards), Penaeus indicus H. Milne Edwards and Parapenaeopsis stylifera (H. Milne Edwards).
From June 1963, regular samples of prawns were collected from the catches of commercial fishing boats operating off Cochin at depths of 9 to 36 m. These samples were sorted into species and their lengths, from tip of rostrum to tip of telson, were recorded separately for both sexes. Observations on the nature, colour, size and texture of ovaries, indicating the degree of maturity, were also recorded. On the basis of these, the season of highest spawning activity was determined, when the highest frequency of ‘light green’ and ‘dark green’ ovaries occur.
Diameters of ova were determined from ovaries hardened in 5 percent formaldehyde for at least two days, using a compound microscope with an ocular micrometer. The diameters were taken parallel to the ocular micrometer in order to avoid errors due to selection, and distortion in preservation.
Preliminary observations did not indicate differential development of ova in the various parts of the ovary. For the purpose of this study, however, samples were taken from the middle lobe only and 300 ova were measured from each ovary.
The fecundity, or total number of ova in a mature ovary, was calculated from the weight of the ovary and the number of eggs in a weighed subsample.
Shaikmahmud and Tembe (1958) have described the anatomy and histology of the male and female reproductive organs of P. stylifera. The general shape and appearance of the ovaries are similar in all the four species studied, and also agree with the descriptions of the ovaries of Penaeus setiferus by King (1948) and P. duorarum by Cummings (1961). The mature ovaries are paired organs, situated dorsally, extending from the base of the rostrum to the last abdominal segment. They are bilaterally symmetrical and partly fused. Each half of the ovary consists of three lobes, of which the slender anterior lobe occupies the anterior region. The middle lobe has 6 or 7 finger-like lateral lobules which entirely fill the area between the anterior region and the posterior border of the carapace. The posterior lobes extend the length of the abdomen. The two halves of the ovary are united by two commissures, one at the base of the anterior lobes and the other at the tip of the posterior lobes in the 6th abdominal segment (Fig. 1).
There is little consistency among the various workers who have studied the maturation of ovaries in prawns as to the number of steps of maturity recognized. Thus, king (1948) recorded five stages in Penaeus setiferus, and Cummings (1961) described only four in P. duorarum. Shaikmahmud and Tembe (1961) differentiated between spent and regenerating P. stylifera, to give six stages, but Rajyalakshmi (1961) included neither spent nor regenerating stages among the five which she recognized for Macrobrachium rosenbergii and M. mirabile (as Palaemon carcinus and P. mirabilis respectively). Renfro and Brusher (1964) classified the developmental stages of white shrimp (Penaeus setiferus) into seven stages, and recently Oka and Shirhata (1965) recognized eight maturity stages in Penaeus orientalis.

Fig. 1 Mature ovary of a typical penaeid prawn, showing the different regions.
Five maturation stages are recognized for the four species of Penaeidae covered by the present paper, and they may be distinguished on the following points:
Immature stage: The ovaries of immature prawns are thin, translucent, unpigmented and confined to the abdomen. They contain oocytes and small spherical ova with clear cytoplasm and conspicuous nuclei.
Early maturing stage: The ovary is increasing in size and the anterior and middle lobes are developing. The dorsal surface is light yellow to yellowish green. Opaque yolk granules are formed in the cytoplasm and partly obscure the nuclei. The developing ova are clearly larger than the immature stock.
Late maturing stage: The ovary is light green and is visible through exoskeleton. The anterior and middle lobes are fully developed. The maturing ova are opaque, due to the accumulation of yolk.
Mature stage: The ovary is dark green and clearly visible through the exoskeleton. The ova are larger than in the preceding stage and the peripheral region becomes transparent. This stage is believed to be the last stage of maturity before actual spawning as the largest ova are encountered only in this stage. Further confirmation is available in M. dobsoni, in which the diameters of the ova in this stage are very close to those of the planktonic eggs (Menon, 1952).
In mature eggs of P. indicus rod-like peripheral bodies radiate from the opaque central region. The presence of these peripheral bodies in the mature ova has not been observed in the other three species; it is considered to be a characteristic feature of the final stage before spawning in several other species of Penaeus (Hudinaga, 1942; King, 1948; Cummings, 1961).
Spent-recovering: It is probable that after the extrusion of eggs, the gonad revert almost immediately to the immature condition. The present stage is therefore distinguishable from that found in immature virgin females only on the size of the prawn (see section 5).
The size distributions of ova at various stages during maturation for each of the four species are given in Figs. 2 to 5. These figures help to emphasize that growth of eggs is a continuous process and that the subdivision of this process into maturation stages is arbitrary. These data are summarized, with respect to the five maturity stages recognized in Table I.
TABLE I
Range in diameter (mm) which includes at least 90% of the ova in each stage, for each of the four species considered
| M. dobsoni | M. affinis | P. indicus | P. stylifera | |
| Immature | < 0.080 | < 0.096 | < 0.080 | < 0.080 |
| Early maturing | 0.08 – 0.19 | 0.10 – 0.25 | 0.08 – 0.24 | 0.10 – 0.19 |
| Late maturing | 0.14 – 0.25 | 0.16 – 0.32 | 0.18 – 0.30 | 0.14 – 0.27 |
| Mature | 0.14 – 0.32 | 0.19 – 0.35 | 0.22 – 0.38 | 0.20 – 0.35 |
| Spent-recovering | < 0.096 | < 0.096 | <0.096 | < 0.096 |
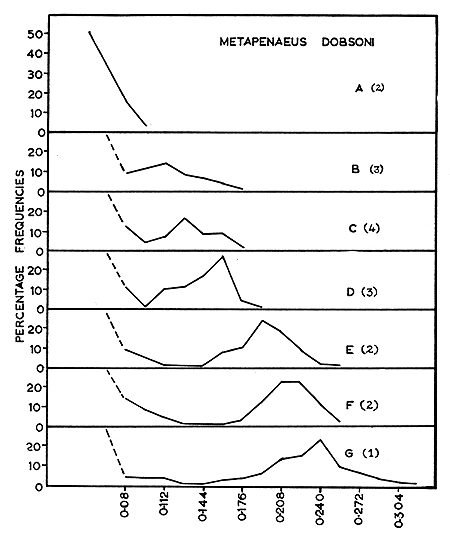 | 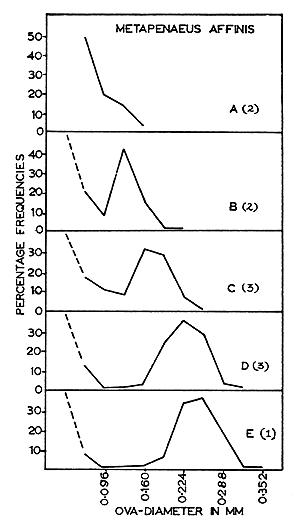 |
Fig. 2 Metapenaeus dobsoni: Size-frequency distribution of maturing ova. | Fig. 3 Metapenaeus affinis: Size-frequency distribution of maturing ova. (A) Immature. (B) (C) Early maturing. (D) Late maturing. (E) Mature. |
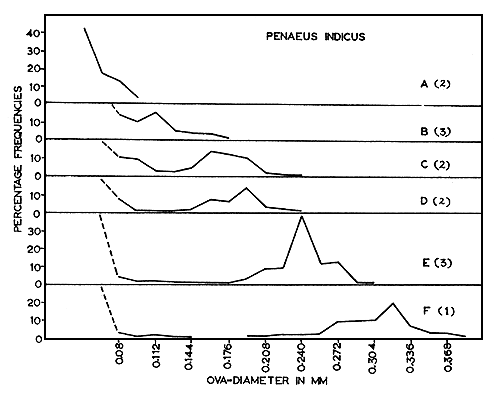
Fig. 4 Penaeus indicus: Size-frequency distribution of maturing ova. (A) Immature. (B) to (D) Early maturing. (E) Late maturing. (F) Mature. Number of specimens examined shown in brackets.
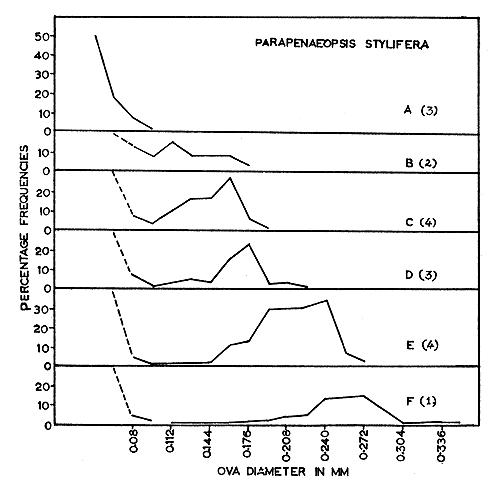
Fig. 5 Penaeopsis stylifera: Size-frequency distribution of developing ova.
(A) Immature. (B) (C) Early maturing. (D) (E) Late maturing. (F) Mature.
Number of specimens examined shown in brackets.
 |  |
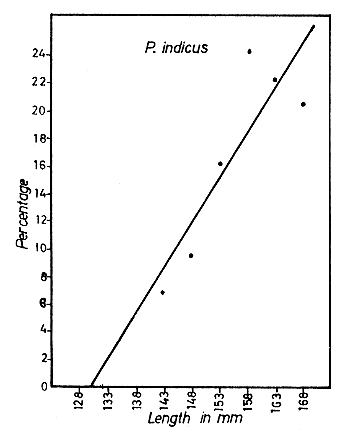 |  |
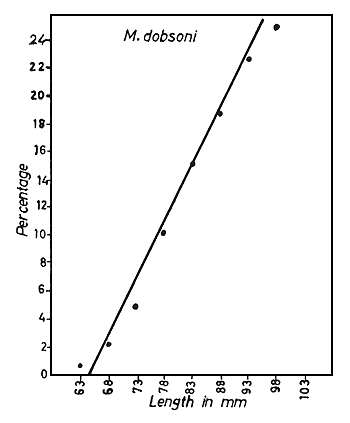
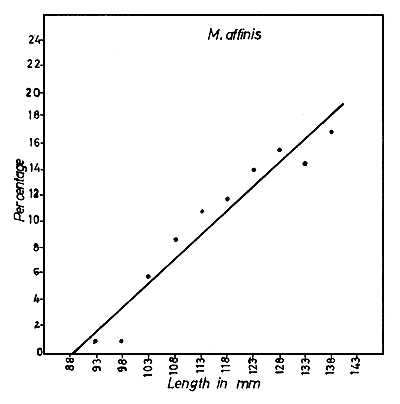
Fig. 6 Percentage of maturing and mature female prawns plotted against total length, for each species. The regression line cuts the length axis at a value corresponding to the minimum length at first maturity.
The size at first sexual maturity was determined for each species by estimating the percentages of all the maturing and mature female prawns in 12 monthly samples and plotting the length (in 5 mm groups) against the percentage of maturing and mature prawns in each size group (Fig. 6). For fitting the regression lines, only sizes up to about 100 mm for M. dobsoni and P. stylifera, up to about 140 mm for M. affinis and up to about 170 mm for P. indicus were taken into consideration. By extrapolation, the estimated minimum size at first sexual maturity was found to be 64.1 mm for M. dobsoni, 88.6 mm for M. affinis, 130.2 mm for P. indicus and 63.2 mm for P. stylifera. The smallest mature specimens of these species collected during the present study were 64, 94, 134 and 70 mm respectively, and these figures are fully consistent with the calculated minimum values.
Present observation on M. dobsoni show that all the maturity stages are represented in all months, except in September, when immature and early maturing prawns only are encountered. The presence of late maturing and mature stages in almost all months suggests that M. dobsoni breeds throughout the year. However, peaks of spawning activity are evident in June, November to December, and April. The seasonal distribution of spawners is depicted in Fig. 7.
Menon (1952; 1955) recorded this species as breeding for at least 6 mo of the year, both on the Malabar coast and in the Cochin area, while Kesteven and Job (1957), George 1962) and George, Raman and Nair (1967) found it to breed throughout the year. It appears to be usual for the species to show two main peaks of breeding activity in the year, but the timing of these peaks may vary from place to place and from year to year.
The seasonal distribution of M. affinis in different stages of maturity (Fig. 7) shows that non-spawning prawns dominate the catches from April to September, and that the species breeds from October to March with a peak in December. George (1961) and George, Raman and Nair (1967) report the breeding time of this species as October to December in the inshore fishery and as November to February in the offshore fishery of this coast. The present observation confirms this.
The seasonal distribution of mature P. indicus is shown in Fig. 7. The species has a prolonged breeding period in Cochin waters, with the greatest breeding activity between October and April. Panikkar and Menon (1956) recorded two breeding periods, in October to November and in May to June. George (1962) and George, Raman and Nair (1967) observed that the species breeds throughout the season with spawning peaks in October to November and May to June. Subrahmanyam (1963) reports that in Madras waters, the highest breeding activity of the species is seen during March and May to September.
The seasonal distribution of mature P. stylifera (Fig. 7) shows that this species also breeds throughout the year in the Cochin area, with peaks during December and June to August. Menon (1953) has observed the breeding of the species on the Malabar coast during October to December, while Shaikmahmud and Tembe (1960) recorded the species breeding throughout the year in Bombay waters, with an intensive period in March to May. George, Raman and Nair (1967) however, observed maximum numbers of maturing and mature females in December, during the 1959 to 1960 season.
Instances of penaeid prawns spawning more than once in a season have been observed in Penaeus japonicus (Hudinaga, 1942), P. setiferus (Lindner and Anderson, 1956) and P. duorarum (Cummings, 1961). During the present investigation, the monthly distribution of late maturing and mature females of each species was studied to determine the number of spawning in different species (Table II a,b,c,d).
TABLE II
Showing the monthly distribution (by numbers) of late maturing and mature female prawns in different sizes. The modes are underlined.
a. Metapenaeus dobsoni
Season: 1963 to 1964
| Months | ||||||||||||
| Size group | Jun. | July | Aug. | Sep. | Oct. | Nov. | Dec. | Jan. | Feb. | Mar. | Apr. | May |
| 61 – 70 mm | - | - | - | - | - | - | - | - | 11 | - | 1 | 2 |
| 71 – 80 " | - | - | - | - | - | - | - | 1 | 24 | 4 | 23 | 3 |
| 81 – 90 " | - | 2 | 3 | - | - | 4 | 6 | 14 | 22 | 11 | 41 | 20 |
| 91 – 100 " | 9 | 5 | 1 | - | - | 5 | 13 | 52 | 34 | - | 43 | 4 |
| 101 – 110 " | 23 | 24 | 1 | - | - | 7 | 10 | 25 | 17 | - | 19 | 3 |
| 111 – 120 " | 3 | 2 | - | - | 2 | 11 | 21 | 6 | 3 | - | 6 | - |
| Season: 1964 to 1965 | ||||||||||||
| 61 – 70 mm | - | - | - | - | 9 | - | - | - | - | - | - | - |
| 71 – 80 " | - | - | - | - | 43 | 1 | 2 | 1 | 4 | - | 1 | - |
| 81 – 90 " | 6 | 1 | - | - | 2 | 1 | 19 | 13 | 35 | 5 | 13 | 2 |
| 91 – 100 " | 57 | 2 | - | - | 4 | 4 | 25 | 32 | 82 | 13 | 68 | 7 |
| 101 – 110 " | 69 | 1 | - | - | 15 | 4 | 9 | 13 | 23 | 3 | 40 | 22 |
| 111 – 120 " | 1 | - | - | - | 24 | 4 | 34 | 17 | 11 | 2 | 7 | 8 |
| 121 – 130 " | - | - | - | - | - | 1 | 11 | - | 1 | 2 | 1 | - |
b. Metapenaeus affinis
Season: 1963 to 1964
| Months | ||||||||||||
| Size group | Jun. | July | Aug. | Sep. | Oct. | Nov. | Dec. | Jan. | Feb. | Mar. | Apr. | May |
| 91 – 100 mm | - | - | - | - | - | - | - | 4 | - | - | - | - |
| 101 – 110 " | - | - | - | - | - | - | 3 | 10 | 3 | 2 | - | - |
| 111 – 120 " | - | 1 | - | - | - | 5 | 1 | 11 | 2 | 10 | 1 | 2 |
| 121 – 130 " | - | 2 | - | - | - | 6 | - | 3 | 3 | 11 | 1 | - |
| 131 – 140 " | - | - | - | - | - | 7 | 2 | 21 | 4 | 7 | - | - |
| 141 – 150 " | - | 1 | - | - | - | 1 | 5 | 18 | 3 | 8 | - | - |
| 151 – 160 " | - | - | - | - | - | - | - | 2 | 1 | 2 | 2 | 1 |
| 161 – 170 " | - | - | - | - | - | - | - | - | - | - | 1 | - |
| Season: 1964 to 1965 | ||||||||||||
| 91 – 100 mm | - | - | - | - | - | - | - | - | 1 | - | - | - |
| 101 – 110 " | - | - | - | - | 3 | - | 3 | 2 | 9 | 2 | 1 | 1 |
| 111 – 120 " | 1 | - | - | - | 6 | 3 | 2 | 4 | 7 | 2 | 3 | 12 |
| 121 – 130 " | - | 1 | - | - | 14 | 21 | 3 | 6 | 11 | 7 | 2 | 3 |
| 131 – 140 " | - | - | - | - | 4 | 14 | 15 | 1 | 9 | 2 | 7 | 1 |
| 141 – 150 " | - | - | - | - | 2 | 1 | 16 | 9 | 2 | 3 | 7 | - |
| 151 – 160 " | 1 | - | - | - | - | 1 | 4 | 19 | 3 | 1 | 1 | - |
| 161 – 170 " | - | - | - | - | - | 1 | - | 8 | 2 | 2 | 1 | - |
c. Penaeus indicus
Season: 1963 to 1964
| Months | ||||||||||||
| Size group | Jun. | July | Aug. | Sep. | Oct. | Nov. | Dec. | Jan. | Feb. | Mar. | Apr. | May |
| 131 – 140 mm | - | - | - | - | - | - | - | - | - | 2 | - | - |
| 141 – 150 " | - | - | - | - | 2 | - | - | - | 3 | - | - | - |
| 151 – 160 " | - | 3 | 1 | 1 | 3 | 1 | - | - | 2 | 1 | - | - |
| 161 – 170 " | 3 | 1 | 6 | - | 2 | 1 | 1 | 1 | 3 | 3 | - | - |
| 171 – 180 " | - | - | 1 | 1 | 4 | 3 | - | 2 | 4 | - | - | - |
| 181 – 190 " | - | - | - | - | 1 | 2 | 1 | 13 | 9 | 2 | - | - |
| 191 – 200 " | - | - | - | - | - | 1 | - | - | - | - | - | - |
| Season: 1964 to 1965 | ||||||||||||
| 131 – 140 mm | - | - | - | - | - | - | - | - | 1 | - | - | - |
| 141 – 150 " | 3 | - | - | - | 3 | - | - | - | 3 | 4 | 3 | - |
| 151 – 160 " | 5 | - | 2 | - | 5 | - | - | - | 2 | 3 | 22 | - |
| 161 – 170 " | - | - | 3 | - | 9 | 1 | 1 | - | - | 2 | 22 | - |
| 171 – 180 " | - | - | - | - | 10 | 4 | 1 | - | 1 | - | 3 | - |
| 181 – 190 " | - | - | - | - | 3 | 4 | 1 | - | 1 | - | 3 | - |
| 191 – 200 " | - | - | - | - | - | 3 | 5 | 1 | - | 5 | 2 | - |
| 201 – 210 " | - | - | - | - | - | 1 | - | - | 1 | - | - | - |
d. Parapenaeopsis stylifera
Season: 1963 to 1964
| Months | ||||||||||||
| Size group | Jun. | July | Aug. | Sep. | Oct. | Nov. | Dec. | Jan. | Feb. | Mar. | Apr. | May |
| 71 – 80 mm | - | - | - | - | 17 | 23 | - | - | 3 | 1 | 6 | 6 |
| 81 – 90 " | 1 | - | 1 | 10 | 29 | 14 | 1 | - | 5 | 10 | 8 | 8 |
| 91 – 100 " | 5 | - | 3 | 12 | 19 | 16 | 3 | 7 | 8 | 9 | 22 | 12 |
| 101 – 110 " | 4 | 5 | 1 | 7 | 1 | 7 | - | 11 | 10 | 9 | 17 | 14 |
| 111 – 120 " | - | 3 | - | - | - | - | - | 3 | 2 | 4 | 5 | 6 |
| 121 – 130 " | - | - | - | - | - | - | - | - | - | 2 | - | - |
| Season: 1964 to 1965 | ||||||||||||
| 61 – 70 mm | - | - | - | - | - | - | - | - | - | - | 2 | - |
| 71 – 80 " | 1 | - | - | - | - | 1 | 2 | 3 | - | 1 | 4 | 9 |
| 81 – 90 " | 3 | 1 | - | 14 | 17 | 26 | 17 | 6 | 5 | 7 | 14 | 44 |
| 91 – 100 " | 5 | 5 | - | 17 | 15 | 38 | 47 | 12 | 4 | 4 | 41 | 53 |
| 101 – 110 " | 6 | 9 | - | 2 | 5 | 4 | 12 | 37 | 13 | 5 | 41 | 38 |
| 111 – 120 " | 2 | - | - | - | - | - | 1 | 7 | 7 | 1 | 12 | 13 |
| 121 – 130 " | - | - | - | - | - | - | - | - | - | - | 1 | 1 |
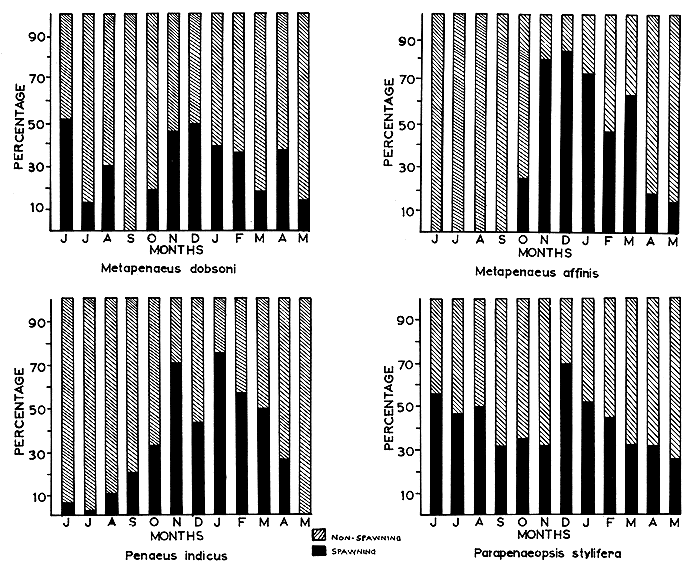
Fig. 7 Monthly percentages of prawns with ovaries in spawning and non-spawning condition.
M. dobsoni: In both years of observation, the 91 to 100 mm length-group provided the greatest number of spawning females between January and April or May, the 101 to 110 mm group were the dominant spawners in June and July, and the 111 to 120 mm group were dominant from October to December (Table II a). The suggested interpretation of these figures is that those females which constituted the 91 to 100 mm length-group in January spawned three times before attaining a length of 111 to 120 mm in December. Such a suggestion is consistent with the growth rate for the species, as determined by Banerji and George (1967).
TABLE III
The length and fecundity relationship and the minimum and maximum number of eggs produced by prawns
| Species | Relationship between fecundity and length | Coefficient of correlation (r) | No. of eggs produced | |
| Minimum | Maximum | |||
| M. dobsoni | Log F = -0.7175+2.8473 Log L | 0.8029 | 34,500 at 70 mm | 160,000 at 120 mm |
| M. affinis | Log F = -0.4306+2.7179 Log L | 0.8281 | 88,000 at 95 mm | 363,000 at 160 mm |
| P. indicus | Log F = -8.1277+6.0808 Log L | 0.9716 | 68,000 at 140 mm | 731,000 at 200 mm |
| P. stylifera | Log F = -1.5746+3.3437 Log L | 0.8079 | 39,500 at 70 mm | 236,000 at 120 mm |
Although minimum size at first maturity is 64 mm, the least modal size observed for mature prawns is 71 to 80 mm and the next mode is 81 to 90 mm. From this, it could reasonably be deduced that prawns spawn twice before attaining the 91 to 100 mm size. Thus an individual prawn of this species may spawn 5 times during its lifetime.
M. affinis: As the larger mature females of this species are encountered only from October to April, the spawning pattern is not as clear as in other species. However, it is probable (Table II b) that this species also spawns more than once during its growth from 91 to 100 mm to 151 to 160 mm.
P. indicus: In June to August the spawners of this species have a modal length of 161 to 170 mm; in October to November the modal group is 171 to 180 mm, and in January to February it is 181 to 190 mm (Table II c). These figures are consistent with spawning taking place thrice between the size 161 and 190 mm. Since the least size at first maturity is in the 131 to 140 mm group, it may be assumed that these prawns spawn at least twice before entering the 161 to 170 mm group, thus making a total of 5 spawnings during the lifetime of the individual.
P. stylifera: In the case of this species, the 91 to 100 mm modal group of mature prawns is seen in August to September, the 101 to 110 mm group in January to February and again in May to July (Table II d). Again 3 spawnings in a year are suggested. Since the smallest size of the mature prawn obtained is 70 mm, it is quite probable that, as in the case of M. dobsoni, this species also may spawn twice before growing to 91 to 100 mm size, thus making a total of 5 times before reaching the maximum size.
In all these species, it appears that there is a gap of nearly 2 mo between successive spawnings. This probably represents the time taken for the development of the immature ova to the final stage of maturity.
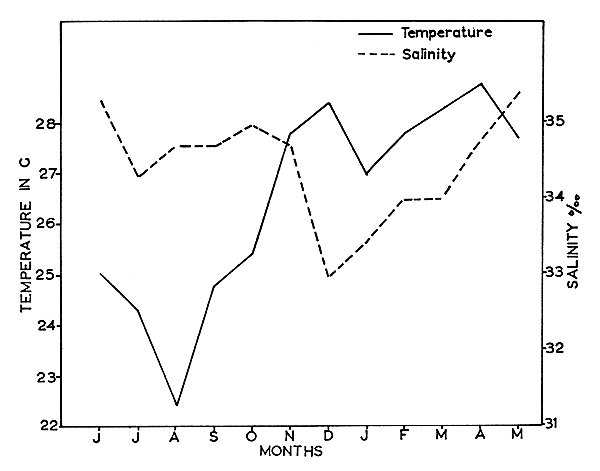
Fig. 8 Seasonal changes in bottom temperature and salinity of the prawn fishing grounds of Cochin during 1963–64.
Correlation between water temperature and spawning has been reported by Lindner and Anderson (1956), Ingle et al. (1959), Eldred et al. (1961), Cummings (1961) and Idyll, Jones and Dimitriou (1963). It has been shown that intensive spawning activity is generally related to rise in water temperature. The temperature and salinity variations of the bottom waters off Cochin for the year 1963 to 1964 are presented in Fig. 8. During July to September the bottom water temperature of the fishing grounds is less than 25.0°C and few mature females occur during this period. From October onwards the temperature increases, and the highest spawning peaks for most of the species are observed during this period.
There seems to be no clear relation between salinity and spawning. However, it is well known that juveniles of M. dobsoni, M. affinis and P. indicus migrate back to sea from their nursery grounds in the estuaries as they approach adulthood.
The number of eggs produced by mature females of each species was determined and the relationship between the length of the prawn and fecundity was estimated. It is found that the number of eggs produced varies with the size of the prawn in a logarithmic manner. The length-fecundity relationship for each species and the minimum and maximum number of eggs extruded by the females are given in Table III.
Fecundity of some of the palaemonid prawns has been estimated by Nataraj (1947), Aiyer (1949), Kunju (1956) and Rajyalakshmi (1961). Heldt (1938) found the fecundity of Penaeus kerathurus (as P. trisulcatus) to be between 800,000 and 1,300,000, while Fujinaga (1963) estimated the number of eggs produced in P. japonicus to be 1,200,000 in an individual weighing 25 g. Anderson (1956) reported the fecundity of P. setiferus as between 500,000 and 1,000,000 and Racek (1959) found the number of eggs extruded in Metapenaeus macleayi and M. mastersii to be 350,000 and 200,000 respectively.
In all the species studied during the present investigation, the distribution of ova in the mature ovaries (Fig. 2(G), 3(E), 4(F) and 5(F)) shows only two groups of ova, representing the immature and the mature stock. Moreover, these two groups are sharply separated from each other in size. As the mature stock of eggs are larger, with transparent peripheral regions, the individuals are either in the spawning condition or very nearly in that condition. Since the mature ova are well differentiated from the immature, spawning is probably restricted to a short and definite period. As the ovaries of spent-recovering specimens contain only small, transparent, yolk-less, immature ova of less than 0.096 mm, it is possible that all the mature ova present in the ovary are liberated at a single spawning act within a short time. Heldt (1938) and Fujinaga (1963) have observed that spawning takes place within 2 to 3 min in other penaeids. The present work suggests that the ova of the immature stock mature in about 2 mo.
The determination of the spawning period and its duration becomes difficult due to the fact that, while breeding is restricted to a short and definite period in the individual prawn, it is rather protracted in the population as a whole. The following explanations may be considered:
There may be different spawning times for younger and older specimens within the parent stock, as has been shown to occur in the decapods Palaemon elegans (as Leander squilla) and Carcinus maenas by Thorson (1946). The data on hand show, however, that in most months prawns of various sizes occur in all stages of maturity.
The population of each species may be broken into separate units and the spawning in these units is not synchronized. From our present knowledge of the population of these prawns, this does not seem likely.
Some females may remain in the early ripe condition for a long time preceding actual spawning, in order to tide over the winter and to spawn in the warmer months. This possibility also seems not to be applicable here as the temperature variation of the tropical waters is not significant.
A prawn may spawn frequently, with only a short duration between successive spawnings.
Prawns of various sizes occur in the samples and it seems likely that individuals of at least 3 of the species are capable of spawning 5 or more times. After each spawning, a group of ova gets separated from the immature stock and attains maturity within about 2 mo. The frequent spawning of individuals of various sizes and the short time between successive spawnings would result in the occurrence of all maturity stages in the fishery, and thereby show a prolonged breeding period in the population as a whole.
Aiyer, R.P., 1949 On the embryology of Palaemon idae Heller. Proc.zool.Soc.Beng., 2(2): 101–48
Anderson, W.W., 1956 Observations upon the biology, ecology and life history of the common shrimp, Penaeus setiferus (Linnaeus), along the South Atlantic and Gulf coasts of the United States. Proc.Indo-Pacif.Fish.Coun., 6(3):399–403
Banerji, S.K. and M.J. George, 1967 Size distribution and growth of Metapenaeus dobsoni (Miers) and their effect on the trawler catches off Kerala. Proc.Symp. Crustacea mar.biol.Ass.India, Part 2: 634–48
Cummings, W.C., 1961 Maturation and spawning of the pink shrimp, Penaeus duorarum Burkenroad. Trans.Am.Fish.Soc., 90(4):462–68
Eldred, B. et al., 1961 Biological observations on the commercial shrimp, Penaeus duorarum Burkenroad, in Florida waters. Prof.Pap.Ser.mar.Lab.Fla., 3:1–139
Fujinaga, M., 1963 Culture of Kuruma-shrimp (Penaeus japonicus). Curr.Aff.Bull.Indo-Pacif. Fish.Coun., (36):10–1
George, M.J., 1961 Studies on the prawn fishery of Cochin and Alleppey coast. Indian J.Fish., 8(1):75–95
George, M.J., 1962 On the breeding of penaeids and the recruitment of their postlarvae into the backwaters of Cochin. Indian J.Fish., 9(1):110–16
George, M.J., K. Raman and P.K. Nair, 1967 Observations on the off-shore prawn fishery of Cochin. Indian J.Fish., 10(2) (Also issued as Advance Abstr.Contr.Fish. aquat.Sci.India, 1(1):24–5
Heldt, J.H., 1938 La reproduction chez les crustacés décapodes de la famille des Penaeides. Annls Inst.océanogr.Monaco, 18(2):31–206
Hudinaga, M., 1942 Reproduction, development and rearing of Penaeus japonicus Bate. Jap.J. Zool., 10:(2):305–93
Idyll, C.P., A.C. Jones and D. Dimitriou, Production and distribution of pink shrimp larvae and postlarvae. Circ.U.S.Fish Wild.Serv., (161):93–4
Ingle, R.M., et al., Preliminary analalysis of Tortugas shrimp data 1957–58. Tech. Ser.Fla St.Bd Conserv., (32):1–45
Kesteven, G.L. and T.J. Job, 1957 Shrimp culture in Asia and the Far East: a preliminary review. Proc.Gulf and Caribb.Fish.Inst., 10:49–68
King, J.E., 1948 A study of the reproductive organs of the common marine shrimp, Penaeus setiferus (Linnaeus). Biol.Bull.mar.biol.Lab.,Woods Hole, 94(3):244–62
Kunju, M.M., 1956 Preliminary studies on the biology of the palaemonid prawn, Leander styliferus M. Edw. Proc.Indo-Pacif.Fish.Coun., 6(3):404–16
Lindner, M.J. and W.W. Anderson, 1956 Growth, migrations, spawning and size distribution of shrimp, Penaeus setiferus. Fishery Bull.Fish Wildl.Serv.U.S., 56(106):555–645
Menon, M.K., 1952 The life-history and bionomics of an Indian penaeid prawn, Metapenaeus dobsoni (Miers). Proc.Indo-Pacif.Fish.Coun., 3(2):80–93
Menon, M.K., 1953 Notes on the bionomics and fishery of the prawn Parapenaeopsis stylifera (M.Edw.) on the Malabar coast. J.zool.Soc.India., 5(1):153–62
Menon, M.K., 1955 Notes on the bionomics and fishery of the prawn Metapenaeus dobsoni (Miers) on the south-west coast of India. Indian J.Fish., 2(1):41–56
Nataraj, S., 1947 Preliminary observations on the bionomics, reproduction and embryonic stages of Palaemon idae Heller. Rec.Indian Mus., 45(1):89–96
Oka, M. and S. Shirhata, 1965 Studies on Penaeus orientalis Kishinouye. 2. Morphological classification of the ovarian eggs and the maturity of the ovary. Bull. Fac.Fish.Nagasaki Univ., (18):30–40
Panikkar, N.K. and M.K. Menon, 1956 Prawn fisheries of India. Proc. Indo-Pacif.Fish.Coun., 6(3):328–44
Racek, A.A., 1959 Prawn investigations in eastern Australia. Res.Bull.St.Fish.N.S.W., (6): 1–57
Renfro, W.C. and H.A. Brusher, 1964 Population distribution and spawning. Fishery Research Galveston Biological Laboratory. Circ.Fish Wildl.Serv.Wash., (183):13–5
Rajyalakshmi, T., 1961 Studies on maturation and breeding in some estuarine palaemonid prawns. Proc.natn.Inst.Sci.India(B), 27(4):179–88
Shaikmahmud, F.S. and V.B. Tembe, 1958 Study of Bombay prawns: the reproductive organs of Parapenaeopsis stylifera (M.Edw.). J.Univ.Bombay, 27(3):99–110
Shaikmahmud, F.S., 1960 Study of Bombay prawns: the seasonal fluctuation and variation in abundance of the commercially important species of Bombay prawns with a brief note on their size, state of maturity and sex ratio. Indian J.Fish., 7(1):69–81
Shaikmahmud, F.S., 1961 A brief account of the changes in the developing ovary of (Penaeid prawns) Parapenaeopsis stylifera (M.Edw.) in relation to maturation and spawning cycle. J.Univ.Bombay., 29(3–5):62–77
Subrahmanyam, C.B., 1963 A note on the annual reproductive cycle of the prawn, Penaeus indicus (M.Edw.) of Madras coast. Curr.Sci., 32(4):165–66
Thorson, G., 1946 Reproduction and larval development of Danish marine bottom invertebrates, with special reference to the planktonic larvae in the Sound (Øresund). Meddr Kommn Danm Fisk.-og.Havunders., 4(1):7–523
Acknowledgements
The author wishes to express his thanks to Mr. K.H. Mohamed and Mr. M.J. George for their invaluable guidance and criticism throughout the course of this work. Thanks are also due to Mr. S.K. Banerji for his advice and valuable suggestions. The data on temperature and salinity have been taken from the Oceanography Section of this Institute and this is gratefully acknowledged.