by
M.N. KUTTY 1
Central Marine Fisheries Research Institute
Mandapam Camp, India
Abstract
The routine oxygen consumption of Penaeus indicus and Penaeus semisulcatus (acclimation and test in seawater at 30°C), starved for 5–10 days, declined sharply by about the second day of starvation, and no marked change from the reduced rate was observed during the subsequent days of starvation in either species. The reduced level of metabolism attained due to starvation was 0.404 mg/g/h, being 32 percent less than the metabolic rate for the first day of starvation in the case of P. indicus, whereas the corresponding values for P. semisulcatus were 0.151 mg/g/h and 57 percent. When the amount of available dissolved oxygen is the same, P. indicus and P. semisulcatus starved for two days or over can be expected to survive for 1.7 and 2.3 times as long, respectively, as those not starved, under the conditions of the present tests.
The present regression of log oxygen consumption on log weight in P. indicus has a slope of 0.501. While the highest routine metabolic rate of P. indicus declined with the decrease in ambient oxygen concentration, such oxygen dependence need not be exhibited in all cases. There is possibly an increase in the standard metabolic rate of P. indicus at an oxygen level of 1–2 ppm when the prawns are allowed to bring down the ambient oxygen concentration by their own respiration.
1 Present address: Reader in Zoology, Madurai University, Madurai-2, S. India
CONSOMMATION D' OXYGENE DES CREVETTES Penaeus indicus H. MILNE EDWARDS ET Penaeus semisulcatus DE HAAN
Résumé
La consommation normale d'oxygène de Penaeus indicus et de Penaeus semisulcatus (acclimatation et épreuve en eau de mer à 30°C) privés de nourriture pendant 5 à 10 jours a nettement diminué à partir du deuxième jour environ, et n'a plus accusé de modification marquée pendant les jours suivants. Le niveau réduit du métabolisme causé par le jeûne (0,404/mg/g/h) était inférieur de 32 p. 100 à celui du premier jour de jeûne dans le cas de P. indicus, les valeurs correspondantes chez P. semisulcatus étant de 0,151 mg/g/h et de 57 p. 100. Lorsque la quantité d'oxygène dissous disponible est la même, on peut s'attendre à ce que P. indicus et P. semisulcatus à jeun pendant deux jours ou plus présentent un taux de survie représentant respectivement 1,7 et 2,3 fois celui des animaux non privés de nourriture, dans les conditions des expériences rapportées.
La courbe actuelle de régression de la consommation d'oxygène (log) par rapport au poids (log) chez P. indicus accuse une pente de 0,501. Si le métabolisme normal plus élevé de P. indicus a diminué avec la teneur en oxygène du milieu cette sujétion n'apparaîtra pas nécessairement dans tous les cas. Il se peut que le taux métabolique normal de P. indicus s'accroisse pour une teneur en oxygène de 1–2 parties par million lorsque les crevettes font baisser la concentration ambiante en oxygène par leur respiration.
EL CONSUMO DE OXIGENO DE LOS LANGOSTINOS Penaeus indicus H. MILNE EDWARDS Y Penaeus semisulcatus DE HAAN
Extracto
El consumo normal de oxígeno de Penaeus indicus y P. semisulcatus (aclimatación y ensayo en agua de mar a 30°C), mantenidos sin alimentar de 5 a 10 días, disminuyó bruscamente al segundo día de la prueba, no observándose en ambas especies durante los días siguientes a ésta ningun marcado cambio en relación con la reducida tasa. El reducido nivel de metabolismo obtenido debido al hambre a que se sometieron fue de 0,404 mg/g/h, siendo un 32 por ciento inferior a la tasa metabólica en el primer día de hambre en el caso de P. indicus, mientras que los valores correspondientes para P. semisulcatus fueron de 0,151 mg/g/h y 57 por ciento. Cuando la cantidad de oxígeno disuelto existente es la misma, P. indicus y P. semisulcatus, mantenidos sin comer durante dos días o más, pueden sobrevivir 1,7 y 2,3 veces más, respectivamente, que los que no se mantuvieron sin comer, dentro de las condiciones de los actuales ensayos.
La actual regresión del consumo logarítmico de oxígeno en relación con el peso logarítmico del P. indicus tiene una pendiente de 0.501. Aunque descendió la elevada tasa normal metabólica de P. indicus al disminuir la concentración del oxígeno en el ambiente, tal dependencia del oxígeno no era preciso que existiera en todos los casos. Posiblemente existe un aumento de la tasa metabólica normal de P. indicus a un nivel de oxígeno de 1 – 2 p.p.m. cuando se deja a los langostinos que hagan descender la concentración del oxígeno del ambiente con su respiración.
Studies on the oxygen consumption of animals of economic value are of special interest since, as well-recognized, an estimate of the energy requirements of an animal can usually be obtained from measurements of its oxygen consumption in an aerobic state. Though the respiratory physiology, especially with reference to oxygen consumption, has been studied extensively in the case of fishes (review by Fry, 1957), only a few workers have paid attention to oxygen consumption in prawns (review by Wolvekamp and Waterman, 1960). Rao (1958) and Subrahmanyam (1962) have made recent studies in this field in India. Whereas the influence of weight and ambient oxygen concentration on the oxygen consumption of Penaeus indicus have been studied by Subrahmanyam (1962), the oxygen consumption of Penaeus semisulcatus has not previously been investigated. There is no study, as it appears, on the influence of starvation on the respiration of crustaceans, even though it is known that a marked reduction in oxygen consumption takes place due to starvation in some other poikilotherms (Berg, Lumbye and Ocklemann, 1958; Mann, 1958; Saunders, 1963; Beamish, 1964a).
The present study provides information on the influence of starvation on the metabolism of P. indicus and P. semisulcatus and also supplements information already available on the influence of weight and ambient oxygen concentration on the respiration of P. indicus.
P. indicus (juveniles) used in the present study were caught at Pullamadam from a brackishwater creek opening into Palk Bay. Larger P. indicus (adults) were taken from a large concrete stocking tank in the marine aquarium of the Central Marine Fisheries Research Institute. The adults were used only for studying the influence of weight and ambient oxygen on metabolism. The experiments designed to study the influence of starvation on the oxygen consumption of adult P. indicus had to be abandoned due to some unforseen difficulties. Only adults of P. semisulcatus were used in the present study. They were caught in an Indo-Norwegian project trawler fishing off Mandapam. The expermental animals were kept in running sea-water of an average salinity of 36.5‰ and an average temperature of 30°C for at least two weeks before they were used in experiments. The oxygen concentrations of the water in the acclimation tanks were close to air saturation. The animals undergoing acclimation were exposed to normal daylight. During the acclimation period the prawns were fed with chopped clam meat (Donax spp.) once daily, as much as they would eat. The prawns held on to the clam meat with their pereiopods for several hours and ate avidly. The remains of the food were removed about 6 hours after it was supplied.
Experiments were conducted at the same level of salinity and temperature to which the prawns were acclimated. Oxygen concentrations during tests declined from near air saturation to about 70 percent air saturation, except in the experiments in which the influence of ambient oxygen on the metabolism was studied, in which case the ambient oxygen concentration in the respirometers was allowed to be reduced by the respiration of the animals themselves until close to the asphyxial level. All the tests were done in the afternoons.
The smaller prawns (juveniles) were kept one each in 400 ml conical flasks and the larger prawns in similar flasks of 1000 ml capacity; these flasks (respirometers) remained submerged in sea water inside a ‘metabolism chamber’ (Job, 1955, 1959), which is a wooden box with galvanised iron sheeting inside, the inner walls of the iron sheeting and the wooden lid being painted black. Water entered the box through an inlet on one side and overflowed through an outlet on the opposite side, maintaining a constant level of water over the respirometers. Each respirometer with the experimental animal inside was arranged as shown in Fig. 1. The arrangement was such that water flowed through the respirometers for extended periods without any apparent harm to the animals, and therefore experiments lasting for several days could be conducted without disturbing the animals. Animals under test were not fed. Water continued to flow through the respirometers throughout the period of the experiment, except during the short intervals when the outlets of the respirometers were closed once a day for the determination of metabolic rate.
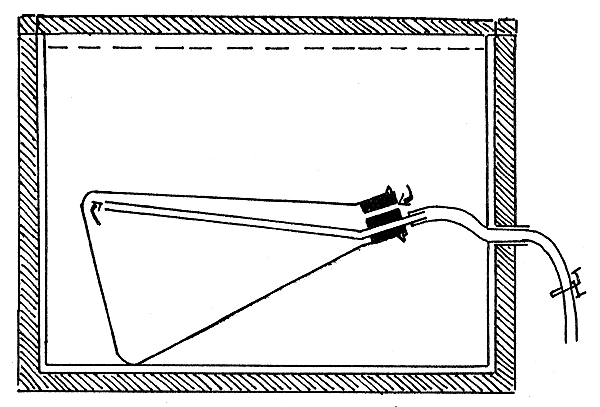
Fig. 1 Schematic section through a single unit of respirometer assembly (Job's apparatus). The broken horizontal line above shows the level of water. The arrows indicate the direction of flow of water when the outlet tube from the respirometer is kept open. The walls of the chamber are of wood (hatched area) with galvanized iron sheeting inside. The level of water is maintained by a constant level device at one end of the box (not shown). Water enters from the end of the chamber opposite to the overflow.
Dissolved oxygen concentrations were measured by using standard Winkler techniques on 20 ml water samples.
2.1 Experimental procedure
The prawns were starved for about 18 h in their acclimation tanks, after which they were removed for weighing. The adhering water on each prawn was carefully removed with a dry towel and filter paper, and then it was put inside a weighed tube about half-full with sea water which just accommodated the animal. After weighing, one prawn was put in each respirometer. The box with the respirometers inside was closed, and after sea water had flowed through the respirometers for 2 to 2½ h, i.e. about 20 h after the animals were last fed, a water sample was taken for oxygen determination and flow through the respirometer was then stopped by clamping the outlet tube. After a measured interval (15 or 20 min unless otherwise stated) a second water sample was taken, then the flow through the respirometer was resumed. The metabolic rate of the prawn was calculated from the oxygen concentrations of the two samples. This sampling procedure was repeated at 24 h intervals. The animals were weighed again at the end of the experiment.
It may be pointed out that the rate of oxygen consumption of each prawn was determined only once a day, except in experiments where the influence of ambient oxygen was studied. External disturbances to the animals were avoided as far as possible. The chamber was normally opened only once a day, after the metabolic rates for the day were measured, to check the state of the animals. The measurements of oxygen consumption made under such conditions can be termed as the ‘routine metabolic rate’ (Job, 1955; Fry, 1957) in that the only activity of the animals under test was spontaneous.
In the case of P. indicus, one extra series of tests was performed in which the prawns were handled every day, as indicated below. In this respect the experimental procedure of the latter series differed from the one described above. On each day the respirometers were removed from the experimental assembly. Subsequently the prawns were taken out by hand, kept for a few minutes in a bucket of sea water and put back again into the respirometers, which were then reassembled as in the original set-up. Each day, the metabolic rate was determined 2 to 2½ after the prawns were handled. The latter series of tests was designed as a supplement to the earlier series, to distinguish, if possible, the influences of starvation and handling on the oxygen consumption of the prawns under study.
The experiments dealing with the influence of ambient oxygen were done on adult P. indicus starved for three days inside the experimental assembly described above. During the tests the respirometers remained closed and water samples for oxygen analyses were taken at intervals of 15 to 30 min until the asphyxial levels of the animals were reached.
3.1 Influence of starvation
3.1.1 Penaeus indicus Table I gives the mean rates of oxygen consumption of starved P. indicus at intervals up to the 10th day. It should be noted that the metabolic rate indicated for the first day of starvation was estimated after the prawns had been starved for about 20 h. As shown in Table I, the mean metabolic rate for the first day (0.695 mg/g/h) was much higher than the means of the rates for each of the subsequent days. This feature is clearly shown in Fig. 2, where the mean rates (closed circles) for each day of starvation are plotted. By about the 2nd day of starvation the metabolic rate declined sharply, and further starvation did not cause any marked change from this reduced level. The mean oxygen consumption calculated from all values determined for the 2nd to the 10th day was found to be 0.404 mg/g/h, indicating a reduction of about 32 percent in the metabolic rate from that of the first day.
Results of experiments of P. indicus ‘handled’ every day (see section 2.1) are presented in Table II. The metabolic rates of oxygen consumption of this group are also plotted in Fig. 2 (open circles). The metabolic rate for the first day is slightly higher than the corresponding value in the first series of experiments. The general trend in the decline of oxygen consumption is markedly similar in both series, as is suggested by the trend line (thick line) drawn considering all the points shown in Fig. 2. A comparison of the mean rates of metabolism presented in Tables I and II indicates negligible differences between the values obtained from prawns which were handled on the first day only and those which were handled on every day of the experimental period. Enhanced metabolism due to handling has been noted in other animals (Winberg, 1956; Fry, 1957). It is possible, however, that in the case of the prawns which were handled daily any enhanced metabolism may have subsided to pre-handling levels within the course of the 2 to 2½ h which elapsed between the actual period of handling and the tests. The results obtained suggest that the metabolic rates of prawns determined in the present study are not influenced significantly by handling.
3.1.2 Penaeus semisulcatus Results of experiments on P. semisulcatus are summarized and presented in Table III. Here again by about the second day of starvation the metabolic rate had declined markedly and stayed at about the same level until the last day of the experimental period (6th day) (see Fig. 3). The mean metabolic rate for the first day was 0.346 mg/g/h, and the mean of all the rates from the second to the last day (6th) of the experiment showed a reduction of about 57 percent from the former. It may be noted that the relative decrease in the metabolic rate is more than that found for P. indicus. The metabolic rate of P. semisulcatus determined after the first day of starvation shows less variation from the steady level than in the case of P. indicus. As pointed out earlier, the individuals of P. semisulcatus tested were adults and larger forms, whereas those of P. indicus were juveniles. These differences may also be due to the quieter nature of P. semisulcatus, which were often seen to bury themselves in the sand in the acclimation tanks and, if sand were provided, inside the respirometers (see below section 3.4). The juveniles of P. indicus did not show this tendency during the several months of observation. Much of the difference in the metabolic rates of the two species may be attributed to the differences in their weights (Subrahmanyam, 1962), but species differences, and the fact that the salinities of the natural environments from which they were collected differ markedly, cannot be ignored (Lofts, 1956).
TABLE I
Influence of starvation on the oxygen consumption of Penaeus indicus (2.70 ± 0.28 g: mean ± one S.E.: n = 4) acclimated to 30°C in sea water (36.5 permille salinity) and tested at the same levels of temperature and salinity
| Day of starvation | Oxygen consumption in mg/g/h (mean ± one S.E.) | Number of determinations |
| 1st day | 0.695 �0.109 | 3 |
| 2nd day | 0.384 ±0.028 | 3 |
| 3rd day | 0.458 ±0.094 | 4 |
| 4th day | 0.406 ±0.045 | 4 |
| 5th day | 0.487 ±0.234 | 3 |
| 8th day | 0.278 ±0.064 | 4 |
| 10th day | 0.450 ±0.146 | 2 |
TABLE II
Influence of starvation on the oxygen consumption of Penaeus indicus (3.99 ± 1.16 g : mean ± one S.E.: n = 4) ‘Handled’ (see text) daily prior to the determination of the rates of oxygen consumption. The conditions of acclimation and test are similar to those indicated in the explanation for Table I
| Day of starvation | Oxygen consumption in mg/g/h (mean ± one S.E.) | Number of determinations |
| 1st day | 0.783 ±0.117 | 4 |
| 2nd day | 0.399 ±0.080 | 4 |
| 3rd day | 0.500 ±0.216 | 4 |
| 4th day | 0.384 ±0.077 | 4 |
| 5th day | 0.344 ±0.113 | 4 |
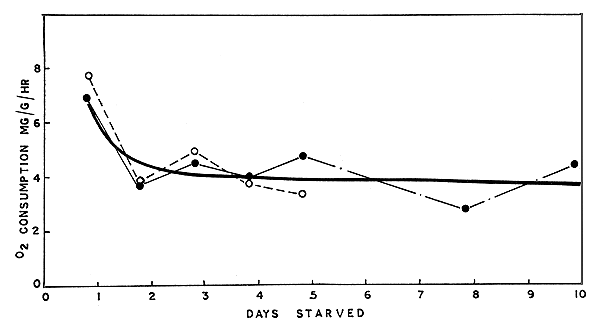
Fig. 2 Oxygen consumption in relation to period of starvation in Penaeus indicus in sea water at 30 C. The closed circles are mean values (taken from Table I) of metabolic rates of prawns ‘handled’ only on the first day of the experiment, and the open circles, denote similar values (taken from Table II) from prawns ‘handled’ on every day of the experiment (see text for details). The trend (thick) line is drawn by eye.
TABLE III
Influence of starvation on the oxygen consumption of Penaeus semisulcatus (17.39 ± 0.93 g : mean ± one S.E. : n = 4) acclimated and tested under similar conditions as those indicated in the explanation for Table I
| Day of starvation | Oxygen consumption in mg/g/h (mean ± one S.E.) | Number of determinations |
| 1st day | 0.346 ±0.070 | 3 |
| 2nd day | 0.161 ±0.019 | 4 |
| 3rd day | 0.111 ±0.007 | 3 |
| 4th day | 0.179 ±0.040 | 4 |
| 5th day | 0.153 ±0.034 | 2 |
| 6th day | 0.132 ±0.008 | 2 |
3.2 Influence of weight
As indicated earlier, the influence of weight was studied in P. indicus only. The results obtained are presented in Fig. 4 (closed circles). The regression line calculated for the values has a slope of 0.501, as against the ‘b’ value of 0.604 obtained by Subrahmanyam (1962) for P. indicus at 14 to 15‰ salinity and 28°C. A combined plot of the present values and those obtained by Subrahmanyam (1962) (open circles) in Fig. 4, shows that the points intermingle; it does not suggest such a difference in the scatter values as the difference in slope values indicate. It is evident that the present values apply to larger animals mainly. The difference in salinity levels of the two sets of experiments may explain the difference in the slopes of the regression lines, but no definite conclusion can be reached from the limited data available. Data obtained in another series of experiments (Kutty, MS) are also plotted (squares) in Fig. 4. These points represent means of 3 to 8 values of metabolic rates of much smaller prawns (see legend for Fig. 4) acclimated to three different salinities and tested at the same three salinities. The influence of salinity on the metabolic rates of P. indicus will be discussed elsewhere, but it appears that all the points shown in Fig. 4 form part of a single scatter in spite of the differences in salinity in the different tests.
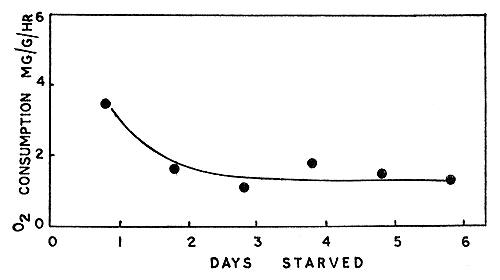
Fig. 3 Oxygen consumption in relation to period of starvation in Penaeus semisulcatus in sea water at 30°C. Mean values of metabolic rates from Table III are plotted.
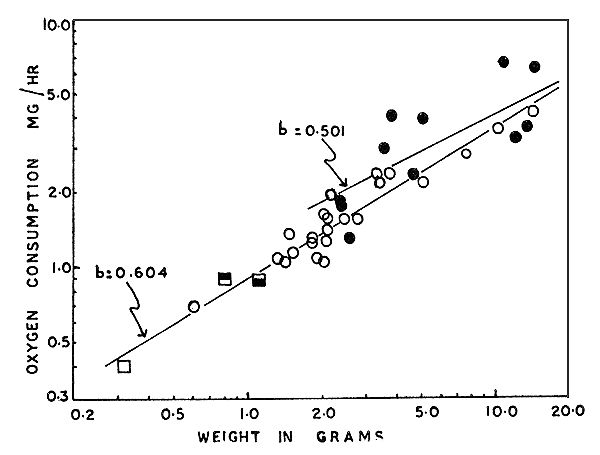
Fig. 4 Oxygen consumption in relation to weight in Penaeus indicus. Present values are shown as closed circles. The open circles are plotted from results obtained by Subrahmanyam (1962) on P. indicus at 28.2°C and 14‰ (salinity); the three large squares indicate values obtained (Kutty, MS) from small P. indicus acclimated to to and tested at the same temp (29°C) and the same 3 levels of salinities (35‰, large open square; 21‰, square-upper half closed; 7‰, square-lower half closed). Each square represents a mean of 3 to 8 determinations, for accommodation of which in the graph both ‘X’ and ‘Y’ values are multiplied by a factor of 10.
3.3 Influence of ambient oxygen
These experiments were also done with P. indicus only. Results obtained from four separate series of runs performed simultaneously are shown in Fig. 5. Data from the four animals used are indicated separately in the figure. A curve is drawn connecting the highest rates obtained. The lowest rates have also been connected similarly. The shape of the curve through the highest routine rates appears to be similar to the metabolic rate - ambient oxygen curves obtained for P. indicus by Subrahmanyam (1962), whereas the values other than the highest, which were obtained during the same test period, do not indicate such marked oxygen-dependence. This feature suggests that these animals kept undisturbed under standard conditions may have exhibited different levels of spontaneous activity (Beamish and Mookherjii, 1964), which, as stressed by Fry (1947, 1957), is a factor of prime importance in metabolism studies. In studies reported earlier, uniformly high rates have been obtained which show clearly the oxygen-dependence of metabolism, but these animals were probably under some stimulus causing excitement and activity, which induced them to respire at near maximum levels.
The line drawn through the lowest values in Fig. 5 can be taken as the closest to the standard metabolic rate of P. indicus. It may be pointed out that the rise shown in the latter curve at the low ambient oxygen level of 1 to 2 ppm is possibly indicative of an increase in the ‘standard metabolism’ of the prawn. A similar rise in the standard metabolism below the critical oxygen level has been observed in certain fishes by Beamish (1964b).
3.4 General considerations
Decrease in oxygen consumption in consequence of partial starvation was reported in the limpet Ancylus fluviatilis by Berg, Lumbye and Ocklemann, (1958). Mann (1958) observed that, after a meal of Tubifex, oxygen consumption of the leach Erpobdella testacea increased three-fold and declined to the previous level over a period of 4 days. Saunders (1963) noted that the oxygen consumption of cod decreased gradually during starvation for about a week. In the above studies the oxygen consumption refers to the routine metabolic rate only, and in estimating metabolic rates no account was taken of the spontaneous activity of the animals. The close relation between the metabolic rate and activity has already been indicated with reference to the influence of ambient oxygen on the metabolic rate. In his studies with speckled trout (Salvelinus fontinalis) and white sucker (Catostomus commersoni) Beamish (1964a) used an apparatus which could take account of spontaneous activity and obtained extrapolated values of standard metabolism of these fishes. He observed that there was a steep decline in the standard oxygen consumption of the trout and white sucker to reach a steady level by about the second day, which was maintained for 10 to 25 days. Simultaneous measurements of routine metabolic rates of these fishes, however, showed a gradual decline due to starvation, as observed in other animals by earlier workers. Beamish suggested that the reduction in the routine metabolic rates over the period of starvation was mainly due to a decline in spontaneous activity, whereas the decline in standard rate reflected an actual reduction in oxygen consumption due to starvation.
In the present study, only ‘routine’ metabolic rates of prawns are available. However, it is suggested that the values of oxygen consumption presented in the starvation experiments, especially those obtained for P. semisulcatus, are not markedly affected by spontaneous activity and are close to the standard metabolic rate. This contention is supported by the fact that when metabolic rates (0.152 ± 0.016 mg/g/h : mean ± one S.E.) of non-buried prawns (P. semisulcatus) were compared with the values (0.150 ± 0.020 mg/g/h) of buried (resting) prawns (cf. Dall, 1958; Egusa, 1961), the difference between the two was found to be not significant (t = 0.073; d.f. = 13). It is also of interest to note that the decline in the oxygen consumption of both species of prawn due to starvation appears to be sharp, as in the case of the standard oxygen consumption of fishes studied by Beamish, and not gradual as in the case of routine rates exhibited by fishes and other poikilotherms. It is thus likely that the decrease in oxygen consumption in starved prawns, especially in the case of P. semisulcatus, is primarily due to starvation and not due to a mere reduction in spontaneous activity.
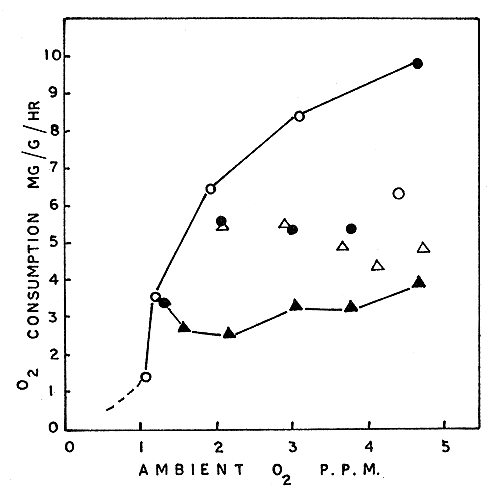
Fig. 5 Oxygen consumption in relation to ambient oxygen in Penaeus indicus in sea water at 30°C. Different symbols designate individual prawns tested: open circle, 14.5g; closed circles, 13.4g; open triangles, 10.6g; closed triangles, 11.4g. The line connecting the highest level of routine metabolism is closest to active metabolic rates; the line connecting the lowest points denotes levels closest to standard metabolism.
Results obtained suggest that when the amoung of available dissolved oxygen is the same, prawns starved for about two days or over can survive for a longer period than those not starved (about 1.7 times as long in the case of P. indicus and 2.3 times in P. semisulcatus under the conditions of the present experiments). The information obtained can be applied profitably when one is faced with the problem of transporting live prawns in water in which the oxygen supply is limited.
Beamish, F.W.H., 1964a Influence of starvation on standard and routine oxygen consumption. Trans.Am.Fish.Soc., 93(1): 103–7
Beamish, F.W.H., 1964b Respiration of fishes with special emphasis on standard oxygen consumption. 3. Influence of oxygen. Can.J.Zool., 42(3):355–66
Beamish, F.W.H. and P.S. Mookherjii, 1964 Respiration of fishes with special emphasis on standard oxygen consumption. 1. Influence of weight and temperature on respiration of goldfish, Carassius auratus L. Can.J.Zool., 42(2): 161–75
Berg, K.J., J. Lumbye and K.W. Ocklemann, 1958 Seasonal and experimental variation in the oxygen consumption of the limpet, Ancylus fluviatilis. J.exp.Biol., 35:43–73
Dall, W., 1958 Observations on the biology of the greentail prawn, Metapenaeus mastersii (Haswell) (Crustacea Decapoda: Penaeidae). Aust.J.Mar.Freshwat.Res., 9(1):111–34
Egusa, S., 1961 Studies on the respiration of the ‘Kuruma’ Prawn, Penaeus japanicus Bate. 2. Preliminary experiments on its oxygen consumption. Bull.Jap.Soc. Scient.Fish., 27(7):650–9
Fry, F.E.J., 1947 Effects of the environment on animal activity. Univ.Toronto Stud.Biol. Ser., (55)
Fry, F.E.J., 1957 The aquatic respiration of fish. In The physiology of fishes; edited by M.E. Brown, New York, Academic Press, Vol. I, pp. 1–63
Job, S.V., 1955 The oxygen consumption of Salvelinus fontinalis. Univ.Toronto Stud.Biol. Ser., (61)
Job, S.V., 1959 The metabolism of Plotossus anguilaris (Bloch) in various concentrations of salt and oxygen in the medium. Proc.Indian Acad.Sci.(B), 50:267–88
Kutty, M.N., MS Studies on the influence of salinity and temperature on the oxygen consumption of Penaeus indicus
Lofts, B., 1956 The effects of salinity changes in the respiratory rate of the prawn Palaemonetes varians (Leach). J.Exp.Biol., 33:730–6
Mann, K.H., 1958 Seasonal variation in the respiratory acclimation of the leech, Erpobdella testacea (Sav.). J.Exp.Biol., 35:314–23
Rao, K.P., 1958 Oxygen consumption as a function of size and salinity in Metapenaeus monoceros Fab. from marine and brackish-water environments. J.exp.Biol., 35:307–13
Saunders, R.L., 1963 Respiration of the Atlantic cod. J.Fish.Res.Bd Can., 20(2):373–86
Subrahmanyam, C.B., 1962 Oxygen consumption in relation to body weight and oxygen tension in the prawn, Penaeus indicus (Milne Edwards). Proc.Indian Acad.Soi.(B), 55:152–61
Winberg, G.G., 1960 Rate of metabolism and food requirements of fishes. Transl.Ser.Fish. Res.Bd Can., (194)
Wolvekamp, H.P. and T.H. Waterman, 1960 Respiration. In The physiology of Crustacea; edited by T.H. Waterman, New York, Academic Press, Vol.I, pp. 35–100
Acknowledgements
I gratefully acknowledge the help rendered by Mr. G. Murugopoopathy in conducting the experiments included in this study.
I am very thankful to Dr. S. Jones for his encouragement and interest taken in this work and to Mr. S.K. Banerji for offering his comments on this paper.