| FAO Fisheries Synopsis No.94 | FRm/S94 SAST - Prawn |
prepared by
K.H. MOHAMED
Central Marine Fisheries Research Institute 1
Mandapam Camp, India
Phylum Arthropoda
Class Crustacea
Subclass Malacostraca
Series Eumalacostraca
Superorder Eucarida
Order Decapoda
Suborder Natantia
Section Penaeidea
Family Penaeidae
Subfamily Penaeinae
Genus Penaeus Fabricius, 1798
Species Penaeus indicus
H. Milne Edwards, 1837
Generic
Genus Penaeus Fabricius, 1798, Suppl. Entomol. Syst.: 408. Type species, by selection by Latreille (1810, Consid. gén. Anim. Crust. Arachn. Ins.: 102, 422): Penaeus monodon Fabricius, 1798. Gender: masculine. Placed on Official List of Generic Names in Zoology as name no. 498.
Rostrum toothed dorsally and ventrally. Carapace without longitudinal or transverse sutures; cervical and orbito-antennal sulci and antennal carinae always present. Hepatic and antennal spines pronounced, pterygostomial angle rounded. Telson with deep median sulcus, without fixed subapical spines, with or without lateral movable spines. First antennular segment without a spine on ventral distomedian border. Antennular flagella shorter than carapace. Maxillulary palp with 2 or 3 segments, usually 3. Basial spines on 1st and 2nd pereiopods; exopods on 1st 4 pereiopods, usually present on 5th. Petasma symmetrical, pod-like with thin median lobes with or without distal protuberances; lateral lobes often with thickened ventral margin. Appendix masculina with distal segment subtriangular or ovoid, bearing numerous spines. Thelycum usually with an anterior process, variable in shape, lying between the coxae of 4th pereiopods; with or without lateral plates on sternite XIV. Pleurobranchiae on somites IX to XIV: a rudimentary arthrobranch on somite VII, and a posterior arthrobranch on somite XIII; mastigobranchiae on somites VII to XII. Zygocardiac ossicle consisting of a principal tooth followed by a longitudinal row of smaller teeth which often end in a cluster of minute teeth. Body glabrous. (After Dall 1957, slightly modified by Pérez-Farfante).
Specific
Species Penaeus indicus H. Milne Edwards, 1837 (Fig. 1a).
The type specimens, if still extant, are preserved in the collection of the Muséum National d'Histoire Naturelle, Paris, France.
Type locality: “Les côtes de Coromandel” (coast of S.E. India north and south of Madras).
Body completely glabrous. Rostrum slender, long, with distinct double curve, 1½ to twice the length of carapace in the juvenile stages, first five dorsal teeth close together, penultimate and distal teeth widely separated, position of latter variable. Rostrum becomes shorter with increasing size, equalling length of carapace in prawns of 80 mm, almost straight and with higher blade. Rostrum extending beyond tip of antennal scale in large prawns, blade high but not forming a triangular crest. Adrostral groove shallow, decreasing in depth backwards up to epigastric tooth. Eight to nine (sometimes seven) dorsal and four to five ventral teeth on rostrum. Carapace glabrous, thin, sulci and carina feebly defined. Gastro-orbital carina occupying the posterior ⅔ distance between hepatic spine and orbital angle. Orbitoantennal sulcus wide and ill-defined. Postantennular spine continued as an oblique ridge to the hepatic spine. Subhepatic ridge absent. Abdominal segments four and five keeled, keel on sixth segment ending acutely. Telson grooved, without lateral spines. Second and third joints of the first pereiopod and second joint of the second pereiopod provided with a spine.
Maxilliped III reaches to the second segment of the antennular peduncle. Dactyl of maxilliped III of adult male as long as the propodus. First, fourth and fifth pereiopods reach the first segment of the antennular peduncle; second pereiopod extending to tip of antennular peduncle and third surpassing the same by half length of chela. Mandibular palp two segmented, last segment subrhomboidal, bluntly pointed at apex, nearly twice as long as wide. Endopodite of maxillula segmented in two. Distal piece of appendix masculina deltoid in outline with rounded apex fringed with thickly set setae. Sixth abdominal somite as long as telson.
Median lobe of petasma rounded at tip, projecting forward to apex of lateral lobe which is covered with sparsely set fine setae on outer surface. Terminal portion of distal margin serrated with 12 well-calcified teeth. Anterior median process of thelycum roughly semicircular and relatively small, situated on sternite between fourth pereiopods; minute apical spines on anterior margin of this process. Two large lateral plates housing sominal receptacles occupy most of last thoracic sternite. Lateral plates meet each other in median line where edges of plates are upcurved to form appearance of a valve. (Fig. 1b and c).
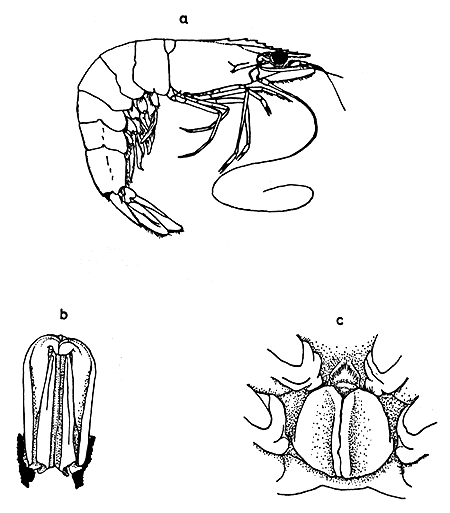
Fig. 1 a. Penaeus indicus; b. Petasma; c. Thelycum
(From Hall, 1962)
Cardiac plate has 21 to 23 equidistant spinules set in a longitudinal row. Zygocardiac ossicle has 8 to 13 conical teeth set in an arc-shaped series. Prepyloric armed with about ten pointed teeth which get successively larger towards tip.
“Colour in life pale buff (body semitranslucent) with blue pigment spots. Crest of carapace and abdomen reddish brown. Eyestalks and antennal scales bluish, margin of uropoda blue with bright red fringe. Antennae not banded, antennulae spotted” (Racek, 1955).
Hall (1962) has shown that variations in the number of spinules in the different components of the stomodial apparatus are more or less similar in P. indicus and P. merguiensis.
P. indicus is one of the about 28 species of the genus Penaeus Fabr. It is well characterized and shows closest resemblance to P. merguiensis De Man and P. penicillatus Alcock, which by some authors were considered to be mere varieties of P. indicus. At present the three are considered distinct species by the majority of authors. P. indicus longirostris De Man proved to be based on immature specimens of the present species. P. durbani Stebbing probably also is nothing but a juvenile stage of the present species.
Keys distinguishing P. indicus from other Indo-Pacific Penaeinae are given by Cheung (1960) and by Racek and Dall (1965).
Penaeus indicus H. Milne Edwards, 1837 (Hist. nat. Crust. 2:415)
Objective synonymy
[None]
Subjective synonymy
?Palaemon longicornis Olivier, 1811, Enc. méth. Hist. nat. Ins. 8: 662
Penaeus indicus longirostris De Man, 1892, Weber's Zoolog. Ergebn., 2: 511.
?Penaeus durbani Stebbing, 1917, Ann. Durban Mus., 1:442.
Penaeus longicornis (Olivier) Stebbing, 1921, Ann. S. Afr. Mus., 18: 463.
| Country | Standard common name | Vernacular name |
| Australia | Indian prawn | Indian prawn |
| East Pakistan | ‘Chapda chingri’ | Chapra, Chamma, Chaga |
| India | ||
Calcutta | - | Chapda chingri |
Madras | - | Yera |
Kerala | - | Naran, Vella chemmeen |
Bombay | - | Jinga |
| Indonesia | - | Udang putik tambak |
| Philippines | - | Hipon-puti |
| West Pakistan | - | Jairo |
No subspecies of P. indicus are recognized at present; (the case of P. indicus longirostris is mentioned in section 1.1.2). Differences between specimens from India and from further east in the setation of the propodus of the third maxilliped are mentioned by Hall (1967).
The occurrence of P. indicus has been reported from New South Wales: off Broadwater; Queensland: Gulf of Carpentaria; New Guinea: Port Moresby; North Borneo: Labuan, Sandakan; Indonesia: Palembang, East Kalimantan, Java; Philippines: Manila Bay, St. Miguel Bay, Bulacan Province, Santa Cruz, Luzon; Singapore: Amoy; Andamans; East Pakistan; coasts of India and Ceylon; West Pakistan; Gulf of Aden; east coast of Africa from the Red Sea to Algoa Bay, including Madagascar. In terms of the FAO areas code (see Holthuis and Rosa, 1965), the species occurs in most coastal regions of sea area ISW and the western part of area ISEW; it also extends a little way into area PSE, on the east coast of Australia. It occurs on the coasts of land areas 154, 155, 415, 421(W), 423, 424, 425, 432, 433, 434, 437, 615 and 616. In India, Ceylon, Malaya, Singapore and much of east Africa the species exists as a commercial fishery but it is reported as of scattered distribution in Australia, New Guinea and the Philippines. Considered rare in waters east and southeast of Borneo.
The species has been recorded from as deep as 43 m (Barnard, 1950) but is not generally common in water of more than about 10 m (see also 2.2.2). It usually occurs on a muddy seabed, occasionally on sand.
Eggs
Eggs of P. indicus have been reported to occur in large numbers in subsurface plankton in Madras waters (Subrahmanyam, 1965). They were obtained from 3 m below surface in February 1964. These eggs have been referred to this species purely on the basis of the circumstantial evidence of simultaneous occurrence of adults in mature condition. Panikkar and Menon (1956) stated that the species preferred deeper waters for spawning, its eggs and larvae having been seldom obtained along with those of other penaeids known to exist in the same areas.
Larvae
Menon (1937) recorded three stages of protozoea and one schizopod (mysis) stage from surface plankton on the Madras coast. Collections made from areas near Cochin indicate that large numbers of larval forms in advanced stages are present in the inshore subsurface waters particularly in the early hours of the morning. In Madagascar, Crosnier (1965) found protozeal stages to be restricted to the shallow parts of certain sheltered bays; later (mysis) stages were rather less restricted in distribution.
Postlarvae
Postlarval stages of the species are well represented in the tow net collections taken from the estuaries near Cochin on the south-west coast of India. George (1962) estimated the seasonal abundance of postlarvae of P. indicus (8 to 14 mm in total length) and studied the recruitment of these into the backwaters of Cochin. He observed that the postlarvae enter the Cochin backwaters in all months except June to September. Hall (1962) observed that penaeid larvae and postlarvae (species not specified) formed only an insignificant portion of the plankton collected from Singapore prawn ponds. He observed that more postlarvae were present in the inflowing waters than in the outflowing waters of the prawn pond.
Juveniles
In India stages of P. indicus (30 to 120 mm total length) spend their life mostly in the estuaries and backwaters. On the south-west coast of India these juveniles support a good commercial fishery in the backwaters and paddy fields where they live till they attain lengths of 100 to 120 mm, after which they go back into the sea (Menon, 1955, Menon and Raman, 1961). Hall (1962) observed the maximum size of the species in the Singapore prawn pond as 27 mm carapace length. This is about 113.4 mm total length as per the conversion rate given by Hall. The entire catch from the prawn ponds are therefore juveniles. The juveniles are bottom living and are obtained from the estuarine environment throughout the year. Crosnier (1965) captured juveniles and adults, ranging in length from 30 to 200 mm, in fixed traps in the intertidal zone of bays in Madagascar, but took only specimens of over 100 mm in fine-meshed trawls a little way offshore, in water of 5–10 m depth.
Sexually mature adults occur only in the sea. They are associated with shallow coastal regions and muddy sea bottom which are subject to changes due to the physical conditions of the coast line and the nutrients obtained from the land and rivers. De Bruin (1965) stated that they prefer sand bottom and shallow waters of the sea within 2 to 6 fm (3.7 to 11 m).
On the coasts of India the adults form part of the prawn fishery within 25 fm (45.8 m) in the sea. The occurrence of the species in the fishery is subject to seasonal fluctuations as detailed in section 5.3. George and Mohamed (1966) observed that the prawn fishery of Kanyakumari district (southern extremity of the west coast of India) is exclusively supported by large-sized mature P. indicus. In Madagascar and other parts of east Africa fishable concentrations of adults are rarely found at depths greater than 10 m (Crosnier, 1965).
The restricted bathymetric range of the species and the factors determining it were discussed by Crosnier (1965). He listed seasonal variations in the temperature and salinity at different depths in Ambaro Bay, Madagascar, where the species is common; oxygen tension was always near saturation. He gave an analysis of the physical and chemical properties of the substrate at different sampling stations, and suggested that turbidity of the water may be an important ecological factor. If turbid water is favorable for the species and attractive to it, the lower turbidity generally associated with deeper waters may largely account for the comparative scarcity of the species in waters of more than about 10 m.
P. indicus is heterosexual. Sexes can be distinguished by external characters such as the presence of morphologically differentiated male and female sex organs. While the male sex organ, petasma, is abdominal in position, being the endopodite of the first pleopod, the female sex organ, thelycum, is a modification of the thoracic sternite. The presence of an appendix masculina on the endopod of the second pair of pleopods is another male character. While the genital openings of the male are situated on the coxa of the fifth pair of pereiopods those of the female are on the coxa of the third pair of pereiopods. Females attain relatively larger sizes than males.
Menon (1957) reported that most of the mature individuals examined by him measured 150 mm and over, and hence he used this size as the limit to determine the proportion of mature and immature prawns in the samples. By observing the nature of the petasmal endopodites Hall (1962) observed that specimens below 23.4 mm carapace length were immature. Rao (1968) has studied the process of maturation of the species by ova diameter measurements and has statistically estimated the size of the females at first maturity as 130.2 mm. The smallest mature female actually observed by him was 134 mm in total length. The age of the species at first maturity has not been precisely estimated. George et al. (1968) have stated that females of 141 to 145 mm size group and males of 126 to 130 mm size group represent the first year class. It could therefore be assumed that the species attains sexual maturity at about 130 mm, when about one year old.
The species is promiscuous. During mating the sperm packs known as spermatheca are deposited by the male in the external genitalia of the female. The females carry the spermatheca and the sperms are dispensed at the time of spawning.
Fertilization is external. As the eggs are extruded from the genital openings of the female the sperms are dispensed from the spermatheca.
No published information is available on the fecundity of the species. Rao (1968) has estimated fecundity as 68,000 in a female of 140 mm total length, to 731,000 eggs in a female 200 mm long. The relationship of gonad size and egg number is not determined but the estimated relationship of body length to number of eggs produced is Log F = -8.1277 + 6.0808 Log L, where F is the fecundity and L the total length of the prawn in mm, with a regression coefficient of 0.9716
Spawning seasons
Panikkar and Menon (1956) indicated the existence of two breeding periods namely October to November and May to June. Based on the occurrence of postlarvae of the species in the Cochin backwaters, George (1962) recorded the spawning season as from October to May with two peak spawning periods in November to December and during February to April. Hall (1962) observed the spawning season of the species as February to April in Singapore waters. Subrahmanyam (1963) studied the gonad index of the species from Madras waters and observed that the breeding activity appeared to be pronounced in the months of May, July, August and September, and that there may be lesser breeding activity in March. George et al. (1968) stated that the species breeds throughout the fishing season with two peaks as observed earlier. Rao (1968) observed that P. indicus has a prolonged breeding period extending from October to April in Cochin waters. The species also has an extended spawning period in Madagascan waters, with a peak in March and April, the months in which the highest water temperatures were recorded (Crosnier, 1965).
Number of spawnings per year, frequency
By closely following the sizes of the spawners during the breeding season Rao (1968) concluded that individuals of P. indicus spawn five times during a life time and that the interval between two successive spawnings is about two months.
Panikkar and Aiyar (1937) observed that the species did not attain sexual maturity in backwaters but that young ones were noticed in large numbers in the Adayar estuary when the bar was open to the sea. Panikkar and Menon (1956) stated that P. indicus seemed to prefer deeper waters for breeding, its eggs and larvae having been seldom obtained along with those of the other prawns of the area, which liberate their eggs in coastal waters not exceeding 10 to 12 fm (18.3 to 22 m) in depth. Shaikhmahmud and Tembe (1960) found the species represented in the Bombay catches by immature specimens although they observed a few mature females in November to December. Hall (1962) demarcated the possible spawning area of the species in the Malayan region east of Singapore in 10 to 20 fm (18.3 to 36.6 m) depth between Lat. 01°20'N and 01°40'N Long. 104°20'E and 104°30'E. Subrahmanyam (1965) collected freshly spawned eggs and nauplii from very close inshore waters of Madras, on the basis of which he suggested that the species might be breeding in the inshore areas. It is, however, probable that on most Indian coasts the species breeds in the sea in relatively deeper waters and the postlarvae migrate into the estuaries and backwaters for feeding and growth. In Madagascar, the eggs are probably laid in fairly shallow water, within the bays (Crosnier, 1965).
The largest ovarian eggs of the species measure 0.304 to 0.384 mm in diameter. They are transparent and spherical. From the opaque central region rod-like periferal bodies radiate towards the outer region. Subrahmanyam (1965) described the eggs of the species obtained from the Madras plankton. The eggs are perfectly spherical with considerable perivitelline space and measure 0.45 to 0.47 mm in diameter. In the blastula stage the embryonic mass encircled by a thin embryonic membrane measures 0.23 mm in diameter.
Four embryonic stages have been described by Subrahmanyam (1965) (Fig. 2a-d). The earliest stage shows only the blastula. In the next stage a definite pattern of limb buds can be discerned. The outline of the nauplius with its appendages is distinct in the third stage, and in the final stage the embryonic membrane ruptures releasing the nauplius within the egg capsule. This nauplius exhibits twitching movements inside the capsule. The newly hatched larva is the first nauplius. He described three nauplius stages (Fig. 2e-g) and stated that after the third nauplius the larva metamorphoses into the first protozoea and this takes place 68 hours after hatching. His description of three nauplius stages, however, does not conform with the nauplius stages of other species of the genus. It is possible that two nauplius stages are missing from his description. The protozoea obtained by rearing from the final stage nauplius by Subrahmanyam resembled the one described by Menon (1937). Menon's description included three protozoeae and the first schizopod stage (Fig. 2h-k). The third protozoea kept alive in the laboratory by Menon metamorphosed directly into the mysis (schizopod) stage in a couple of days, and he was of the opinion that there are only three protozoea stages in the life history of the species. Details regarding subsequent stages of development are wanting.
Feeding
No information is available regarding the food habits of the larval stages.
Rates of development and survival
The earliest developmental stage seen by Subrahmanyam (1965) was a blastula which was collected in the morning, and he presumed that spawning had taken place in the early hours of the morning. The eggs hatched out in the afternoon. On the basis of this the time taken for development is as follows:
| 12 (?) h after spawning | hatch into nauplius I |
| 20 h after hatching | nauplius II |
| 44 h after hatching | nauplius III |
| 66 h after hatching | protozoea I |
Menon's (1937) collection of protozoea stages and first schizopod stage were obtained from plankton samples and hence the time required for the development from one stage to the other was not given.
Parental care
There is no parental care of eggs or larvae.
Parasites and predators
As the larvae are pelagic some amount of predation by plankton feeders can be expected. There is no precise record on the magnitude of predation although fishery biologists who have worked on the food of fishes of the Indian region have recorded ‘Penaeus larvae’ and ‘prawn larvae’ as important constituents of the food of several fishes.
Panikkar and Aiyar (1939) stated that praw of this species enter the backwaters and lakes as larval or postlarval stages and grow there for about a year, after which they go back to the sea to breed. George et al. (1968) observed what they considered to be a third year class in the offshore catches of Cochin in certain years, but most species of Penaeus do not live for more than 1½ years.
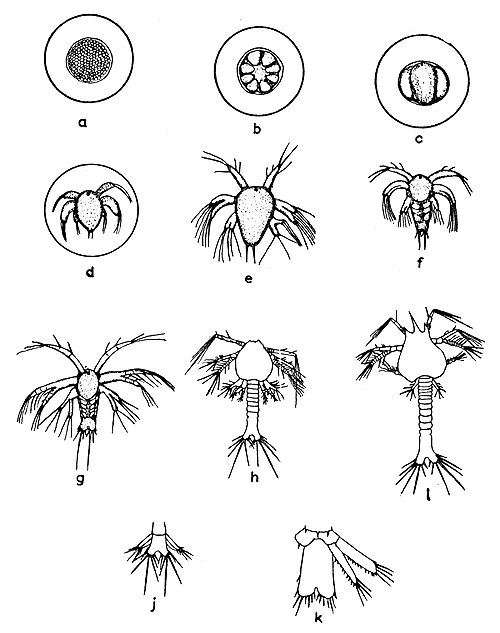
Fig. 2 a-d. Development stages of egg; e. Nauplius I; f. Nauplius II; g. Nauplius III (Subrahmanyam, 1965) h. Protozoea I; i. Protozoea II; j. Uropod and telson of protozoea III; k. Uropod and telson of Schizopod I (Menon, 1937)
P. indicus is capable of withstanding a wide range of salinity. Perhaps this tolerance of salinity changes is more pronounced in the younger stages. To some extent the species is eurythermal as seen from the wide gradient of temperature of its natural habitats. It cannot live out of water for long. Hall (1962) reported that mashed tea-seed-cake spread on water to poison fishes did not have any effect on the penaeids of the Malayan prawn ponds.
Penaeids in general are preyed upon by the demersal fishes of the area where they exist. In most cases fishery biologists have recorded ‘penaeids’, ‘prawns’, etc. as forming major portions of food of several species of demersal fishes.
The greatest recorded size is a female of 9 in (228.6 mm) from the south-west coast of India (Panikkar and Menon, 1956). The largest male and female taken in Madagascar were 184 and 202 mm respectively (Crosnier, 1965).
Gopalakrishnan (1952) observed that the species does not show any significant difference in food habits in different months of the year. Analysis of the food of different sizes also showed no variations. Based on the periodic fluctuations of the various items of food consumed, he observed that these prawns feed on whatever suitable material they come across. From laboratory observations he remarked that in the state of nature the species is partly predatory and chases smaller creatures of a size which can be seized between the appendages. Larger crustaceans, fish and others are attacked only in dead condition. They usually prefer small particles of food, which are grasped by the chelae of the pereiopods and passed on to the mouth. When bigger articles are taken up, more than one pereiopod, with or without the assistance of the third maxilliped, is used to catch hold of the prey. While capturing small ctenophores and medusae the pereiopods hold the prey close to the mouth so that the mouth parts can act on it. The actual ingestion of the material is accomplished very slowly and sometimes the prawn releases its hold to take up smaller food items available. Even though many living forms are kept together for a number of days in the laboratory tanks, no instance of smaller prawns being attacked and killed by the bigger ones was noticed, even after starving them for days together. However, when an individual in the tank died or was about to die, it was immediately attacked by the living ones and eaten. Crosnier (1965) found that, in the aquarium, P. indicus would attack and eat moulting specimens of the same species.
Panikkar (1952) stated that the food of young penaeids consists of organic detritus found in the mud, algal material and other extremely small organisms contained in the mud. Gopalakrishnan (1952) analyzed the gut contents of 380 specimens and found that vegetable matter and crustaceans formed the bulk of food consumed. Presence of other animal matter indicated omnivorous habits. The vegetable matter included diatoms like Coscinodiscus, Pleurosigma, Rhizosolenia, the planktonic alga Trichodesmium and cuttings of sea weeds. The crustaceans included copepods, ostracods, amphipods, tiny decapods and their larval stages. Molluscan shell pieces, polychaetes, echinoderm larvae, hydroids, trematodes (living) and foraminifera were occasionally met with. Panikkar and Menon (1956) stated that the food of prawns (Metapenaeus dobsoni and P. indicus) consists of detritus, both animal and plant, that accumulate at the bottom of their habitats which are usually areas with muddy bottom. They seem to consume large quantities of algal matter when available, some of the stomachs examined having been practically full of it. Small living creatures, like molluscs and worms, living at the bottom may also be taken in. Hall (1962) found that the food of the juveniles of the species from Malayan prawn ponds consisted of crustacea (small and large), vegetable matter and polychaeta. Larger specimens took in more crustaceans including penaeids and brachyurans.
Hall (1962) estimated a growth rate of 0.102 mm in carapace length per day in prawns caught from Malayan prawn ponds, and he considered this a fast rate of growth since limiting factors such as extreme temperatures and scarcity of food are nonexistent in that habitat. Temperatures varied between 28 and 34°C. While studying the offshore prawn fishery of Cochin, George et al. (1968) stated that the modes seen in the size frequency distribution at 126 and 130 mm for males and 141 to 145 mm for females represent the first year class, those at 161 to 165 mm for males and 171 to 175 mm for females represent the second year class and those above 195 mm represent the third year class. By following the progression of modes they also estimated a growth rate of 20 mm in males and 15 mm in females during four months at the end of the first and beginning of the second year.
Hall (1962) has estimated the length weight relationship of the species as
W = 0.6918 C2.922
where W is weight of prawn and C is carapace length in cm.
The rate of growth of juvenile prawns is relatively high when they are in the estuaries and backwaters, which act as a sort of nursery ground for the species.
In many parts of India the life cycle of the species is completed after passing through two distinct environments - the sea and the estuary. The larval development takes place in the sea, and the migration into the estuaries, lakes and backwaters commences when they are in late mysis or early postlarval stages. According to Panikkar and Menon (1956) large numbers of young ones pass into the backwaters before they are 10 mm long and this process of migration is continuous throughout the breeding period. The size attained by the species in the backwaters does not exceed 120 mm. Kemp (1915) recorded the highest size observed by him in the Chilka Lake as 120 mm. The seaward migration begins after this size is attained. The seaward movement is described as a passive process as the prawns are usually carried by the large influx of rain water flowing into the sea during the monsoon period. Further growth, attainment of sexual maturity and other life processes take place in the sea. Shaikhmahmud and Tembe (1960) recorded that the species moves into Bombay waters in September to December and February to June, and that these movements mostly take place after heavy rains.
Hall (1962) demarcated the spawning ground of P. indicus about 50 to 60 mi (80 to 96 km) from the Singapore prawn ponds and remarked that “migration of 40 to 50 n mi should be well within the capabilities of the mature P. indicus”. Regarding the offshore prawn fishery of Cochin, George et al. (1968) stated that the females of the species belonging to the larger size groups move to still deeper waters or other areas for spawning activities and never return.
In Madagascar any migration which takes place appears to be over very short distances (Crosnier, 1965).
(See Sections 3.1.1 to 3.1.7)
The sex ratio of the species obtained from the backwaters and from the inshore marine catches from Narakkal was studied in detail by Menon (1957). (Data from Menon's work reproduced in Tables I and II). His studies showed that the sexes were more or less equally distributed both in marine and in backwater environments. Shaikhmahmud and Tembe (1960) observed larger numbers of females in September and November in the commercial catches of Bombay. George and Rao (1967) found the distribution of sexes in the trawl catches of Cochin significantly different from what could be accounted for by the binomial theory. Crosnier (1965) found the proportion of males in seven samples from Madagascar to range from 35 to 55%, with an average of 45%.
In south-west India, late larvae and early juveniles enter the backwaters in November and December and move out in September and October of the following year, when almost 1 year old (George, 1962). The prawns which occur in Singapore prawn ponds for 10 mo of the year (Hall, 1962) are also less than 1 year old. The catches of intertidal traps in Madagascar (Crosnier, 1965) appear to consist mostly of prawns in their first year but include some specimens likely to be over 1 year old. Catches from the open sea in all areas where the species occurs appear to consist almost entirely of prawns of more than 1 year old.
In the backwater catches of Cochin the maximum size recorded is 140 mm. More than 80 percent of these catches were below 100 mm and the modal size groups observed were between 81 and 90 mm (Menon and Raman, 1961). In the inshore marine catches of the Alleppey coast George (1961) observed the modal length shifting from 113 (i.e. 111–115) mm in July to 133 mm in October and at Chellanam from 113 mm to 128 mm from January to May and from 153 to 163 mm in September to December. At Narakkal the predominant size group was observed at 98 mm in January to February and at 133 and 143 mm in June, July and October. In the offshore trawl catches George et al. (1968) observed 163 mm in males and 173 mm in females predominating the catches during the early part of the season. Towards the close of the season the mode was seen at 148 mm for males and 158 mm for females. In Singapore prawn ponds the maximum size was 27 mm carapace length (ca. 114 mm total length), the majority size was between 10 and 20 mm carapace length (ca. 42 to 84 mm total length). In the commercial catches of Bombay, Shaikhmahmud and Tembe (1960) recorded 45–125 mm length range and 180–200 mm length range, the latter occurring only occasionally. In Madagascar (Crosnier, 1965), intertidal barrage traps took specimens ranging from 30 to 200 mm (total length), with the modal length varying between 100 and 150 mm in different catches. The length of specimens in trawl catches ranged from 100 to 210 mm, with the mode usually about 150 mm.
TABLE I
Sex ratio of P. indicus in Cochin waters (Menon, 1957)
| Year | All size | Over 120 mm | Over 150 mm | |||||||
| Ratio | Ratio | % in total | Ratio | % in total | ||||||
| M | F | M | F | M | F | M | F | M | F | |
| 1952 | 41.6 | 58.4 | 41.4 | 58.6 | 82.9 | 83.5 | 36.5 | 63.5 | 29.9 | 37.2 |
| 1953 | 49.0 | 51.0 | 46.2 | 53.8 | 31.7 | 35.4 | 34.8 | 65.2 | 4.4 | 8.0 |
| 1954 | 51.0 | 49.0 | 51.0 | 49.0 | 77.0 | 77.0 | 49.4 | 50.6 | 29.0 | 30.9 |
| 1955 | 48.7 | 51.3 | 49.5 | 50.5 | 54.6 | 52.9 | 40.0 | 60.0 | 7.5 | 10.7 |
| Average | 49.0 | 51.0 | 48.8 | 51.2 | 59.8 | 60.2 | 44.6 | 55.4 | 16.5 | 19.7 |
TABLE II
Sex ratio of different sizes of P. indicus (Menon, 1957)
| Size groups | Sex ratio | Percentage in total | ||
| M | F | M | F | |
| Backwater catches | ||||
| Less than 120 mm | 50.0 | 50.0 | - | - |
| Sea catches | ||||
| Up to 120 mm | 49.3 | 50.7 | 40.2 | 39.7 |
| Between 120 and 150 mm | 50.6 | 49.4 | 43.4 | 40.5 |
| Over 150 mm | 44.6 | 55.4 | 16.5 | 19.7 |
| All sizes | 49.0 | 51.0 | - | - |
In the backwaters of Kerala the species is caught in large quantities in the stake nets, cast nets, drag nets, dip nets and small scoop nets. On the Kerala coast there are conical sluice nets specially designed to catch all the prawns entering the paddy fields situated near the backwaters. All these nets are made of cotton twine. Some ingenious contraptions like ‘Changala pachil’ (Panikkar, 1937), bamboo screen traps, etc. are also in use in different parts of the estuaries and backwaters.
In the Indian inshore marine fishery the principal gear employed in the capture of prawns is the boat seine and shore seines. In Gujarat and Maharashtra States on the west coast of India large stake nets are used in the inshore prawn fishery. Along the Kerala coast and on the southern end of the west coast of Indian cast nets of various dimensions form an important gear for capture of prawns.
A description of the intertidal barrage trap, or ‘valakira’, employed in parts of Madagascar is given by Crosnier (1965). The valakira is v-shaped, with the arms 150–300 m long, made of plaited raffia or bamboo supported on wooden posts.
From the deeper regions prawns are caught in trawls and stake nets only. Mechanized Indian vessels use the common 2 or 4 seam shrimp trawls having 13 to 18 m headline. The mesh sizes of the various parts of the shrimp trawl are: the wings 76 mm, the belly 50 mm, the batings 38 mm and the codend 25 mm. These nets are mostly made of cotton twine but in some cases synthetic fibres are also used. The bigger trawlers, however, use nets with longer headline. A modified ‘Spanish trawl’ used in Madagascar is figured by Crosnier (1965).
Small dug-out canoes (4 to 6 m long) are the principal craft in use in the Indian backwaters. Larger dug-outs (6 to 10 m long), canoes and catamarans are used in the inshore prawn fishery in the west coast of India. In the east coast plank built canoes and catamarans are in use. The shrimp trawls are operated from 7 to 11 m pablo type wooden hull boats powered by 10 to 30 hp diesel engines. A few larger steel built boats are also operating shrimp trawls.
P. indicus is fished commercially in India, Pakistan, Ceylon, Singapore, Malaya, Aden, Egypt, Kenya, Tanzania, Mozambique and Madagascar (Malagasy Republic); i.e. in land areas 122, 131, 133, 155, 156, 421(W), 423, 424, 425 and 433 and in sea area ISW (Holthuis and Rosa, 1965).
The estuarine and backwater fishery for the juveniles of the species is carried out in very shallow waters not exceeding 10 m in depth. The depth of water in the paddy fields of Kerala, from where the species is caught in commercial quantities, and of the Singapore prawn ponds, is less than 1.5 m. In India the commercial fishery for adults is generally carried out in the coastal waters up to a depth of 50 m, but in east Africa commercial quantities are rarely found in water of more than 10 m.
In the backwaters of Kerala the species is fished almost throughout the year. The observations of Menon (1955) and Menon and Raman (1961) do not clearly indicate any seasonal preponderance of the species in the backwaters. The monthly percentages of the species in the paddy field catches and in the backwater catches are shown in Table III.
In the Singapore prawn ponds Hall (1962) observed two peaks in relative abundance, in March to April and again in September. According to Panikkar and Menon (1956): “Though a few prawns may be caught throughout the year at various points along the west coast (of India), the marine fishery is largely seasonal. On the west coast the season generally coincides with the monsoon period, June to September, so far as the southern region is concerned”. This is a general observation based on the overall prawn catches and not concerning a particular species. In the offshore catches of Cochin, George et al. (1968) found the maximum abundance of the species during January to April. Early in the season, during the September to October period, the species was conspicuously rare in the offshore catches. In the commercial catches of Bombay, Shaikhmahmud and Tembe (1960) found the species occurring throughout the year except during the months of January, July and August. Mohamed (1967) observed the species contributing substantially to the prawn landings at Sassoon Dock (Bombay) in certain months but there was no regularity in its appearance.
TABLE III
Showing the percentages of P. indicus in the commercial catches from the backwaters of Cochin
(Data for 1952 and 1953 from Menon (1955) and for 1956 to 1958 from Menon and Raman (1961))
| 1952 | 1953 | 1956 | 1957 | 1958 | |
| January | 11.7 | 9.0 | - | 15.5 | - |
| February | 8.4 | 7.0 | - | 11.7 | - |
| March | 12.5 | 29.8 | - | 28.5 | 4.8 |
| April | 2.0 | 48.0 | - | 15.0 | - |
| May | - | - | - | 10.7 | 2.2 |
| June | - | - | - | 25.0 | 8.0 |
| July | - | - | - | 8.8 | 20.4 |
| August | - | - | - | 3.1 | 4.1 |
| September | - | - | - | - | - |
| October | - | - | - | 1.9 | - |
| November | 1.6 | - | 4.3 | 15.6 | - |
| December | 8.7 | - | 2.5 | 7.6 | - |
Shaikmahmud and Tembe (1960) observed that large numbers of P. indicus were obtained usually after heavy rains and comparatively small quantities before heavy rains. Menon and Raman (1961) found correlation between prawn catches and lunar periodicity as well as rainfall.
In most east African countries the main intertidal fishery for P. indicus and other prawns is during the rainy season, the months involved varying from country to country (Hall, 1967). In Madagascar, trawling by research vessels has produced the highest catches in April, May and June, at the beginning of the dry season (Crosnier, 1965).
The only available figures relating catches to fishing effort are for research vessels trawling in Madagascan waters (Crosnier, 1965). Catches per h varied from 0 to 1312 kg.
George (1961) observed the species forming 1 to 5% of the inshore marine catches at Alleppey; 10.3 to 75% at Chellanum and 3.5 to 33.3% at Narakkal, on the west coast of India. In the offshore fishery at Cochin George et al. (1968) record the highest value of 48% for the species in the total catches. In the Singapore prawn ponds Hall (1962) recorded P. indicus as forming 27.99% of the catches. In the marine fisheries of India this species forms approximately 10% of the prawn catches (Mohamed, 1967 a). On the basis of this the total catch of the species per year in India is estimated as 8,000 tons.
P. indicus is the most important species in the prawn catches of many east African countries, but records of landings are not available for any of these countries.
On the southwest coast of India the only regulation now in existence pertains to the paddy field fishery, which is allowed to operate only from the middle of November to the middle of April. According to Panikkar and Menon (1956) this restriction is imposed more in the interest of the paddy cultivation than of the fishery. They state that “the methods of fishing now in vogue do not involve the destruction on any appreciable scale of prawn fry and leave sufficient numbers of breeding females to replenish the stock. The fear of depletion has not therefore arisen anywhere and thus no serious problem in management, requiring regulation of the fishery, has confronted the Government of the various states”.
Fixed engines like stake nets and Chinese dip nets are licensed by the Government authorities in the backwaters of Kerala.
Farming and culturing of this species is not reported from any part of the world. Trapping of the younger stages of this prawn, along with other species, is extensively practised in the paddy fields of Kerala (Panikkar, 1937; Menon, 1955; Gopinath, 1956; Panikkar and Menon, 1956; Kesteven and Job, 1957). Soon after the rice cultivation is over in October the paddy fields lying close to the backwaters and connected canals are prepared for prawn filtration. These preparations include strengthening of the bunds and refixing of the sluices which control the flow of water into the fields. The water is let in during the high tide and let out during the low tide. When the water is let out a bamboo screen is placed inside the sluice to prevent prawns from escaping. During the night a petromax lamp (Ca. 300 candle power) is kept over the mouth of the sluice in order to attract the prawns when the water is let in. Fishing is generally carried out during ebb tide at night when there is maximum tidal gradient due to the spring tide. A conical net (sluice net) is fixed to the mouth of the sluice and the water from the field is let off virtually filtering through the net. The prawns are collected from the bag end of the net. The practice is described in detail by Menon (1955) and Gopinath (1956). Culturing, in the strict sense, is not involved in this practice as the prawns remain in the fields only for a few days.
Barnard, K.H., 1950 Descriptive catalogue of South African decapod crustacea. Ann.S.Afr.Mus., 38: 1–837
Cheung, T.S., 1960 A key to the identification of Hong Kong penaeid prawns with comments on points of systematic interest. Hong Kong Univ.Fish.J., (3): 61–9
Crosnier, A., 1965 Les crevettes penaeides du plateau continental Malgache. Cah. O.R.S.T.O.M. (Océanogr.) 3, Suppl. 3: 158p.
Dall, W., 1957 A revision of the Australian species of Penaeinae (Crustacea Decapoda: Penaeidae). Aust.J.mar.Freshwat.Res., 8(2): 136–231
De Bruin, G.H.P., 1965 Penaeid prawns of Ceylon (Crustacea Decapoda, Penaeidae). Zoöl. Meded., Leiden, 41(4): 73–104
De Man, J.G., 1892 Decapoden des Indischen Archipels. In Zoologische Ergebnisse einer Reise in Niederländisch Ostindien, by Max Weber. Leyden, Netherlands, vol.2, pp.265–527
George, M.J., 1961 Studies on the prawn fishery of Cochin and Alleppey coast. Indian J.Fish., 8(1): 75–95
George, M.J., 1962 On the breeding of penaeids and the recruitment of their postlarvae into the backwaters of Cochin. Indian J.Fish., 9(1): 110–6
George, M.J., and K.H. Mohamed, 1966 An assessment of marine prawn fishery resources of Kanyakumari District - southwest coast of India. Proc.Indo-Pacif.Fish.Coun., 12(2): 210–5
George, M.J., and P.V. Rao, 1967 Distribution of sex ratios of penaeid prawns in the trawl fishery of Cochin. Symp.ser.mar.biol.Ass.India, 2(2): 698–700
George, M.J., K. Raman and P.K. Nair, 1968 Observations on the offshore prawn fishery of Cochin. Indian J.Fish., (1963), 10A(2): 460–99
Gopalakrishnan, V., 1952 Food and feeding habits of Penaeus indicus, J.Madras Univ. (B) 22(1): 69–75
Gopinath, K., 1956 Prawn culture in the rice fields of Travancore-Cochin, India. Proc.Indo-Pacif.Fish. Coun., 6(3): 419–24
Hall, D.N.F., 1956 The Malayan Penaeidae (Crustacea Decapoda). Part I. Introductory notes on the species of the genera Solenocera, Penaeus and Metapenaeus. Bull.Raffles Mus., 27: 68–90
Hall, D.N.F., 1962 Observations on the taxonomy and biology of some Indo-west Pacific Penaeidae (Crustacea, Decapoda). Fish.Publs colon.off., 17: 229p.
Hall, D.N.F., 1967 Penaeidae of the east coast of Africa. Publs scient.tech.res.Commn Orgn Afr.Unity, (96): 89–101
Holthuis, L.B., and H. Rosa, 1965 List of species of shrimps and prawns of economic value. FAO Fish.tech.Pap., (52): 21p.
Kemp, S., 1915 Fauna of the Chilka Lake. Crustacea decapoda. Mem.Indian Mus., 5: 201–325
Kesteven, G.L., and T.J. Job, 1957 Shrimp culture in Asia and the Far East: a preliminary review. Proc.Gulf Caribb.Fish.Inst., 10: 49–68
Kubo, I., 1949 Studies on the penaeids of Japanese and its adjacent waters. J.Tokyo Coll.Fish., 36(1): 1–467
Menon, M.K., 1937 Decapod larvae from the Madras plankton. Bull.Madras Govt Mus.(nat.Hist.), 3(5):1–55
Menon, M.K., 1955 On the paddy field prawn fishery of Travancore-Cochin and an experiment in prawn culture. Proc.Indo-Pacif.Fish.Coun., 5(2):131–5
Menon, M.K., 1957 Contributions to the biology of penaeid prawns of the southwest coast of India. I. Sex ratio and movements. Indian J.Fish., 4(1):62–74
Menon, M.K., 1965 Life history of prawns - A review of recent studies with special reference to Indian species. Fishery Technol., Ernakulam, 2(1):12–8
Menon, M.K., and K. Raman, 1961 Observations on the prawn fishery of the Cochin backwaters with special reference to the stake net catches. Indian J.Fish., 8(1):1–23
Milne Edwards, H., 1837 Histoire naturelle des Crustacés, comprenant l'anatomie, la physiologie et la classification de ces animaux. Paris, vol.2, 532 p.
Mohamed, K.H., 1967 Penaeid prawns in the commercial shrimp fisheries of Bombay with notes on species and size fluctuations. Symp.ser.mar.biol.Ass.India, 2(4):1408–18
Mohamed, K.H., 1967a Prawn Fisheries. In Central Marine Fisheries Research Institute, Government of India, 20th Anniversary Souvenir. Mandapam Camp, pp.75–81
Panikkar, N.K., 1937 The prawn industry of the Malabar coast. J.Bombay nat.Hist.Soc., 39(2): 343–53
Panikkar, N.K., 1952 Possibilities of further expansion of fish and prawn cultural practices in India. Curr.Sci., 21:29–33
Panikkar, N.K., and R.G. Aiyar, 1939 Observations on breeding in brackish-water animals of Madras. Proc.Indian Acad.Sci.(B), 25(9):343–64
Panikkar, N.K., and M.K. Menon, 1956 Prawn fisheries of India. Proc.Indo-Pacif.Fish.Coun., 6(3): 328–44
Racek, A.A., 1955 Littoral Penaeinae from New South Wales and adjacent Queensland waters. Aust.J.mar.Freshwat.Res., 6(2):209-41
Racek, A.A., and W. Dall, 1965 Littoral Penaeinae (Crustacea Decapoda) from Northern Australia, New Guinea and adjacent waters. Verh.K.ned.Akad.Wet.(B), 56(3):1–119
Rao, P.V., 1968 Maturation and spawning of the penaeid prawns of the southwest coast of India. FAO Fish.Rep., (57) vol.2: 285–302
Shaikhmahmud, F.S., and V.B. Tembe, 1960 Study of Bombay prawns: the seasonal fluctuation and variation in abundance of the commercially important species of Bombay prawn with a brief note on their size, state of maturity and sex ratio. Indian J.Fish., 7(1): 69–81
Subrahmanyam, C.B., 1963 A note on the annyal reproductive cycle of the prawn, Penaeus indicus (M.Edw.) of Madras coast. Curr.Sci., 32(4): 165–6
Subrahmanyam, C.B., 1965 On the unusual occurrence of penaeid eggs in the inshore waters of Madras. J.mar.biol.Ass.India, 7(1): 83–8