| FAO Fisheries Synopsis No. 95 | FRm/S95 SAST - Prawn |
prepared by
T. H. BUTLER
Fisheries Research Board of Canada
Biological Station
Nanaimo, British Columbia, Canada
Phylum Arthropoda
Class Crustacea
Subclass Malacostraca
Order Decapoda
Suborder Natantia
Section Caridea
Superfamily Pandaloida
Family Pandalidae
Genus Pandalus Leach 1814
Species Pandalus platyceros
Brandt, 1851
Generic
Pandalus Leach, 1814, Brewster's Edinburgh Encyclopaedia, 7:432. Gender: masculine. Type species, by monotypy: Pandalus montagui Leach 1814.
The generic concept as used by Holthuis (1955) has been adopted. The following definition of the genus Pandalus is based on his ‘Key to the Pandalidae’.
Carpus of 2nd pereiopods consisting of more than 3 joints. No longitudinal carinae on the carapace except the postrostral crest. Rostrum not movable. Eyes well developed, cornea much wider than eyestalk. Third maxilliped without exopod. Laminar expansion of the inner border of the ischium of the 1st pair of pereiopods wanting or inconspicuous. The first 4 pereiopods with epipods. Arthrobranchs present at the bases of the first 4 pereiopods. Posterior lobe of scaphognathite acutely produced. Upper margin of rostrum with movable spines only.
Nine species of the genus Pandalus occur along the west coast of North America. Five species, P. platyceros, P. danae Stimpson, P. stenolepsis Rathbun, P. gurneyi Stimpson, and P. hypsinotus Brandt, appear to be closely related.
The following key is based on Rathbun (1904) and Schmitt (1921):
Dorsal spines on rostrum and carapace not reaching behind middle of carapace, P. platyceros
Dorsal spines extending behind middle of carapace
Dorsal spines more than 15, P. hypsinotus
Dorsal spines less than 15
Rostrum at least 1½ times as long as carapace, P. gurneyi
Rostrum less than 1½ times as long as carapace
Antennal scale very narrow, distal half of blade narrower than adjacent spine, P. stenolepis
Antennal scale of moderate width, distal half of blade wider than adjacent spine, P. danae
Specific
The holotype of P. platyceros, if still extant, may be in the Zoological Museum at Leningrad. Type locality: “bei der Insel Unalaschka” (Unalaska Island, Aleutian Islands, Alaska) (Brandt, 1851).
The species is illustrated in Fig. 1.
The following description of P. platyceros is taken from Schmitt (1921).
Body stout. Carapace covered with dense, short pubescence. Rostrum 1½ to 1 ⅔ times length of carapace, provided with broad, entire, laminar crest on each side; dorsal spines 14 to 17, extending to middle of rostrum, anterior 1 to 5 spines fixed and rest movable; usually a single spine near acute tip; ventral fixed spines 7 or 8; anterior ½ to ⅔ ascending, tip above level of carapace. Antennal scale 4/5 to ⅞ length of carapace, oblong, distal part of blade subtruncate, slightly exceeded by spine. Right second pereiopod reaches distal end of 3rd maxillipeds, carpus with 8 or 9 segments; left pereiopod 2/5 length of right, carpus with 27 or 28 segments. Abdomen more than 2 times length of carapace, smooth, not carinated. Colour of adults light tan to reddish brown, juveniles green or dark brown; horizontal white striping on carapace, 2 white spots on 1st and 5th abdominal segments.
Pandalus platyceros Brandt, 1851, in; Middendorff, Reise N.O. Sibiriens, vol. 2 pt. 1, p.123.
Objective synonymy
None
Subjective synonymy
Pandalus pubescentulus Dana, 1852, Crust. U.S.Explor.Exped (1838–42), 13(1).
In Alaska and Washington the species is known as the spot shrimp, or spot. The common name in British Columbia and California is the prawn.
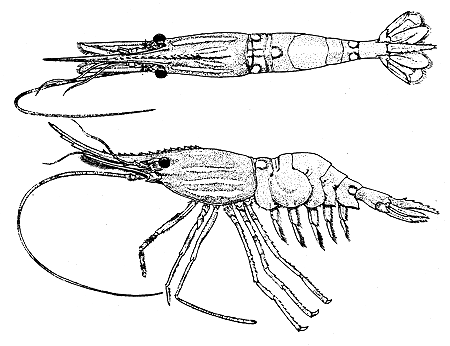
Fig. 1 Pandalus platyceros Brandt. Dorsal and side views of female, carapace length 46.4 mm.
Subspecies or varieties have not been established for P. platyceros.
The species is distinct from its several congeners and is recognized by all specialists as a valid species.
The prawn occurs in the North Pacific Ocean, along the west coast of North America from Unalaska to San Diego; and in Asian waters: Hokkaido, Toyama Bay, Nagasaki, Korea, Vladivostok. In terms of the FAO areas code (see Holthuis and Rosa, 1965) it occurs in parts of sea areas INE and INW, and on the coasts of land areas 211, 212, 220, 231, 232, 444, 451 and 710. The species lives in bays and inlets, on the continental shelf, and continental slope. It has a wide bathymetric range from 4 to 487 m.
In general, the area of distribution is characterized by (1) relatively large continental shelf, (2) low to high precipitation and runoff, (3) relatively low to high surface salinity, (4) surface temperatures 2°–20°C in the eastern Pacific with a seasonal range of 3°–7°C; 10°–25°C in the Sea of Japan, (5) surface currents changing with seasonal winds and (6) basic organic production: medium to high.
Bottom temperature and salinity values for the species were recorded by Butler (1964) as:
| T °C | S ‰ | |
| Maximum | 10.97 | 30.83 |
| Minimum | 7.61 | 26.42 |
Prawns breed in relatively deep water (73 m, and deeper) in the autumn. Eggs are carried over the winter months by the females which appear to remain in the breeding area through hatching of larvae.
Berkeley (1930) found the first and second larval stages in deep water near the habitat of adult prawns. The earlier larval stages may inhabit the lower half of the water column. Larvae, determined as the 4th and 6th stages, were collected by the same author from water of 4 to 6 m in depth.
Metamorphosis in early summer occurs mainly in shallow water, particularly in bays and shallow inlets, from the sublittoral to about 55 m. The postlarval prawns remain in this zone for most of the first year of life, but leave the sublittoral before the winter.
By the time of first maturity, in the 2nd yr, prawns have migrated to relatively deep water.
Evidence of annual variations in differential distribution is not available.
The species is hermaphroditic (Berkeley, 1930). Each individual matures and functions first as a male, then passes through a transition or intersexual phase to become a female. Primary or early maturing females which occur in other pandalids (Allen, 1959), have not been reported. Apart from females bearing external eggs, the sex of prawns may be determined by examining the endopodites of the first 2 pleopods. In the male the endopodite of the 1st pleopod or organ of copulation is tapered, with a slight “shoulder” or protrusion, and has at its tip a series of small hooks or cincinnuli; lower on this structure is a group of well-developed, stout spines. On the inner margin of the endopodite of the 2nd pleopod, in addition to the appendix interna, is the appendix masculina which is distinctly longer than the former and is armed with about 15 thornlike setae. In the female, the endopodite of the 1st pleopod is elongate and niblike, following atrophy of the tip with cincinnuli and development of the protrusion mentioned above as the new tip; the spines and the setae proximal to them disappear. On the 2nd pleopod the appendix masculina is absent, having atrophied through intersexual moults.
In southern British Columbia the species matures first as a male at about 1½ yr, having a mean carapace length of about 28 mm. Most individuals appear to remain as males for another year; the rest change sex and function as females at 2½ yr when the mean carapace length is about 33 mm. Those females which mature at 3½ yr have attained a carapace length of about 38 mm (Butler, 1964).
Near Vancouver Island the relationship between fecundity and carapace length has been calculated. Egg counts from 21 females, 33.5 to 41.4 mm, ranged from 1,393 to 3,162; the equation was: Log F = 3.3967 log L - 2.0564 (F = number of eggs, L = carapace length in mm). Near Petersburg, Alaska, Hynes (1930) found an average count of 3,900 eggs, based on 5 individuals.
The prawn breeds in the autumn. In southern British Columbia all females examined around the end of October were ovigerous (Butler, 1964).
There is no evidence that the prawn breeds more than once a year. Presumably relatively few Vancouver Island female prawns breed more than once in their lives.
The evidence is that prawns breed in their normal adult habitat at depths greater than 73 m, where the bottom is rocky.
The egg structure has not been studied. In southern British Columbia on 25 February 1964, 138 “eyed” eggs from 23 ovigerous females, 33.5 to 41.4 mm carapace length had a mean length and diameter of 2.0 mm and 1.5 mm, respectively. There is no information available on parasites of developing eggs.
According to Butler (1964) the embryonic period lasts from 5 to 5½ mo with hatching in late March or early April. The eggs are carried on the first 4 pairs of pleopods, as in other caridean shrimps. The colour of the egg changes from a dark orange when freshly extruded to brown at time of hatching.
Berkeley (1930), including the prawn with other pandalids studied in the laboratory, found that most larvae hatch at night. She reported that the female moves the pleopods vigorously for about a minute to release from 5 to 25 larvae, while clinging to some object or while swimming freely. After release of each batch, the female rests for 10 min or longer and then the procedure is continued until all larvae are free. Hatching is frequently completed in one night but sometimes takes two or more days.
The 1st larval stage of the prawn was reared by Berkeley (1930). She collected 1st and 2nd stages and others, determined as 4th and 6th, from the plankton. The 4 known stages are illustrated in Fig. 2 and 3, and the characters are summarized in Table I.
The same author described a later stage, 2 cm long, undoubtedly postlarval, which has mainly adult characters except for the presence of the ocellus, rudimentary development of the antennules, and the podobranchia on the 2nd maxilliped, small arthrobranchiae, and the lack of secondary sex characters.
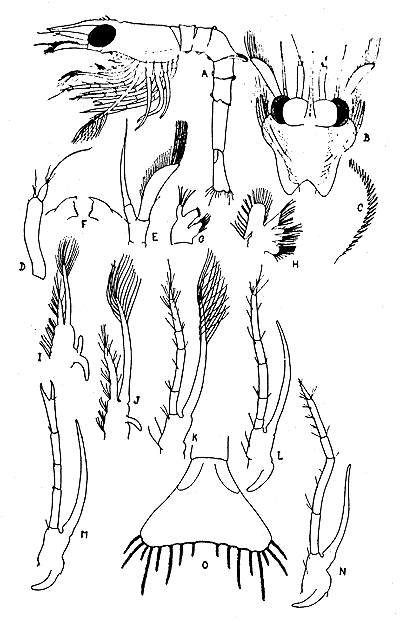
Fig. 2 Pandalus platyceros, first stage larva. (A) Entire larva, lateral view. (B) Dorsal view of cephalothorax and appendages. (C) Lateral expansion of carapace much enlarged. (D) Antennule. (E) Antenna. (F) Mandibles. (G) (H) First and 2nd maxillae respectively. (I) (J) (K) First, 2nd and 3rd maxillipeds respectively. (L) (M) (N) First, 2nd and 3rd pereiopods respectively. (O) Telson. (After Berkeley, 1930)
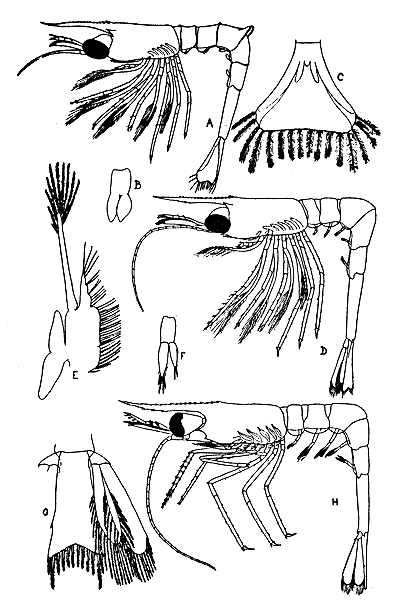
Fig. 3 Pandalus platyceros Second, 4th and 6th (?) stage larvae. (A) Second stage larva, lateral view. (B) Third pleopod of same. (C) Telson of same. (D) Fourth stage larva, lateral view. (E) Second maxilla of same. (F) Third pleopod of same. (G) Telson and uropod of same. (H) Sixth (?) stage larva, lateral view. (After Berkeley, 1930)
TABLE I
Larval development of the prawn (based on Berkeley, 1930)
| Stage | Characters |
| 1 | 8 mm long, brightly coloured. Lateral margins of carapace and posterior margins of abdominal segments expanded and denticulated. Rostrum large, with 9 dorsal spines. Inner flagellum of antennule with 3 simple setae. Scale of antenna with 6 plumose setae and 19–20 simple setae on inner margin; 2 simple setae on outer margin. Eyes immobile. First maxilliped with bilobed epipodite; 2nd with simple, tablike epipodite. Second pereiopod with functional chela; maxillipeds and pereiopods 1 – 3 bear exopodites; buds of pleurobranchiae at bases of pereiopods. Pleopods present as simple or bilobed buds. Telson with 16 setae. |
| 2 | 11 mm long. Lateral margins of carapace still expanded and serrated. Supraorbital spine present, rostrum with 15–16 dorsal spines, and 3–4 ventral spines. Eyes stalked. Flagellum of antenna with 2 segments. Epimeral plates developing on abdominal somites. Denticles on abdomen not as well developed as in first stage. Pleopods biramous. Telson longer and with stouter setae than in first. |
| 4 | 15 mm long. Lateral expansions and serrations of carapace nearly disappeared. Supraorbital spine present, rostrum bears about 17 strong dorsal spines and 4 ventral spines. Antenna near adult, with segmented flagellum. Mastigobranchiae on 3rd maxilliped and pereiopods. Pleopods jointed, setose, and appendices internae present. Uropods separated, with rami well developed. Telson wider at tip, 6 pairs terminal and 2 pairs lateral setae. |
| 6 | 16 mm long. Supraorbital spine absent, rostrum with 16 dorsal spines, 5 ventral spines. Mandibles, maxillae, maxillipeds approaching adult form. Pereiopods have vestiges of exopodites, otherwise all adult except 2nd. Arthrobranchiae present as buds on first 4 pereiopods, all pleurobranchiae present. Pleopods well developed. |
The smallest postlarval or juvenile prawn collected by Butler (1964) late in August 1961, had a carapace length of 9.5 mm (total length about 48 mm); mean carapace length of others in the same group, estimated at 1½ to 2 mo after metamorphosis, was 14.4 mm; 2 mo later this group showed a mean increment of almost 4 mm. It is not known at which length the external sex characters form.
Age estimates of older prawns, as in other crustaceans, leave much to be desired. The work of Butler (1964), however, indicates that relatively few prawns live longer than 4 yr.
Prawns appear to be very hardy. Fishermen keep them alive for as long as 10 days in floating pens or sunken boxes under conditions of overcrowding and variations of temperature and salinity are likely.
The prawn is considered a benthic feeder, so it is likely that some associated animals are competitors for food; for example, other decapod crustaceans, Lopholithodes foraminatus (Stimpson), Acantholithodes hispidus (Stimpson), and Chorilia longipes Dana.
Presumably a number of demersal, and even pelagic, fishes are predators of the prawn, but no published record is known. Investigators have found unidentified “shrimps” in the stomachs of several gadids (Hart, 1949) and dogfish (Chatwin and Forrester, 1953). The lingood, Ophiodon elongatus Girard feeds on P. danae, a closely related commercial shrimp (Wilby, 1937).
There is no record of epicaridean isopods parasitic on the prawn.
A male prawn, 32.5 mm carapace length, infested with a rhizocephalan, Sylon sp, was found near the Queen Charlotte Islands on 20 June 1966.
The species is said to reach 10 in (254 mm) in total length in Alaskan waters (Barr, 1964). The largest specimen measured from British Columbia was an ovigerous female, 61.1 mm carapace and 253 mm total length.
Prawns have been observed feeding in aquaria day and night. Baited traps set during daylight hours and overnight will catch prawns, but no definite difference in catching rates has been demonstrated.
In August 1927, Miss Berkeley examined prawn stomachs collected at 2 localities near Vancouver. Her unpublished data are tabulated as:
| Burrard Inlet 110 m | Howe Sound 196 m | |
| Total number of stomachs | 8 | 16 |
| Total with Crustacea | 6 | 13 |
Unidentified | 6 | 12 |
Amphipod | 0 | 1 |
| Total with Polychaeta | 5 | 4 |
Sabellaridae, Pallasia sp | 5 | 3 |
Polynoidae, possibly Lagisca sp | 0 | 1 |
Nephthydidae, Nephthys sp | 3 | 0 |
Maldanidae | 0 | 4 |
| Others | ||
Fish scale | 0 | 1 |
Siliceous sponge spicules | 0 | 6 |
Butler (1964) found that the “conversion factor”, i.e. the relative length of the carapace to total length (tip of rostrum to tip of telson), varied indirectly with size increase and concomitant sex change as follows:
| Sex | Factor |
| M | 5.05 |
| Transition | 5.04 |
| F | 4.76 |
Recent work (Butler, MS) shows that the decrease in value of the conversion factor may be attributed to the rostral length. After sex change takes place, the growth of the rostrum, relative to the carapace, is less than in the male phase and the carapace accounts for a relatively greater part of the total length.
Butler (1964) calculated the length-weight relationship of the prawn as:
Log W = 2.93148 log L - 3.07787,
where W = total weight in g, and L = carapace length in mm.
Berkeley (1930) was the first to study growth of the species. By analysis of (total) length frequencies of specimens sampled periodically near Vancouver, she determined the growth pattern which is summarized as:
| Age in months | Sex | Mean total length mm |
| 4 | M (immature) | 45 |
| 12 | M " | 100 |
| 18 | M | 150 |
| 24 | M | 160 |
| 30 | M | 180 |
| 36 | Trans. | 195 |
| 38 | F | 200 |
| 42 | F | 220 |
On the basis of carapace lengths obtained near Vancouver Island, Butler (1964) plotted the growth curve shown in Fig. 4. In Table II, mean carapace and total lengths, and mean total weights at age intervals through 4 years of life are summarized.
The migration of young prawns from shallow coastal waters during the first year of life is well established (Berkeley, 1930; Butler, 1964). Small prawns have been caught in midwater trawls but there is no clear evidence of diel migration.
Mating of the prawn has not been observed. It is likely, however, that mating behaviour is similar to that described by Needler (1931) for Pandalus danae. The female generally moults at night and apparently mating and oviposition occur within 36 h after this moult. The male locates the nubile female through a kinesis reminiscent to that when the animal is near food. The first action of the male is an attempt to run up the back of the female, but he may be shaken off by the larger female. When the male succeeds in holding the female he assumes a position with the anterior part of his abdomen under the posterior part of her cephalothorax; sometimes the female rolls over and the two lie side by side. Copulation generally takes a minute or less, leaving the female with a loose mass of spermatozoa between the bases of the last 2 pairs of pereiopods. Oviposition is carried out while the female is on the bottom, resting on the dactyli of the 3rd periopods and on the telson. The 4th and 5th pairs of pereiopods are bent under the body and are kept active in an “elbowing” motion. The pleopods move gently and continuously, and the eggs pass from the oviducts in a steady stream between the pereiopods to the abdomen, becoming attached to the anterior pleopods first. Oviposition lasts about half an hour, but afterwards, the female may remain in position for the same period before moving about normally.
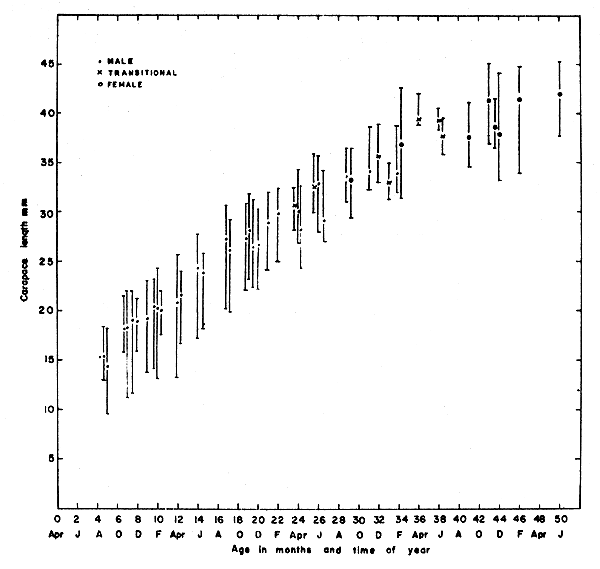
Fig. 4 P. platyceros. Means and ranges of carapace lengths according to age.
TABLE II
Pandalus platyceros. Mean carapace and total lengths, and body weights, at intervals from 5 to 50 months of age
| Month | Age (in months) | Sex | Mean carapace length (mm) | Mean total length (mm) | Mean total weight (g) |
| August | 5 | M | 14.4 | 73 | 2.1 |
| November | 7 | M | 18.3 | 92 | 4.2 |
| April | 12 | M | 21.2 | 107 | 6.4 |
| August | 17 | M | 26.7 | 135 | 12.7 |
| November | 19 | M | 27.7 | 140 | 14.2 |
| April | 24 | M + ( | 28.5 | 144 | 15.4 |
| November | 31 | M | 32.9 | 166 | 23.4 |
| November | 31 | F | 33.2 | 158 | 24.1 |
| April | 36 | Trans | 33.7 | 170 | 25.1 |
| November | 42 | F | 37.9 | 180 | 35.5 |
| June | 50 | F | 42.1 | 200 | 48.3 |
In a hermaphroditic animal such as the prawn, sex ratio appears to have little meaning. Due to natural and fishing mortality one would expect to find fewer of the older females in a population.
No sampling data of commercial catches are available. Prawns caught in traps similar to commercial gear near Vancouver Island were composed of 4 and, at times including the O-group, 5 age groups (Butler, 1964). The dominant age groups were 1+, 2+, and 3+ yr olds.
As stated above, information on commercial catches is lacking. Using experimental trap gear Butler (1964) found throughout the year that the carapace lengths of males ranged from 13.0 to 38.8 mm, transitionals from 28.2 to 40.3 mm, and females from 30.8 to 45.5 mm.
Dahlstrom (1963), fishing experimental traps near Monterey, California, in late January, calculated the mean carapace lengths of males, transitionals, and females as 37.0, 41.8, and 47.9 mm, respectively. Counts of whole shrimps ranged from 15.8 to 29.5 per kg. A year later Odemar (1964), fishing in the same region, noted practically the same mean carapace lengths and a similar range of counts.
Recruitment to the trap fishery in southern British Columbia takes place at about 1½ yr. In the same region younger prawns caught in trawls are utilized.
It is assumed that British Columbia prawn stocks are exploited at a low level, and there is no evidence that it is otherwise in other parts of the range.
The gear used specifically for catching prawns is a small, single-chambered trap, also known as a “pot”. Trap design varies from area to area because practically all fishermen build their own traps. The common basic type is oblong with a conical entrance at each end (Fig. 5). Invariably the entrances are made of netting, but traps may be constructed of different materials and dimensions vary. A welded frame of iron rod is usually covered with netting or wire mesh (Fig. 6); and a wooden frame has sides covered with spaced wooden laths or pieces of plywood. Oblong traps vary in size from 61 cm × 30 cm × 30 cm to 91 cm × 45 cm × 45 cm.
The prawn trap fished near Monterey, California, has been described by Phillips (1931). This trap is between 1.2 and 1.5 m long, is 91 cm in diameter at one end, tapering in a curve to about 23 cm in diameter at the other end. There is one conical entrance, at the large end, which extends for about 60 cm into the trap to an opening of 152 mm in diameter. The trap is woven of rattan cane, with circular reinforcing wire.
Recently experimental prawn traps have been fished in California (Dahlstrom, 1963; Odemar, 1964), in British Columbia (Butler, 1963) and in Alaska (U.S. Bur.Comm.Fish., 1966, 1967). The main finding is that traps having sides covered with solid materials (e.g. sheet metal or plastic) catch more prawns than traps covered with net or screening.
Prawn traps are fished on the bottom by means of a groundline. Each trap has a short leader line, about 2 m in length, which has a snap hook or clip at the end to attach the trap to the groundline. Up to 40 traps may be put on a groundline, spaced 9 or 18 m apart. A light anchor, or a small weight of concrete or scrap iron, is placed at each end of the groundline where the lighter buoy lines are attached. In the past, treated manilla lines were used but now polypropylene lines are used throughout the fishery. Inflatable plastic buoys mark the position of the gear, but, where there is much boat traffic, fishermen may dispense with surface markers; they are able to grapple without difficulty the polypropylene groundline, which in places is off the bottom. When wooden traps are new or have been ashore for a time they must be weighted separately so as to go to the bottom satisfactorily.
Traps are lifted once or twice daily, and the bait is renewed each time. Dogfish, shark, herring or scrap fish are satisfactory bait for prawns.
P. platyceros is caught incidentally in trawling operations for other pandalids. Large prawns may be sorted from the catch and sold separately but the bulk is processed with other smaller species.
There is no vessel type designed specifically and used exclusively for prawn trap fishing. The type operating in the fishery is a small combination vessel, generally the salmon gill-netter (Fig. 7) and to a lesser extent the troller. These vessels are from 9 to 12 m in length.
The power drum on a gill-netter will haul prawn traps without any special modification. One man normally operates a prawn boat, and stands in the stern cockpit to handle traps when setting and hauling. The line may lead from the drum over a block attached to a gantry, or over a roller on the taffrail. A foot control for the drum leaves the fisherman's hands free. Bait is renewed after the trap is unfastened, and then the catch is dumped into a container. After emptying, the baited traps are closed and stacked midships, ready for resetting.
Vessels without power drums lead the line through a block on a davit mounted midships and haul with a gypsy or warping head, coiling the line by hand. Two men are required for this operation. By either method a string of 40 traps may be hauled in 1 to 1½ h. Prawn boats will fish a total of from 50 to 200 traps. Normally these vessels fish daily but some are fitted with refrigeration and make trips lasting from 10 to 14 days.
Table III summarizes known prawn fishing areas. Evidently there is little, if any, prawn fishing in Alaskan regions other than the southeastern area. Ronholt (1963) states that P. platyceros was not caught west of Port Dick (Kenai Peninsula) by trawling in the course of exploratory cruises.
Latitudes (approximate) 58°N 136°W to 47°N 122°W plus 36°N 122°W.
The only prawn trap fishing ground not located in a coastal inlet or sound is the region near Monterey, California.
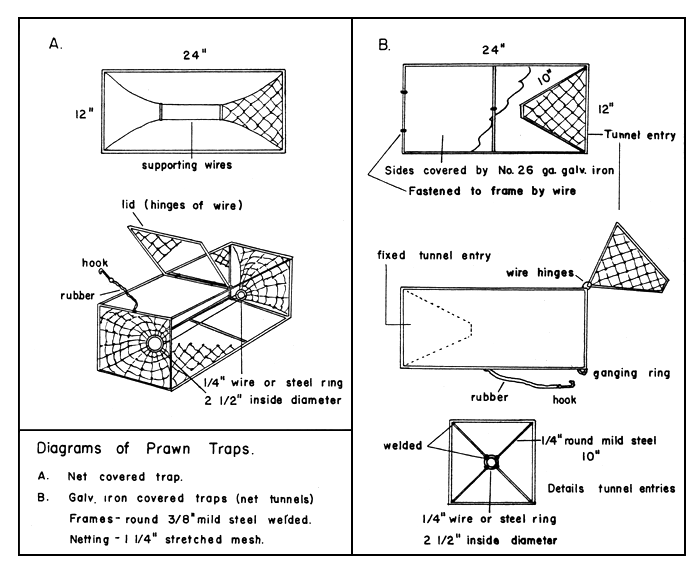
Fig. 5 Diagrams of prawn traps. (A) Net covered trap. (B) Galvanized iron covered traps (net tunnels). Frames - round 3/8" mild steel welded. Netting - 1¼" stretched mesh.

Fig. 6 Frame trap, covered with cotton netting.

Fig. 7 Typical British Columbia gill-netter.
TABLE III
General geographic distribution
| Alaska | British Columbia | Washington | California | |
| Southeastern (Dixon Entrance to Cape Spencer) | 1. | Mainland inlets, (Smith Sound - Howe Sound) | Puget Sound (Hood Canal) | Monterey Peninsula |
| 2. | Vancouver Island (East and west coast) | |||
Alaska: 91 to 110 m (Harry, 1964). British Columbia: 73 to 165 m (Butler, 1964). Washington: unavailable, but probably within ranges above. California: around 274 m (Phillips, 1931).
Trap fishing for the species takes place almost entirely during the autumn and winter months. The reason for this pattern is that fishermen pursue prawn fishing as an operation secondary to salmon gill-netting, trolling, or other major fisheries.
In Butler's (1964) trap sampling data for the prawn (his Fig. 16), there is an indication of an apparent decline in abundance, at least of females, during summer months. Some British Columbia fishermen have reported this disappearance which follows the hatching of larvae in spring.
According to the Canada Department of Fisheries the numbers of prawn traps owned and operated by British Columbia in recent years were:
| Year | Number of traps |
| 1960 | 1,240 |
| 1961 | 1,341 |
| 1962 | 2,582 |
| 1963 | 2,590 |
| 1964 | 2,620 |
| 1965 | 2,710 |
British Columbia fishing effort for the species, from 1962 to 1966, is shown in Table IV as “trap days”. These Canada Department of Fisheries statistics are collected by a sales slip system, and a “trap day” is a day's operation by a prawn boat (without account of the number of traps hauled). The catch per day has varied from 42 to 57 kg.
The selectivity of commercial prawn traps is undetermined. Trap experiments by the U.S. Bureau of Commercial Fisheries in southeastern Alaska have shown that the size of prawns caught apparently depends on the size of trap entrances. In traps with circular entrances of 51 mm, inside diameter, average counts of whole prawns per kg ranged form 32.8 to 38.9; while traps with larger entrances, of various shapes and dimensions, had prawns with averaging counts from 26.6 to 28.8 (U.S. Bur.Comm. Fish., 1966). In a later report (U.S. Bur. Comm.Fish., 1967), average counts of prawns in traps with entrances of 51 and 76 mm (inside diameter) are compared; one kind of trap with the smaller entrance size caught prawns averaging 37.2 per kg and two trap types with the larger entrances had prawns with separate average counts of 24 and 29.5 per kg.
Recent British Columbia prawn catches, by trap and trawl gear, are shown in Table IV. Also included in this table, by way of comparison, are trawl catches of other pandalid species.
Total trap catches from other regions, Alaska (Chitwood, 1964, 1965) and California (Greenhood and Mackett, 1965, 1966), are given in Table V. Statistics of the Puget Sound fishery are shown in Table VI (R.E. Westley, personal communication).
Although no fishery for the species is being managed by measures based on the results of scientific research, there are regulations in existence which curtail fishing seasons and restrict the design of traps.
Pertinent regulations are as follows:
Alaska (Alaska Dep. Fish Game, 1966). Shrimp fishing (by definition of legal gear, includes traps and trawls) is prohibited in the Petersburg-Wrangell area from 15 February to 1 May.
Washington (Pacif.mar.Fish Commn., 1965). Shrimp fishing, trapping and trawling, is prohibited in Puget Sound from 1 November to 31 March; fishing is restricted further in the Lopez Island area to 30 June, and in Skagit Bay from 16 June to 31 August.
California (Gates, 1965). North of Point Conception; traps not to exceed 183 cm in greatest dimension and no opening from exterior to interior may be greater than 127 mm in any dimension.
From Point Conception south to the southern boundary of Ventura County; in waters not less than 91 m in depth. No openings on traps, through which prawns enter, shall exceed 76 mm in greatest dimension.
TABLE IV
British Columbia prawn catches, and trap fishing effort
| Year | Catch (kg) | Fishing effort (trap days) | |
| Trawl | Trap | ||
| 1962 | 4,631 | 34,459 | 609 |
| 1963 | 8,036 | 30,917 | 581 |
| 1964 | 5,584 | 34,731 | 628 |
| 1965 | 4,631 | 18,659 | 405 |
| 1966 | 3,223 | 36,229 | 863 |
TABLE V
Prawn trap catches in kg, Alaska and California
| Year | California | Alaska |
| 1962 | 315 | - |
| 1963 | 3,834 | 386 |
| 1964 | 2,622 | 2,461 |
| 1965 | 316 | - |
TABLE VI
Prawn catches in kg, trap and trawl, Puget Sound, Washington
| Year | Trap | Trawl | |
| Hood Canal | Other areas | Areas, excluding Hood Canal | |
| 1962 | 7,911 | 499 | 913 |
| 1963 | 12,347 | 2,719 | 1,135 |
| 1964 | 25,652 | 1,516 | 1,366 |
| 1965 | 24,305 | 507 | 906 |
| 1966 | 18,740 | 229 | 88 |
Alaska Department of Fish and Game, 1966 Regulations of the Alaska Board of Fish and Game for commercial fishing in Alaska, Juneau, 123 p.
Allen, J.A., 1959 On the biology of Pandalus borealis Krøyer, with reference to a population off the Northumberland coast. J.mar.biol.Ass.U.K., 38(1):189–220
Barr, L., 1964 Characteristics of the commercial shrimp landings at Wrangell, Alaska. Rep.U.S. Commnr Fish., MS Report (MR64-4): 11 p.
Berkley, A.A., 1930 The post-embryonic development of the common pandalids of British Columbia. J.Fish.Res.Bd Can., 21(6):1403–52. Issued also as: Contr.Can.Biol.Fish., 6(6):79–163
Brandt, J.Y., 1851 Krebse. In Dr. A. Th. von Middendorff's Reise in den äussersten Norden und Osten Sibiriens. 2. Zoologie, I.
Butler, T.H., 1963 An improved prawn trap. Circ.biol.Stn, Nanaimo, (67):7 p.
Butler, T.H., 1964 Growth, reproduction, and distribution of pandalid shrimps in British Columbia. J.Fish.Res.Bd Can., 21(6):1403–52
Butler, T.H., MS Relative growth of the rostrum in several pandalid species.
Chatwin, B.M. and R.C. Forrester, 1953 Feeding habits of dogfish (Squalus suckleyi (Girard)). Prog. Rep.Pacif.Cst Stns, (95):35–8
Chitwood, P.E., 1964 1963 Alaska commercial fisheries catch and production statistics. Statist. Leafl.Alaska Dep.Fish Game, (7):26 p.
Chitwood, P.E., 1965 1964 Alaska commercial fisheries catch and production statistics. Statist.Leafl. Alaska Dep.Fish Game, (9):28 p.
Dahlstrom, W., 1963 Cruise report 63-A-1, prawn-shrimp. California Department of Fish and Game Marine Resources Operations, 3p. (mimeo)
Dana, J.D., 1852 Crustacea. In United States exploring expedition during the years 1838, 1839, 1840, 1841, 1842, under the command of Charles Wilkes, U.S.N. Philadelphia, Pa., vol.13
Gates, D.E., 1965 Digest of 1965–67 commercial fish laws. California Department of Fish and Game Resources Agency, 31 p.
Greenhood, E.C. and D.J. Mackett, 1965 The California marine fish catch for 1964. Calif.Fish Game, (132):45 p.
Greenhood, E.C., 1966 Statistical report of fresh, canned, cured and manufactured fishery products for 1965. Circ.Dep.Fish Game Calif., (40):15 p.
Harry, G.Y., 1964 The shrimp fishery of Alaska. Proc.Gulf Caribb.Fish.Inst., 16:64–71
Hart, J.L., 1949 Food of fish of the cod family. Prog.Rep.Pacif.Cst Stns, (79):35–6
Holthuis, L.B., 1955 The recent genera of caridean and stenopodidean shrimps (Class Crustacea, Order Decapoda, Supersection Natantia) with keys for their determination. Zool.Verh.,Leiden, 26:157 p.
Holthuis, L.B. and H. Rosa, Jr., 1965 List of species of shrimps and prawns of economic value. FAO tech.Pap., (52):21 p.
Hynes, F.W., 1930 Shrimp fishery of southeast Alaska. Rep.U.S.Commnr Fish., 1929 (Appendix 1): 18 p.
Needler, A.B., 1931 Mating and oviposition in Pandalus danae. Can.Fld Nat., 45(5): 107–8
Odemar, M., 1964 Cruise report 64-A-1, prawn. California Department of Fish and Game Marine Resources Operations, 2 p.
Pacific marine fisheries commission, 1965 Data series: crab and shrimp section, 139 p.
Philipps, J.B., 1931 Prawn fishery started at Monterey. Calif.Fish Game, 17(2): 159–62
Rathbun, M.J., 1904 Decapod crustaceans of the northwest coast of North America. In Crustaceans, Harriman Alaska Expedition, 10: 3–211. Reprinted 1910. Publs.Smithson.Instn, (1997):1–337
Ronholt, L.L., 1963 Distribution and relative abundance of commercially important pandalid shrimps in the northeastern Pacific Ocean. Spec.scient.Rep.U.S. Fish Wildl.Serv., (449):28 p.
Schmitt, W.L., 1921 The marine decapod Crustacea of California. Univ.Calif.Publs Zool., (23):470 p.
United States Bureau of Commercial Fisheries, 1966 Cruise report, exploratory fishing and gear research cruise 66-1 M/V LITTLE LADY, April 18–June 13, 1966. 4p.(mimeo)
United States Bureau of Commercial Fisheries, 1967 Cruise report, Alaska exploratory cruise 66-4, M/V JOHN R. MANNING, October 31–December 16, 1966. 5 p.(mimeo)
Wilby, G.V., 1937 The ling cod, Ophiodon elongatus Girard. Bull.biol.Bd Can., 54:24 p.