by
James Ogari
K.M.F.R.I.
Kisumu, Kenya
| ABSTRACT |
| The distribution and the food and feeding habits of L. niloticus occurring in Nyanza Gulf of Lake Victoria, were studied during the period August 1981 – July 1982. Lates niloticus was found to have colonized the whole of Nyanza Gulf. The postlarvae and juvenile Lates inhabited mainly the shallow inshore areas and there was an increase in Lates size with an increase in depth. Highest catches were realized within the mid-deep offshore areas. |
| Lates niloticus is a carnivore which preys upon pisces, insects, Crustacea and molluscs. The post-larvae preferred microscopic crustacea and insects, whereas the juveniles fed mainly on prawn, Caridina nilotica. With the increase in size L. niloticus became more piscivorous. The type of prey taken depended on both food availability and the size of predator. The variations in the composition of the gut contents and feeding intensity are discussed in relation to the size groups and water depth. |
INTRODUCTION
Lates niloticus is an exotic species which was introduced into Lake Victoria in the early 1960s, Gee (1965). The purpose for the introduction into Lake Victoria was to utilize the abundant but relatively uneconomic haplochromine species, so as to provide biomass more appropriate for food. The introduction was further aimed at managing the fishery, by permitting an extension of the fishing grounds into the deeper offshore waters, and the use of a wider variety of gear for exploitation. These management aims were geared toward releasing the fishing pressure on “tilapias” whose habitat was within the shallow inshore areas of the Lake, Anderson (1961).
Hamblyn (1960) reported the appearance of Nile perch in the commercial landings in Lake Victoria. Gee (1965a) observed an increase of between 500% and 700% in landings within the hinterland of Masese in Uganda. The author also noted the spread of Lates round the northern and eastern shore of Lake Victoria from west of Entebbe to South of Musoma in Tanzania.
In 1966 a 34.5-kg Lates was caught within the Kenya waters of Lake Victoria just off Rusinga Island, Arunga (1981).
In terms of commercial importance, Lates has the highest landing in the Kenya waters of Lake Victoria where in 1983 it constituted 68% of the total catch.
Hamblyn (1960) noted Nile Perch in Lake Victoria to be feeding on haplochromine species and Clarias sp. Gee (1969) observed the dominant prey to be Cichlidae and Mormyridae. Okedi (1971a) reported the dominant food of Lates in Lake Victoria to be haplochromines. The author reported a progressive decrease in Lates abundance with an increase in depth up to a limit of 25 metres. Kudhongania and Cordone (1974) observed a progressive decrease in Nile perch catches with an increase in water depth from 2.0 kg/hr within the 4–9 m depth zone to 0.4 kg/hr within the 20–29 m depth range in Lake Victoria. Benda (1981) caught the species exclusively in the 10–19 m depth range with a mean catch of 23.8 kg/hr in Lake Victoria.
The success of the introduced Lates aroused great interest, and investigations into various aspects of its biology were initiated to throw more light on its future performance and management. Some of the information that is useful in more intuitive management methods include basic trophic investigation of systems, i.e., determination of who eats whom, and how often. Such studies reveal the impact of Lates on population control, and how such impact is influenced by exploitation.
The present investigation is based on the work carried out in Nyanza Gulf (Figure 1) during the period August 1981 – July 1982.
Materials and methods
The gulf was divided vertically into five sampling zones (Figure 1). Each zone had a depth range of about 4 metres. The main method used for sampling was bottom trawling using a 34-foot fibreglass boat powered with an 85 hp caterpillar engine. The trawler had a head rope of 15.4 m, a bottom rope of 18.5 m and cod-end mesh size of 38 mm. Trawling was maintained at 3 knots, with each haul lasting 30 minutes. The other methods used to obtain specimen samples included beach-seining and gillnetting.
After every haul the catch was sorted into species and weighed. Samples of Lates niloticus taken were measured in centimetres and weighed in grammes individually on board. The depth at which each haul was done was noted.
The fish were each dissected and the stomach removed for stomach content identification and counting of the food items. The recording of the food items was based on the occurrence method described by Hynes (1950). The percentage frequency of occurrence of each food item was obtained by dividing the number of stomachs containing specific food items by the total number of stomachs examined.
RESULTS
Distribution
Lates niloticus was found to be ubiquitous in Nyanza Gulf. The post-larvae appeared to be restricted within the inshore areas, though juveniles above 3.0 cm total length were caught at all depths in the Gulf. Despite the presence of juveniles in the deeper offshore areas, there was a tendency of Lates size to progressively increase with increase in water depth (Figure 2).
Data on relative abundance of L. niloticus within the five different depth zones in the Gulf are presented in Table 1, Figure 3. The figure shows an increase in relative abundance with increase in water depth especially up to the 12–15.9 m depth zone. Statistical reports from the Kenya Fisheries Department from 1976 to 1983 is presented in Table 2, Figure 4. Results from both the Table and the Figure indicate a decline in the commercial landings of most of the fish stocks and an increase in landings for Lates and Oreochromis niloticus.
Food and feeding habits
The diet of Lates niloticus in Nyanza Gulf consisted of fish, insects, crustacea and molluscs. The type of prey ingested by the predator depended on both predator size and prey availability and abundance within a given habitat.
The post-larvae and juveniles preyed upon crustacea (Cladocera, chironomids and Caridina nilotica), insects (Povilla adusta and Odonata nymph), and fish (Rastrineobola argentea). The percentage occurrence of the four main prey items is shown in Figure 5. It is also revealed that up to a predator size of 70 cm TL, crustacea was the main prey sought. However, with increase in predator size above 70 cm TL, the utilization of crustacea as prey declined and was replaced by fish as the dominant prey. Insects made a small but significant contribution to the food of Lates of up to 50 cm TL. Molluscs contributed a small but significant proportion of the prey ingested especially by Lates of 30–90 cm TL. The large Lates had a high percentage of empty stomachs and those which had food in their stomach fed mainly on fish.
Within the 0–3.9 m depth zone, the fish as diet formed the bulk of food (37.0%). The dominant fish species was R. argentea (17.0%), and L. niloticus (13.0%). Crustacea was represented mainly by C. nilotica (21.6%).
Between 4 and 7.9 m depth range, fish as diet once more formed the bulk of the diet constituting 38.1%. The dominant fish species being L. niloticus (16.7%), followed next by R. argentea (13.7%). Crustacea was represented by C. nilotica constituting 32.3%. Among the insects, Odonata nymph was the predominant prey (15.6%).
At depths of 8.0–11.9 m C. nilotica formed the bulk of the diet constituting 56.1%. The next significant food item in the diet was fish, represented by L. niloticus (18.7%). Insects as prey were represented by Odonata nymph (8.3%). Molluscs were recorded though they made an insignificant contribution to the diet within this depth range.
Above 12.0 metres depth zone C. nilotica continued to form the bulk of the diet constituting approximately 73.0%. Fish as diet was represented by L. niloticus (13.0%). Both insects and molluscs were noted in the diet but in negligible numbers.
A scatter plot of the total length of each of the three major fish prey (Oreochromis niloticus, haplochromine and Lates itself) against the total length of Lates niloticus (predator) is provided (Figure 6). It appears from the figure that most of the food items were less than 1/3 of the predator size. It was only in two Lates stomachs, in which the length of prey approached 50% of the length of the predator. This occurred in L. niloticus of 167.0 cm TL and 68.0 cm TL which had ingested a Clarias sp. of 85.0 cm TL and a Lates of 34.5 cm TL respectively. The graph shows a general increase in prey size with increase in predator size.
DISCUSSION
Gee (1969) described L. niloticus as a carnivorous fish. Hamblyn (1966), Gee (1969), Okedi (1971a) and Hopson (1972 and 1975) studied the food of post-larvae L. niloticus in various lakes in East and West Africa, and observed the predominant prey item as planktonic crustacea. Coulter (1976) noted the food of the four Lates species under 3.0 cm TL in Lake Tanganyika as being composed of zooplankton.
Hamblyn (1966) in Lake Mobutu observed invertebrates as the dominant prey among Lates less than 60 cm TL, but with increase in predator size above 60 cm TL, the latter became piscivorous.
Hopson (1975) and Hunter (1970) in Lakes Turkana and Mobutu noted a tendency of food items of L. niloticus to change with water depth.
Gee (1969) and Coulter (1976), observed a general increase of prey size with increase in predator size. Gee (1969) noted the proportion of prey body length to predator body length to be in the region of 25%, and rarely exceeding 30%. Okedi (1971a) and Coulter (1976) observed the proportion to be approximately 35%.
In Lake Kyoga (EAFFRO Annual Report 1968) cichlids and mormyrids which formed the bulk of the diet were later replaced by Rastrineobola and Odonata nymph as the dominant prey, but with both haplochromine cichlids and Oreochromis occurring. Okedi (1971a) observed haplochromine species as the dominant prey of Lates in Lake Victoria. During the present investigation the dominant prey items were Lates and Rastrineobola in the shallow inshore waters and crustacea (C. nilotica) in the deeper waters. The change in diet with time may be related to the availability and relative abundance of the prey items.
CONCLUSION
Lates niloticus has colonized the whole of the Nyanza Gulf, and the species apparently is moving out of the Gulf into the main Lake. Its success has been due partly to the fact that Lates is both a potential predator and a superior competitor of the local fish species. The other reason for its success has been probably due to its access to an ample food supply comprising invertebrates and fish species, which apparently had not evolved in the presence of a vigorous predator and which have consequently been particularly vulnerable.
With the high rate of multiplication and the high degree of inter-specific competition Lates appears to have exhausted its resources and has not succeeded in striking a balance and be able to co-exist with the other fish in Nyanza Gulf except for Oreochromis niloticus. Due to this hypothesis there is a tendency for Lates to be involved in cannibalism and an apparent drift out to the gulf into the main lake in pursuit of its prey.
The flexibility of the diet of Lates suggests that the species is an opportunistic feeder. In Nyanza Gulf there is little size segregation by depth, suggesting that non-selective fishing gear will capture a mixture of young and adult Lates.
REFERENCES
Anderson, A.M., 1961. Further observations concerning the proposed introduction of Nile perch into Lake Victoria. E.Afr.Agric.For.J., 36(4):195–201
Arunga, J., 1981. A case study of the Lake Victoria Nile perch (Lates niloticus) fishery. In Proceedings of the Workshop on Aquatic resources of Kenya, July 1981. Mombasa, Kenya, Kenya Marine and Fisheries Research Institute, pp. 165–83
Benda, R.S., 1981. A comparison of bottom trawl catch rates in the Kenya waters of Lake Victoria. J.Fish Biol., 18:609–13
Coulter, G.W., 1976. The biology of Lates species (Nile perch) in Lake Tanganyika, and the status of the pelagic fishery for Lates species and Luciolates stappersii (Blgr.) J.Fish Biol., 9:235–59
Fryer, G., 1960. Concerning the proposed introduction of Nile perch into Lake Victoria. E.Afr.Agric.For.J., 25(4):267–70
Fryer, G. and T.D. Iles, 1972. The Cichlid fishes of the Great Lakes of Africa. Edinburgh, Oliver and Boyd, 641 p.
Gee, J.M., 1965. The Nile perch investigation. Annu.Rep.E.Afr.Freshwat. Fish.Res.Org., (1964):13–7
Gee, J.M., 1965a. The spread of Nile perch (Lates niloticus) in East Africa with comparative biological notes. J.Appl.Ecol., 2(27):407–8
Gee, J.M., 1969. A comparison of certain aspects of the biology of Lates niloticus (Linné) in endemic and introduced environment in East Africa. In Man-made lakes: the Accra Symposium, edited by L.E. Obeng. Accra, Ghana University Press for Ghana Academy of Sciences, pp. 251–9
Hamblyn, E.L., 1960. The Nile perch investigation. Annu.Rep.E.Afr. Freshwat.Fish.Res.Org., (1959):23–8
Hamblyn, E.L., 1960a. Preliminary notes on Lates from Bulaka, Lake Albert. E.Afr.Freshwat.Fish.Res.Org., (1959):30–1
Hamblyn, E.L., 1960b. The Nile perch project. Annu.Rep.E.Afr.Freshwat. Fish.Res.Org., (1960):26–32
Hamblyn, E.L., 1966. The food and feeding habits of Nile perch Lates niloticus (Linné) Pisces:Centropomidae. Rev.Zool.Bot.Afr., 74(1–2):28 p.
Hopson, A.J., 1972. A study of the Nile perch (Lates niloticus (L.) Pisces:Centropomidae) in Lake Chad. Overseas Res.Publ.Lond., (19):93 p
Hopson, A.J., 1975. Preliminary observations on the biology of Lates niloticus in Lake Rudolph. Paper presented at the Symposium on the Hydrobiology and fisheries of Lake Rudolf (Turkana). Molo 25–29 May 1975
Hunter, J.B., 1970. Observations on the taxonomy and biology of Lates (Cuvier 1828) in Lake Albert. Occas.Pap.Fish.Dep.Uganda, (3)
Hynes, H.B.N., 1950. The food of the freshwater sticklebacks (Casterosteus aculeatus and Pygosteus pungitius) with a review of the methods used in the studies of the food of fishes. J.Anim.Ecol., 19:36–58
Kudhongania, A.W. and A.J. Cordone, 1974. Bathospatial distribution patterns and biomass estimates of major demersal fishes in Lake Victoria. Afr.J.Trop.Hydrobiol.Fish., 3(1):15–31
Ogari, J., 1984. The biology of Lates niloticus (L.) in Nyanza Gulf of Lake Victoria (Kenya) with special reference to the food and feeding habits. M.Sc. Thesis, University of Nairobi
Okedi, J., 1971. The food and feeding habits of the small mormyrid fishes of Lake Victoria. Afr.J.Trop.Hydrobiol.Fish., 1(1):1–12
Okedi, J., 1971a. Further observations on the ecology of the Nile perch, Lates niloticus (Linné) in Lake Victoria and Lake Kioga. Annu.Rep.E.Afr.Freshwat.Fish.Res.Org., (1970):42–55
Okemwa, E.N., 1984. Potential fishery of Nile perch L. niloticus Linné (Pisces: Centropomidae) in Nyanza Gulf of Lake Victoria, East Africa. Hydrobiologia, 108(2): 121–6
Wanjala, B. and G. Marten, 1975. Survey of the Lake Victoria fishery in Kenya. Annu. Rep. E.Afr. Freshwat. Fish. Res.Org., (1974): 81–5
Stoneman, J. and J.F. Rogers, 1970. Exotic fishes. Occas. Pap. Fish. Dep. Uganda, (3):16–9
Table 1: Mean catch of Lates niloticus per unit effort (kg/hr) in Nyanza Gulf for the period August 1981 to July 1982
| Depth Zones (m) | Mean Catch rate kg/h | Standard deviation | 95% confidence limit | |||||
| 0 | – | 3.9 | (21)* | 102.3 | 92.4 | 102.3 | ± | 41.8 |
| 4.0 | – | 7.9 | (72) | 131.0 | 183.7 | 131 | ± | 43.2 |
| 8.0 | – | 11.9 | (21) | 204.0 | 169.5 | 204 | ± | 77.2 |
| 12.0 | – | 15.9 | (24) | 502.7 | 259.9 | 502.7 | ± | 109.3 |
| 16+ | (13) | 264.8 | 179.6 | 264.8 | ± | 108.6 | ||
* Figures in parentheses indicate numbers of hauls.
Table 2: Annual Catches for Kenya Waters of Lake Victoria Catch and (%) by species
| Species | 1976 | 1977 | 1978 | 1979 | 1980 | 1981 | 1982 | 1983 | ||||||||
| O. esculenta | 49 | (0.3) | 42 | (0.2) | 180 | (0.8) | 94 | (0.3) | 90 | (0.3) | 166 | (0.4) | 399 | (0.7) | 64 | (0.1) |
| O. niloticus | 421 | (2.3) | 465 | (2.4) | 972 | (1.1) | 962 | (3.1) | 1184 | (4.4) | 2213 | (4.8) | 2581 | (4.2) | 2516 | (3.3) |
| O. variabilis | ||||||||||||||||
| O. leucostictus | 537 | (2.9) | 928 | (4.8) | 1454 | (6.1) | 1683 | (5.5) | 3739 | (13.9) | - | - | - | |||
| T. zillii Bagrus | 1025 | (5.5) | 1141 | (5.9) | 1396 | (5.9) | 1769 | (5.8) | 642 | (2.4) | 435 | (1.0) | 2532 | (4.2) | 1243 | (1.6) |
| Lates | 94 | (0.5) | 203 | (1.1) | 1066 | (4.5) | 4286 | (14.0) | 4310 | (16.0) | 27259 | (59.7) | 33134 | (54.4) | 52377 | (67.7) |
| Protopterus | 935 | (5.0) | 773 | (4.0) | 612 | (2.6) | 472 | (1.5) | 370 | (1.4) | 251 | (0.5) | 239 | (0.4) | 108 | (0.1) |
| Haplochromines | 6368 | (34.1) | 6255 | (32.4) | 6621 | (27.8) | 6599 | (21.6) | 3636 | (13.5) | 968 | (2.1) | 2546 | (4.2) | 612 | (0.8) |
| Clarias | 2507 | (13.4) | 1755 | (9.8) | 1729 | (7.2) | 3029 | (9.9) | 1223 | (4.5) | 1126 | (2.5) | 2062 | (3.4) | 893 | (1.2) |
| Barbus | 182 | (1.0) | 183 | (0.9) | 199 | (0.8) | 417 | (1.4) | 421 | (1.6) | 381 | (0.8) | 682 | (1.1) | 100 | (0.1) |
| Syndontis | 191 | (1.0) | 310 | (1.6) | 155 | (0.6) | 482 | (1.6) | 388 | (1.4) | 159 | (0.3) | 232 | (0.4) | 91 | (0.1) |
| Mormyrus | 89 | (0.5) | 102 | (0.5) | 132 | (0.6) | 359 | (1.2) | 333 | (1.2) | 269 | (0.6) | 2678 | (4.4) | 218 | (0.3) |
| Labeo | 123 | (0.1) | 62 | (0.3) | 148 | (0.6) | 443 | (1.4) | 482 | (1.8) | 159 | (0.3) | 918 | (1.5) | 81 | (0.1) |
| Schilbe | 57 | (0.3) | 129 | (0.7) | 120 | (0.5) | 320 | (1.0) | 117 | (0.4) | 58 | (0.1) | 78 | (0.1) | 22 | (0) |
| Rastrineobola | 5652 | (30.3) | 6704 | (34.7) | 8710 | (36.5) | 9321 | (30.5) | 9443 | (35.1) | 9326 | (20.4) | 10419 | (17.1) | 16444 | (21.3) |
| Alestes | - | - | - | - | - | 1 | (0) | 2 | (0) | 4 | (0) | |||||
| Others | 447 | (2.4) | 280 | (1.4) | 362 | (1.4) | 356 | (1.1) | 536 | (2.0) | 2289 | (5.0) | 1495 | (2.5) | 1658 | (2.1) |
| Small mixed | - | - | - | - | - | 607 | (1.3) | 961 | (1.6) | 894 | (1.2) | |||||
| Total | 18 677 | 19 332 | 23 856 | 30 592 | 26 914 | 45 667 | 60 958 | 77 325 | ||||||||
Note: no records

Figure 1 Map of Nyanza Gulf showing depth contours and sampling zones A-E
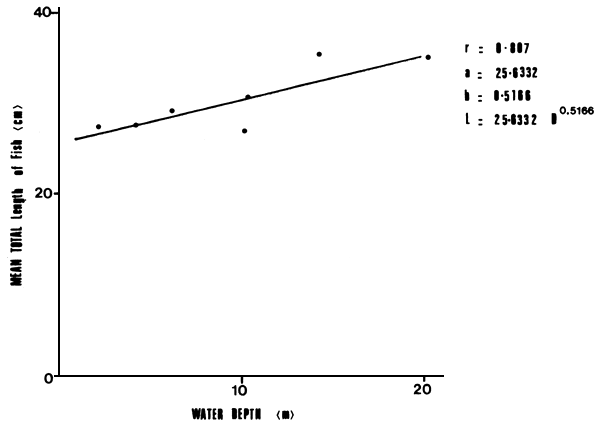
Figure 2 Distribution of Lates niloticus of different sizes by depth
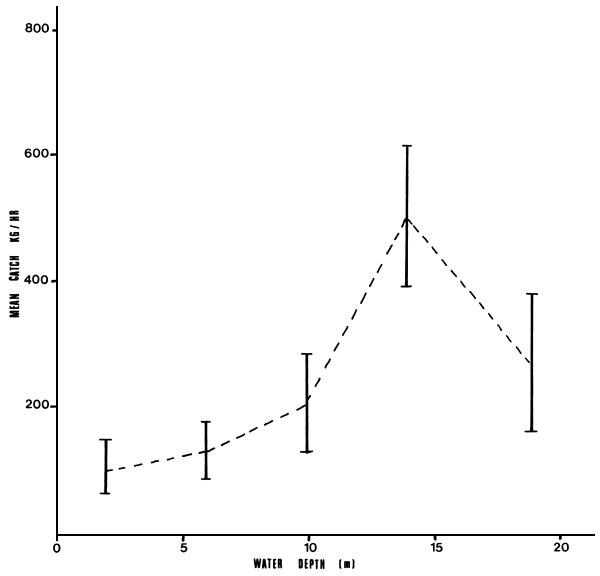
Figure 3 Mean catch per unit effort of Lates niloticus in Nyanza Gulf from five depth zones between August 1981 and July 1982 (vertical bars indicate the 96% confidence limits)
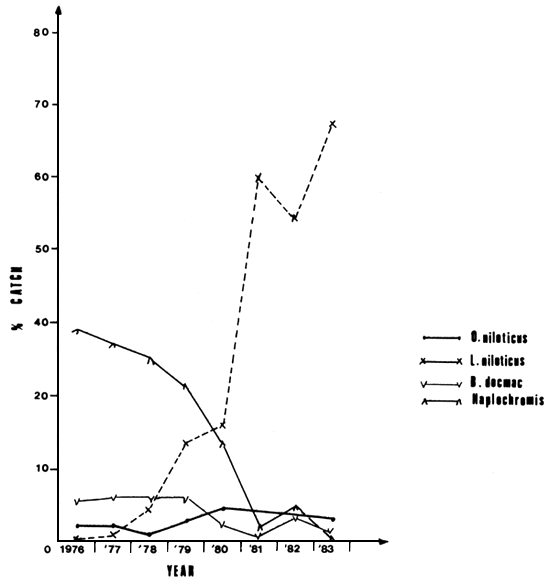
Figure 4 Total landings of B. docmac, Haplochromis, O. niloticus and Lates niloticus from Kenya waters of Lake Victoria during the period 1976–83
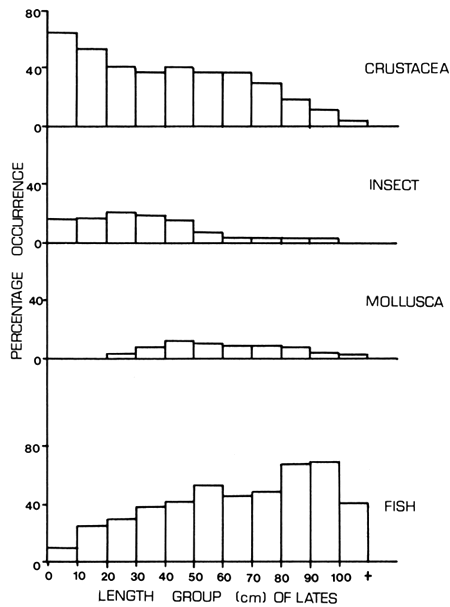
Figure 5 Percentage occurrence of four food items in relation to the size of Lates niloticus
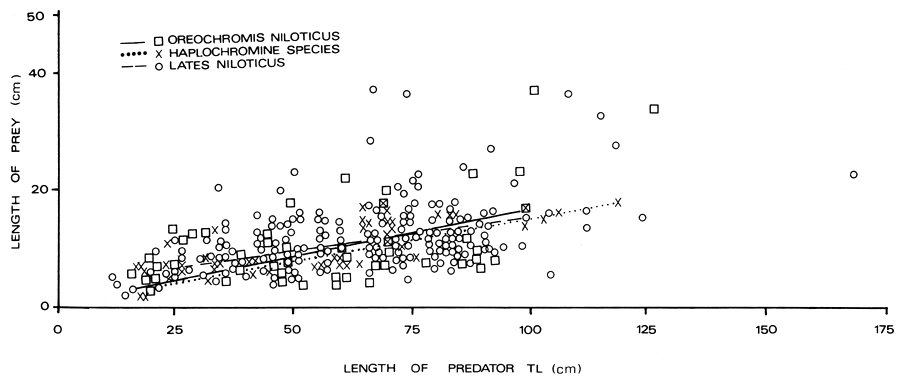
Figure 6 Relationship between size of Lates (a predator) and size of three prey species in the Nyanza Gulf of Lake Victoria
by
F. Witte and P.C. Goudswaard
Haplochromis Ecology Survey Team (HEST)
Leiden, The Netherlands
and Mwanza, Tanzania
INTRODUCTION
Research in the 1960s revealed that 80% of the demersal fish stock of Lake Victoria consisted of haplochrimine cichlids (Kudhongania and Cordone, 1974). In the following decade attempts were made to increase the exploitation of this vast amount of protein and until recently the prospects for a fishery on haplochromine fishes was regarded as very promising (CIFA, 1982; Kudhongania and Cordone, 1974). At present, however, there are signs of local overfishing of haplochromine stocks due to intensive trawl fishing. Moreover, in certain areas the haplochromines have been reduced to very low population levels as a result of the explosive increase of the Nile perch which predates heavily on these fish (Arunga, 1981; Okemwa, 1981, 1984). As a result of these recent developments the prospects for the haplochromine fishery in Lake Victoria obviously have to be reconsidered.
RESULTS
A summary of research results based on the work of the Haplochromis Ecology Survey Team (HEST) over the past seven years in the Mwanza area of Lake Victoria is as follows:
1. Haplochromines can be classified into 11 trophic groups (Greenwood, 1974; oijen, Witte and Witte-Maas, 1981; Witte, Goudswaard and Katunzi (in prep.)) which to some extent can be used as ecological guilds, for example the zooplanktivore migrates to the water surface during the night, while most other trophic groups remain at the bottom (Witte 1984; Goldschmidt, pers.comm.).
2. Trophic groups are not evenly distributed over the Lake; each area has its own trophic composition (Table 1; Oijen, 1979; Witte, 1981, 1984). In the areas suitable for bottom-trawl fisheries the four most important trophic groups are detritivores, zooplanktivores, insectivores and molluscivores.
3. Because of behavioural (e.g., spawning patterns) and morphological (e.g., size) characteristics of trophic groups, the different trophic composition at various areas (or habitats) of the Lake, have consequences for fisheries in those areas: for example, where molluscivores dominate the catches, a larger mesh size should be used than in areas where the smaller detritivores are abundant (Witte, 1981).
4. Many species are strongly habitat restricted (Hoogerhoud and Witte, 1983; Witte, 1981, 1984). As a result, overfishing may result from heavy, localized fishing pressure which then cannot easily be repopulated by migration of fishes from other areas (oijen, 1979).
5. A number of species show migration to spawning areas and to nursery grounds (oijen, Witte and Witte-Maas 1981, Witte, Goudswaard and katunzi (in prep.)).
6. Many species important to the trawl fishery, have a seasonal breeding pattern and spawn during approximately the same period (Witte, 1981, Witte, Goudswaard and katunzi (in prep.)). This makes it desirable to introduce a closed season for fishing.
7. The growth rate of smaller species (adult size 5.0–7.5 cm SL) is approximately 5–6 cm per year. This means that the generation time of these species approximates to one year (Witte, 1981, Witte, Goudswaard and Katunzi (in prep.)). For larger species (e.g., molluscivores and piscivores whose standard length is generally between 10 and 20 cm) the generation time may be longer. The life span of these fishes is not yet known but some of the zooplanktivores seem to live at least 2–3 years (Goldschmidt, pers.comm.).
8. Since the start of the trawl fishery in the Mwanza Gulf in 1973, distinct changes in catch composition of the haplochromines have been observed:
The larger haplochromines (SL>15 cm) virtually disappeared from the catch (Tables 2, 3; Kukowski 1978, Witte, Goudswaard and Katunzi (in prep.)). This caused a change in the trophic composition.
The percentage of detritivores increased at the cost of zooplanktivores (Table 5; Witte, Goudswaard and Katunzi (in prep.)).
The mode of the length frequency distribution of small species, such as the detritivore H. “nigrofasciatus” (maximum SL 7.5 cm), decreased significantly over the period 1978 until 1982. Over this period the females of this species start breeding at a smaller size than before the increased fishing pressure (Table 4; Witte, Goudswaard and katunzi (in prep.)).
The average catch rate per hour decreased from 1753 kg/h in 1976 to 680 kg/h in 1982 (Table 6; Witte, Goudswaard and katunzi (in prep.)).
9. Recent trawl catches in the as yet unexploited deep offshore areas (40–60 m) of the Lake revealed catch-rates of approximately 150 kg haplo-chromines per hour. This is four times lower than the present catch rate of the same trawler in the Mwanza Gulf (4–18 m).
DISCUSSION
Since 1973, when the first trawler started regular fishing in the Mwanza area, the trawl fishery gradually increased and at the moment approximately ten small trawlers (10–15 m; 60–170 hp) are operating in the Mwanza Gulf and its surroundings. This fishery had distinct effects on the composition of the haplochromine stock in the Mwanza Gulf. Similar effects (changes in species composition from large to small species) were observed for demersal cichlid stocks in Lake Nyasa (=Malawi) (Turner, 1977, 1977a). In Lake Nyasa these changes did not seem to affect the catch per effort nor the total yield (Turner, 1977a). In the Mwanza Gulf of Lake Victoria the catch per effort of the R.V. MDIRIA decreased, but it is not known if and how the total yield has changed during the past years. In a trawl fishery on a multi-species stock such as the haplochromines, overfishing of the larger species can hardly be avoided. As a result the trophic composition will change. What effects these changes will have in the long term is difficult to foresee. It might lead to a less efficient ecosystem, that is ecosystem overfishing. The indications that the smallest species are also seriously affected by the trawl fishery in the Mwanza Gulf seems more alarming at present. It is possible that overfishing of even the smallest species occurs. To avoid this larger mesh sizes should be used. The change from a 25 mm to a 38 mm cod end was reported to have a favourable effect on the cichlid stock of Lake Nyasa (= Malawi). After an initial decrease, the catches finally became larger than before this change (Turner and Tweddle, pers. comm.).
Stock recruitment overfishing is also a serious danger for haplo-chromine species because the standing stock per species is often low, they produce relatively few eggs (most of the smaller species produce less than 50 eggs per time; Goldschmidt, pers. comm.) and probably not more than two broods per season can be raised (Witte, 1984). The sensitivity to stock recruitment overfishing may differ from species to species as both the standing stocks of the species and the number of eggs which they produce differ: e.g., zooplanktivores produce approximately half as many eggs as detritivores.
The apparent relative decrease of the zooplanktivores in the catch composition in the Mwanza Gulf might be due to such a relatively high sensitivity to stock recruitment overfishing because, with the mesh sizes presently in use, they are probably less sensitive to growth overfishing as the detritiphytoplanktivores (zooplanktivores are more slender than detritivores but they mature at a somewhat larger size).
Fishing during the spawning season probably has a negative influence on the haplochromine stock. Haplochromines are mouth brooders, the females carry the eggs for approximately three weeks in the mouth. With each brooding female caught, its young are also destroyed. Moreover, many species are known to build nests at the bottom of the Lake though it is not certain whether mud dwelling species do likewise (Hoogerhoud and Witte, 1983; Witte, 1981). If so, each time a trawl net passes by all nests will be swept away. This may seriously hamper successful spawning. A closed fishing season during the period at which most species breed in a certain area seems desirable.
Although the proposed measures will probably be useful for a number of areas, they are unfortunately rendered out of date by a recent, even more serious danger in other parts of the Lake: the rapidly increasing stock of the Nile perch (Lates niloticus). This fish was introduced into the Lake at the beginning of the 1960's (Arunga, 1981; Okemwa, 1984). During the past seven years a sudden strong increase in catch rate of Lates was observed in the Nyanza Gulf (Arunga, 1981; Okemwa, 1981; 1984) and subsequently near Ukerewe and in the Speke Gulf. In a number of habitats of the above mentioned regions haplochromines are almost depleted (Arunga, 1981; Okemwa, 1981; 1984). Nile perch densities in the Mwanza Gulf increased strongly here also since 1983 and there is a chance that a large part of the present haplochromine fishery will vanish in the near future. However, many open questions remain: (i) According to some authors (Arunga, 1981; Hopson, 1972; Okemwa, 1984) Lates niloticus has a preference for shallow oxygenrich habitats, but now it is also found in deeper offshore areas. The question remains whether it will be able to establish itself in such areas to the same extent as in the shallow Nyanza Gulf; (ii) Zooplankton and phytoplankton-feeders which are partly (or mainly) pelagic might be less sensitive to predation by the demersal Nile perch. Catches from other areas revealed for instance that Rastrineobola argentea (dagaa), a pelagic zooplanktivorous cyprinid, is able to coexist with Lates (Arunga, 1981; Hopson, 1972; Okemwa, 1984). Although for the time being the strong increase of Lates seems a favourable development, the final consequences may be very serious for the fish production of the Lake. In the first place adding one step to a food chain generally causes an energy loss of 80%. Secondly a large number of haplochromines are primary consumers (detritus and phytoplankton). When these are depleted a major part of the energy input in the Lake may be cut off for fish production. The same holds for food sources such as molluscs, that are fed on by specialized haplochromines. These effects may finally result in a strong decrease of the total fish yield of the Lake.
From the foregoing it will be clear that the haplochromine stock in the Mwanza Gulf will not withstand the pressures of both overfishing and the predation of the Nile perch. A depletion of the haplochromine stock, the major food source of Nile perch, must inevitably have its effects on the Nile perch stock and in the long term, on the whole ecosystem. All recent data point to the necessity that management measures should be taken, although it is difficult to predict the results of these measures. One of the options which should be seriously considered at the moment is to stop the trawl fishery for haplochromines at short notice and instead put a heavy fishing pressure on Nile perch. This might lead to a system in which not all primary consumers (detritiphytoplanktivorous haplochromines) are eradicated so that this input to the food chain is not cut off completely. This seems the only way to guarantee reasonable Nile perch catches at longer terms. If the haplochromine stock is depleted Nile perch is forced to switch to its own progeny as a food source. This implicates the establishment of an equilibrium at a much lower level. Depending on the results of the suggested measure, it should be decided at a later stage if a limited haplochromine fishery could be reintroduced again. Whether the haplochromine fishery is possible in other areas of the Lake remains to be investigated. Preliminary catch results in the deeper offshore waters (40–60 m) revealed lower catch rates than expected from the earlier trawl surveys of Kudhongania and Cordone, 1974. These authors show that the catch rates at a depth of 40–60 m were approximately half as high as in the shallow waters, at that time not yet overfished. Our preliminary results revealed catch rates that were four times lower than the present catches in shallow water. However, as our catch data is all from one month, it is possible that catch rates increase at other periods of the year. Furthermore, the samples were partly made in an area for which Kudhongania and Cordone, 1974 also found exceptional low catches. Exploration of these deeper offshore areas and other yet uninvestigated areas are obviously needed.
REFERENCES
Arunga, J., 1981. A case study of the Lake Victoria Nile perch Lates niloticus fishery. In Proceedings of the Workshop on the Aquatic resources of Kenya. July 1981, Mombasa, Kenya, Kenya Marine and Fisheries Research Institute, pp. 165–83
Barel, C.D.N., et al., 1977. An introduction to the taxonomy and morphology of the haplochromine cichlidae from Lake Victoria. Neth.J. Zool., 27:333–89
Cordone, A.J. and A. Kudhongania, 1972. Observations on the influences of cod end mesh size on bottom trawl catches in Lake Victoria, with emphasis on the haplochromis population. Afr.J.Trop.Hydrobiol. Fish., 2(1):1–19
CIFA, (Committee for Inland Fisheries of Africa) 1982. Report of the first session of the Sub-Committee for the Development and Management of the fisheries of Lake Victoria. Mwanza, Tanzania, 12–14 October, 1981. FAO Fish. Rep., (262):73 p. Issued also in French
Greenwood, P.H., 1974. The cichlid fishes of Lake Victoria, East Africa: the biology and evolution of a species flock. Bull.Brit.Mus.(Nat. Hist.) (Zool.), Suppl. 6:134 p.
Hoogerhoud, R.J.C. and F. Witte 1983. The morphoecological differentiation of two closely resembling haplochromine species (Pisces: Cichlidae) from Lake Victoria. Neth.J.Zool., in press
Hopson, A.J., 1972. A study of the Nile perch (Lates niloticus (L.) Pisces: Centropomidae) in Lake Chad. Overseas Res.Publ.,Lond., 19:93 p.
Kudhongania, A.W. and A.J. Cordone, 1974. Bathospatial distribution patterns and biomass estimate of the major demersal fishes in Lake Victoria. Afr.J.Trop.Hydrobiol.Fish., 3(1): 15–31
Kukowski, G., 1978. Trawling results in the Tanzanian waters of Lake Victoria by the Freshwater Fisheries Institute Nyegezi, 1973–1977. Report. East African Freshwater Fisheries Research Institute.
Okemwa, E.N., 1981. Changes in fish species composition of Nyanza Gulf of Lake Victoria. In Proceedings of the Workshop on Aquatic resources of Kenya. July 1981, Mombasa, Kenya, Kenya Marine and Fisheries Research Institute, pp. 138–56
Okemwa, E.N., 1984. Potential fishery of Nile perch Lates niloticus Linne (Pisces: Centropomidae) in Nyanza Gulf of Lake Victoria, East Africa. Hydrobiologia, 108(2): 121–6
Oijen, M.P.J. van, 1979. Prospects of an intensive trawl fishery in the Mwanza area of Lake Victoria. Reports from the Haplochromis Ecology Survey Team (HEST) 7.
Oijen, M.P.J. van, F. Witte and E.L.M. Witte-Maas, 1981. An introduction to ecological and taxonomic investigations on the haplochromine cichlids from the Mwanza Gulf of Lake Victoria. Neth.J.Zool., 31:149–74
Turner, J.L., 1977. Some effects of demersal trawling in Lake Malawi (Lake Nyasa) from 1968–1974. J.Fish Biol., 10:261–71
Turner, J.L., 1977a. Changes in the size structure of cichlid populations of Lake Malawi resulting from bottom trawling. J.Fish.Res.Board Can., 34(2):232–8
Witte, F., 1981. Initial results of the ecological survey of the haplochromine cichlid fishes from the Mwanza Gulf of Lake Victoria (Tanzania): breeding patterns, trophic and species distribution. Neth.J.Zool., 31:175–202
Witte, F., 1984. Ecological differentiation in Lake Victoria haplochromines: a contribution to the comparison of cichlid species flocks. In Evolution of fish species flocks, by A.A. Echelle and I. Kornfield, Orono, Maine University of Maine, 155–67.
Witte, F., P.C. Goudswaard and E.F.B. Katunzi. Ecological data for fishery on haplochromines in the southern Lake Victoria; (in prep.)
Table 1: Trophic composition of haplochromine catches (20 mm mesh cod end) in different areas of Lake Victoria. Mean percentages of total numbers and (in addition) for the Mwanza Gulf of total weight are presented.
| Mwanza Gulf 3–16 m 6 trawl shots June – October 1982 | Mwanza Gulf entrance 18–22 m 4 trawl shots April–August 1981 | Nafubo Island 8–17 m 2 trawl shots December 1981 | Bukoba Area 15–20 m 8 trawl shots February–April 1981 | |||||||||
| % of number ± s. dev. | % of weight ± s. dev. | % of number ± s. dev. | % of number | % of number ± s.dev. | ||||||||
| detriti/phyto-planktivores | 77.9 | ± | 4.5 | 68.6 | ± | 4.7 | 75.0 | ± | 5.5 | 76.1 | 58.4 | ? |
| zooplanktivores | 19.6 | ± | 5.5 | 24.3 | ± | 5.0 | 21.3 | ± | 7.8 | 16.4 | 10.7 | ? |
| insectivores | 1.6 | ± | 1.0 | 4.8 | ± | 3.0 | 1.9 | ± | 1.2 | 1.1 | 1.6 | ? |
| molluscivores | 0.2 | ± | 0.3 | 0.3 | ± | 0.3 | 0.2 | ± | 0.2 | 4.7 | 25.5 | 5.5 ± 1.6 |
| piscivores | 0.4 | ± | 0.1 | 1.2 | ± | 0.5 | 0.5 | ± | 0.9 | 1.1 | 0.4 | 1.1 ± 0.9 |
| other groups | 0.4 | ± | 1.0 | 0.9 | ± | 2.1 | 1.6 | ± | 1.5 | 0.2 | 3.3 | - |
Table 2: Decrease of large haplochromines from catches in the Mwanza Gulf (0–19 m depth) with a 89 mm cod end mesh size (from Kukowski, 1978)
| Year | 1974 | 1975 | 1976 | 1977 |
| % frequency occurrence | 85 | 78 | 33 | 9 |
| % of total catch | 3 | 2 | 1 | + |
| catch rate kg/hr | 8 | 3 | 1 | + |
Table 3: Frequency of occurrence of the paedophagous species H. microdon and H. “black cryptodon” in trawl catches in the Mwanza Gulf in 1978 and 1982
| occurrence | H. microdon + | H. microdon - | total |
| period | |||
| 1978 Jan.–Aug. | 32 | 8 | 40 |
| 1982 April–July | 3 | 15 | 18 |
| 35 | 23 | 58 |
x2 test p < 0.001
| occurrence | H. “black cryptodon” + | H. “black cryptodon” - | total |
| period | |||
| 1978 Jan.–Aug. | 25 | 15 | 40 |
| 1982 April–July | 1 | 17 | 18 |
| 26 | 32 | 58 |
x2 test p < 0.001
Table 4: Length frequency distribution of brooding females of H. “nigrofasciatus” caught in March, April and June 1979 and 1982 at station E and G in the Mwanza Gulf
| Standard length | 4.0 | 4.5 | 5.0 | 5.5 | 6.0 | 6.5 |
| 1979 | - | 1 | 3 | 8 | 3 | 1 |
| 1982 | 2 | 8 | 7 | 2 | 1 | - |
Table 5: Mean trophic composition in the Mwanza Gulf from catches in 1978–79 and 1982
| 30 trawl shots Jan. 1978–Feb. 1979 | 6 trawl shots June–Oct. 1982 | |||||
| % of number ± s.dev. | ||||||
| detriti/phyto-planktivores | 61.4 | ± | 10.5 | 77.9 | ± | 4.5 |
| zooplanktivores | 30.4 | ± | 12.2 | 19.6 | ± | 5.5 |
| insectivores | 3.1 | ± | 1.7 | 1.6 | ± | 1.0 |
| molluscivores | 0.2 | ± | 0.3 | 0.2 | ± | 0.3 |
| piscivores | 0.7 | ± | 0.6 | 0.4 | ± | 0.1 |
Table 6: Annual mean catch rates of haplochromines in the Mwanza Gulf, with a 20-mm stretched mesh cod end (depth range 0–19 m)
| year * | mean catch rate kg/h | catch effort ** |
| 1973 | 1220 | ≤ 72 hauls |
| 1974 | 1513 | ≤ 76 hauls |
| 1975 | 1532 | ≤ 137 hauls |
| 1976 | 1753 | ≤ 105 hauls |
| 1977 | 1248 | ≤ 135 hauls |
| 1982 | 682 | 511 hours |
* Data of 1973–77 from Kukowski (1978)
by
J.O. Okaronon
T.O. Acere
D.L. Ocenodongo
Uganda Freshwater Fisheries Research Organization Jinja, Uganda
| ABSTRACT |
| A study of species catch composition, average size and geographical distribution was carried out in Napoleon Gulf, Buvuma Channel and Lingira Bay in Lake Victoria from 1981 to 1983. |
| In the experimental trawl catches, Haplochromis species declined from 543.3 to 270.8 kg per hour in 1981 and 1983 respectively, while Lates niloticus increased from 0.5 to 57.5 kg per hour in the same period. Bagrus docmac exhibited an increase, whereas the other remaining species showed a decline. In the commercial catches, however, only Lates niloticus increased, contributing 13.4, 75.1 and 68.4 percent in 1981, 1982 and 1983 respectively. The remaining fish species either fluctuated or declined. |
| It was observed that the trend in average weights of the different fish species over time in the trawl and commercial catch did not agree. For instance, while Oreochromis niloticus, Protopterus aethiopicus and Lates niloticus increased in average weight in the trawl fishery they showed no change in the commercial fishery where only Oreochromis leucostictus increased and Bagrus docmac declined. |
| All species were represented in Lingira Bay trawl catch which was not the case in Napoleon Gulf and Buvuma Channel. Haplochromis species appeared predominantly in all the areas with 49 percent of the total catch coming from Ingira. Lates niloticus was second in abundance being caught in all the three areas with Napoleon Gulf producing the largest proportion (4 percent). The Oreochromis species were more or less confined to Lingira. |
INTRODUCTION
The current state of the fisheries in the northern part of the Uganda waters of Lake Victoria with particular reference to species composition, average size and temporal and spatial distribution has been studied during 1981 through 1983. The area covered in this study includes Napoleon Gulf, Thruston Bay, Buvuma Channel, Lingira Bay, Hannington Bay, Itome Bay and waters around Igwe and Lufu islands (see Figure 1 in Annex 1.2). This is mostly the shallow (0–30 m) inshore part of the Lake. This area is in the hinterland of Jinja town. Though to a limited extent this is a follow up of the UNDP/EAFFRO research work carried out during 1968–71 covering the whole Lake (Bergstrand and Cordone, 1971; Kudhongania and Cordone, 1974).
The purpose of this study was to monitor any possible changes (including individual fish sizes) that might have occurred in both the trawl and commercial catches since the last report.
MATERIALS AND METHODS
Trawling was carried out from the R.V. IBIS which is fully rigged for bottom trawling but now stripped of its former navigational and echo-sounding equipment. The rest of the information on IBIS remains as reported earlier by Bergstrand and Cordone. The trawl nets used during this study were (except for the codend) made by the UFFRO gear technologist and the specifications of these 26 m (headrope) bottom trawls were:
Headrope: 24 m; 38 mm circumference combination rope.
Groundrope: 34 m; 12 mm diameter wire rope served with 64 mm circumference
manila rope.
Breastline: 4.8 m; 40 mm circumference polythylene rope.
Weight of Groundrope: 60 kg.
The codend used during the study was of 19 mm mesh. Haul durations were between 1 and 1 h 30 min. Fishing was concentrated to daylight hours during which a maximum of 5 hauls were made in a day. Every effort was made to trawl along the same transects.
The commercial catch data (for fresh fish) was obtained from Masese Fish Landing (Figure 1) where the Fisheries Department has permanently stationed staff to take records of the fish landed. Most of the fish landed at Masese on a daily basis is brought by motorized transport canoes from the various areas covered by this study. The major gear used by the commercial fishermen are gillnets, beach seines and longlines.
RESULTS
Species Composition and Distribution
Twenty fish species excluding the haplochromine cichlids were recorded in the trawl catches in the study area (Table 1). All these species were represented in Lingira Bay. Oreochromis leucostictus and Labeo victorianus were not encountered in Napoleon Gulf while O. leucostictus, Tilapia zillii and Astatoreochromis spp. were not caught by trawl in Buvuma Channel. In the commercial catches landed at Masese only twelve species were recorded, namely Oreochromis esculentus, O. variabilis, O. niloticus, O. leucostictus, Tilapia zillii, Bagrus docmac, Clarias mossambicus, Protopterus aethiopicus, Lates niloticus, Barbus altianalis, Mormyrus kannume, and haplochromine cichlids.
Haplochromines contributed 85.7 percent of total trawl catch and was caught predominantly in all the areas with Lingira Bay alone contributing more than half (Table 2). Lates niloticus was second to Haplochromis; it contributed 6.8 percent of total catch with 3.7 percent of total catch coming from Napoleon Gulf. The Oreochromis species were predominantly caught in Lingira Bay and contributed only 2.4 percent of total trawl catch during the period in all areas. In the commercial catches landed at Masese during the period, the average distribution of the catch (percentage by weight) was as follows: Lates niloticus (56.8%), Oreochromis niloticus (22.5%), O. variabilis (6.4%), Protopterus aethiopicus (5.8%), Clarias mossambicus (2.7%), Barbus altianalis (1.6%), Bagrus docmac (1.3%), haplo-chromines (1.1%), Tilapia zillii (0.8%), Mormyrus kannume (0.7%), Oreochromis leucostictus (0.3%) and S. esculentus (0.1%).
The bulk of trawl catches occurred between September and November (Table 3). Haplochromine peak catches were recorded between September and December while two separate peak catches of Oreochromis variabilis, O. niloticus, and Lates niloticus occurred during April to July and October and November.
There was no clear seasonal pattern in the commercial catch on the basis of catch (in kg) per day (Table 3). However, on the basis of observed monthly commercial catches, the general peaks occurred during August to November and April to June in 1981–82 and 1983 respectively and contributed by Lates niloticus. There were shifts in peak commercial catches of the Oreochromis species from year to year. Oreochromis niloticus displayed peaks during June to November 1981, February to March and September 1983. O. variabilis which was on the decline during that period, displayed peak catches during September to November 1981 and February 1982.
Status of Fish Stocks
Table 4 presents the mean catch rates (kg per hour) for 12 fish species collected in 581 trawl hauls (in a total time of 637.4 hours) in the northern part of the Uganda waters of Lake Victoria. Haplochromines, Oreochromis esculentus, O. variabilis, O. niloticus, Clarias mossambicus and Synodontis victoriae catch rates declined 2.0, 15.0, 8.1, 2.7, 3.6 and 2.6 times, respectively, from 1981 to 1983. Bagrus docmac and Lates niloticus catch rates increased 2.8 and 11.5 times, respectively, from 1981 to 1983. Protopterus aethiopicus catch rate remained more or less the same. However, O. leucostictus, Tilapia zillii, Barbus altianalis, Synodontis afrofischeri, Mormyrus kannume, Schilbe mystus, Xenoclarias sp. and Astatoreochromis alluandi were only trace species if considered individually.
Similarly, the percentage catch composition (by weight) of the various fish species landed at Masese by the commercial fishermen indicates a similar pattern (Figure 1). In the commercial catches, O. esculentus, O. variabilis, O. niloticus, Bagrus docmac and Protopterus aethiopicus catches declined 1.7, 11.8, 3.2, 3.5 and 1.6 times respectively, from 1981 to 1983. Lates niloticus catches increased 5.1 times from 1981 to 1983 while the catches of Clarias mossambicus remained more or less unchanged. O. leucostictus and Tilapia zillii catches which are combined in Figure 1 individually showed differing trends; O. leucostictus catches declined 6.0 times and T. zillii catches increased 1.6 times from 1981 to 1983. Barbus altianalis and Mormyrus kannume which are the only two species presented in Figure 1 as ‘other species’, indicated varying trends when considered separately. Barbus altianalis catches increased 2.9 times from 1981 and 1983 and the catches of M. kannume remained unchanged during the same period. Haplochromines were landed in trace quantities. The available records on the commercial catches landed at Masese did not, however, indicate the presence (in the catch) of Labeo victorianus, Schilbe mystus, Xenoclarias sp., Synodontis sp., Mastacembelus frenatus and Astatoreochromis.
Average Size of Fish
Mean weights (in kg) of individual fish taken from the trawl and commercial catches are shown in Figure 2. In the trawl catches, the mean weights of Oreochromis niloticus, Bagrus docmac, Protopterus aethiopicus and Lates niloticus increased over the period. Both O. niloticus and B. docmac increased from 0.6 to 0.9 kg from 1981 to 1983 respectively. Protopterus aethiopicus mean weight went up from 5.5 kg in 1981 to 6.5 kg in 1983 while the mean weight of L. niloticus rose from 4.5 kg in 1981 to a stable 5.0 kg in 1982 and 1983. On the contrary, the mean weights of Clarias mossambicus fell from 4.9 to 4.2 kg in 1981 and 1983 respectively, while those of Oreochromis esculentus and S. variabilis remained unchanged at 0.4 and 0.3 kg respectively (Figure 2).
In the commercial catch, the mean weights of O. niloticus, and Bagrus docmac fell from 1.2 and 1.1 kg in 1981 to a stable 1.0 and 0.7 kg in 1982–83 respectively (Figure 2). Lates niloticus and C. mossambicus first increased in mean weight from 4.8 and 5.4 kg in 1981 to 8.6 and 5.9 kg in 1982, then decreased to 5.8 and 4.7 kg in 1983 respectively. The mean weights of O. esculentus and O. variabilis remained more or less unchanged at 0.3 kg each, while P. aethiopicus exhibited a decline in mean weight initially from 9.1 kg in 1981 to 7.8 kg in 1982 then a rise to 8.6 kg in 1983 (Figure 2).
DISCUSSION AND CONCLUSION
The study by Kudhongania and Cordone (1974) revealed: (1) 24 fish species excluding the haplochromine cichlids and that there appeared a well-defined trend in number of species by depth with maximum species diversification occurring in the shallow inshore waters of the lake; (2) at least 80 percent of the ichthyomass was composed of haplochromines, and Lates niloticus contributed less than 0.1 percent, and (3) the mean catch rate of 797 kg per hour for waters between 0 and 30 m deep. The two reports of Bergstrand and Cordone (1971) and Kudhongania and Cordone, however, indicated an increasing trend in catches of haplochromines from 54.3 to 83.0 percent between 1968–70 and 1970–71 respectively, which was purely the result of the codend meshes used (Cordone and Kudhongania 1972).
All the 24 fish species encountered by Kudhongania and Cordone (op. cit) in the 1968–71 survey appeared again in the present study period except two, namely Gnathonamus longibarbis and Alestes sp. Okedi (1967) reported the presence of G. longibarbis in the shallow inshore waters over muddy bottoms adjacent to extensive papyrus swamps and also in deep water (30–50 m) near rocky islands. Gee and Gilbert (1967) recorded Alestes jacksoni and A. sadleri while surface trawling in the Jinja area of Lake Victoria. Alestes sp. were also occasionally recorded in Napoleon Gulf, after poisoning, between January and March 1962 (Hamblyn, 1966). The absence of records for some of the fish species in the commercial catch may be contributed by: (1) lack of suitable gear for some species like Mastacembelus frenatus and Xenoclarias spp.; (2) retention for consumption by the fishermen of some species, especially Synodontis sp., Alestes sp. and Labeo sp., and (3) lack of market for certain species in fresh form and thus only their processed products were marketed and were not included in the data used for this report. The processed fish landed at Masese include Rastrineobola argentea, Bagrus docmac, and Haplochromis. The results in this study regarding species distribution by depth indicated that there has been no change since the 1968–71 study by Kudhongania and Cordone (op. cit.).
There has been a decline in catch rates for all the fish species combined from 797 kg per hour in 1968–71 to 595, 363 and 355 kg per hour in 1981, 1982 and 1983 respectively. The haplochromine group which still continued to dominate the trawl catches, contributing on average 85 percent of the total catch by weight, showed a decline from 91.4 to 76.2 percent (or 543.3 to 270.8 kg/hr) in 1981 and 1983 respectively. However, Lates niloticus which was insignificant in 1968–71 (at 1 kg/hr) has been increasing, and contributed 5, 42, and 58 kg per hour in the trawl catches in 1981, 1982 and 1983 respectively, and a similar pattern is reflected in the commercial catches where it constituted 13, 75 and 68 percent of the catch landed in 1981, 1982 and 1983 respectively. Although Oreochromis niloticus catch rate increased from 3.4 to 13.6 kg per hour between 1971 and 1981, it subsequently declined to 5.0 kg per hour by 1983. The increased trend shown in 1981 and subsequent decline is also reflected in the commercial catches. The catch rates of O. esculentus, P. aethiopicus and C. mossambicus have drastically and continuously declined over the period. On the other hand, the catch rate of B. docmac which had drastically declined from a high of 33.3 kg per hour in 1968–71 to a low of 4.1 kg per hour in 1981, showed subsequent progressive increases. Thus on the basis of the catch rates, one is inclined to conclude that the formerly pre-dominant haplochromine cichlids are decreasing while Lates niloticus which was then insignificant is now the dominant fish. Seasonal patterns were visible in trawl catches which was not the case in the inconsistantly recorded commercial catches.
The average size (kg) in the trawl catches for the individuals of O. niloticus, B. docmac, P. aethiopicus and L. niloticus increased over the period. In the commercial catches these species declined in average size over the period except for Lates niloticus whose average size fluctuated. In the case of O. niloticus the decline may be due to economic factors forcing fishermen to continuously change to cheaper and smaller mesh gillnets and beach seines. However, in 1981 L. niloticus appears to have been harvested in the tilapia gear which may have been replaced with much larger meshed gillnets in 1982 designed specifically for it, thus the increase in average size. But the apparent combined use of this gear and beach seines may have caused the fall in average size of L. niloticus caught in 1983.
ACKNOWLEDGEMENTS
This study was possible through the cooperation of R.V. IBIS's crew and UFFRO scientific staff on Lake Victoria. We are grateful to the Fisheries Department staff in Jinja for their cooperation and to Dr. T. Twongo of UFFRO for the useful suggestions. We wish to thank the Director and staff of UFFRO for contributing in various ways to this study. Miss H. Nakiridde typed the manuscript.
REFERENCES
Bergstrand, E. and A.J. Cordone, 1971. Exploratory bottom trawling in Lake Victoria. Afr.J.Trop.Hydrobiol.Fish., 1(1):13–23
Cordone, A.J. and A.W. Kudhongania, 1972. Observations on the influence of codend mesh size on bottom trawl catches in Lake Victoria with emphasis on the Haplochromis population. Afr.J.Trop. Hydrobiol.Fish., 2(1):1–19
Gee, J.M. and M.P. Gilbert, 1967. Experimental trawling operations on Lake Victoria. Annu.Rep.E.Afr.Freshwat.Fish.Res.Org., (1966): 33–46
Hamblyn, E.L. 1966. A note on the inshore fish population of Napoleon Gulf (Lake Victoria). Annu.Rep.E.Afr.Freshwat.Fish.Res.Org., (1965):23–35
Kudhongania, A.W. and A.J. Cordone, 1974. Bathospatial distribution patterns and biomass estimate of the major demersal fishes in Lake Victoria. Afr.J.Trop.Hydrobiol.Fish., 3(1):15–32
Okedi, J.Y.O. 1967. The biology of some mormyrid fishes in Lake Victoria basin. Thesis submitted for the Degree of Doctor of Philosophy at Makerere University College, University of East Africa
Table 1: Percentage frequency of occurrence of fishes caught during trawling in the northern part of the Uganda waters of Lake Victoria during 1981 to 1983.
| Fish species | Napoleon Gulf 6–24 m | Buvuma Channel 10–27 m | Lingira Bay 3–12 m |
| Haplochromis spp. | 100.0 | 100.0 | 100.0 |
| Sarotherodon esculentus | 11.7 | 0.7 | 10.9 |
| S. variabilis | 54.7 | 10.6 | 59.2 |
| S. niloticus | 80.3 | 12.0 | 74.5 |
| S. leucostictus | - | - | 2.2 |
| Tilapia zillii | 6.6 | - | 22.3 |
| Bagrus docmac | 92.7 | 93.0 | 71.2 |
| Clarias mossambicus | 29.9 | 64.8 | 58.15 |
| Xenoclarias spp. | 1.5 | 16.9 | 16.3 |
| Protopterus aethiopicus | 13.1 | 12.7 | 32.6 |
| Lates niloticus | 78.1 | 72.5 | 68.5 |
| Synodontis victoriae | 2.9 | 45.1 | 22.8 |
| S. afrofischeri | 4.4 | 6.3 | 3.8 |
| Barbus altianalis | 81.0 | 4.2 | 11.4 |
| Labeo victorianus | - | 0.7 | 1.1 |
| Mormyrus kannume | 11.0 | 1.4 | 2.7 |
| Schilbe mystus | 7.3 | 7.3 | 2.2 |
| Engraulicypris spp. | 14.1 | 6.5 | 12.6 |
| Barbus spp. | 5.7 | 1.1 | 3.4 |
| Astatoreochromis spp. | 0.0 | - | 0.0 |
| Mastacembelus frenatus | 0.0 | 0.0 | 0.0 |
Table 2: Percentage catch composition (by weight) of fish caught in the trawl in the Northern portion of Lake Victoria (U) during 1981 to 1983
| Species | 1981 | 1982 | 1983 | 1981–1983 | ||||||||
| Napoleon Gulf | Buvuma Chan. | Lingira Bay | Napoleon Gulf | Buvuma Chan. | Lingira Bay | Napoleon Gulf | Buvuma Chan. | Lingira Bay | Napoleon Gulf | Buvuma Chan. | Lingira Bay | |
| Haplochromis spp. | 1.82 | 28.39 | 62.09 | 16.32 | 51.15 | 16.83 | 7.29 | 16.04 | 57.52 | 7.46 | 29.37 | 49.01 |
| Sarotherodon esculentus | - | - | 0.02 | 0.01 | - | 0.00 | 0.00 | - | 0.00 | 0.00 | 0.00 | 0.01 |
| O. variabilis | 0.00 | 0.00 | 1.42 | 0.21 | 0.01 | 0.33 | 0.09 | 0.02 | 0.09 | 0.23 | 0.01 | 0.72 |
| O. niloticus | 0.00 | 0.02 | 1.40 | 0.96 | 0.01 | 0.85 | 0.57 | 0.08 | 0.89 | 0.45 | 0.04 | 1.08 |
| O. leucostictus | - | - | 0.02 | 0.01 | - | 0.00 | - | - | 0.00 | 0.00 | - | 0.01 |
| Tilapia zillii | - | - | - | - | - | - | - | - | - | - | - | - |
| Bagrus docmac | 0.10 | 0.25 | 0.30 | 0.79 | 0.93 | 0.38 | 1.05 | 0.86 | 0.97 | 0.62 | 0.64 | 0.56 |
| Clarias mossambicus | 0.01 | 0.88 | 1.78 | 0.49 | 0.51 | 0.47 | 0.16 | 0.38 | 0.69 | 0.19 | 0.61 | 1.05 |
| Protopterus aethiopicus | 0.00 | 0.02 | 0.37 | 0.03 | 0.03 | 0.22 | 0.15 | 0.05 | 0.30 | 0.06 | 0.03 | 0.31 |
| Lates niloticus | 0.00 | 0.13 | 0.79 | 5.28 | 1.72 | 1.39 | 6.59 | 2.42 | 2.92 | 3.71 | 1.36 | 1.71 |
| Synodontis victoriae | - | 0.09 | 0.04 | 0.01 | 0.07 | 0.00 | 0.00 | 0.06 | 0.04 | 0.00 | 0.08 | 0.03 |
| S. afrofischeri | - | - | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Other spp. | 0.03 | 0.00 | 0.01 | 0.48 | 0.00 | 0.00 | 0.60 | 0.00 | 0.00 | 0.35 | 0.00 | 0.00 |
| Total | 1.97 | 29.79 | 68.24 | 24.58 | 54.44 | 20.98 | 16.51 | 19.92 | 63.57 | 12.92 | 32.46 | 54.62 |
| 100.00 | 100.00 | 100.00 | 100.00 | |||||||||
| Total weight (kg) | 1740.9 | 26388.4 | 60448.5 | 14218.0 | 31483.7 | 12135.0 | 13620.2 | 16429.7 | 52429.9 | 29579.1 | 74301.8 | 12501.0 |
| 88577.8 | 57836.7 | 82479.8 | 228894.3 | |||||||||
Table 3: Catch rates on monthly basis in the northern part of the Uganda waters of Lake Victoria between 1981 and 1983
| Period (month) | Trawl catches (kg/h) | Commercial catches (kg/day) | ||||
| 1981 | 1982 | 1983 | 1981 | 1982 | 1983 | |
| January | - | 775.1 | 231.4 | - | 769.4 | 2514.5 |
| February | - | - | 253.2 | - | 885.3 | 2014.3 |
| March | - | 294.4 | 397.8 | 546.5 | 817.9 | 2014.8 |
| April | - | 231.3 | 427.7 | 613.0 | 1330.7 | 5928.8 |
| May | - | 418.7 | 178.0 | 614.7 | 1568.3 | 5520.5 |
| June | 228.8 | 618.5 | 277.0 | 820.5 | 971.9 | 5380.8 |
| July | - | 381.7 | 252.9 | 937.4 | 1226.4 | 1211.2 |
| August | 371.3 | 179.6 | 168.5 | 899.0 | 6538.4 | 1335.0 |
| September | 586.3 | 180.6 | 624.6 | 1079.0 | 6331.8 | 1766.8 |
| October | 625.9 | 402.5 | 1033.6 | 977.9 | 7179.1 | 834.3 |
| November | 650.1 | 348.9 | 781.7 | 1212.1 | 7241.8 | 2095.9 |
| December | 747.1 | - | 202.7 | 607.0 | 3958.0 | 1059.6 |
| Annual mean | 613.1 | 363.3 | 361.7 | 832.9 | 2290.8 | 2491.1 |
Table 4: Trawl mean catch rates (Kg/hr) of the various fishes in the northern part of the Uganda waters of Lake Victoria
| Species | 1968–71 | 1981 | 1982 | 1983 |
| 510 hauls ca. 510 hrs | 127 hauls 144.5 hrs | 191 hauls 223.4 hrs | 263 hauls 269.5 hrs | |
| Haplochromis spp. | 668.20 | 543.30 | 294.34 | 270.84 |
| Sarotherodon esculentus | 29.79 | 0.15 | 0.04 | 0.01 |
| O. variabilis | 1.04 | 8.70 | 1.97 | 1.07 |
| O. niloticus | 3.36 | 13.60 | 6.56 | 5.03 |
| O. leucostictus | 0.18 | 0.11 | 0.02 | 0.01 |
| Tilapia zillii | - | - | - | - |
| Bagrus docmac | 33.26 | 4.09 | 8.37 | 11.24 |
| Clarias mossambicus | 32.60 | 15.07 | 7.16 | 4.32 |
| Protopterus aethiopicus | 22.08 | 2.66 | 1.09 | 2.23 |
| Lates niloticus | 0.96 | 5.02 | 42.08 | 57.47 |
| Synodontis victoriae | 4.77 | 0.91 | 0.27 | 0.35 |
| S. afrofischeri | 0.10 | 0.01 | 0.00 | 0.01 |
| Other species | 2.56 | 0.32 | 1.40 | 2.69 |
| Total | 796.72 | 594.94 | 363.30 | 355.28 |
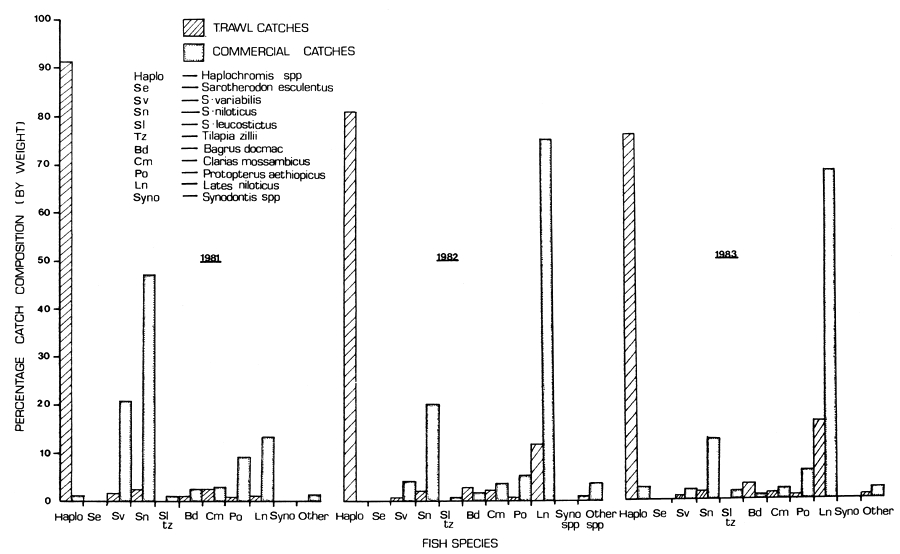
Figure 1 Percentage species composition (by weight) of various species in trawl and commercial gillnet catches for the northern part of the Uganda sector of Lake Victoria for the period 1981–1983 (S. esculentus = O. esculentus; S. variabilis = O. variabilis; S. niloticus = O. niloticus; and S. leucostictus = O. leucostictus)
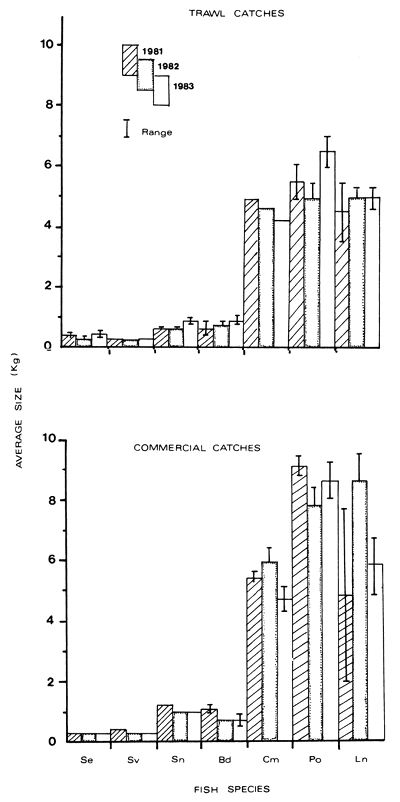
Figure 2 The average size (kg) of various fish species in trawl and commercial gillnet catches in the northern part of the Uganda sector of Lake Victoria