by
T.O. Acere
Uganda Freshwater Fisheries Research Organization
Jinja, Uganda
| Abstract |
| The age, growth, size at first maturity and sexuality of Lates niloticus in the northern part of Lake Victoria, within the hinterland of Jinja, have been studied between 1960 and 1984. The first officially recorded stocking of the Lake was in May 1962 with 35 Nile perch off Entebbe Pier. However, in May 1960 a perch was first caught at Bugungu just above the Ripon Falls, and another in Waigala Hannington Bay in November of the same year. Nile perch grow very fast attaining a length of 52 cm TL (mean length of 36.38 cm TL) by the end of the first year of the cohort. Growth rate gradually decreased between year 2 and 5 and then became almost linear up to the observed length of 190 cm TL at age 13+. The maximum or asymptotic mean length is 256.67 cm TL, the growth rate k is 0.09 and the hypothetical time to is between -1 and -1.09 years. |
| Lates niloticus exhibit sexual dimorphism and unequal sex ratio. The males dominate up to 120 cm TL and thereafter females become more populous than males. The smallest sexually mature individuals observed were 53.5 cm TL male (age 1+) and 67.5 cm TL female (age 1+). By age 2 (>70 cm TL) all the Nile perch examined were sexually mature. Older and larger females had significantly larger ovaries. The gonad maturity stages in samples examined varied among size classes, age groups, years sampled and from one time interval to another. |
| Studies on survival rates, mortality rates, food and growth of the fishery of Lates niloticus in the northern part of Lake Victoria have been undertaken since 1960. Survival rate during the first four years of the cohort was 2.0037 which subsequently varied from year to year (0.0269-0.504) when the population's age increased. During the early part of the first year Nile perch feed on, among other things, prawns, Caridina, fish fry and small gastropods and bivalves, and later change to prey predomimantly on haplochromine cichlids, Rastrineobola (Engraulicypris) in proportions to their abundance. They occasionally feed on Xenoclarias, Mastacembelus, Barbus spp, gastropods and bivalves. By 1984 the species was contributing over 50 percent of the total commercial tonnage while in the experimental trawling catch it constituted 68 and 16 percent of the table fish bulk and total catch respectively. Nile perch are expanding rapidly at the expense of some of the indigenous or endemic species. |
INTRODUCTION
The survival rates, mortality rates, food and growth of the fishery of Nile perch in the northern part of Lake Victoria are based on data collected as far back as 1959.
The growth rate, age at first maturity and sexuality of Nile perch (Lates niloticus) in the northern part of Lake Victoria were studied between 1960–71, 1974–77 and 1981–84. The data for estimating these parameters were collected during the monitoring of Nile perch catches in commercial gear and experimental trawling for stock assessment. The first recorded catch of Nile perch was at Bugungu just above the Ripon Falls in May 1960 and another in Waigala Hannington Bay in November of the same year (Gee, 1964). Up to December 1960 eight Nile perch had been caught by commercial fishermen, the last of which was caught in North Buvuma channel and the rest above the Owen Falls dam (Van Somerene, 1960).
Nile perch, however, were for the first time officially introduced into Lake Victoria in May 1962 when 35 fish between 16 and 43.5 cm TL were stocked off Entebbe Pier in May 1962 amid unsettled controversy for and against it (Anderson 1961, Fryer 1960, Hamblyn 1960, 1960a, and 1962, Simpson 1960, Stoneman 1960, Beverton 1959, Corbet 1959, Worthington 1932 and Graham 1929). In September 1963 the Lake was stocked with a further 339 fish. The purpose of the introduction of Nile perch to Lake Victoria was to utilize Haplochromis which were otherwise of little direct commercial importance.
Previous studies on Lates niloticus in various lakes, dams and ponds in different areas have been mostly on descriptive biology. There have been others which demonstrated great variability in growth, size at age and maturity among the different populations (Kenchington 1933, Hamblyn 1960, 1962, 1966, Reidel 1962, Hopson 1964 and 1972, Gee 1964 and 1965 and Coulter 1976). The purpose of the present study is to determine parameters on mortality, age, growth and size at first maturity of Nile perch toward the establishment of a management strategy for its fishery in Lake Victoria.
It was conceived that Nile perch would utilize the vast quantities of haplochromines which were occurring in the Lake but then considered too small in individual size to merit commercial exploitation on any but subsidiary scale. Stocking with Nile perch was considered a re-introduction rather than an introduction, for fossils of Lates had been found in Miocene deposits on Rusinga Island showing that this genus occurred in Lake Victoria at this time.
Knowledge of the vital statistics of a fish species is an essential tool in its management, development and exploitation. Since its re-introduction, into the Lake, Lates niloticus has spread throughout the Lake. The purpose of this paper, therefore, is to provide some parameters such as mortality rates, survival rates and food of Nile perch, and to describe the status of its fishery in the northern part of Lake Victoria. Mortality rates, combined with age and mean rates of growth in length and weight are the necessary ingredients in the computation of yield, for purposes of rational exploitation of the species.
METHODS AND MATERIALS
The data was obtained from commercial gillnets (20–250 mm stretched mesh), beach seines, longlines and experimental trawling all of which were operated in Napoleon Gulf, Thruston Bay, Buvuma Channel, Ingira Bay, Hannington Bay, Itome Bay and Lufu fishing grounds. Commercial catches were recorded at Masese Fish Landing Jinja between 28 October 1964 and 9 December 1977. In the late 1960s trawl data was obtained using the research vessel R.V. ‘IBIS’ which is 17 m long and is fully rigged for a variety of fishing gear. It was equipped with two electronic echosounders for recording both depth and biological targets within the water column and had a 2-ton capacity hydraulic winch for deploying and retrieving the gear. Different codends of varied mesh sizes (83, 76, 64, 57, 51, 38 and 19 mm) were used (Bergstrand and Cordone, 1971).
Fish were measured to the nearest millimetre total length (TL) from the tip of the snout to the end of the longest caudal fin ray and were weighed to the nearest 10 grammes. The abdomen was dissected to determine the sex and gonad maturity stage as well as stomach contents and fullness. Age composition was determined by plotting length frequency histograms for 9 200 fish at 1 cm class intervals. Age class boundaries were set by the method of Buchanan-Wollaston and Hodgson (1929), Harding (1949) and Cassie (1954) using probability paper. On the probability paper a normal curve becomes a straight line, thus separating polymodal length frequency data into its component groups or ages. Another graphic method used was to plot frequency polygons on semi-logarithmic paper which transformed the normal distribution to a parabola (Tanaka, 1962).
The von Bertalanffy (1933, 1938) growth equation was fitted to the data in 2 stages. The first operation involves plotting length differences, Lt+1 - Lt, between two consecutive ages, t and t+1 years, against length Lt at respective ages. This is similar to the Walford plot (Ford, 1933 and Walford, 1946). This regression has a slope (k - 1), its ordinate intercept is equal to L∞ (1 - k) and its abscissal intercept is therefore L, which are the parameters for Ford (1933) and Walford (1946) equation:
| Lt + 1 = L∞ (1 - k) + k Lt | (1.1) |
where k = Ford's growth coefficient, L∞ is the mean asymptotic length and Lt = length at time (age) t.
The second operation is the regression of ln (L∞ - Lt) against t, which provides a slope of the natural log line k and also the value to since the Y - axis intercept is equal to ln L∞ + Kto. These are the parameters required in the generalised von Bertalanffy growth equation:
| Lt = L∞ [l - e-k (t-to)] | (1.2) |
where Lt, L∞ and t are as before, K is the Brody (1927, 1945) growth coefficient determining the rate of increase or decrease in length increments, and to is the hypothetical time when the fish would have been zero length if it had always grown in the manner described by this equation.
The sex ratio was determined using 1 950 fish which were also used for gonad maturity stages. The data was gathered from commercial and trawl catches between 1969–77 and 1982–83. Females were considered mature if the ovaries were either cream-yellow, firm to the touch with full appearance and contained well-developed eggs for the ready stage, or were yellow-orange, soft to the touch with well-developed eggs for the ripe stage, or were generally large and pink containing dark-red contents for the spent or recycling stage (Hamblyn, 1962). Males were considered mature if the testes were thickened and contained milt, which in the running gonad stage, was freely discharged on even slightly pressing the abdomen or on cutting and pressing the testes.
RESULTS
Growth
The growth of the Nile perch was found to be very fast attaining a mean total length of 52 cm during the first year, and showing four growth stanzas at 26, 36, 46 and 52 cm TL (Figure 2). Growth rate decreased during the 2nd, 3rd, 4th and 5th year with annual increments of 24.3, 23.02, 17.2, 18.72 and 9.84 cm TL and then remained almost constant (9 cm TL) for the rest of the cohort's life (Table 1, Figures 2, 3 and 4). The mean lengths at any given age as derived separately by the use of probability paper (Cassie, 1954) and the semi-logarithmic paper (Tanaka, 1962) were not significantly different even at 5 percent level by analysis of variance.
The longest fish observed measured 190 cm TL. The mean asymptotic or mean maximum length, L was 256.67 and 245.46, k was 0.913 and 0.9091, K was 0.0856 and 0.087 and to was equal to -0.9979 and -1.0865 years as estimated using mean lengths at ages provided by the probability paper and semi-logarithmic paper respectively. From the paired mean age lengths the Ford-Walford and the von Bertalanffy growth equations were fitted and took the forms respectively:
| Lt + 1 = 22.3194 + 0.913 Lt | (2.1) | |
| Lt + 1 = 22.3019 + 0.9091 Lt | (2.2) | |
| and | Lt = 256.67 [1 - 0.918(t+1)] | (3.1) |
| Lt = 245.46 [1 - 0.917(t+1.09)] | (3.2) |
Age Composition
Thirteen distinct age groups were distinguished by length frequencies plotted separately on probability paper and semi-logarithmic paper (Figures 2 and 3; tables 1 and 2). The probability paper groups were represented by straight lines. The first line between 0 and 52 cm were treated as one age group displaying four growth stanzas on account of the mode being at 33.5 cm TL class. On the semi-logarithmic paper 13 parabolas were portrayed with distinct modes. The age boundaries were set as indicated in Table 1 and Figures 2 and 3.
Maturity and Sexuality
Lates niloticus is sexually dimorphic. The male has only anal and urinogenital openings just anterior to the anal fin, whereas the female has a genital orifice separate from the urinary opening. The sex ratio of females to males among the 1 950 fish examined was on average 1 to 0.77. However, up to 120 cm TL males were, on average, more than females with a ratio of 1 female to 2.35 males, but thereafter the reverse was the case with 11 females to one male (Table 4).
The smallest sexually mature individuals observed were a 53.5-cm male (ages 1+ or year 2) and a 67.5-cm female (age 1+ or year 2). By the age 2+ (>70 cm TL) all individuals examined were sexually mature. The gonad maturity stages for individuals in age class 1+ to 13 and size classes between 70 and 190 cm TL varied from sample to sample, place to place and period to period. All stages of gonad maturity were observed at any one time in a sample.
Survival Rate
The fish were grouped into 13 years (Table 1). Estimates of survival rate were low between 1964 and 1969 ranging from 0.0037 to 0.2479 by the best estimate and 0.0037 to 0.2686 by Heinke's method. From 1969/70 to 1976/77 there was increased survival rate (average 0.5) during the successive years with a slight drop in 1976/77 when it was 0.4189 (Table 5). Survival rates during the first and second years were very low S = 0.063 using the graphic method (Figure 4).
Mortality Rate
Annual mortality rate was very high in the first years of life. The estimated annual mortality rate for age 1 was 0.9963 with an instantaneous rate of 5.5993. The high mortality continued from 1964/65 to 1968/69 when survival rates were very low. After 1968/69 annual mortality rates more or less equalled survival rate at 0.5 (Table 2). Similarly the instantaneous rate of mortality stabilized at a value of 0.69.
Food
Lates niloticus when a fingerling feeds on prawns (Caridina), fish fry, small gastropods and bivalves. Later after moving into deeper water haplochromine cichlids constitute over 95 percent of the food consumed. Every Nile perch stomach opened was either empty or contained haplochromines. A Nile perch, 92 cm TL, had eaten up to 60 haplochromines and 2 Rastrineobola sp. A perch, 159.2 cm TL, had in its stomach 1 Xenoclarias (20 cm TL), 37 digested haplochromines and 11 fresh haplochromines. Among the occasional items in the diet of Nile perch are included small Barbus spp., Xenoclarias, sand grains, gastropods and bivalves. Rastrineobola is common in the diet in terms of occurrence, and is second to haplochromines.
Parasites
Nile perch have been observed to be infested with Lernea in the region after the operculum. In the gills have been found argulids. Nematodes have occurred on the body.
Fishery
Nile perch catches both in the commercial gillnets, beach seines and longlines and in experimental trawling have been on the increase since 1960 when 8 fish (weights <1.5 kg) were caught near Jinja. Today it is contributing over 50 percent of the total commercial tonnage (Table 3). Lates niloticus is second only to haplochromine species contributing 6.8 and 85.7 percent of the trawl catch weight respectively. The mean size has also increased from 1.5 kg in 1960 to 16.63 kg in 1977. Fish weighing over 100 kg are now a common sight. Table 3 gives data taken as sample records at Masese Fish Landing based on a small fraction of canoes, about 5 canoes per day out of a total active fishing fleet of over 40 canoes. There are two peaks in the catches of Nile perch, one is in January to March and the other in November to December though in some years it has extended from July to December (Table 3).
DISCUSSION AND CONCLUSION
Growth is variable among the populations of Lates described in the literature. Hopson (1972) found that Lates niloticus in Lake Chad attained a length of 51.3 cm during the third year. Coulter (1976) reports Lates marie in Lake Tanganyika as attaining a length of 51.3 cm in the third year of life. In the 4-acre Dam at Sagama Kenya, Lates niloticus of 1 cm grew to 13.5 cm between September and November 1961, which amounts to attaining a length of 50 cm in the first year (Midgley, 1968). Hamblyn (1966) suggested that in the Luwala Sugar Estate Dam, Jinja, the parent stock of Lates niloticus introduced to the Dam during October 1959 could have achieved a length of 80–100 cm by February 1962 at age 2+. Moore (1979) reports that in Papua New Guinea, a female Lates calcarifer 42 cm TL and 73 cm was a 2 year old and 5 year old respectively. In the present study, in Lake Victoria Lates niloticus attained a length of 52 cm during the first year of life.
The growth of Lates niloticus becomes almost linear after age 3+. This phenomenon is reported by Ricker (1958) who notes that arithmetic growth occurs in many long-lived freshwater and marine fishes in cool temperate to sub-arctic waters. The values of 93.07 cm L∞ (asymtotic length) for Lates niloticus in Lake Chad (Hopson, 1972), 95.02 cm L∞ for Lates niloticus in Lake Albert (Holden, 1963) and 72 cm L∞ for L. marie in Lake Tanganyika (Coulter, 1976) are rather unrealistic because they are less than the observed maximum lengths for the respective lakes. Their common error seems to arise from dealing with only the data up to the first inflection point, demarcating the first stanza of free life. This provided them with the regression line that immediately and naturally forced only a small portion of their data to fit into one of the many growth models.
Schnute (1981) summarized a list of growth models:
generalized von Bertalanffy growth
(1) Y(t) = y∞ (l - e-9(t - to))p,
Richards growth
(2) Y(t) = y∞ (l - e-g(t - to))p,
Gompertz growth
(3) Y(t) = y∞ e-e-g(t - to),
logistic growth (Richards growth with p = 1)
(4) Y(t) = y∞ (l - e-g(t - to))-1,
and linear growth
(5) Y(t) = g(t - to),
where Y(t) represents fish size at age t, and y , g, p and to are parameters, usually with y∞>0, g>0, and p>0. He notes that in (1) and (5) the parameter to represents a time when the growth curve crosses the t-axis. By contrast in (2)–(4) to corresponds to an inflection point on the curve. Indeed the curves (3) and (4) never cross the t-axis and neither does (2) if p:0. Furthermore, if g 0, then y∞ in (1)–(4) is a theoretical limiting size. Schnute asks why should or should not axis crossings and inflection points be important and why should a limiting size be important or well defined. In addition, models (1) and (2) have four parameters, while (3) and (4) have three parameters, and (5) has only two. So the question arises as to what is to be considered a reasonable number of parameters to be used, which is the dilemma invariably faced by the practitioner. This dilemma is solved by a proposed new comprehensive model which includes (1)–(5) as special cases and many other models as well. In the new model the apparent problem of comparing parameters in models (1)–(5) disappear. Axis crossing, inflection points and asymptotic size no longer play an essential or dominant role, although they can be identified easily if they occur. Finally it illustrates how a data set can suggest Gompertz growth (3) as a limiting version of von Bertalanffy growth (1). In the present study the von Bertalanffy growth model (1) with p = 1 described very closely the growth of Lates niloticus without need for resorting to the Schnute (1981) model. It should be borne in mind that in a real fisheries study for management aspect, like this one, it would not really be philosophically valid to try several statistical analyses on the same data. One should instead pick the best for the situation in advance and stick to it, so long as it meets the basic assumptions.
The first year of Lates niloticus in Lake Victoria is marked by growth stanzas with inflection points at 25, 36, 46 and 52 cm TL. These may be correlated with change in habitat and feeding habit. Gee (1964) reported that young fish, most under 20 cm SL (which is approximately less than 25 cm TL), appeared to inhabit Ceratophyllum zone of the Lake shore, and after attaining this size they moved outside the weedy-bed.
From the age results 10-year-old Lates niloticus appearing in 1967/68 population, must belong to the 1957/58 year class. Nile perch were introduced to Luwala Sugar Estate Dam near Jinja on 16 October 1959. Since this Dam drains into Lake Victoria at Nyenga, some of these fish could have escaped into the Lake (Gee, 1964; Hamblyn, 1966). Lates niloticus seems to have got access to Lake Victoria between 1957 and 1960 when it belonged to at least 1957/58 year class. This could have happened at the time of stocking of the Nile below the Owen Falls Dam, when some adventurous official put some fish above the Dam, resulting in the first catch being taken above the dam by December 1960 (Van Somerene, 1960). The other possibility and in addition to the above could be the escape of Nile perch from Luwala Sugar Estate Dam.
Lates has been found to display sexual dimorphism. This phenomenon is also extended to Protopterus aethiopicus whose cloacal opening is on the left for female and on the right for male. The drastic change in sex-ratio toward more females at the size greater than 120 cm TL (age 7+) may be attributed to one or several reasons: (1) congregation of members of one sex in one secluded area after segregating from the opposite sex, (2) selective mortality for males after that size, and (3) sex inversion by some males to females. Lates calcarifer in the Northern Territory and south eastern Gulf of Carpentaria exhibits sexual inversion when some males change to females (Moore, 1979; Davis, 1982).
The high mortality rate of between 0.937 and 0.996 during the first and second years of life may be attributed to the small mesh gillnets below 125 mm, which were in use in the early part of Nile perch fishery in the 1960s. In the 1970s there was a shift in the gear in favour of larger meshed gillnets that were more profitable in terms of catch per net. It is now apparent that the exploitation of this new fishery has stabilized with a total annual mortality of 0.5. The fishery is attacking Lates at all ages with full recruitment being achieved in the first year of a cohort. The estimates of survival rates provided by the method of Heinke (1913) for the years 1968–69 to 1976–77 are excessively high as, in a situation where both fishing and natural mortalities are operating, it is absurd to talk of a survival rate equal to 1.0. The method of Robson and Chapman (1961) provides more reliable estimates than those obtained using the model of Heinke (1913). Estimates similar to those of Robson and Chapman (1961) are also obtained using the estimators of Jackson (1939), Rounsefell and Everhart (1953), Beverton and Holt (1957), as demonstrated by Manooch and Huntsman (1977) on the porgy (Pagrus pagrus).
Nile perch is doing exactly what it was introduced to perform in Lake Victoria. It is utilizing the vast quantities of Haplochromis occurring in the Lake, estimated by Kudhongania and Cordone (1974) as having a biomass of approximately 600 000 metric tons, but formerly considered of little commercial value. It is not preying on the highly prized table fish such as Bagrus, Clarias, Oreochromis esculentus, etc. The decline of Oreochromis esculentus may be caused by O. niloticus which has also increased in number and average size and probably has out-competed the former. Oreochromis niloticus has been so successful in its population increase that it is now ranked third in commercial catches. I am inclined to believe that Nile perch population will continue to increase but eventually will stabilize just as it did in the other lakes, Rudolf, Albert, Chad, Tanganyika etc., where it is endemic. However, as the main prey of Nile perch, haplochromine cichlids will continue to decline beyond a level at which commercial trawling for fish meal will be a paying venture. The Rastrineola argentea fishery stands threatened. These two genera tend to school thus attracting attention from Nile perch.
Hamblyn (1960a) reported that Nile perch would not take dead prey objects in still water but might be induced to do so by animating the object artificially. In Lake Albert the prawn Caridina forms a most important element in the food of Lates. Caridina is an abundant element of the benthos in Lake Albert where they occur in great numbers between the bottom and 27 m level (Hamblyn, 1962). Gee (1964) reported that in Lake Victoria haplochromines formed the main basis of the food of Nile perch, being found in over half the fish with any stomach contents while Alestes, Barbus and Rastrineobola also made a significant contribution. It is then apparent that Nile perch goes for the most abundant food item. The absence of Alestes in the stomachs examined in the 1980s may be an indicator of the disappearance of this genus in this part of the Lake, and yet it was relatively abundant in the 1960s.
RECOMMENDATIONS
1. The use of length data for aging has great potential for stock assessment in the tropics (Morgan, 1983; Ssentongo, 1972). This is more so because rings appearing in scales and hard skeletal structures of tropical fish are not necessarily associated with age. However, in young fish these rings have been associated with daily growth. Haplochromine cichlids of up to 180 days old have been found to have equivalent daily rings (Basasibwaki pers. comm.).
2. Data collection at fish landing should be diligently and accurately recorded. These should among others include length and weight of sample individual fish. This cry was raised in 1959 (EAFFRO, 1959) but to no avail or with only partial success.
3. Since Lates niloticus has spread throughout Lake Victoria it is imperative to evolve management regulations bearing in mind that by age 2+ (>70 cm TL) the fish have spawned or are almost doing so for the first time.
ACKNOWLEDGEMENTS
I wish to thank the Laboratory Assistants particularly Messrs Moini and Mugerenge for data recorded at Masese Fish Landing and Drs. J.M. Gee and J.O. Okedi and M. E.L. Hamblyn who pioneered this early exercise of monitoring Nile perch and its spread. I am greatly indebted to my colleagues in UFFRO Lake Victoria Research team in persons of Messrs D.L. Ocenodongo, J.O. Okaronon, J. Kamanyi, Miss G. Namulemo, the supporting staff, among whom mention must again be made of Mr. Moini, and the crew of R.V. IBIS who despite the very difficult conditions have continued to provide me with data on Nile perch and other species. I wish to extend my appreciation to Mr. S.N. Sowobi; Miss I. Kampire; and Miss H. Nakiridde for assisting in various ways.
My sincere thanks also go to the staff of the Uganda Fisheries Department and Uganda Freshwater Fisheries Research Organisation who collected and availed me the data for Masese Fish Landing. I wish to thank Miss Florence Kakayi for typing the manuscript.
REFERENCES
Anderson, A.M., 1961. Further observations concerning the proposed introduction of Nile perch into Lake Victoria. E.Afr.Agric.For.J., 26(4): 195–201
Bergstrand, E. and A.J. Cordone, 1971. Exploratory bottom trawling in Lake Victoria. Afr.J.Trop.Hydrobiol.Fish., 1(1): 13–23
Beverton, R.J.H., 1959. Report on the state of the Lake Victoria fisheries. Lowestoft, England, Ministry of Agriculture, Fisheries and Food, Fisheries Laboratory (mimeo)
Beverton, R.J.H. and S.J. Holt, 1957. On the dynamics of exploited fish populations. Fish.Invest.Minist.Agric.Fish.Food G.B. (2 Sea Fish.), (19): 533 p.
Brody, S., 1927. Growth rates. Bull.Univ.Missouri Agric.Exp.Stn., (97)
Brody, S., 1945. Bioenergetics and growth. New York, Reinhold, 1023 pp.
Buchanan-Wollaston, N.J. and J.W. Hodgson, 1929. A new method of treating frequency curves in fishery statistics with some results. J.Cons.CIEM, 4:207–25
Cassie, R.M., 1954. Some uses of probability paper in the analysis of size frequency distributions. Aust.J.Mar.Freshwat.Res., 5: 513–22
Corbet, P.S., 1959. The food of the non-cichlid fish in Lake Victoria. Annu.Rep.E.Afr.Fish.Res.Org., (1958): 21–6
Coulter, G.W., 1976. The biology of Lates species (Nile perch) in Lake Tanganyika, and the status of the pelagic fishery for Lates species and Luciolates stappersii (Blgr) J.Fish.Biol., 9:235–59
Davis, T.L.O., 1982. Maturity and sexuality in barramundi, Lates calcarifer (Bloch) in the Northern Territory and south-eastern Gulf of Carpentaria. Aust.J.Mar.Freshwat.Res., 33(3): 529–45
EAFFRO, 1959. A note on the value of determining the length frequency distribution of fish caught commercially in gill nets. Annu.Rep. E.Afr.Fish.Res.Org., (1959): 15–7
Ford, E., 1933. An account of the herring investigations conducted at Plymouth during the years 1924–1933. J.Mar.Biol.Assoc.U.K., 19:305–84
Fryer, G., 1960. Concerning the proposed introductions of Nile perch into Lake Victoria. E.Afr.Agric.For.J., 25(4): 267–70
Gee, J.M., 1964. Nile perch investigations Annu.Rep.E.Afr.Freshwat.Fish. Res.Org., (1962–63): 14–24
Gee, J.M., 1965. The spread of Nile perch, Lates niloticus in East Africa, with comparative biological notes. J.Appl.Ecol., 2(27): 407–8
Graham, M., 1929. A report on the fishing survey of Lake Victoria, 1927–28. London, Waterlaw and Sons Ltd., 255 pp.
Hamblyn, E.L., 1960. The Nile perch project. Annu.Rep.E.Afr.Freshwat. Fish.Res.Org., (1960): 26–32
Hamblyn, E.L., 1960a. The food and feeding behaviour of Nile perch. Annu.Rep.E.Afr.Freshwat.Fish.Res.Org., (1960): 33–4
Hamblyn, E.L., 1962. Nile perch investigation. Annu.Rep.E.Afr.Freshwat. Fish.Res.Org., (1961): 23–8
Hamblyn, E.L., 1966. Some notes on the Nile perch, Lates niloticus in the role of predator in fish-farm ponds. Annu.Rep.E.Afr.Freshwat. Fish.Res.Org., (1965): 14–7
Harding, J.P., 1949. The use of probability paper for the graphical analysis of polymodal frequency distributions. J.Mar.Biol.Assoc. U.K., 28: 141–53
Heinke, F., 1913. Investigations on plaice. General report. First part. Rapp.Cons.CIEM, (17A): 1–153
Holden, M.J., 1963. Report on the fisheries of Lake Albert. Report to Uganda Government (mimeo)
Hopson, A.J., 1964. Federal Fisheries Service Lake Chad Research Station, Malamfatori, Report for 1963. Rep.Fed.Fish.Serv.Lake Chad Res. Stn.,Malamfatori, (1963): 34 p.
Hopson, A.J., 1972. A study of the Nile perch, (Lates niloticus (L.) Pisces: Centropomidae) in Lake Chad: Overseas Res.Publ., Lond., (19): 93 p.
Jackson, C.H.N., 1939. The analysis of an animal population. J.Anim. Ecol., 8:238–46
Kenchington, F.E., 1933. Observations on the Nile perch (Lates niloticus) at Senmar. Sudan Notes Rec., 16(1): 73–81
Kudhongania, A.W. and A.J. Cordone, 1974. Bathospatial distribution patterns and biomass estimates of the major demersal fishes in Lake Victoria. Afr.J.Trop.Hydrobiol.Fish., 3(1): 15–31
Manooch III, C.S. and G.R. Huntsman, 1977. Age growth and mortality of the red porgy, Pagrus pagrus. Trans.Am.Fish.Soc., 106(1): 26–33
Midgley, S.H., 1968. A study of Nile perch in Africa and consideration as to its suitability for Australian tropical inland waters. Fellowship Rep.Winston Churchill Memorial Trust, (3): 20 p.
Moore, R., 1979. Natural sex inversion in the giant perch (Lates calcarifer). Aust.J.Mar.Freshwat.Res., 30:803–13
Morgan, G.R., 1983. Application of length-based stock assessments to Kuwait's fish stocks. ICLARM Newsl., 6(4): 3–4
Reidel, D., 1962. A giant Nile perch from Lake Margherita (Abaya) Ethiopia. Arch.Hydrobiol., 58:339–42
Robson, D.S. and W.H. Chapman, 1961. Catch curves and mortality rates. Trans.Am.Fish.Soc., 90:181–9
Ricker, W.E., 1958. Handbook of computations for biological statistics of fish populations. Bull.Fish.Res.Board.Can., (119): 300 p.
Rounsefell, G.A. and W.H. Everhart, 1953. Fishery science: its methods and applications. New York, John Wiley and Sons, 444 p.
Schnute, J., 1981. A versatile model with statistically stable parameters. Can.J.Fish.Aquat.Sci., 38(9):1128–40
Simpson, C.J.H., 1960. Kajansi fish farm. Uganda Game and Fisheries Department. Unpublished department report
Ssentongo, G., 1972. Yield isopleths of Tilapia esculenta Graham 1928 in Lake Victoria and T. nilotica (Linnaeus) in Lake Albert. Afr. J.Trop.Hydrobiol.Fish., 2(2): 121–8
Stoneman, J.S., 1960. Studies on Nile perch. Uganda Game and Fisheries Department. Unpublished department report
Tanaka, S., 1962. A method of analysing a polymodal frequency distribution and its application to the length distribution of the porgy, Taius tumiformis (T. and S.). J.Fish Res.Board.Can., 19(6): 1143–59
Van Somerene, V.D., 1960. Nile perch studies. Ann.Rep.E.Afr.Freshwat. Fish.Res.Org., (1960): 7–8
von Bertalanffy, L., 1933. Untersuchungen über die Gesetzlichkeit des Wachstums. 1. Wilhelm Roux Arch.Entwicklungsmech., 131:613
von Bertalanffy, L., 1938. Aquantitative theory of Organic growth. Hum.biol., 10(2): 181–213
Walford, L.A., 1946. A new graphic method of describing the growth of animals. Biol.Bull.Mar.Biol.Lab.,Woods Hole, 90(2): 141–7
Worthington, E.B., 1932. A report on the Fisheries of Uganda investigated by the Cambridge Expedition to East African Lakes. 1930–31. Chap. 4. Lake Nabugabo. London, Crown Agents for the Colonies
Table 1: Age composition of Lake Victoria Lates niloticus, its length ranges, modes, mean L, and mean annual length increments between 1964 and 1977 estimated using probability paper (pp) and semi logarithmic paper (LP).
| Length in cm. | ||||||||||
| Age | Number | Range | Mode | Mean | 2L | |||||
| PP | LP | PP | LP | PP | LP | PP | LP | PP | LP | |
| to = -1.0-1.09 | - | - | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| 0 | - | - | 15.42 | 15.47 | - | - | - | - | ||
| 1 | 7400 | 7400 | 52.00 | 52.00 | 33 | 32 | 36.38 | 36.38 | 36.38 | 36.38 |
| 2 | 458 | 504 | 52–68 | 52–75 | 60 | 60 | 59.41 | 60.68 | 23.03 | 24.30 |
| 3 | 101 | 49 | 68–90 | 75–90 | 68 | 76.61 | 83.70 | 17.20 | 23.02 | |
| 4 | 58 | 36 | 10–100 | 90–97 | 99 | 95.33 | 93.25 | 18.72 | 9.55 | |
| 5 | 105 | 101 | 100–110 | 97–108 | 105 | 100 | 105.17 | 102.88 | 9.84 | 9.63 |
| 6 | 206 | 111 | 110–120 | 108–114 | 118 | 110 | 114.86 | 111.05 | 9.69 | 8.17 |
| 7 | 175 | 183 | 120–130 | 114–123 | 121 | 120 | 124.53 | 118.63 | 9.67 | 7.58 |
| 8 | 243 | 217 | 130–140 | 123–134 | 137 | 128 | 134.95 | 128.86 | 10.41 | 10.23 |
| 9 | 219 | 248 | 140–150 | 134–144 | 141 | 136 | 144.67 | 138.86 | 9.78 | 10.00 |
| 10 | 145 | 199 | 150–160 | 144–154 | 151 | 150 | 153.90 | 148.91 | 9.23 | 10.05 |
| 11 | 64 | 104 | 160–170 | 154–164 | 161 | 156 | 163.20 | 158.58 | 9.30 | 9.67 |
| 12 | 18 | 39 | 170–180 | 164–174 | 171 | 166 | 172.50 | 168.38 | 9.30 | 9.79 |
| 13 | 8 | 9 | 180–190 | 174–189 | 180 | 176 | 184.50 | 181.33 | 11.58 | 12.96 |
Table 2: Catch and age composition of Lates niloticus in the northern part of Lake Victoria between 1964 and 1977. Age is computed from length frequency distribution using probability paper.
| Year | Catch No. | I | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII | XIII |
| 1964/65 | 1872 | 1865 | 7 | |||||||||||
| 1965/66 | 1339 | 1334 | 4 | 0 | 1 | |||||||||
| 1966/67 | 2601 | 2529 | 35 | 21 | 4 | 5 | 7 | |||||||
| 1967/68 | 1087 | 795 | 251 | 29 | 5 | 3 | 2 | 1 | 0 | 0 | 1 | |||
| 1968/69 | 1041 | 650 | 155 | 20 | 3 | 6 | 3 | 2 | 1 | 1 | 0 | |||
| 1969/70 | 28 | 1 | 2 | 14 | 0 | 2 | 1 | 3 | 2 | 3 | 0 | |||
| 1970/71 | 63 | 0 | 1 | 4 | 12 | 4 | 10 | 10 | 12 | 3 | 5 | 2 | ||
| 1974/75 | 940 | 0 | 0 | 10 | 27 | 61 | 142 | 133 | 200 | 183 | 116 | 50 | 15 | 3 |
| 1975/76 | 142 | 1 | 2 | 2 | 4 | 21 | 23 | 20 | 20 | 23 | 14 | 8 | 1 | 3 |
| 1976/77 | 87 | 25 | 1 | 1 | 2 | 3 | 18 | 6 | 8 | 6 | 9 | 4 | 2 | 2 |
| Total | 9200 | 7400 | 458 | 101 | 58 | 105 | 206 | 175 | 243 | 219 | 145 | 64 | 18 | 8 |
| Mean Percentage | 80.44 | 4.98 | 1.1 | 0.63 | 1.14 | 2.24 | 1.9 | 2.64 | 2.38 | 1.58 | 0.70 | 0.20 | 0.09 |
Table 3: Observed catch in numbers and weight (kg), mean size in weight, (W), percentage by weight of total catch and peak seasons for Lates niloticus in Lake Victoria between 1964 and 1984
| Year | No. | Kg. | Catch W | % wt. | Peak months | |
| 1960 | 8 | - | 1 .5 | - | - | |
| 1964 | 2 170 | 1 | 000 | 0.4243 | - | Nov.–Dec. |
| 1965 | 1 188 | 563 | 0.4736 | - | Jan.–Mar., July–Dec. | |
| 1966 | 2 270 | 1 | 070 | 0.4715 | - | Jan.; Aug.–Dec. |
| 1967 | 1 882 | 1 | 957 | 1.0396 | - | Jan.–Mar., Sept.–Dec. |
| 1968 | 1 045 | 1 | 942 | 1.8586 | - | Jan.–Mar., Nov.–Dec. |
| 1969 | 1 205 | 1 | 913 | 1.5875 | - | Jan.–Mar. |
| 1970 | - | - | - | - | - | |
| 1971 | - | - | - | - | - | |
| 1972* | - | 5 566 | 000 | - | 0.62 | - |
| 1973 | - | - | - | - | - | |
| 1974* | - | 366 | 000 | 42.14 | 19.38 | - |
| 1975 | - | 292 | 808 | 72.27 | - | Jan.–Mar. |
| 1976* | - | 77 021 | 000 | - | 20.75 | - |
| 1977 | - | - | 16.63 | - | - | |
| 1978 | - | - | - | - | - | |
| 1979 | - | 3 060 | 000 | - | 2.34? | - |
| 1980* | - | 13 830 | 000 | - | 4.70? | - |
| 1981* | - | 37 000 | 000 | 4.82 | 13.4 | Jan.–Dec. |
| 1982* | - | 49 800 | 000 | 8.64 | 75.17 | Jan.–Dec. |
| 1983* | - | 367 869 | 000 | 5.79 | 68.36 | Nov.–Dec. |
* Fisheries Department Estimates for Masese Fish Landing (Jinja)
Table 4: The sex ratio of Lates niloticus between 1969 and 1983 in Lake Victoria
| Length | Sex | Year | Total | ||||||
| 1969/70 | 1970/71 | 1974/75 | 1975/76 | 1976/77 | 1982 | 1983 | |||
| < 120 | 9 | 12 | 54 | 13 | 10 | 108 | 127 | 133 | |
| 9 | 19 | 107 | 42 | 14 | 126 | 366 | 783 | ||
| Ratio | 1:1 | 1:1.83 | 1:3.83 | 1:3.23 | 1:1.4 | 1:17 | 1:2.88 | 1:2.35 | |
| > 120 | 7 | 30 | 616 | 72 | 34 | 1 | 6 | 766 | |
| 0 | 1 | 53 | 10 | 2 | 1 | 1 | 68 | ||
| Ratio | 7:0 | 30:1 | 11.62:1 | 7.2:1 | 17:1 | 1:1 | 6:1 | 11.26:1 | |
| Pooled | 16 | 42 | 670 | 85 | 44 | 109 | 133 | 1099 | |
| total | 9 | 20 | 260 | 52 | 16 | 127 | 367 | 251 | |
| 1:0.56 | 1:0.48 | 1:0.39 | 1:0.61 | 1:0.36 | 1:1.7 | 1:2.76 | 1:077 | ||
Table 5: Estimates of annual survival rates (by the method of Robson and Chapman and the method of Heinke), annual total mortality rate and instantaneous mortality rate Z, corresponding to Best S, of Lates niloticus in northern Lake Victoria between 1964 and 1977.
| Survival Rate | Mortality Rate | |||
| Year | Best S | Heinke's S | Annual A | Instantaneous Z |
| 1964/65 | 0.0037 | 0.0037 | 0.9963 | 5.5993 |
| 1965/66 | 0.0037 | 0.0037 | 0.9963 | 5.5993 |
| 1966/67 | 0.0269 | 0.0277 | 0.9731 | 3.6155 |
| 1967/68 | 0.2479 | 0.2686 | 0.7521 | 1.3942 |
| 1968/69 | 0.1552* | 0.1835* | 0.8448 | 1.8644 |
| 1969/70 | 0.5* | 0.9643* | 0.5 | 0.6931 |
| 1970/71 | 0.504* | 1.0* | 0.496 | 0.6852 |
| 1974/75 | 0.5003* | 1.0* | 0.4997 | 0.6931 |
| 1975/76 | 0.5* | 0.993* | 0.5 | 0.6931 |
| 1976/77 | 0.4189* | 0.7126* | 0.58 | 0.8699 |
* means the difference is statistically significant
Figure 1 Map of the study area (Uganda waters of Lake Victoria)

Figure 2 Cumulative length frequency distribution of Lates niloticus plotted on probability paper
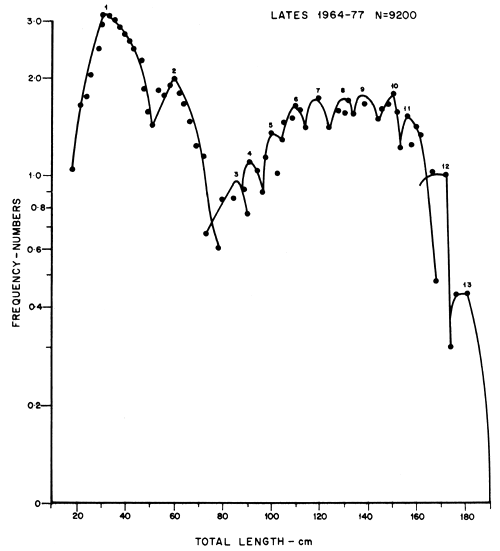
Figure 3 Logarithms of length frequency distribution

Figure 4 Logarithms of length frequency distributions of Lates niloticus in Uganda waters of Lake Victoria
P.C. Goudswaard
HEST, Mwanza, Tanzania
and
F. Witte
HEST, Leiden, The Netherlands
INTRODUCTION
Although the major aim of the research of the Haplochromis Ecology Survey Team concerns the stock of haplochromine cichlids (furu) in the Tanzanian part of Lake Victoria, observations on other fishes caught simultaneously with haplochromines are also made. Of these Nile perch (Lates niloticus) is of particular interest because it is a voracious predator on haplochromines and other fishes. In areas where Nile perch has become established a strong decline and sometimes even a virtual depletion of the haplochromine stock followed (Arunga, 1981; Okemwa, 1981, 1984). In this paper our preliminary observations on Lates niloticus made during our survey on haplochromines are discussed.
ORIGIN OF THE DATA
Data were obtained from catches made by the HEST trawler R.V. KIBOKO, which became operational in May 1984 and by a number of other trawlers operating in the Mwanza Gulf and the Speke Gulf (Table 1). As not all catch reports have been fully analysed only estimates of catch rates can be given.
RESULTS
Distribution and catch rates
Observations on catches from different areas of the Tanzanian part of Lake Victoria are as follows (Figure 1):
Mwanza Gulf: depth - 5–18 m; bottom - soft mud Catch reports of the R.V. MDIRIA of the Freshwater Fisheries Research Institute at Nyegezi show that Nile perch was caught in the Mwanza Gulf from 1972 onwards, when the ship became operational and when large specimens occasionally entered the catch. In the period 1974 through 1976 the percentage of occurrence of Nile perch in trawl catches as well as mean catch rate per hour increased (Table 2, Kukowski, 1978). In 1977 there was a decline. Since that time a slow increase of Lates niloticus in the catches was observed, with a sudden acceleration during the past two years. Nile perch now occurs in virtually every trawl catch in the Mwanza Gulf and is the second important fish (approx. 170 kg/h) after the haplochromines (approx. 600 kg/h). Until recently the Nile perch catches comprised only large specimens of more than 10 kg but in the middle of 1984 large numbers of small perches (15–39 cm SL, approx. 0.5–2 kg) suddenly appeared. During the second half of 1983 juveniles of approximately 2 cm SL were caught with a mosquito net beach seine along the shores of the Mwanza Gulf (Nyegezi Bay). In beach seine catches of local fishermen Nile perch are now common. Such catches are normally made in shallow areas with sandy bottoms.
Magu area (Speke Gulf): depth - 6–30 m; bottom - mud in shallow areas, sand and gravel in deeper water Sixteen trawl shots of the R.V. MDIRIA in the Magu area in November 1983 yielded average Nile perch catch rates of 200–250 kg/h. Haplo-chromine catches were exceptionally low (40–300 kg/h, depending on area).
Nafubo area (Speke gulf): depth - 8–12 m; bottom - probably sand Until the end of 1983 catches in the Nafubo area were still dominated by halpochromines. Catch rates were comparable to those in the Mwanza Gulf. In the course of 1984 the catch composition changed dramatically in favour of Lates niloticus. As a result, the 35-ft wooden trawler from Nansio (Ukerewe) which exploits the Nafubo area, changed its fishery from haplochromines to Nile perch. In July 1984 the R.V. NINGU of the Mwanza Fisheries Research Centre caught approximately 20 kg/h haplochromines in the Nafubo area. In the Mwanza Gulf the same vessel had an average catch rate of 150–200 kg haplochromines per hour.
North of Ukerewe: Grant Bay: depth - 6–8 m; bottom - mud; near Ukara Island: depth - 6–8 m; bottom - mud In June 1984 four trawl shots were made with the R.V. KIBOKO in the area north of Ukerewe. These catches yielded 500–550 kg Nile perch per hour and less than 10 kg of haplochromines.
Deep, offshore areas north and west of Ukerewe and north-north west of the Mwanza Gulf: depth - 50–60 m; bottom - probably mud Approximately 20 trawl shots were made with the R.V. KIBOKO in these areas in June 1984. Each catch contained 80–150 kg Nile perch per hour. The sizes of these fishes ranged from 0.5 up to 40 kg. The average catch rate of haplochromines at these depths was approximately 150 kg/h.
Food of the Nile perch
Stomach contents of approximately 200 specimens have been examined and in all haplochromines are the major prey items. However, in deep water (50–60 m) most fishes probably fed on the shrimp, Caridina niloticus. In these deep-water catches the stomachs of the Nile perches were generally squeezed through the mouth by the swimbladder, which expanded due to the large pressure difference between bottom and surface. In such catches the frequently observed lumps of shrimps were probably the stomach contents of Nile perch, which was nearly the only large fish in the catch.
DISCUSSION
Habitat preference
The present observations on Nile perch suggest that it occurs in virtually every habitat of the Lake, with the possible exceptions of rocks, swamps and the pelagic zone. According to other authors Lates niloticus was mainly restricted to shallow waters over sandy bottoms where the oxygen concentration is relatively high (Arunga, 1981; Okemwa, 1984). Greenwood (1966) and Hopson (1971) mention mass mortalities of Lates niloticus in Lake Albert and Lake Chad respectively, which were probably due to low oxygen concentrations. Greenwood (op.cit.) quoted the experimental work of Fish (1956) which proved that L. niloticus has a relatively high oxygen demand compared to other fresh water fishes. Due to stratification during approximately six months of the year the oxygen concentration is very low (less than 10 percent of saturation) within the lowermost 5 m of the water column in the deep offshore areas (Talling, 1966). In other periods the oxygen concentration is still low (often less than 3 mg/l) as compared to the littoral areas (6–7 mg/l). It is therefore remarkable that Nile perch occurs in the deep-water catches. Two possible explanations for this phenomenon can be given:
The catches were made in the dry season during which the stratification normally breaks down (Talling, op.cit.). So at that period the oxygen concentration could have been sufficient for Lates niloticus. During periods of low oxygen concentration the fishes would die or migrate back to shallower areas.
There are two species of Lates present in the Lake, one preferring shallow water, the other living in deep water. The Nile perch was introduced from Lake Turkana and Lake Albert (Arunga, 1981) and in both lakes a shallow and a deep water species of Lates occurs (L. niloticus and L. longispinus in Lake Turkana; L. niloticus and L. macrophthalmus in Lake Albert; Greenwood, 1976).
Expansion of the distribution area
Although within ten years after the introduction small numbers of large Nile perches were reported occasionally in catches throughout the Lake, observations of the vast expansion of these fishes cover only the past seven years. In 1978 a sudden strong increase in the Nyanza Gulf was observed (Arunga, 1981) and in the following years a similar increase was reported near Ukerewe and subsequently in the Speke Gulf. Apparently the expansion of the Nile perch moves from north to south. The mechanism of the “sudden” colonization of an area is not yet clear but probably it is the result of an expansion from area to area rather than the result of reproduction of a number of immigrants in a certain area.
Impact of Nile perch on other fish species
The impact of Nile perch on almost all other fish species within its area of distribution is dramatic. In the Nyanza Gulf stocks of almost all other fishes declined or virtually disappeared, with the exception of the zooplanktivorous Rastrineobola argentea (dagaa) and Oreochromis niloticus (Arunga, 1981; Muller and Benda, 1981; Okemwa, 1981, 1984). The latter species is only abundant in shallow areas near the papyrus fringes where Nile perch is not very abundant (Arunga, 1981). R. argentea appears to coexist with Lates in both Lake Kyoga and the Nyanza Gulf (Arunga, op.cit.). Catches by the R.V. KIBOKO near Ukerewe also yielded relatively large amounts of R. argentea (pers.obs.). Possibly these pelagic fishes are less sensitive to predation by the demersal Nile perch than benthic fishes. For the same reason, zooplanktivorous and phytoplanktivorous haplochromines which are partly (or mainly) pelagic might be better suited to coexist with Nile perch than the benthic haplochromines.
Although for the time being the strong increase of Lates seems a favourable development, the final consequences may be very serious for the fish production of the Lake. In the first place, adding one step to a food chain generally causes an energy loss of 80 percent. Secondly, a large number of haplochromines are primary consumers (detritus and phytoplankton), when these are depleted, a major part of the energy input in the Lake may be cut off for fish production. The same holds for special food sources like molluscs that are fed on by specialized haplochromines. The above-mentioned effects may finally result in a strong decrease of total fish yield of the Lake.
REFERENCES
Arunga, J., 1981. A case study of the Lake Victoria Nile perch Lates niloticus fishery. In Proceedings of the Workshop on Aquatic resources of Kenya, July, 1981. Mombasa, Kenya, Kenya Marine and Fisheries Research Institute, pp. 165–83
Fish, G.R., 1956. Some aspects of the respiration of six species of fish from Uganda. J.Exp.Biol., 33:186–95
Greenwood, P.H., 1966. The fishes of Uganda. Kampala, The Uganda Society Publications, Kampala, 367 p.
Greenwood, P.H., 1976. Review of the family Centropomidae (Pisces, Perciformes). Bull.Brit.Mus.(Nat.Hist.)(Zool.), 29:3–81
Hopson, A.J., 1971. A study of the Nile perch in Lake Chad. London, Her Majesty's Stationery Office, 93 p.
Kukowski, G., 1978. Trawling results in the Tanzanian waters of Lake Victoria by the Freshwater Fisheries Institute, Nyegezi, 1973–77. Jinja, East African Freshwater Fisheries Research Organization
Muller, R.G. and R.S. Benda, 1981. Comparison of bottom trawl stock densities in the inner Kavirondo Gulf of Lake Victoria. J.Fish Biol., 19:399–401
Okemwa, E.N., 1981. Changes in fish species composition of Nyanza Gulf of Lake Victoria. In Proceedings of the Workshop on Aquatic resources of Kenya, July 1981. Mombasa, Kenya, Kenya Marine and Fisheries Research Institute, pp. 138–56
Okemwa, E.N., 1984. Potential fishery of Nile perch, Lates niloticus Linne (Pisces, Centropomidae) in Nyanza Gulf of Lake Victoria, East Africa. Hydrobiologia, 108(2):121–6
Talling, J.F., 1966. The annual cycle of stratification and phytoplankton growth in Lake Victoria (East Africa). Int.Rev.Gesamt. Hydrobiol., 51:545–621
Table 1: Trawlers from which catch data on Nile perch were obtained
| Name | Length in m | hp | Headrope net in m | Cod end mesh size in mm |
| R.V. MDIRIA | 14.8 | 120 | 25 | 89,19 |
| R.V. KIBOKO | 12.0 | 105 | 25 | 19 |
| R.V. NINGU | 10.0 | 60 | ? | 19 |
| Trawlers fish-meal factory | 16.8 | 170 | 21 | 19 |
Table 2: Nile perch catches in the Mwanza Gulf - 0–18 m depth
with a 89-mm cod end mesh size
(data of 1974 through 1977 from Kukowski, 1978)
| Year | 1974 | 1975 | 1976 | 1977 | 1984 |
| % frequency occurrence | 5 | 6 | 15 | 10 | 100 |
| % of total catch weight | + | + | 2 | 1 | approx. 20% |
| catch rate kg/hr | 1 | 1 | 2 | 1 | approx. 175 |
+ indicates presence; less than 0.5%

Figure 1 Map of the southeast part of Lake Victoria with locations (*) at which Nile perch catches discussed in the present paper were made