Richard Ogutu-Ohwayo
Uganda Freshwater Fisheries Research Organization
Jinja, Uganda
| ABSTRACT | |
| The outcome of the UNDP/Lake Victoria stock assessment project was the interest in the commercial exploitation of haplo-chromine cichlid populations. Nile perch had earlier been introduced into Lake Victoria to feed on haplochromines and convert them into a more acceptable fish for human consumption. Trial introduction of Nile perch into Lake Kyoga was partly intended to show the possible impact of the Nile perch on a predominantly cichlid fauna. Following the introduction of the Nile perch into Lake Kyoga, populations of some fish species declined and others completely disappeared. The food of the Nile perch has been examined to estimate the extent of predation upon the populations of fish in the Lake. Nile perch has been observed to feed on invertebrates when young, changing to a piscivorous diet with increasing age. Rastrineobola argentea and Oreochromis niloticus were the most important fish prey with haplochromines and L. niloticus forming a less significant contribution to the diet. Since its introduction, L. niloticus has shifted from one type of fish prey to another, cannibalism has increased and the condition factor of the predator decreased. It appears that Nile perch has caused considerable damage to the populations of fish in Lake Kyoga and is capable of depleting stocks of other fish in a few years. The Nile perch is now well established in Lake Victoria and occupies the most commercially and biologically productive shallow inshore areas of the Lake. Since Nile perch in Lake Victoria feeds more on haplochromines than any other fish, plans for a mechanized trawl fishery for haplochromines in the same area should be re-evaluated. | |
| For the Lake Kyoga fishery it is therefore recommended that: | |
| (i) | Selective fishing for Nile perch larger than 80 cm be encouraged to reduce predation pressure on the commercially important O. niloticus; |
| (ii) | Commercial exploitation of R. argentea in Lake Kyoga should not be encouraged, in view of its current and predictable future importance as food for L. niloticus. |
INTRODUCTION
There have been plans for a long time to utilize the abundant haplo-chromine populations of Lake Victoria either directly or indirectly for human food. One of the problems has been that haplochromines are small fishes with many bones so that consumers have tended to avoid them. As early as 1927, it had been suggested that a more commercially desirable predator such as the Nile perch (Lates niloticus) or the tiger fish (Hydrocynus) should be introduced into the Lake to feed on these “trash” fishes and convert them into a more commercially desirable table fish (Graham, 1929; Anderson, 1961). Later, direct exploitation of haplo-chromine populations, either for human food or fish meal, was proposed. Since the FAO/UNDP Lake Victoria Fisheries Research Project estimated that at least 80 percent of the demersal ichthyomass of Lake Victoria consisted of the less used haplochromines, there have been plans to tap this abundant resource through a mechanized trawl fishery.
As well as increasing the production of fish that could be directly consumed by the human population, the introduction of Lates was also expected to extend the traditional, predominantly inshore fishery to open waters. It was argued that since L. niloticus coexist with Oreochromis and other species in Lakes Turkana and Albert, it would have no harmful effects on similar species in Lake Victoria. Arguments against the introduction of L. niloticus were advanced, however, based upon fears that it might cause serious damage by eating up commercially important fish, especially Oreochromis species (Fryer, 1960). Existing predators, such as Bagrus docmac (Forsk.) would be affected further through competition for the same food with the Nile perch.
The Nile perch was introduced into Lake Kyoga partly to find out what effects such an introduction could have on the cichlid dominated fauna of Lake Victoria (Gee, 1964; Stoneman and Rogers, 1970). But before this trial could be tested to estimate the effects of the Nile perch upon the fishes of Lake Kyoga, L. niloticus was discovered to have gained access to Lake Victoria before May 1960 (Gee, 1964). It was later intentionally introduced into the Lake in 1962 and 1963.
THE LAKE KYOGA FISHERY
The major commercial fish species of Lake Kyoga, from the time of Worthington (1929) to the early 1950s were, in the order of their importance: Oreochromis variabilis, O. esculentus, Protopterus aerthiopicus, Bagrus docmac, Clarias mossambicus, Barbus species and Schilbe mystus. Members of the genus Haplochromis were abundant in the Lake, for Worthington who accompanied Graham (1929) on the Lake Victoria survey observed that species of the genus Haplochromis in Lake Kyoga were as numerous as they were in Lake Victoria.
The Nile perch, together with Tilapia zillii, Oreochromis niloticus and O. leucostictus, were introduced into Lake Kyoga between 1954 and 1957 (Stoneman and Rogers, 1970). These introduced species established themselves fast and started appearing among commercial catches as early as 1958. From 1964, the population of L. niloticus increased steadily and has stabilized at about half of the total commercial landings since 1968. The introduced O. niloticus also increased to contribute over 20 percent of the commercial landings by 1968 and has also stabilized at about half the total catch since 1970. During this period, the native oreochromus sp. declined in importance to less than 5 percent of the total catch by 1968. The catch of B. docmac also fell from 233 t per annum in 1961 to only 4.8 t in 1965 (Stoneman and Rogers, 1970).
A preliminary survey of the fish stocks of Lake Kyoga during this study has shown that L. niloticus, O. niloticus and R. argentea are presently the most abundant and widely distributed fish in the Lake. P. aethiopicus, C. mossambicus, S. mystus and even Haplochromis species which were originally abundant are rare with relict populations being restricted either inshore or to areas of aquatic macrophytes. The originally most important O. esculentus, O. variabilis and the native predator Bagrus docmac were not encountered at all. Analysis of fish catch statistics has shown that as the populations of the Nile perch increased, that of the native Oreochromis species decreased.
There have been lamentations that the introduced predator, through its feeding, has depleted stocks of fish in Lake Kyoga. Population changes in a fishery can, however, be caused by other factors such as fluctuations in fishing effort and fishing gear. In Lake Victoria, for example, the decline in catch per unit effort has been attributed to an increase in fishing effort due to introduction of gillnets (Jackson, 1971).
At the time of Worthington's survey, Lake Kyoga was fished only by the natives around it. The fishing gear consisted of locally made basket traps, hooks and seine nets of papyrus. The fishing effort was, therefore, low and caused little damage to the fishery. Before 1950, successful use of gillnets on Lake Kyoga was difficult because of the abundance of crocodiles which destroyed the nets. The other problem was the large expanse of aquatic vegetation and floating islands. When the population of crocodiles was reduced by trapping, the use of gillnets on the Lake increased. Unfortunately, there was no limit to the size of nets used. As a result, nets of varying sizes, 2.5–4 inches were freely used. The 3.5 and 4 inch nets were most suitable for the native Oreochromis spp., but the 2.5 inch nets, though aimed at species like Schilbe mystus and Labeo victorianus could harm larger species by cropping immature fish. As early as 1950, the catch per unit effort of the gillnets had fallen. Initially, it had been possible to catch up to 30 fish in each net per night. But by 1950 this catch rate had dropped as low as 7.7 fish per net in some places after only a few years of exploitation (Uganda Game and Fisheries Department, Annual Report, 1958).
The introduced species started appearing among commercial catches at an early stage. By July 1958 some of these were so numerous at some landings that fishermen found it more profitable to set nets specifically for them. As a result, some fishermen discarded the 2.5 to 3.5 inch nets in favour of those of 4, 4.5 and 5 inch mesh in order to catch the larger size O. niloticus and T. zillii which grew up to 4 lb (1.82 kg) compared to the native Oreochromis spp. which rarely exceeded 0.5 lb (0.23 kg) (Uganda Game and Fisheries Department Annual Report, 1960). This could have favoured a recovery in the populations of native species. Unfortunately, this did not happen. As the population of the introduced species increased, that of the native species continued to decline. It is surprising that, despite the shift in fishing pressure toward the larger introduced species as early as 1958, the original commercially important species still declined and were replaced by the introduced O. niloticus and L. niloticus. In this paper, the food and feeding habits of the Nile perch in Lake Kyoga have been examined to estimate how predation could have influenced these changes. These are discussed in the light of the recent increase in the Nile perch population in Lake Victoria and its possible effects on the haplochromine populations upon which a trawl fishery is envisaged.
THE FOOD OF THE NILE PERCH
Study area, materials and methods
These data were collected from Lake Kyoga in central Uganda (Figure 1). The Lake is situated between longitude 32°E to 34°E and latitutde 1°N to 2°N. It is at an altitude of 1 037 m above sea level and covers an area of 2 350 km2. The lake is shallow with an average depth of 3 to 4.5 m.
Specimens of L. niloticus came from commercial catches and experimental gillnets and seine nets. The total length (TL) and standard length (SL) of each specimen were measured. After recording the weight of each specimen, the fish was opened and the gonads assigned to a maturity state on the basis of the key of Hopson (1972).
The stomachs with food were removed and preserved in a 5 percent formalin solution to be examined later but those which contained large fish prey were examined on the spot. For each stomach, the total weight of food, after being blotted dry of moisture, was measured.
The food items were then identified as far as possible to various taxonomic groups and the number of each type of prey recorded. The standard length of the fish prey that were found whole in the stomach were measured to estimate the size of prey ingested. The length of the invertebrates was taken from the anterior tip of the head to the posterior end of the last abdominal segment using a vernier calliper.
RESULTS
The type of prey
During the period April 1978 to December 1982, 5 502 stomachs of Nile perch of 2.0–170 cm SL from Lake Kyoga were examined for food. The frequency of occurrence of the different types of prey ingested by L. niloticus of different sizes is shown in Table 1. The Nile perch was observed to feed on invertebrates changing to a piscivorous diet with age. The invertebrate prey included: Chaoborus, Povilla and smaller ephemeropterans, Zygoptera, Anisoptera, Hemiptera, chironomids, prawns (Caridina nilotica) and molluscs. The fish prey consisted of Rastrineobola argentea (= Engraulicypris argenteus), haplochromine species Oreochromis niloticus, Protopterus aethiopicus, Clarias spp. and L. niloticus itself.
The type of prey varied with the size of the predator. The relative importance of different types of prey to various sizes of the predator is illustrated in Figure 2. Prey were dropped sequentially from the diet as the size of the predator increased. Invertebrates declined in importance until they were completely dropped by L. niloticus of more than 50 cm SL; beyond which size the predator turned exclusively piscivorous. The prawn, Caridina nilotica, was the most important invertebrate in the diet of the Nile perch. Among predators of less than 20 cm when invertebrates were most prominent, 54 percent of the invertebrate diet by weight was made up of C. nilotica, 38.5 percent by anisopteran nymphs, 3 percent by chironomids and the remaining 4.5 percent by the other invertebrates. Rastrineobola argentea was the most important fish prey in L. niloticus of 10–79 cm beyond which O. niloticus formed the most important component of the food of the predator. Haplochromines and L. niloticus contributed less to the diet of the Nile perch and both appeared to merely bridge the gap between R. argentea and O. niloticus. Both P. aethiopicus and Clarias spp. occurred in less than 0.5 percent of the stomachs with fish.
It is interesting to note that haplochromine species, which were expected to form the bulk of the food of the Nile perch by virtue of its introduction in Lakes Victoria and Kyoga, now contribute little to its diet. As the proportion of R. argentea in the diet in L. niloticus of about 40–59 cm decreased, that of haplochromines could have increased but failed to do so. The failure to bridge the gap caused by the declining importance of R. argentea might have been the cause of increased cannibalism in Nile perch of 40–59 cm.
The number of prey
The predator could meet its food requirements either by increasing the number or size of prey. The maximum number of the main types of prey found in the stomach of a single Nile perch were as follows: O. niloticus - 6, L.niloticus - 16; haplochromines - 33; R. argentea - 134; Anisoptera - 42; C. nilotica - 163; and chironomid - 280. Among invertebrates and the generally small fish (R. argentea and haplochromine species), the predator usually ingested many prey specimens. However, for O. niloticus and L. niloticus, the predator normally consumed either one or two specimens. For instance, of all the Nile perch which had fed on O. niloticus, 81.1 percent of the stomachs contained only one or two prey specimens, yet in those which had fed on chironomids, the same proportion of fish had eaten more than five specimens each.
The study showed that there is variation in the average number of prey for successive size groups of the predator (see Figure 3). For inverte-brates, R. argentea and haplochromine species, the average number of prey eaten first increased with predator size to a peak and then declined as the prey was dropped from the diet. The peak for each prey occurred at a different size of the predator with larger predators consuming larger prey. This suggests that preference for a particular type of prey breaks down either when the size of the prey relative to that of the predator becomes too small, or when the density of prey falls below a certain level. The predator would then have to switch to another preferably larger and more profitable prey.
The size of prey
The sizes of each type of fish ingested was analysed to estimate the extent of predation upon individual types of fish. The mean length of prey ingested by predator of different sizes is shown in Figure 4. The available data indicate that the average length of R. argentea ingested initially increased with the size of the predator and then stabilized between 3.2 and 3.6 cm in L. niloticus of more than 20 cm length. The size range of R. argentea ingested was 1.3–4.6 cm though Rastrineobola larvae of 0.7–1.1 cm were eaten by predators of 2–3 cm. The largest R. argentea caught from the Lake was 5 cm and the modal size of this fish in Lake Kyoga was about 3.2–3.8 cm. L. niloticus, therefore, feeds on most size groups of R. argentea in Lake Kyoga.
The average size of haplochromines ingested changed with the size of the predator in a pattern similar to that of R. argentea with the average size of prey eaten stabilizing between 3.5 and 4.2 cm. The size range of haplochromine cichlids ingested was 1.0–6.4 cm but rarely exceeded 5 cm. The largest Haplochromis caught in the Lake was 8 cm. Though the length of R. argentea and that of Haplochromis eaten were similar, there was a difference in their weight. For a given length, haplochromine cichlids were more than twice as heavy as R. argentea. For instance, R. argentea of 3.4 cm had an average weight of 0.50 g, while haplochromines of the same length weighed 1.2 g. The Nile perch would, therefore, get more food for each haplochromine eaten compared to R. argentea for any given length.
Both O. niloticus and L. niloticus grow much bigger than Haplochromis and R. argentea. The size range of O. niloticus eaten was 2.6–43.0 cm, while that of L. niloticus was generally 2.0–26.0 cm though larval L. niloticus of about 0.8 cm were also occasionally eaten. The mean length of O. niloticus and L. niloticus ingested increased over the entire size range of the predator. The largest O. niloticus recorded from Lake Kyoga during this study was 48.0 cm SL. L. niloticus, however, exploits only a small segment of its own population as food. In Lake Kyoga, L. niloticus can grow to 170 cm SL, but the maximum size of L. niloticus eaten was only 26 cm. Nevertheless, L. niloticus can feed on relatively large individuals of its own kind, for instance, a Nile perch of 60 cm SL was recorded from the stomach of a 117-cm L. niloticus from Lake Chad (Hopson, 1972). If the populations of O. niloticus and R. argentea are considerably reduced, the size of L. niloticus eaten could probably increase.
The overall change in the size of the prey with that of the predator was also examined. The relationship between the length of Lates and size of prey is illustrated in Figure 5. The average size of prey increased with that of the predator in distinct phases. The first phase in L. niloticus of less than 35 cm corresponded to a phase of a predominantly invertebrate diet and the second, in L. niloticus of about 35–75 cm, to a diet dominated by small fish particularly R. argentea. The abrupt rise in the average size of prey ingested by L. niloticus of 76–90 cm coincided with the shift from R. argentea to O. niloticus, which grew to a larger size. Analysis of the relative size of prey gives the same picture as that of average size.
There was a wide range in the size of individual prey ingested by L. niloticus of a given size group. The maximum size of prey increased steadily over the entire size range of the predator but the minimum did not. There was high correlation (r = 0.988, p < 0.001) between the maximum length of prey and that of the predator for the relationship:
1max = 0.549 + 0.289L
where 1max is the maximum length of the prey and L that of the predator. This showed that L. niloticus was capable of feeding on prey up to a third their own length. The minimum length of prey increased steadily only in L. niloticus of less than 80 cm followed by an abrupt rise in Lates of 80–89 cm. L. niloticus of more than 80 cm did not generally feed on prey of less than 14.5 cm. The size ranges of O. niloticus and Lates ingested by Lates niloticus is given in Figure 6.
It should be noted that L. niloticus of more than 80 cm mainly ate O. niloticus. L. niloticus of less than 60 cm generally ingested small O. niloticus and L. niloticus while those of more than 80 cm never ate O. niloticus or L. niloticus smaller than 14.5 cm. There was a general overlap in the sizes of prey ingested by L. niloticus of less than 60 cm and another overlap in those consumed by larger predators of more than 80 cm with a 60–80 cm intermediate group which overlapped with the other two. The distribution of the 60–80 cm groups was slightly bimodal suggesting that at about this size the predator switched to larger prey. The complete absence of the small O. niloticus and L. niloticus in the diet of Lates of more than 80 cm suggests that these sizes of prey were lacking in the area occupied by Nile perch of more than 80 cm length. This supports the assumption that there was spatial separation between the large predators and certain sizes particularly of O. niloticus. A tentative survey on the distribution of L. niloticus and O. niloticus also supports this view. However, a more detailed survey is needed to confirm the principle of segregation which might throw some light on why L. niloticus and O. niloticus have managed to coexist in the Lake. The present deduction, however, is supported by the observations of Hopson (1972) that L. niloticus progressively move offshore with increase in size so that there is size segregation within the species. The young of Oreochromis spp. are also known to move progressively offshore with increasing size (Graham, 1929; Welcomme, 1966).
CONDITION FACTOR OF THE NILE PERCH
Available data on the condition factor of the Lates (Figure 7) shows that the highest values for K were recorded in the smallest fish of less than 10 cm length. The condition factor was generally uniform at about 2.15 in fish of 10–99 cm, apart from the marked decline in the intermediate size group. Above 100 cm, K increased slightly and stabilized at about 2.30 in larger fish. The higher condition factor of the small fish which depend on invertebrates for food suggests that this size of fish had relatively more food available to them than the larger piscivorous individuals.
DISCUSSION
Food and feeding behaviour
A comparison of the food of the Nile perch in Lake Kyoga over the years shows that the Nile perch has shifted from one prey to another. A few years after its introduction into Lake Kyoga, Hamblyn (1962) reported that haplochromine species, small mormyrids and P. aethiopicus were the main fish prey ingested by L. niloticus. Later, Gee (1964, 1969) observed that cichlids including Oreochromis (= Sarotherodon = Tilapia) spp. formed the main food of the predator in the Lake. Examining the food of L. niloticus, Okedi (1971) observed that R. argentea which had not been previously recorded was even more prominent than the cichlids. Presently, the predator does not only feed on R. argentea but also on commercially valuable O. niloticus. The Nile perch in Lake Kyoga has, therefore, shifted from haplochromine species which were expected to form the bulk of its food to other prey. This is probably caused by a change in the type of prey available. Haplochromines are now virtually absent from the Lake.
The prey-size data given by Gee (1969) soon after introduction of the Nile perch into Lakes Victoria and Kyoga, and that of Hopson (1972) for Lake Chad where the Nile perch is endemic and the present data for Lake Kyoga, have been compared.
It is evident from Figure 8 that:
L. niloticus takes larger prey as its size increases. The average size of prey may vary, probably depending on the sizes of prey available in the Lake.
L. niloticus of less than 80 cm has shifted to smaller prey since it was introduced into Lake Kyoga (from 2A to 1A). This demonstrates that the predator is capable of shifting to other sizes of prey probably when more suitable sizes become scarce.
The maximum size of prey ingested is similar indicating that there is an upper limit to the maximum size of prey that can be ingested. The tendency to feed on larger fish prey is, however, more prominent where the Nile perch has lived longest. The predator might attempt to feed on slightly larger prey as suitable sizes of prey become scarce.
Lastly, when the data of Hopson (1972) are compared with the present data, it is interesting to note that there is, in both cases, an abrupt increase in the size of prey consumed by fish of about 80 cm. It is not clear if Gee's data could have shown a similar pattern because, apart from two specimens, his predatorprey length data was based on Lates of less than 80 cm. But when the average length for Gee (2A) is extrapolated upward, it merges with the present data (1A) and is close to 3A. If all sizes of prey were abundant, the size of prey consumed would probably increase evenly with that of the predator in a pattern similar to 2A. The abrupt shifts occurring at different sizes of the predator in 1A and 3A suggests that there are distinct size groups of prey in the habitat. Predation has probably eliminated certain sizes of prey from the lakes where the Nile perch has lived for a long time.
The sudden increase in the size of prey presently consumed by L. niloticus suggested that prey of about 4.5–15 cm were not available to the predator of more than 80 cm. Soon after its introduction into the Lake, the Nile perch preferred prey of 3–17.5 cm (Gee, 1969). This corresponds to the gap that now exists in its diet. Thus the populations of the fish growing in this size range, notably the haplochromines may have been eliminated by predation some time after the arrival of the predator. This would imply that the predator shifted to R. argentea and O. niloticus after eliminating the intermediate size of prey. But both Lates niloticus and O. niloticus which are abundant in the Lake, grew through this size. They could have escaped being eaten by large Nile perch because their juvenile stages appear not to occupy the same areas as predators of a size that would feed on them.
When the values of condition factor (K) obtained in this study were compared with previous observations (Gee, 1969), L. niloticus was found to be in poorer condition than soon after its introduction in the Lake (Table 2). The decline in K was more prominent during the piscivorous phase (over 43 cm) suggesting that there has been a reduction in the quantity of fish available to the predator. The values of K are, however, still higher than those for the lakes from which the fish were introduced. Hamblyn (1966) and Gee (1969) attributed the higher values of K of L. niloticus in Lakes Victoria and Kyoga, compared to Albert and Turkana to the superabundance of suitable prey especially haplochromine species. There was more food for the predator in the new than the original habitats which had already been subjected to heavy predation pressure. The deterioration in the condition of L. niloticus in Lake Kyoga over the years supports the view that there had been a decrease in the abundance of suitable prey species.
Management of Lake Kyoga fishery
The value of a fishery may be measured in terms of the amount of protein food it provides to the human population. Though there are present commercial benefits attributed to the Nile perch, there are yet unpredictable ecological consequences some of which dangers are seen in the decline in populations of the native fish species, the apparent reduction in food supply and the shift from one prey to another. There is need therefore to formulate management policies aimed at reducing destruction of populations of other fish by the Nile perch and a firm decision to conserve the fishery.
The main objection to the introduction of L. niloticus into Lakes Victoria and Kyoga was the effect it would have on the resident species of these lakes. It was feared that the Nile perch would feed on the commercially desirable Oreochromis (Tilapia) species and further affect the native predators through competition for the same food. The Nile perch in Lake Kyoga has been observed to feed on Oreochromis species, thus justifying the arguments that were advanced against its introduction into the Lake. Although the native Oreochromis species are now so scarce that they could not be prominent in the food of the Nile perch, the fact that it feeds on the larger introduced O. niloticus seems to indicate that L. niloticus might have earlier depleted stocks of these fish because they used to grow to a smaller size than the introduced Oreochromis species. The native O. variabilis and O. esculentus in Lake Kyoga grew to a maximum size of 25 and 26 cm, respectively (Worthington, 1929). Since L. niloticus can ingest O. niloticus up to 43 cm all sizes of the native Oreochromis species were vulnerable to predation.
The Nile perch seems to be confined to waters of less than 30 cm deep in Lake Victoria (Okedi, 1971; Kudhongania and Cordone, 1974) and has, therefore, not fulfilled the expectation advanced by proponents of its introduction that it would extend the traditional predominantly inshore fishery to open waters. However, there has been considerable increase in commercial fish landings of Lake Kyoga from an estimated 4 500 t in 1956 to over 100 000 t since 1967 (Uganda Game and Fisheries Department Annual Report 1958, 1960). The introduced O. niloticus and L. niloticus have replaced the native fish fauna and form the most important component of the commercial fishery. It is not absolutely clear whether the introduced species have found the new environment suitable for their survival or have been better competitors. Nevertheless, L. niloticus is depleting the commercial fishery by feeding on Oreochromis populations. It is, however, difficult to evaluate at this apparently transitional stage, what final benefits or hazards will come out of this introduction. What is important at the moment is to design measures aimed at sustaining the fishery.
Predation by the Nile perch is doing some damage by feeding on the populations of the commercially important O. niloticus in Lake Kyoga. There is need, therefore, to consider measures that might reduce this predation pressure. One plausible suggestion would be to remove the predator from the Lake but this would be difficult. Also, O. niloticus and L. niloticus are currently the only two commercially important fish species in Lake Kyoga. Whereas O. niloticus as a herbivore is useful in converting the energy fixed in plants into human food, L. niloticus by virtue of its feeding on R. argentea and several aquatic invertebrates, plays an important role in converting the energy from the zooplankton and the detritus food chains into useful human food. Any management measure, therefore, should for the moment be geared toward the conservation of both these species in the Lake.
The greatest impact of predation upon O. niloticus is caused by Nile perch greater than 80 cm standard length. A reduction in populations of the predators larger than this would reduce predation pressure upon O. niloticus and subsequently increase the populations of the prey. Observations made on Lates species in some parts of Lake Tanganyika, have shown that the abundance of the prey can be increased following intensive exploitation of the predator (Coulter, 1976). Similarly, in Lake Victoria, Marten (1979) observed that fish catches can be improved by reducing the populations of the predator through fishing. Hence, increasing the fishing pressure on the predators may increase the yield of its prey.
In view of this, it is suggested that the populations of L. niloticus larger than 80 cm should be reduced by selectively fishing for them. Fortunately, the Nile perch grows to a much larger size than any other fish found in Lake Kyoga. In a parallel study, which will be reported elsewhere, it has been observed that gillnets of more than eight inches stretched mesh predominantly catch Nile perch. Also, hooks of certain sizes will catch Nile perch in exclusion of other species. It might, therefore, be possible using suitable size of nets and hooks to selectively crop the Nile perch without damaging other fish.
There is another advantage of cropping very large fish; they use much of the food they eat in merely maintaining themselves and convert only a small portion of its food into new flesh. The efficiency of utilization of the basic food source in the production of new flesh is, therefore, low. The younger and faster growing fish make more efficient use of the food that they consume. For this reason, it has been suggested that proper fishery management should keep the proportion of larger fish in the stock as low as possible (Gulland, 1970). In addition, increased fishing pressure on the large individuals may result in higher condition factor for the remaining ones due to a possible increase in the relative amount of food available. Selective fishing for the large Nile perch would, therefore, not only reduce predation pressure on the prey but would also increase the efficiency of utilization of the available food.
In Lakes Albert and Turkana, where the Nile perch is endemic, it has been reported that large L. niloticus feed on relatively smaller prey, such as Rastrineobola, Odonata and Caridina nilotica (Hamblyn, 1966; Gee, 1969), than has yet been recorded for the habitats to which the predator was recently introduced. Since the time of its introduction into Lake Kyoga, the Nile perch has shifted from relatively larger fish prey such as haplo-chromine species (Hamblyn, op.cit., Gee, op.cit.) to small prey like R. argentea (Okedi, 1971; Ogutu-Ohwayo, 1984). The predator, therefore, seems to be capable of shifting to smaller or larger prey as certain sizes of prey become scarce.
At the time when more suitable sizes of prey were available, R. argentea could have been neglected by L. niloticus because of its pelagic position in the water column which might have made its capture difficult. This is supported by laboratory experiments of Hamblyn (1966) which demonstrated that L. niloticus was least successful in capturing pelagic fish or those swimming near the surface. R. argentea may, at that time, have also been too small to meet the energy requirements of the predator in the presence of more suitable prey. Otherwise, pelagic species are known to be very important in the diet of Lates, especially in those habitats where it has been for a long time. Three small pelagic forms: Micralestes acutidens, Alestes dageti and Eutrophicus niloticus have been reported to be important in the diet of L. niloticus in Lake Chad (Hopson, 1972). In Lake Tanganyika, two pelagic clupeids, Limnothrissa miodon and Stolothrissa tanganicae form the bulk of the diet of Lates mariae (Coulter, 1976). Rastrineobola stella has also been observed to be important in the diet of Lates in Lake Turkana (Gee, 1969). R. argentea has also been reported in the diet of L. niloticus in Lake Victoria and it is possible, as predicted by Hopson (1972) and supported by the present results for Lake Kyoga, that when Lates is well established in Lake Victoria and the haplo-chromine populations considerably reduced, pelagic species like R. argentea will be even more prominent in its diet.
The small pelagic cyprinid, R. argentea, presently forms a very important component of the diet of the medium-sized L. niloticus in Lake Kyoga. It is possible that as alternative prey become scarce, L. niloticus larger than are currently feeding on this species might revert to it. Rastrineobola is not presently commercially exploited in Lake Kyoga but fishing for it is widespread in neighbouring Lake Victoria and could easily extend to Lake Kyoga. In view of its current and predictable future importance as food of L. niloticus it is suggested that the exploitation of Rastrineobola in Lake Kyoga should not be encouraged until the population dynamics of the prey and its rate of turnover in the Lake is well understood.
Nile perch in relation to Lake Victoria fishery
The present population magnitude and distribution of L. niloticus in Lake Victoria are not well known. After its introduction at Jinja in 1960 and Entebbe in 1962, L. niloticus spread along the northern and eastern shores of the Lake (Gee, 1964). There are strong indications from commercial catches that this shoreline spread of the Nile perch is now complete. The outward extent of the predator into the Lake is, however, still largely unknown. In its native habitat of Lake Albert, L. niloticus is restricted to waters less than 20 m deep (Hamblyn, 1966). In Lake Victoria, trawling surveys during the early 1970s showed that, at that time, the species was generally limited to waters less than 30 m deep (Okedi, 1971; Kudhongania and Cordone, 1974). Recent trawl surveys in the Mwanza Gulf indicate that the species might have spread to deeper waters (HEST, pers.comm.).
When the stocks of Lake Victoria fishes were defined during a joint survey conducted by UNDP/FAO and EAFFRO, the contribution of L. niloticus to the total demersal ichthyomass of the Lake was insignificant. L. niloticus had not shown the success it had by that time shown in Lake Kyoga. At present there is strong evidence from commercial and experimental fishing that L. niloticus is well established in Lake Victoria. At Masese fish landing, for example, the proportion of L. niloticus in the commercial catches increased from 18.6 percent of the total catch by weight in 1981 to 67.8 percent in 1983 (Fishery Department statistics). Trawl catches from UFFRO research vessel IBIS, apart from showing a similar trend in catch composition, also indicate that the population of haplochromine species in the area occupied by the Nile perch is declining.
The significance of L. niloticus to the Lake Victoria fishery is well illustrated by the commercial catches from the Kenya waters of the Lake (Table 3). The proportion of L. niloticus in the commercial catches in this area alone rose from 0.5 percent in 1976 to 67.7 percent in 1983. The population of the Nile perch has increased to make it the most important commercial fish species in the Kenya waters of Lake Victoria over a period of only seven years.
The ecological consequences of such a sudden rise in the population of a voracious predator to the resident species not previously exposed to such a predator is partly demonstrated by the corresponding decline in the commercial landings of its main fish prey (haplochromines) from 34.1 percent of the total commercial landings in 1976 to less than 1 percent in 1983 (Table 3). The proportion of other indigenous commercially important species notably Bagus docmac, P. aethiopicus and C. mossambicus has also declined considerably over this period. Information from scientists working in the Nyanza Gulf of Lake Victoria (Ogari, pers.comm.) indicate that this area of the Lake is like Lake Kyoga, evolving into a three species fishery dominated by the two introduced nilotic species, L. niloticus and O. niloticus and the native pelagic cyprinid R. argentea.
The maximum species diversity in Lake Victoria is supposed to be in the shallow inshore areas of the Lake. Kudhongania and Cordone (1974), for example, observed that half of the demersal ichthyomass of the Lake inhabited waters less than 30 m deep. Most of the commercially desirable species such as Bagrus docmac, Clarias mossambicus and all the Oreochromis species were most abundant in this area and their catch rates decreased with depth. Although the haplochromine species were observed to be eury-benthic, their catch rates were also highest in waters less than 30 m deep. The Nile perch is therefore in contact with the most productive and commercially valuable areas of the Lake.
In Lake Victoria, L. niloticus has been observed to feed more on haplochromines than any other fish (Gee, 1969; Okedi, 1971). The reduction in the population of other fish particularly the originally abundant haplo-chromine populations in Lake Kyoga and the Nyanza Gulf of Lake Victoria following increases in the Nile perch population there, suggests that this large predator is capable of depleting populations of other fish in a few years. Heavy mechanized exploitation of haplochromine stocks, which are already under heavy predation pressure from an introduced predator and other native predators like B. docmac, is likely to exert a double force on the populations of haplochromines. In fact, given our present ignorance of the magnitude of haplochromine stocks of Lake Victoria, plans for a mechanized fishery may be made on an already diminishing resource.
Observations in the Mwanza Gulf of Lake Victoria indicate that haplo-chromine species may not endure intensive exploitation. Though they are usually regarded as one group, they have a wide range of trophic groups which may not have a wide range of ecological tolerance. It may, therefore, be impossible for species normally resident in the deeper waters to replenish diminishing stocks in the shallower areas outside their ecological range (Witte, pers. comm.).
A mechanized trawl fishery could initially be confined to areas outside the known distribution of L. niloticus. But the present distribution of L. niloticus is not known. Also because the diversity of species and the magnitude of trawl catches decrease with depth, trawling the central offshore zone of the Lake may not be as productive as the shallow inshore areas. For this and other economic reasons, trawl operators may be tempted to fish in the shallower waters of less than 30 m depth which are within the known range of the Nile perch. Any reduction in the haplochromine population by trawling to a small density may force the Nile perch to switch to other and probably more commercially desirable species as in the case of Lake Kyoga.
ACKNOWLEDGEMENTS
During the time I did this work, I was a student at the University of Dar-es-Salaam under the sponsorship of the Uganda Government and the Commonwealth Fund for Technical Cooperation, as well as a member of the staff of the Uganda Freshwater Fisheries Research Organization (UFFRO). I am grateful to all these organizations for providing funds and facilities for this project. I am greatly indebted to Professor D. Griffiths of the Department of Zoology, University of Dar-es-Salaam, for supervising the project. Many thanks also go to Miss F. Bazanya for typing this manuscript; Messrs. Sowobi and Msuya for cartographic work; Messrs. P. Mugerenge and J. Were for laboratory and field assistance and to many colleagues at UFFRO who may have read and commented on the manuscript. Part of this work was submitted as a thesis for an M.Sc. degree at the University of Dar-es-Salaam.
REFERENCES
Anderson, A.M., 1961. Further observations concerning the proposed introduction of Nile perch into Lake Victoria. E.Afr.Agric.For.J., 26(4):195–201
Coulter, G.W., 1976. The biology of Lates species (Nile perch in Lake Tanganyika, and the status of the pelagic fishery for Lates species and Luciolates stappersii (Blgr.) J.Fish Biol., 9:235–59
Fryer, G., 1960. Concerning the proposed introduction of Nile perch into Lake Victoria. E.Afr.Agric.For.J., 25(4):267–70
Gee, J.M., 1964. Nile perch investigations. Annu.Rep.E.Afr.Freshwat.Fish. Res.Org., (1962–63):14–24
Gee, J.M., 1969. A comparison of certain aspects of the biology of Lates niloticus (Linne) in some East African lakes. Rev.Zool. Bot.Afr., 80(3–4): 244–61
Graham, M., 1929. The Victoria Nyanza and its fisheries. A report on the fish survey of Lake Victoria 1927–28, London, Crown Agents for the Colonies
Gulland, J.A., 1970. Food chain studies and some problems in world fisheries. In Marine food chains, edited by J.H. Steele. Edinburgh, Oliver and Boyd, pp. 296–315
Hamblyn, E.L., 1962. Nile perch investigation. Rep.E.Afr.Freshwat.Fish. Res.Org., (1961):23–8
Hamblyn, E.L., 1966. The food and feeding habits of the Nile perch, Lates niloticus (Linne) (Pisces: Centropomidae). Rev.Zool.Bot.Afr., 74(1–2):28 p.
Hopson, A.J., 1972. A study of the Nile perch (Lates niloticus (L.) Pisces: Centropomidae) in Lake Chad. Res.Publ., (19):93 p.
Jackson, P.B.N., 1971. The African Great Lakes fisheries, past present and future. Afr.J.Trop.Hydrobiol.Fish., 1:35–49
Kudhongania, A.W. and A.J. Cordone, 1974. Bathospatial distribution patterns and biomass estimate of the major demersal fishes in Lake Victoria. Afr.J.Trop.Hydrobiol.Fish., 3(1):15–31
Marten, G.F., 1979. Predator removal: effects on the fisheries yield in Lake Victoria (East Africa). Science, Wash., 203:646–8
Ogutu-Ohwayo, R., 1984. Predation by the Nile perch, Lates niloticus introduced into Lake Kyoga (Uganda) and its effects on the populations of fish in the Lake. M.Sc. Thesis. University of Dar-es-Salaam, Tanzania
Okedi, J., 1971. Further observations on the ecology of the Nile perch (Lates niloticus Linne) in Lake Victoria and Lake Kyoga. Annu.Rep.E.Afr.Freshwat.Fish.Res.Org., (1970):42–55
Stoneman, J. and J.F. Rogers, 1970. Increase in fish production achieved by stocking exotic species (Lake Kyoga, Uganda). Occas.Pap. Fish.Dep.Uganda, (3):16–9
Uganda Game and Fisheries Department, 1958. Annual report for 1956/57. Entebbe, Uganda Government Printers, 67 p.
Uganda Game and Fisheries Department, 1960. Annual report for 1959. Entebbe, Uganda Government Printers, pp. 54–5
Welcomme, R.L., 1966. The biological and ecological effects of climatic change on some fishes in the Lake Victoria basin. Ph.D. Thesis. Makerere University, Kampala.
Worthington, E.B., 1929. A report on the fishing survey of Lakes Albert and Kyoga. London, Crown Agents for the Colonies
Table 1: The frequency of occurrence of prey types within the stomachs of various sizes of Lates niloticus arranged by standard length classes to show the change of food with size of predator. Data obtained from Lake Kyoga over the period April 1978 to December 1982
| Standard length group of predator (cm) | 0 to 1.9 | 2.0 to 5.9 | 6.0 to 9.9 | 10.0 to 19.9 | 20.0 to 39.9 | 40.0 to 59.9 | 60.0 to 79.9 | 80.0 to 99.0 | 100 to 139 | 140 to 179 | Total | |
| Food item: | ||||||||||||
| Invertebrates | 7 | 757 | 431 | 480 | 295 | 34 | 1 | - | - | - | 2 005 | |
| Copepods | 3 | - | - | - | - | - | - | - | - | - | 3 | |
| Chaoborus | 1 | 13 | - | - | - | - | - | - | - | - | 14 | |
| Povilla | - | 23 | 37 | - | 1 | - | - | - | - | - | 61 | |
| Other | 1 | 88 | 24 | - | - | - | - | - | - | - | 113 | |
| Ephemeroptera Zygoptera | - | 44 | 21 | 2 | - | - | - | - | - | - | 67 | |
| Hemiptera | - | 22 | 5 | 2 | 1 | - | - | - | - | - | 30 | |
| Chironomid | 3 | 70 | 73 | 48 | 26 | - | - | - | - | - | 220 | |
| Caridina | - | 490 | 196 | 202 | 49 | - | - | - | - | - | 937 | |
| Anisoptera | - | 7 | 70 | 225 | 201 | 29 | - | - | - | - | 532 | |
| Molluscs | - | - | - | - | 16 | 5 | 1 | - | - | - | 22 | |
| Insect remains | - | 13 | 5 | 1 | 1 | - | - | - | - | - | 20 | |
| Fish: | 2 | 80 | 156 | 366 | 642 | 234 | 182 | 39 | 45 | 31 | 1 777 | |
| Rastrineobola | 1 | 50 | 97 | 217 | 408 | 95 | 56 | 3 | - | - | 927 | |
| Haplochromis | - | 3 | 16 | 26 | 69 | 45 | 30 | 1 | - | - | 190 | |
| Lates | - | 2 | 2 | 23 | 50 | 46 | 38 | 6 | 6 | 1 | 174 | |
| Oreochromis | - | 2 | 6 | 28 | 63 | 35 | 50 | 29 | 37 | 28 | 278 | |
| Protopterus | - | - | - | - | - | - | - | - | 2 | 2 | 4 | |
| Clarias | - | - | - | - | 1 | - | 1 | - | - | - | 2 | |
| Fish remains | 1 | 23 | 35 | 72 | 51 | 13 | 7 | - | - | - | 202 | |
| Others: | - | - | 5 | 10 | 10 | 6 | 2 | - | - | - | 33 | |
| Plant material | - | - | 2 | 5 | 10 | 5 | 2 | - | - | - | 24 | |
| Gravel | - | - | 3 | 5 | - | 1 | - | - | - | - | 9 | |
Table 2: A comparison of condition factor in L. niloticus in its native habitats with that introduced in Lakes Victoria and Kyoga. The figure in brackets shows the number of observations
| Lake | Year | Length class of Lates | Source of information | |||||
| Under 20 cm | 20–42.9 | Over 43 cm | ||||||
| Turkana | 1969 | - | 2.03 | (2) | 2.11 | (14) | Gee 1969 | |
| Albert | 1969 | 1.90 | (10) | 1.94 | (85) | 1.92 | (5) | Gee 1969 |
| Victoria | 1969 | 2.09 | (79) | 2.25 | (401) | 2.65 | (208) | Gee 1969 |
| Kyoga | 1969 | 1.82 | (1) | 2.19 | (12) | 2.36 | (32) | Gee 1969 |
| Kyoga | 1982 | 2.20 | (370) | 2.10 | (828) | 2.17 | (1 045) | Present study |
Table 3: The percentage contribution of the different fish species to the total weight of fish landed from the Kenya waters of Lake Victoria between 1976 and 1983
| 1976 | 1977 | 1978 | 1979 | 1980 | 1982 | 1983 | |
| O. esculentus | 0.3 | 0.2 | 0.8 | 0.3 | 0.3 | 0.7 | 0.1 |
| O. niloticus | 2.3 | 2.4 | 1.1 | 3.1 | 4.4 | 4.2 | 3.3 |
| O. variabilis | |||||||
| O. leucostictus | 2.9 | 4.8 | 6.1 | 5.5 | 13.9 | 2.5 | 2.1 |
| T. zillii | |||||||
| Bagrus | 5.5 | 5.9 | 5.9 | 5.8 | 2.4 | 4.2 | 1.6 |
| Lates | 0.5 | 1.1 | 4.5 | 14.0 | 16.0 | 54.4 | 67.7 |
| Protopterus | 5.0 | 4.0 | 2.6 | 1.5 | 1.4 | 0.4 | 0.1 |
| Haplochromis | 34.0 | 32.4 | 27.8 | 21.6 | 13.5 | 4.2 | 0.8 |
| Clarias | 13.4 | 9.8 | 7.2 | 9.9 | 4.5 | 3.4 | 1.2 |
| Barbus | 1.0 | 0.9 | 0.8 | 1.4 | 1.6 | 1.1 | 0.1 |
| Synodontis | 1.0 | 1.6 | 0.6 | 1.6 | 1.4 | 0.4 | 0.1 |
| Mormyrus | 0.5 | 0.5 | 0.6 | 1.2 | 1.2 | 0.4 | 0.3 |
| Labeo | 0.1 | 0.3 | 0.6 | 1.4 | 1.8 | 1.5 | 0.1 |
| Schilbe | 0.3 | 0.7 | 0.5 | 1.0 | 0.4 | 0.1 | 0.1 |
| Rastrineobola | 30.3 | 34.7 | 36.5 | 30.5 | 35.1 | 17.1 | 21.3 |
| Others | 2.4 | 1.4 | 1.4 | 1.1 | 2.0 | 1.6 | 1.2 |
| Total catch (metric tonnes) | 18 680.0 | 19 332.0 | 23 856.0 | 30 592.0 | 26 914.0 | 60 958.0 | 77 328.0 |
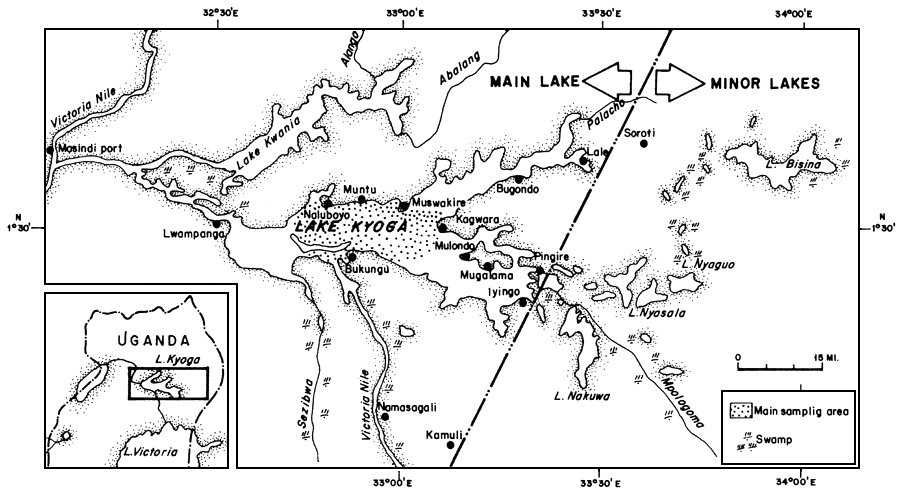
Figure 1 Map of Lake Kyoga showing the major fish landings and the main sampling area with a map showing its location in Uganda
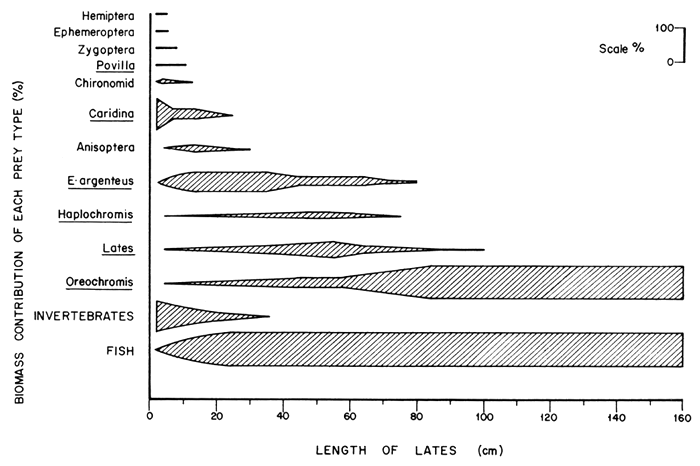
Figure 2 The relative importance of the different types of prey eaten by Lates niloticus expressed as a percentage of the total biomass of food consumed by predator of different size groups

Figure 3 The average number of prey consumed by L. niloticus of different sizes, illustrating how the number for different types of prey changed with predator size

Figure 4 The average length of different types of fish prey ingested by L. niloticus of different sizes for R. argenteus, Haplochromis spp., L. niloticus and O. niloticus. The average number of prey consumed showing that both length and nymber of prey varied with the size of the predator. The lines in each case are fitted by eye

Figure 5 The relationship between the length of L. niloticus and the size of prey. The average, maximum and relative length of prey are given. The vertical bars represent the size range of prey consumed
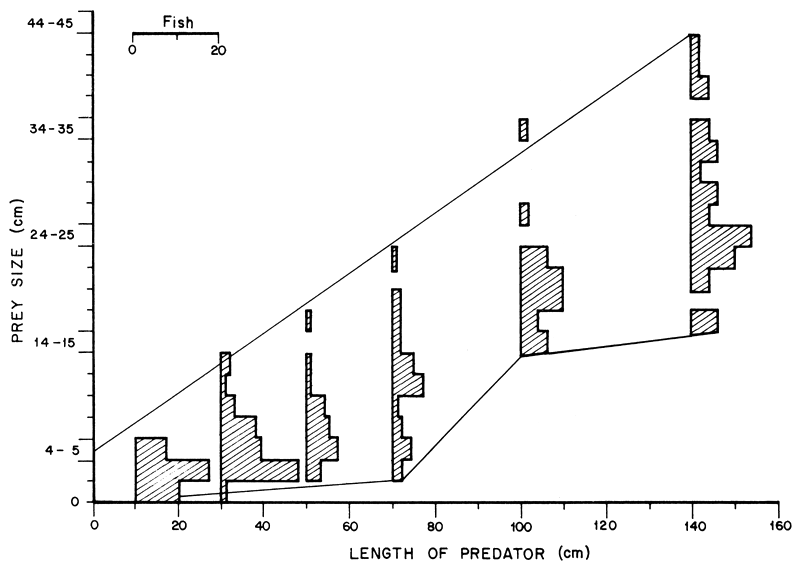
Figure 6 The length frequency distribution of O. niloticus and L. niloticus ingested by the predator. The data is grouped in 20-cm length groups for predators of less than 80 cm and 40-cm groups in the larger ones

Figure 7 The change in condition factor (K) with change in size of L. niloticus
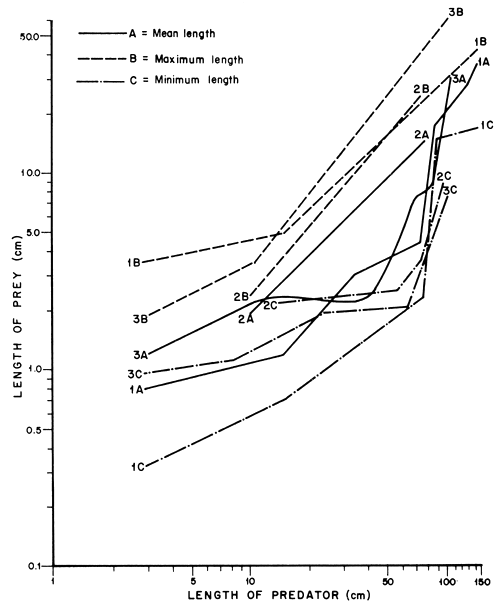
Figure 8 A comparison of prey size data in different habitats (based on (1) author; (2) Gee, 1969; and (3) Hopson, 1972)