(Z. Svobodová, B. Vykusová, J. Máchová)
The following physico-chemical changes in the aquatic medium are most frequently recorded as the immediate causes of fish injury and intoxication in fish culture practice.
Water temperature
Fish are poikilothermic animals, which means that their body temperature is the same as, or 0.5 to 1°C above or below, the temperature of the water they live in. The intensity of the metabolism of the fish is closely associated with water temperature: the higher the water temperature (the closer to the optimum values), the higher the metabolic rate. This fully applies to the warm-water fishes. The cold-water fishes, e.g. salmonids, whitefish, or burbot, have a different type of metabolism: their metabolism continues at comparatively low temperatures, whereas at high water temperatures, usually above 20°C, they lose much of their activity and eat much less food. Water temperature also has a great influence on the rise and course of a number of fish diseases. The immunity system of the majority of fishes shows optimum function at water temperatures about 15°C.
In their original area, fish easily tolerate seasonal change in temperature, e.g. a decrease to 0°C in winter and increase to 20–30°C (depending on species) in summer under Central European conditions, but these changes should not be abrupt. Temperature shock occurs if the fish are put into a new environment in which the temperature is 12°C colder or warmer (8°C in the case of salmonids). Such fish die, showing symptoms of paralysis of the respiratory and cardiac muscles with early fry problems may even arise when the difference in temperature is as low as 1.5 – 3°C. If fish are fed and then abruptly transferred to water colder by 8°C or more, their digestion process will slow down or stop. The food will remain undigested or half-digested in the digestive tract and the gases produced bloat the fish which lose balance and die. In carp, given high-nitrogen food (natural food, high-protein pellets), abrupt transfer to much colder water highly increases the level of ammonia nitrogen in the blood serum and the decrease in metabolic rate reduces ammonia elimination through the gills. This leads to ammonia autointoxication and death.
Great progress has recently been recorded in warm water fish culture. Water temperature control offers good conditions for optimally encouraging the fish to grow and to utilize their growth potential for maximum weight gains.
Water pH
The pH levels optimum to the fish range from 6.5 to 8.5. Levels of pH above 9.2 and below 4.8 damage and kill salmonids (mainly brown and rainbow trout) and pH values above 10.8 and below 5.0 may be fatal to cyprinids (especially carp and tench). Salmonids, compared with cyprinids, are more vulnerable to high pH and more resistant to low pH. American char is especially resistant to low pH: it tolerates pH levels as low as 4.5–5.0.
Low water pH most frequently occurs in spring when the snow thaws, mainly in peat bog areas. High water pH in turn, occurs in eutrophicated reservoirs (ponds) where the green plants (the blue-green algae, green algae and higher aquatic plants) consume too much CO2 for their intensive photosynthetic assimilation. This affects the neutralizing capacity of the water and increases its pH to 9.0–10.0. Water pH also changes when acids, hydroxides or other acid or alkaline substances leak into the water courses, ponds or lakes.
To defend itself against the effect of a low or high water pH, the fish body produces an increased amount of mucus on the skin and on the inner side of the gill covers. Extremely high or low pH values cause damage to the tissues, especially the gills, and haemorrhages may occur on the gills and on the lower part of the body. Excess amounts of mucus, often containing blood, are observed post mortem on the skin and gills. The mucus is dull-coloured and watery.
Water pH also has a significant influence on the toxic action of a number of other substances (ammonia, sulphane, cyanides, toxic metals and others) on the fish.
Oxygen
Oxygen enters the water in diffuse form from the air and also through the photosynthetic assimilation of aquatic plants. On the other hand, oxygen is removed by anaerobic decomposition of organic substances, by the oxidation of some organic compounds and through the respiration of the organisms present in the water. The concentration of the oxygen dissolved in water can be expressed in mg per litre or as percentage of saturation. Water temperature, atmospheric pressure and contents of salts dissolved in water have to be taken into account when the values in mg per litre are converted to saturation % or vice versa.
Different fishes have different requirements for the concentration of oxygen dissolved in water. Salmonids are especially particular about oxygen in the water they live in: for them the optimum concentration is 8–10 mg per litre, and if the level declines below 3 mg per litre they show symptoms of suffocation. Cyprinids are less demanding: they feel best in water containing 6–8 mg per litre and show symptoms of suffocation when oxygen concentration falls to 1.5–2 mg per litre.
Oxygen demand in fish also depends on water temperature, pH, CO2 level, stress, metabolic rate and other factors. The major criteria in oxygen demand in fish include temperature, average individual weight and total weight of the fish per unit volume of water. Oxygen demand increases at a higher temperature and higher total weight of fish per unit volume of water (e.g. an increase in water temperature from 10 to 20°C doubles the oxygen demand).
Oxygen demand significantly declines with increasing individual weights. In carp this decline may be expressed by the following indices: yearling = 1, two-year-old carp = 0.5 – 0.7, market carp = 0.3 – 0.4. Significant differences in oxygen demand are also recorded between different species. Putting coefficient 1 as expressing oxygen demand in common carp, the converted values for some other fishes will be as follows: trout 2.83, peled 2.20, pike-perch 1.76, roach 1.51, sturgeon 1.50, perch 1.46, bream 1.41, pike 1.10, eel 0.83, tench 0.83.
The factor most frequently responsible for decreases in oxygen concentration in water (oxygen deficiency*) is water pollution with organic substances (waste waters from agriculture, food industry, public sewage). The organic substances decompose in water and take from the water the oxygen they need for the decomposition. The concentration of organic substances in water and their capacity of taking oxygen from the water are evaluated by means of the chemical oxygen demand (COD) and biochemical oxygen demand for five days (BOD5). The COD level, determined by the Kubela method and considered as optimum for cyprinids in pond or river waters (CODMn), is up to 20–30 mg O2 per litre, and the optimum BOD5 for cyprinids is up to 8–15 mg O2 per litre, depending on the intensity of the culture. For salmonids the respective levels are up to 10 mg O2 per litre and up to 5 mg O2 per litre.
In winter, fish are most frequently killed by suffocation in polluted storage ponds and in summer this most frequently happens in polluted water courses at a high temperature and low flow rate. In severely eutrophicated ponds, oxygen deficiency often occurs in summer early in morning as a result of increased oxygen consumption for the bacterial decomposition of organic substances and for dissimilation in aquatic plants. In highly nutritive ponds (e.g. the sewage ones) with a constant inflow of decomposed organic substances, oxygen deficiency is also caused by excess development of zooplankton: the zooplankton itself needs plenty of oxygen and, in addition, its feeding pressure considerably suppresses oxygen producers (phytoplankton).
* Oxygen deficiency is the additional oxygen concentration needed to reach saturation
Oxygen deficiency elicits symptoms of suffocation and fish of different species successively die, depending on how much oxygen each species needs. Fish exposed to oxygen deficit do not take food, move near the water surface, gasp for air (cyprinids), gather at the inflow (in ponds), are torpid, fail to react to irritation, lose escaping reflex and die. The major pathologico-anatomic changes include a very pale skin colour, congested to the cyanotic gills, the gill fringes stuck together, small haemorrhages in the front chamber of the eye and in the skin of the gill covers. In the majority of predatory fishes the mouth is spasmodically open and the opercles of the gills loosely stick out.
Damage caused to fish by too much oxygen in water is seldom encountered. This may happen, for example, when fish are transported in polythene bags with oxygen atmosphere. The oxygen saturation of water critical to fish is 250 to 300 %. The fish may be injured at saturation values above this level: the gills of the affected fish have a conspicous light red colour and the ends of the gill fringes fray. When such fish are used for stocking they suffer from secondary fungus infestation and some of them may die.
Ammonia
Ammonia pollution of water courses, ponds and lakes may be of organic origin (public sewage, focal and large-scale agricultural pollution, biochemical reduction of nitrates and nitrites contained in water) or of inorganic origin (industrial waste waters from gas works, coking plants and generator stations). In water or in biological liquids, ammonia is present either in its molecular (nondissociated) form (NH3) or in the form of the ammonia ion (dissociated) (NH4+). The ratio of these two forms depends on the pH and on water temperature (Table 8). For the toxic action it is important that the cell wall is comparatively insoluble to the ammonia ion (NH4+), but molecular ammonia (NH3) penetrates the tissue barriers very easily, so it is toxic to fish. Under normal conditions there is an acidobasic balance on the tissue barriers. If this balance is broken, the side on which the pH is lower intercepts molecular ammonia. This explains how molecular ammonia passes from water through the epithelium of the gills to the blood and also how it passes from the blood to the tissues. Ammonia possesses special affinity for the brain: this is why nervous symptoms are so strong in cases of ammonia intoxication of fish.
Water quality monitoring in water courses, lakes and fish culture facilities includes determination of total ammonia. From the viewpoint of toxic action it is important to know the concentration of nondissociated toxic ammonia (NH3). This is determined from the measured values of total ammonia (NH4++NH3), temperature (T) and water pH, using the formula:
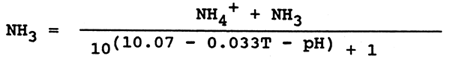
or the values are read from a table 8 compiled from calculations on the basis of that formula. Besides water temperature and pH, the factors that influence ammonia toxicity include the concentration of oxygen dissolved in water. The lower the oxygen concentration in water the greater the toxicity of ammonia (Fig. 19).
Nondissociated ammonia is highly to very highly toxic to fish. The LC50 value, determined in the acute toxicity test, ranges between 1 and 1.5 mg NH3 per litre in cyprinids and between 0.5 and 0.8 mg NH3 per litre in salmonids. The maximum admissible ammonia (NH3) concentration is 0.05 mg per litre for cyprinids and 0.0125 mg per litre for salmonids.
Table 8 : Dependence of NH3 content (as % of total ammonia) on water pH and temperature
| pH | t °C | |||||
| 0 | 5 | 10 | 15 | 20 | 25 | |
| 7.0 | 0.082 | 0.12 | 0.175 | 0.26 | 0.37 | 0.55 |
| 7.2 | 0.13 | 0.19 | 0.28 | 0.41 | 0.59 | 0.86 |
| 7.4 | 0.21 | 0.30 | 0.44 | 0.64 | 0.94 | 1.36 |
| 7.6 | 0.33 | 0.48 | 0.69 | 1.01 | 1.47 | 2.14 |
| 7.8 | 0.52 | 0.75 | 1.09 | 1.60 | 2.32 | 3.35 |
| 8.0 | 0.82 | 1.19 | 1.73 | 2.51 | 3.62 | 5.21 |
| 8.2 | 1.29 | 1.87 | 2.71 | 3.91 | 5.62 | 8.01 |
| 8.4 | 2.02 | 2.93 | 4.23 | 6.06 | 8.63 | 12.13 |
| 8.6 | 3.17 | 4.57 | 6.54 | 9.28 | 13.02 | 17.95 |
| 8.8 | 4.93 | 7.05 | 9.98 | 13.95 | 19.17 | 25.75 |
| 9.0 | 7.60 | 10.73 | 14.95 | 20.45 | 27.32 | 35.46 |
| 9.2 | 11.53 | 16.00 | 21.79 | 28.95 | 37.33 | 46.55 |
| 9.4 | 17.12 | 23.19 | 30.36 | 39.23 | 48.56 | 57.99 |
| 9.6 | 24.66 | 32.37 | 41.17 | 50.58 | 59.94 | 68.63 |
| 9.8 | 34.16 | 43.14 | 52.59 | 61.86 | 70.34 | 77.62 |
| 10.0 | 45.12 | 54.59 | 63.74 | 71.99 | 78.98 | 84.60 |
| 10.2 | 56.58 | 65.58 | 73.59 | 80.29 | 85.63 | 89.70 |
| 10.4 | 67.38 | 75.12 | 81.54 | 86.59 | 90.42 | 93.24 |
| 11.0 | 89.16 | 92.32 | 94.62 | 96.26 | 97.41 | 98.21 |
The first symptoms of ammonia intoxication include slight restlessness, accelerated respiration, the fish stay close to the water surface, intoxicated cyprinids gasp for air, the restlessness increases, motion accelerates, respiration becomes irregular, and then follows the excitation stage. The fish intensely react to outside stimuli, lose balance, leap above the water surface and their muscles twitch in tonic-clonic spasms. The affected fish lie on one side and keep the mouth and gill covers wide open in spasms. Then follows a short period of apparent recovery. The fish return to normal gait and look slightly restless. This stage is later replaced by another severe excitation; the body surface becomes pale and the fish die.

Fig. 19: If there is not enough oxygen in the water, nondissociated ammonia kills the fish: fatal cases; + waters with high concentration of nondissociated ammonia in which no cases of injury to fish did occur in March to April; xxx - lethal boundary of nondissociated ammonia
The skin of ammonia-intoxicated fish is light coloured, covered with plenty or excess of mucus. In some cases there are small haemorrhages, mainly at the bases of the pectoral fins and in the fore chamber of the eye. The gills are heavily congested and filled with plenty of mucus; the gills of fish exposed to high ammonia concentrations may slightly to severely bleed. Intense production of mucus can be observed on the inner side of the gill covers, mainly on the end flap of the gill cover arch. The organs inside the body cavity are congested and parenchymatous, showing dystrophic alterations.
In recent years, great losses in carp culture have been caused by the so-called toxic necrosis of the gills. The factors responsible for the rise of this disease include ammonia intoxication at which the ammonia level in the blood is highly increased. Ammonia is, at the same time, the final product of nitrogen metabolism in carp and most of it is eliminated through the gills to the water. If this elimination is reduced for some reason or another (high water pH, oxygen deficit, damaged gills etc.), the ammonia level in the blood is highly increased, causing a condition called autointoxication of the fish.
A very interesting case of autointoxication of carp yearling (C1) with extremely high ammonia N levels in the blood serum was diagnosed after the transfer of the fish from the pond to well water in large aquarium tanks. Part of the fish caught and transferred during the forenoon exhibited typical symptoms of ammonia intoxication the following morning. These symptoms included great restlessness, accelerated respiration, leaping above the water surface, inco-ordination of motion, and tonic-clonic spasms of the muscles. The skin of the affected fish was light in colour, the gills were heavily congested, dark red and oedematously swollen (the swelling was particularly severe on the edges of the gill fringes). The histopathological changes in the gills corresponded with what was described with toxic necrosis of carp gills. The digestive tract of the fish with severe symptoms of intoxication was filled with undigested food. On the other hand, fish that had evacuated (faeces on the bottom of the tank, gut almost empty), were free of symptoms of toxic damage. The average blood serum level of ammonia N in the fish with symptoms of intoxication was 3054 (2400 – 3600) μg per 100 ml of serum, whereas in the fish free of such symptoms the ammonia level was 825 (750 – 900) μg per 100 ml of serum. In the affected fish the autointoxication, caused by an enormous increase in the ammonia N level in the blood serum, was probably due to the persistence and absorption of the digesta (natural food, high-protein feed pellets) in the digestive tract of the carp exposed to stress (transport, reduced oxygen level during the transport, water temperature reduced by about 5°C).
Considering this case of ammonia autointoxication of carp, some other unexplained abrupt deaths of fish may be ascribed to a similar cause. Such events may happen mainly on carp farms with a high intensity of feeding high-nitrogen feeds if the fish are exposed to stresses such as abrupt oxygen deficit, abrupt change in water temperature and others.
Nitrites, nitrates
Nitrites as a rule accompany nitrates and ammonia nitrogen in surface waters but their concentrations are low because of their low stability. They readily oxidize or are readily reduced, both chemically and biochemically. Nitrates are the final product of the decomposition of organic nitrogen compounds in aerobic medium. They are present at small concentrations in all surface waters. There is almost no nitrate interception in the soil, so nitrates are washed down to water courses, ponds and lakes. The main source of nitrate pollution of surface waters is the application of nitrogenous fertilizers and manure to farm soil.
Nitrites have a variable toxicity to fish: the variability depends on a number of internal and external factors (fish species and age, water quality and others). The importance and role of many of the factors are continuously checked and reviewed. Different authors often assert contradictory conclusions and still fail to offer a definitive explanation of either the mechanism of nitrites toxic action on fish or the involvement of different environmental factors.
According to the latest findings, nitrite ions get into the fish bodies with the help of chloride cells and the main route of their penetration is via the gills. In the blood, nitrites are bound by haemoglobin, giving rise to methaemoglobin: this reduces the oxygen-transporting capacity of the blood. The increase in the amount of methaemoglobin manifests itself as the brown colour of the blood and gills. If the amount of methaemoglobin in the blood does not exceed 50 % of the total haemoglobin, the fish usually survive. If the fish have more methaemoglobin in their blood (70–80 %) they become torpid, and with a further increase in the methaemoglobin level they lose orientation and are unable to react. Nevertheless, in spite of all that, the fish may survive because the erythrocytes in their blood contain the enzyme reductase which converts methaemoglobin to haemoglobin. This process can return haemoglobin to its normal level in 24–48 hours if the fish are put into nitrite-free water.
A great differentation in nitrite toxicity to fish kept in water having different properties is asserted by LEWIS and MORRIS (1986). In their investigations the 96h LC50 for rainbow trout ranged from 0.24 to 12.20 mg per litre, depending on chloride content in the diluting water (in the given case the chloride content in water ranged from 0.35 to 40.9 mg per litre). The effect of chlorides on nitrite toxicity is so significant that the results of tests conducted without recording chloride concentrations in water cannot be compared with other tests.
Nitrite toxicity is also influenced (reduced) by hydrogen carbonates, potassium, sodium, calcium and other ions, but the effect is not so great as that of chlorides. Among these ions, those of potassium are most significant; the importance of sodium and calcium is lower. These monovalent ions compete with nitrites in their penetration into the fish body and inhibit the passage of nitrites through the gills.
The pH value was also considered to be important for nitrite toxicity: pH and temperature control the balance between NO2- and nondissociated HNO2 and it was believed that the amount of nitrites in the blood plasma of fish depends on the penetration of nondissociated HNO2 to the fish body through the gills. Nevertheless, the results of later experiments denied these theories and demonstrated that within the acidity /alkalinity/ range normally encountered in waters the pH is not so important for the toxicity of nitrites.
Another factor that influences nitrite toxicity is the amount of dissolved oxygen in combination with water temperature. This is associated with the fact that fish need more oxygen when their blood contains methaemoglobin which cuts the oxygen-carrying capacity of the blood.
Long exposure to sublethal concentrations of nitrites causes no great damage to the fish. Concentrations corresponding to 20–40 % of the levels that have a lethal action on the fish may slightly depress growth but no serious damage has ever been recorded.
For estimating the safe nitrite concentration in each particular case it is recommended to monitor the weight ratio of chloride and nitrite concentrations. These ratios (mg.1-1 C1- : mg.1-1 N-NO2-) are recommended to be around 17 for rainbow trout and around 8 for fish of low economic importance.
As to nitrates, their toxicity to fish is very low. Toxic action of nitrates is only recorded when their concentration is above 1000 mg per litre. 80 mg per litre is considered to be the maximum admissible nitrate concentration for carp and 20 mg for rainbow trout. In surface waters and in fish farming facilities where the water contains plenty of oxygen with no danger of denitrification (i.e. conversion of NO3- to NO2- and then to elementary nitrogen or N2O and NO), it is not so important to monitor the concentrations of nitrates.
Sulphane (hydrogen sulphide)
Sulphane occurs in organically polluted waters where proteins decompose. Sulphane is also present in industrial waste waters (from metallurgical and chemical works, effluents from pulp plants, tanneries and other plants). Sulphane is highly to very highly toxic to fish. The lethal concentrations to different fishes range from 0.4 mg H2S per litre (salmonids) to 4 mg per litre (crucian carp, tench and eel). The toxicity of sulphane decreases with increasing water pH (nondissociated toxic H2S changes into the less toxic HS- ions). The concentration of nondissociated sulphane is determined from the measured total sulphane values (HS- + H2S + S2-) and from the pH value of the water. The formula is:
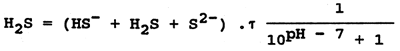
where τ = activity coefficient depending on the ionic strength of water. For natural water it is ± 0.92.
Carbon dioxide
Carbon dioxide is dissolved in water in its molecular state; only 10 % of it forms carbonic acid H2CO3. These two forms in which carbon dioxide occurs constitute what is called free CO2. The ionic forms, i.e. fixed carbon dioxide, are represented by the hydrogen carbonate and carbonate ions (HCO3- and CO32-, respectively). Their presence in the aquatic medium is important for the neutralization capacity of water. The amounts of CO2 present in flowing surface waters are typically in the order of mg per litre, seldom above 20 to 30 mg per litre. In stagnant surface waters the CO2 levels are stratified because of phytoplankton photosynthetic assimilation: the upper strata usually have less free CO2 than the lower strata. It may happen in the surface strata that all the free CO2 may be consumed for photosynthesis and the water pH may thus increase above 8.3. Common underground waters usually contain several tens of mg of free CO2 per litre.
The action of carbon dioxide is either direct or indirect. The indirect action of both free and bound CO2 is exerted on the fish through its influence on water pH. A direct adverse effect takes place when there is an excess or lack of free CO2. In low-oxygen waters in which intensive microbial processes take place when the fish are stored or transported, and also when poorly aerated ground waters are used, high concentrations of free CO2 may be a danger to the fish. In such cases the fish are unable to excrete as much CO2 as they should, their blood loses its acidobasic balance, and acidosis develops. CO2 and O2 exchange in the blood is limited, the fish accelerate their respiration, become restless, stagger, and may die. Twenty mg free CO2 per litre is considered the maximum permissible free CO2 concentration for trout and 25 mg free CO2 per litre is the maximum for carp (if the acid capacity is 0.5 mmol per litre at a pH of up to 4.5). The sensitivity of fish to free carbon dioxide declines with increasing acid capacity of water.
However, what happens much more frequently is a lack of free carbon dioxide in water. Carbon dioxide deficiency occurs when too much free CO2 is consumed in the phytoplankton photosynthetic assimilation, when the water used in thermal power plants is artificially softened, when water is aerated more intensively than needed and in other cases. Free carbon dioxide concentrations below 1 mg per litre break the acidobasic balance in the fish bodies and cause alkalosis. A lack of free carbon dioxide is particularly dangerous for cyprinid fry when they pass from endogenous to exogenous nutrition. Cyprinid fry breathe through their body surface and are unable to regulate their acidobasic balance by respiration. Low partial pressure of free CO2 in water is conducive to a high CO2 output from the body, to alkalosis and finally to death. If the fry of cyprinids suffer from free CO2 deficiency, they gather close to the water surface and exhibit symptoms of suffocation though the concentration of oxygen in the water is high enough.
Chlorine
Active chlorine* enters into the water courses, lakes and ponds with the effluents discharged from the textile and paper plants. Chlorine and the coumpounds that release active chlorine are used as dissinfectants in both human and veterinary medicine. Chlorinated lime serves for total dissinfection of pond bottoms (application rate of 600 kg per ha), storage ponds and other facilities for fish culture and transport. If fish suffer from a disease of the gills it is recommended to spread chlorinated lime on the surface of the pond at a rate of 10–15 kg per ha (if the average depth of the pond is 1 m). Overdosage or improper handling of chlorine or chlorine-releasing compounds may damage or kill the fish. Fish may also be damaged by chlorine if retailers keep them in tanks with intensive flow of chlorinated tapwater which contains 0.05 to 0.3 mg active chlorine per 1 litre.
Active chlorine is very toxic to fish. Its toxicity largely depends on water temperature: for example, an active chlorine concentration of 3.5 mg per litre has a sublethal action on carp at a water temperature of 3–7°C but when carp are exposed to the same concentration of active chlorine at a temperature of 15–20°C they die in 1 to 2 hours. Generally it can be said that long exposure to active chlorine concentrations of 0.04 to 0.2 mg per litre are toxic to the majority of fishes.
Active chlorine may affect the fish both locally (the skin and gills) and systemically (when chlorine is absorbed into the blood). The systemic affection manifests itself mainly as nervous disorders. The clinical symptoms of chlorine intoxication include great restlessness, leaping above the water surface, cramps, lying on the side and spastic movements of the mouth, fins and tail. The spasms of the mouth hinder respiration, the fish suffocate, fall into depression and die. The skin and gills of the intoxicated fish are covered with a thick layer of mucus and if the concentration of active chlorine is very high the gills are congested and may bleed. The body surface of the poisoned fish is pale and the margins of the gill fringes and fins are covered with a grey-white coating. Histopathologically, there is a marked dystrophy, necrobiosis to necrosis, with desquammation of the respiratory epithelium of the gills and the epidermis of the skin.
Cyanides
As far as cyanides occur in waters, they are not of natural origin: they come from different industrial effluents and/or from plating shops and from thermal processing of coal. Cyanides may be present in waters either as simple compounds (nondissociated HCN, simple CN- ions) or as complex compounds (e.g. complex iron, cobalt, nickel cyanides and others). Simple cyanides are very toxic up to extremely toxic to fish. The lethal concentrations for the majority of fishes range between 0.03 and 0.5 mg per litre. The toxicity of these substances is conditioned by the pH value of the water: if the water pH is low the proportion of nondissociated HCN increases and so does the toxicity (Table 9). Cyanide toxicity is also markedly enhanced by increasing water temperature and decreasing concentration of oxygen dissolved in water. In complex cyanides the toxicity varies considerably with their ability to split off HCN. For example, the complex iron cyanides are of low to very low toxicity to fish but the complex cyanides of zinc, cadmium, copper and mercury are highly toxic. The concentrations of different cyanide compounds proposed as the maximum admissible levels in fish culture range between 0.002 and 0.02 mg per litre.
Table 9: The dependence of HCN content (% of simple cyanide content) on water pH
| pH | % HCN | % CN- |
| 7 | 100 | 0 |
| 8 | 93 | 7 |
| 9 | 60 | 40 |
| 10 | 10 | 90 |
| 11 | 2 | 98 |
The mechanism of the toxic action of cyanides is based on the inhibition of the respiratory enzymes (cytochromoxidase). This blocks the transfer of oxygen from the blood to the tissues, damages tissue respiration and leads to tissue asphyxia. The clinical symptoms of the cyanide poisoning of fish include laboured respiration, nervous disorders and a long agony. If the fish are transferred to clean water while they are in the stage of loss of balance they will recover in 1 to 2 hours. The characteristic features of the patho-anatomic finding in cases of cyanide poisoning include cherry red colour of the gills and sometimes also the presence of transsudate with an admixture of blood in the body cavity.
Metals and metal salts
Trace quantities of metals present in waters are of natural origin. If waters are polluted with metals at greater concentrations, the metals can be traced back to ore mining and processing, to smelting plants, rolling mills plants for the surface treatment of metals, film, textile and leather industries and other sources. Atmospheric precipitation polluted by fumes generated by the burning of fossil fuels and by the exhausted gases of motor vehicles may be another source.
The mechanism of the toxic action of metals on fish varies. Most of the metals have a great affinity for amino acids and the SH groups of proteins: as such, they act as enzymic poisons. The toxicity of metals to fish is significantly influenced by the form of their occurrence in water. Inorganic or organic insoluble or hardly soluble complexes are usually less toxic than simple ions. The adverse action of metals is particularly strong in the early stages of development of the fish. Another important adverse property of many metals is their ability of amply accumulating in the sediments and in the aquatic flora and fauna (biological accumulation). This property is quantitatively described by the accumulation (concentration) coefficient whose values range from several hundred to hundreds of thousands. Mercury, selenium and some other metals have a particularly high bioaccumulating capacity. Hence, the concentration of these metals in water does not provide a true indication of the actual over-all pollution of the aquatic medium: it is better to use the content of metals in the sediments and especially in the bodies of predatory fishes, which are the final link in the food chain in the aquatic medium.
The metals of the highest ichthyotoxicological importance include aluminium, chromium, iron, nickel, copper, zinc, arsenic, cadmium, mercury and lead.
Aluminium
The toxicity of aluminium to fish depends, to a considerable extent, on the physico-chemical properties of the water and particularly on its pH, underlying the form of occurrence of aluminium: at a higher water pH there is a higher concentration of the dissolved forms and a higher toxicity of aluminium to fish. The concentration of the dissolved forms of aluminium is also great when the pH is very low, about 4.
In aluminium toxicity tests, rainbow trout fry were exposed to different aluminium concentrations at pH 7. A concentration as low as 0.52 mg aluminium per litre was recorded to markedly reduce the growth of these fish. When a still lower concentration, 0.05 mg per litre, was tested no adverse effect was exerted on the growth of rainbow trout fry, so it can be regarded as safe. A mass kill of maraena and peled fry, reared in water clarified by means of aluminium sulphate, can be mentioned as an example of practical experience. Aluminium concentration in that water was up to 0.3 mg per litre and the pH value was between 7 and 7.5. All the fry of maraena and peled died in about 10-14 days of hatching.
Chromium
In surface waters, chromium can be present in oxidation states III and VI. Chromium compounds in oxidation state III are more toxic to fish and other aquatic organisms than are those in oxidation state VI. According to the LC50 for different fishes, chromium compounds in oxidation state III can be included among substances of a high toxicity to fish (LC50 2 to 7.5 mg per litre) and chromium compounds in oxidation state VI can be included among substances of medium toxicity (LC50 35 to 75 mg per litre). The toxicity of chromium compounds to fish is also considerably influenced by the physico-chemical properties of water, especially by the pH value and the concentrations of calcium and magnesium. At a low pH and at high concentrations of calcium and magnesium the toxicity of chromium to aquatic organisms is low. At acute intoxications by chromium compounds, the body surface of the fish is covered with mucus, the respiratory epithelium of the gills is damaged and the fish die with symptoms of suffocation. Fish suffering from chronic chromium intoxication accumulate an orange yellow liquid in their body cavity.
Iron
In surface waters, iron occurs in oxidation state II (soluble compounds) or oxidation state III (mostly insoluble compounds). The ratio of these two forms of iron depends on oxygen concentration in water, on water pH and on other chemical properties of the water where the fish are kept. Cases of damage caused to fish by iron compounds may occur in low-oxygen waters of a low pH where the iron is present mainly in the form of soluble compounds. On the gills of the fish, showing an alkaline reaction, iron in oxidation state II oxidizes into insoluble compounds of oxidation state III, which cover the gill fringes and hinder respiration. At a low water temperature and in the presence of iron, iron-depositing bacteria will propagate at a high rate on the gills and contribute to the oxidation of iron compounds in oxidation state II. Their filamentous colonies cover the gills: first they are colourless but later the deposited iron colours them brown. The precipitated iron compounds and tufts of the iron bacteria reduce the gill area available for respiration, damage the respiratory epithelium and may choke the fish. Like on the gills of adult fish, iron compounds act on the eggs which die of the lack of oxygen.
The lethal concentration of iron for fish is not easy to determine because it depends on the physico-chemical properties of the water. It is generally required in cyprinid culture that the concentration of the soluble forms of iron should not be greater than 0.2 mg per litre; for salmonids this limit is 0.1 mg per litre.
Nickel
Nickel can get into the surface waters with the effluents from the plants where metals are surface-treated. Nickel compounds are of medium toxicity to fish. In short exposure, the lethal concentration is between 30 and 75 mg per litre. Like the toxicity of other metals, the toxicity of nickel compounds to aquatic organisms is markedly influenced by the physico-chemical properties of water. For example, in low-calcium and low-magnesium waters the lethal concentrations of nickel compounds for the stickle-back were below 10 mg per litre. In such cases nickel is highly toxic to fish. After intoxication with nickel compounds, the gills of the poisoned fish are filled with slime and are dark red in colour.
Copper
Though copper is highly toxic to fish, its compounds are used in fish culture and fisheries as algicides and as substances that are useful in the prevention and therapy of some fish diseases. The physical and chemical properties of water exert a strong influence on the toxicity of copper to fish. In water containing organic substances at a high concentration copper produces insoluble complex compounds. In high-pH water it produces low-solubility compounds of alkaline nature (hydroxides), and in waters of a high acid capacity (alkalinity) copper precipitates as low-soluble or insoluble cupric carbonate. For compounds that are reluctant to dissolve or are insoluble it is difficult to penetrate into the fish body, so their toxicity to fish is low. It will be a good example to compare the different LC50 levels for carp as recorded during 48 hours exposure to CuSO4.5H2O in pond water [ph 7.6, acid capacity (up to a pH of 4.5) 2.2 mmol per litre, CODMn 32 mg O2 per litre] and in well water [pH 6.2, acid capacity (up to a pH of 4.5) 0.6 mmol per litre, CODMn 2.2 mg O2 per litre]: in the pond water the LC50 was 8.1 mg per litre and in well water it was 1.5 mg per litre.
The highest copper concentration in water still admissible from the viewpoint of the safety of fish ranges from 0.001 to 0.01 mg per litre, depending on the physical and chemical properties of water and on the species of the fish. The characteristic clinical symptoms of fish intoxication with copper and copper compounds include laboured breathing and, in cyprinids, gasping for air on the water surface. The typical patho-anatomic finding includes a large amount of mucus on body surface, under the gill covers and in the gills. Acute copper intoxication can be diagnosed on the basis of a chemical analysis of the gills in which the concentration of copper is much greater than in other parts of the body of the fish. The gills of fish caught in waters free of copper contamination contain up to 10 mg of copper per 1 kg of dry matter.
Zinc
Zinc intoxication of fish is most frequently encountered in trout culture. Rainbow trout and brown trout, and especially their fry, are extremely sensitive to zinc and its compounds. The lethal concentrations are around 0.1 mg per litre in salmonids (some authors even assert a level of 0.01 mg per litre) and 0.5 to 1.0 mg per litre in cyprinids. Resistance to zinc compounds increases with age. The toxicity of zinc to fish is influenced by the chemical characteristics of water. The clinical symptoms and patho-anatomic picture of the zinc intoxications in fish are similar to those in exposure to copper. The best prevention of the frequent intoxications with zinc compounds in trout culture is to avoid using galvanized pipes for the supply of water and to avoid using galvanized containers and equipment.
Arsenic
Arsenic as a rule occurs in water in oxidation state V but some of it may also be present there in non-stable forms, i.e. in oxidation state III. Like with mercury, biological activity may lead to the formation of organic methyl derivatives of arsenic. The main sources of arsenic pollution in surface waters include industrial effluents e.g. from tanneries, ore processing plants and dyestuff production plants. Arsenic is able to cumulate in large quantities in sediments on the bottom of water courses and reservoirs and in the aquatic organisms. Arsenic compounds in the third oxidation state (arsenites) are absorbed fairly fast into the fish body and are more toxic than arsenic compounds in oxidation state V (arsenates). According to lethal concentrations during 48 hours of exposure of different fishes, diarsenic trioxide, for example, can be included among substances of a medium to high toxicity to fish. Lethal concentrations are between 3 and 30 mg per litre.
Cadmium
Cadmium in waters accompanies zinc but its concentrations are much lower. The cadmium present in surface waters may be either dissolved or undissolved. As to the dissolved forms, those which may be poisonous to fish include the simple ion and the different inorganic and organic complex ions. Apart from the toxic action similar to that of other poisonous metals (damage to the central nervous system and parenchymatous organs), cadmium - though in trace amounts - may have some specific effects if exposure to its action is long. Chief among the specific effects are those exerted on the reproduction organs. An adverse influence of long exposure to cadmium upon the maturation, hatchability and development of larvae in rainbow trout was recorded at concentrations as low as 2 to 20 mg per litre. Cadmium toxicity declines with increasing contents of calcium and magnesium in water. For salmonids the highest admissible cadmium concentration in water is 0.0002 mg per litre and for cyprinids 0.001 mg per litre.
Mercury
Mercury is carried to the aquatic medium mainly with industrial effluents and atmospheric precipitation. Unpolluted waters permanently contain a trace amount of mercury which does not exceed 0.1 μg per litre.
Mercury concentration determined in surface waters is not a true measure of either the actual amount of mercury or the mercury pollution of the aquatic medium: mercury passes from water to the sediments on the bottoms of water courses, lakes and reservoirs and accumulates there mostly in the sulphide form. Elementary mercury and its organic and inorganic compounds undergo methylation (a process induced by the activity of microorganisms) in the bottom sediments. The toxic products of this methylation (methyl mercury) enter the food chains and accumulate in increased amounts in aquatic organisms.
Mercury penetrates into the fish bodies with food via the alimentary tract and the other routes are through the gills and skin. Absorption from the alimentary tract has proved to be of the greatest importance in mercury accumulation process: evidence is provided by the results of investigation at sites in the drainage area of the Berounka river in Central Bohemia. The total mercury content in the flesh of fish in these localities is about 10 times that recorded in the food organisms. This coefficient of cumulation can be compared with the food efficiency coeficient of the fish living in open waters and feeding on the aquatic invertebrates. Of the other aquatic organisms in the drainage area of the Berounka river, the greatest mercury levels were recorded in leeches: this can be ascribed to their exclusively predatory mode of feeding. With their wide distribution in different types of waters, leeches (Helobdella stagnalis) may also be considered as good indicators of mercury contamination of the aquatic medium.
Fish as the final link in the food chain in water contain the largest amounts of mercury. The issue of mercury in the aquatic medium is important not only from the environmental and hygienic viewpoints but also from the viewpoint of fish culture. It has been demonstrated that mercury compounds damage some important tissues and organs in the fish body and may also have a harmful effect on fish reproduction. At very low concentrations they reduce the vitality of spermatozoa, depress the production of eggs and affect the survival rate of fecundated eggs and fry. Acute lethal concentratios of inorganic mercury compounds range from 0.3 to 1.0 mg per litre for salmonids and from 0.2 to 4.0 mg per litre for cyprinids, depending on the physical and chemical properties of the water. The acute lethal concentrations of organic mercury compounds are 0.025 to 0.125 mg per litre for salmonids and 0.20 to 0.70 mg per litre for cyprinids. For salmonids the highest admissible concentration of mercury in the form of inorganic compounds is about 0.001 mg per litre and for cyprinids about 0.002 mg per litre. For fish in general, the highest admissible concentration of mercury in organic compounds is claimed to be 0.0003 mg per litre.
Lead
A significant source of the lead contamination of atmospheric water, and thereby also surface waters, are the exhausted gases of motor vehicles which contain the products of decomposition of tetraethyl lead. In the aquatic medium, lead largely accumulates in bottom sediments where the lead content is about four orders larger than in water. Lead toxicity to fish and other aquatic organisms is significantly influenced by water quality: it depends on the solubility of lead compounds and on the concentration of calcium and magnesium in water. The water solubility of lead compounds declines with increasing acid capacity (alkalinity) and pH value of the water. Further, the toxicity of lead is known to decline with increasing calcium and magnesium concentrations in water. The acute toxic and lethal concentrations in different types of water range between 1 and 10 mg per litre for salmonids and between 10 and 100 mg per litre for cyprinids. Acute lead intoxication is characterized, first of all, by damage to the gill epithelium: the affected fish are killed by suffocation. The characteristic symptoms of chronic lead intoxication include changes in the blood picture, with severe damage to the erythrocytes and leucocytes, degenerative alterations of the parenchymatous organs and damage to the nervous system. The highest admissible lead concentration in water is 0.004 to 0.008 mg per litre for salmonids and 0.07 mg per litre for cyprinids.
Phenols
Phenols penetrate into surface waters with industrial effluents, especially the waste waters from the thermal processing of coal, from petroleum refineries, from the production of synthetic fabrics and other industrial segments. Analytically, phenols are either monobasic (phenol, cresol, naphtol, xylenol) or polybasic (pyrocatechol, resorcine, hydroquinon, pyrogalol, floroglucin).
Surface waters may also contain badly smelling chlorophenols, which arise as a result of the chlorination of phenols. The highest concentrations admissible from the point of view of fish culture are 0.001 mg per litre for phenol, 0.003 mg per litre for cresol, 0.004 mg per litre for resorcine, and 0.001 mg per litre for hydroquinon. Concentrations of 0.1 mg of phenols per litre of water and 0.02 mg of chlorophenols per litre of water are high enough to cause changes in the sensory properties of fish flesh. Long exposure to a concentration of 0.2 mg of phenols per litre of water was observed to drive the fish out of the drainage area of the polluted water course. According to the lethal concentrations to fish, the different phenol compounds will rank as follows: hydroquinon (0.2 mg per litre), naphthols (2 to 4 mg per litre), phenol, cresol, pyrocatechol and xylenol (2 to 20 mg per litre), resorcine and pyrogalol (10 to 50 mg per litre), floroglucin (400 to 600 mg per litre).
Phenols are typical nerve poisons which damage the central nervous system. The clinical signs of intoxication are characterized by great restlessness, increased irritability, leaping over the water surface, loss of balance and muscular spasms.
The post-mortem findings include conspicuous whitening of the water surface, amply covered by mucus; exposure to high temperatures cause haemorrhages on the lower side of the body. Long exposure to low concentrations leads to dystrophic to necrobiotic changes in the brain, parenchymatous organs, circulation system and gills.
Polychlorinated biphenyls
Polychlorinated biphenyls (PCBs) have recently become a very important factor of environmental pollution. PCBs are among organic compounds of the highest stability. Their solubility in water is low but they readily dissolve in nonpolar solvents and fats. A mixture of PCBs isomers with different ingredients is used in the electrotechnical industry (fillings of power capacitors and high-voltage transformers), mechanical engineering (incombustible liquids for heat transfer, fillings of hydraulic equipments and lubricants for compressors) and in the chemical industry (production of synthetic varnishes, dyestuffs and plastics). The widespread brandnames of PCBs include Delor (CSFR), Aroclor (USA), Clophen (Germany), Kaneclor (Japan), and Savol and Sovtol (USSR). As a response to many warning signals, drawing attention to the environmental hazards and excess spread of the PCBs, the production of polychlorinated biphenyls has been successively restricted since 1971.
In surface waters, PCBs occur at concentrations from 1.10-8 to 1.10-4 mg per litre. Polychlorinated biphenyls have a high capacity of accumulation in the bottom sediments and in the aquatic organisms in which the accumulation coefficient is 103 to 105.
PCBs are very toxic to extremely toxic to fish, mainly their young developmental stages. Their 48 hours LC50 are below 1 mg per litre.
As to the mechanism of the toxic action of PCBs, these substances have been found to adversely interfere with the enzyme systems of the microsomal fraction of the liver. If fish are exposed for long to low sublethal PCBs levels the contaminants accumulate in their bodies and cause, mainly in the fry, deformations of the skeleton, damage to the skin and fins (the fins disintegrate), damage to the parenchymatous organs, mainly liver (hypertrophy, local dystrophic, necrobiotic to necrotic changes), damage to the gonads and subsequent great losses during hatching, high mortality of early fry and increased occurrence of different deformations in the early fry.
The highest admissible PCBs concentrations in water range from 1.10-6 to 5.10-6 mg per litre for salmonids and from 2.10-6 to 1.10-5 mg per litre for cyprinids. Lower admissible concentration levels are recommended during hatching and rearing of the early stages of the fry.
Tensides
Tensides are either natural or synthetically produced surfactants used in many segments of the economy. They are traditionally used for their detergent and cleaning action and also for their emulsifying, dispersing and other effects.
From the chemical point of view, tensides are either ionic (liable to electrolytic dissociation) or nonionic (not dissociating in water). Ionic tensides are subdivided into anion-active (dissociating to surface-active anion and inactive cation), cation-active (dissociating to surface-active cation and inactive anion), and ampholytic (assuming either anion-active or cation-active properties, depending on ambient conditions). The anion-active tensides are ionic tensides most widely used in industry.
As to the mechanism of the toxic action of tensides on fish and other aquatic organisms, it is stated in literature that besides the toxicity ensuing from the chemical structure of the tenside, its physico-chemical efect also contributes to the harmful action. The surface tension of the water decreases and this leads to hydration and to enlargement of the cell volume. At lower tenside concentrations this enlargement is reversible. High concentrations suppress metabolic processes in the cells. Long exposure damages the cells which necrotize in the later stages. These changes manifest themselves mainly in impairment of the respiratory epithelium of the gills. In addition, exposure of fish to some tensides changes the activity of the respiratory enzymes, especially cytochromoxidase. Tensides also damage the protective layer of mucus on the skin: the layer loosens and the infection resistance of the fish decreases. Sublethal tenside concentrations also damage the eggs and sperm.
The toxicity of tensides to fish is influenced by a number of biotic and, especially, abiotic factors. The age of the fish is a particularly important biotic factor.
During embryonal and larval development, the sensitivity of fish to tensides is sometimes greater by an order, in comparison to the juvenile and adult stages. Of the abiotic factors, the tensides molecular structure and the physico-chemical properties of water exert the greatest influence on their toxicity. The results of investigation of the relationship between toxicity and molecular structure indicate, for example in linear alkylbenzene sulphonates, that toxicity to fish markedly increased with the length of the chain. Similar dependence of toxicity on chain length was observed in other tensides. Among the physico-chemical properties of water, calcium and magnesium concentrations have the greatest influence on tenside toxicity and some influence is also exerted by the pH: toxicity declines with increasing calcium and magnesium concentrations in water. Where cation-active and anion-active tensides are contained in the waste waters the toxicity is much lower. This is due to the formation of insoluble complex between the anion-active and cation-active tensides.
The acute toxicity of tensides varies greatly with the species of the fish. Nevertheless, these substances and preparations are highly toxic to fish in the majority of cases, the 48-hour LC50 ranging between 1 and 10 mg per litre. A minor part of the tenside group can be classified as medium toxic substances (48-hour LC50 between 10 and 100 mg per litre) down to substances of very low toxicity (48-hour LC50 to 10 000 mg per litre). No significant differences in toxicity to fish were recorded between the anion-active, cation-active and nonionic tensides.
Tenside intoxication of fish cause, in particular, damage to the respiration epithelium of the gills (enlargement and vacuolation, dystrophic to necrobiotic changes in cells). Hence, the clinical signs of intoxication include respiration disorders (accelerated respiration; cyprinids gasp for air on the water surface) and later by depression. The characteristic findings in the patho-anatomic picture are an increased amount of mucus on the skin and in the gills and congestion to oedematous swelling of the gill apparatus. The mucus easily falls off the body surface and gills.
Pesticides
The use of pesticides has considerably increased (both the total quantity and the spectrum of pesticides used).
At present, pesticides are among the main factors responsible for intoxication of fish. At lower concentration they may not alvays have a direct effect on the fish stock but may exert a long-continued adverse effect on the earliest developmental stages of the fish. Besides these acute and chronic direct effects, importance is also attached to their indirect action. Inexpert application of herbicides or algicides to the water or contamination of surface waters with these chemicals may kill the aquatic plants and algae. The decomposition of this organic matter requires plenty of dissolved oxygen and takes it from the water. This leads to oxygen deficit and the fish may die of suffocation. Another very serious indirect consequence of pesticide contamination of the aquatic medium is the reduction or complete destruction of the natural food base for the fish. Many of the organisms the fish feed on are much more sensitive than the fish themselves. The LC50 levels of organo-phosphorus pesticides, determined during the acute toxicity test, may be a good example: for common carp the LC50 is 545 mg of Soldep per litre of water and for Daphnia magna it is 0.0002 to 0.001 mg per litre (the active ingredient of Soldep is 25 % trichlorphon).
Besides their active ingredient, pesticides introduce in the surface waters a number of other chemicals which may sometimes be much more toxic to fish than the active ingredient itself.
When a pesticide penetrates into the aquatic medium the active ingredient undergoes chemical and biological decomposition. In some cases the decomposition products may be more toxic to the fish than is the original active ingredient. For example, biological oxidation of parathion gives rise to paraoxon, which is more toxic; decomposition of trichlorphon produces more toxic dichlorvos and the like. From this it follows that absence of a pesticide in water cannot guarantee the absence of the harmful products of its decomposition.
Some pesticides are used in fish culture and water management to kill the aquatic plants (Gramoxone S, Reglone). Trichlorphone-based organo-phosphorus pesticides, e.g. Soldep, Masoten, Neguvon and others, serve to reduce the coarse Daphnia zooplankton, to counter the oxygen deficit in the pond, to kill predatory cyclopids before stocking the pond with the sac fry of fishes, to control the parasites that vex cyprinids, and for other useful purposes. Pesticides based on copper oxichloride may be used for antiparasitic treatment of the fish, for the control of water slugs and to kill water bloom.
However, in the majority of cases pesticides cause damage to the fish. The most dangerous of them are those based on chlorohydrocarbons, organic phosphorus compounds, carbamates and thiocarbamates, carboxylic acid derivatives, substituted urea, triazines and diazines, synthetic pyrethroids, metallic compounds and others.
Pesticides based on chlorohydrocarbons
These pesticides act as nerve poisons. They are highly to extremely toxic to fish (48-hour LC50 > 10 mg per litre).
The clinical signs of fish intoxication by pesticides on the basis of chlorohydrocarbons include excitation, followed by a long stage of depression. There is no specific patho-anatomic picture in these cases of intoxication; dystrophic alterations are recorded in the liver and kidneys.
Pesticides based on organo-phosphorus compounds
The mechanism of the toxic action of pesticides containing organo-phosphorus compounds upon fish has the same pattern as their toxic action on homoiothermic animals. Some hydrolytic enzymes, particularly acetylcholine hydrolase, are inhibited. The degree of inhibition of cerebral acetylcholine hydrolase in fish varies with the organo-phosphorus substances by which the inhibition is caused. Phenitrothion-based pesticides reduce the enzymes activity to 60 %, dichlorvos- and imidane-based pesticides cause a reduction as great as to leave only 22 % of physiological activity. The toxicity of these preparations to fish also varies: according to the values of 48h LC50 they are included among substances of medium to very high toxicity to fish (91 – 100 mg per litre). Salmonids are very sensitive to pesticides containing organic phosphorus compounds. The typical sign of fish intoxication with organo-phosphorus-based pesticides is the darkening of body surface at the onset of inco-ordination of motion. The patho-anatomic picture of such intoxications is characterized by much mucus on the body surface and in the gills, great congestion of the gills and dot-like haemorrhages in the gills when the pesticides concentration is high.
The water fleas Daphnia magna are most sensitive to organic phosphorus pesticides in water. According to the values of 48h LC50 these substances are extremely toxic to fish. It is interesting to note that the water fleas were found to be as sensitive to trichlorphon and dichlorvos as the gas chromatograph. Daphnia magna can be regarded as the most reliable indicator of pollution of the aquatic medium with organo-phosphorus pesticides.
Pesticides based on carbamates and thiocarbamates
Carbamate- and thiocarbamate-based preparations inhibit the activity of the acetylcholine hydrolase. Unlike in the fish poisoned by organo-phosphorus compounds, the inhibited enzyme soon regenerates in cases of carbamate and thiocarbamate intoxication. The toxicity levels of these substances to fish vary from low to wery high toxicity (48h LC50 ranging from 1 to 1000 mg per litre). The clinical and patho-anatomic picture of intoxication of fish with carbamate-based pesticides are not specific.
Pesticides based on carboxylic acid derivatives
A number of these pesticides are based on phenoxyacetic acid. The main representative of this group is 2-methyl-4-chlorphenoxyacetic acid (MCPA). Most of the MCPA-based products are of low to medium toxicity to fish (the 48h LC50 ranges from 10 to 1000 mg per litre). The clinical signs of intoxication are mostly characterized by the stages of depression. The patho-anatomic picture of fish intoxication with these herbicides is not very pronounced.
Pesticides based on substituted urea
Herbicides formulated on the basis of substituted urea are of low to high toxicity to fish (the 48h LC50 ranges from 1 to 1000 mg per litre). The clinical symptoms of intoxication are not specific and include restlessness, irregular respiration, inco-ordination of motion and a long period of agony. The patho-anatomic picture is characterized by increased amount of mucus on the darkened body surface, hyperemia of the gills and presence of a small amount of transsudate in the body cavity of the fish.
Pesticides based on diazines and triazines
Triazine-based pesticides are of medium to high toxicity to fish (48h LC50 ranging from 1 to 100 mg per litre). The clinical signs of fish intoxication with these chemicals are largely characterized by depression stages. Formation of transudate in the body cavity and in the digestive tract is an especially characteristic patho-anatomic sign, mainly in rainbow trout. The presence of transudates causes a marked enlargement of the body cavity; in rainbow trout it was conducive to a rupture of the body wall in some cases.
Diazine-based herbicidal preparations are less toxic to fish than are triazine-based preparations. Most of the former are of low to very low toxicity to fish (48h LC50 ranging from 100 to 10 000 mg per litre). The clinical course of intoxication is characterized by stages of depression. The patho-anatomic picture is not specific.
Pesticides based on synthetic pyrethroids
With their 48h LC50, these pesticides rank among substances of high toxicity (up to 10 mg per litre) to extreme toxicity (less than 0.1 mg per litre) to fish. The clinical signs of intoxication are not specific and include respiration disorders. The most conspicuous patho-anatomic change is the presence of a small amount of transsudate in the body cavity.
Pesticides based on metal compounds
These include, first of all, fungicides formulated on the basis of copper, mercury and aluminium. In the majority of cases, the toxicity to fish and the clinical and patho-anatomic symptoms correspond to those recorded in fish intoxicated with the respective metals.
Petroleum and petroleum products
Petroleum and the products of its processing are responsible for most of the recent pollution of surface and underground waters. Over 1970 to 1990 these materials were responsible for the majority of water pollution accidents that happened worldwide. These accidents were not caused by problems in sewage water treatment plants: most of them were due to negligent storage and handling, transport accidents, defects in equipment and the like - hence, all can be ascribed, either directly or indirectly, to the human factor.
However, petroleum and products of its processing also penetrate into the aquatic medium with waste waters. Petrochemical industry is responsible for most of the pollution. Other important sources of pollution include engineering and metallurgical works and the car and truck repair and service shops. Most of these sources have been polluting waters for a long time.
None of the petroleum processing products will readily solve in water. There are large differences between petroleum and its different products as to their toxicity to fish. Most of them have 48h LC50 values between 0.5 and 200 mg per litre. Toxicity varies with the chemical composition of the different products, with the water solubility of the different petroleum hydrocarbons, with the degree of emulsification and other factors. It is generally asserted that the lighter petroleum fractions (kerosene, petrol) are much more toxic to fish than the heavy fractions (oils). There are also differences in the sensitivity to petroleum and its products in different fishes. The fry of the predatory fishes (asp, pike-perch, trout) show the greatest sensitivity to petroleum products.
When petroleum products penetrate to rivers or ponds they spread on the surface, thus reducing (especially in stagnant waters) the passage of oxygen from the air to water. In cases of pollution of flowing waters the pollutant does not form a compact layer on the water surface: the petroleum products emulsify, the gills of the fish are mechanically contaminated and their respiration capacity is reduced. Petroleum products also contain various highly toxic substances, soluble in water, which penetrate into the bodies of the fish and cause straight intoxication. These include the naphthenic acids which are acute nerve poisons and are able to kill fish at concentrations as low as 0.03 to 0.1 mg per litre.
Symptoms of disturbances of the function of the nervous system and respiration prevail in cases of acute fish intoxication by petroleum and products of its processing. The main clinical symptoms include great restlessness, accelerated respiration, loss of balance (the fish lie on the side), loss of reactivity to irritation, depression, slow-down of respiratory movements, and death.
The scales of the dead fish are dull in colour and are covered with mucus, the skin shows local congestion, the epidermis breaks and peels off and surface wounds may occur in some cases. Damage to the cornea of the eyes may lead to blindness. The gills suffer from severe dysthrophic changes and necrosis and there may also be proliferation of the respiratory epithelium and hyperthrophy of the mucus cells. Long-continued exposure to petroleum at low concentrations is conducive to severe degenerative necrobiotic changes in the kidneys of the fish and in the eggs. The dead fish have a petroleum smell and taste.
Toxicity is not the only danger associated with petroleum and the products of its processing: the aquatic ecosystems and fish farming are badly affected by the oil smell and taste of the water and of organisms that live in such water.
For this reason, sensory assessment is preferred to toxicological analyses in determining the highest admissible amounts of petroleum and petroleum products that can be contained in water. For the different substances derived from petroleum, the highest admissible concentrations range from 0.002 to 0.025 mg per litre.
Dyes
Chemical dyestuffs have also been attracting toxicologists attention in recent years. They are present in the waste waters discharged from textile plants, food plants and paper mills. Though the waste waters containing dyes are very conspicuous even at very great dilutions they seldom cause severe damage to the fish. The toxicity of dyes depends on the physico-chemical composition of the water. Where the water contains much organic matter the dyes are bound to these organic substances and their toxicity decreases.
The mechanism of the toxic action of dyestuffs on fish is not direct in the majority of cases. If the water is polluted with organic dyes, the increase in the content of organics leads to oxygen deficit. Other dyes increase or decrease water pH. Still others, e.g. anilline, act as methaemoglobin poisons and as cancerogenic substances.
The acute toxicity of different dyestuffs to fish considerably varies. Most of the dyes are among the substances of low to wery low toxicity to fish (48h LC50 ranging from 100 to 10 000 mg per litre). This group includes colouring agents used in the food industry and selected organic dyestuffs. Another group, including e.g. acriflavin, rhodamin and also anilline and methylene blue, comprises substances of medium toxicity to fish. Their LC50 values at 48h exposure range from 10 to 100 mg per litre. The group of dyes of very high toxicity to fish includes, for example, malachite green.
The clinical symptoms of fish intoxication by the different dyes are not specific. The patho-anatomic changes that signal such intoxication may include a change of body colour by the dyestuffs tested, and the organs inside the fish body may also take on an intensive colouring, e.g. from malachite green.
Phytoplankton toxins
Increasing eutrophication of surface waters brings mass development of phytoplankton and higher aquatic plants. This raises water pH to levels above 10 and the breakdown of the mass of dead phytoplankton and higher aquatic plants causes oxygen deficit. Further, some representatives of these aquatic organisms produce substances (toxins) that may affect not only fish but also farm animals and man. These include, in particular, the blue green algae of the genera Microcystis, Aphanizomenon and Anabaena. An endotoxin, having the nature of cyclic polypeptides, was isolated from the alga Microcystis aeruginosa.
In exposed fish the action of the products of these aquatic organisms (blue green algae) increases thiaminase activity and reduces thiamine content in organs and tissues; this leads to B1 avitaminosis. The toxic products get into water during the period of development of water bloom (algae) and particularly when the water bloom dies and breaks down. These toxins penetrate into the fish body through the gills and body surface. Part of it is also ingested with food. The clinical symptoms of intoxication include a damage to the central nervous system. First there is increased restlessness and accelerated respiration, followed by inco-ordination of motion, then the fish lie flat on the bottom and die. The major patho-anatomic signs include haemorrhages on the skin and gills and in the internal organs.
Some phytoplankton representatives have been found to generate hydroxylamine as a product of their metabolism. The occurrence of hydroxylamine in the heavily eutrophicated waters of some ponds is also supported by a high concentration of organic substances which reduces the oxide reduction potential of the environment, thus allowing the hydroxylamine to accumulate. This is why the highest hydroxylamine concentrations in surface waters are usually recorded during the periods of mass decomposition of the water bloom (blue-green algae), when the concentrations may reach toxic levels for a short time. Hydroxylamine is highly toxic to fish, its LC50 in acute exposure being less than 10 mg per litre (in sensitive fishes it may be as low as about 1 mg per litre). The toxic action of this substance causes severe methaemoglobinaemia and damage to the central nervous system. Though the cases of damage to fish are seldom ascribable to the action of phytoplankton toxins, this danger should not be underrated, especially in the warm regions.