It is a difficult and complicated task to diagnose fish poisoning because the mortality is often recorded with a delay and the fish and water are not sampled in time. In such cases the patho-anatomic changes in the fish are obscured by the starting post-mortem changes and the toxic substances that poisoned the fish have been carried away with the water flow. Hence, it is necessary to use all the available information and possible analytic methods to detect the cause of the death of the fish and aquatic invertebrates. The analytical work should start from examining the anamnestic data and performing the physico-chemical and hydrobiological analyses of the water, and if necessary also the bottom sediments and periphytes and then the fish themselves should be examined. Bioassay for water toxicity is an important item in the diagnosis of fish poisoning.
Examination in situ
When the fish are observed to exhibit strange behaviour, or to die, the following major steps should be taken on the spot.
If it is suspected that the fish might die or change their behaviour owing to the application of a chemical in the vicinity of the affected water course or pond or lake, detailed information should be gathered, concerning the time and method of application and about the kind and amount of the chemical applied. An example of notes serving as document on local inquiry in cases of abrupt change of behaviour or mortality of fish.
| 9. Status of zooplankton | killed | yes | no | |
| phytoplankton | killed | yes | no | |
| benthos | killed | yes | no | |
| aquatic plants | killed | yes | no | |
13. Place, hour of sampling, designation of samples
water
sediments
periphytes
fish to be examined:
If the fish are killed by the application of a chemical in the vicinity of a water course or reservoir (e.g. a pesticide), the following data should also be included in the document on local inquiry:
Map: A brief chart of situation, indicating the area where fish died and the places where water samples were taken.
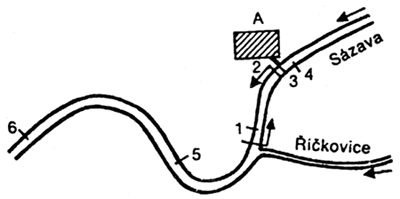
A - Fenolana plant
 part of river where fish died
part of river where fish died
Water sampling sites:
Hydrochemical examination
Choice of the right sampling sites and the right water sampling method is the main prerequisite for successful work, so they must be given maximum attention. In flowing waters, water sampling sites are laid out as follows:
In reservoirs and fishponds, the places of water sampling should be specified according to the actual situation; in some cases the samples have to be taken from different heights of the water column. Special sampling bottles (e.g. the Hrbáček displacement bottle, Fig. 20) are used to take water samples from different depths and from just above the bottom.
The samples are poured in clean 1- to 2-litre bottles. It is not recommended to fill the bottle with water near the shore: the samples are as usual taken 1–2 m off shore. From near the bottom, the water samples must be taken with care not to raise mud and other sediments. For maximum objectivity of the data on the water examined, the time between sampling and analysis must be as short as possible. The samples are shipped with advantage in thermally insulated bags. In the laboratory the bottles with the samples should be placed in refrigerators and kept at 3–4°C. in thermally insulated bags. In the laboratory the bottles with the samples should be placed in refrigerators and kept at 3–4°C. However, all this may not be sufficient for some water components. In such cases the samples must be analyzed as soon as they are collected, or may be preserved with a small amount of preservative. Detailed data on the preservation and treatment of the samples are given in the Table 10.
Table 10: Preservation and treatment of water samples
| Water Characteristics | Preservative (amount per 1 litre of sample) | Method of sample treatment |
| Temperature | - | Measure during sampling |
| Colour | - | 1. Determine during sampling |
| 2.Store at 4°C - determine within 24 h | ||
| Transparency | - | 1. Determine during sampling (field determination) |
| 2.Determine in 24 hours (laboratory determination) | ||
| Odour | - | Identify some smells during sampling (e.g.chlorophenol), others in 24 h at the maximum |
| pH | - | 1.Determine during sampling |
| 2. Collect into oxygen bottle, determine within 24 h | ||
| Oxygen capacity | - | 1.Determine during sampling |
| 2.Collect into oxygen bottle, determine within 24 h | ||
| Dissolved oxygen | - | Collect into oxygen bottles, fix after collecting |
| Chemical oxygen demand (CODMn,CODCr) | a) 1 ml H2SO4 | Determine as soon as possible after sampling, in 24 h at the maximum |
| b) store at 4°C | ||
| Biochemical oxygen demand (BOD5) | - | Maintain at 4°C, process in 24 h |
| Ammonia | a) 1 ml H2SO4 | 1.Determine during sampling |
| b) 3 ml CHCl3 | 2. Store at 4°C - determine within 24 h | |
| 3.Determine after preservation | ||
| Nitrates, nitrites | a) 1 ml H2SO4 | 1.Determine on sampling day |
| 2.Maintain at 4°C - -determine in 24 h | ||
| 3.Determine after preservation | ||
| Chlorine | Collect into brown bottle, determine immediately after sampling | |
| Cyanides | solid NaOH up to pH 11 at the minimum | Determine immediately, several hours after sampling at the maximum |
| Copper, zinc | 5 ml HNO3 | Cannot be preserved if sample contains cyanides |
| Iron (total) | 5 ml HNO3 | Completely filled bottles: prevent contact with air |
| Phenols | NaOH added to reach pH 11 (about 4 g per litre) | 1.Determine within 24 h |
| 2.Determine after preservation | ||
| 3.No phenol preservation needed at phenol levels above 150 mg per litre | ||
| Tensides: | ||
| anion active | 3 ml CHCl3 | Collect to glass bottles, |
| cation active | - | determine within 72 h |
| nonionic | - | |
| Petroleum and its products | The collected sample size is 1 to 5 litres, depending on the pollution. Use glass bottles (never use polythene bottles). Determine as soon after sampling as possible | |
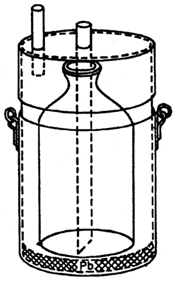
Fig. 20: Hrbáček's displacement-type bottle for water sampling to determine the concentration of dissolved oxygen
The basic hydrochemical analysis includes the determination of the colour, odour, pH, acid capacity (alkalinity), concentration of dissolved oxygen, chemical oxygen demand (COD), biochemical oxygen demand (BOD5), ammonia, nitrites, nitrates, phosphates and total phosphorus. The method of determination of other chemicals is chosen on the basis of the results of local investigation (anamnestic data) so as to make it possible, from the results of the investigation, to identify the causes of the mortality or damage of the fish. When assessing the results of the physico-chemical analysis of water samples as to the causes of mortality, the parameters should not be evaluated in isolation: a complex approach has to be used. The toxicity of the different chemicals and products to fish and aquatic invertebrates is influenced by the nature of the aquatic environment as a whole.
Hydrochemical examination of the water is performed at site during the local investigation and also in chemical laboratories. In the field analyses, the Combi kit, produced by the Central Laboratories of the Fish Culture and Hydrobiology Research Institute at Vodnany, Czechoslovakia, can be used with advantage. The Combi kit allows to determine the following: water transparency (using the Secci disc), concentration of dissolved oxygen, pH, acid capacity (alkalinity) and total concentrations of ammonia and phosphates in water.
The physico-chemical characteristics of the water are determined according to Czechoslovak Standard ČSN 83 0530 Chemical and Physical Analysis of Surface Water, Parts 1–50, chlorine level is determined on the basis of ČSN 83 0520 Physico-chemical Analysis of Drinking Water, Part 18. The amount of metals contained in water is measured by the atomic absorption spectrophotometry method (AAS). The gas and liquid chromatography methods are used for the determination of organic compounds, e.g. the active ingredients of pesticides, tensides, organic dyes, PCBs and others.
Examination of bottom sediments
In justified cases, bottom sediments may be sampled, apart from collecting the samples of water. This is usually the case when there is suspicion that a water course or reservoir is polluted with petroleum products, metals, pesticides and some other substances which accumulate in the bottom sediments.
There is no standardization of sediment sampling: local conditions must always be respected. The main principle is to take the samples mainly from the top layer of the sediments, to take them at several sites within the locality investigated, and to mix the samples for analysis. The bottom sediments are analyzed by modifications of the methods used for determination of pollutants in waters and other (mainly biological) materials.
Hydrobiological examination
Hydrobiological examination of water is very important for the diagnosis of intoxication of fish and lower aquatic organisms. This examination includes evaluation of the qualitative and quantitative structure of the lower aquatic organisms and recording of the damage these organisms might suffer, and of the changes in the behaviour of the fish, or their death. Exposure to a specific group of poisons can be estimated from the changes in the composition of the biocoenosis after the intoxication. For example, crustaceans and insect larvae are very sensitive to insecticides, aquatic plants are sensitive to herbicides, algae react sensitively to algicides etc. In cases of accidents on water courses and in reservoirs, aquatic invertebrates are usually the first indicator of pollution of the aquatic environment, and the fish follow afterwards. This is especially characteristic of the pollution of water courses and reservoirs with pesticides and some metals. Substances of surface activity (tensides) exhibit about the same toxicity to fish and to aquatic invertebrates. On the other hand, fish are the main indicators of pollution in the cases of accidents at which the water courses or reservoirs are polluted with organic substances.
Examination of periphytes
Periphytes form a mat consisting of an association of aquatic organisms (bacteria, fungi, algae, protozoans, bryozoans, rotifers and others) growing on, or attached to, surfaces such as the stems of higher aquatic vegetation, stones, structures built in water, immersed logs and the like. In surface waters, periphytes are important as food for many water animals, including fish. They make a significant contribution to the self-cleaning processes and their quality and quantity indicate the average quality of water at the given site: short-term and insignificant changes in water quality usually have an influence on the association of organisms making up the periphytic mat. This is of great importance in water analyses. At accidents, mostly characterized by a short toxic wave killing the free-living organisms and carrying them away, the damaged periphytes may provide information on the past wave as well as on the quality of water in the preceding period. Periphyte analyses are an important part of the over-all examination of water quality. Their importance is particularly high in places with flowing water where the analyses of single samples from single sampling sites usually fail to provide representative results. Periphyte samples are usually obtained by plucking them off the different surfaces by means of a pair of long tweezers. A scraper held on a long handle will be of help in greater depths. The periphytes attached to underwater structures of concrete or other materials can be easily scraped by means of a laboratory brush (normally used in washing laboratory glassware) on a long pole: the material attached to the bristles of the brush are then put into the sampling phial, using a pair of tweezers. In the laboratory the periphyte samples are subjected to hydrobiological analysis in normal microscopic preparations, the organisms present in the samples are identified and their number is estimated by means of an estimation scale. The procedure is described in Czechoslovak Standard CSN 83 0532 Biological Analysis of Surface Water (its Part 5 Periphyton Determination).
Biological assay for water toxicity testing
Examination of the source and cause of an accident that happens in a water course or reservoir includes, besides hydrochemical and hydrobiological analyses, also a bioassay for water toxicity. The water used for the bioassay is taken from part of the water sample (free of preservatives) sent to laboratory for physico-chemical analysis. The aquatic organisms serving for the assay include aquarium fishes, especially poecilia reticulata, and aquatic invertebrates, especially the water fleas of the genus Daphnia, which are among the aquatic organisms of the highest sensitivity to the majority of pollutants. As to poecilia reticulata, it is not an extremely sensitive fish but its advantage like in the majority of aquarium fish is its easy availability all the year round.
The procedure of the bioassay for water toxicity is as follows: Ten water fleas (Daphnia) or two or three aquarium fish (Poecilia reticulata) are put in 100-200 ml of water sample. If the water samples are large enough, the assay is done simultaneously on both organisms. Parallel to the tested sample, the same number of organisms are put in uncontaminated water for control. The best containers are, by our experience, crystallizing dishes where the water column is shallow, allowing sufficient oxygen to diffuse from air to the water. The behaviour and state of the organisms are monitored during the bioassay, which may last 24 to 48 hours.
If the result of the assay is negative (the behaviour of the fish or water fleas does not differ from the control), it may be assumed with some probability that the tested water sample does not contain toxic substances at harmful or lethal concentrations. If the testing organisms change their behaviour or die, physico-chemical and/or other methods should be immediately started to find the cause of intoxication.
Though the use of the bioassay for water toxicity testing cannot result in a direct identification of the cause of the accident or the mortality of the fish and other aquatic organisms, it can be of much help in the diagnostic process. Other aquatic organisms, e.g. rainbow trout, common carp or the cyclopes, may also be used for the bioassays, but these are not available all the year round, and rainbow trout and common carp have the additional disadvantage of needing more water in the sample and the water has to be aerated. It is a general feature of all water toxicity bioassays that their methodology is considerably variable: in any particular case the method must be chosen with respect to the actual conditions and to the equipment and capacity of the laboratory.
Examination of the fish
The number of the fish that have to be examined and sent to diagnostic examination is not always the same. If there is any suspicion that fish might have been poisoned or suffocated by water pollution, 3 to 5 fish of each of several species (those most frequently occurring among the killed or dying fish) are taken as samples. If such a situation occurs in a reservoir (pond) with a single-species stock, the number of fish in the sample should be 5 to 20, depending on weight, age and other circumstances.
Success of the examination depends on the state of the fish. It is of no use to send fish that start decaying or even are decayed. The sample fish should best be delivered alive, with clinical symptoms of damage. If this is impossible, it is absolutely necessary that the fish be fresh and intact when they reach the lab. They should never be sent in water in which they died. The fish sent for general examination should be free of preservatives (formalin, spirit or others) because such substances would make the diagnosis impossible. In those cases when the fish are only sent for chemico-toxicological examination for the contents of metals and residues of pesticides and other pollutants, it is recommended to send the samples frozen.
Fish that have just died or those with clinical symptoms of damage are subjected to detailed health examination to eliminate diseases of infection or invasion type. If such diseases are eliminated, it is necessary to specify the cause of the abrupt change in the environment that caused the death of the fish or the change in their behaviour. The clinical symptoms given in the document from the local investigation are evaluated in the first stage of the examination, then follows the patho-anatomic dissection, in justified cases the organs and tissues of the fish are subjected to chemico-toxicological examination, and further examinations are performed if necessary.
Chemico-toxicological examination of the organs and tissues is one of the most objective, and also most difficult, methods of diagnosis of intoxication. In the case of suspicion of intoxication by methals or in the inspection of the content of metals in fish as human food, the chemico-technological examination has its justified position. Such examinations are also performed when phenols and pesticides may be involved, and if certain conditions are met, chemico-toxicological analysis may be of help in diagnosing ammonia intoxiacation of fish. On the other hand, in diagnosis of intoxications of fish by some substances (chlorine, sulphane, cyanides) this method cannot be used.
The presence of metals in fish organs and tissues is determined by the atomic absorption spectrophotometry (AAS). An increase in the concentration of metals is most frequently recorded in the parenchymatous organs and in the gills of the fish. For example, acute copper intoxication can be diagnosed on the basis of chemical analysis of the gills, where the concentration of the metal increases several times. Copper content in the gills of the fish in water not contaminated with copper is up to 10 mg per 1 kg of dry matter.
Fish intoxication with pesticides based on organo-phosphorus compounds can be diagnosed either by direct determination of these substances or their metabolites in the organs and tissues or by indirect determination, based on the inhibition of acetylcholine hydrolase mainly in the brain of the fish. The same method is applicable to the diagnosis of fish intoxication by pesticides on the basis of carbamates. Diagnoses of the intoxication of fish by other pesticides and also other organic compounds are based on the determination of these substances in the organs and tissues of the fish by gas chromatography methods.
Another chemico-toxicological method used in the diagnoses of intoxication is the detection of phenols in the fish. The chemicals are detected in the flesh and skin of the fish by the photometric method and by means of 4-amino-antipyrin (Czechoslovak Standard CSN 83 0530, part 33) after repeated distilling of the tissue used for analysis.
Ammonia intoxication of the fish can be diagnosed, if some conditions are maintained (fish blood and brain sampling during intoxication or immediately after the death of the fish, performance of the analysis within 48 hours at the maximum of freeze-storage of the samples), by determining ammonia nitrogen in the blood serum and brain of the fish. The level of ammonia nitrogen in the blood serum and the fish brain homogenate is best determined by the Blood Ammonia Test kit, made by HYLAND. However, ammonia nitrogen level in the serum and brain of the fish varies with metabolic rate and increases under the action of the various adverse factors, especially O2 deficiency. Owing to these circumstances it is impossible to diagnose ammonia intoxication of fish by merely determining the ammonia N level in the grain or in the blood serum: the final diagnosis must be based on a thorough quality examination of the water and detailed examination of the state of health of the fish.
The presence of petroleum and its products in fish can be easily detected by changes in sensory characteristics: the “petroleum smell” can be detected at concentrations of an order as low as 102 mg per litre of water. Contamination of aquatic medium with phenols and chlorophenols can be detected in the same way at or above the respective concentrations of 0.1 mg per litre and 0.02 mg per litre. This method of detection needs no sophisticated laboratory equipment, though its sensitivity compares favourably with that of chemico-toxicological examination.
General principles of preventing fish poisoning
All persons who handle substances that may pollute the environment should regularly check their equipment and take measures to prevent accidents: this is a moral obligation, which is in many countries supported by law. Strict discipline and high responsibility in handling such substances provide the most effective, most readily available and cheapest prevention.
The primary measure that can save surface water from pollution is the building of treatment plants, both for the treatment of sewage and industrial effluents. In principle, any used water must have been treated before it is discharged back to the environment. An important role in the protection of the aquatic medium is also played by the so-called biological ponds which are able to intercept and eliminate mainly the organic pollutants generated in agricultural production (livestock fattening and rearing facilities, including those for waterfowl) and in the food industries (slaughterhouses, poultry processing plants and others). To a lesser degree these biological ponds may intercept and remove the sewage from residential areas, if such waste waters are not polluted with petroleum products, PCB, pesticides and other dangerous contaminants.
In industrial production, especially in those industries where there is a danger of leakage of petroleum products and other very toxic substances which do not easily yield to biological degradation, it is necessary to build special leak-proof pits to protect water sources against contamination.
Much attention must also be paid to some technological processes in agriculture. Particularly great dangers are involved in spray application of chemicals, including fertilizers and pesticides. Any direct drift of the chemicals to water areas during application must of course be avoided and precautions should be taken to prevent later washing of the chemicals with rainwater down to the rivers and ponds. Such precautions are incorporated in technological regulations for application (no chemical sprays in rain or wind and 24 hours before the rain is expected etc.) and they are also reflected in some principles of soil cultivation: grassy strips around water reservoirs, the use of adequate crops and adequate systems of tillage in exposed fields, and some other pollution-control practices.
It is required by current regulations that any newly developed chemical must have undergone a complex of toxicological testing and evaluation before it is introduced in practice. Its biological degradability, acute and chronic toxicity and/or teratogenicity, mutagenicity and the like should be known. Priority should be given to substances of a high selectivity, low active concentration, low toxicity and easy degradability. The natural environment should not be contaminated with toxic substances of low degradability.
The toxicity of substances, preparations and effluents to the aquatic organisms is influenced, first of all, by their nature (e.g. chemical composition, water solubility, pH), sensitivity of the fish on which these substances act (salmonids are most sensitive, cyprinids are somewhat more resistant, older fish are more resistant than younger fish; an important role is also played by the momentary condition and state of health of the fish, and in early fry also by the state of their feeding) and, last but not least, by the nature of the aquatic medium (water temperature, pH, content of dissolved oxygen and the like).
Evaluation of the chemicals, preparations and effluents
For the fish culturist, the most important trait of any new product or chemical is its toxicity to aquatic organisms. Toxicity tests are carried out, in the essence, at three levels:
The cell- and tissue-level tests are often used for theoretical explanation of findings obtained in trials conducted at the organisms level. Good reproducibility is their advantage, but unfortunately, the results obtained in vivo often considerably differ from those in vitro. Tests at the level of biocoenoses offer the advantage of the toxic actions being studied in the natural environment or on a model which can simulate reality with good authenticity. However, biocoenosis-level trials have drawbacks: the changes in the composition of the biocoenosis may not always be a consequence of direct toxic action on a given species but may be due to a disturbance in the food chain or other factors; the reproducibility of such tests is often very limited because it is impossible in authentic natural environment to prepare exactly the same conditions as in the preceding test.
Most of the trials, especially the acute toxicity tests, are performed at the organisms' level. Though some reproducibility problems and risks are involved in applying their results to actual natural conditions, these tests represent a practical compromise, acceptable to all: the fish culturist, conservationist, technologist and economist.
Distinction is drawn between acute toxicity and chronic toxicity; hence, substances, preparations and/or waste waters (effluents) are subjected to acute toxicity tests and chronic toxicity tests. Almost all these tests are carried out at the organisms level.
Acute toxicity tests
Determination of the acute toxicity of chemical substances, preparations and effluents to aquatic organisms is among the main duties of the producers. The majority of the acute toxicity tests are performed on fish and some aquatic invertebrates. Different methods of toxicity tests have been standardized in different countries. Some methodical procedures are recognized internationally, including the most widespread standards issued by the International Standards Organization (ISO), the OECD methodical manuals and some others. In determining the acute toxicity to aquatic organisms, the following ISO methodical manuals are most widely used:
It follows from this survey that the basic tests include the acute toxicity tests on the highly sensitive water flea Daphnia magna and on the aquarium fish Brachydanio rerio. The standard also allows to use some other species, e.g. Poecilia reticulata, pimephales promelas, Oryzias latipes and others. The basic data are those on LC50 for a period of 24, 48, 72 or 96 hours. The representatives of primary producers used for the acute toxicity tests include, first of all, those of algae.
Apart from these standard methods of toxicity testing, laboratories worldwide use many modified toxicological methods. In using the modified methods, the laboratories respect the actual natural and economic conditions prevailing in each country, in each particular region. Some countries have standardized the methods of determining the tests of acute toxicity of waste waters to freshwater fishes (e.g. the USA, UK), others have introduced special tests for the determination of the toxicity of pesticides to fishes (e.g. Japan, the UK). The national methodologies differ in the test organisms used, in the time of exposure, in the water used for dilution, but in the majority of cases the main output is the LC50 value as the most accurately determinable quantity.
The division standard currently used in Czechoslovakia, ON 46 6807 Acute Toxicity Test on Fish and Other Aquatic Organisms, also derives from the above-mentioned ISO and OECD standards. In the test methodology (2 levels: the orientation test and basic test), as well as in the selection of the test organisms (the basic test organisms are Daphnia magna and Poecilia reticulata and the auxiliary ones include common carp, rainbow trout, Brachydanio rerio, Rasbora heteromorpha, Cyclopidae, Tubificidae and also the larvae of the amphibians, Xenopus laevis and the green frog (Rana temporaria), which serve in special tests.
The selection of these aquatic organisms for the acute toxicity tests should be adjusted to the purpose for which the tested substances are evaluated. For example, when evaluating a substances or preparation to be used in fish culture or for direct application to the aqautic environment, toxicity tests will have to be performed on all the above-mentioned representatives of aqautic fauna. On the other hand, for classification of the tested substances, e.g. newly developed preparations to be introduced in practical use, the basic evaluation will be sufficient (compulsory tests on Daphnia magna and Poecilia reticulata). Tests on Rasbora heteromorpha is required in chemicals to be exported.
In accordance with the internationally agreed methods, the Czechoslovak division standard, ON 46 6807, recommends to use artificially prepared dilution water. Further, it recommends to use a reference substance, i.e. a control preparation analyzed for LC50 alongside the tested substance. The changes in the LC50 of the standard reflect the variability in the circumstances in which the trials are Performed and in the condition of the organisms tested. K2Cr2O7 is the most frequently used reference substance.
According to the level of 48h LC50, determined on the basis of ON 46 6807, the tested substances (preparations) are classified (in Czechoslovakia) into the following toxicity classes:
| 0 - | substances of almost no toxicity: substances in which the 48h LC50 is higher than 10 000 mg per litre, |
| 1 - | substances of very low toxicity: substances in which the determined 48h LC50 is between 1000 and 10 000 mg per litre, |
| 2 - | substances of low toxicity: substances in which the determined 48h LC50 is between 100 and 1000 mg per litre, |
| 3 - | substances of medium toxicity: substances in which the determined 48h LC50 ranges between 10 and 100 mg per litre, |
| 4 - | substances of high toxicity: substances in which the determined 48h LC50 is between 1 and 10 mg per litre, |
| 5 - | substances of very high toxicity: substances in which the determined 48h LC50 ranges from 0.1 to 1 mg per litre, |
| 6 - | substances of extreme toxicity: substances in which the determined 48h LC50 is smaller than 0.1 mg per litre. |
Any tested sample (substance, preparation) is definitively included in the appropriate toxicity class according to its 48h LC50 level for the most sensitive organism tetsed.
The acute toxicity test also yields data on the following parameters, included in the result:
Chronic toxicity tests
The maximum concentration of substances in water still admissible for the fish (MAC) serves as a basis for evaluation of the water that flows to the fish culture facilities, for diagnostic analyses when the causes of damage or mortality are sought, or for permitting the discharge of effluents, containing different contaminants, to the rivers or ponds. The MAC levels are determined in the chronic toxicity tests. A standard procedure of a chronic toxicity test for examination of toxicity in different substances, products and effluents was proposed, for example, by Lesnikov (1976). By Lesnikov the MAC is a concentration of a substance or its metabolites in waters at which a permanent exposure has no adverse effect on:
The MAC methodical manuals recommend first to perform acute toxicity tests, then tests of detoxication of the substances and their metabolites into safe products, and finally, long-term observations, using the results of acute toxicity tests and detoxication tests. The resultant MAC should then be determined according to a parameter which is adversely affected at the lowest concentration of the substance or its metabolite. Materials of high stability (96 % decomposition into non-toxic compounds in summer lasting longer than half a year) with a high accumulation capacity and with a MAC below 0.0001 mg per litre are regarded as particularly dangerous.
Salmonids, especially one-year-old rainbow trout, are most frequently used for the chronic toxicity tests. The main parameters examined when the long-term toxicity test is being finished include the state of nourishment of the fish, the individual and total gain of the fish, the change in the sensory assessment of the flesh and the degree of accumulation of toxic substances in the fish body. The supplemental parameters include the behaviour of the fish during the test, the patho-anatomic and histo-pathologic picture, the physiological, biochemical and haematological changes in the fish after the termination of the test. Together with the basic parameters under study, the histologic examination of the fish organs and tissues after the long-term tests is among the most important criteria of evaluation, because its results usually reduce the MAC of the substances and products determined according to the basic parameters.
These principles are also fully applied in the methodical manual Determination of the Maximum Admissible Concentrations of substances in Water from the Viewpoint of Fish Culture, issued by the Research Institute of Fish Culture and Hydrobiology, Vodňany, in 1989. According to this manual, the chronic toxicity test is to last 90–100 days and the test is performed on the fry of common carp and rainbow trout. A comparatively stable concentration of the tested substance is obtained by putting the fish into a new fresh bath of the toxic substance every day. After computation and evaluation of all the results of the chronic toxicity test, the concentration which, compared with the control group, did not cause any significant changes in the experimental fish is denoted as the maximum admissible concentration (MAC).
of the aquatic invertebrates, the chronic toxicity tests are conducted mainly on the water fleas Daphnia magna. All the individuals of this species used in the test must be of the same age (3–7 days), produced in synchronized culture. Both the basic parameters (survival, release of the young from the germ sac, viability of the young, changes in the biomass) and supplemental parameters and criteria (behaviour of the water fleas, symptoms of death, state of the gonads and contents of the germ sac, filling of the intestine and the colour of the intestinal contents, body colour, amount and colour of the fat droplets) are evaluated during the course of the test. A detailed description of this method is given in the OECD standard.
Bêsides the chronic toxicity test, water fleas are also subjected to the reproduction toxicity test, based on the examination of the reproduction capacity of several generations of the tested water fleas, kept in a solution of the substance being tested. The evaluated parameters include the release of the young, the number of the young, their survival, and the ability of parthenogenetic reproduction.
Other toxicity tests
Efforts to replace the lengthy and costly chronic toxicity tests by other tests, which would be both fast and sensitive, have intensified in recent years. The use of cell cultures is one of the promising methods. These tests are based on the observation of the toxic action straight on the primary cell cultures from the different fish tissues or on stable cell lines (e.g. FMH, PG, RTG-2 and others). At present they are being elaborated on.
The method of embryolarval toxicity tests is also being examined (the technique after Birge et al. is regarded as an ISO method). The influence of toxic substance is monitored until the yolk sac is exhausted. Unfortunately, the first results of experiments conducted in our laboratories do not confirm a high sensitivity of this technique; it is rather comparative with the acute toxicity tests. To reach a greater accuracy, the technique was extended to include a fasting test. This provided a slight improvement of sensitivity but in spite of this improvement the technique cannot replace the traditional chronic toxicity test. In this case, the time of killing of the experimental fish is evaluated in comparison with the control fish.
Another interesting method is the bacterial bioluminiscence inhibition technique (the Microtox test: ISO Standard N110/1988, draft elaborated in France). Of course, this technique also has to be adjusted to local conditions.
Degradability of substances in aquatic environment
Besides toxicity, another important criterion of evaluation of substances and products is their biological degradability in the aquatic medium. It may be evaluated by determining the biological degradability itself (the non-specific method) or by determining the time of persistence of the residues of the tested substances in the aquatic medium (specific method).
Biological degradation means a sequence of processes taking place in removing the organic substances by micro-organisms. The degradation involves both dissimilation and assimilation processes.
Czechoslovak ichthyotoxicologists, conservationists and water management experts widely use the technique proposed by pitter (1974) as a standard test of biological degradability of organic substances. This is a single-act kinetic test performed in an open system. The decrease of the amount of the tested substance is measured by the determination of the chemical oxygen demand (CODCr), total organic carbon (Corg), or by specific reactions. The results are compared with a blank test and with the degradability of a standard substance. Environmental importance is attached not only to the degree of decomposition but also to the rate at which the substance is degraded. For practical reasons it is recommended to indicated biological degradability as percentage of removal of CODCr or Corg in the days of incubation.
When the results of biological degradability tests are applied to actual natural conditions, it should be taken into account that the test simulates the conditions existing in the sewage plants. A number of other factors (temperature, microbiological life, pH, amount of dissolved oxygen in water and others) influence the rate of degradation of the tested substances under natural conditions, outside the sewage treated plants. Nevertheless, it generally holds that a material of good biological degradability in non-adapted activated sludge is also most likely to decompose well under natural conditions.
The techniques of determining the residues of the various pollutants and their metabolites in the aquatic medium are not easy to perform; for this reason primary attention is focused on really toxic substances and those which are not readily degradable. The residues are determined by chemical analyses (metals, DDT and its metabolites, HCH, PCB, triazines and others) and also by bioassays for toxicity testing (e.g. test on Daphnia magna serves to determine the residues of organo-phosphates).
Legislation
Today special legislation is being developed in each country where due efforts are taken to maintain and improve the state of the aquatic environment. In addition to this, international agreements are being prepared and signed to extend the possibilities of conservation of aquatic resources and natural environment as a whole (e.g. agreements on the Baltic Sea).
The legislation of every country should take into account the adverse aspects of the action of various chemicals, products, waste waters and solid wastes on the aquatic medium. The duties of the producers and users of such substances as well as those of the producers of the wastes must be clearly defined, including the penalties entailed by violation or circumventing the legislation, strict and regular inspection, and emphasis on personal responsibilities. A whole complex of mutually related preventive measures should be developed and introduced in practice to reduce the number of accidents in water courses, to eliminate the sources of pollution and to minimize the consequences of accidents on the aquatic biocoenoses.
Recomended literature
Alabaster J.S., Lloyd R. (1980): Water quality criteria for freshwater fish. Butterworths. pp. 297.
Baier Ch., Hurle K., Kirchhoff J. (1985): Datensammlung zur Abschätzung des Gefãhrdungspotentials von pflanzenschutzmittel-Wirkstoffen für Gewãsser. Verlag paul parey, Hamburg, Berlin, pp. 294.
Horáková M., Lischke p., Grünwald A. (1989): Chemical and physical metods of water analyses, SNTL, ALFA, Praha, pp. 389 (In Czech).
Hrbáček J. et al. (1974): Limnological methods. SPN, Praha pp. 208 (In Czech).
Lesnikov L.A. (1979): Elaboration of standards of acceptable concentration of pollutants in the water of fish culture units, Sbornik naučnych trudov, GosNIORCH, Leningrad, 144, pp. 3–41 (In Russian).
Lewis W.M., Morris D.P.(1986): Toxicity of nitrite to fish: A review. Trans. Amer. Fish. Soc., 115, No. 2, 183–195.
Liebmann H. (1960): Handbuch der Frischwasser-und Abwasserbiologie. VEB Gustav Fischer Verlag, Jena, pp. 1149.
Lukjanenko V.I. (1967): Fish toxikology. Piščevaja promyšlennost, pp. 216 (In Russian).
Marchetti R. (1965): Critical review of the effects of synthetic detergents on aquatic life. Stud. Rev. Gen. Fish. Coun. Medit., No. 26, pp. 25.
Mattheis Th., Taege M. (1982): Datensammlung der Grenzkoncentrationen von Schadstoffen für die Fischproduction. II. Tenside. Institut Für Binnefischerei, Berlin, pp.54.
Mattheis Th., Sommer M. Ch, Grahl K. (1984); Datensammlung der Grenzkoncentrationen von Schadstoffen für die Fischprodution. III. Polychlorierte Biphenyle. Institut für Binnenfischerei, Berlin, pp. 58.
Pitter p. (1974): Design of standard test for assessment of biological degradability of organic substances. Vod. Hospod., B, 24, 247–250 (In Czech).
Pitter P. (1981):The effect of pH, temperature and conductivity on relative share of different forms of ammonium nitrogen in water. vod. Hospod., B, 31, 217–219 (In Czech). Pitter P. (1990): Hydrochemistry. SNTL, Praha, pp. 565 (In Czech).
Schäperclaus W. et al. (1979): Fischkrankheiten. Akademie-Verlag, Berlin, pp.1089. Schreckenbach K. (1982): Die bedeutung von Umweltfaktoren bei der Fischproduction in Binnengewässer. Mh. Vet. Med., 37, 220–230.
Svobodová Z., Máchová J. (1985): Causes, diagnostics and prevention of fish intoxication. ÚVO, Pardubice, pp. 136 (In Czech).
Svobodová Z., Vykusová B. (1991): Determining the maximum admissible concentrations of substances in water from the point of view of fish culture requirements. Research Institute of Fish Culture and Hydrobiology, Vodňany, pp. 39
Svobodová Z. et al. (1987): Toxicology of water animals. SZN, Praha, pp. 231 (In Czech).
Vámos R., Szöllözy G. (1974): There is not a danger of ammonia intoxication of fish if there is enough oxygen in water. Halázat, 20, No. 4, 124 (In Hungarish).
Revised report on fish toxicity testing procedures. FAO-EIFAC, Rome, Techn. Paper, 24, 1983, pp. 37.
Wasserschadstoff-Katalog. Institut für Wasserwirtschaft, Berlin, 1981.
ČSN 83 0520 Fyzikálně chemický rozbor pitné vody. ÚNM, Praha, 1979
ČSN 83 0530 Chemický a fyzikálni rozbor povrchové vody, část 1 – 50, ÚNM, Praha, 1979
ČSN 83 0532 Biologický rozbor povrchové Vody, část 4–6, ÚNM, Praha, 1979
ISO 6341 Water quality-Determination of the inhibition of the mobility of Daphnia máagna Straus (Cladocera, Crustacea), 1982.
ISO 7346/1 Water quality-Determination of the acute lethal toxicity of substances to a freshwater fish [Brachydanio rerio Hamilton-Buchanan (Teleostei, Cyprinidae) - Part 1: Static method of 1984.
ISO 7346/2 Water quality-Determination of the acute lethal toxicity of substances to a freshwater fish [Brachydanio rerio Hamilton-Buchanan (Teleostei, Cyprinidae)] - Part 2: Semi-static method of 1984.
ISO 7346/3 Water quality-Determination of the acute lethal toxicity of substances to a freshwater fish [Brachydanio rerio Hamilton-Buchanan (Teleostei, Cyprinidae)] - Part 3: Flow-through method of 1984.
ISO 8692 Water quality freshwater algal growth inhibition the with Scenedesmus subspicatus and Selenastrum capricornutum, 1989.