(Z. Svobodová, J. Ruprich)
Mycotoxicoses
Mycotoxicoses that occur in animals are caused by administration of feeds affected by toxinogenic fungi and contaminated with their toxic metabolites. Fungi living on the dead plant substrate are most frequently responsible for such disturbances. These are fungi of the saprophytic group, comprising many species of the genera Aspergillus, Fusarium and others.
Aflatoxicosis
Aflatoxicosis is a disease of domestic animals sensitive to the toxic action of the metabolites of the fungi Aspergillus flavus, Aspergillus parasiticus and some others. These fungi grow best on plants containing oil. However, a fungus present on the feed may not always produce a toxic metabolite, an aflatoxin: aflatoxin production as well as the growth of the fungi themselves are influenced by a number of environmental factors (humidity, temperature and others) of the place where the feed is stored. Aflatoxins have a high thermal stability, withstanding temperatures up to 300°C. There are various kinds of aflatoxins of which aflatoxin B1 has the greatest toxicity. Aflatoxins action is mainly hepatotoxic: they damage the liver of the animals affected. For some animals, long-continued administration of sublethal doses of aflatoxins has a cancerogenic effect.
Of all fishes, rainbow trout is most vulnerable to the cancerogenic action of aflatoxins. Tumorous nodes develop on the surface as well as inside the liver of rainbow trout given these substances for several months. The nodes are gray-yellow, tough when palpated, well supplied with blood, and on section they look like connective tissue. Large tumours occur in brood trout old 3–6 years: these fish often have metastases in the kidneys, spleen, gut, gonads and other organs. The tumours may be as large as a chestnut. The big ulcers are palpable through the body wall. They often compress the heart and cause disorders in blood circulation. Ascites often occurs and organs often coalesce. The hepatomata are of the nature of hepatocellular carcinoma. Literature provides evidence that aflatoxin B1 also has a strong acute hepatotoxic action on rainbow trout fry. Feed containing about 5 μg aflatoxin B1 per 1 kg killed 75–100 % of a rainbow trout fry stock in a short time. The liver of the affected rainbow trout was pale, with heavily injected blood vessels and with degenerative changes. Hepatotoxic action (circulation disorders in parenchymatous organs, represented by venostasis, reactive processes around intrahepatal bile ducts, dystrophic alterations of liver cells) prevails in the effect of aflatoxins on carp.
Prevention of aflatoxicosis is based on thorough inspection of the peanuts and meals, inspection of produced feeds, and cool and dry storage of all feedstuffs, especially those for salmonids, with particular emphasis on the feeds for rainbow trout. For the rainbow trout the highest admissible amount of aflatoxin B1 in feed is 0.1 μg per 1 kg and for the remaining fishes 5μg per 1 kg.
From the point of view of the hygiene of human food, the maximum tolerable B1 aflatoxin concentration is 5 μg per 1 kg of fish flesh.
Fusariotoxicosis
This ailment is caused by fungi of the genus Fusarium. The production of these moulds toxic metabolites is influenced by the composition of the substrate parasitized by them, and by the environmental conditions. Fusarium fungi frequently attack fodder cereals during the growing season and also infest stored fodder grain and feeds produced by grain processing. The fungi produce fusariotoxins, mainly T2 toxin (12,13-epoxytrichothecen) while parasitizing these substrates. Carp exhibits the greatest sensitivity to this toxin. At a 72 hour exposure, the lethal doses are 0.46 mg per 1 kg live weight for carp and 6.1 mg per 1 kg of live weight for rainbow trout. Carp orally treated with lethal doses of T2toxin exhibit depression, poor reactivity to stimulation, accelerated respiration, dark body surface, the fish refuse food, excrete undigested food, later replaced by long strings of white mucus. The fish keep close to the water surface and gasp for air. Then follow ataxia, inco-ordination of motion, the fish stop moving and die. The patho-anatomic picture includes dark body surface and marked changes on the gills (oedematous swelling and marbling). Inside the body cavity the blood vessels are heavily injected, parenchymatous organs change colour and size, the mucous membranes of the digestive tract are inflamed with dots of haemorrhages. Long-continued administration of T2 toxin together with other fusariotoxins at sublethal concentrations to carp causes depression, the fish excrete half-digested food, reduce their food intake and show much reduced weight gains.
Determination of mycotoxins in feeds and in fish as human food
1/ Sampling
A high importance is attached to the taking of samples for the detection of mycotoxins. If a feed was produced from contaminated raw materials, its contamination may be uniform, but if the substrate itself is infested by toxinogenic microscopic fungi it often happens that the contamination is of focal nature, with mycotoxin concentrations sometimes higher by an order in the foci than in the rest of the substrate. This of course must be taken into account in the sampling. As a practical solution, an average sample is taken, having a final weight of 1 kg. The whole sample is homogenized and the weight of its portion used for analysis is 20–100 g (most frequently 50 g).
2/ Analytical methods
In principle, there are several different techniques available for the analysis of mycotoxins. Because of the importance of aflatoxins, further text will deal with this group of mycotoxins.
Biological determination
There are a number of organisms on which the toxicity of mycotoxins can be tested. However, the method is not very accurate and usually fails to identify actual mycotoxins. Tests are most frequently conducted on micro-organisms such as Bacillus brevis or Bacillus megaterium, on aquatic organisms such as Artemia salina, and sometimes also on higher organisms, e.g. chick embryos, ducklings and others.
Chromatographical determination
Chromatography is among the most frequently used techniques and among the most accurate ones. The main chromatographic techniques include thin layer chromatography (TLC, HPTLC), high performance liquid chromatography (HPLC) and gas chromatography (GC). Of these, TLC is employed in the majority of cases because it is simple and requires no hi-tech equipment. Where great accuracy is required, instrumentalized HPTLC, HPLC and GC are of help, but they need costly equipment.
Immunochemical determination
Immunochemical techniques were developed for fast tentative determination of some mycotoxins. The principle of these techniques is that the sample mycotoxin and standard mycotoxin are left to compete for a bond to the specific antibody. The mycotoxins are low-molecular organic compounds and do not possess the nature of antigen. However, if they are bound to a suitable proteinaceous carrier they have the nature of an antigenous determinant against which the immunized organism produces antibodies. The mycotoxin then can react with them as a haptene. The substances used most frequently for labelling in the immunochemical determination are enzyme (EIA, ELISA) or radioisotope (RIA). Such techniques are now commercially available e.g. for the determination of aflatoxin B1, ochratoxin A and T2 toxin (TRANSIA, France), aflatoxin B1 and ochratoxin A (RIEDEL-DE HAEN, Germany) etc.
Determining Aflatoxin B1, B2, G1 and G2 by the HPTLC Method
The method is designed for quantitative determination of aflatoxin and is based on the HPTLC technique with visual or densitometric evaluation.
Material:
Methanol, chloroform, acetone, diethylether (peroxide-free),
petrolether, all of a p.a. purity grade.
Sulphuric acid, trifluoroacetic acid, sodium chloride,
anhydrous sodium sulphate, distilled water and other
ingredients.
Chromatographic HPTLC Merck (5547) plates or others of
adequate properties. Developing chamber for vertical
development of TLC plates.
Pipettes, glas capillaries, atomizer and other aids.
Densitometer.
Preparation of sample:
Chromatographic separation:
If extracts are contaminated, subject them first to purification straight on the chromatographic plate. Mark the start 5 cm from the end on the HPTLC plate about 12 cm high. Apply to the start the individual aflatoxins standard (mixed standard of 100 picogrammes of each aflatoxin in 1 microlotre) in amounts of 100, 500 and 1000 pg, together with the sample in amounts of 1, 5 and 10 microlitres (0.04, 0.2 and 0.4 g of sample per 1 spot). It is advantageous to repeat the application of sample with an addition of standard (internal standard). Develop the chromatogramme in pure diethyl ether in the direction from the start to the nearest border of the plate in a saturated chamber. After development, cut off the washed impurities about 1 cm above the start, turn the plate and develop in the reverse direction in a mixture of chloroform + acetone + water (88 + 12 + 0.2) in a saturated chamber.
Chromatogramme evaluation:
Evaluate the chromatogramme in long-wave UV light (366 nm). Aflatoxins fluoresce blue and green. The positions of the spots must be identical with the standards. Determine the quantity tentatively by visual comparison of fluorescence intensity with the standards. Use densitometer (e.g. CAMAG TLC SCANNER II) for accurate evaluation. In visual evaluation, the sensitivity of determination is about 1μg per 1 kg of sample. With the use of the densitometer (employing a greater number of standards) the sensitivity is about 0.1 μg per 1 kg of sample. The recovery is around 90 %.
Aflatoxin identification:
Ceroid degeneration of liver
This ailment occurs in intensive fish culture (especially in salmonids) where the feeding is not much varied. Rainbow trout is particularly vulnerable. As known, unlike other salmonids, rainbow trout shows no great food preference and is able for long to consume food of low value and poor quality, even containing rancid fats. Wild rainbow trout does not deposit store fat in liver but rainbow trout in intensive culture, given low-value pellets, suffers from considerable adiposis of the liver. As fat gathers in the liver cells, there is a considerable increase in the amount of unsaturated fatty acids which are very susceptible to self-oxidation and to the formation of ceroid. Ceroid is a brown-yellow pigment, produced as a result of self-oxidation of unsaturated fatty acids accumulated in the liver of the fish.
Ceroid accumulation in the liver cells leads to a serious specific disease of the rainbow trout: ceroid liver degeneration. It occurs most frequently in those fish populations which are given poor quality feed pellets containing fish and meat-and-bone meals high in rancid fats. Such pellets contain large quantities of unsaturated fatty acid peroxides which destroy vitamins A and E in the fish bodies. (These two vitamins, especially vitamin E, are strong natural antioxidants and, under normal conditions, provide significant protection of the fatty acids of cell membranes).
The ceroid degeneration syndrome is characterized by very conspicuous clinical symptoms. The first of them are dark pigmentation, anaemia and inappetence of the affected fish: this may easily occur even in fish showing a good state of nourishment. The liver is enlarged, sand yellow in colour, with a positive ceroid finding in the hepatocytes. The gall bladder is filled with transparent fluid, the gut is grey-white, its wall is thin and sagged. The stomach is empty or may contain a small amount of transparent whitish fluid. The gill system is markedly anaemic, which is associated with the considerable changes that occur in the blood picture; these changes are characterized by a reduction of the erythrocyte count and corpuscular haemoglobin. As a result, the growth of the fish is retarded and their mortality is high.
Prevention of this disease includes through veterinary inspection of the feed pellets, inspection of feed freshness, adequacy of feed, storage and of the feed administration technique. Feed inspection includes determination of three important parameters: fat acid number as a measure of the hydrolytic breakdown of the fat, peroxide number, and 2-thiobarbituric acid number.
The values of these parameters should not exceed the following levels: fat acid number 45 mg KOH per 1 g, peroxide number 0.30 % iodine, 2-thiobarbituric acid number 1.7 % absorbance of one-percent solution.
Preventive liver examination and haematological investigation of rainbow trout may also be of help in recognizing the first signs of damage and in preventing the disease from spreading. In the early stages the disease can be countered by stopping the administration of the faulty pellets and by replacing them by a sound food fortified with vitamin E (fresh beef spleen has been found to be the best for this purpose). Vitamin E is administered in the form of germ oil with vitamin E forte (at a rate of 10 ml per 1 kg of feed). In the Rt1 category (rainbow trout yearling), such a feed is administered every other day, in the Rt2 category (two-years-old rainbow trout) the feed is administered once in three days until the fish are restored to health. At the present time the rainbow trout pellets are stabilized by antioxidant supplementation (e.g. with ethoxycholine-based preparations).
Feed safety inspection by determining the fat acid number, peroxide number and 2-thiobarbituric acid number
In intensive fish culture, the point always is to evaluate the state and quality of the fat contained in the feed pellets. The samples must be taken so as to be representative of the average quality of the feed batch inspected. They have to be kept in the dark and cold to prevent changes (deviations from the original state) and the laboratory analysis should be performed within as short a time as possible.
The acid number, the peroxide value and the 2-thiobarbituric acid number are used for evaluating the state and quality of fat.
Fat is extracted by means of chloroform for the above-mentioned methods: grind the pellets but do that with care not to generate heat. Mix 50 g of the ground material with 70 ml of chloroform. After 2 hours of frequent stirring, filter the mixture through filter paper into a beaker and leave the chloroform to evaporate in a fume chamber overnight. Then dry the material in a drier at a temperature of up to 40°C. For determination of the peroxide value and the 2-thiobarbituric acid number, do the extraction at a room temperature to avoid further oxidation or loss of volatile oxidation products. Before the analysis, carefully melt the extracted fat on water bath and stirr it with a glass rod.
The acid number of fat expresses the consumption of KOH (in mg) for the neutralization of free fatty acids contained in 1 g of fat. Weigh 30 to 50 mg of fat and add to it, at a 1:1 ratio, 20 ml of alcohol ether (alcohol denaturated with 1 % benzine), neutralized with 0.1 M KOH to phenolphthalein (use about 1 ml of one-percent solution of phenolphthalein in alcohol per 100 ml of alcohol ether and titrate until first pink colouring occurs). Titrate against a white background of 0.1 N KOH.
The acid number (A) of fat in mg KOH per 1 gramme has the following formula:
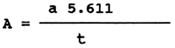
where a = consumption of 0.1 N KOH
t = weighed amount of fat in g (number having 4 decimal
places)
The peroxide value expresses the amount of iodine (in grammes) released from potassium iodide by the peroxide compounds contained in 100 g of fat. Filter the fat dissolved in chloroform into dried and weighed Erlenmayer flasks. Dissolve the fat (0.5 to 0.9 g), weighed with an accuracy of 4 decimals, in 50 ml of a mixture of acetic acid and chloroform (3:2). Add 1 ml of saturated solution of potassium iodide, close the flask and put it in refrigerator. Five to ten minutes later add 50 ml distilled water cooled to 4°C, thoroughly shake the flask (0.5 min), add 1 ml of 1% starch and titrate with 0.002 N sodium thiosulphate until original colour is obtained (when starch is added the solution becomes blue).
The peroxide value (per), expressed as the % of iodine, has the following formula:
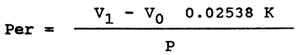
| Where V1 | = | consumption of 0.002 N sodium thiosulphate in ml |
| VO | = | consumption of 0.002 N sodium thiosulphate in blank sample |
| K | = | sodium thiosulphate factor (1) |
| P | = | weighed amount of fat in g |
For purposes of international expression in mE per 1 kg
of fat, the amount of peroxides, calculated by the formula
800 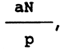 should be divided by 8.
should be divided by 8.
| In the formula, a | = | consumption of Na2S2O3 in ml |
| N | = | normality of this solution |
| P | = | Weight of fat in g. |
The 2-thiobarbituric acid number offers a good method to study the early stages of rancidification of fats and fat-containing feeds, as far as these fats contain polyenic fatty acids.
To determine the 2-thiobarbituric acid number by the direct method without distilling, weigh 30–50 mg of fat in a volumetric flask and dissolve it in 5 ml chloroform. Add 10 ml of 20 % solution of trichloroacetic acid in 2-propanol (isopropyl alcohol) and 10 ml of saturated solution of 2-thiobarbituric acid in 2-propanol (shake 1 g of acid with 100 ml of isopropanol and leave to stand overnight to let the solution saturate). Warm to 60°C and keep that temperature for 24 hours, then measure the intensity of colouring at 530 nm. Amyl alcohol can be used instead of 2-propanol: in such a case, warm the test material to 120°C and maintain the temperature for an hour.
The calibration curve is drawn with the help of tetraethoxypropane (malondialdehyde diacetal), which hydrolyzes into malondialdehyde. Pipette 0.2 to 1.6 ml of 10-4 M solution of tetraethoxypropane in 40 % alcohol and then follow the procedure as in the determination itself. Prepare the 10-5 M solution by 10-4 dilution at a ratio of 1:9.
The 2-thiobarbituric acid number is expressed as malondialdehyde per 1 g of fat (μmol MDA per 1 g).
Damage caused to fish by high-mercury feed
such damage may be caused
The administration of high-mercury feeds to fish affects the hygienic value of the fish flesh. Mercury compounds have also been found to damage some fish organs and tissues. Mercury accumulates in the fish bodies and is strongly fixed to the SH-groups of amino acids. Evidence that mercury fixed in the organs and tissues of fish practically fails to be eliminated from the body is provided by the results of many experiments. It is therefore important for prevention to check for mercury content the feeds and feed mixture pellets intended for fish. The maximum admissible amount is 0.1 mg of total mercury per 1 kg of feed. The technique of feed checking for mercury content is shown in detail in the chapter on the health safety of fish flesh from the viewpoint of contaminants content.
Recommended literature
Halver J.E. (1976): Aflatoxicosis and advetitionis toxins for fish. Fish Patol., 10, 199–219.
Galaš V.T., Ivanova N.S. (1978): About the necessity of research of fish mycotoxicoses. Ryb. Choz., CNNTEIRCH, Moskva, 4, 17 (In Russian).
Ruprich J., Piskač A. (1983): Treatment, purification and identification of some types of aflatoxins. Veterinářstvi, 33, 555–557 (In Czech).
Řehulka J. (1990): Effect of hydrolytically changed and oxidized fat in dry pellets on the health of rainbow trout, Ocorhynchus mykiss (Richardson). Aquaculture and Fisheries Management, 21, 419–434. Svobodová Z. et al. (1987): Toxicology of water animals. SZN, Praha, pp. 231 (In Czech).
Zanini E. et al. (1974): Riserche sulla possibilita di inquinomento dietetico de mercurio in piscicoltura. Riv. Ital. Piscic. Ittiopatol., 9, 7–12.
Manuals of Food Quality Control. 10. training in Mycotoxins Analysis. 14/10, FAO Rome 1990, pp. 113.