The world ecosystem is becoming unstable through an ever increasing reliance on fossil fuels, which are in limited supply. Both terrestrial agriculture and fisheries in developed countries are energy intensive and are thus not suitable for application in developing countries on a large scale. Western agriculture is efficient at producing relatively high yields of food per unit land area, but this is achieved through an intensive, highly mechanized system which depends on large inputs of fossil fuels, for machinery, commercial fertilizers and pesticides. Fish production using Western style methods is usually energy intensive. Most fish consumed in the West are caught by trawlers, and aquaculture in Europe, Israel and the USA is also energy intensive since most fish farms use pelleted feed, the cost of which is often more than 50% of the total fish farm operating costs. Clearly, an alternative strategy to an increasing reliance on fossil fuel for the development of aquaculture in developing countries is needed. This should be based on locally available sources of renewable energy and resources.
A more stable fish production system is based on the recycling of organic wastes, as is practiced on a large scale in China today. The Chinese have developed a most efficient system of aquaculture over the centuries involving waste recycling, because of the need to feed a large population on limited areas of arable land. With soaring populations in most developing countries today, the amount of land available per farmer is shrinking rapidly so there is a need to apply the Chinese “model” of integrated farming to alleviate poverty and malnutrition in these countries. Wastes, by-products which are not fully utilized in the production system, should not be discarded but should be regarded as “resources out of place”.
In integrated farming systems in China, with aquaculture as the main emphasis, livestock are reared primarily to provide manure as a fish pond fertilizer. Crops are grown on the pond dikes for fish feed, and a major source of crop fertilizer is accumulated pond mud which is periodically removed. Aquatic weeds or macrophytes are either cultivated or gathered from natural water bodies as pond inputs. In addition, a variety of other agricultural wastes or by-products are used in the fish production system.
The rationale of adding manure to a fish pond is to encourage the growth of natural pond organisms which serve as feed for fish. While there may be direct consumption of manure by fish, the major sources of nutrition for fish in such a system are generally the natural pond organisms which develop as a result of the fertilizing effect of the added waste (Fig.41). There are two major food chains in a waste fed pond, the autotrophic and heterotrophic systems. In the former, bacteria decompose the waste with the uptake of dissolved oxygen to release inorganic nutrients, which are taken up by plants during the process of photosynthesis. Dense growths of phytoplankton, which normally turn the water a greenish colour, are characteristic of waste fed ponds. Rooted aquatic macrophytes are less common in waste loaded ponds since the growth of phyto-Plankton normally prevents sufficient light penetration into the water to enable them to grow. Phytoplankton may be consumed by zooplankton. The heterotrophic system does not depend on light since it does not involve the process of photosynthesis. Instead, the bacteria which proliferate with the addition of waste to the pond, may be consumed by zooplankton or benthic animals. All the natural pond organisms provide food for various species of fish. It should be emphasized that the autotrophic and heterotrophic food chains are not mutually exclusive; on the contrary, both systems operate simultaneously in waste fed ponds although the relative importance of the two has led to a considerable amount of recent discussion in the fish biology literature.
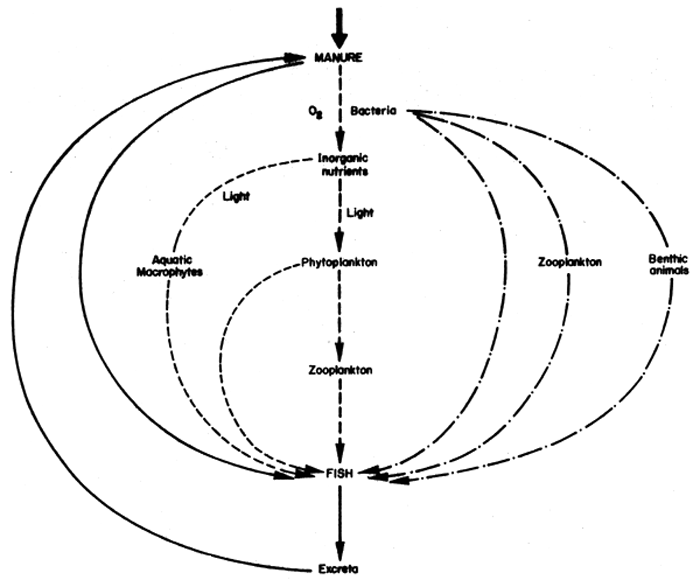
Fig. 41 Possible food chains in a waste loaded fish pond. Solid line, direct consumption of manure by fish; dashed line, autotrophic food chain involving photosynthesis; dashed and dotted line, heterotrophic food chain in which bacteria are consumed by larger organisms.
There is considerable controversy concerning the role of phytoplankton as fish food. It has been stated that fish cannot derive nutrition from certain phytoplankton since certain species are either toxic, too small to be removed from the water, or are indigestible.
There are reports of blue green algae toxic to livestock and water fowl from several parts of the world, particularly the mid western states of the USA. Toxic chemicals have been isolated from Microcystis and Anabaena in the USA, but there is no evidence of Microcystis, a common phytoplankton in waste fed ponds, being toxic to fish in Asia. Toxic strains of these blue-green algae have not been reported from fish ponds and Microcystis has been identified as a component in the feed of silver carp, rohu, and tilapia. A yellow-brown alga, Prymnesium parvum, has been reported to cause fish kills in Israel, but it does not appear to be widespread. Certain dinoflagellates which cause “red tides” may be toxic, but these are largely restricted to coastal waters.
The majority of phytoplankton in waste loaded fish ponds are nannoplankton i.e., so small, usually less than 20 μm, that they pass through nets used to sample phytoplankton. The most well known method used by fish to remove phytoplankton from pond water is by straining, using gill rakers, which in the phytoplankton feeding silver carp can filter particles from the water only larger than about 20 μm. Phytoplankton feeding tilapia species do not have fine gill rakers, but remove phytoplankton from the water by entrapping the algal cells in mucus. There is copious secretion of mucus by cells in the oral and pharyngeal epithelium, in which the phytoplankton become entangled; the mucus and feed aggregate is carried posteriorly in the pharyngeal cavity but is prevented from escaping with the outgoing current by the gill rakers, and is swallowed. Recent Russian work suggests that silver carp may also use mucus secretion, from the labyrinthiform organ, to entrap phytoplankton, in addition to mechanical straining by the gill rakers. Mucus secretion by phytoplankton feeding fish provides an explanation for the presence of nannoplankton in their digestive tracts.
For several years it was believed that many of the more important phytoplankton in waste loaded fish ponds e.g., blue green algae, coccoid green algae, and euqlenoids, were not digested by fish, in particular tilapia. This conclusion was reached because fish biologists observed live phytoplankton in the faeces of fish; motile species were seen to emerge from the digestive tract moving, whilst non motile species could be cultivated from faeces in the laboratory. Such observations even led one fish biologist to advise against using animal manure in fish ponds since he believed that it caused the development of indigestible blue green and euglenoid algae. However, the digestibility of blue green algae by tilapia was re-investigated in the early 1970's after it was observed in certain E. African lakes with large densities of tilapia, that the predominant phytoplankton species was the blue-green alga, Microcystis. It was discovered that there is a diurnal secretion of acid by the fish, which increases throughout the day. Acid secretion does not commence until the stomach is full so that phytoplankton consumed early in the morning, when most of the earlier observations were presumably made, passed through the intestine unchanged. Later in the day when the pH falls to less than pH 2, occasionally to pH 1 – 1.25, the blue green algae are lysed. During the night when feeding does not take place, the pH of the stomach rises to pH 5 – 7. Since the pH of the silver carp stomach does not fall low enough to lyse prokaryotic cell walls, and vertebrates normally do not possess gastric enzymes capable of digesting muramic acid, a major constituent of blue green algal cell walls, further studies are needed on the digestion of phytoplankton by silver carp.
The traditional view of the food web in ecosystems, including aquatic ecosystems, is that energy and materials are passed up to higher trophic levels only through grazers cropping plants. In a waste loaded fish pond, this would mean that the main pathway would be phytoplankton grazed by zooplankton, which in turn would be grazed by fish. It is now known that much of the energy and materials pass along the heterotrophic chain, either from decaying plant matter, or from organic wastes such as manure added to the pond. This is often referred to as the detrital food web. Detritus, particles of non living organic matter, consisting mainly of a matrix of plant fragments, which are being decomposed by bacteria, which in turn are grazed by Protozoa, is an important food source in waste fed fish ponds. In this respect, a waste fed fish pond has been compared to the stomach of a ruminant, in which microbial activity on coarse, fibre rich foods in the rumen provides the animal with food in an easily assimilatable form. Thus, the fish pond may be regarded as an environment in which fish food is grown, as well as a medium for the fish to live. Bacteria may constitute at least 1–5% of the dry weight of detritus and provide a souce of protein rich food for the fish.
Solitary bacteria are not common in fish ponds, most bacteria being aggregated into flakes, films, glomeruli, or composite colonies. There is a high degree of aggregation around detrital particles as discussed above, but also in the surface water film. Aggregates from 6–20 μm can be trapped best by zooplankton, from 21–60 μm by silver carp, and greater than 60 μm by bighead carp. About 50% of all bacteria may be consumed by silver carp and 25–33% by bighead carp. Since bacterial aggregates in the front section of the guts of silver carp are almost always surrounded by mucus, perhaps secreted by the labyrinthiform organ, isolated bacteria and small bacterial aggregates evidently adhere to the mucus, are aggregated into larger masses, and swallowed. Since tilapia also secrete mucus, perhaps they also can trap and feed on bacteria in waste loaded ponds.
Zooplankton feed mainly on phytoplankton or on small bacterioplankton aggregates less than 20 μm. Although zooplankton may be important in waste loaded ponds as fishfeed, there is evidence that they may be suppressed in ponds with dense phytoplankton communities caused by high loadings of organic matter. The reasons for the scarcity of zooplankton in ponds with high phytoplankton densities are not known, but there is evidence that certain algae secrete chemicals that inhibit zooplankton. Furthermore, the elevated pH in ponds with dense algal growths, often exceeding pH 9.5, may inhibit zooplankton. A more simple explanation to explain such a phenomenon, which has been observed in flow-through sewage stabilization ponds, is that with a high organic loading of waste and therefore a small detention time (the meantime a water molecule is present in the pond), while rapidly growing phytoplankton flourish, the water may be in the pond for an insufficient period of time for zooplankton to develop due to the short detention time.
Probably the most important benthic animals in waste loaded fish ponds are the filter feeding larvae of midges, chironomids or blood worms. They exist in tens of thousands per m2 in the sediment of ponds with low dissolved oxygen due to the microbial decomposition of organic matter, and are a high protein feed for bottom feeding fish.
The data from Israel in the following table, indicate the importance of both zooplankton and chironomids as fish food in a fish pond receiving fluid cow shed manure, stocked with a polyculture of silver carp, tilapia and common carp. In summer, all four 400 m2 ponds were stocked with fish, which heavily grazed the food organisms; the biomass of zooplankton and chironomids were only 0.2 – 0.8 g dry weight/m3 and 200 – 1,000 individuals/m2, respectively. However, in winter an experimental design incorporating presence or absence of fish, and presence or absence of manure, clearly indicates the importance of pond manuring for the production of food organisms and their utilization by fish.
Table 1. The effect of manure on the production of zooplankton and chironomids and their consumption by fish
Food organism | Fish absent | Fish present | ||
| Manure | No manure | Manure | No manure | |
| Zooplankton (g dry weight/m3) Chironomids (number × 102/m2) | 3.3 - 42.4 79 - 215 | < 0.1 1 - 7 | 0.3 - 1.3 1 - 4 | < 0.1 1 - 2 |
Many species of fish consume manure directly, but apparently grain little nutritional benefit because manure is a low quality feedstuff. Metabolizable energy in cow and chicken manure is only 600 – 800 and 900–1,200 kcal/kg, respectively, compared to 3,000 and 3,000 – 4,000 kcal/kg for conventional pelleted feed and zooplankton, respectively. In addition, more than 50% of the crude protein content of manure (determined by Kjeldahl nitrogen x 6.25) is non protein nitrogen such as uric acid. Thus, from aspects of both available energy and protein, cow and chicken manure are inferior feeds. In experiments with common carp and channel catfish, in which dried manure was incorporated into standard feed pellet as a replacement for higher quality components, fish growth decreased with increasing concentrations of manure incorporated into the pelleted feed. In the above experiments, the fish were grown in cages or tanks so that they did not have access to natural food produced as a result of the fertilization of decayed products of uneaten pellets or faeces.
However, in ponds where fish had access to natural food organisms which developed as a result of the fertilizer effect of the feed pellet decay products, pelleted feed replaced by up to 30% manure produced fish growth equivalent to that of fish fed conventional feed pellets. It would appear that the high quality natural food produced in the pond was able to compensate for the decrease in pelleted feed given.
It should be pointed out however, that when tilapia was fed commercial trout diet incorporating up to 30% dried poultry waste, in running water troughs where the fish did not have access to natural food organisms produced from the waste, there was no statistically significant difference in growth with the control, 100% trout diet. Tilapia was able to derive considerable nutrition from the poultry waste, possibly due to acid hydrolysis of manure in its stomach due to low pH. Thus, tilapia may be able to derive more nutrition from the direct consumption of manure than other types of fish.
The theoretical basis for applying organic wastes to water has been developed by sanitary engineers for the treatment or disposal of human waste. However, this knowledge can be applied to the study of waste loaded fish ponds.
When organic wastes are added to water, they are degraded or broken down by bacteria, with the release of inorganic nutrients and the uptake of oxygen. The amount of oxygen required to oxidize the organic matter in the waste is referred to as the oxygen demand and is expressed in terms of the concentration of oxygen required to oxidize a given volume or weight of organic waste. There are two basic ways to measure the oxygen demand, chemical and biological oxygen demand.
The waste is oxidized chemically with a boiling acid dichromate solution and the reaction normally proceeds to more than 95% completion. Since the test takes only about 3 hours, it is used frequently for monitoring wastes, but it does not give any information on the rate of bio-oxidation by bacteria, nor on the proportion of the waste that can be oxidized by bacteria.
The theoretical oxygen demand, the theoretical amount of oxygen required to completely oxidize the organic fraction of the waste to carbon dioxide and water is close to the COD, as mentioned above. The theoretical oxygen demand for an organic substance can be calculated using the atomic weights of the elements involved, providing that the chemical formula is known. The theoretical oxygen demand of a 500 mg/l solution of glucose is:
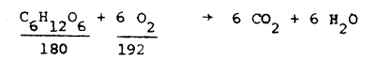
i.e., the ratio of glucose to O2 required to completely oxidize it is about 1:1.
The test normally used by sanitary engineers is the BOD5 i.e., the amount of O2 required to oxidize the waste by bacteria at 20°C for 5 days. The test was established by the United Kingdom Royal Commission on Sewage Disposal of 1898-1915 to safeguard rivers in Britain from deoxygenation by organic waste pollution. The criteria for the test were chosen because British rivers do not have a flow time to the open sea greater than 5 days, and the mean summer temperature in the United Kingdom is approximately 20°C. Clearly the values chosen for the test have limited applicability for most other areas of the world, but the test is now standard for sanitary engineers and BOD5 data are used as design criteria for waste treatment systems.
The BOD5 test at 20°C has limited value for waste loaded ponds. A more appropriate test for aquaculture would be BOD1 (24 hours) or BOD0.5 (12 hours) at pond temperature, since the most critical aspect of waste loaded ponds is the decrease of dissolved oxygen in the pond water during the night.
The organic loading is the rate at which biodegradable organic waste is added to a pond and is normally measured in kg BOD5 or COD/ha/day. The principle in treating organic wastes in oxidation or stabilization ponds, ponds designed by sanitary engineers to treat or dispose of wastes, is to utilize the smallest land area possible to minimize costs. This means that high organic loadings are employed, but these decrease the oxygen to such an extent that much of the pond system is anaerobic, at least during the night, and the system is unsuitable for fish culture.
Since the organic matter in a waste loaded fish pond provides nutrients for the growth of natural fish food in the pond, the principle in waste loaded fish ponds is to add sufficient waste to provide adequate nutrition for the food organisms, but not enough to lead to dangerously low dissolved oxygen levels for fish growth and survival. The addition of waste to fish ponds is still very much an art learned through experience, but research is in progress to quantify organic loading in fish ponds to determine bioengineering design criteria for the construction of integrated fish farms.
The rationale behind the use of livestock manure in fish culture is evident when it is realised that approximately 72–79% of the nitrogen, 61–87% of the phosphorus, and 82–92% of the potassium, present in the feed fed to the animal is recovered in their waste.
The amount and composition of livestock waste (urine and faeces) varies with the species and the total live weight of the animal (Table 2). Larger animals obviously produce more waste than smaller ones of the same species, but younger animals void more waste per total live weight than mature animals since the former have a higher metabolic rate than the latter. Climate, feed and water intake, and management practice all affect the waste characteristics. There have been few studies relating the composition of the diet to the nutrient characteristics of the waste, but livestock fed higher quality feed have a higher concentration of nutrients in their waste. Livestock fed with large amounts of feed would also tend to produce waste with a higher nutrient content since digestion is less efficient than livestock fed with low levels of feed because of a faster rate of passage of food through the gut. The data presented in Table 2, from Taiganides (1977) are based on well fed animals in feed lots in the USA and should only be used as a general guideline. For specific design purposes, actual measurements should be made of livestock with relevant management practices.
Urine contains less than 40% of the total wet weight of the waste, but nitrogen and potassium are more concentrated than in the faeces. Phosphorus is mainly present in the faeces, except in pigs which have considerable phosphorus in the urine.
From Table 2 it can be seen that dairy cattle produce considerably more waste on a wet weight basis as a percentage of total live animal weight per day than do pigs and hens, but that hens produce the most total solids on a dry weight basis in their waste. In terms of N, P, K nutrients, hen waste has the highest concentration, followed by pigs with cattle waste having the lowest concentration.
The management of the wastes will have a marked effect on their characteristics. The following three factors are of particular importance:
Table 2 Bio-engineering parameters of animal wastes. TLW = total live weight. (modified from Taiganides, 1977).
| Parameter | Symbol | Units | Pigs | Laying hens | Dairy Cattle |
| Wet waste | TWW | % TLW/d | 5.1 | 6.6 | 9.4 |
| Total solids | TTS | % TWW | 13.5 | 25.3 | 9.3 |
| % TLW/d | 0.69 | 1.68 | 0.89 | ||
| Volatile solids | TVS | % TTS | 82.4 | 72.8 | 80.3 |
| % TLW/d | 0.57 | 1.22 | 0.72 | ||
| Biochemical oxygen demand (5 day, 20°C) | BOD5 | % TTS | 31.8 | 21.4 | 20.4 |
| % TVS | 38.6 | 29.4 | 25.4 | ||
| % TLW/d | 0.22 | 0.36 | 0.18 | ||
| Chemical oxygen demand: biochemical oxygen demand ratio | COD/BOD5 | Ratio | 3.3 | 4.3 | 7.2 |
| Total nitrogen | N | % TTS | 5.6 | 5.9 | 4.0 |
| N | % TLW/d | 0.039 | 0.099 | 0.036 | |
| Phosphate | P2O5 | % TTS | 2.5 | 4.6 | 1.1 |
| P2O5 | % TLW/day | 0.017 | 0.077 | 0.010 | |
| P | % TTS | 1.1 | 2.0 | 0.5 | |
| Potash | K2O | % TTS | 1.4 | 2.1 | 1.7 |
| K2O | % TLW/day | 0.010 | 0.035 | 0.015 | |
| K | % TTS | 1.2 | 1.7 | 1.4 | |
| Nitrogen/Phosphorus ratio | N/P | Ratio | 5.1 | 3.0 | 8.0 |
Whether or not the waste collected includes the urine.
The amount of inclusion of foreign materials in the waste. Water used to wash livestock quarters may considerably dilute the waste. It is not uncommon to find waste in pits under cow milking sheds with only a 1% concentration of solids, whereas fresh cow manure should have a dry matter content of about 10%. Poultry manure scraped from pen floors may include considerable amounts of soil so that its ash content (total solids minus volatile solids) can exceed 50% of the total dry solids, whereas the ash content should not exceed 30%. Thus, weights of livestock waste should be expressed as both total solids and volatile solids to determine the amounts of both washing water and soil contamination.
Storage in situ. Considerable losses of nitrogen, from about 20–90% depending on the methods of collection and handling, are possible due mainly to volatilization of ammonia. Losses would increase with both temperature and wind strength. The loss of phosphorus is less of a problem since it is bound to or is part of the solid matter.
The COD/BOD5 ratios of livestock wastes (Table 2) is useful measure of the bio-degradability, or the potential rate of microbial breakdown of the wastes. Low values indicate a higher rate of degradation. The ratios for pig, hen, and dairy cattle are 3.3, 4.3, and 7.2, respectively. These are considerably higher than domestic sewage for which the ratio is approximately 2 and which is therefore considerably more bio-degradable.
Terrestrial plants and aquatic macrophytes are added to fish ponds in China in large amounts, largely as green fodder for the consumption of macrophyte feeding fish such as grass carp and wuchang fish. The term “green manure” is sometimes used in fish culture for macrophytes added to fish ponds, but should be avoided if the main reason for adding the vegetation is as supplementary feed. There will of course be a fertilization effect from unconsumed and from partially digested vegetation in fish faeces, particularly in grass carp; the inefficient digestion of this fish has led to it being called a “living manuring machine”. The term “green manure” has been borrowed from the field of agronomy, in which it is used to describe plants which are either cultivated or collected as a crop fertilizer. The use of the term “green manure” would be valid to describe the addition of vegetation to a fish pond devoid of herbivorous macrophyte feeders, since in such a case the vegetation would decay and function as a fertilizer. Vegetation is used as an organic fertilizer in Chinese nursery ponds.
A variety of terrestrial crops are cultivated on pond dikes in China as green fodder. These include elephant or napier grass in the Pearl River area and English rye and sudan grass in the Yangtze River area. A variety of vegetables, largely belonging to the cabbage family, Brassica spp., are cultivated in both areas. Where natural bodies of water exist, such as Lake Taihu in the lower Yangtze valley, aquatic macrophytes are collected as green fodder. In addition, aquatic macrophytes may be cultivated in rivers and canals adjacent to fish farms, particularly the three aquatic plants: Eichhornia, Pistia, and Alternanthera.
It might appear to be advantageous to grow aquatic macrophytes and herbivorous fish together in the same pond so that fish could harvest the vegetation as it grows, Unfortunately, this would not be feasible with either submersed or floating macrophyte species. To provide for adequate growth of submersed macrophytes, it would be necessary to fertilize the water to increase their growth rate; however, phytoplankton would also respond to the increase in fertilization and would reduce light penetration into the water, which would eliminate the submersed vegetation through shading; dense growths of submersed macrophytes and phytoplankton are seldom observed to co-exist in the same system. Floating macrophytes would in theory respond to added fertilizer since they themselves would shade the phytoplankton; however, in practice it would be difficult to maintain an appropriate equilibrium between floating macrophytes and fish; should the floating macrophytes provide a substantial vegetation cover on the water surface, it could lead to anaerobic conditions in the water with concomitant stress to the fish; alternatively, the herbivorous fish population could consume the vegetation at a faster rate than its replenishment by growth and lead to its elimination from the pond. It would be difficult to manage such a system from a practical point of view.
There is considerable variation between species in the chemical composition of both dried aquatic macrophytes and terrestrial fodder crops. While many aquatic macrophytes are inferior to terrestrial fodder as animal feed, several are better. Alfalfa hay, a conventional USA terrestrial forage crop, has a crude protein value of about 17%, whereas crude protein values of aquatic macrophytes range from about 6–24% on a dry weight basis. Higher crude protein values have been reported for aquatic macrophytes grown on nutrient rich media, even up to 40% crude protein for duckweed. Perhaps the major difference between terrestrial green fodder and aquatic macrophytes is their water content. Pasture grass is about 80% water, whereas aquatic macrophytes are often at least 90% water. This means that approximately twice as much fresh plant material of an aquatic macrophyte is required to obtain the same amount of dry plant matter of pasture grass.
To effectively feed herbivorous fish with green fodder, it is necessary to understand both their feeding habits and feeding efficiency. Unfortunately, herbivorous fish do not eat all species of aquatic macrophytes with equal relish, but certain generalizations concerning vegetation preference can be made. The most favoured plants are filamentous algae, soft submersed macrophytes, and duckweed. Among the least favoured are rushes, sedges, water cress, water lettuce and water hyacinth. Although herbivorous fish prefer more succulent plants, taste appears to be involved since in a list of sixteen plants eaten by grass carp in order of preference, water cress, which is fairly succulent, was the fourteenth species listed. It is unfortunate that water hyacinth is not readily consumed by herbivorous fish since it grows so prolifically. In China the plant is macerated by machine into a liquid slurry or paste, which is added to the pond as a fertilizer.
To evaluate the value of vegetation for herbivorous fish feed, it is necessary to know the FCR or food conversion ratio (the weight of fresh vegetation consumed divided by the increase in the weight of the fish). There is a tremendously wide range of FCR values reported in the literature, but in general they are large i.e., the conversion of vegetation to fish is a highly inefficient process. The single, largest factor for the inefficiency of the food conversion is the high water content of the vegetation. The FCR of terrestrial napier grass has been reported to be 27:1, but the FCR's of aquatic macrophytes range from about 50–100, due to their higher water content.
Green fodder unconsumed by fish in the pond would exert an oxygen demand. The COD of three aquatic macrophytes ranged from 0.88–1.09 mg O2/mg dry weight of plant material, with a mean value of 1.02. The rates of oxygen consumption, measured by BOD at 30°C at increasing intervals of time, indicated that the rate of oxygen consumption was the highest on the first day, declined by about 50% on the second day, and afterwards the rate of oxygen uptake decreased gradually as the amount of substrate available declined (Table 3). However, the mean COD/BOD5 ratio for the three aquatic macrophytes of 5.25 indicates a relatively slow rate for complete biodegradation. In fact, after 5 days only 14% of Typha, 18% of Eichhornia and 35% of Najas had been oxidized, which indicates that the remains of these plants would accumulate on the pond bottom and break down slowly. There is evidence for a refractory portion of aquatic macrophytes, which resists decomposition, and which would build up on the pond bottom. The data presented in Table 3 are for aquatic macrophytes, but terrestrial fodders would have oxygen demand characteristics within the range reported, since one of the three aquatic macrophytes, Typha, has a coarse, emergent life form with a high amount of biodegradable resistant matter. The accumulated, decaying plant matter on the pond bottom is periodically removed in China for crop fertilizer.
Since fish and most of the other pond biota require oxygen for respiration, the basic energy providing biochemical reactions, it is essential that adequate levels of dissolved oxygen (DO) be maintained in fish ponds.
The concentration of oxygen in water depends on both physical and biological factors. The solubility of oxygen in water varies with temperature, salinity and atmospheric pressure but temperature has the most marked affect on the concentration of dissolved oxygen. The concentration of oxygen in water is inversely proportional to temperature i.e., warm water in equilibrium with air contains less dissolved oxygen than cool water; the solubility of oxygen in pure water at 1 atmosphere pressure at 20°C and 30°C is 8.84 and 7.53 mg/l, respectively (Fig.42). Problems caused by a decrease in the concentration of oxygen in water at higher temperatures are compounded by the increase in the metabolic demand for oxygen and a decreased affinity of haemoglobin for oxygen with an increase in temperature. Oxygen concentrations are expressed in either mg oxygen/1 or as percentage saturation in water.
Table 3 Chemical oxygen demand (COD) and biological oxygen demand (BOD) at 30°C of aquatic macrophytes (modified from Almazan and Boyd, 1978).
| Aquatic macrophyte | Life form | COD (mg O2/mg dry weight) | BOD (mg O2/mg dry weight/day) | COD/BOD5 | |||||
| BOD0.5 | BOD1 | BOD2 | BOD3 | BOD4 | BOD5 | ||||
| Typha latifolia | Emergent | 1.08 | 0.045 | 0.067 | 0.105 | 0.121 | 0.145 | 0.148 | 7.30 |
| Eichhornia crassipes | Floating | 0.88 | 0.044 | 0.079 | 0.109 | 0.128 | 0.145 | 0.157 | 5.61 |
| Najas guadalupensis | Submersed | 1.09 | 0.077 | 0.107 | 0.187 | 0.283 | 0.313 | 0.383 | 2.85 |
| Mean | 1.02 | 0.055 | 0.084 | 0.134 | 0.177 | 0.201 | 0.229 | 5.25 | |
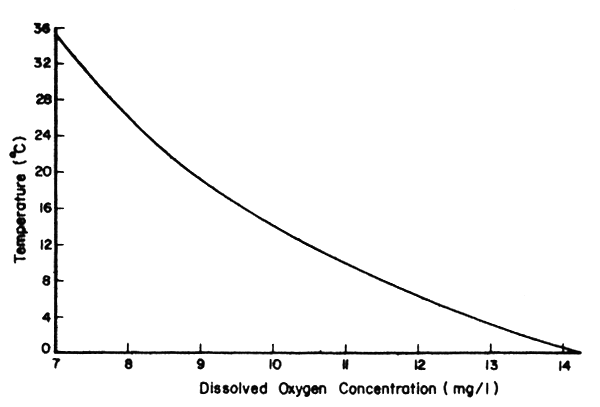
Fig. 42 Solubility of oxygen in pure water at different temperatures at a pressure of 1 atmosphere.
The concentration of oxygen in water can temporarily become supersaturated and exceed its solubility under the prevailing conditions. Diffusion of oxygen into air would then take place, and this would be accelerated by wind and water turbulence. Conversely, if the concentration of oxygen in water fell below its solubility, oxygen would diffuse into the water from the air.
Biological activity in the pond accounts for the greatest variations in dissolved oxygen. The main source of oxygen is the photosynthesis of phytoplankton during daylight hours, which can be represented by the following simplified general equation:
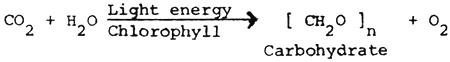
The major cause of oxygen depletion is the respiration of the pond biota, including the phytoplankton, which can be represented by a general equation which is essentially the reverse of photosynthesis:
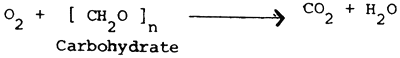
Diurnal changes in dissolved oxygen in a series of hypothetic ponds are presented in Fig.43a. In ponds with infertile water, there would be only slight changes in dissolved oxygen due to low amounts of biological activity. A fertile or eutrophic fish pond, well managed to prevent deoxygenation during the early morning hours, would have a diurnal dissolved oxygen range from only a few mg DO/1 in the early morning to approximately double super-saturation or about 12–18 mg DO/1 in the late afternoon. High rate algal ponds designed specifically to produce phytoplankton, and not containing fish, would be anaerobic at night and have triple supersaturated levels of dissolved oxygen during the afternoon due to intense growth of phytoplankton. A diagrammatic representation of respiration which is assumed to be constant which decreases, and diurnal changes in photosynthesis leading to an increase in dissolved oxygen, are presented in Fig. 43b. The net changes in dissolved oxygen are responsible for the diurnal curve of dissolved oxygen in fertile fish ponds (Fig. 43a).
Fish species vary in their tolerance to low levels of dissolved oxygen in water. Air breathing fish such as snakehead, Channa, and catfish, Clarias and Pangasius, are least affected since they do not depend on dissolved oxygen for their oxygen supply. However, most fish species depend on the oxygen dissolved in the water for their requirements. The most sensitive fish are the cold water salmonids, followed by cyprinids and finally cichlids. Data in the literature concerning fish tolerance to dissolved oxygen levels are unsatisfactory since they mainly represent fish survival in constant dissolved oxygen concentrations. These data have limited relevance to waste loaded fish ponds with widely fluctuating dissolved oxygen concentrations since fish may be able to survive low concentrations of dissolved oxygen for short periods of time; thus, it is not only the level to which the dissolved oxygen concentration falls, but the length of time at which it remains at low levels. Furthermore, there may be sublethal effects; fish may be able to survive a given low concentration of dissolved oxygen but perhaps growth and/or reproductive capacity are impaired.
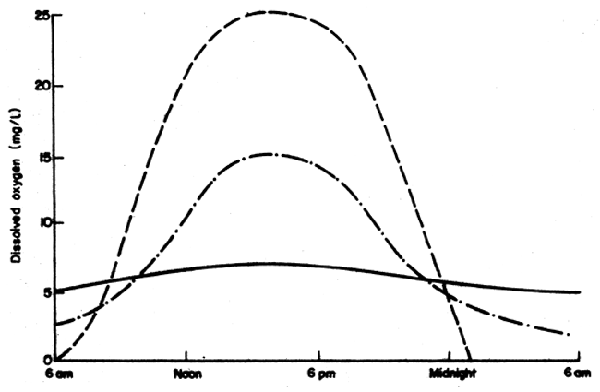
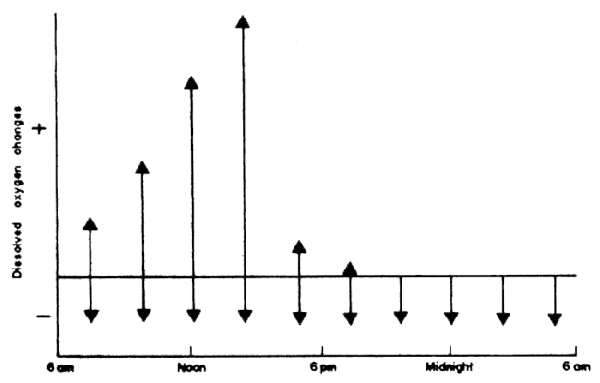
Fig.43 (a) Diurnal changes in dissolved oxygen. Solid line, infertile pond; dashed and dotted line, fertile fish pond; dashed line, high rate algal pond. (b) Positive (photosynthesis) and negative (respiration) oxygen changes in a fertile fish pond.
The critical period for dissolved oxygen in a waste fed fish pond is during the early morning hours (Fig.43a). The following equation includes those factors which influence dissolved oxygen levels:
| DOdn | = | DOdk ± DOdf - DOf - DOm - DOp | |
| where: | DOdn | = | DO concentration at dawn |
| DOdk | = | DO concentration at dusk | |
| DOdf | = | DO gain or loss due to diffusion | |
| DOf | = | DO consumed by fish | |
| DOm | = | DO consumed by mud respiration | |
| DOp | = | DO consumed by plankton |
During the night the only source of oxygen is diffusion, which is seldom as great as the respiratory loss due to the pond biota, so the dissolved oxygen concentration declines. Waste loaded fish ponds are usually supersaturated with oxygen at dusk, because of intense photosynthetic activity during the daylight hours, and there will be a loss of oxygen to the air during the early part of the night. However, during the early morning hours, when the respiration of the pond biota has decreased the oxygen concentrations to less than saturation level, oxygen will diffuse into the pond from the air. Rates of oxygen loss by diffusion, which are greatest with high supersaturation and turbulence, are difficult to determine, but if the pond dissolved oxygen is more than 120% saturated in the late afternoon, the net overnight transfer of dissolved oxygen across the pond water-air interface will be negative i.e., more oxygen will be lost in the late afternoon and early evening than will be gained in the early morning. It has been estimated that the net loss of oxygen to the atmosphere by diffusion in a pond with double supersaturation in the late afternoon is greater than 4 mg DO/1.
Values for mud respiration in fish ponds in Israel and the USA have been reported to vary from 8–125 mg O2/m2/hour. If a medium value of 60 mg O2m2/ hour is taken, and if a pond 1 m2 deep uses oxygen at the above rate for an overnight period of 12 hours, then 720 mg O2/m2 of mud surface, would be consumed. Since a volume of 1 m3 of water overlies a 1 m2 area of mud in a 1 m deep fish pond, the above rate of oxygen consumption would only lower the dissolved oxygen in the water in 12 hours by 0.72 mg O2/1.
Fish respiration varies with species, size, activity and temperature. A general predictive equation valid over a temperature range of 20–30°C is as follows:
| Y | = | 0.001W0.82 | |
| where: | Y | = | g O2 consumed/fish/hour |
| W | = | mean weight of fish (g) |
If a pond were stocked with 10,000 individuals/ha weighing 260 g/fish, the total weight of the fish population would be 2,600 kg/ha. If the pond were 1 m deep, fish respiration according to the above equation would decrease the oxygen in the water by only 1.15 mg O2/1 in 12 hours.
The most important factor in the overnight consumption of dissolved oxygen in a waste loaded fish is the respiration of the plankton (bacterioplankton, phytoplankton, and zooplankton). The mean BOD of water in intensive fish ponds in Israel varied from 3–17 mg O2/1/24 hours, or a maximum overnight consumption of 8.5 mg O2/1. Thus, the major danger to oxygen depletion is the development of heavy blooms of phytoplankton, which cause a far greater reduction in dissolved oxygen than the other parameters involved. Prolonged periods of cloudy weather may lead to dissolved oxygen problems; due to restricted phytoplankton photosynthesis caused by low light intensity, dissolved oxygen concentrations at dusk are not as high as after a clear, sunny day, and this leads to a further reduction in early morning dissolved oxygen concentrations.
The biological oxygen demand of the organic waste is not included in the equation for the factors affecting dissolved oxygen levels; it is accounted for in the BOD of the pond water (plankton) and the pond mud since the waste is either suspended in the water column or settled on the pond bottom. However, the oxygen demand of manure added to the pond is only a minor factor in the overnight consumption of oxygen. In Israel, fluid cowshed manure (12.5% dry weight) has been applied to fish ponds at rates of up to 1 ton or 1 m3/ha/day. Since the BOD, of fluid cowshed manure is 7 g O2/kg/24 hours at 30°C, 1 m3 of manure would consume 7,000 g O2/24 hours or 3,500 g O2/12 hours. Thus, the overnight oxygen demand in a 1 ha pond, 1 m deep, (containing 10 million 1 of water), would be only 0.35 mg O2/1. It appears that it is not the oxygen demand of the manure itself that causes the greatest reductions in dissolved oxygen, but the respiration of the phytoplankton that develop as a result of the release of nutrients contained in the manure.
The major reason for adding organic wastes to a fish pond is to provide substrates for bacteria, the degradation of which releases inorganic nutrients for phytoplankton growth. For the design of waste loaded fish pond systems, a knowledge of the major nutrients required for the growth of bacteria and phytoplankton is needed. Generally, the C:N:P ratio of bacterial growth media is about 100:5:1. Most phytoplankton species have a remarkably similar chemical composition when grown in a nutrient rich medium in which light is growth limiting, about 45–50%C, 8–10%N, and 1%P i.e., a C:N:P ratio of about 50:10:1. Clearly, the major nutrient in aquatic ecosystems is carbon, which is not normally considered as a fertilizer in land crops because the CO2 content of the air is for practical purposes more or less constant. However, in a fish pond with dense growths of phytoplankton, carbon could become a limiting factor. It should also be noted that N is required in considerable excess of P for both bacterial and phytoplankton growth.
Carbon dioxide is highly soluble in water, but since it is only a minor part of the air, about 0.03% by volume, concentrations of CO2 in water are small. Carbon dioxide dissolves in water to form carbonic acid, H2CO3, which dissociates to form bicarbonate, HCO3-. Natural pond waters usually contain more bicarbonate than that derived from the ionization of carbonic acid in water saturated with CO2; the CO2 in natural waters reacts with bases in rocks and soil such as calcite, CaCO3, and dolomite, CaMg (CO3)2, to form bicarbonate. Total alkalinity is the total titratable bases in water, and in most waters the predominant bases are bicarbonate and carbonate derived from carbonate rocks and soils. Total alkalinity levels for natural waters range from a few mg/l (expressed as equivalent CaCO3) to a few hundred mg/l. Waters with a high alkalinity are generally the most productive due to high concentrations of C, but other essential elements such as N and P must be present also in adequate amounts.
The equation relating the various forms of C in water is as follows:
| H2O + CO2 | → ← | H2CO3 | → ← | H+ + HCO3- | → ← | 2H+ + CO3 |
| carbon dioxide | carbonic acid | bicarbonate | carbonate |
The equilibrium between the various forms of C depends on pH as indicated in Fig.44. At pH less than 5, only free CO2 is important; at pH 7–9, bicarbonate is most significant; whereas at pH greater than 9.5, only carbonate is important.
Phytoplankton growth is controlled by the total concentration of inorganic C in an inorganic C limited medium:
CT = CO2 + H2CO3 + HCO3- + CO3--
where CT = total carbon.
During photosynthesis, phytoplankton take up CO2 and H2CO3 with a corresponding rise in pH. However, the marked rises in pH in waste loaded ponds in excess of pH 9, and decrease in total alkalinity, are due to the uptake of bicarbonate and the release of strongly basic hydroxyl ions during photosynthesis:
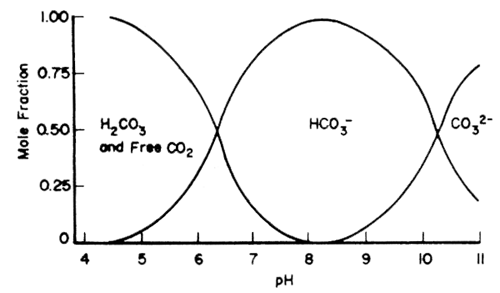
Fig.44 Effect of pH on the relative proportions of H2CO3 and free CO2 (total CO2), HCO3-, and CO32-
HCO3- → CO2 + OH-
Bacterial respiration using organic wastes as substrates is undoubtedly a major source of CO2 for photosynthesis in waste loaded ponds.
Fish can sense small differences in the free CO2 concentration of water and attempt to avoid water with high CO2 levels. Free CO2 levels in water in intensively cultivated fish ponds varies from O mg/l in the afternoon to 5–10 mg/l at dawn. Concentrations of 10 mg CO2/1 or more are tolerated if the concentration of DO is high, but may cause stress to the fish if DO levels are critically low.
The nitrogen cycle is the most complex of the nutrient cycles (Fig. 45). The pathways are relatively well defined, but the rates of conversion of nitrogen from one form to another are poorly understood. Organic nitrogen in the form of waste added to the pond, or decaying organisms in the pond, is decomposed by bacteria to form ammonium (NH4+). The ammonium is oxidized by bacteria in the process of nitrification, first to nitrite (NO2-) and then to nitrate (NO3-). Phytoplankton are able to absorb both nitrate and ammonium, and probably nitrite to a lesser extent. The nitrogen absorbed by plants is metabolized into organic nitrogen and is either passed along the food chain via herbivores and carnivores, or decays at the various trophic levels.
Losses of nitrogen from the system can occur by the volatilization of ammonia or by denitrification which partially explain the relatively rapid loss of nitrogen fertilizers when added to fish ponds. When ammonia dissolves in water, the following equilibrium is established:
| NH3 + H2O | → ← | NH4+ + OH- |
The ratio of the ammonium ion, NH4+, to ammonia, NH3 is inversely related to pH. Thus, there is a strong tendency for volatile NH3 to be lost from the pond system to air during periods of high photosynthetic activity when the pH rises due to uptake of carbon.
The denitrification of nitrate to nitrogen gas occurs in the anaerobic portion of the pond sediments. Nitrate diffuses across the oxidized zone of the sediment to the oxidized-reduced interface where reduction takes place. Maximum losses occur in sediments of high biological activity, which are commonly found in waste loaded fish ponds, where there is only a relatively thin oxidized zone. Nitrate losses would tend to be minimal in water bodies with less biological activity, possessing thick oxidized zones in the sediments.
Nitrate may be gained by the pond system through the process of nitrogen fixation. The process, in which inert nitrogen gas is converted to nitrate, is carried out mainly by filamentous species of blue-green algae possessing heterocysts, which contain the enzyme nitrogenase responsible for the biochemical transformations, e.g., Anabaena and Nostoc. Nitrogen fixation normally does not occur in the presence of excess nitrogen available for phytoplankton growth in the water. However, there is evidence that if the N:P ratio of the waste added to the system is such that there is an excess of P relative to N for phytoplankton growth, then nitrogen fixation will occur. Most livestock manures have an N:P ratio less than the optimal ratio of 8–10:1 for phytoplankton growth (Table 2), which suggests that nitrogen fixation may proceed in waste loaded ponds.
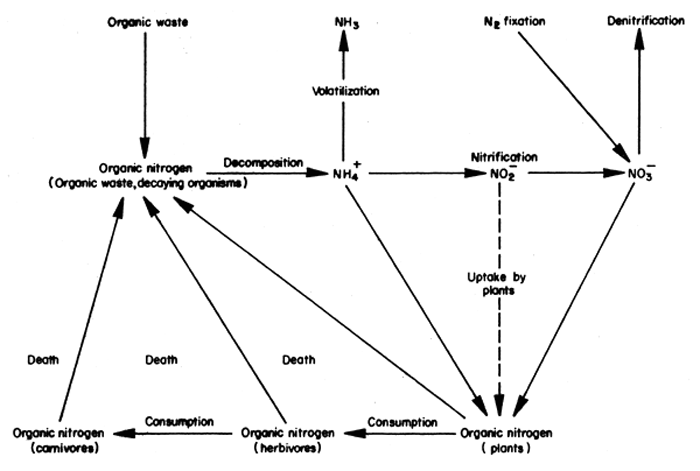
Fig. 45 The nitrogen cycle in a waste loaded fish pond.
Ammonia at certain concentrations is toxic to fish. Ionized ammonia, NH4+, is not toxic since it is a large ion due to hydration, and is charged, so it does not easily pass through the charge lined micropores of the hydrophobic gill membrane. Un-ionized ammonia (NH3) however, is toxic, since it is lipid soluble and has no charge, and thus can readily diffuse across the gill membrane. The percentage of un-ionized ammonia increases with both pH and temperature (Fig. 46). Toxic levels of total ammonia to fish vary from 0.6 – 2.0 mg/l, according to short term bio-assay experiments, but the effects of ammonia in fish ponds are largely unknown. In channel catfish ponds in the USA, total ammonia concentrations often reach 0.5 mg/l as nitrogen. However, at pH 9 and 30°C, NH3 would be only 0.22 mg/l. There is evidence that tilapia can survive concentrations of un-ionized ammonia as high as 3.4 mg/l with no mortality in 48 hours, following acclimation to sublethal concentrations of 0.43 – 0.53 mg NH3/1 for 35 days. The concentration of ammonia in the pond water would depend on equilibria between rate of addition or production, and rate of removal. In a highly fertile fish pond it is unlikely that ammonia would reach high concentrations due to uptake by phytoplankton and loss to the atmosphere due to volatilization at the characteristically high pH values.
There is recent evidence that nitrite, NO2-, may be toxic to fish due to inhibition of oxygen uptake by fish blood through the conversion of haemoglobin to methaemoglobin.
The interactions of phosphorus in waste loaded ponds are less complex than those of nitrogen, but the rates of conversion of the various forms of phosphorus are still not well understood.
Most of the phosphorus in waste loaded ponds, which seldom exceeds 1 mg total P/1, is contained as organic phosphorus in seston i.e., living and dead particulate matter. Organic phosphorus in seston is degraded by bacteria to soluble organic phosphorus, and subsequently to orthophosphate, in which from it is readily available to plants. Concentrations of soluble orthophosphate are usually in the range 5–20 μg PO4-P/1, and seldom more than 100 μg PO4-P/1 in highly eutrophic waters.
When orthophosphate is added to a fish pond it rapidly disappears, 90% or more in a few days. Some is absorbed by bacteria and phytoplankton, but much is precipitated as insoluble calcium phosphate in ponds with calcareous mud or as iron or aluminium phosphates in ponds with non calcareous mud.
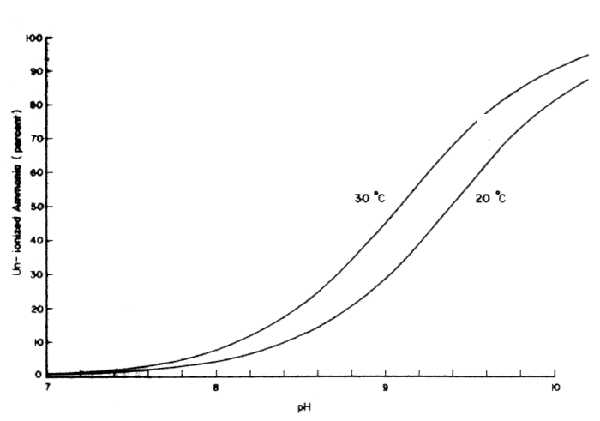
Fig. 46 Percentage of Un-ionized ammonia in aqueous solution at different pH values and temperatures
The nutrient of the waste is its most important characteristic since the nutrients contained in the waste eventually become constituents of pond food organisms. Furthermore, it is the respiratory activities of the plankton rather than the oxygen demand of the waste itself, that cause the most severe depressions in dissolved oxygen during the early morning hours (section 6).
With adequate nutrients in a waste loaded pond, the primary productivity of the phytoplankton reaches a maximum rate determined by the amount of light which can penetrate the pond water. In the subtropics and tropics, this may be about 10 g C/m2/day, equivalent to about 20 g phytoplankton dry weight/m2/ day assuming that the algae are about 50% carbon on a dry weight basis. Primary productivity in Israeli fish ponds in summer reaches 4–8 g C/m2/day, but there is considerable turbidity due to the bottom sediments continuously being resuspended in the water column by high densities of common carp, which reduces the amount of light available for photosynthesis.
The addition of nutrients to a fish pond can result in the production of dense growths of phytoplankton in the surface layers, with the result that the algae in the upper water layer shade those in the lower water layer. Without the presence of filter feeding fish in the pond to consume phytoplankton, it is possible that a higher rate of fertilization could actually lead to a lower net production of algae than a lower rate of fertilization due to shading by the algae. In a heavily fertilized fish pond with dense growths of phytoplankton, it is essential to maintain a sufficiently high density of phytoplankton feeding fish to exploit the high level of primary productivity.
A major reason for adding waste to a fish pond is to provide nutrients for bacterial and phytoplankton growth. Unfortunately, nitrogen and phosphorus added to the pond rapidly disappear from the water in a matter of a few days. Some of the nutrients are taken up by the pond biota, as intended, but there may be considerable losses of nitrogen by volatilization of ammonia and denitrification of nitrate, and losses of phosphorus by precipitation. According to work on pond fertilization in Israel, the maximum concentrations of nitrogen and phosphorus that can be attained in pond water, due to chemical considerations, are approximately 2.0 mg/l and 0.5 mg/l, respectively. The addition of nutrients to a pond in an attempt to attain water nutrient concentrations in excess of the above values would lead to the excess nutrients added being effectively lost from the system.
In a well designed integrated livestock-fish farming system, it would be necessary to know how much waste to add for growth of bacteria and phytoplankton to provide adequate nutrition for filter feeding fish, yet at the same time not produce excess growth of phytoplankton which would depress dissolved oxygen to dangerously low levels in the pre-dawn hours and stress the fish. Due to poor knowledge of rates of nutrient uptake by pond biota and losses from the pond system, rates of nutrient addition have still not been formulated. The nutrient contents of various livestock manures (Table 2) would need to be considered in determining the optimum number of livestock/ unit area for a given system.
The number of livestock/unit pond area reported in the literature vary widely: 15–300 pigs/ha; 150–13,125 ducks/ha, although summer fish kills occurred in excess of 3,000 ducks/ha; 1,000–10,000 chickens/ha. Variations are due to climate, water management, and fish species. Higher numbers of livestock/unit pond area are reported in the tropics than in temperate climates; in ponds with water exchange or movement than in static water ponds; and in ponds stocked with air breathing fish than with fish which breathe dissolved oxygen. According to Israeli work, there is a maximum amount of manure that a pond can digest/unit area/unit time. The addition of manure above the maximum would lead to the accumulation of organic matter on the pond bottom with the development of anaerobic, interstitial conditions. The maximum loading rate is estimated to be 100–200 kg manure dry weight/ha/day or 70–140 kg organic matter/ha/day. These values correspond approximately to waste from 100-200 pigs weighing 100 kg each/ha/ day, 15–30 cows weighing 500 kg each/ha/day, or 2,000–4,000 poultry each weighing 2 kg each/ha/day. The numbers of livestock/ha have been calculated from data presented in Table 2. With such highly loaded ponds, fish yields from 7,000–10,000 kg/ha/year are feasible.
If the aim is to maximize fish production in a waste fed pond, it may not be possibly to add large amounts of waste on a regular basis due to the unpredictability of phytoplankton growth. Eutrophic or fertile ponds with large phytoplankton growths are notoriously unstable; occasionally, excessive growths of algae or blooms develop, for reasons which are still poorly understood, but which can cause serious problems in fish culture. Perhaps the only feasible way to load waste into a pond is empirically or by “trial-and-error” as has been practised in China for centuries. In China, the farmer observer the amount of phytoplankton growth by visually inspecting the intensity of the colour of the water caused by algal growth; he may also assess the amount of light penetration by inserting his arm into the water and observing the depth at which his hand is visible. Surfacing behaviour of fish at dawn is also used to indicate excessive phytoplankton growth.
Turbidity caused by the suspension of silt particles in the water is normally not a problem in waste loaded fish ponds since biological activity in the water leads to a coagulation and settling of silt. However, the pond mud should not be mixed with the water to a large extent, since penetration of light would be reduced with a lowering of photosynthesis. High stocking densities of bottom feeding fish, livestock wading into shallow ponds, and the removal of large amounts of pond mud for crop fertilizer during fish growth, would all lead to a resuspension of silt in the water column and should be avoided. However, small disturbances of pond mud should be advantageous since they would speed up the rate of mineralization of sedimented organic matter and increase the nutrient content of the water.
Since the major reason for adding manure to a fish pond is to provide nutrients for bacteria and phytoplankton, it follows that the waste should be distributed as uniformly as possible throughout the pond water. To avoid large losses of nutrients and to obtain more effective nutrient uptake by bacteria and phytoplankton, it is better to add a given amount of manure frequently in small doses. Ideally, manure should be added daily if phytoplankton densities are low, or at least twice a week. Although the oxygen demand of the manure itself is not great if the manure is widely distributed over the water surface, it is best to apply manure to a pond during mid morning when oxygen levels are rising rapidly due to photosynthesis. This would minimize the oxygen demand caused by bacterial degradation of the manure itself during the critical pre-dawn hours.
In the old European method of manuring, the manure was spread in a layer over the pond bottom once a year after the pond was drained at the end of the growing season. This was unsatisfactory since it led to anaerobic conditions in the pond mud, which inhibited the development of benthic fauna and led to a loss of carbon and nitrogen as CH4, and NH3 and N2, respectively. A modification of the method in which the manure was made into 2–4 piles/ha before filling the ponds in spring, led to an improvement in bottom fauna, but anaerobic conditions around the manure piles still caused nutrient losses. A third method was developed in which the manure was added to the ponds at frequent intervals during the growing season, at definite places where the fish were fed. It was observed that the fish lost their apetite, presumably due to lack of dissolved oxygen because of anaerobic conditions at the manuring - feeding sites; anaerobic conditions would have led to nutrient losses also. The method used in the several hundred hectare fish ponds of E. Europe today involves spreading the manure more evenly over the pond surface by manuring boats. Wet manure is shovelled into a hopper through which water is pumped at high speed to distribute the manure slurry over the pond surface. Alternatively, a screen basket made of parallel iron rods 2–2.5 cm apart, suspended 10–20 cm below the water line, is attached to the side of the boat; manure is shovelled into the basket and dispersed as the boat moves and forces water into the basket. By distribution of manure daily, or at least every two days, far greater amounts of manure can be added to the pond than by the previously used methods, and the effectiveness of manuring in terms of fish production is increased.
In the smaller fish ponds of Asia it is not necessary to use manuring boats. In small ponds, less than 0.5 ha, wind and water currents would probably provide acceptable manure distribution, providing that the livestock quarters were suitably placed. However, with large numbers of animals, particularly large species such as pigs and cattle, mixing by wind alone would be inadequate. Quarters to house ducks and chickens may be constructed either over the pond surface, which would facilitate manure distribution, or on the pond dikes. Pig sties and cow sheds should be constructed on the dikes to minimize construction costs, since they need to be of more substantial construction. In the latter case, manure distribution techniques generally need to be employed to avoid the development of anaerobic conditions in the pond adjacent to the livestock quarters. Several options are available: manual distribution using buckets; the construction of channels or pipes of brick or concrete to distribute the manure to various locations in the pond by gravity or by pumping; or the use of a spray or sprinkler system to widely distribute manure over the pond surface.
Waste loaded fish ponds normally develop deposits of pond mud, which are a rich source of nutrients. Pond deposits accumulate because the rate of addition of waste to the pond is greater than the capacity of the pond to degrade the material to soluble inorganic nutrients. Since many manures and plant materials have high COD:BOD5 ratios their rate of breakdown is relatively slow, which leads to their accumulation on the pond bottom. According to experience in China, ponds with a high stocking density of grass carp, which receive large amounts of green fodder, develop the thickest pond mud deposits.
Two main methods of pond mud removal are used in China; at the end of the growing season when the pond is drained; or two to three times a year when the pond contains water and fish. The latter method provides a more frequent supply of mud to fertilize the crops and is less labour intensive since the mud, which is scooped up from the pond bottom, is transported to the pond dikes by boat. To avoid resuspending an excessive amount of mud in the water column, which would decrease light penetration and reduce photosynthesis, only one section of the pond mud is removed at any one time. Some disturbance of pond mud is probably beneficial since it leads to a remineralization of some of the fertile mud deposits as they are suspended in the aerobic water column.
Since pond mud is essential to maintain pond fertility, not all the pond mud is removed each season in China; some is left to provide a basal pond fertilizer. In fact, pond mud deposits may not be removed from newly constructed fish ponds for the first few years.
To obtain the maximum benefit from pond mud deposits not removed from the pond, the pond bottom should be allowed to remain exposed to the air for a period of at least a week, depending on the temperature. This is to allow the anaerobic mud deposits time to dry out and become oxidized. On refilling the pond, the mineralized pond deposits should have a marked fertilization effect on the water.
The term composting refers to the decomposition or stabilization of organic matter by bacteria, fungi, and other organisms. It occurs whenever animal or plant waste is added to a fish pond. There are two basic processes, aerobic and anaerobic composting or decomposition.
The decomposing organisms use oxygen in their breakdown of the organic matter. Much of the carbon serves as an energy source and is burned or respired with the production of CO2 and heat. Carbon is also a major element in cell protoplasm, about one third of the carbon being combined with nitrogen and other elements in new cellular material. Thus, about two thirds of the carbon in the waste is respired as CO2.
The speed of the process is dependant on the C:N ratio of the raw materials. If there is a high C:N ratio with a great excess of C compared to N in the waste, biological breakdown is slow and several growth cycles of micro-organisms are needed to burn up most of the carbon and to reduce the C:N ratio of the final compost. When some of the organisms die, their C and N are made available again, but mainly the C is lost while the N is conserved and incorporated into new microbial biomass. If the C:N ratio is low, with an excess of N relative to C, N may released as NH3 which will tend to be lost from the system. Some nitrogen, however, may be oxidized to nitrate. The optimal C:N ratio of waste for maximal degradation with conservation of N is about 20–35:1.
A second important factor in aerobic composting is the moisture content of the waste materials. An optimal moisture content is about 50–70%. A moisture content higher than this range would lead to anaerobic conditions, whereas a lower one would provide insufficient water for rapid microbial growth.
The technique is widely used to China, particularly in Sichuan and Hopei Provinces, for the sanitary disposal of human and livestock wastes. The rise in temperature due to microbial activity, normally up to 65–70°C, leads to marked pathogen die-off. The compost in China is used to fertilize crops rather than for fish culture.
The Chinese ground-surface continuous aerobic composting method is currently being used in Thailand to produce low cost fish pond inputs. Mixtures of nightsoil, water hyacinth, and rice straw, compost in 3–4 weeks and lead to significant yields of Tilapia nilotica. The compost is a relatively poor fertilizer, containing less than 3% nitrogen on a dry weight basis but the fish consume the compost as a feed. Presumably the low pH values in the tilapia stomach cause acid hydrolysis of the biota in the compost and the non living detrital matter so that the fish can digest these products. The C:N ratio of water hyacinth, about 25–30:1, is in the optimal range, and this aquatic macrophyte is being composted alone as fish feed, in about 4 weeks. The C:N ratio of fresh rice straw exceeds 100:1, but if it is kept at the correct moisture content, it produces a compost with a C:N ratio of less than 20 in about 3–4 months in the tropics. Experiments are currently underway to evaluate rice straw compost as feed for tilapia.
Aerobic composting techniques provide useful methods for the conversion of plant materials that normally degrade slowly into products suitable for fish pond inputs. These methods may provide low energy inputs suitable for fish farming by the rural poor in developing countries. Since China has considerable practical expertise in composting, it would be useful to study the various systems in detail and assess their relevance for fish culture.
Anaerobic composting is the decomposition of organic wastes in the absence of oxygen. Nutrients are again used to make new cell protoplasm, but under anaerobic conditions carbon not utilized in growth of micro-organisms is released as methane, CH4, and nitrogen as NH3. Disagreeable odours of H2S and reduced sulphur containing compounds are usually produced. Un-ionized H2S is extremely toxic to fish and any detectable concentration is detrimental to fish production. The process is slower than aerobic composting. There is insufficient heat generated to raise the temperature significantly so as a consequence, anaerobic composting is less suitable for the treatment of insanitary wastes than aerobic composting.
The following practical aspects of anaerobic composting need to be considered:
1. Fermentation of livestock manure
It is commonly believed in China that the storage of manure under anaerobic conditions, commonly referred to as fermentation, is beneficial for a number of reasons:
the waste is more sanitary after storage due to pathogen attenuation. This is true but the waste still may be hazardous to health (see Section 4.11).
the decomposition of the organic waste makes for easier handling and distribution since the material becomes partially liquified.
the decomposition of the waste reduces its oxygen demand and therefore there is less chance to stress the fish. This is only partially true since the oxygen demand of manure well distributed in a pond is only a minor cause of low dissolved oxygen levels during the early morning hours (see Section 4.6). Furthermore, anaerobic conditions would increase the rate of loss of C & N nutrients through the production of CH4 and NH3 which would reduce the quality of the manure.
In China, it appears that the main reason for the construction of manure fermentation tanks is to store the manure, since the water quality does not always permit the addition of manure to the pond. During the early part of the fish growing season when the need for manure is highest, there is little time for fermentation to occur. Only during the later part of the growing season, when significant phytoplankton densities in the ponds preclude regular manure inputs, does manure accumulate in the storage tanks and ferment to any appreciable extent.
2. Fermentation of plant matter
Aquatic macrophytes which are not readily consumed by herbivorous fish e.g. water hyacinth, may be piled into one corner of the pond and allowed to decompose with the release of nutrients. Rice straw is used in the same way in Guangdong Province in China when other ponds inputs are scarce.
Due to a greater nutrient loss under anaerobic conditions compared to aerobic conditions, it may be better to finely chop the plant matter and distribute it evenly over the pond surface to promote aerobic decomposition. This is done with water hyacinth in certain Communes in China.
3. Biogas
Recently in Asia there has been revived interest in the production and collection of methane, the carbonaceous gaseous product of anaerboic composition. In the past more interest centred on the biogas, but now there is emphasis on integrated biogas technology (IBT) in which both the gas and the slurry are utilized.
Most of the early work was carried out in Europe, and the first major activity in Asia was in India with the construction of small, cow dung, or gobar biogas digesters. However, China now has the world's largest biogas programme. Experimentation began in the 1950's in China, but the installation of large numbers of digesters did not start until the 1970's. Now there are over 7 million small scale digesters to provide biogas for cooking and lighting, but also large digesters to run engines for electricity generation.
Biogas is a colourless, odourless, inflammable gas consisting of about ⅔ CH4, ⅓ CO2, and with traces of H2S. The calorific value of the gas is about 4,500–6,000 calories/m3. One m3 of gas is equivalent to about 0.6 1 crude oil or 2.2 kilowatt-hours of electricity. A l m3 digester produces an average of 0.15 m3 gas/day. Since biogas consumption is estimated at 0.2–0.3 m3/capita/ day, a small sized digester of less than 10 m3 will serve a family of 5 people.
The Chinese digester and gas holder are combined into one, and are made of brick or concrete. The “three-in-one” concept is widely used i.e., a digester, latrine, and pig sty are built close together so that nightsoil, pig manure, and other household wastes can flow into the digester. However, a variety of organic matter can be used for gas generation, including crop residues, grass, and aquatic macrophytes. For optimum gas production a C:N ratio of 25–30:1 is required; thus, materials with a low C:N ratio such as manure and nightsoil need to be mixed with straw and plant matter with a high C:N ratio. The mixture in the digester should be about 90% water for optimum biogas production. The Chinese digester is a short cylinder with a domed roof for gas collection, and has inlet and outlet pipes. The contents of the digester are mixed to enhance gas production by using a bamboo pole in the inlet or by violently pouring liquid into the digester.
The effluent is collected regularly during digester operation, but twice a year the digesters are emptied of accumulated sludge, both of which are used as fertilizer. Most biogas digester effluents are used to raise land crops but in certain areas in China, particularly in Guangdong Province, they are used as fish pond inputs; in Xinfu Production Brigade, Le Liu People's Commune, 223 out of 292 households have family biogas units with an additional 12 units collectively owned. More than 80% of the biogas slurry or effluent is added to fish ponds and is considered to be an effective fertilizer.
There is the possibility that livestock manure used in fish culture may present a health problem for humans, since some diseases of animals are transmissable to human beings. However, there are relatively few data in the literature on the potential for disease transfer in waste loaded fish ponds. The fish themselves are generally healthy in well managed, manure loaded systems, with the production of high yields of fish.
Fish are not known to suffer from infections of Salmonella and other intestinal bacteria of warm blooded livestock and humans, although there is the possibility that these pathogens may be carried passively in the fish intestines and infect humans. The same applies to viruses and the nematode worm, Ascaris. However, there are helminth infections in which the fish are intermediate hosts of stages in the life cycle of the parasite, and would infect man if infected fish were consumed either raw or not well cooked e.g., Fasciola, the liver fluke.
To minimize potential risks of disease transfer, the livestock themselves should be kept in sanitary conditions, provided with adequate nutrition, and inspected by veterinarians to reduce the incidence of disease in the livestock.
The treatment of livestock waste before application to the fish pond would reduce the density of pathogenic organisms. Aerobic composting of manure slurry is an effective method of eliminating pathogens due to the development of elevated temperatures as high as 70 °C. The storage of manure in fermentation tanks is less effective, because of anaerobic conditions which do not lead to a rise in temperature. However, there is evidence that storage of slurry significantly reduces the density of Salmonella bacteria over an extended period of time. The addition of livestock manure to biogas digesters and the use of the slurry in fish ponds leads to an increase in sanitation. However, even with the use of fresh livestock manure, the high pH and high oxygen levels which develop in waste loaded fish ponds probably help to reduce the density of any potential pathogens that are added to the system. It appears that the risk of disease being transmitted to humans via fish grown in fish ponds fertilized with livestock manure is not great, but efforts should be made to reduce the risks further.
ALMAZAN, G., and C.E. BOYD. 1978. Effects of nitrogen levels on rates of oxygen consumption during decay of aquatic plants. Aquatic Botany 5: 119–126.
EDWARDS, P. 1980a. Food Potential of aquatic macrophytes. ICLARM Studies and Reviews 5, 51 pp. Manila, Philippines.
EDWARDS, P. 1980b. A review of recycling organic wastes into fish, with emphasis on the tropics. Aquaculture 21: 261–279.
GRAY, K.R., K. SHERMAN, and A.J. BIDDLESTONE. 1971. Review of composting. Part 2. The practical process. Process Biochem., October, pp. 22–28.
KUZNETSOV, Y.A. 1977. Consumption of bacteria by silver carp (Hypophthalmichthys molitrix). J. Ichthyol., 17: 398–403.
ROMAIRE, R.P., C.E. BOYD, and W.J. COLLIS. 1978. Predicting night-time dissolved oxygen decline in ponds used for Tilapia culture. Trans. Am. Fish. Soc., 107: 804–808.
SHIAN, S., M. CHANG, Y. YE, and W. CHANG. 1979. The construction of simple biogas digesters in the Province of Szechwan, China. Agricultural Wastes 1:247–285.
TAIGANIDES, E.P. 1977. Bio-engineering properties of feedlot wastes. In: E.P. Taiganides (editor), Animal Wastes, pp. 131–153, Applied Science Publishers Ltd., London.