One of the constraints for the further development and expansion of coastal fishpond culture in the region is the problem associated with acid sulfate soils. Approximately, 5 million hectares of coastal area in the South and Southeast Asia are known to be potential or actual acid sulfate soils (Poernomo and Singh, 1982).
Acid sulfate soils are those that contain a high proportion of hydrogen and aluminum ions compared to the hydroxyl ions. Soils characterized by this condition result to poor productivity when utilized as fishponds. High acidity conditions cause slow fish growth and mortalities, poor fertilizer response, low natural food production, and erosion of dike soils because of poor grass cover.
1. FORMATION OF ACID SULFATE SOIL
Figure C.1 illustrates the origin and development of acid sulfate soils. Basically, this type of soils results from the formation of pyrite. The process involves the accumulation and deposition of sedimentary parent materials of mangrove soils on coastal edges resulting in the establishment of swamp forests. During the development of swamp forests, organic and inorganic debris are trapped by the rooting activities of trees giving rise to bacterial decomposition. As a result, abundant sulfate from seawater is reduced to sulfides which undergo chemical reactions to form crystals known as mineral pyrites.
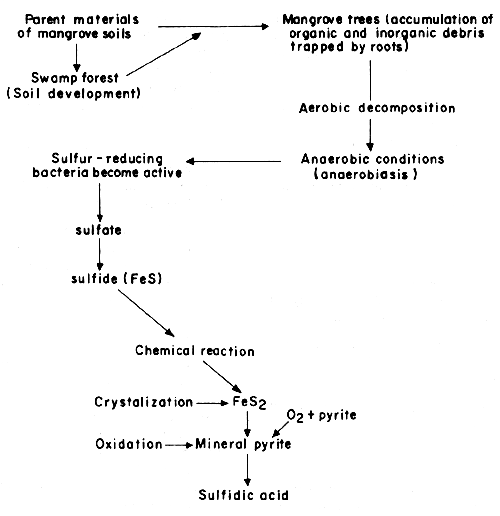
Fig. C.1 Formation of acid sulfate soils
(From Potter, 1976)
2. PHYSICAL AND CHEMICAL PROPERTIES
Poernomo and Singh (1982) cited the following physical and chemical properties of acid sulfate soils:
Bulk density, 1.0 g/cm3 to 1.4 g/cm3
Texture, generally clayey and rarely loamy or sandy
pH, 3 to 6.5
Organic carbon, 1.5% to 18%
Cation/Exchange capacity, 10 to 25 meq/100 g
Total sulfur, 0.1% to 0.75%
3. FIELD IDENTIFICATION
Below is a practical and simple guide in identifying actual and potential acid sulfate soils.
| Criteria | Characteristics/Indications |
|---|---|
| Occurrence | Tidal mangrove, back swamps of marine and estuarine areas with slight depressions; low terraces on landward side, and criss-crossed by tidal creeks. |
| Soil characteristics: | |
Colour | Pale yellow mottles overlying the subsoil. Red colouration of pond bottom when drained. |
Odour | Strong muddy odour due to ferrous and hydrogen sulfide |
| Organic matter: | |
Content | High |
| Land form | Variation in elevation Occurrence of mounds, especially of mud lobster (Thalassina anomala) |
| Vegetation | Dominance of dense rooting vegetation especially Rhizophora, Nypa and Melaleuca |
| Poor or spotly growth or absence of vegetation on fishpond dikes |
4. MANAGEMENT
The potential adverse effect of acid sulfate soils can be prevented with proper engineering and construction work and good management practices. However, most of the suggested solutions are associated with the problem of economics. The following practices have been found to be effective.
4.1 Reducing the volume of exposed dike soil in relation to pond area
A hectare of fish farm that has five compartments of 2 000 m2 each has more volume of exposed dike soil than one with two compartments of 5 000 m2 each. During heavy rains, the acid soil in the dike reacts with runoff which mixes with the pond water. Due to smaller volume of water in the 2 000 m2 ponds, the resulting concentration of acidic water will be higher than in the 5 000 m2 pond with greater volume of water and less amount of acidic runoff mixing with it due to smaller volume of dike soil.
Observations of pH in ponds at Gelang Patah, Malaysia indicate that smaller ponds are more severely affected during rainfall than larger ones. With this, it is recommended that size of ponds in acid sulfate soils should not be smaller than 0.5 ha and with water depth of 1.0 meter or more at all times (Ti, et al., 1982).
4.2 Liming
The effectiveness of lime application to control soil and water acidity has been well established. Lime improves water pH and phosphate fertilizer response. Recommendations on the rate of application range from the minimum to prohibitive amounts. To determine the lime requirement, the following procedure by Boyd (1967) and by the ASEAN National Coordinating Agency of the Philippines (1978) are as follows.
(a) Soil samples should be taken from the ponds. Twelve samples should be collected from the ponds of one hectare or less and 25 samples from ponds of 2 to 20 hectares. Samples should be taken in the pond 5 cm from the top.
(b) The composite soil sample is mixed thoroughly and spread in a thin layer to air dry. After drying, the sample is crushed gently into a powder and sieved through a screen with 0.85 mm openings. A p-nitrophenol buffer of pH 0.8 + 0.1 is prepared by diluting 20 g p-nitrophenol, 15 g boric acid, 74 g potassium chloride, and 10.5 g potassium hydroxide to one liter with distilled water.
Place 20 g of dry soil in a 100 ml beaker, add 20 ml of distilled water and stir periodically for one hour. Then measure the pH of the mud-distilled water mixture with a glass electrode pH meter. Add 20 ml of the p-nitrophenol buffer and stir periodically for 20 minutes. Set the pH meter at 8.0 by using a mixture of one part p-nitrophenol buffer and one part distilled water. Next read the pH of the sample (soil, distilled water, buffer mixture) while stirring vigorously. The pH value of the soil in water and the soil in buffer solution is used to obtain the lime requirement from the following table (Table C.1). If the pH of the soil in the buffered solution is below 7, repeat the analysis with 10 g of dry soil and double the amount of lime required given in the table (Boyd, 1976).
Table C.1
Lime requirement in kg/ha of calcium carbonate (neutralizing value of 100)
to increase total hardness and total alkalinity of pond water above 20 mg/1 (after Boyd, 1976)
| Mud in buffered solution | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mud pH in water | 7.9 | 7.8 | 7.7 | 7.6 | 7.5 | 7.4 | 7.3 | 7.2 | 7.1 | 7.0 |
| (kg/ha of calcium carbonate required) | ||||||||||
| 5.7 | 121 | 242 | 363 | 484 | 605 | 726 | 847 | 968 | 1 089 | 1 210 |
| 5.6 | 168 | 336 | 504 | 672 | 840 | 1 008 | 1 176 | 1 344 | 1 512 | 1 680 |
| 5.5 | 269 | 538 | 806 | 1 075 | 1 344 | 1 613 | 1 881 | 2 150 | 2 419 | 2 688 |
| 5.4 | 386 | 773 | 1 159 | 1 546 | 1 932 | 2 318 | 2 705 | 3 091 | 3 478 | 3 864 |
| 5.3 | 454 | 907 | 1 361 | 1 814 | 2 268 | 2 722 | 3 175 | 3 629 | 4 082 | 4 536 |
| 5.2 | 521 | 1 042 | 1 562 | 2 083 | 2 064 | 3 125 | 3 646 | 4 166 | 4 687 | 5 208 |
| 5.1 | 588 | 1 176 | 1 764 | 2 353 | 2 940 | 3 528 | 4 116 | 4 704 | 5 292 | 5 880 |
| 5.0 | 672 | 1 344 | 2 016 | 2 688 | 3 360 | 4 032 | 4 704 | 5 376 | 6 048 | 6 720 |
| 4.9 | 874 | 1 747 | 2 621 | 3 494 | 4 368 | 5 242 | 6 115 | 6 989 | 7 974 | 8 736 |
| 4.8 | 896 | 1 792 | 2 688 | 3 584 | 4 480 | 5 376 | 6 272 | 7 186 | 8 064 | 8 960 |
| 4.7 | 941 | 1 882 | 2 822 | 3 763 | 4 704 | 5 645 | 6 586 | 7 526 | 8 467 | 9 408 |
4.3 Drying and flushing acid sulfate soils with seawater
This is one of the most promising methods of improving acid sulfate soil conditions. The acid formed through pyrite oxidation during drying is removed through a process of repeated flooding and draining of the ponds.
4.4 Covering acid sulfate soil with suitable soil
This is done to provide a non-acid substrate. An evaluation of the cost involved is necessary prior to the adoption of this method.
4.5 Appropriate water management
Careful water management practices can improve acid sulfate soil conditions. Water in the ponds should be maintained at an equal level or higher than that of the adjacent canal to limit inflow through seepage.
4.6 Control of dike soil erosion
Dikes should be provided with vegetative cover such as the acid-resistant African star grass (Cynodon plectostachyus) and other Cynodon species such as the Bermuda grass.