Two TRACOR-550 gas-chromatographs have been installed, one equipped with a flamephotometric detector (FPD) and the other equipped with an electroncapture detector (ECD). The ECD had as a source of electrons the radioactive material Ni-63 (beta-emmitor) of 14.5 mCi (milliCurie).
Both gas-chromatographs were bought with two glass columns containing solid phase Chromosorb W, 80/100 mesh, loaded with the liquid phase OV-1 at 3 percent concentration. The detectors can be connected to each column separately through a 4-port valve.
The FPD equipped gas-chromatograph is specific for the analysis of organo-sulphur and organo-phosphorus compounds, where the elements sulphur and phosphorus are reduced in an oxygen deficient hydrogen flame to their elemental state, and the light emission is measured by a photometric detector. Specificity for compounds containing phosphorus and sulphur is achieved by insertion of glass-filters which pass emission wave lengths characteristic of those elements. Provision is made for the detection of the flame ionization response (FID), which also makes it possible to investigate any organic substance, in particular hydrocarbons, that can be handled by a gas-chromatograph (but, at higher concentrations than applicable for pesticide residue analysis). The sensitivity of the FPD equipment ensures the detection of organo-phosphorus pesticides from the level of about 1 nanogramme (10-9 gramme) up to about 500 nanogramme per injection, depending on what kind of compound is injected. For organosulphur compounds the response is of the same order of magnitude or slightly higher.
The equipment with ECD is particularly specific for organo-chlorine compounds at very low concentrations, although the detector also records any other compounds at higher concentrations. The range of amount per injection should lie between 10 picogramme (10-11 gramme) to about 50 ng, also depending, as for the FPD, on the kind of compound being injected.
The carrier-gases required for the gas-chromatographs are nitrogen for the FP (and FI) detector and Argon/Methane (95 percent/5 percent) for the EC detector. The EC detector will also work on nitrogen, but in order to improve its stability, the system with Argon/Methane was chosen. Therefore the electronics of the ECD equipment contained an Electron Capture Linearizer, the FPD equipment contained a simple Electrometer. The Temperature Programme Unit was inter-replaceable with an Isothermal Temperature Controller.
Each gas-chromatograph is equipped with a Westronics MT recorder of 1 mV full-scale sensitivity.
The various conditions of gas-chromatograph were:
| (a) | FPD equipment (Flame Photometric Detector): | ||
| Carrier gas: | Nitrogen 99.5–99.9 % purity, 80 ml/min | ||
| Burner gases | Hydrogen, trade quality, 75 ml/min | ||
| Oxygen, trade quality, 10 ml/min | |||
| Nitrogen, as above, 80 ml/min | |||
| Column: | Glass column, containing Chromosorb-W 80/100 mesh, + 3 % OV-1 | ||
| Temperatures: | Detector: | 175° | |
| Outlet/Inlet: | 210° | ||
| Oven: | 180° for Diazinon analysis 220° for Leptophos analysis | ||
| Recorder speed: | 1/4 inch per minute | ||
| (b) | ECD equipment | (Electron Capture Detector); Ni-63 as source (14.5 mCi) | |
| Carrier gas: | Argon/Methane (95 %/5 %) (highest purity), 80 ml/min | ||
| Column: | Glass column, containing Chromosorb-W 80/100 mesh, + 3 % OV-1 (also used 10 % DC-200 and 5 % QF-1 on Gas Chrom Q 80/100 mesh) | ||
| Temperatures: | Detector: | 280° | |
| Inlet/outlet: | 210° (220° if Leptophos was present) | ||
| Oven: | 210° (organo-chlorine compounds in general) 220° for Leptophos and breakdown products | ||
| Recorder speed: | 1/4 inch per minute. | ||
In order to optimize the installation of the pesticide laboratory the following items were installed: 6 Soxhlet extraction units with electrical heating, 2 vacuum-rotating evaporators with vacuum pump, 2 all-glass distillation units with electrical heaters, 1 freeze-drier complete with vacuum pump, centrifuge, small chromatographic columns for clean-up procedures, large driving oven up to 250° for making glassware pesticide-free, and various miscellaneous facilities such as pipettes, micropipettes, erlemeyers, sample bottles with screw caps, centrifuge tubes, and a stock of aluminium paper.
A stock of pestigrade organic solvents, mainly hexane, acetonitrile, ethyl ether, acetone, chloroform and benzene was also procured. For clean-up materials, aluminium oxide, silicagel and Florisil were bought or ordered.
The virtual lack of specific laboratory facilities locally, necessitated careful advance planning in order to procure the necessary equipment in time. Surface transportation and associated custom clearance facilities at Jakarta usually cause great delays, and considering the duration and nature of the assignment the equipment was sent by airfreight.
Another concern was that for such new equipment, such as gas-chromatographs, no help from representatives of the supply companies could be obtained since they had no experience yet with the equipment. Thus all problems of installation, whether technical or electronic had to be solved locally.
Concerning the carrier gases, only hydrogen, oxygen and nitrogen could be obtained locally but the nitrogen was found to be of insufficient purity for the ECD equipment. For this equipment, nitrogen of a purity of 99.995 percent is required, and the local nitrogen was quoted to have only a purity of 99.5 percent. Thus, either high-grade nitrogen or Argon/Methane had to be imported, and in view of the Electron-Capture Linearizer of the ECD equipment Argon/Methane was preferred. This was imported from Japan. Also of special concern were the organic solvents, since several of them are volatile (ethyl ether boils at 36°C) at elevated tropical temperatures. However those solvents which were sent by airfreight in rigid metal containers arrived without loss.
Gas-chromatographs are normally never turned off in order to ensure constant conditions for the columns and detectors. The Jepara centre at present supplies stable 220 or 110 V electricity, only during the working hours and from 18–24 hours, when the large generator was on. The installation of a second large generator should solve this problem.
Airconditioning was installed in the pesticide analytical laboratory, in particular to reduce the humidity of the air, a serious danger for highly sensitive electronic equipment.
Routine laboratory facilities like glassware, sample bottles, chemicals, etc., have all to be imported in Indonesia, and consequently are very costly at the local market. To ensure continued working of the laboratory within the counterparts operational budget, utmost economy and care were exercised in the use of equipment and chemicals.
Initial limited experience of counterpart personnel in the field of pesticide analysis necessitated simple operation and adaptation of methods consistent with precision. At various stages of the analytical procedures the number of handlings have been reduced with the advantage that also errors causing contamination are reduced, which is a serious problem for pesticide residue analysis at low concentration.
The way of taking samples in the field required adaptation to the tropical conditions where decomposition of biological material is rapid. The usual methods require the mixing of ground material with sodium sulphate or deep freeze of the samples, but both these methods meet with difficulties in the field, due to absence of necessary facilities. A simple additional method was therefore applied, where the biological samples were dissected on the spot, and small amounts (usually a few grammes) of material were placed in pesticide-clean bottles to which 5 ml of hexane was added. The bottles were closed with a screw cap, using aluminium foil as a seal.
A review of the applied methods is further presented in Table 1, concerning the analysis of pesticides in water, sediments and organisms. For most of these methods, a clean-up procedure was used as follows: 2 ml hexane extraxt is passed over 2 cm Florisil column (6 mm φ), followed by 8 ml 10 % ether/90 % hexane mixture. The complete elute plus first run-off is reduced to again 2 ml with an air current and slight heating in warm water. 10 μl is injected. Than 8 drop H2SO4 is added, shaken for 5 min, and centrifuged. Again 10 μl is injected. Dieldrin and Endrin are found to be present in the first elute, but absent in the elute treated with H2SO4. DDT, its metabolites, BHC were present in both. (Florisil is regularly preheated to 130°C.)
Additional alkaline treatment can be: 1 pellet KOH, 0.2 ml double distilled water, 1 ml absolute ethyl alcohol, and 1 ml of the “after H2SO4” extract of hexane. Shake for 30 min, and add 2 ml distilled water. Centrifuge and inject 10 μl of hexane. This treatment shifts peaks of DDT and metabolites to other metabolites, thus a distinction with PCB peaks is possible. Also 2,4 D esters are saponified.
The simplified methods for the analysis of pesticides have been tested against the more sophisticated (and more organic solvent using) traditional methods, using Soxhlet extraction as the basic extraction method.
Not all these methods produce the same results, and for a variety of material a number of these methods have been tried out (Table 2). The methods I and V have in majority been applied for the analysis of pesticides in marine organisms and sediments, respectively. Since the methods I and V are considerably more simple than the sophisticated methods using Soxhlet extractions (IV and VI), their reliability was tested against the sophisticated methods (Table 2). A mean ratio between the results of I/IV and I/VI has been determined for organisms, and of V/IV and V/VI for sediments. Only taken into consideration were the values, where for each of the methods the concentrations were above detectable level.
For the marine organisms the mean ratio was 0.88, with a standard deviation of 0.62, or 70% (27 ratios). For Mackerel, Kuro and Bandeng of pond E4, with higher levels of pesticides, the mean ratio was 0.83 with a standard deviation of 0.40 or 48% (17 ratios).
For sediments, the results were less favourable, namely a mean ratio of 0.52 with a standard deviation of 0.53 or 104%, although for the higher concentrations of BHC the results matched better between V, IV and VI.
The accuracy of the sophisticated methods themselves have not been determined during this programme, but some literature data are available on the statistics of pesticide analysis. Holden (1970) reports from an intercalibration exercise that the standard deviation of the results obtained between analysts with wildlife samples requiring maximum processing is between 30 and 60%. Supposing this range is valid for both the methods I, IV, V and VI, the ratio of one to the other should have a standard deviation between 42 and 85%, calculated according to
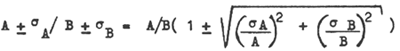
Our results for marine organisms are within this range, those for the marine sediments not. We have therefore characterized the results obtained on the sediments as semiquantitative.