by
D.J.W. Moriarty1, H.L. Cook2, Rosly bin Hassan2 and M. Thanabal2
2 Coastal Aquaculture Development Project, Gelang Patah, Johore Bahru, Malaysia
Penaeid prawns are being grown to a marketable size in ponds at the Coastal Aquaculture Development Project at Gelang Patah, Malaysia. In an attempt to lower production costs, experiments are underway to determine whether part or even all of an expensive pelleted feed can be substituted by chicken manure. Schroeder (1978) estimated that micro-organisms comprised about half of the food supply for fish in intensely-manured fish ponds. He pointed out that the manure itself was not a useful food source, but that it supplied nutrients, particularly N and P which promoted a high production of bacteria on the organic matter present (mainly straw particles). Bacteria comprise a small part of the food of penaeid prawns; meiofauna and small macrofauna are the major items of their diet in their natural habitat (Hall, 1962; Chong and Sasekuumar, 1981; Moriarty and Barclay, 1981). In manured ponds the food chain would be manure-bacteria-meiofauna-prawns and also might include protozoa.
Studies on the density and distribution of meiofauna and the effects of prawn predation are reported here. The work reported here is part of a preliminary study on the role of bacteria and meiofauna in the prawn ponds. Other work has shown that there is a high biomass and production of bacteria in these ponds, which is stimulated by adding chicken manure (Moriarty, in prep.). It was found that the bacterial productivity in ponds that were stocked with prawns was so high that most of the added feed pellets were being utilized directly by the bacteria. The work indicated that bacterial respiration would be intense, so independent studies were needed to check this. In this paper, data on respiration and primary production are presented. Nutrient concentrations were also measured.
Details of the pond layout, sizes and soil types are given by Ti and Rajamanickam (1981) and Ti (1980). The ponds were constructed a few years ago on acid-sulphate mangrove soil. Due to problems with alkalinity and pH, about half the water in each pond was exchanged daily on a tidal cycle. A number of fish and penaeid prawn species are cultured in the ponds. Salinity was between 26 × 10-3 and 28 × 10-3 and water temperature ranged from 28° to 32°C.
The ponds had a peripheral canal about 3 mm wide with a deep layer of soft sediment. The centre of the ponds was higher, with a thin (20–30 mm) layer of soft sediment over a heavy clay substrate. Water depth was about 1 m in the centre and was lowered to a depth of about 50 cm at low tide once per day, and then refilled on the next high tide from a river fringed by extensive mangrove forests.
Pond 11 (0.5 ha) was stocked with P. merguiensis and supplied with pelleted food at a rate of 10 kg d-1. Pond 29 (0.25 ha) was stocked with P. monodon and supplied with pelleted food at a rate of 16 kg d-1. Pond 23 (0.25 ha) contained a nursery pen 10 × 20 m. Only the pen was studied here. It was stocked with larval P. merguiensis and supplied with a food mash at a rate of 5 g m-2d-1 initially, and then 8 g mm-2d-1 shortly before the measurements reported here were made. Pond 22 (0.15 ha) was unstocked. Chicken manure had been added for two weeks at the rate of 0.6 g dry weight m-2d-1. The manure contained 400 mgC g-1 and 27 mg organic N g-1. Pond 21 (0.15 ha) was also unstocked. Chicken manure was supplied at the same rate as pond 22 for three weeks. Pond 25 (0.1 ha) had not been used for some months, nor had water been exchanged, as it was particularly prone to acidity problems. Pond 32 (1.0 ha) contained 3 pens, each 3 m in diameter, constructed with plastic mesh (5 mm squareholes) in the central area of the pond. Pen 1 was supplied with chicken manure at a rate of 0.6 g dry weight m-1d-1, for one week prior to sampling for bacterial biomass, organic C and N determinations and meiofauna numbers. Pen 2 was treated with chicken manure for two weeks and pen 3 was treated with manure for three weeks at the same rate as pen 1.
An integrated column of water was removed using a long plastic tube, which was pushed down to about 5 cm from the bottom, and then it was stoppered and removed. Water was brought back to the laboratory for analysis of nutrients.
Primary production and respiration were measured using the light and dark bottle technique; changes in oxygen concentration were determined using a Winkler titration procedure (Strickland and Parsons, 1968). Water was sampled at 3 depths (surface, midwater (40–60 cm) and within 20 cm of the bottom (80–90 cm)); bottles were incubated in situ at the appropriate depth. A simple water sampling method was used.
Ammonia and phosphate were measured soon after water was collected and filtered through Whatman GF/F filters, according to procedures in Strickland and Parsons (1968). Nitrate and nitrite were measured about one week later, in water that was filtered through Whatman GF/F filters, and preserved with mercuric chloride. Organic C and N were measured with a Perkin Elmer model 240 CHN analyser. Particulate matter in water samples (50 ml) was collected on Whatman GF/F filters.
For meiofauna enumeration 3 cores 20 mm diameter and 20 mm deep were taken and combined for each sample. The cores were extruded into beakers containing formalin (3% v/v) and rosebangal. Clay was washed out through a fine sieve (50 μm) and 30% colloidal silica (Ludox, Dupont) was used to separate the meiofauna from the remaining sediment and detritus-(de Jonge and Bouwman, 1977). Two or three treatments with Ludox were used. Very few, or no animals were found to be left in the sediment when it was checked after the Ludox treatment.
The average depth of the euphotic zone in the ponds was about 60 to 70 cm. No net primary production occurred below 70 cm (Figure 1a). There was considerable variation between ponds in the rate of net primary production at the surface (Figure 1a). Primary production in the ponds increased from dawn until noon and then decreased (Figure 1b).
More carbon was required to support respiration in the total water column than was generated by gross primary production (Table 1). In other words, more oxygen was consumed than was produced by photosynthesis. Net production was measured in the euphotic zone only, whereas respiration was measured over the full water column. In terms of oxygen, gross production ranged from 2.6 mg 02 1-1d-1 in pond 22 and respiration was 7.6 mg02 1-1d-1 in pond 26 and 12 mg 02 1-1d-1 in pond 22. At the surface, respiration was less than gross production and thus there was a measurable net production, but in the lower part of the water column, respiration was higher (Table 2). Oxygen concentration was quite low at the bottom of the water column in some ponds (Table 2).
Between one week and two weeks after chicken manure was added to the pens in pond 32, meiofauna numbers increased markedly from an average total of 300 to over 1 250 per 10 cm-2 (Table 3). There was no significant change in numbers after three weeks of manure treatment. When prawns were placed in the pens, the numbers of meiofauna dropped to very low values (Table 3). Some nematodes remained, but in most samples polychaetes and copepods were completely absent.
With the exception of polychaetes, the numbers of meiofauna were greater in the ponds that were receiving chicken manure. Nematodes were particularly abundant after two weeks, and harpacticoid copepods after three weeks of manure treatment (Table 4). Very large numbers of nematodes were found in the sediment of the nursery area in pond 32, although they were patchily distributed. A comparison of meiofauna numbers in various ponds with the meiofauna in a natural mangrove habitat is given in Table 4. Polychaetes, and to a lesser extent harpacticoid copepods, were more common in the mangrove sediment on a creek bank, than in the ponds.
Bacteria were insignificant in the food of the prawns in the pens (Table 5). The C:N rations of the gut contents were low (5.4–6.8), an indication that protein was a large part of their food. This suggests that animals (meiofauna) were being eaten. These prawns were not fed pellets.
Levels of inorganic nitrogen were low in the water column, even in ponds that were being fertilized with chicken manure (Table 6). Phosphate concentration was low in the water column. Organic N concentration was higher, particularly in ponds 11, 29, 23 (receiving pelleted food) and ponds 21 and 22 (chicken manure for two or three weeks). Pond 26, the control pond, and pond 20, which had been treated with chicken manure for only one week, had low concentrations of organic N compared to other ponds or river water. Pond 5, which was not treated in any way, had a high concentration of N, but the water level was low and thus exchange with the sediment would be facilitated.
The work reported in this paper was carried out as part of a preliminary study in the role of microbial food chains in aquaculture ponds. It is aimed at showing the direction more detailed studies should take. Bacterial productivity is high in the water column and sediments of these ponds, and it seemed that much of this productivity resulted from the food pellets thrown into the ponds (Moriarty, in prep.). One question which arose was how much algal production contributed to the pond carbon budget. It was possible to carry out only two measurements of primary production at the same time as bacterial production was measured. From the measurements made, it seems that primary production is high (Table 1). As half of the water was exchanged each day, only half the production would contribute to pond production over a longer term. Primary production in the river water was not measured. For future work, chlorophyll a concentration should be measured or algal cells counted, so that comparisons of primary production can be made on a biomass rather than volumetric basis.
There are problems in determining what proportion of primary production is available for eventual use by bacteria or other organisms. True values for net production are difficult to measure, especially with the oxygen technique. Algal respiration depends on previous light history; also, photorespiration may occur. These points have been discussed by Ganf (1974), who reported that there were quite large diurnal fluctuations in respiration rates in a shallow tropical lake. The dark bottle measures total community respiration, which is primarily due to algae and bacteria; respiration of zooplankton and meiofauna is likely to be very small. An independent estimate of bacterial respiration is obtained from the thymidine technique for measuring bacterial growth rates. Assuming a growth efficiency of 50%, respiration is equal to production. (Although the estimate of 50% can be used for approximate calculations, it is possible that 30% may be more usual for planktonic bacteria if the organic matter is not readily degradable (Koop et al., 1982).
From the data for the whole water column (Table 1), it is obvious that respiration considerably exceeds photosynthesis and thus there can be no net production in the pond as a whole. For pond 29 on 16 February, bacterial production was 1.3 gCm-2d-1 (Moriarty, in prep). Thus bacterial respiration would be the same, assuming 50% efficiency. Subtracting this from respiration, the remaining community respiration is 1.2 gCm-2d-1, which is the same as the gross primary production. Thus after removing the bacterial component, algal respiration in the whole water column throughout 24 hours will use up all the net primary production. The euphotic zone is limited to the upper part of the water column by turbidity in the water, and brown colour probably due to humic compounds leached from the mangrove forest.
In pond 21 on 26 February, bacterial respiration was 0.76 mgCm-2d-1. Subtracting this from total repiration, there is a deficit of about 0.7 gCm-2d-1 in respiration which is not made up by gross primary production. As pointed out above, bacterial growth efficiencies may be closer to 30% than 50%, in which case bacterial respiration would be 1.6 gCm-2d-1 and net production for the whole water column would be 0.17 gCm-2d-1.
The conclusion from this discussion is that in general, there is no overall net production in these ponds by the algal community itself. In other words, primary production does not contribute significantly to other levels of production in ponds. Bacterial production must be dependent on other sources of organic matter. These sources are the pelleted food supplied to prawns in ponds 11, 29 and 23; chicken manure in ponds 20, 21 and 22; and river water in all ponds. A comparison of inputs of organic matter and bacterial requirements for the water column shows a reasonably close correspondence (Table 7). In ponds 29 and 23, receiving large amounts of pellets of mash, the bacteria in the water column did not use all carbon supplied, but the bacterial production in the sediments in those two ponds was high. Within the limits of the methods used, and uncertain conversion efficiencies, there is a very good agreement between values for bacterial requirements for carbon, and carbon supplied. It is clear, therefore, that most of the organic matter being supplied to these ponds was being utilized directly by bacteria. The binder used in the pellets at that time was poor, allowing pellets to break up rapidly. A better quality binder is now used, and pellets are stable in the water for at least 12 hours. Particles of food are likely to be lost from the pellets while the prawns were feeding, so it is not possible to prevent wastage entirely.
Some bacterial growth in the sediments may be at the expense of organic matter in the sediment. In ponds being fertilized with manure this is most likely. The manure contained sawdust, which would not be utilized by bacteria. As it seems likely that N (and perhaps P) are limited in the ponds (see below), the organic N and P in the manure would stimulate bacterial decomposition of organic matter in the sediment. Some evidence for this is seen in the experiment with pens in pond 32, in which the C:N increased due to a decrease in organic N content, even though manure was added (Table 8). An explanation for this is that bacteria were decomposing a part of the organic matter with a consequent loss of N, probably due to denitrification. The effect on organic C is not obvious, because the accumulating sawdust would obscure it. In ponds 20, 21 and 22, however, there was a noticeable loss of C as well as N. As only two samples were taken, statistically valid conclusions cannot be drawn. The results do suggest, however, that the high levels of organic matter in the pond sediments are due in part to a low rate of bacterial decomposition controlled by low availability of N. Further evidence for a limiting role for N and P is seen in the low concentrations of inorganic N and P in the water column. The high rates of bacterial production and primary production indicate that the nutrients must be rapidly recycled and thus may control the rates of production. Most nitrogen in the water column was present as organic N. Simpson et al. (1983) also found that phosphate concentration was very low; their values for NH4 + were higher (10–50 μM) in these ponds.
A build-up of organic matter in the pond sediments is generally regarded as undesirable, as it promotes anaerobic processes particularly sulphate reduction. Sulphate reduction is the chief terminal process of carbon mineralization in marine systems, including brackish water ponds, because sulphate is present at high concentrations. Its end product, H2S, is toxic. In these ponds, iron pyrite also forms, and there are considerable problems with water quality due to acid production during oxidation of iron pyrite (Ti, 1980; Simpson et al., 1983). Anaerobic conditions keep iron pyrite in the reduced state and thus lessen problems due to low pH and alkalinity. From this point of view, maintenance of anaerobic conditions in the pond sediment may be beneficial where acid-sulphate soils are a problem.
The feed pellets were primarily the base of a microbial food chain, rather than being used as food for the prawns. Bacteria comprised a minor part of the prawns' diet (Table 5). Under the microscope fragments of animals, benthic microalgae and unrecognizable material were seen in the foreguts. The productivity studies were not refined enough to show whether a small proportion of the feed pellets was being used directly. Studies with labelled food (e.g., by using a dye or polystyrene beads) would be needed to show this.
Meiofauna as well as small macrofauna are eaten by penaeid prawns (Hall, 1962; Chong and Sasekumar, 1981; Moriarty and Barclay, 1981). The work reported here demonstrates that the meiofauna is a link between bacteria and the prawns in these ponds. In the pen experiments, the number of meiofaunal animals decreased after the prawns were added. Polychaetes and copepods were particularly preferred, as almost all of them were eaten. A few nematodes remained (Table 3). There were marked differences in the meiofauna community structures in the various ponds. These differences were probably due mainly to different levels of predation. Bell and Coull (1978) showed that Palaemonetes pugio fed on meiofauna, causing a marked reduction in numbers in a salt marsh habitat. In the ponds, polychaetes were almost entirely absent. Although only ponds 11 and 29 were stocked, a small population of wild prawns was present in other ponds and presumably were preying on polychaetes. As polychaetes are larger than nematodes and copepods, the prawns would gain more energy per unit effort expended by feeding on polychaetes. When small areas of pond 32 were enclosed in pens, and prawns excluded, the number of polychaetes quickly increased. Harpacticoid copepods and nematodes were much less numerous in ponds 11 and 29 than in pond 23 or the ponds receiving manure. Bacterial productivity was high in ponds 11 and 29, and thus food was not scarce for the meiofauna. We conclude therefore that the prawns in the ponds were feeding on the meiofauna. It is probable that only a small proportion of their food consisted of meiofauna. The biomass and production of meiofauna in ponds containing prawns was low (Table 9), and was not sufficient to support prawn production. Obviously, the prawns must have been eating some of the pelleted food.
An estimate of the food requirements of the prawns may be obtained from the yield at harvest (Table 8). If we assume that the prawns utilize the food eaten with a 10% efficiency, which is within the range measured for P. merguiensis in culture (Sedgwick, 1979), the estimated feeding rate is 10 times the growth rate. Thus in pond 11, the prawns ate about 150 mgCm-2d-1, which is 15% of the pelleted food, but more than the meiofaunal production and less than the bacterial production. In pond 29, the prawns required an amount of carbon equivalent to about 7% of the pelleted food. It is not surprising, therefore, that bacterial production did account for most of the added food. Severe mortality of prawns sometimes occurred, due to acidity from the acid-sulphate soils on which these ponds are constructed. It was difficult, therefore, to balance feeding levels with stocking rates, as mortality was difficult to measure before harvest time.
Interactions between manure additions, bacteria and meiofauna are best seen in the pen experiments, where prawns were excluded. One week after adding manure, bacterial biomass had increased (Table 9). Bacterial biomass decreased twofold after two weeks of manure treatment and was five times lower after three weeks. The marked decline in bacterial biomass occurred at the same time as a marked increase in meiofauna (Table 3). Bacterial growth rates were stimulated, presumably in response to grazing by the larger number of meiofauna. Protozoa were not examined in this study, but their role should be looked at in future work, as they also control bacterial populations. The greatest density of nematodes was found in the nursery area of pond 23, which also had the highest bacterial biomass. There seems to be no doubt that the meiofauna density is directly correlated to bacterial biomass.
From the changes in number of meiofauna in the pens and ponds 20, 21 and 22 with time after manure addition, it is possible to estimate growth rates of meiofauna.
The relative or specific growth rate (r) was calculated using the following equation:
r = l/t ln (Nt/No)
where t is time (days); Nt is the final number and N the original number of animals. Growth rates were found to range from 0.06 to 0.19 do1 for nematodes; from 0.10 to 0.17 d-1 for harpacticoid copepods; and from 0.07 to 0.20 d-1 for polychaetes. These values are only approximate. The number of samples taken was small, and patchiness in distribution was pronounced. There is no doubt, particularly in the pen experiments, that rapid growth of the meiofauna really occurred. True generation times may be slower than indicated by these values, because large numbers of eggs or larvae may already have been present. Thus these growth rates may reflect only the growth to adult stages, and not include full life cycles. These growth rates are consistent with rates reported by others. Growth rates of 0.06 to 0.14 d-1 have been measured for nematodes in culture (Alongi and Tietjeu, 1980). Values ranging from 0.005 to 0.24 have been reported for harpacticoid copepods (Hicks and Coull, 1983).
Both the biomass and production of meiofauna were low compared to bacteria in the pond sediments (Table 10). In the ponds stocked with prawns, meiofaunal biomass was 0.4 to 2% of bacterial biomass; in other ponds it was 2 to 4% of bacterial biomass. Where the meiofaunal density was not limited by predation, i.e., in the pens (pond 32), the estimated requirement of carbon for production, respiration and ingestion is similar to the measured value for bacterial production. An example is given for pen 2 (Table 11) where the total daily carbon requirement was 177 mgCm-2, the bacterial production was 182 mgCm-2d-1 (Table 9). The effect of protozoans on the bacterial has not been measured but must also be considerable. Thus bacterial population density would be expected to decline under such grazing pressure, and this did happen (Table 9). In the stocked ponds, where meiofauna density was limited by grazing, bacterial density and production was higher than in the pens.
The work discussed here shows how the ways in which interactions between food supply to ponds, and bacteria, meiofauna and prawns can be analysed. It is clear that modern methods in microbial ecology have an important role to play in aquaculture research.
REFERENCES
Alongi, D.M., and Tietjen, J.H., 1980. Population growth and trophic interactions among free-living marine nematodes.
Bell, 'S.S., and Coull, B.C., 1978. Field evidence that shrimp predation regulates meiofauna. Oecologia (Berl.), 35: 141–148.
Chong, V.C., and Sasekumar, A., 1981. Food and feeding habits of the white prawn P. merguiensis. Mar. Ecol. Prog. Ser., 5: 185–191.
Coull, B.C., and Vernberg, W.B., 1970. Harpacticoid copepod respiration: Enhydrosoma propinquum and Longipedia helgolandica. Mar. Biol., 5: 341–344.
Dall, W., and Moriarty, D.J.W., 1983. Functional aspects of nutrition and digestion. pp. 215–261. In: The Biology of Crustacea. Vol. V. Internal Anatomy and Physiological Regulation. Ed. L.H. Mantel. Academic Press, New York.
de Jonge, V.N., and Bouwman, L.A., 1977. A simple density separation technique for quantitative isolation of meiobenthos using the colloidal silica Ludox-TM. mar. Biol., 42: 143–148.
Duncan, A., Schiemer, F., and Klekowski, R.Z., 1974. A preliminary study of feeding rates on bacterial food by adult females of a benthic nematode, Plectus palustris de Man 1880. Polski Archiwum Hydrobiologii, 21(2): 249–258.
Ganf, G.G., 1974. Rates of oxygen uptake by the planktonic community of a shallow equatorial lake (Lake George, Uganda). Oecologia (Berl.), 15: 17–32.
Hall, D.N.F., 1962. Observations on the taxonomy and biology of some Indo-West Pacific Penaeidae (Crustacea, Decapoda). Fish. Publ. Colonial Off. (London), No. 17. 229 pp.
Hicks, G.R.F., and Coull, B.C., 1983. The ecology of marine meiobenthic harpacticoid copepods. Oceanogr. Mar. Biol. Ann. Rev., 21: 67–175.
Koop, K., Newell, R.C., and Lucas, M.I., 1982. Biodegradation and carbon flow based on kelp (Ecklonia maxima) debris in a sandy beach microcosm. Mar. Ecol. Prog. Ser., 7: 315–326.
Moriarty, D.J.W., in press. The productivity of bacteria in aquaculture ponds. Aquaculture.
Moriarty, D.J.W. and Barclay, M.C., 1981. Carbon and nitrogen content of food and the assimilation efficiencies of penaeid prawns in the Gulf of Carpentaria. Aust. J. Mar. Freshwater Res., 32: 245–251.
Sedgwick, R.W., 1979. Effect of ration size and feeding frequenty on the growth and food conversion of juvenile Penaeus merguiensis de Man. Aquaculture, 16: 279–298.
Simpson, H.J., Ducklow, H., Deck, B., and Cook, H.L., 1983. Brackish-water aquaculture in pyrite-bearing tropical soils. Aquaculture, 34: 333–350.
Ti, T.L., 1980. Problems associated with the use of mangrove swamps for aquaculture: a first observation recorded at Gelang Patah. Malaysian Agricultural Journal, 52(3): 298–307.
Ti, T.L., and Rajamanickam, L.D., 1981. Observations on the effects of rainfall on the pH of pond water in Gelang Patah. Fisheries Bulletin No. 25, Ministry of Agriculture, Malaysia. 19 pp.
Table 1. Primary production and respiration in pond water.
All values in gC m-2d-1.
| Pond | Date | Gross Primary Production | Total Respiration | Bacterial Respirationa |
| 29 | 16 February | 1.20 | 2.5 | 1.3 |
| 29 | 24 April | 0.81 | 2.4 | |
| 20 | 24 February | 1.40 | 2.30 | |
| 21 | 26 February | 1.53 | 2.9 | 0.7 |
| 21 | 24 April | 1.28 | 3.6 | |
| 22 | 23 April | 1.56 | 3.47 | |
| 26 | 26 April | 0.77 | 2.27 |
Table 2. Oxygen production and consumption in pond water.
| Pond | Depth | Gross Primary Production | Respiration | Oxygen Concentration |
| cm | mgO2 1-1h-1 | mgO2 1-1h-1 | mgO2 1-1 | |
| 29 | 10 | 0.49 | 0.34 | 4.61 |
| 60 | 0.43 | 0.39 | 3.38 | |
| 80 | 0.08 | 0.20 | 0.92 | |
| 22 | 10 | 0.95 | 0.36 | 7.63 |
| 44 | 1.1 | 0.49 | 6.50 | |
| 75 | 0.28 | 0.54 | 1.84 | |
| 21 | 10 | 0.69 | 0.21 | 7.17 |
| 65 | 0.69 | 0.49 | 7.63 | |
| 120 | 0.23 | 0.36 | 3.17 |
Table 3. Effect of manure treatment and prawn predation on meiofauna in Pond 32a.
| Pen | Date | Sample | Nematodes | Harpacticoid Copepods | Polychaetes | Others | Total |
| 1 | 6 Feba | 1 | 104 | 40 | 112 | 136 | 392 |
| 2 | 96 | 24 | 16 | 64 | 200 | ||
| 1 | 19 Feb | 1 | 32 | 0 | 0 | 8 | 40 |
| 2 | 40 | 0 | 0 | 0 | 40 | ||
| 2 | 6 Feb | 1 | 200 | 480 | 256 | 320 | 1256 |
| 2 | 328 | 560 | 520 | 464 | 1872 | ||
| 2 | 19 Feb | 1 | 48 | 8 | 0 | 8 | 64 |
| 2 | 48 | 8 | 0 | 8 | 64 | ||
| 3 | 6 Feb | 1 | 160 | 640 | 440 | 208 | 1448 |
| 2 | 368 | 652 | 304 | 200 | 1524 | ||
| 3 | 19 Feb | 1 | 10 | 0 | 0 | 0 | 16 |
| 2 | 0 | 0 | 0 | 16 | 16 |
Table 4. Meiofauna in pond sediments. All values are number/10cm-2a.
| Pond | Treatment | Nematodes | Harpacticoid Copepods | Polychaetes | Others | Total |
| 11 | pellets, adult prawns | 622 (185) | 134 (52) | 4 (2) | 52 (26) | 812 |
| 29 | pellets, adult prawns | 132 (45) | 8 (8) | 0 | 4 (4) | 144 |
| 23 | nurseryb, mash larvae | 1388 (564) | 624 (48) | 0 | 184 (96) | 2196 |
| 20 | manure, 1 week | 91 (33) | 198 (132) | 6 (6) | 42 (16) | 337 |
| 22 | manure, 2 weeks | 892 (251) | 624 (157) | 0 | 186 (64) | 1702 |
| 21 | manure, 3 weeks | 338 (122) | 978 (179) | 4 (4) | 142 (64) | 1462 |
| 26 | none | 28 (7) | 412 (158) | 0 | 4 (4) | 444 |
| Mangrove, intertidal creek bank | 252 | 516 | 96 | 0 | 864 | |
a Means and SE for 4 samples are shown.
b Mean and range for duplicates only.
Table 5. Analysis of foregut contents of prawns in pens.
Values are in terms of dry weight.
| Pen | Species | Organic C | Organic N | C:N | Bacteria |
| mg g-1 | mg g-1 | ||||
| A | Penaeus monodon | 177 | 29 | 6.1 | 1.3 |
| P. merguiensis | 185 | 34 | 5.4 | 0.7 | |
| B | P. monodon | 198 | 35 | 5.7 | 1.2 |
| P. merguiensis | 181 | 28 | 6.6 | 0 | |
| C | P. monodon | 223 | 33 | 6.8 | 0.7 |
| P. merguiensis | 143 | 25 | 5.6 | 0 |
Table 6. Particulate organic C and N in water column retained on Whatman GF/F filters and total inorganic Na.
| Pond | NO3- | NH4+ | Organic C | Organic N | C:N |
| μM | μM | mg 1-1 | or g m-2 | ||
| 11 | 10 | <0.2 | 4.0 | 0.58 | 6.9 |
| 29 | 3 | <0.2 | 5.5 | 0.72 | 7.6 |
| 20 | 3 | <0.2 | 3.9 | 0.25 | 15.6 |
| 22 | 6 | <0.2 | 4.3 | 0.64 | 6.7 |
| 21 | ndb | 0.5 | 3.7 | 0.44 | 8.4 |
| 26 | nd | <0.2 | 3.4 | 0.19 | 17.9 |
| 5 | 3 | 0 | 3.3 | 0.50 | 6.6 |
| 23 nursery | 3 | 1 | 5.4 | 0.80 | 6.7 |
| 23 open water | nd | <0.2 | 5.4 | 0.46 | 11.7 |
| River | 3 | <0.2 | 4.6 | 0.58 | 7.9 |
a NO2- was below the level of detection (<2μM).
b nd = not detectable (<2μM).
Table 7. Comparison of organic C input to ponds with organic C required to support bacterial production and respiration.
All values are gC m-2d-1.
| Pond | Input | Bacterial requirementa | |||
| Feed or Manure | River waterb | Total | Water | Sediment | |
| 11 | 1.0 | 0.76 | 1.76 | 1.84 | 0.8 |
| 29 | 3.2 | 0.76 | 3.96 | 2.64 | 1.36 |
| 23 (nursery) | 4.0 | 0.76 | 4.76 | 4.2 | 1.67 |
| 21 | 0.24 | 0.76 | 1.0 | 1.4 | 1.07 |
| 22 | 0.24 | 0.76 | 1.0 | 1.78 | 1.2 |
| 26 | 0 | 0.76 | 0.76 | 0.86 | 0.93 |
a Calculated from production values Moriarty, in preparation), using a growth efficiency of 50% for water column and 30% for sediments.
b From Moriarty (in preparation).
Table 8. Prawn production.
| Pond | Area | Number | Stocking size Total Length | Harvest size Total Length | Growth Rate | Feeding Rate |
| ha. | Harvested | mm | mm | mgC m-2d-1 | mgC m-2d-1 | |
| Penaeus merguiensis | ||||||
| 11 | 0.5 | 6120 | 20–25 | 120 | 15 | 150 |
| Penaeus monodon | ||||||
| 29 | 0.25 | 6000 | 70 | 136 | 23 | 230 |
Table 9. Effect of chicken manure on organic C and N content and bacterial production in sediment.
| Pond Treatment | Organic C | Organic N | C:N | Bacteria | |||
| Biomass | Productionb | Doubling | |||||
| g m-2 | g m-2 | gC m-2 | mgC m-2 d-1 | time (d) | |||
| Outside Pensc | 101 ± 14 | 7.8 ± 0.2 | 13.1 ± 1.5 | 3.4 ± 0.0 | 444 ± 54 | 6 | |
| 1 | Manure, 1 week | 140 ± 12 | 10.1 ± 1.2 | 14.0 ± 0.8 | 4.3 ± 0.20 | 346 ± 69 | 8 |
| 2 | Manure, 2 weeks | 184 ± 8 | 10.2 ± 1.4 | 18.6 ± 1.6 | 1.9 ± 0.20 | 182 ± 39 | 6 |
| 3 | Manure, 3 weeks | 160 ± 25 | 7.6 ± 1.0 | 20.7 ± 0.8 | 0.8 ± 0.07 | 230 ± 57 | 2 |
| 20 | Manure, 1 week | 243 ± 25 | 19.5 ± 3.9c | 12.7 ± 1.3c | 2.4 ± 0.1c | 300 ± 20 | 5.5 |
| 22 | Manure, 2 weeks | 136 ± 11c | 12.4 ± 0.2c | 11.0 ± 0.6c | 2.5 ± 0c | 370 ± 30 | 3.5 |
| 21 | Manure, 3 weeks | 110 ± 18c | 10.0 ± 1.6c | 11.0 ± 0c | 1.8 ± 0.1 c | 360 ± 50 | 4 |
a The experiment was carried out in 3 pens in Pond 32. All values are mean standard error, n=4, except as noted (from Moriarty, in preparation).
b Values are mean S.E., n=3.
c Values are mean range of duplicate determinations.
Table 10. Comparison of biomass and production of bacteria and meiofauna in some ponds.
| Pond | |||||
| 11 | 29 | 23 (nursery) | 21 | 22 | |
| Biomass (mgC m-2) | |||||
| Bacteriaa | 2700 | 2600 | 6400 | 1800 | 2500 |
| Nematodesb | 46 | 10 | 104 | 25 | 67 |
| Copepodsb | 6 | 0.4 | 28 | 44 | 28 |
| Production (mgC m-2d-1) | |||||
| Bacteriaa | 240 | 410 | 500 | 320 | 360 |
| Nematodes | 7 | 1 | 16 | 4 | 10 |
| Copepods | 1 | 0 | 4 | 6 | 4 |
a From Moriarty (in preparation).
b Based on carbon content of 0.75 μgC nematode-1 and 0.45 μgC copepod-1 (unpublished work).
Table 11. Approximate carbon budget for meiofauna production in some ponds.
| Nematodes | Copepods | Polychaetes | Others | |
| Specific growth rate (d-1) | 0.14 | 0.13 | 0.14 | 0.17 |
| Biomassa (μgC animal-1) | 0.75 | 0.45 | 1.83 | 2.3 |
| Respiration (30M C) (μgC animal-1d-1) | 0.04b | 0.03c | ||
| Pen 2 | ||||
| Production (mgC m-2d-1) | 3.0 | 3.2 | 10.8 | 16.5 |
| Respiration (mgC m-2d-1) | 1.1 | 3 | ||
| Assimilation efficiency | 15%b | 50%d | ||
| Growth efficiencye | 20% | 20% | ||
| Consumption (mgCm-2d-1) | 28 | 13 | 54 | 82 |
| Pond 11 Consumption (mgC m-2d-1) | 64 | 3 | 0 | 9 |
| Pond 29 Consumption (mgC m-2d-1) | 14 | 0.2 | 0 | 0 |
a Biomass: average values from unpublished work.
b From Duncan et al (1974), assuming Q10=2.
c From Coull & Vernberg (1970), value calculated from data for small
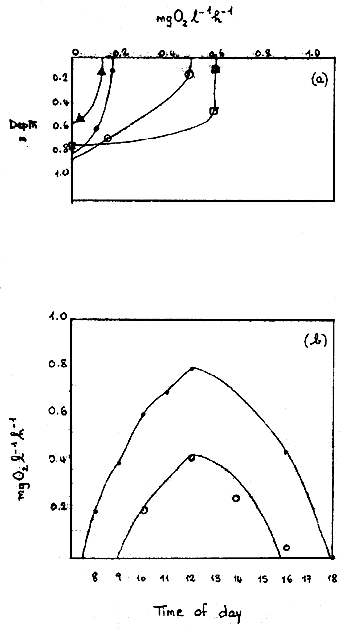
Fig. 1 Variations with depth and time of day in net primary production. (a) Effect of depth on net production in 4 ponds; (b) diurnal variation in net production in pond 11. • = production near surface (10 cm); o = production at 60 cm