APPENDIX I: List of Participants - Liste des Participants - Lista de Participantes
APPENDIX II: Draft General Standard for the Use of Dairy Terms (Advanced to Step 8 of the Codex Procedure)
APPENDIX III: Common Labelling Provisions in Milk Product Standards
APPENDIX IV: Draft Revised Standard for Butter (A-1) (Advanced to Step 8 of the Codex Procedure)
APPENDIX V: Draft Revised Standard for Milkfat Products (A-2) (Advanced to Step 8 of the Codex Procedure)
APPENDIX VI: Draft Revised Standard for Evaporated Milks (A-3) (Advanced to Step 8 of the Codex Procedure)
APPENDIX VII: Draft Revised Standard for Sweetened Condensed Milks (A-4) (Advanced to Step 8 of the Codex Procedure)
APPENDIX VIII: Draft Standard for Milk Powders and Cream Powders (A-5/A-10) (Advanced to Step 8 of the Codex Procedure)
APPENDIX IX: Draft Revised Standard for Cheese (A-6) (Advanced to Step 8 of the Codex Procedure)
APPENDIX X: Draft Revised Standard for Whey Cheese (A-7) (Advanced to Step 8 of the Codex Procedure)
APPENDIX XI: Draft Group Standard for Cheeses In Brine (A17) (Advanced to Step 8 of the Codex Procedure)
APPENDIX XII: Methods of Analysis and Sampling for Milk Products
|
CHAIRPERSON: |
Dr. Peter O´Hara |
|
PRÉSIDENT: |
Deputy Director General |
|
PRESIDENTE: |
Ministry of Agriculture and Forestry |
|
|
PO Box 2526 Wellington |
|
|
New Zealand |
|
Phone: |
64.4.4744211 |
|
Fax: |
64.4.4744287 |
|
Email: |
Ing. Agr. Adán Traverso
Dirección Promoción de la Calidad
Alimentaria
de la Sagpya, área Codex Alimentarius
Phone: 541.349.2044
Fax: 541.349.2041
E-mail: [email protected]
Ing. Gabriel Esteban Pons
Coordinación de Establecimientos
Lácteos
SENASA Av. Paseo Colón 367 (1063)
Buenos Aires
Phone: 541 342-2781
Fax: 541.3422781
Lic. Qím. Susana Beatriz Fattori
Ministerio de Salud y Acción Social ANMAT –
INAL
Estados Unidos 25 (1101) Capital Federal
Phone: 54-1-340-0800
Fax: 54-1-331-6418
E-mail: [email protected]
Mr. Federico Silva Garretón
Consejero Económico y Comercial
Adjunto
Embajada de Argentina en Uruguay
Andés 1365 Piso 10 Uruguay
Phone: 600.3031
Fax: 908.7154
E-mail: fsg1957.yahoo.com
Lic. Juan Carlos Pagano
Asesor Bioquímico del Centro de la Industria
Lechera
Carlos Pelegrini 704
Quilmes
Phone: 541.2572565
Fax: 541.9830587
E-mail: [email protected]
Ing. Quím. María Rosa Rabanal
Consejo de Administración SENASA
La Pampa 2768 1 A
Buenos Aires
Phone: 541.3298100
Fax: 541.3298200
Ing. Ricardo Julián Weill
Director Investigación y Desarrollo de
DANONE ARGENTINA S.A.
Moreno 877 (1091) Buenos Aires
Phone: 541.3414243
Fax: 541.3414244
E-mail: tweill@danon
Ing. Agr. Analía Castellani
Asesora Centro de la Industria Lechera
Medrano 281 (1178) Buenos Aires
Phone: 541-983-1865
Fax: 541-958-4056
E-mail: [email protected]
Lic. Roberto Urrere
Coordinador de Calidad
CEC – SANCOR Cooperativas U. Ltda.
Quintana 2962 – Gral. Pacheco 1617
Buenos Aires
Phone: 541.6654081
E-mail: [email protected]
Dr. Hugo Guillermo Pérez
Director Técnico
Mastellone Hnos. S.A.
Acem 720 (1001)
Buenos Aires
Phone: 541.3185000
Fax: 541.3136822
AUSTRALIA
AUSTRALIE
Mr. Peter Miller
Counsellor (Veterinary) AQIS
Embassy of Australia
Washington, D.C. 2 00 36
USA
Phone: 1.202.797 3319
Fax: 1.202.797 3037
E-mail: [email protected]
Mr. James Gruber
Principal Food Technologist
Australia New Zealand Food Authority
55 Blackall St. Barton
Phone: 026.271.2226
Fax: 026.271.2278
E-mail: [email protected]
Mr. Christopher Chan
Senior Policy & Technical Advirer NSW Dairy
Corporation 140 Myrtle Street – Chippendale NSW
Phone: 61.2.9699 0573
Fax: 61.2.9699 0586
E-mail: [email protected]
Mr. Peter Mitchell
Manager, Regulatory Affairs
KRAFT
162 Salmón St. Port
Melbourne, Victoria, 3150
Phone: 61.03.9676 5814
Fax: 61.03.9676 5881
AUSTRIA
AUTRICHE
Dipl. Ing. Karl Schober
Division VI A4 (Organization of Markets, Milk
and Milk products) in the Federal Ministry of
Agriculture and Forestry
Stubenring 1. A 1012 Vienna
Phone: 43.1.71100.2844
Fax: 43.1.71100.2901
BELGIUM
BELGIQUE
BELGICA
ir. Josiane Houins-Roulet
Inspecteur principal - chef de service à l’
inspection
générale des denrées
alimentaires
Ministère de la Santé publique
C.A.E. Quartier Esplanade. 11º - B. 11.18
Bd. Pacheco, 19 Bta. 5
1010 Bruxelles
Phone: 322 210 4865
Fax: 322 210 4816
E-mail: [email protected]
Mr. Herman Hooyberghs
Director Ministry of Agriculture
WTC 3 – 22 nd Floor, Simon Bolivarlaan 30 1000
Brussels
Phone: 322 208 4920
Fax: 322 208 4925
E-mail: [email protected]
Dr. Guido Kayaert
Nestle co-ordination Center
European Regulatory and Environmental Affairs
Birmingham Street 221
B 1070 Brussel
Phone: 322 529 5330
Fax: 322 529 5620
E-mail: [email protected]
BRAZIL
BRESIL
BRASIL
Mr. Sergio Taam
Consejero, Diplomático
Ministerio de Relaciones Exteriores
Embajada de Brasil Uruguay
Phone: 709.6821/23
E-mail: [email protected]
Dr. Rose Mary Rodrígues
Asesor Técnico
Ministerio de Agricultura y Abastecimiento de Brasil
Shis QI 27 Conj.6 Casa No.1 – Lago Sul –
Brasilia
Phone: 367.2023/2182680
E-mail: [email protected]
Dr. Adauto Lima Rodrigues
Jefe de División
Ministerio de Agricultura y Abastecimiento de Brasil
Shis QI 27 Conj. 6 Casa No.1
Phone: 55.61.2182315
Fax: 55.61.2243995
E-mail: [email protected]
Mrs. Mariela Berezovsky
Asesora Técnico Científica
Asociación Brasilera Industrias de
Alimentación
R. Conselheiro Brotero, 823 Ap.113
Phone: 55.11.37419051
Fax: 55.11.37419157
E-mail:
[email protected]
Dr. Orlando Soares Júnior Abogado
Asociación Brasilera Industrias de
Alimentación
Rua Conego Eugenio Leite 665
Sao Pablo
Phone: 5511 374 19091
Fax: 5511 374 19666
E-mail: [email protected]
Farm. Bioc.Yasumi Kimura Ozawa
Asociación Brasilera Industrias de
Alimentación
Rua Bertioga 132 ap. 74
Sao Paulo sp
Phone: 5511.759 9437
Fax: 5511.751 9339
E-mail: [email protected]
Dr. Paulo Aoki Abogado
Asociación Brasilera Industrias de
Alimentación
Yakult s/a Industria e Comercio
Fax: 5511.281 9943
E-mail: [email protected]
CANADA
Mr. John Wakelin
Agrologist Manger Dairy Program
Canadian Food Inspection Agency
59 Camelot Crt. Nepean
Ontario – Kiaoy 9
Phone: 613 225-2342
Fax: 613 228-6632
E-mail: [email protected]
Ms. Helene Couture
Acting Chief, Evaluation Division, Bureau of Microbial
Hazards
Food Directorate, Health Protection Branch
Health Canada
Sir Frederick Banting 2204AI
Tunney’s Pasture Ottawa K1A 0L2
Phone: 613 957-1742
Fax: 613 952-6400
E-mail: [email protected]
Mr. Kempton L. Matte
President and CEO
National Dairy Council of Canada
221 Laurier Ave East Ottawa
Phone: 613 238-4116
Fax: 613 238-6247
E-mail: [email protected]
Mr. Timothy Finkle
Vice President
National Dairy Council of Canada
221 Laurier Avenue E., Ottawa
Phone: 613 238-4116
Fax: 613 238-6247
E-mail: [email protected]
Mr. Rejean Bouchard
Assistant Director
Policy and Dairy Production
Dairy Farmers of Canada
75 Albert Street, Suite 1101
Ottawa, Ontario K1P 5E7
Phone: 613 236-9997
Fax: 613 236-0905
E-mail: [email protected]
Mr. Dale A. Tulloch
Commissioner
Canadian Dairy Commission
1525 Carling Ave
Ottawa, K1A 0Z2
Phone: 613 230-1070
Fax: 613 230-8756
E-mail: [email protected]
CHILI
CHILE
Dr. Claudio Poblete
Jefe Sub-Departamento Industria Pecuaria M.
Veterinario
Av. Bulnes 140 7º Piso - Santiago
Phone: 56.2.671.4047
Fax: 56.2.6716184
E-mail: [email protected]
COLOMBIA
COLOMBIE
Dr. Víctor Raul Orozco Quintero
Veterinario Zootecnista
Ministerio de Agricultura
Calle 22 B # 62-30 Torre 2 ap. 903
Phone: 57.1.286 7630
Fax: 284 1735
COSTA RICA
Lic. Marco Aguilar B.
Representante del Ministerio de Economía y
CACIA
Barrio Córdoba, San José Apartado 605
1000 San José
Phone: 506.233.9783
Fax: 506.223.2302
E-mail: [email protected]
CROATIA
CROATIE
CROACIA
Prof. Dr. sci. Jasmina Lukac Havranek
President of National Codex Committee on Milk and Milk
Products
Faculty of Agriculture University of Zagreb
10000 Zagreb, Svetošimunska cesta 25
Phone: 385 1 239 3848
Fax: 385 1 239 3988
E-mail: [email protected]
CUBA
Lic. Juana Rodríguez Montero
Especialista en Aseguramiento de la Calidad de la Leche y
Productos Lácteos
Ministerio de Industria Alimenticia
Av. Rancho Boyeros Km.3 ½
Cerro – Habana
Phone: (537) 414848
Fax: (537) 577171
E-mail: [email protected]
Ing. José Z. Capdevila Valera,
Especialista en Producción y Calidad de la
Leche
Centro Nacional de Sanidad Agropecuaria
“CENSA”
Carretera de Jamaica y 8 Vías, San José de las
Lajas
C.P. 32700, La Habana
Phone: 53.064.63653- 63014. Ext.24
Fax: 53.064.63897
E-mail: [email protected]
CYPRUS
CHYPRE
CHIPRE
Dr. Phrosso Hadjilucas
Food Technologist Standards Officer
Cyprus Organization of Standards and Control of
Quality
Ministry of Commerce, Industry and Tourism Nicosia
Phone: 357 2 867 173
Fax: 357 2 375 120
E-mail: [email protected]
Mr. Loukis Charalambides
Charalambides Dairies / Han. Director
Member of the Board of Cyprus Milk Industry
Organization
K. Lakatamia
Phone: 357 2 387241
Fax: 357 2 383310
Mr. Panicos Hadjicostas
Christis Dairies Ltd/Cyprus Man. Director
Member of the Board of Cyprus Milk Industry
Organization
P. O. Box 1148, Limassol
Phone: 3575 726280
Fax: 3575 726260
Mr. Andreas Marangos Director
Cyprus Milk Industry Organization
39, Dem. Severis Ave 1521 Nicosia
Phone: 357 2 665697
Fax: 357 2 667313
E-mail: [email protected]
Mr. Savvas Evangelou
Chairman of Farmers Association
Member of the Board of Cyprus Milk Industry
Organization
Phone: 357.2.665697
Fax: 357.2.667313
DENMARK
DANEMARK
DINAMARCA
Mr. Knud Østergaard
Head of Division
Not. Veterinary and Food Services
Rolighedsvej 25
DK-1958 Frederiksberg C
Phone: 45.339.56120
Fax: 45.313.52976
E-mail: [email protected]
Mr. Jørgen Hald Christensen
Head of Division
Danish Dairy Board
Frederiks Alle 22, 8000 Århus C
Phone: 45.873.12000
Fax: 45.873.12001
E-mail: [email protected]
Mr. Michael Strube
Scientific Adviser
Danish Veterinary and Food Administration
Rolighedsvej 25, DK-1958 Frederiksberg C
Phone: 45.339.56000
Fax: 45.313.62976
E-mail: [email protected]
Ms. Charlotte Rulffs Klausen
Legal Counsellor
Danish Dairy Board
Frederiks Alle 22, DK-8000 Århus C
Phone: 45.873.12000
Fax: 45.873.12001
E-mail: [email protected]
DOMINICAN REPUBLIC
REPUBLIQUE DOMINICAINE
REPUBLICA DOMINICANA
Dr. Rafael Cabrera Hernández
Embajador de la República Dominicana en
Uruguay
Julio M. Sosa 2275
Montevideo, Uruguay
Phone: 711.4265
Fax: 916.5447
FINLAND
FINLANDE
FINLANDIA
Esko Uusi-Rauva
Professor
National Veterinary and Food Research Institute
Box 368 Fin. 00231 Helsinki
Phone: 358 9 393 1961
Fax: 358 9 393 1811
E-mail: [email protected]
FRANCE
FRANCIA
Mr. Jean-Pierre Doussin
Chargé de Mission to the Director-General
Ministère de l’Economie
59, Boulevard Vincent Auriol Télédoc
051
75013 Paris
Phone: 331 44 97 34 70
Fax: 331 44 97 30 37
E-mail:
[email protected]
Mrs. Marie-Hélène Le Henaff
Chef de Bureau des Industries Lactières
Ministère de l’Agriculture et de la
Pêche
8 Rue Jean Daudin 75015 - Paris
Phone: 01 49 55 58 93
Fax: 01 49 55 49 25
Mr. Jean-François Roche
Charge de Mission Codex
Ministère de l’Agriculture et de la
Pêche
95 Rue du Chevaleret Appt 133B
75013 Paris
Phone: 33 1 49 55 58 81
Fax: 33 1 49 55 59 48
Mrs. Catherine Vincent-Race
Veterinaire Inspecteur Hygiene du Lait et Produits
Laitiers
Ministère de l’Agriculture et de la
Pêche
87 Rue de la Sablece
91120 Papaseau
Phone:. 33 1 49 55 84 94
Fax: 33 1 49 55 56 80
Mr. Laurent Laloux
Ingenieur, chef d’unité “Qualité du
Lait et des Produits Laitiers”
Ministère de l’Agriculture et de la Pêche
-CNEVA
10 rue Pierre Curie
94704 Maisons Alfort
Phone: 33 1 49 77 27 40
Fax: 33 1 49 77 26 85
E-mail: [email protected]
Mr. Benoît Tarche
Agregado Agrícola
Ambassade de France en Argentine
Av. del Libertador
498 1001 Buenos Aires
Argentina
Phone: 541 819 24 22
Fax: 541 819 24 01
Mrs. Isabelle Gilles
Chargée de Mission
Association Internationale Fabricants Yogourt
34, Rue de Saint-Pétersbourg
75008 Paris
Phone: 33 1 49 70 72 30
Fax: 33 1 42 80 63 90
Mr. Eric Grande
Directeur Reglementation
BONGRAIN
42, Rue Rieussec
78223 Viroflay Cedex
Phone: 33 1 34 58 66 18
Fax: 33 1 34 58 54 26
E-mail: [email protected]
Mr. Jean Chibon
Assistant de ONILAIT
2, Rue Saint-Charles
75740 Paris 15
Phone: 33 1 40 58 72 27
Fax: 33 1 40 59 04 58
Mrs. Dominique Burel
Chef de Service de CNIEL
34 rue de St. Petersbourg
75008 Paris
Phone: 33 1 49 70 71 15
Fax: 33 1 42 80 63 45
Mr. Jean Maurin
Secretary General of SILC
34, Rue de Saint-Pétersbourg
Paris 8ème 75388
Phone: 33 1 49 70 72 65
Fax: 33 1 42 80 63 62
E-mail: [email protected]
Mr. Arnaud de Miollis
Délégué général
Syndifrais-Syndilait
34, Rue de Saint-Pétersbourg
75008 Paris
Phone: 33 1 49 70 72 30
Fax: 33 1 49 80 63 90
Mr. Jean-Claude Gillis
Ingenieur ATLA
34, Rue de Saint-Pétersbourg
75008 Paris
Phone: 33 1 49 70 72 68
Fax: 33 1 42 80 63 62
E-mail: [email protected]
Mr. André Kozlovsky
SODIAAL
170 bis Boulevard du Montparnasse
75014 Paris
Phone: 33 1 44 10 90 46
Fax: 33 1 44 10 90 24
Mr. Antoine Lefranc
Juriste
BESNIER
10-20 Rue Adolphe Beck
53089 Laval Cedex 09
Phone: 33 2 43 59 40 73
Fax: 33 2 43 59 42 08
Mr. Jean-François Molle
Directeur Reglementation, Sécurité,
Environnement
DANONE
7, Rue de Téhéran 75008 Paris
Phone: 33 1 44 35 24 52
Fax: 33 1 44 35 24 69
Mrs. Huguette Meyer-Caron
Chef Department Qualité
F. BEL
4, Rue d’Anjou
75008 Paris
Phone: 33 1 40 07 72 82
Fax: 33 1 40 07 72 98
GERMANY
ALLEMAGNE
ALEMANIA
Dr. Deterd Goeman
Deputy Director
Federal Ministry Food, Agriculture and Forestry
Roschusstraße 1
D-53123 Bonn
Phone: 49228.529.3587
Fax: 49228.529.3353
Mrs. Dorothea Kellen
Assistant Head of Unit
Federal Ministry Food, Agriculture and Forestry
Rochusstraße 1 In 53123 Bonn
Phone: 49228.5293388
Fax: 49228.5294407
Dr. Juliane Bräunig
Bundesinstitut für gesundheitlichen
Verbraucherschutz ud Veterinärmedizin
Diedrsdorfer Weg 1
D-12277 Berlin
Phone: 49.30.84122142
Fax: 49.30.84122157
Mr. Thomas Kützemeier
Verband der Deutschen Milchwitrschaft
Meckenheimer Allee 137
53115 Bonn
Phone: 49.228.982430
Fax: 49.228.9824320
E-mail: [email protected]
Mr. Gernot Werner
Milchindustrie-Verband
Godesberger Allee 157
D-53175 Bonn
Phone: 49.228.959690
Fax: 49.228.373780
E-mail: [email protected]
Mr. Jörg W. Rieke
Milchindustrie-Verband
Godesberger Allee 157, 53175 Bonn
Phone: 49.228.959690
Fax: 49.228.371535
E-mail: [email protected]
Ms. Astrid Jakobs de Padua
Embajada de Alemania en Uruguay
La Cumparsita 1417
Uruguay
Phone: 902.5222
Fax: 902.3422
Mrs. Eva María Fohrmann
Embajada de Alemania en Buenos Aires
Villanueva 1055
Buenos Aires
Argentina
Phone: 01.7782500
HUNGARY
HONGRIE
HUNGRIA
Dr. Nora Toth
Comité Codex National Millk and Milk Product
1145 Budapest – Torontal u 31
Phone: 36.1.261 7172
Fax: 36.1.261 8766
INDIA
INDE
Mr. Jacob Babu
Joint Secretary to Government of India
Ministry of Agriculture
Room 245; Krishi Bhavan
New Delhi 110001
Phone: 91.11.338 7804
Fax: 91.11.338 6115
E-mail: [email protected]
Mr. N. K. Chawla
Executive Director
National Dairy Development
Board, Anand – 388001
Phone: 91.2692.40166
E-mail: [email protected]
I.K. Narang
Assistant Commissioner (Dairy)
Ministry of Agriculture
Room No. 415 Krishi Bhavan
New Delhi 11001
Phone/Fax: 91.11.338 4509
E-mail: [email protected]
INDONESIA
INDONESIE
Mr. Iwan Wijaya Mulyatno
Tercer Secretario
Encargado de la Sección Económica
Embajada de la República de Indonesia
Ramón Castilla 2901
Buenos Aires, Argentina
Phone: 541.807.2211
Fax: 802.448
IRELAND
IRLANDE
IRLANDA
Mr. John Ferris
Deputy Chief Veterinary Officer
Department of Agriculture and Food
Agriculture House 6C
Kildare St Dublin 2
Phone: 343.1.6702000
Fax: 353.1.6767100
Mr. Denis Kiely
Agricultural Inspector
Department of Agriculture and Food
Agriculture House 1E
Kildare St
Dublin 2
Phone: 35.1.6072154
Fax: 353.1.676.7100
ITALY
ITALIE
ITALIA
Dr. Brunella Lo Turco
Dirigente Ministero delle Politiche Agricole, Alimentari e
Forestali
Via XX Settembre 20
Roma
Fax: 39 06.4880273
Dr. Roberta Lodi
Ricercatore CNR - Assolatte
Milano – Via Celoria, 2
Fax: 39.02.2666257
E-mail: [email protected]
Mr. Renzo Mora
Consorzio Parmiggiano Reggiano
Via Kennedy, 18
42100 Reggio Emilia
Phone: 0522 - 302337
Prof. Bruna Bianchi Salvadori
Esperto Codex Italiano
Centro Sperimentale del Latte
Strada per Merlino, 3
Zelo Buon Persico (LO)
Phone: 02.90696.1
Fax: 02.9069699
Dr. Vittorio Camilla
Dirigente Ministero delle Politiche Agricole, Alimentari e
Forestali
Via XX Settembre 20
Roma
Phone: 39.6.4819968
Fax: 39.6.483998
JAPAN
JAPON
Mr. Hiroshi Umeda, D.V.M
Section Chief for Milk and Milk Products
Veterinary Sanitation Division
Environmental Health Bureau
Ministry of Health and Welfare
1-2-2 Kasumigaseki Chiyoda-ku
Tokyo
Phone: 81.3.3595.2337
Fax: 81.3.3503.7964
E-mail: [email protected]
Mrs. Tsujiyama Yayoi
Deputy Director
Milk and Dairy Products Division
Livestock Industry Bureau
Ministry of Agriculture, Forestry and Fisheries
1-2-1 Kasumigaseki chiyoda-ku
Tokyo
Phone: 81.3.3501.1018
Fax: 81.3.3506.9578
E-mail: [email protected]
Mr. Bunji Kanzaki
Technical Advisor
Japan Dairy Technical Association
1-14-19 Kudankita chiyoda-ku
Tokyo
Phone: 3.3264.1921
Fax: 3.3264.1569
Mr. Akitoshi Ito
Technical Advisor
Japan Fermented Milk and Fermented Milk
Drinks Association
1-1, Sadohara – Cho, Ichigaya
Hoken- kaikan-Bekkan, Shinjuku-ku
Tokyo 162
Phone: 81.3.3267.4686
Fax: 81.3.3267.4663
Mr. Yoshiichi Kihara
Technical Advisor
The Japanese National Committe of IDF
1-14-19, Kudankita, Chiyoda-ku
Tokyo
Phone: 81.3.3264.3731
Fax: 81.3.3264.3732
Mr. Hideki Suzuki
Technical Advisor
The Japanese National Committe of IDF
1-14-19, Kudankita , Chiyoda – Ku
Tokyo
Phone: 81.3.3264.3731
Fax: 81.3.3264.3732
Mr. Goro Hanagata
Technical Advisor
The Japanese National Committe of IDF
Nyugyo-Kaikan 1-14-19 Kudankita
Chiyoda – Ku
Tokyo 1020073
Phone: 81.3.3264.3731
Fax: 43.3269.3732
Mr. Osamu Suganuma
The Japanese National Committe of IDF
Nyugyo Kaikan, 1 – 14-19 Kudankita
Chiyoda-Ku, Tokyo, 102 – 0073
Phone: 81.3.3264.3731
Fax: 81.3.3264.3732
LEBANON
LIBAN
LIBANO
Dr. Fouad El-Khoury
Embajador de Líbano en Uruguay
Av. Italia 7032
Montevideo, Uruguay
Phone/Fax: 408.6365
MEXICO
MEXIQUE
Ing. Rafael Nuñez Domínguez
SAGAR
Magallanes 12, Colinas de San Diego
Texcoco 56230
Phone: 52.5.534.7712
E-mail: Rafael.nuñ[email protected]
Dr. Pablo Hernández
José Martí 206-3 – Cel. Escandon
México 11800 D.F.
Phone: 525.5248203
Fax: 525.5349744
E-mail: [email protected]
Dr. Oscar Vázquez Bustamante
CANILEC
Centro Cívico 27, CD, Satélite
Naucalpan, CP 53100
Phone: 525.7298207
Fax: 525.5625882
Mr. José Luis Villicaña
Presidente del Grupo Alimentaria - SAGAR
Pedro Santacilia 260 D.F. CP.03520
Phone: 525.5793172
Fax: 525.6961835
E-mail: [email protected]
NETHERLANDS
PAYS-BAS
PAISES BAJOS
Mr. Nicolas Schelling
Ministry of Agriculture, Nature Management and
Fisheries
P O Box 20401
2500 EK The Hague
Phone: 31.703784235
Fax: 31.703786123
E-mail: [email protected]
Mr. Marinus. J. A. Bouwman
COKZ
P O Box 250
3830 AG Leusden
Phone: 31.33965696
Fax: 31.33940674
Dr. Ludwig Bercht
Dutch Dairy Association
PO Box 165, 2700 AD Zoetermeer
Phone: 31.79.3430304
Fax: 31.79.3426185
Mr. Joris Francken
Ministry of Health, Welfare and Sport
P.O. Box 5406, 2280
HK Rijswijk
Phone: 3170.3406848
Fax: 3170.3405554
E-mail: [email protected]
Mr. Rob Oost
Productschap Zuivel
P O Box 5806 – 2280 HV Rijswijk
Phone: 070.3409423
Fax: 070.3409943
E-mail: [email protected]
NEW ZEALAND
NOUVELLE-ZELANDE
NUEVA ZELANDIA
Mr. Philip Fawcet
MAF Regulatory Authority
Ministry of Agriculture and Forestry
P.O Box 2526, Wellington
Phone: 64.4.498.9874
Fax: 64.4.474.4196
E-mail: [email protected]
Dr. Joan Wright
New Zealend Dairy Board
PO Box 417 Wellington
Phone: 64.4.471.8553
Fax: 64.4.471.8539
E-mail: [email protected]
NORWAY
NORVEGE
NORUEGA
Mr. Frode Baumann
Executive Officer
Norwegian Food Control Authority
P O Box 8187 Dep N-0034 Oslo
Phone: 47.22.246650
Fax: 47.22.246699
E-mail: [email protected]
Dr. Anders Oterholm
Professor
Norwegian Dairies’ Association
P O Box 9051 Grønland, N-0133 Oslo
Phone: 47.22.93.8800
Fax: 47.22.17.2299
PARAGUAY
Srta. Jorgelina Brizuela
MAG
Coor. Sanitario de control Médico Veterinario
Diagonal Molas 950
Asunción
Phone: 201-517 – 582224 – 585212
PERU
PEROU
Mr. Carlos Pastor Talledo
Director Ejecutivo
Ministerio de Salud
Amapolas 350
Phone: 511.4406871
Fax: 511.4406797
E-mail: cpastor@digesa sld.pe
POLAND
POLOGNE
POLONIA
Mrs. Elzbieta Markowicz
Agricultural and Food Quality Inspection
Warsaw Zurawia 32/34
Phone: 48.22.625.2028
Fax: 48.22.6214858
Mr. Jan Szymborski
Veterinary Surgeon
Ministry of Agriculture and Food Economy
30 Wspólna St
00-930 Warsaw
Phone: 623.22.03
Fax: 623.14.08
Ms. Anna Wolska
Agriculture and Food Quality Inspection
Zurawia 32/34 00-515 Warsaw
Phone: 48-22 6216421
Fax: 48-22 6214858
PORTUGAL
Mrs. Antonieta Queimada
Ministerio de Agricultura
Rua Pedro Jose Pezerat Lote 236 6º.C
1900 Lisboa
Phone: 351.1.7983730
Eng. Joao Borges
Presidente da Commissao de Normalizaçao de Leite e
Produtos Lácteos
R. Prof. Domingos Matos
Vàlega, 3880 Ovar
Phone: 351.34.884105
Fax: 351.34.884630
ROMANIA
ROUMANIE
RUMANIA
Mr. Tudorel Balta
Director of Standards and Agro-Food Products
Quality Division
Ministry of Agriculture and Food
Bucharest
Bd, Carol I
No. 1, sector 3
Phone: 40 1.613.5089
Fax: 40.1.312.1249
Prof. Dr. Gheorghe Mencinicopschi
Director of Food Research Institute
Bucharest
Garlei St
No 1, sector 1
Fax: 40 1 2300311
E-mail: [email protected]
SPAIN
ESPAGNE
ESPAÑA
Mrs. María Luisa Aguilar
Jefe de Sección de la Subdirección General de
Higiene de Alimentos
Dirección General de Salud Pública Ministerio de
Sanidad y Consumo
Fernán González 39
28009 Madrid
Phone: 91.5961997
Fax: 91.5964409
E-mail: [email protected]
Mr. José Antonio Mateos
Asuntos Reglamentarios
Representante de la Federación Nacional de Industrias
Lácteas
C. Alloza, 8-12 Sat 08016 Barcelona
Phone: 34.3291.2220
Fax: 34.3419.6592
E-mail: [email protected]
SWEDEN
SUEDE
SUECIA
Mrs. Eva Lonberg
National Food Administration
Liusmedelverkef Box 622
75126 Uppsala
Phone: 46.18.175500
Fax: 46.18.105848
E-mail: [email protected]
Mrs. Karin Winberg
National Food Administration
Box 622
S 75126 Uppsala
Phone: 46.18.175609
Fax: 46.18.123275
E-mail: [email protected]
Mrs. Gunilla Johansson
Swedish Dairies Association
S-10546 Stockholm
Phone: 46.8.677.3244
Fax: 46.8.20.3329
E-mail: [email protected]
SWITZERLAND
SUISSE
SUIZA
Mrs. Eva Zbinden
Head of Codex Section
Swiss Federal Office of Public Health
3003 Berne
Phone: 41.31.322.9572
Fax: 41.31.322.9574
E-mail: [email protected]
Mr. Frédéric Brand
Swiss Federal Office of Agriculture
3003 Berne
Phone: 41.31.322.26.29
Fax: 41.31.322.2634
E-mail: [email protected]
Mr. Jean Vignal
Nestec Ltd
1800 Vevey
Phone: 41.21.924.3501
Fax: 41.21.924.4547
E-mail: [email protected]
THAILAND
THAILANDE
TAILANDIA
Miss Sukhontha Naekamanurak
Senior Scientist
Department of Science Service
Phone: 662.644.5431
Fax: 245.8939
Mr. Suphsorn Chayovan
Director of the National Food Institute
53912 Gipsum Metropolitan Tower 11th.
Sri-Ayudhya Rd.
Rajdhevee Bangkok 1044
Phone: 662.642.5334-40
Fax: 662.642.5342
Mr. Pisit Rangsaritvuthikul
Standards Officer 7
Thai Industrial Standards Institute
Ministry of Industry
Rama VI St.
Ratchathewi Bangkok
Phone: 662.2023.438
Fax: 662.248.7987
E-mail: [email protected]
UNITED KINGDOM
ROYAUME-UNI
REINO UNIDO
Dr. Dorian Kennedy
Head of Branch
Food Labelling and Standards Division
Joint Food Safety and Standards Group
Ministry of Agriculture, Fisheries and Food
Room 316, Ergon House, 17 Smith Square
London, SW1P 3JR
Phone: 44 171 238 5574
Fax: 44 171 238 6763
E-mail: [email protected]
Mr. Christopher Pratt
Head of Branch B
Food Hygiene Division
Joint Food Safety and Standards Group
Ministry of Agriculture, Fisheries and Food
Room 416, Ergon House, 17 Smith Square
London, SW1P 3JR
Phone: 44 171 238 6466
Fax: 44 171 238 6745
E-mail: [email protected]
Miss A. P. Najran
Food Labelling and Standards Division, Branch C
Joint Food Safety and Standards Group
Ministry of Agriculture, Fisheries and Food
Room 325c, Ergon House, 17 Smith Square
London, SW1P 3JR
Phone: 44 171 238 6182
Fax: 44 171 238 6763
E-mail: [email protected]
Dr. Ed Komorowski
Dairy Industry Federation
19 Cornwall Terrace, London NWL 4QP
Phone: 44 171 486 7244
Fax: 44 171 487 4734
E-mail: [email protected]
Mr. Richard Ross
Unigate European Foods
St Ivel House
Interface Business Park
Wootton Bassett, Swindon
Wiltshire, SN4 8QE
Phone: 44 1793 843429
Fax: 44 1793 843454
E- mail: [email protected]
UNITED STATES OF AMERICA
ETATS-UNIS D’AMERIQUE
ESTADOS UNIDOS DE AMERICA
Mr. Duane R. Spomer
Dairy Standards USDA Government
U.S. Department of Agriculture
1400 Independence Avenue
Washington, DC 20090-6456
Phone: 202 720-7473
Fax: 202 720-2643
E-mail: [email protected]
Mr. John C. Mowbray
Consumer Safety Officer
U.S. Food and Drug Administration
200 C Street, SW, Washington, DC 20204
Phone: 202 205-1731
Fax: 202 205-4422
E-mail: [email protected]
Mr. Syed Amjad Ali
Food Technologist
U.S. Codex Office
1400 Independence Avenue
Washington, DC 20250
Phone: 202 205-7760
Fax: 202 720-3157
E-mail: [email protected]
Mr. Thomas M. Balmer
Vice President
National Milk Producers Federation
2101 Wilson Boulevard, Suite 400
Arlington, Virginia 22201
Phone: 703 243-6111
Fax: 703 841-9328
E-mail: [email protected]
Dr. Russell J. Bishop
Director
Center for Dairy Research
1605 Lindon Drive, Babcock Hall
Madison, Wisconsin 53760
Phone: 608 265-3696
Fax: 608 262-1578
E-mail: [email protected]
Mr. Ramesh Chandan
Consultant
Yoplait-Colombo - USA
3257 Rice Creek Rd
New Brighton, Mn 55112
Phone: 612 540-4681
Fax: 612 531-0083
E-mail: [email protected]
Dr. Warren Clark
Executive Director
American Dairy Products Institute
300 West Washington Street, Suite 400
Chicago, Illinois 60606
Phone: 312 782-4888
Fax: 312 782-5299
Mr. Robert L. Garfield
National Yogurt Association
2000 Corporate Ridge, Suite 1000
McLean, Virginia 22124
Phone: 703 821-0770
Fax: 703 821-1350
E-mail: [email protected]
Ms. Diane D. Lewis
Vice-President of Technical Services
U.S. Dairy Export Council
2101 Wilson Boulevard, Suite 400
Arlington, Virginia
Phone: 703 528-3049
Fax: 703 528-3705
E-mail: [email protected]
Dr. Timothy A. Morck
Director of Nutrition & Regulatory Affairs
The Dannon company, Inc.
120 White Plains Road
Tarrytown, New York 10591
Phone: 914 366-2891
Fax: 914 366-2752
E-mail: [email protected]
Mr. Johnnie G. Nichols
Director Technical Services
National Milk Producers Federation
2101 Wilson Boulevard, Suite 400
Arlington, Virginia
Phone: 703 243-6111
Fax: 703 841-9328
E-mail: jnichols@nmpf. org
Mr. Allen R. Sayler
Director, Regulatory Affairs and International
Standards
International Dairy Foods Association
1250 H Street, N.W., Suite 900
Washington, DC 20050
Phone: 202 737-4332
Fax: 202 331-7820
E-mail: [email protected]
Ms. Deborah Van Dyke
Director of Legal Affairs
Schreiber Foods, Inc
P.O. Box 19010
Green Bay, Wisconsin
Phone: 920 437-7601
Fax: 920 436-2700
E-mail: [email protected]
URUGUAY
Ing. Ana Berti
Dirección Gral. Servicios Agrícolas
Ministerio de Ganadería, Agricultura y Pesca
Representante del Comité Nacional del Codex
Díaz 1028 Ap. 901
Fax: 309.3069
Dr. María Luisa Blanco
Dirección Gral. de Servicios Ganaderos
Ministerio de Ganadería, Agricultura y Pesca
Coordinadora del Sub Comité de la Leche y Productos
Lácteos
Gonzalo Ramírez 2033 Ap. 001
Fax: 402.6302
E-mail: DSA.leche.com.uy
Ing. Quím. Jorge Castro
LATU
Co-coordinador del Sub Comité de
Lácteos
Capurro 918
Phone: 307.2293
E-mail: [email protected]
Dr. Delvey Anchieri
Ministerio de Salud Pública
Representante del Sub Comité de Higiene
P. Campbell 1483 Ap. 001
Phone: 409.8302
Fax: 622.1740
E-mail: [email protected]
Dr. Mónica Larrechart
Ministerio de Ganadería, Agricultura y Pesca
Sub Comité de Leche y Productos
Lácteos
Américo Vespucio 1313
Phone: 209.1198
E-mail: [email protected]
Ing. Agr. Eduardo Davyt
Escuela de Lechería
Sub Comité Leche y Productos Lácteos
Ruta 52 Km. 123.500 – Colonia Suiza
Phone: 055-44587
Fax: 055-44588
Ing. Agr. Silvia Gaimari
CILU
Representante de la Industria Láctea
Tomás Gomensoro 3072
Phone: 924.7171
Fax: 924.2517
Ing. Quím. Andrés Gutman
CILU
Representante de la Industria Láctea
V. Ledesma 2381/501
Phone: 711.4786
E-mail: [email protected]
Quim. Farm. Annamaría Narizano
LATU
Sub-Comité de la Leche y Productos
Lácteos
Av. Italia 6201
Phone: 601.3724
Fax: 601.8554
E-mail: [email protected]
Dr. Martha Illa
Comisión Técnica Asesora de Alimentos
MSP
Fco. Vidal 723
Fax: 400.9022
E-mail: [email protected]
Dr. Juan C. Resbani
CONAPROLE
Cno. Carrasco 4680 B. 508
Phone: 924.7171
Dr. Angel Grillo
MSP
Br. España 2575/402
Phone: 709.4903
Esc. María de Pilar Lozano Bonet
MEF
J. Campana 2809
Phone: 4807467 – 9004106
Fax: 9021043
Mrs. Beatriz Ramos
Directora de la Dirección Defensa del
Consumidor
Ministerio de Economía y Finanzas
Acevedo Díaz 1680
Phone: 900.4106
Fax: 902.1043
Dr. Graciela Oficialdegui
MGAP
Scosería 2910
Phone: 402.6302
Téc. Agrop. Rómulo Silva
MGAP
Constituyente 1476
Phone: 402.6302
Analista Fabiana Rey
LATU
Havre 1909
Phone: 601.3724
E-mail: [email protected]
Dr. Alicia Guidini
MEF
Havre 2016
Phone: 902.1043
Ing. Cristina Vaz
MGAP
Yaguarón 1348/201
Phone: 901.5423
Ing. Ruperto Long
LATU
Mr. Nelson Avdalov
INFOPESCA
Graseras 809/207
Phone: 711.50.69
E-mail: [email protected]
Mrs. Mabel Lorenzo
Pta. Liga Amas de Casa Consumidores y Usuarios
Lanza 1358/901
Phone: 908.22.80
Fax: 924.4052
Dr. Graciela Mosquera
MGAP
Grito de Gloria 1789
Fax: 402.6302
Nelly Costa
Vice-Presidente
Liga Amas de Cons. y Usuarios
Javier Barrios Amorín 1017
Phone: 902.1112
Carlos Pimienta
Licenciado en Administración y Gerencia
Av. Brasil 2860 Ap. 702
Phone: 709.7306
Quím. Elizabeth Ferreira
LATU
Bruno Mendez 3470
Phone: 601.3724
Fax: 601.8554
E-mail: [email protected]
Dr. Héctor Lazaneo
MGAP
Iturriaga 3389 Ap. 201
Phone: 402.6346
Fax: 402.6317
Roberto Galli
Asociación Obreros y Empleados CONAPROLE
J. Suarez 2878
Phone: 209.7244
Ing. Roberto Claramount
Ministerio de RR.EE.
Millán 3822 – Montevideo
Phone: 902.0618
Fax: 901.7413
Mrs. Alicia Rappeti
Subcomité de la Leche
San Martín 2652 bis apto.301
Phone: 200.9014
INTERNATIONAL ORGANIZATIONS
ORGANISATIONS INTERNATIONALES
ORGANIZACIONES INTERNACIONALES
AOAC INTERNATIONAL
Mrs. Margreet Lauwaars
AOAC International
POB 153
6720 AD Bennekom
The Netherlands
Phone: 31 318 418 725
Fax: 31 318 418 359
E-mail: [email protected]
CONSUMERS INTERNATIONAL
Mr. Héctor Villaverde
Consumers International
Juan José de Amézaga 1441 esq San
Martin
11800 Montevideo Uruguay
Fax: 598.2.208.4533
E-mail: [email protected]
EUROPEAN COMMUNITY
Mr. Paul Culley
Council of Ministers of the EU
175 Rue de la Loi
1048 Brussels
Belgium
Phone: 32.2.285.6197
Fax: 32.2.285.7928
Mr. Walter Cancela
Asesor Económico
Bvar. Artigas 1257
Phone: 400.7580
Fax: 401.2008
E-mail: [email protected]
EUROPEAN DAIRY ASSOCIATION
Mr. Christophe Wolff
Officer Legal Affairs
European Dairy Association
14 rue Montoyer
B-1000 Brussels
Belgium
Phone: 32.2.549.5040
Fax: 32.2.849.5049
E-mail: [email protected]
FEDERACION PANAMERICANA DE LECHERIA
Dr. Eduardo Fresco León
Secretario General
Federación Panamericana de Lecheria
Ituzaingo 1324 /305
Phone: 916.89.97
Fax: 915.76.70
E-mail: [email protected].
Ing. Agr. Aldo A Ibarra
Coordinador de la Comisión de Normalización y
Control
Santa Mónica 2261
Phone: 600.6486 (private)
Mr. José Pedro Urraburu
Gerente de Información
Maldonado 1397
Phone: 916.8997
Fax: 915.7670
E-mail: [email protected]
INTERNATIONAL DAIRY FEDERATION
Mr. Edward Hopkin
Secretary General
41 Square Vergote, B-1030 Brussels Belgium
Phone: 32.2.733 9888
Fax: 32.2.733 0413
E-mail: [email protected]
Mr. Jörg Seifert
Technical Assistant
41 Square Vergote, B-1030 Brussels
Belgium
Phone: 32.2.733.9888
Fax: 32.2.733.0413
E-mail: [email protected]
Mr. Claus Heggum
President
Commission D Legislation
Danish Dairy Board, Frederiks Alle 22
8000 AA Rhus
Denmark
Phone: 45.873.12000
Fax: 45.873.12001
E-mail: [email protected]
Mr. Jerome Kozak
President IDF
2101 Wilson Blvd. Suite 460
Phone: 703.243.6111
Fax: 703.841.9328
PAHO/INPPAZ
Dr. Juan Cuellar
Advisor in Food Safery
Talcahuano 1660 Bs.As.
Phone: 541.7920087
Fax: 541.793.0927
E-mail: [email protected]
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
Mr. M. Négrin
FAO Representative in Uruguay
Casilla de Correo 1368
11000 Montevideo
Uruguay
Phone: 901.7340
Fax: 902.1202
E-mail: [email protected]
Mr. Horacio Brugnini
RR.PP FAO Uruguay
Jose M. Montero 3017
Uruguay
Phone: 901.73.40
Fax: 90.12.03
Dr. Gabriel Rodríguez Marquez
FAO Uruguay
Lazaro Gadea 971 Ap. 1
Montevideo, Uruguay
Phone: 901.7340
Fax: 902.1203
E-mail: [email protected]
JOINT FAO/WHO SECRETARIAT
Dr. Alan Randell
Senior Officer
Joint FAO/WHO Food Standards Programme
Food and Nutrition Division
Viale delle Terme di Caracalla
Rome, Italy
Phone: 39.6.57054390
Fax: 39.6.57054593
E-mail: [email protected]
Dr. David Byron
Food Standards Officer
Joint FAO/WHO Food Standards Programme
Food and Nutrition Division
Viale delle Terme di Caracalla
Rome, Italy
Phone: 39.6.57054419
Fax: 396.57054593
NEW ZEALAND SECRETARIAT
Mr. Brian Burgess
MAF Regulatory Authority
Ministry of Agriculture and Forestry
PO Box 2425 Wellington
Phone: 64.4.498.9875
Fax: 64.4.474.4239
E-mail: [email protected]
Ms. Fiona Duncan
Policy Analyst
Ministry of Agriculture and Forestry
PO Box 2425 Wllington
Phone: 64.4.4744.298
Fax: 64.4.4744.163
E-mail: [email protected]
URUGUAYAN SECRETARIAT
Cra. Adriana Guido
Directora Adscripta M.G.A.P.
Phone: 402.63.25
Ing. Agr. Ana Berti
Integrante Comité Nacional del CODEX
Phone: 309.20.74
Dr. Maria Luisa Blanco
Integrante del Sub Comité de Leche
Phone: 402.6369I
Mrs. Rosario Molinari
Secretaría Comisión Organizadora
1. SCOPE
This General Standard applies to the use of dairy terms in relation to foods to be offered to the consumer or for further processing.
2. DEFINITIONS
2.1 Milk is the normal mammary secretion of milking animals obtained from one or more milkings without either addition to it or extraction from it, intended for consumption as liquid milk or for further processing.
2.2 Milk product is a product obtained by any processing of milk, which may contain food additives, and other ingredients functionally necessary for the processing.
2.3 Composite milk product is a product of which the milk, milk products or milk constituents are an essential part in terms of quantity in the final product, as consumed provided that the constituents not derived from milk are not intended to take the place in part or in whole of any milk constituent.
2.4 A reconstituted milk product is a product resulting from the addition of water to the dried or concentrated form of the product in the amount necessary to re-establish the appropriate water to solids ratio.
2.5 A recombined milk product is a product resulting from the combining of milkfat and milk-solids-non-fat in their preserved forms with or without the addition of water to achieve the appropriate milk product composition.
2.6 Dairy terms means names, designations, symbols, pictorial or other devices which refer to or are suggestive, directly or indirectly, of milk or milk products.
3. GENERAL PRINCIPLES
Foods shall be described or presented in such a manner as to ensure the correct use of dairy terms intended for milk and milk products, to protect consumers from being confused or misled and to ensure fair practices in the food trade.
4. APPLICATION OF DAIRY TERMS
4.1 GENERAL REQUIREMENTS
4.1.1 The name of the food shall be declared in accordance with Section 4.1 of the Codex General Standard for the Labelling of Prepackaged Foods (CODEX STAN 1-1985, Rev. 1-1991; Codex Alimentarius, Volume 1A).
4.1.2 A word or words denoting the animal or, in the case of mixtures, all animals from which the milk has been derived shall be inserted immediately before or after the designation of the product. Such declarations are not required if the consumer would not be misled by their omission.
4.2 MILK
4.2.1 Only a food complying with the definition in Section 2.1 may be named “milk”. If such a food is offered for sale as such it shall be named “raw milk” or other such appropriate term as would not mislead or confuse the consumer.
4.2.2 Milk which is modified in composition by the addition and/or withdrawal of milk constituents may be identified with a name using the term “milk”, provided that a clear description of the modification to which the milk has been subjected is given in close proximity to the name.
4.2.3 Notwithstanding the provisions of Section 4.2.2 of this Standard, milk which is adjusted for fat and/or protein content and which is intended for direct consumption, may also be named “milk” provided that:
4.3 MILK PRODUCTS
4.3.1 Only a product complying with the provisions in a Codex standard for a milk product may be named as specified in the Codex standard for the product concerned.
4.3.2 Notwithstanding the provisions of Section 4.3.1of this Standard and Section 4.1.2 of the Codex General Standard for the Labelling of Prepackaged Foods (CODEX STAN 1-1985, Rev. 1-1991), a milk product may be named as specified in the Codex standard for the relevant milk product when manufactured from milk, the fat and/or protein content of which has been adjusted, provided that the compositional criteria in the relevant standard are met.
4.3.3. Products that are modified through the addition and/or withdrawal of milk constituents may be named with the name of the relevant milk product in association with a clear description of the modification to which the milk product has been subjected provided that the essential product characteristics are maintained and that the limits of such compositional modifications shall be detailed in the standards concerned as appropriate.
4.4 RECONSTITUTED AND RECOMBINED MILK PRODUCTS
Milk and milk products may be named as specified in the Codex Standard for the relevant milk product when made from recombined or reconstituted milk or from recombination or reconstitution of milk products in accordance with Section 4.1.2 of the Codex General Standard for the Labelling of Prepackaged Foods (CODEX STAN 1-1985, Rev. 1-1991), if the consumer would not be misled or confused.
4.5 COMPOSITE MILK PRODUCTS
A product complying with the description in Section 2.3 may be named with the term “milk” or the name specified for a milk product as appropriate, provided that a clear description of the other characterizing ingredient(s) (such as flavouring foods, spices, herbs and flavours) is given in close proximity to the name.
4.6 USE OF DAIRY TERMS FOR OTHER FOODS
4.6.1 The names referred to in Sections 4.2 to 4.5 may only be used as names or in the labelling of milk, milk products or composite milk products.
4.6.2 However, the provision in Section 4.6.1 shall not apply to the name of a product the exact nature of which is clear from traditional usage or when the name is clearly used to describe a characteristic quality of the non-milk product.
4.6.3 In respect of a product which is not milk, a milk product or a composite milk product, no label, commercial document, publicity material or any form of point of sale presentation shall be used which claims, implies or suggests that the product is milk, a milk product or a composite milk product, or which refers to one or more of these products.
4.6.4 However, with regard to products referred to in Section 4.6.3, which contain milk or a milk product, or milk constituents, which are an essential part in terms of characterization of the product, the term “milk”, or the name of a milk product may be used in the description of the true nature of the product, provided that the constituents not derived from milk are not intended to take the place, in part or in whole, of any milk constituent. For these products dairy terms may be used only if the consumer would not be misled.
If however the final product is intended to substitute milk, a milk product or composite milk product, dairy terms shall not be used.
For products referred to in Section 4.6.3 which contain milk, or a milk product, or milk constituents, which are not an essential part in terms of characterization of the product, dairy terms can only be used in the list of ingredients, in accordance with the Codex General Standard for the Labelling of Prepackaged Foods (CODEX STAN 1-1985, Rev. 1-1991). For these products dairy terms cannot be used for other purposes..
5. LABELLING OF PREPACKAGED FOODS
Prepackaged milk, milk products and composite milk products shall be labelled in accordance with Section 4 of the Codex General Standard for the Labelling of Prepackaged Foods (CODEX STAN 1-1985, Rev. 1-1991), except to the extent otherwise expressly provided in a specific Codex standard or in Section 4 of this Standard.
LEADING PARAGRAPH
In addition to the provisions of the Codex General Standard for the Labelling of Prepackaged Foods (CODEX STAN 1-1985, Rev. 1-1991) and the General Standard for the Use of Dairy Terms, the following specific provisions apply.
NAME OF THE FOOD
The Name of the Food shall be ....
ORIGIN OF MILK
To be deleted (Covered by the Draft General Standard for the Use of Dairy Terms).
DECLARATION OF MILKFAT CONTENT[28]
If the consumer would be misled by the omission, the milkfat content shall be declared in a manner acceptable in the country of sale to the final consumer, either :
LABELLING OF NON-RETAIL CONTAINERS
Information required in Section 7 of this Standard and Sections 4.1 to 4.8 of the General Standard for the Labelling of Prepackaged Foods and, if necessary, storage instructions, shall be given either on the container or in accompanying documents, except that the name of the product, lot identification and the name and address of the manufacturer or packer shall appear on the container. However, lot identification and the name and address of the manufacturer or packer may be replaced by an identification mark, provided that such a mark is clearly identifiable with the accompanying documents.
1 SCOPE
This Standard applies to butter intended for direct consumption or for further processing in conformity with the description in Section 2 of this Standard.
2 DESCRIPTION
Butter is a fatty product derived exclusively from milk and/or products obtained from milk, principally in the form of an emulsion of the type water-in-oil.
3 ESSENTIAL COMPOSITION AND QUALITY FACTORS
3.1 RAW MATERIALS
Milk and/or products obtained from milk.
3.2 PERMITTED INGREDIENTS
Sodium chloride and food grade salt
Starter cultures of harmless lactic acid and/or flavour producing bacteria Potable water.
3.3 COMPOSITION
|
Minimum milkfat content |
80% m/m |
|
Maximum water content |
16% m/m |
|
Maximum milk solids-not-fat content |
2% m/m |
Only those food additives listed below may be used and only within the limits specified.
|
INS No. |
Name |
Maximum Level |
|
|
Colours |
|
|
160a(i) |
b-Carotene (synthetic) |
25 mg/kg |
|
160a(ii) |
Carotenes (natural extracts) |
600 mg/kg |
|
160b |
Annatto |
20 mg/kg (bixin/norbixin basis) |
|
160e |
b-apo-Carotenal |
35 mg/kg |
|
160f |
b-apo-8'-Carotenoic acid, methyl
or ethyl ester |
35 mg/kg |
|
|
Acidity Regulators |
|
|
339 |
Sodium phosphates |
2 g/kg |
|
500(i) |
Sodium carbonate |
) |
|
500(ii) |
Sodium hydrogen carbonate |
) Limited by GMP |
|
524 |
Sodium hydroxide |
) |
|
526 |
Calcium hydroxide |
) |
5.1 HEAVY METALS
The products covered by this Standard shall comply with the maximum limits established by the Codex Alimentarius Commission.
In particular, the following limit applies:
|
Metal |
Maximum Level |
|
Lead |
0.05 mg/kg |
The products covered by this Standard shall comply with the maximum residue limits established by the Codex Alimentarius Commission.
6 HYGIENE
6.1 It is recommended that the products covered by the provisions of this standard be prepared and handled in accordance with the appropriate Sections of the Recommended International Code of Practice - General Principles of Food Hygiene (CAC/RCP 1-1969, Rev.3-1997), and other relevant Codex texts such as Codes of Hygienic Practice and Codes of Practice.
6.2 From raw material production to the point of consumption, the products covered by this standard should be subject to a combination of control measures, which may include, for example, pasteurization, and these should be shown to achieve the appropriate level of public health protection.
6.3 The products should comply with any microbiological criteria established in accordance with the Principles for the Establishment and Application of Microbiological Criteria for Foods (CAC/GL 21-1997).
7 LABELLING
In addition to the provisions of the Codex General Standard for the Labelling of Prepackaged Foods (CODEX STAN 1-1985, Rev.1-1991; Codex Alimentarius, Volume 1A) and the General Standard for the Use of Dairy Terms[29], the following specific provisions apply:
7.1 NAME OF THE FOOD
The name of the food shall be “Butter”. The name "butter" with a suitable qualification shall be used for butter with more than 95% fat.
[A word or words denoting the animal or, in the case of a mixture, all the animals from which the milk has been derived, should be inserted immediately before or after the designation of the product. Such declarations are not required if the consumer would not be misled by their omission.] [30]
7.1.1 Butter may be labelled to indicate whether it is salted or unsalted according to national legislation.
7.2 DECLARATION OF MILKFAT CONTENT
If the consumer would be misled by the omission, the milkfat content shall be declared in a manner found acceptable in the country of sale to the final consumer, either (i) as a percentage by mass or volume, or (ii) in grams per serving, provided that the number of servings is stated.
7.3 LABELLING OF NON-RETAIL CONTAINERS
Information required in Section 7 of this Standard and Sections 4.1 to 4.8 of the General Standard for the Labelling of Prepackaged Foods (CODEX STAN 1-1985, Rev.1-1991; Codex Alimentarius, Volume 1A), and, if necessary, storage instructions, shall be given either on the container or in accompanying documents, except that the name of the product, lot identification, and the name and address of the manufacturer or packer shall appear on the container. However, lot identification, and the name and address of the manufacturer or packer may be replaced by an identification mark, provided that such a mark is clearly identifiable with the accompanying documents.
8 METHODS OF SAMPLING AND ANALYSIS
8.1 SAMPLING
According to IDF Standard 50C:1995/ISO 707:1997/AOAC968.12.
8.2 DETERMINATION OF MILKFAT, SOLIDS-NOT-FAT AND WATER CONTENT
According to IDF Standard 80:1977/ISO 3727:1977/AOAC 920.116 & 938.06.
8.3 DETECTION OF VEGETABLE FAT
According to either IDF Standard 32:1965/ISO 3595:1976/AOAC 955.34A, “Detection of vegetable fat by the phytosteryl acetate test” or IDF Standard 54:1979/ISO 3594:1976/AOAC 970.50A, “Detection of vegetable fat by gas-liquid chromatography of sterols”.
8.4 DETERMINATION OF SALT CONTENT
According to IDF Standard 12B:1988/ISO 1738:1980/AOAC 960.29.
8.5 DETERMINATION OF LEAD CONTENT
According to AOAC 972.25 (Codex general method).
The Annex to this Standard contains provisions which are not intended to be applied within the meaning of the acceptance provisions of Section 4.A(I)(b) of the General Principles of the Codex Alimentarius.
1 SCOPE
This Standard applies to Anhydrous Milkfat, Milkfat, Anhydrous Butteroil, Butteroil and Ghee, which are intended for further processing or culinary use, in conformity with the description in Section 2 of this Standard.
2 DESCRIPTION
2.1 Anhydrous Milkfat, Milkfat, Anhydrous Butteroil and Butteroil are fatty products derived exclusively from milk and/or products obtained from milk by means of processes which result in almost total removal of water and non-fat solids.
2.2 Ghee is a product exclusively obtained from milk, cream or butter, by means of processes which result in almost total removal of water and non-fat solids, with an especially developed flavour and physical structure.
3 ESSENTIAL COMPOSITION AND QUALITY FACTORS
3.1 RAW MATERIALS
Milk and/or products obtained from milk.
3.2 PERMITTED INGREDIENTS
Starter cultures of harmless lactic acid producing bacteria.
3.3 COMPOSITION
|
|
Anhydrous milkfat/ |
Milkfat |
Butteroil |
Ghee |
|
Minimum milkfat (% m/m) |
99.8 |
99.6 |
99.6 |
99.6 |
|
Maximum water (% m/m) |
0.1 |
- |
- |
- |
Only those food additives listed below may be used and only within the limits specified.
4.1 Inert gas with which airtight containers are flushed before, during and after filling with product.
4.2 ANTIOXIDANTS
The following are permitted with or without antioxidant synergists in all products except Anhydrous Milkfat:
|
INS No. |
Name |
Maximum Level |
|
|
Antioxidants |
|
|
310 |
Propyl gallate |
100 mg/kg |
|
321 |
Butylated hydroxytoluene (BHT) |
75 mg/kg |
|
320 |
Butylated hydroxyanisole (BHA) |
175 mg/kg |
|
|
Any combination of propyl gallate, BHA and BHT providing
limits above are not exceeded |
200 mg/kg |
|
306 |
Mixed tocopherols concentrate |
) |
|
307 |
a-Tocopherol |
) 500 mg/kg individually or |
|
304 |
Ascorbyl palmitate |
) in combination |
|
305 |
Ascorbyl stearate |
) |
|
|
Antioxidant Synergists |
|
|
330 |
Citric acid |
Limited by GMP |
|
331 |
Sodium citrate |
Limited by GMP |
5.1 HEAVY METALS
The products covered by this Standard shall comply with the maximum limits established by the Codex Alimentarius Commission.
5.2 PESTICIDE RESIDUES
The products covered by this Standard shall comply with the maximum residue limits established by the Codex Alimentarius Commission.
6 HYGIENE
6.1 It is recommended that the products covered by the provisions of this standard be prepared and handled in accordance with the appropriate Sections of the Recommended International Code of Practice - General Principles of Food Hygiene (CAC/RCP 1-1969, Rev.3-1997), and other relevant Codex texts such as Codes of Hygienic Practice and Codes of Practice.
6.2 From raw material production to the point of consumption, the products covered by this standard should be subject to a combination of control measures, which may include, for example, pasteurization, and these should be shown to achieve the appropriate level of public health protection.
6.3 The products should comply with any microbiological criteria established in accordance with the Principles for the Establishment and Application of Microbiological Criteria for Foods (CAC/GL 21-1997).
7 LABELLING
In addition to the provisions of the Codex General Standard for the Labelling of Prepackaged Foods (CODEX STAN 1-1985, Rev.1-1991; Codex Alimentarius, Volume 1A) and the General Standard for the Use of Dairy Terms[31], the following specific provisions apply:
7.1 NAME OF THE FOOD
The name of the food shall be:
|
Anhydrous Milkfat |
) |
|
|
Milkfat |
) |
According to description specified in Section 2, |
|
Anhydrous Butteroil |
) |
composition specified in 3 and the use of |
|
Butteroil |
) |
antioxidants (see Section 4). |
|
Ghee |
) |
|
7.2 LABELLING OF NON-RETAIL CONTAINERS
Information required in Section 7 of this Standard and Sections 4.1 to 4.8 of the General Standard for the Labelling of Prepackaged Foods (CODEX STAN 1-1985, Rev.1-1991; Codex Alimentarius, Volume 1A), and, if necessary, storage instructions, shall be given either on the container or in accompanying documents, except that the name of the product, lot identification, and the name and address of the manufacturer or packer shall appear on the container. However, lot identification, and the name and address of the manufacturer or packer may be replaced by an identification mark, provided that such a mark is clearly identifiable with the accompanying documents.
8 METHODS OF SAMPLING AND ANALYSIS
8.1 SAMPLING
According to IDF Standard 50C:1995/ISO 707:1997/AOAC 968.12
8.2 DETERMINATION OF MILKFAT CONTENT
According to IDF Standard 24:1964/ISO CD 8852.
8.3 DETERMINATION OF WATER CONTENT
According to IDF Standard 23A:1988/ISO DIS 5536.
8.4 DETECTION OF VEGETABLE FAT
According to either IDF Standard 32:1965/ISO 3595:1976/AOAC 955.34A “Detection of vegetable fat by the phytosteryl acetate test” or IDF Standard 54:1979/ISO 3594:1976/AOAC 970.50A, “Detection of vegetable fat by gas-liquid chromatography of sterols”.
8.5 DETECTION OF ANIMAL FAT OTHER THAN MILKFAT
To be developed.
ANNEX
This text is intended for voluntary application by commercial partners and not for application by governments.
1 Other Quality Factors
|
|
Anhydrous milkfat |
Milkfat |
Butteroil |
Ghee |
|
Maximum free fatty acids (%m/m as oleic acid) |
0.3 |
0.4 |
0.4 |
0.4 |
|
Maximum peroxide value(milli-equivalents of oxygen/kg
fat) |
0.3 |
0.6 |
0.6 |
0.6 |
|
Taste and odour |
Acceptable for market requirements after heating a sample to
40-45_C |
|||
|
Texture |
Smooth and fine granules to liquid, depending on
temperature |
|||
HEAVY METALS
The following limits apply to Anhydrous Milkfat, Milkfat, Anhydrous Butteroil and Butteroil
|
Metal |
Maximum Level |
|
Copper |
0.05 mg/kg |
|
Iron |
0.2 mg/kg |
3.1 DETERMINATION OF FREE FATTY ACIDS CONTENT (expressed as oleic acid)
According to IDF Standard 6B:1989/ISO 1740:1991/AOAC 969.17.
The free fatty acids content can be calculated by multiplying the fat acidity by 0.282.
3.2 DETERMINATION OF PEROXIDE VALUE
According to IDF Standard 74A:1991/ISO 3976:1977 or AOAC 965.33.
3.3 DETERMINATION OF COPPER CONTENT
According to AOAC 971.20 (Codex general method) or IDF Standard 76A:1980/ISO 5738:1980/AOAC 960.40 (Codex general method).
3.4 DETERMINATION OF IRON CONTENT
According to NMKL 139:1991 (Codex general method) or IDF Standard 103A:1986/ISO 6732:1985.
1 SCOPE
This Standard applies to evaporated milks, intended for direct consumption or further processing, in conformity with the description in Section 2 of this Standard.
2 DESCRIPTION
Evaporated milks are milk products which can be obtained by the partial removal of water from milk by heat, or by any other process which leads to a product of the same composition and characteristics. The fat and/or protein content of the milk may have been adjusted, only to comply with the compositional requirements in Section 3 of this Standard, by the addition and/or withdrawal of milk constituents in such a way as not to alter the whey protein to casein ratio of the milk being adjusted.
3 ESSENTIAL COMPOSITION & QUALITY FACTORS
3.1 RAW MATERIALS
Milk and milk powders*, cream and cream powders*, milkfat products*.
* For specification, see relevant Codex standard.The following milk products are allowed for protein adjustment purposes:
|
- milk retentate |
Milk retentate is the product obtained by concentrating milk
protein by ultrafiltration of milk, partly skimmed milk, or skimmed
milk; |
|
- milk permeate |
Milk permeate is the product obtained by removing milk
proteins and milkfat from milk, partly skimmed milk, or skimmed milk by
ultrafiltration; and |
|
- lactose *. |
|
* For specification, see relevant Codex standard.3.2 PERMITTED INGREDIENTS
Potable water
Sodium chloride.
3.3 COMPOSITION
|
Evaporated milk |
|
|
Minimum milkfat |
7.5% m/m |
|
Minimum milk solids** |
25% m/m |
|
Minimum milk protein in milk solids-not-fat** |
34% m/m |
|
|
|
|
Evaporated skimmed milk |
|
|
Maximum milkfat |
1% m/m |
|
Minimum milk solids** |
20% m/m |
|
Minimum milk protein in milk solids-not-fat** |
34% m/m |
|
Evaporated partly skimmed milk |
|
|
Milkfat |
More than 1% and less than 7.5% m/m |
|
Minimum milk solids** |
20% m/m |
|
Minimum milk protein in milk solids-not-fat** |
34% m/m |
|
|
|
|
Evaporated high-fat milk |
|
|
Minimum milkfat |
15% m/m |
|
Minimum milk solids-not-fat** |
11.5% m/m |
|
Minimum milk protein in milk solids-not-fat** |
34% m/m |
** The milk solids and milk solids-not-fat content include water of crystallization of the lactose.4 FOOD ADDITIVES
Only those food additives listed below may be used and only within the limits specified.
|
INS No. |
Name |
Maximum Level |
|
|
|
Firming agents |
|
|
|
508 |
Potassium chloride |
) |
2 g/kg singly or 3 g/kg in combination, expressed |
|
509 |
Calcium chloride |
) |
as anhydrous substances |
|
|
Stabilizers |
|
|
|
331 |
Sodium citrates |
) |
2 g/kg singly or 3 g/kg in combination, expressed |
|
332 |
Potassium citrates |
) |
as anhydrous substances |
|
333 |
Calcium citrates |
) |
|
|
|
Acidity Regulators |
|
|
|
500 |
Sodium carbonates |
) |
|
|
501 |
Potassium carbonates |
) |
|
|
170 |
Calcium carbonates |
) |
|
|
339 |
Sodium phosphates |
) |
2 g/kg singly or 3 g/kg in combination, expressed |
|
340 |
Potassium phosphates |
) |
as anhydrous substances |
|
341 |
Calcium phosphates |
) |
|
|
450 |
Diphosphates |
) |
|
|
451 |
Triphosphates |
) |
|
|
452 |
Polyphosphates |
) |
|
|
|
Thickener |
|
|
|
407 |
Carrageenan |
150 mg/kg |
|
|
|
Emulsifier |
|
|
|
322 |
Lecithin |
Limited by GMP |
|
5.1 HEAVY METALS
The products covered by this Standard shall comply with the maximum limits established by the Codex Alimentarius Commission.
5.2 PESTICIDE RESIDUES
The products covered by this Standard shall comply with the maximum residue limits established by the Codex Alimentarius Commission.
6 HYGIENE
6.1 It is recommended that the products covered by the provisions of this standard be prepared and handled in accordance with the appropriate Sections of the Recommended International Code of Practice - General Principles of Food Hygiene (CAC/RCP 1-1969, Rev.3-1997), and other relevant Codex texts such as Codes of Hygienic Practice and Codes of Practice.
6.2 From raw material production to the point of consumption, the products covered by this standard should be subject to a combination of control measures, which may include, for example, pasteurization, and these should be shown to achieve the appropriate level of public health protection.
6.3 The products should comply with any microbiological criteria established in accordance with the Principles for the Establishment and Application of Microbiological Criteria for Foods (CAC/GL 21-1997).
7 LABELLING
In addition to the provisions of the Codex General Standard for the Labelling of Prepackaged Foods (CODEX STAN 1-1985, Rev.1-1991; Codex Alimentarius, Volume 1A) and the General Standard for the Use of Dairy Terms[33], the following specific provisions apply:
7.1 NAME OF THE FOOD
The name of the food shall be
|
Evaporated milk |
) |
|
|
Evaporated skimmed milk |
) |
according to the composition specified in |
|
Evaporated partly skimmed milk |
) |
Section 3 |
|
Evaporated high-fat milk |
) |
|
[A word or words denoting the animal or, in the case of a mixture, all the animals from which the milk has been derived, should be inserted immediately before or after the designation of the product. Such declarations are not required if the consumer would not be misled by their omission.] [34]
7.2 DECLARATION OF MILKFAT CONTENT
If the consumer would be misled by the omission, the milkfat content shall be declared in a manner found acceptable in the country of sale to the final consumer, either (i) as a percentage by mass or volume, or (ii) in grams per serving, provided that the number of servings is stated.
7.3 DECLARATION OF MILK PROTEIN
If the consumer would be misled by the omission, the milk protein content shall be declared in a manner acceptable in the country of sale to the final consumer, either as (i) a percentage by mass or volume, or (ii) grams per serving, provided the number of servings is stated.
7.4 LIST OF INGREDIENTS
Notwithstanding the provision of Section 4.2.1 of the General Standard for the Labelling of Prepackaged Foods (CODEX STAN 1-1985, Rev.1-1991; Codex Alimentarius, Volume 1A), milk products used only for protein adjustment need not be declared.
7.5 LABELLING OF NON-RETAIL CONTAINERS
Information required in Section 7 of this Standards and Sections 4.1 to 4.8 of the General Standard for the Labelling of Prepackaged Foods (CODEX STAN 1-1985, Rev.1-1991; Codex Alimentarius, Volume 1A), and, if necessary, storage instructions, shall be given either on the container or in accompanying documents, except that the name of the product, lot identification, and the name and address of the manufacturer or packer shall appear on the container. However, lot identification, and the name and address of the manufacturer or packer may be replaced by an identification mark, provided that such a mark is clearly identifiable with the accompanying documents.
8 METHODS OF SAMPLING & ANALYSIS
8.1 SAMPLING
According to IDF Standard 50C:1995/ISO 707:1997/AOAC 968.12.
8.2 DETERMINATION OF MILKFAT CONTENT
According to IDF Standard 13C:1987/ISO 1737:1985/AOAC 945.48G or AOAC 920.115F.
8.3 DETERMINATION OF TOTAL SOLIDS CONTENT
According to IDF Standard 21B:1987/ISO 6731:1989/AOAC 945.48D, AOAC 925.23A or AOAC 920.107.
8.4 DETERMINATION OF PROTEIN CONTENT
Protein content is 6.38 multiplied by total Kjeldahl nitrogen determined by IDF Standard 20B:1993/ISO/CD8968/AOAC 991.20-23 or AOAC 945.48H.
1 SCOPE
This Standard applies to sweetened condensed milks, intended for direct consumption or further processing, in conformity with the description in Section 2 of this Standard.
2 DESCRIPTION
Sweetened condensed milks are milk products which can be obtained by the partial removal of water from milk with the addition of sugar, or by any other process which leads to a product of the same composition and characteristics. The fat and/or protein content of the milk may have been adjusted, only to comply with the compositional requirements in Section 3 of this Standard, by the addition and/or withdrawal of milk constituents in such a way as not to alter the whey protein to casein ratio of the milk being adjusted.
3 ESSENTIAL COMPOSITION & QUALITY FACTORS
3.1 RAW MATERIALS
Milk and milk powder*, cream and cream powders*, milkfat products*.
* For specification, see relevant Codex standard.The following milk products are allowed for protein adjustment purposes:
|
- milk retentate |
Milk retentate is the product obtained by concentrating milk
protein by ultrafiltration of milk, partly skimmed milk, or skimmed
milk; |
|
- milk permeate |
Milk permeate is the product obtained by removing milk
proteins and milkfat from milk, partly skimmed milk, or skimmed milk by
ultrafiltration; and |
|
- lactose * |
(Also for seeding purposes) |
* For specification, see relevant Codex standard.3.2 PERMITTED INGREDIENTS
Potable water
Sugar
Sodium chloride.
In this product, sugar is generally considered to be sucrose, but a combination of sucrose with other sugars, consistent with Good Manufacturing Practice, may be used.
3.3 COMPOSITION
|
Sweetened condensed milk |
|
|
Minimum milkfat |
8% m/m |
|
Minimum milk solids** |
28% m/m |
|
Minimum milk protein in milk solids-not-fat** |
34% m/m |
|
|
|
|
Sweetened condensed skimmed milk |
|
|
Maximum milkfat |
1% m/m |
|
Minimum milk solids** |
24% m/m |
|
Minimum milk protein in milk solids-not-fat** |
34% m/m |
|
|
|
|
Sweetened condensed partly skimmed milk |
|
|
Milkfat |
More than 1% and less than 8% m/m |
|
Minimum milk solids-not-fat** |
20% m/m |
|
Minimum milk solids** |
24% m/m |
|
Minimum milk protein in milk solids-not-fat** |
34% m/m |
|
|
|
|
Sweetened condensed high-fat milk |
|
|
Minimum milkfat |
16% m/m |
|
Minimum milk solids-not-fat** |
14% m/m |
|
Minimum milk protein in milk solids-not-fat** |
34% m/m |
** The milk solids and milk solids-not-fat content include water of crystallization of the lactose.For all sweetened condensed milks the amount of sugar is restricted by Good Manufacturing Practice to a minimum value which safeguards the keeping quality of the product and a maximum value above which crystallization of sugar, may occur.
4 FOOD ADDITIVES
Only those food additives listed below may be used and only within the limits specified.
|
INS No. |
Name |
Maximum Level |
|
|
|
Firming agents |
|
|
|
508 |
Potassium chloride |
) |
2 g/kg singly or 3 g/kg in combination, expressed |
|
509 |
Calcium chloride |
) |
as anhydrous substances |
|
|
Stabilizers |
|
|
|
331 |
Sodium citrates |
) |
2 g/kg singly or 3 g/kg in combination, expressed |
|
332 |
Potassium citrates |
) |
as anhydrous substances |
|
333 |
Calcium citrates |
) |
|
|
|
Acidity Regulators |
|
|
|
500 |
Sodium carbonates |
) |
|
|
501 |
Potassium carbonates |
) |
|
|
170 |
Calcium carbonates |
) |
|
|
339 |
Sodium phosphates |
) |
2 g/kg singly or 3 g/kg in combination, expressed |
|
340 |
Potassium phosphates |
) |
as anhydrous substances |
|
341 |
Calcium phosphates |
) |
|
|
450 |
Diphosphates |
) |
|
|
451 |
Triphosphates |
) |
|
|
452 |
Polyphosphates |
) |
|
|
|
Thickener |
|
|
|
407 |
Carrageenan |
150 mg/kg |
|
|
|
Emulsifier |
|
|
|
322 |
Lecithin |
Limited by GMP |
|
5.1 HEAVY METALS
The products covered by this Standard shall comply with the maximum limits established by the Codex Alimentarius Commission.
5.2 PESTICIDE RESIDUES
The products covered by this Standard shall comply with the maximum residue limits established by the Codex Alimentarius Commission.
6 HYGIENE
6.1 It is recommended that the products covered by the provisions of this standard be prepared and handled in accordance with the appropriate Sections of the Recommended International Code of Practice - General Principles of Food Hygiene (CAC/RCP 1-1969, Rev.3-1997), and other relevant Codex texts such as Codes of Hygienic Practice and Codes of Practice.
6.2 From raw material production to the point of consumption, the products covered by this standard should be subject to a combination of control measures, which may include, for example, pasteurization, and these should be shown to achieve the appropriate level of public health protection.
6.3 The products should comply with any microbiological criteria established in accordance with the Principles for the Establishment and Application of Microbiological Criteria for Foods (CAC/GL 21-1997).
7 LABELLING
In addition to the provisions of the Codex General Standard for the Labelling of Prepackaged Foods (CODEX STAN 1-1985, Rev.1-1991; Codex Alimentarius, Volume 1A) and the General Standard for the Use of Dairy Terms[35], the following specific provisions apply:
7.1 NAME OF THE FOOD
The name of the food shall be:
|
Sweetened condensed milk |
) |
|
|
Sweetened condensed skimmed milk |
) |
according to the composition specified in |
|
Sweetened condensed partly skimmed milk |
) |
Section 3 |
|
Sweetened condensed high-fat milk |
) |
|
[A word or words denoting the animal or, in the case of a mixture, all the animals from which the milk has been derived, should be inserted immediately before or after the designation of the product. Such declarations are not required if the consumer would not be misled by their omission.] [36]
7.2 DECLARATION OF MILKFAT CONTENT
If the consumer would be misled by the omission, the milkfat content shall be declared in a manner found acceptable in the country of sale to the final consumer, either (i) as a percentage by mass or volume, or (ii) in grams per serving, provided that the number of servings is stated.
7.3 DECLARATION OF MILK PROTEIN
If the consumer would be misled by the omission, the milk protein content shall be declared in a manner acceptable in the country of sale to the final consumer, either as (i) a percentage by mass or volume, or (ii) grams per serving, provided the number of servings is stated.
7.4 LIST OF INGREDIENTS
Notwithstanding the provision of Section 4.2.1 of the General Standard for the Labelling of Prepackaged Foods (CODEX STAN 1-1985, Rev.1-1991; Codex Alimentarius, Volume 1A), milk products used only for protein adjustment need not be declared.
7.5 LABELLING OF NON-RETAIL CONTAINERS
Information required in Section 7 of this Standard and Sections 4.1 to 4.8 of the General Standard for the Labelling of Prepackaged Foods (CODEX STAN 1-1985, Rev.1-1991; Codex Alimentarius, Volume 1A), and, if necessary, storage instructions, shall be given either on the container or in accompanying documents, except that the name of the product, lot identification, and the name and address of the manufacturer or packer shall appear on the container. However, lot identification, and the name and address of the manufacturer or packer may be replaced by an identification mark, provided that such a mark is clearly identifiable with the accompanying documents.
8 METHODS OF SAMPLING & ANALYSIS
8.1 SAMPLING
According to IDF Standard 50C:1995/ISO 707:1997/AOAC 968.12.
8.2 DETERMINATION OF MILKFAT CONTENT
According to IDF Standard 13C:1987/ISO 1737:1985/AOAC 920.115F or AOAC 945.48G.
8.3 DETERMINATION OF TOTAL SOLIDS CONTENT
According to IDF Standard 15B:1982/ISO 6734:1991/AOAC 920.115D.
8.4 DETERMINATION OF PROTEIN CONTENT
Protein content is 6.38 multiplied by total Kjeldahl nitrogen determined by IDF Standard 20B:1993/ISO CD8968/AOAC 991.20-23 or AOAC 920.115G.
The Annex to this Standard contains provisions which are not intended to be applied within the meaning of the acceptance provisions of Section 4.A(I)(b) of the General Principles of the Codex Alimentarius.
1 SCOPE
This Standard applies to milk powders and cream powders, intended for direct consumption or further processing, in conformity with the description in Section 2 of this Standard.
2 DESCRIPTION
Milk powders and cream powders are milk products which can be obtained by the partial removal of water from milk or cream. The fat and/or protein content of the milk or cream may have been adjusted, only to comply with the compositional requirements in Section 3 of this Standard, by the addition and/or withdrawal of milk constituents in such a way as not to alter the whey protein to casein ratio of the milk being adjusted.
3 ESSENTIAL COMPOSITION & QUALITY FACTORS
3.1 RAW MATERIALS
Milk and cream
The following milk products are allowed for protein adjustment purposes:
|
- milk retentate |
Milk retentate is the product obtained by concentrating milk
protein by ultrafiltration of milk, partly skimmed milk, or skimmed
milk; |
|
- milk permeate |
Milk permeate is the product obtained by removing milk
proteins and milkfat from milk, partly skimmed milk, or skimmed milk by
ultrafiltration; and |
|
- lactose *. |
|
* For specification, see relevant Codex standard3.2 COMPOSITION
|
Cream powder |
|
|
Minimum milkfat |
42% m/m |
|
Maximum water** |
5% m/m |
|
Minimum milk protein in milk solids-not-fat** |
34% m/m |
|
|
|
|
Whole milk powder |
|
|
Milkfat |
Minimum 26% and less than 42 % m/m |
|
Maximum water** |
5% m/m |
|
Minimum milk protein in milk solids-not-fat** |
34% m/m |
|
|
|
|
Partly skimmed milk powder |
|
|
Milkfat |
More than 1.5% and less than 26% m/m |
|
Maximum water** |
5% m/m |
|
Minimum milk protein in milk solids-not-fat** |
34% m/m |
|
|
|
|
Skimmed milk powder |
|
|
Maximum milkfat |
1.5% m/m |
|
Maximum water** |
5% m/m |
|
Minimum milk protein in milk solids-not-fat** |
34% m/m |
** The water content does not include water of crystallization of the lactose; the milk solids-not-fat content includes water of crystallization of the lactose.4 FOOD ADDITIVES
Only those food additives listed below may be used and only within the limits specified.
|
INS No. |
Name |
Maximum Level |
|
|
|
Stabilizers |
|
|
|
331 |
Sodium citrates |
) |
5 g/kg singly or in combination, |
|
332 |
Potassium citrates |
) |
expressed as anhydrous substances |
|
|
Firming agents |
|
|
|
508 |
Potassium chloride |
Limited by GMP |
|
|
509 |
Calcium chloride |
Limited by GMP |
|
|
|
Acidity Regulators |
|
|
|
500 |
Sodium carbonates |
) |
|
|
501 |
Potassium carbonates |
) |
|
|
452 |
Sodium phosphates |
) |
5 g/kg singly or in combination |
|
340 |
Potassium phosphates |
) |
expressed as anhydrous substances |
|
450 |
Diphosphates |
) |
|
|
451 |
Triphosphates |
) |
|
|
452 |
Polyphosphates |
) |
|
|
|
Emulsifiers |
|
|
|
471 |
Mono- and diglycerides of fatty acids |
2.5 g/kg |
|
|
322 |
Lecithin (or phospholipids from natural sources) |
Limited by GMP |
|
|
|
Anti-caking Agents |
|
|
|
341(iii) |
Tricalcium orthophosphate |
) |
|
|
170(i) |
Calcium carbonate |
) |
|
|
530 |
Magnesium oxide |
) |
|
|
504(i) |
Magnesium carbonate |
) |
|
|
343 |
Trimagnesium phosphate |
) |
|
|
559 |
Aluminium silicate |
) |
10 g/kg singly or in combination |
|
552 |
Calcium silicate |
) |
|
|
553 |
Magnesium silicates |
) |
|
|
554 |
Sodium aluminosilicate |
) |
|
|
551 |
Silicon dioxide, amorphous |
) |
|
|
555 |
Potassium aluminium silicate |
) |
|
|
556 |
Calcium aluminium silicate |
|
|
|
|
Antioxidants |
|
|
|
301 |
Sodium L-ascorbate |
) |
|
|
300 |
L-Ascorbic acid |
) |
0.5 g/kg expressed as ascorbic acid |
|
304 |
Ascorbyl palmitate |
) |
|
|
320 |
Butylated hydroxyanisole (BHA) |
0.01% m/m |
|
|
|
Antifoaming agent |
|
|
|
900 |
Polydimethylsiloxane |
Limited by GMP |
|
5.1 HEAVY METALS
The products covered by this Standard shall comply with the maximum limits established by the Codex Alimentarius Commission.
5.2 PESTICIDE RESIDUES
The products covered by this Standard shall comply with the maximum residue limits established by the Codex Alimentarius Commission.
6 HYGIENE
6.1 It is recommended that the products covered by the provisions of this standard be prepared and handled in accordance with the appropriate Sections of the Recommended International Code of Practice - General Principles of Food Hygiene (CAC/RCP 1-1969, Rev.3-1997), and other relevant Codex texts such as Codes of Hygienic Practice and Codes of Practice.
6.2 From raw material production to the point of consumption, the products covered by this standard should be subject to a combination of control measures, which may include, for example, pasteurization, and these should be shown to achieve the appropriate level of public health protection.
6.3 The products should comply with any microbiological criteria established in accordance with the Principles for the Establishment and Application of Microbiological Criteria for Foods (CAC/GL 21-1997).
7 LABELLING
In addition to the provisions of the Codex General Standard for the Labelling of Prepackaged Foods (CODEX STAN 1-1985, Rev.1-1991; Codex Alimentarius, Volume 1A) and the General Standard for the Use of Dairy Terms[37], the following specific provisions apply:
7.1 NAME OF THE FOOD
The name of the food shall be:
|
Cream powder |
) |
|
|
Whole milk powder |
) |
according to the composition in Section 3.2 |
|
Partly skimmed milk powder |
) |
|
|
Skimmed milk powder |
) |
|
If allowed by national legislation or otherwise identified to the consumer in the country where the product is sold, “whole milk powder” may be designated “full cream milk powder” and “skimmed milk powder” may be designated “low fat milk powder”.
[A word or words denoting the animal or, in the case of a mixture, all the animals from which the milk has been derived, should be inserted immediately before or after the designation of the product. Such declarations are not required if the consumer would not be misled by their omission.] [38]
7.2 DECLARATION OF MILKFAT CONTENT
If the consumer would be misled by the omission, the milkfat content shall be declared in a manner found acceptable in the country of sale to the final consumer, either (i) as a percentage by mass or volume, or (ii) in grams per serving, provided that the number of servings is stated.
7.3 DECLARATION OF MILK PROTEIN
If the consumer would be misled by the omission, the milk protein content shall be declared in a manner acceptable in the country of sale to the final consumer, either as (i) a percentage by mass or volume, or (ii) grams per serving, provided the number of servings is stated.
7.4 LIST OF INGREDIENTS
Notwithstanding the provision of Section 4.2.1 of the General Standard for the Labelling of Prepackaged Foods (CODEX STAN 1-1985, Rev.1-1991; Codex Alimentarius, Volume 1A), milk products used only for protein adjustment need not be declared.
7.5 LABELLING OF NON-RETAIL CONTAINERS
Information required in Section 7 of this Standard and Sections 4.1 to 4.8 of the General Standard for the Labelling of Prepackaged Foods (CODEX STAN 1-1985, Rev.1-1991; Codex Alimentarius, Volume 1A), and, if necessary, storage instructions, shall be given either on the container or in accompanying documents, except that the name of the product, lot identification, and the name and address of the manufacturer or packer shall appear on the container. However, lot identification, and the name and address of the manufacturer or packer may be replaced by an identification mark, provided that such a mark is clearly identifiable with the accompanying documents.
8 METHODS OF SAMPLING & ANALYSIS
8.1 SAMPLING
According to IDF Standard 50C:1995/ISO 707:1997/AOAC 968.12.
8.2 DETERMINATION OF MILKFAT CONTENT
According to IDF Standard 9C:1987/ISO 1736:1985/AOAC 936.06, AOAC 920.115F or 945.48G.
8.3 DETERMINATION OF PROTEIN CONTENT
Protein content is 6.38 multiplied by total Kjeldahl nitrogen determined by IDF Standard 20B:1993/ISO CD 8968/AOAC 991.20-23.
8.4 DETERMINATION OF WATER CONTENT
According to IDF Standard 26A:1993/ISO CD 5537.2.
ANNEX
This text is intended for voluntary application by commercial partners and not for application by governments.
Additional Quality Factors
|
Requirements |
Whole milk powder |
Partially skimmed milk powder |
Skimmed milk powder |
Method |
|
TITRATABLE ACIDITY |
max 18.0 |
max 18.0 |
max 18.0 |
IDF Standard 86:1981 |
|
Scorched particles |
max Disc B |
max Disc B |
max Disc B |
IDF Standard 107A:1995 |
|
Solubility index |
max 1.0 |
max 1.0 |
max 1.0 |
IDF Standard 129A:1988 |
1. SCOPE
This Standard applies to all products, intended for direct consumption or further processing, in conformity with the definition of cheese in Section 2 of this Standard. Subject to the provisions of this Standard, standards for individual varieties of cheese, or groups of varieties of cheese, may contain provisions which are more specific than those in this Standard and in these cases, those specific provisions shall apply. In such cases, those specific provisions shall apply.
2. DESCRIPTION
2.1 Cheese is the ripened or unripened soft or semi-hard, hard and extra hard product, which may be coated, and in which the whey protein/casein ratio does not exceed that of milk, obtained by:
(a) coagulating wholly or partly the following raw materials: milk and/or products obtained from milk, through the action of rennet or other suitable coagulating agents, and by partially draining the whey resulting from such coagulation; and/or2.1.1 Ripened cheese is cheese which is not ready for consumption shortly after manufacture but which must be held for such time, at such temperature, and under such other conditions as will result in the necessary biochemical and physical changes characterizing the cheese in question.(b) processing techniques involving coagulation of milk and/or products obtained from milk which give an end-product with similar physical, chemical and organoleptic characteristics as the product defined under (a).
2.1.2 Mould ripened cheese is a ripened cheese in which the ripening has been accomplished primarily by the development of characteristic mould growth throughout the interior and/or on the surface of the cheese.
2.1.3 Unripened cheese including fresh cheese is cheese which is ready for consumption shortly after manufacture.
3. ESSENTIAL COMPOSITION AND QUALITY FACTORS
3.1 RAW MATERIALS
Milk and/or products obtained from milk.
3.2 PERMITTED INGREDIENTS
- Starter cultures of harmless lactic acid and/or flavour producing bacteria and cultures of other harmless microorganisms4. FOOD ADDITIVES- Safe and suitable enzymes
- Sodium chloride
- Potable water
Only those food additives listed below may be used and only within the limits specified.
Unripened cheeses
As listed in the Standard for Unripened Cheese Including Fresh Cheese (Codex Stan. A-19).
Cheeses in Brine
As listed in the Standard for Cheeses in Brine (Codex Stan. A-17).
Ripened cheeses, including mould ripened cheeses
Additives not listed below but provided for in individual standards for varieties of ripened cheeses may also be used for similar types of cheese within the limits specified within those standards.
|
INS No. |
Name |
Maximum Level |
|
|
|
Colours |
|
|
|
100 |
Curcumins (for edible cheese rind) |
Limited by GMP |
|
|
101 |
Riboflavins |
Limited by GMP |
|
|
141 |
Copper chlorophylls |
15 mg/kg |
|
|
160a(ii) |
Carotenes(vegetable) |
600 mg/kg |
|
|
160a(i) |
Carotenes(synthetic) |
25mg/kg |
|
|
160c |
Paprika oleoresins |
Limited by GMP |
|
|
160b |
Annatto extracts |
|
|
|
|
|
- normal coloured |
10 mg/kg (on bixin/norbixin basis) |
|
|
|
- orange coloured |
25 mg/kg (on bixin/norbixin basis) |
|
|
|
- deep orange coloured |
50 mg/kg (on bixin/norbixin basis) |
|
160e |
b-apo-Carotenal |
35 mg/kg |
|
|
160f |
b-apo-8-Carotenoic acid, ethyl
ester |
35 mg/kg |
|
|
171 |
Titanium dioxide |
Limited by GMP |
|
|
120 |
Carmines (for red marbled cheeses only) |
Limited by GMP |
|
|
140 |
Chlorophylls (for green marbled cheeses only) |
Limited by GMP |
|
|
153 |
Vegetable carbon (For layered cheeses only) |
Limited by GMP |
|
|
163 |
Anthocyanines (for red marbled cheeses only) |
Limited by GMP |
|
|
162 |
Beet red |
Limited by GMP |
|
|
|
Bleaching Agents |
|
|
|
928 |
Benzoyl peroxide |
1g/kg (used to bleach dairy ingredients on weight of bleached
milk) |
|
|
|
Acidity regulators |
|
|
|
170 |
Calcium carbonates |
) |
|
|
504 |
Magnesium carbonates |
) Limited by GMP |
|
|
575 |
Glucono delta-lactone |
) |
|
|
|
Preservatives |
|
|
|
200 |
Sorbic acid |
) |
|
|
201 |
Sodium sorbate |
) 3000 mg/kg calculated as sorbic |
|
|
202 |
Potassium sorbate |
) acid |
|
|
203 |
Calcium sorbate |
) |
|
|
234 |
Nisin |
12.5 mg/kg |
|
|
239 |
Hexamethylene tetramine (Provolone only) |
25 mg/kg, expressed as formaldehyde |
|
|
251 |
Sodium nitrate |
50 mg/kg, expressed as NaNO3 |
|
|
252 |
Potassium nitrate |
) |
|
|
280 |
Propionic acid |
) 3000 mg/kg, calculated as |
|
|
281 |
Sodium propionate |
) propionic acid |
|
|
282 |
Calcium propionate |
) |
|
|
1105 |
Lysozyme |
Limited by GMP |
|
|
|
For surface/rind treatment only: |
|
|
|
200 |
Sorbic acid |
) 1 g/kg singly or in combination, |
|
|
202 |
Potassium sorbate |
) calculated as sorbic acid |
|
|
203 |
Calcium sorbate |
) |
|
|
235 |
Pimaricin (natamycin) |
2 mg/dm2 of surface. Not present in a depth of 5 mm |
|
|
|
Miscellaneous additive |
|
|
|
508 |
Potassium chloride |
Limited by GMP |
|
|
Sliced, cut, shredded or grated cheese |
|
||
|
|
Anti-caking agents |
|
|
|
460 |
Cellulose |
Limited by GMP |
|
|
551 |
Silicon dioxide, amorphous |
) |
|
|
552 |
Calcium silicate |
) |
|
|
553 |
Magnesium silicates |
) 10 g/kg singly or in combination. |
|
|
554 |
Sodium aluminosilicate |
) Silicates calculated as silicon dioxide |
|
|
555 |
Potassium aluminosilicate |
) |
|
|
556 |
Calcium aluminium silicate |
) |
|
|
559 |
Aluminium silicate |
) |
|
|
560 |
Potassium silicate |
) |
|
|
|
Preservatives |
|
|
|
200 |
Sorbic acid |
) 1 g/kg singly or in combination, |
|
|
202 |
Potassium sorbate |
) calculated as sorbic acid |
|
|
203 |
Calcium sorbate |
) |
|
|
235 |
Pimaricin (natamycin)* |
10 mg/kg, for surface treatment, calculated on weight of
cheese |
|
* Referred to the Codex Committe on Food Additives and Contaminants (see para. 70 of this report)5. CONTAMINANTS
5.1 HEAVY METALS
The products covered by this Standard shall comply with the maximum limits established by the Codex Alimentarius Commission.
5.2 PESTICIDE RESIDUES
The products covered by the provisions of this standard shall comply with those maximum residue limits established by the Codex Alimentarius Commission.
6. HYGIENE
6.1 It is recommended that the products covered by the provisions of this standard be prepared and handled in accordance with the appropriate Sections of the Recommended International Code of
Practice - General Principles of Food Hygiene (CAC/RCP 1-1969, Rev.3-1997), and other relevant Codex texts such as Codes of Hygienic Practice and Codes of Practice.
6.2 From raw material production to the point of consumption, the products covered by this standard should be subject to a combination of control measures, which may include, for example, pasteurization, and these should be shown to achieve the appropriate level of public health protection.
6.3 The products should comply with any microbiological criteria established in accordance with the Principles for the Establishment and Application of Microbiological Criteria for Foods (CAC/GL 21-1997).
7. LABELLING
In addition to the provisions of the Codex General Standard for the Labelling of Prepackaged Foods (CODEX STAN 1-1985, Rev.1-1991; Codex Alimentarius, Volume 1A) and the General Standard for the Use of Dairy Terms[39], the following specific provisions apply:
7.1 NAME OF THE FOOD
The name of the food shall be cheese. However, the word “cheese” may be omitted in the designation of an individual cheese variety reserved by a Codex standard for individual cheeses, and, in the absence thereof, a variety name specified in the national legislation of the country in which the product is sold, provided that the omission does not create an erroneous impression regarding the character of the food.
7.1.1 In case the product is not designated with a variety name but with the designation "cheese" alone, the designation may be accompanied by the appropriate descriptive terms in the following table:
|
Designation according to firmness and ripening
characteristics |
||
|
According to firmness: Term 1 |
According to principal ripening: Term 2 |
|
|
MFFB % |
Designation |
|
|
< 51 |
Extra hard |
Ripened |
|
49-56 |
Hard |
Mould ripened |
|
54-69 |
Firm/Semi-hard |
Unripened/Fresh |
|
> 67 |
Soft |
In Brine |
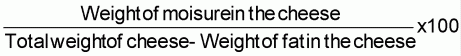
Example:
The designation of a cheese with moisture on a fat-free basis of 57% which is ripened in a manner similar in which Danablu is ripened would be:
"Mould ripened firm cheese or firm mould ripened cheese."[A word or words denoting the animal or, in the case of a mixture, all the animals from which the milk has been derived, should be inserted immediately before or after the designation of the product. Such declarations are not required if the consumer would not be misled by their omission.] [40]
7.2 DECLARATION OF MILKFAT CONTENT
The milkfat content shall be declared in a manner found acceptable in the country of sale to the final consumer, either (i) as a percentage by mass or volume, (ii) as a percentage of fat in dry matter, or (iii) in grams per serving, provided that the number of servings is stated.
Additionally, the following terms may be used:
|
High fat |
(if the content of FDM is above or equal to 60%); |
|
Full fat |
(if the content of FDM is above or equal to 45% and less than
60%) |
|
Medium fat |
(if the content of FDM is above or equal to 25% and less than
45%) |
|
Partially skimmed |
(if the content of FDM is above or equal to 10% and less than
25%) |
|
Skim |
(if the content of FDM is less than 10%) |
Notwithstanding the provisions of Section 4.7.1 of the General Standard for the Labelling of Prepackaged Foods (CODEX STAN 1-1985, Rev.1-1991; Codex Alimentarius, Volume 1A), the date of minimum durability need not be declared in the labelling of firm, hard and extra hard cheese which are not mould/soft-ripened and not intended to be purchased as such by the final consumer: in such cases the date of manufacture shall be declared.
7.4 LABELLING OF NON-RETAIL CONTAINERS
Information required in Section 7 of this Standard and Sections 4.1 to 4.8 of the General Standard for the Labelling of Prepackaged Foods (CODEX STAN 1-1985, Rev.1-1991; Codex Alimentarius, Volume 1A), and, if necessary, storage instructions, shall be given either on the container or in accompanying documents, except that the name of the product, lot identification, and the name and address of the manufacturer or packer shall appear on the container, and in the absence of such a container on the cheese itself. However, lot identification, and the name and address of the manufacturer or packer may be replaced by an identification mark, provided that such a mark is clearly identifiable with the accompanying documents.
8. METHODS OF SAMPLING AND ANALYSIS
8.1 SAMPLING
According to IDF Standard 50C:1995/ISO 707:1997/AOAC 968.12.
8.2 DETERMINATION OF MILKFAT CONTENT
According to IDF Standard 5B:1986/ISO 1735:1987/AOAC 933.05
8.3 DETERMINATION OF TOTAL SOLIDS CONTENT
According to IDF Standard 4A:1982/ISO 5534:1985.
8.4 DETERMINATION OF MOISTURE CONTENT
According to AOAC 926.08.
1. SCOPE
This Standard applies to all products intended for direct consumption or further processing, in conformity with the definition of whey cheese in Section 2 of this Standard. Subject to the provisions of this Standard, standards for individual varieties of whey cheese may contain provisions which are more specific than those in this Standard.
2. DESCRIPTION
Whey Cheese is the solid or semi-solid product obtained by the concentration of whey, with or without the addition of milk, cream or other raw materials of milk origin, and the moulding of the concentrated product.
3. ESSENTIAL COMPOSITION AND QUALITY FACTORS
3.1 RAW MATERIALS
Only raw materials specified in Section 2 of this Standard are permitted.
4. FOOD ADDITIVES
Only those food additives listed below may be used and only within the limits specified.
|
INS No. |
Name |
Maximum level |
|
|
200 |
Sorbic acid |
) |
|
|
201 |
Sodium sorbate |
) |
1 g/kg calculated as sorbic acid |
|
202 |
Potassium sorbate |
) |
|
5.1 HEAVY METALS
The products covered by this Standard shall comply with the maximum limits established by the Codex Alimentarius Commission.
5.2 PESTICIDE RESIDUES
The products covered by this Standard shall comply with the maximum residue limits established by the Codex Alimentarius Commission.
6. HYGIENE
6.1 It is recommended that the products covered by the provisions of this standard be prepared and handled in accordance with the appropriate Sections of the Recommended International Code of Practice - General Principles of Food Hygiene (CAC/RCP 1-1969, Rev.3-1997), and other relevant Codex texts such as Codes of Hygienic Practice and Codes of Practice.
6.2 From raw material production to the point of consumption, the products covered by this standard should be subject to a combination of control measures, which may include, for example, pasteurization, and these should be shown to achieve the appropriate level of public health protection.
6.3 The products should comply with any microbiological criteria established in accordance with the Principles for the Establishment and Application of Microbiological Criteria for Foods (CAC/GL 21-1997).
7. LABELLING
In addition to the provisions of the Codex General Standard for the Labelling of Prepackaged Foods (CODEX STAN 1-1985, Rev.1-1991; Codex Alimentarius, Volume 1A) and the General Standard for the Use of Dairy Terms[41], the following specific provisions apply:
7.1 NAME OF THE FOOD
The name of the food shall be whey cheese. However, the word “whey cheese” maybe omitted in the designation of an individual whey cheese variety reserved by a Codex standard for individual cheeses, and, in the absence thereof, a variety name specified in the national legislation of the country in which the product is sold, provided that the omission does not create an erroneous impression regarding the character of the food.
The designations may be combined with an indication of the fat content as follows:
|
|
Fat on the dry basis* |
|
Creamed whey cheese |
minimum 33% |
|
Whey cheese |
minimum 10% and less than 33% |
|
Skimmed whey cheese |
less than 10% |
*) The dry matter content of whey cheese includes water of crystallization of the lactose.
[A word or words denoting the animal or, in the case of a mixture, all the animals from which the milk has been derived, should be inserted immediately before or after the designation of the product. Such declarations are not required if the consumer would not be misled by their omission.] [42]
7.2 DECLARATION OF MILKFAT CONTENT
The milkfat content shall be declared in a manner found acceptable in the country of sale to the final consumer, either (i) as a percentage by mass or volume, (ii) as a percentage of fat in dry matter, or (iii) in grams per serving, provided that the number of servings is stated.
7.3 LABELLING OF NON-RETAIL CONTAINERS
Information required in Section 7 of this Standard and Sections 4.1 to 4.8 of the General Standard for the Labelling of Prepackaged Foods (CODEX STAN 1-1985, Rev.1-1991; Codex Alimentarius, Volume 1A), and, if necessary, storage instructions, shall be given either on the container or in accompanying documents, except that the name of the product, lot identification, and the name and address of the manufacturer or packer shall appear on the container. However, lot identification, and the name and address of the manufacturer or packer may be replaced by an identification mark, provided that such a mark is clearly identifiable with the accompanying documents.
8. METHODS OF SAMPLING AND ANALYSIS
8.1 SAMPLING
According to IDF Standard 50C:1995/ISO 707:1997/AOAC 968.12.
8.2 DETERMINATION OF MILKFAT CONTENT
According to IDF Standard 59A:1986/ISO 1854:1987/AOAC 974.09.
8.3 DETERMINATION OF DRY MATTER CONTENT
According to IDF Standard 58:1970/ISO 2920:1974.
1. SCOPE
This Standard applies to Cheeses in Brine, intended for direct consumption or further processing, in conformity with the description in Section 2 of this Standard. Subject to the provisions of this Group Standard, standards for individual varieties of Cheeses in Brine may contain provisions which are more specific than those in this Standard.
2. DESCRIPTION
Cheeses in Brine are semi-hard to soft ripened cheeses in conformity with Standard A-6. The body has a white to yellowish colour and a compact texture suitable for slicing, with none to few mechanical openings. The cheeses have no actual rind and have been ripened and preserved in brine until delivered to, or prepacked for, the consumer. Certain individual Cheeses in brine contain specific herbs and spices as part of their identity.
3. ESSENTIAL COMPOSITION AND QUALITY FACTORS
3.1 RAW MATERIALS
Milk and/or products obtained from milk.
3.2 PERMITTED INGREDIENTS
- Starter cultures of harmless lactic acid and/or flavour producing bacteria and cultures of other harmless microorganisms3.3 COMPOSITION- Safe and suitable enzymes
- Sodium chloride
- Potable water
- Herbs and spices where part of the identity of the Cheese in Brine.
|
|
Soft |
Semi-hard |
|
Minimum fat in dry matter, %: |
40 |
40 |
|
Minimum dry matter, %: |
40 |
52 |
Only those food additives listed may be used and only within the limits specified.
Additives not listed below but provided for in individual standards for varieties of Cheeses in Brine may also be used for similar types of cheese within the limits specified within those standards.
|
INS No. |
Name |
Maximum level |
|
|
Acidity regulators |
|
|
270 |
Lactic acid (L-, D- and DL-) |
Limited by GMP |
|
575 |
Glucono delta-lactone |
Limited by GMP |
5.1 HEAVY METALS
The products covered by this Standard shall comply with the maximum limits established by the Codex Alimentarius Commission.
5.2 PESTICIDE RESIDUES
The products covered by this Standard shall comply with the maximum residue limits established by the Codex Alimentarius Commission.
6. HYGIENE
6.1 It is recommended that the products covered by the provisions of this standard be prepared and handled in accordance with the appropriate Sections of the Recommended International Code of Practice - General Principles of Food Hygiene (CAC/RCP 1-1969, Rev.3-1997), and other relevant Codex texts such as Codes of Hygienic Practice and Codes of Practice.
6.2 From raw material production to the point of consumption, the products covered by this standard should be subject to a combination of control measures, which may include, for example, pasteurization, and these should be shown to achieve the appropriate level of public health protection.
6.3 The products should comply with any microbiological criteria established in accordance with the Principles for the Establishment and Application of Microbiological Criteria for Foods (CAC/GL 21-1997).
7. LABELLING
In addition to the provisions of the Codex General Standard for the Labelling of Prepackaged Foods (CODEX STAN 1-1985, Rev.1-1991; Codex Alimentarius, Volume 1A) and the General Standard for the Use of Dairy Terms[43], the following specific provisions apply:
7.1 NAME OF THE FOOD
The name of the food shall be Cheese in Brine. However, the word “Cheese in Brine” may be omitted in the designation of an individual Cheese in Brine variety reserved by a Codex standard for individual Cheese in Brine, and, in the absence thereof, a variety name specified in the national legislation of the country in which the product is sold, provided that the omission does not create an erroneous impression regarding the character of the food.
[A word or words denoting the animal or, in the case of a mixture, all the animals from which the milk has been derived, should be inserted immediately before or after the designation of the product. Such declarations are not required if the consumer would not be misled by their omission.] [44]
7.2 DECLARATION OF MILKFAT CONTENT
The milkfat content shall be declared in a manner found acceptable in the country of sale to the final consumer, either (i) as a percentage by mass or volume, (ii) as a percentage of fat in dry matter, or (iii) in grams per serving, provided that the number of servings is stated.
Additionally, the following terms may be used:
|
High fat |
(if the content of FDM is above or equal to 60%); |
|
Full fat |
(if the content of FDM is above or equal to 45% and less than
60%) |
|
Medium fat |
(if the content of FDM is above or equal to 25% and less than
45%) |
|
Partially skimmed |
(if the content of FDM is above or equal to 10% and less than
25%) |
|
Skim |
(if the content of FDM is less than 10%) |
Information required in Section 7 of this Standard and Sections 4.1 to 4.8 of the General Standard for the Labelling of Prepackaged Foods (CODEX STAN 1-1985, Rev.1-1991; Codex Alimentarius, Volume 1A), and, if necessary, storage instructions, shall be given either on the container or in accompanying documents, except that the name of the product, lot identification, and the name and address of the manufacturer or packer shall appear on the container. However, lot identification, and the name and address of the manufacturer or packer may be replaced by an identification mark, provided that such a mark is clearly identifiable with the accompanying documents.
8. METHODS OF SAMPLING AND ANALYSIS
8.1 SAMPLING
According to IDF Standard 50C:1995/ISO 707:1997/AOAC 933.12.
Special requirements for cheese in brine: A representative piece of cheese is placed on a cloth or on a sheet of non-absorbent paper for 5 to 10 min. A slice of 2-3 cm is cut off and sent to the laboratory in a sealed insulated box for analysis.
8.2 DETERMINATION OF MILKFAT CONTENT
According to IDF Standard 5B:1986/ISO 1735:1987/AOAC 933.05.
8.3 DETERMINATION OF DRY MATTER CONTENT
According to IDF Standard 4A:1982/ISO 5534:1985 or AOAC 926.08.
1. Requirements/Specifications in standards (except food additives)
|
COMMODITY |
PROVISION |
METHOD |
PRINCIPLE |
Note |
|
Milk Products |
Copper |
IDF Standard 76A:1980 |
Photometry, diethyldiethiocarbamate |
Type III |
|
Milk Products |
Copper |
AOAC 971.20 (Codex general method) |
Atomic absorption spectrophotometry |
Type II |
|
Milk Products |
Fat |
IDF Standard 126A:1988 |
Gravimetry (Weibull- Berntrop) |
|
|
Milk Products |
Iron |
IDF Standard 103A:1986 |
Photometry, bathophenanthroline |
Type IV |
|
Milk Products |
Iron |
NMKL 139.1991 (Codex general method) |
Atomic absorption spectrophotometry |
Type II |
|
Milk Products |
Sampling |
IDF Standard 50C:1995 |
General instructions |
- |
|
Milk Products |
Sampling |
IDF Standard 113A:1990 |
Inspection by attributes |
- |
|
Milk Products |
Sampling |
IDF Standard 136A:1992 |
General instructions |
- |
|
Milk Products |
Sampling of milk from Bulk Tanks |
AOAC 970.26 |
|
- |
|
Butter |
Lead |
AOAC 972.25 (Codex general method) |
Atomic absorption spectrophotometry |
Type II |
|
Butter |
Milk solids-not-fat |
IDF Standard 80:1977 |
Gravimetry |
Under revision |
|
Butter |
Milkfat |
IDF Standard 80:1977 |
Gravimetry |
Under revision |
|
Butter |
Sampling |
IDF Standard 50C:1995 |
General instructions |
- |
|
Butter |
Water |
IDF Standard 80:1977 |
Gravimetry |
Under revision |
|
Cheese |
Fat |
IDF Standard 5B:1986 |
Gravimetry (Röse- Gottlieb) |
Under revision |
|
Cheese |
Moisture |
AOAC 926.08 |
Gravimetry, vacuum oven |
|
|
Cheese |
Sampling |
IDF Standard 50C:1995 |
General instructions |
Under revision |
|
Cheese |
Solids |
IDF Standard 4A:1982 |
Gravimetry, drying at102°C |
|
|
Cheese |
Solids |
AOAC 926.08 |
vacuum oven |
|
|
Cheese, C |
Dry matter |
Method to be determined |
|
|
|
Cheeses in Brine |
Dry matter |
IDF Standard 4A:1982 |
Gravimetry, drying at102°C |
|
|
Cheeses in Brine |
Dry matter |
AOAC 926.08 |
Gravimetry, vacuum oven |
|
|
Cheeses in Brine |
Fat in dry matter |
IDF Standard 5B:1986 |
Gravimetry (Röse- Gottlieb) |
Under revision |
|
Cream |
Caseinates |
NOT SUSCEPTIBLE TO ANALYSIS |
|
|
|
Cream |
Gelatin and starch |
AOAC 920.112 |
|
qualitative test for presence or absence |
|
Cream |
Heat treatment |
Method to be determined |
|
when provision is specified |
|
Cream |
Milk solids-not-fat |
TO BE CHECKED |
|
|
|
Cream |
Milkfat |
IDF Standard 16C:1987 |
Gravimetry (Röse- Gottlieb) |
Under revision |
|
Cream |
Sampling |
IDF Standard 50C:1995 |
General instructions |
- |
|
Dairy Spreads |
Fat |
TO BE CHECKED |
|
|
|
Dairy Spreads |
Lead |
Method to be determined |
|
|
|
Edible Casein Products |
Ash (including P2O5) |
IDF Standard 90:1979 |
Furnace, 825°C |
|
|
Edible Casein Products |
Casein in protein |
Method in development |
|
|
|
Edible Casein Products |
Free acid |
IDF Standard 91:1979 |
Titrimetry, aqueous extract |
Type IV |
|
Edible Casein Products |
Lactose |
IDF Standard 106:1982 |
Photometry, phenol and H2SO4 |
Type IV |
|
Edible Casein Products |
Lead |
IDF Standard 133A:1992 |
Spectrometry, 1,5- diphenylthiocarbazone |
|
|
Edible Casein Products |
Lead |
AOAC 972.25 (Codex general method) |
Atomic absorption spectrophotometry |
Type II |
|
Edible Casein Products |
Lead |
AOAC 982.23 (Codex general method) |
Anodic Stripping Voltammetry |
Type III |
|
Edible Casein Products |
Lead |
NMKL 139.1991 (Codex general method) |
Atomic absorption spectrophotometry |
Type III |
|
Edible Casein Products |
Milkfat |
IDF Standard 127A:1988 |
Gravimetry (Schmidt- Bondzynski-Ratslaff) |
|
|
Edible Casein Products |
Moisture |
IDF Standard 78C:1991 |
Gravimetry, drying at102°C |
Under revision |
|
Edible Casein Products |
pH |
IDF Standard 115A:1989 |
Electrometry |
Type IV |
|
Edible Casein Products |
Protein (total N x 6.38 in dry matter) |
IDF Standard 92:1979 |
Titrimetry, Kjeldahl |
Type IV |
|
Edible Casein Products |
Sampling |
IDF Standard 50C:1995 |
General instructions |
- |
|
Edible Casein Products |
Sediment (scorched particles)(in 25 g) |
IDF Standard 107A:1995 |
Visual comparison with standard disks, after
filtration |
Type IV |
|
Evaporated Milks |
Milk solids-not-fat |
Method to be determined |
|
|
|
Evaporated Milks |
Milkfat |
IDF Standard 13C:1987 |
Gravimetry (Röse- Gottlieb) |
Under revision |
|
Evaporated Milks |
Protein (in milk solids-not-fat) |
AOAC 945.48H |
Kjeldahl |
|
|
Evaporated Milks |
Sampling |
IDF Standard 50C:1995 |
General instructions |
- |
|
Evaporated Milks |
Solids |
IDF Standard 21B:1987 |
Gravimetry, drying at 98-100°C |
|
|
Fermented Milks |
[Milk solids-not-fat |
Method to be determined |
|
when provision is specified |
|
Fermented Milks |
[Protein in milk solids-not-fat |
Method to be determined |
|
|
|
Fermented Milks |
Lactic acid |
IDF Standard 150:1991 |
Potentiometry |
|
|
Fermented Milks |
Lactic acid |
AOAC 937.05 |
Spectrophotometric |
|
|
Fermented Milks |
Protein |
IDF Standard 20B:1993 |
Titrimetry (Kjeldahl) |
|
|
Fermented Milks |
Sampling |
IDF Standard 50C:1995 |
General instructions |
- |
|
Fermented Milks |
Lactobacillus acidophilus |
Method in development |
|
|
|
Fermented Milks (Cultured milk, Cultured buttermilk) |
Mesophilic lactic acid producing bacteria, either single
culture or mixed cultures |
Method(s) to be determined |
|
|
|
Fermented Milks |
Bifidobacteria |
Method in development |
|
|
|
Fermented Milks (Kefir) |
Kluyveromyces marxianus, Saccharomyces omnisporus, S.
cerevisiae & S. exiguus |
Method to be determined |
|
|
|
Fermented Milks (Kefir) |
Lactobacillus kefir and species of
Leuconostoc, Lactococcus & Acetobacter |
Method to be determined |
|
|
|
Fermented Milks (Kumys) |
Kluyveromyces marxianus |
Method to be determined |
|
|
|
Fermented Milks (Kumys) |
Lactobacillus delbrueckii subsp.
bulgaricus |
Method to be determined |
|
|
|
Fermented Milks |
Streptococcus thermophilus & Lactobacillus
delbrueckii subsp. bulgaricus |
IDF Standard 117A:1988 |
Colony count at 37°C |
|
|
Fermented Milks |
Streptococcus thermophilus & Lactobacillus
delbrueckii subsp. bulgaricus |
IDF Standard 146:1991 |
Test for identification |
Under revision |
|
Milk and Cream Powders |
Milkfat |
IDF Standard 9C:1987 |
Gravimetry (Röse- Gottlieb) |
Under revision |
|
Milk and Cream Powders |
Protein in milk solids-not-fat |
IDF Standard 20B:1993 |
Titrimetry, Kjeldahl |
Type IV |
|
Milk and Cream Powders |
Sampling |
IDF Standard 50C:1995 |
General instructions |
- |
|
Milk and Cream Powders |
Water |
IDF Standard 26A:1993 |
Gravimetry, drying at102°C |
Type IV |
|
Milk Products obtained from Fermented Milks Heat-Treated after
Fermentation |
[Milk solids-not-fat(no level specified)] |
Method to be determined |
|
when provision is specified |
|
Milk Products obtained from Fermented Milks Heat-Treated after
Fermentation |
[Protein in milk solids-not-fat>=34%] |
Method to be determined |
|
|
|
Milk Products obtained from Fermented Milks Heat-Treated after
Fermentation |
[Solids-not-fat(no level specified)] |
Method to be determined |
|
|
|
Milk Products obtained from Fermented Milks Heat-Treated after
Fermentation |
Protein |
IDF Standard 20B:1993 |
Titrimetry (Kjeldahl) |
|
|
Milk Products obtained from Fermented Milks Heat-Treated after
Fermentation |
Sampling |
IDF Standard 50C:1995 |
General instructions |
- |
|
Milkfat Products |
Certain antioxidants |
IDF Standard 165:1993 |
Reversed phase gradient light chromatography |
Under revision |
|
Milkfat Products |
Free fatty acids (expressed as oleic acid) |
IDF Standard 6B:1989 |
Titrimetry |
|
|
Milkfat Products |
Milkfat |
IDF Standard 24:1964 |
Gravimetry (calculation for solids-not-fat and water
content) |
Type IV |
|
Milkfat Products |
Peroxide value (expressed as milliequivalents of oxygen/kg
fat) |
IDF Standard 74A:1991 |
Photometry, |
Type IV |
|
Milkfat Products |
Peroxide value |
AOAC 965.33 |
Titrimetry |
|
|
Milkfat Products |
Sampling |
IDF Standard 50C:1995 |
General instructions |
- |
|
Milkfat Products |
Vegetable fat |
IDF Standard 32:1965 |
Phytosteryl acetate test |
Under revision |
|
Milkfat Products |
Vegetable fat (sterols) |
IDF Standard 54:1970 |
GLC |
Under revision |
|
Milkfat Products |
Water |
IDF Standard 23A:1988 |
Titrimetry (Karl Fischer) |
|
|
Processed Cheese Products |
Dry matter |
IDF Standard 4A:1982 |
Gravimetry, drying at102°C |
|
|
Processed Cheese Products |
Dry matter |
AOAC 926.08 |
Gravimetry, vacuum oven |
|
|
Processed Cheese Products |
Gelatin and starch |
AOAC 940.24 (cottage cheese) |
|
qualitative test for presence or absence |
|
Processed Cheese Products |
Milkfat (dry basis) |
IDF Standard 5B:1986 |
Gravimetry (Röse- Gottlieb) |
Under revision |
|
Sweetened Condensed |
Milk solids-not-fat |
Method to be determined |
|
|
|
Sweetened Condensed |
Milkfat |
IDF Standard 13C:1987 |
Gravimetry (Röse- Gottlieb) |
Under revision |
|
Sweetened Condensed |
Protein (in milk solids-not-fat) |
AOAC 920.115G |
Titrimetry, Kjeldahl |
|
|
Sweetened Condensed |
Sampling |
IDF Standard 50C:1995 |
General instructions |
- |
|
Sweetened Condensed |
Solids |
IDF Standard 15B:1991 |
Gravimetry, drying at102°C |
Type IV |
|
Sweetened Condensed |
Solids |
AOAC 920.115D |
Gravimetry, vacuum oven |
|
|
Sweetened Yoghurt |
Ethanol |
Method to be determined |
|
|
|
Unripened Cheese |
Glassine and starch |
Method to be determined |
|
|
|
Unripened Cheese |
Dry matter |
IDF Standard 4A:1982 |
Gravimetry, drying at102°C |
|
|
Unripened Cheese |
Dry matter |
AOAC 926.08 |
Gravimetry, vacuum oven |
|
|
Unripened Cheese |
Fat in dry matter |
Method to be determined |
|
|
|
Unripened Cheese |
Protein |
IDF Standard 20B:1993 |
Titrimetry, Kjeldahl |
|
|
Whey Cheese |
Fat (dry basis) |
IDF Standard 59A:1986 |
Gravimetry (Röse- Gottlieb) |
Under revision |
|
Whey Cheese |
Sampling |
IDF Standard 50C:1995 |
General instructions |
- |
|
Whey Powders |
Ash |
IDF Standard 90:1979 |
Furnace, 825°C |
|
|
Whey Powders |
Fat |
IDF Standard 9C:1987 |
Gravimetry (Röse- Gottlieb) |
Under revision |
|
Whey Powders |
Lactose (expressed as anhydrous lactose) |
IDF Standard 79B:1991 |
Enzymatic method; |
Under revision |
|
Whey Powders |
Lead |
AOAC 972.25 (Codex general method) |
Atomic absorption spectrophotometry |
|
|
Whey Powders |
Moisture, “Free” |
IDF Standard 58:1970 |
Gravimetry, drying at88°C |
|
|
Whey Powders |
pH (in 10% solution) |
TO BE CHECKED |
|
|
|
Whey Powders |
Protein (Total N x 6.38) |
IDF Standard 92:1979 |
Titrimetry, Kjeldahl |
Type IV |
|
Whey Powders |
Sampling |
IDF Standard 113A:1990 |
Inspection by attributes |
- |
|
Whey powders |
Sampling |
IDF Standard 50C:1995 |
General instructions |
- |
|
COMMODITY |
PROVISION |
METHOD |
PRINCIPLE |
Note |
|
Processed Cheese |
Added phosphate (expressed as phosphorus) |
IDF Standard 51B:1991 |
Calculation |
Type IV |
|
Processed Cheese |
Citrate emulsifying agents |
IDF Standard 52A:1992 |
Calculation from citric acid & lactose contents |
Type IV |
|
Cheese and Processed |
Citric acid |
IDF Standard 34C:1992 |
Enzymatic |
|
|
Cheese and Processed |
Citric acid |
AOAC 976.15 |
Photometry |
|
|
Cheese (and cheese rind) |
Natamycin |
IDF Standard 140A:1992 |
Molecular absorption spectrometry & HPLC |
|
|
Processed Cheese |
Phosphorus |
IDF Standard 33C:1987 |
Spectrophotometry, molybdate-ascorbic acid |
|