8a
| Strategy | θt | θt-1 | Atθt & θt-1 |
|---|---|---|---|
| 8 | G | G | H |
| P | H | ||
| P | G | L | |
| P | L |
8b
| Pr (θtθt& At) | |||||
|---|---|---|---|---|---|
| θt | |||||
| Strategy | θt | At | G | P | C |
| 8 | G | H | .90 | .10 | .00 |
| L | .90 | .10 | .00 | ||
| Z | .00 | .00 | .00 | ||
| P | H | .10 | .90 | .00 | |
| L | .10 | .90 | .00 | ||
| Z | .00 | .00 | .00 | ||
| C | H | .05 | .95 | .00 | |
| L | .05 | .95 | .00 | ||
| Z | .00 | .00 | .00 | ||
9a
| Strategy | θt | θt-1 | Atθt & θt-1 |
|---|---|---|---|
| 8 | G | G | H |
| P | H | ||
| C | H | ||
| P | G | L | |
| P | L | ||
| C | L | ||
| C | G | Z | |
| P | Z | ||
| C | Z |
9b
| Pr (θtθt& At) | |||||
|---|---|---|---|---|---|
| θt | |||||
| Strategy | θt | At | G | P | C |
| 9 | G | H | .70 | .28 | .02 |
| L | .70 | .28 | .02 | ||
| Z | .70 | .28 | .02 | ||
| P | H | .30 | .65 | .05 | |
| L | .30 | .65 | .05 | ||
| Z | .30 | .65 | .05 | ||
| C | H | .10 | .20 | .70 | |
| L | .10 | .20 | .70 | ||
| Z | .10 | .20 | .70 | ||
10a
| Strategy | θt | θt-1 | Atθt & θt-1 |
|---|---|---|---|
| 10 | G | G | H |
| P | H | ||
| C | H | ||
| P | G | L | |
| P | L | ||
| C | L | ||
| C | G | Z | |
| P | Z | ||
| C | Z |
10b
| Pr (θtθt& At) | |||||
|---|---|---|---|---|---|
| θt | |||||
| Strategy | θt | G | P | C | |
| 10 | G | H | .90 | .09 | .01 |
| L | .90 | .09 | .01 | ||
| Z | .90 | .09 | .01 | ||
| P | H | .10 | .88 | .02 | |
| L | .10 | .88 | .02 | ||
| Z | .10 | .88 | .02 | ||
| C | H | .05 | .10 | .85 | |
| L | .05 | .10 | .85 | ||
| Z | .05 | .10 | .85 | ||
Table 11: Summary of the characteristics and expected payoff, EMV (in units of net present value), for ten hypothetical strategies under each of three stock-recruitment hypotheses (see Table 1). These expected payoffs were computed for a three year time horizon with a 10% annual discount rate. The payoff in each year, given a partic- ular 0 and A, was the same as for the one year example (see Figure 2).
| EMV (as net present value) | ||||||||
|---|---|---|---|---|---|---|---|---|
| S-R Hypothesis | ||||||||
| Characteristics of Strategy | 1 | 2 | 3 | |||||
| Strategy | Prescriptive (P) or Adaptive (A) | Timing of Estimate of θ | Allows for Catastrophic Recruitment | Sampling Intensity | Relative Cost | No Catastrophes No S-R Relationship | No Catastrophes S-R Relationship | Catastrophes S-R Relationship |
| 1 | P | - | - | 27 | 8 | -1 | ||
| 2 | P | - | 21 | 18 | 15 | |||
| 3 | A | t-1 | no | low | 1 | 20 | 16 | 11 |
| 4 | A | t-1 | no | high | 2 | 17 | 15 | 10 |
| 5 | A | t-1 | yes | low | 1 | 19 | 15 | 10 |
| 6 | A | t-1 | yes | high | 2 | 17 | 14 | 9 |
| 7 | A | t | no | low | 2 | 25 | 15 | 10 |
| 8 | A | t | no | high | 3 | 29 | 19 | 13 |
| 9 | A | t | yes | low | 2 | 24 | 14 | 9 |
| 10 | A | t | yes | high | 3 | 29 | 19 | 14 |
Information on the sampling procedure and the estimation method is contained in the conditional sampling distribution of the estimate Ôt, Viz, Pr (ÔtÔt, At). Further, the decision rules may depend upon Ôt, and Ôt-1, Viz, At= f (Ôt, Ôt-1). To show the possibilities of these analyses, the results are shown in Table 11 and from the Table we can see that the decision maker has many options and approaches which he can use to structure the management process. While the results are presented in terms of EMV it should be easy to see that they can be presented in terms of utility as well.
DISCUSSION
There is a growing perception that traditional fisheries management does not work as well. It appears to be inefficient. That is, social and economic benefits produced as a result of management are not sufficient to warrant costs, and management schemes are often associated with political strife and contention.
Various causes have been ascribed to this malaise. For example the “open-access” regime was thought to be a major obstacle preventing efficient and contention-free management. While the importance of the nature of access cannot be denied, it is now generally thought that the key constraint toward effective management is the inefficient way in which governments exert their management authorities.
The problem is quite complex. The critical difficulty is of course to change the socio-economic conditions that foster poor management. Many believe that an improvement in our understanding of fishery dynamics will be a prerequisite to improving the socio-economic conditions. On the other hand without improvement in these conditions it will be difficult to obtain the resources to improve our understanding of fishery dynamics.
This dilemma is further strengthened because managers are constrained by traditional models. The traditional production-model, yield-per-recruit, and stock and recruitment approaches produce results which are only useful for the “average year” and do not provide needed guidance for year-to-year strategies. Extraordinarily large and small year classes occur typically and the conventional management algorithms are not structured to account for these aberrancies. Further the ultimate goals of management are generally economic in nature and yet the deficiencies in biological models are certainly no worse than the deficiencies in economic models, particularly those of estimating supply curves. It is obvious that the development of obtaining new data and information which can be used to structure and formulate the new models.
Since the traditional models cannot predict more than, “on the average events”, generally based upon past environmental conditions, the nature of which are generally not understood, the critical problems of stockcollapse,effectsof environmental modification, and stock rebuilding are addressedonly superficially.
It appears that one major constraint in solving these problems is the optimal allocation of budgetary and human resources to each management activity. Obviously the most efficient approach for allocating resources to the variuos components of management is to examine management under uncertainty. An examination and analysis of the entire process will reveal those elements that are relatively important and those that need special attention.
The beauty of the decision-theory approach is that it enables such a “first-cut comparison” (i.e. magnitude of the costs and benefits) of various management structures *e.g. timing of management activities, hypotheses on population dynamics, and sampling schemes) for different management philosophies (e.g. EMV and expected utility) for various probability structures (i.e. varying degrees of entropy).
The first cut comparison can then be used to initialize iterative improvement of the management activities posed in Figure 1.
It is easy to see that in addition to formalizing the structure the decision theory approach requires a particular examination of 1) states of Nature, 2) alternative actions, 3) varius metrics of performance such as catch or economic values, 4) discount rates, 5) utility functions, 6) hypotheses on population dynamics including those on stock-and rectruitment, 7) and the identification of appropriate planning horizons.
Thus to address technical issues of management we must consider management as an entity in its own right. More specifically, the task of fishery managers involves the design of fishery management systems. The ten strategies articulated in this paper are in fact rudimentary fishery management systems which can be applied in real world fisheries, not only for management in the more narrow sense, but for management in the broader sense where the kinds and quality of information are carefully specified.
ACKNOWLEDGEMENTS
This paper was motivated by my (BJR) study of the Namibian pilchard fishery. I am indebted to John Gulland for his assistance.
REFERENCES
FAO. 1980. Report of the ACMRR Working Party on the Scientific Basis of Determining Management Measures. Rome, 1980. FAO Fish.Rep. (236):149 p.
Keeney, R.L. and H. Raiffa. 1976. Decisions with Multiple Objectives: Preferences and Value Tradeoffs. John Wiley and Sons, New York. 569 p.
by
Loh-Lee Low
Northwest and Alaska Fisheries Center
National Marine Fisheries Service
National Oceanic and Atmospheric Administration
2725 Montlake Boulevard East
Seattle, Washington 98112
USA
Resumen
En la evaluación y ordenación de los recursos de especies de fondo del Mar de Bering se ha dado un gran valor práctico al uso de modelos de ecosis- temas como medio para mejorar el análisis tradicional por especies de los datos de la pesquería. En este trabajo se muestra que el modelo de Laevastu- Larkins, el modelo PROBUB, puede ser utilizado para evaluar las tendencias a largo tiempo de los niveles de población de los varios grupos de peces de fondo y que su uso puede determinar cambios en el establecimiento de las re- gulaciones de cuotas de captura. El uso del modelo ha dado por resultado que al establecer cuotas de capturas se tomen en consideración aspectos que son diferentes de aquéllos provenientes de estimados basados en análisis mono- específicos de cuatro grupos de especies. El modelo sugiere que puede ser poco realísta tratar de reconstruir dos stocks a sus niveles altos debido a que los niveles bajos actuales corresponden al nivel normal a largo tiempo. El modelo también ha mostrado que las comunidades de peces de fondo fluctuan en promedio en un 22% en un ciclo de cinco a seis años. La mayor fluctuación para una de las especies componentes es tan alta como el 50% en tres años. Todos los otros grupos de especies, a excepción del grupo de peces de rocas de vida larga, muestran fluctuaciones del 8 al 30% en ciclos de cinco a diez años. El modelo sin embargo no puede todavía remplazar los métodos de análi- sis tradicional mono-específicos para la ordenación de la pesquería, ya que estos análisis proporcionan un gran número de detalles esenciales sobre los cambios de año a año en la tendencia general, en la composición por especies, la estructura por edades, y la característica de la dinámica de población de cada stock.
INTRODUCTION
Large variations in production of neritic fish resources have been well illustrated throughout this meeting. Similar variations, perhaps of smaller amplitude and notoriety, occur commonly in the demersal and semi-demersal fisheries resources as well. A case to be illustrated here is the Bering Sea groundfish resource. Over the past thirty years, catches of groundfish have fluctuated through several distinct cycles (Bakkala et al., 1979). A peak catch of 750,000 t in 1961 dominated by yellowfin sole Limanda aspera was attained in the first obvious cycle (Figure 1). This was followed by a record catch of 2.2 million t in 1972 when the dominant species had switched to walleye pollock Theragra halcogramma. Since 1977, catches have varied in a relatively narrower range between 1.2 and 1.4 million t, largely as a result of imposed catch quotas. The quotas stabilized catches that would otherwise follow wide natural fluctuations in abundance of the stocks.
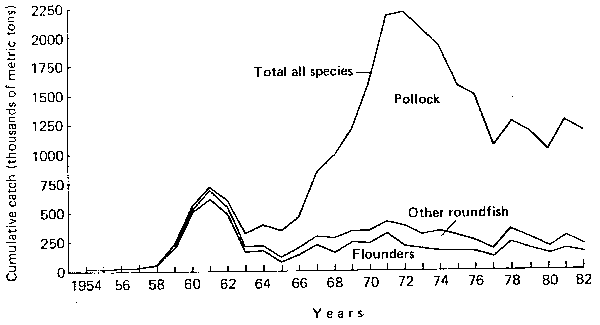
Figure 1. Groundfish catches in the Boring Sea, 1954-82.
General major causes for such natural variations in the production of groundfish in the Bering Sea have been discussed by Laevastu et al. (1982). They noted that besides external factors, such as effects of temperature anomalies and fishing; ecosystem internal factors like predation, cannibalism, competition, and migration can contribute signif- icantly to the fluctuations of population biomass.
More specific year-to-year variations can be illustrated from status of stocks assessments made on a species-by-species basis as typically reported in Bakkala and Low (1982)1. These assessments depend largely on single species analytical techniques like empirical evaluations of catch-per-unit-effort trends, and the more sophisticated population dynamics analyses of the data. They provide estimates of potential yield from the resources that largely reflect current and short-term trends, but lack the longer-term effects of fisheries exploitation from an ecosystem context.
This paper will illustrate the use of an ecosystem model developed at the Northwest and Alaska Fisheries Center in Seattle by Dr. Taivo Laevastu (Laevastu and Larkins, 1981) to evaluate some of these effects of fisheries exploition. The model has been used to supplement traditional species-by-species assessments and its results have led to alternative considerations for catch quotas that are derived through single species analyses of the Bering Sea groundfish resources.
1 Bakkala, R.G., and L.L. Low (eds.). 1982. Condition of groundfish resources of the eastern Bering Sea and Aleutian Islands region in 1982. Northwest and Alaska Fish. Cent., Natl. Mar. Fish. Serv., 2725 Montlake Blvd. E., Seattle, WA 98112. (Doc submitted to the Intl. North Pacific Fish. Comm. in Oct. 1982).
STOCK ASSESSMENT AND MANAGEMENT
Although the Bering Sea groundfish fishery is largely a trawl fishery and is multispecies in character, assessment and management of the resources have been approached on a species-by-species basis. The status of individual stocks have been determined for each species or species group and an optimum yield (OY) set for each unit. These OY's eventually become annual catch quotas for management. When the catch quota for a species is reached, the entire fishery or that of a particular group may be subject to closure, even though abundance of the species may have changed to dictate otherwise from the time the quota was set a year or two earlier. This management process under the present fishery management plan (FM Plan) does not, therefore, allow a quick change of the regulations. Instead, the FM Plan requires a lengthy and elaborate review process for a proposed change to be made.
In order to improve on this management system, an amendment to the FM Plan has been developed to manage the groundfish complex as a unit while allowing adjustments to the catch of individual species according to fluctuations in their abundance. The amendment allows the annual OY of the groundfish complex to be set within a 1.4 to 2.0 million t range at the beginning of each year without a lengthy amendment process each time. The OY range has been set to reflect the present yield potential of the resource and the current socio-economic preference of the fishing industry and management to harvest at that range. At the time annual OY is set, catch quotas for the individual component species within the complex are also set to reflect their current status of stocks.
The latest status of stocks analyses reported by Bakkala and Low (1982) 1 show that the annual surplus production (ASP) of the individual species groups add up to more than 2 million t (Table 1). The term ASP is defined as the annual yield that may be taken without changing the biomass of the stock the following year. The estimates, therefore, reflect the current and short term yield from the resources.
| Annual surplus | ||
|---|---|---|
| Species group | production | Abundance trend |
| (1,000 t) | ||
| Pollock | 1,300.0 | Average abundance, on declining |
| trend | ||
| Pacific cod | 298.0 | Historic high abundance, |
| expected to decline rapidly | ||
| Yellowfin sole | 200.0 | Historic high abundance, |
| stable | ||
| Turbots | 85.0 | Average abundance, stable |
| Other flatfish | 120.0 | Average abundance, stable |
| Sablefish | 2.9 | Low abundance, stable |
| Other rockfish | 14.1 | Average abundance, stable |
| Atka mackerel | 26.0 | Average abundance, stable |
| Total groundfish | 2,057.8 | High abundance, expected to |
| decline slightly |
Pollock is clearly the most dominant and productive species in the goundfish complex. Its ASP of 1.3 million t for 1983 accounts for 63% of the total production. The ASP for Pacific cod Gadus macrocephalus is next highest (298,00 t) due to the presence of unusually strong year classes and accounts for 15% of the groundfish production. Yellowfin sole production is a close third at 200,000 t, or 10% of total production. The rest of the flatfishes (turbots and other flatfish) account for an ASP of 205,000 t, or another 10% of total production. The turbot group is made up of arrowtooth flounder Atheresthes stomias and Greenland turbot Reinhardtius hippoglossoides. The other flatfish group is mainly made up of Alaska plaice Pleuronectes quadrituberculatus, rock sole Lepidopsetta bilineata, and flathead sole Hippoglossoides elassadon. The rest of the species--Pacific ocean perch Sebastes alutus, other rockfishes Sebastes spp, and Sebastolobus spp., stable- fish Anaplopoma fimbria, and Atka mackerel Pleurogrammus monopterygius -- account for the remaining 2% of the total groundfish production.
Normally, the species-by-species analyses on the status of stocks and ASP's summarized in Table 1 are sufficient basis for setting catch quotas. However, since ecosystem models have been developed to the extent that they can now be used to evaluate long term trends of the groundfish complex, they have been applied to supplement the species-by-species analyses for stock assessment and management.
ECOSYSTEM ANALYSIS
Various computer simulation models have been developed to describe the interrela- tionship and dynamics of the Bering Sea ecosystem. Two widely tested models are the Prognostic Bulk Biomass (PROBUB) and the Dynamical Numerical Marine Ecosystem (DYNUMES) models developed at the Northwest and Alaska Fisheries Center and described by Laevastu and Larkins (1981). Both models compute the transfer of biomass between trophic levels given natural and man-induced processes that occur in the ecosystem. The PROBUB model is a more detailed representation of its dynamics. To evaluate the long-term consequences of harvesting in this paper, the PROBUB model was used because of its greater simplicity over the DYNUMES model.
PROBUB simulates the dynamics of the ecological components (marine birds, marine mammals, groundfish, pelagic fish, and benthic organisms) and the principal individual species within these components. The model takes into account the biomass of each species group, the prey-predator relationships among these groups and tracks their change in biomass in specific areas and time steps. The input data required to initiate the simulations were described by Laevastu et al. (1980) 2. These input data are abundance of each species group, their food requirements in terms of species and quantities, and the effects of growth and mortality on the biomass and distribution of species groups.
Several series of simulations were made to evaluate the natural equilibrium and effects of fishing on the ecosystem. There were three series of basic simulations which provided the long-term equilibrium bounds for the populations.
Equilibrium Biomass
Starting with the present species make-up and their prey-predator relationships. PROBUB was run until the species composition (with emphasis on the groundfish species) reached equilibrium. These simulations produce three sets of equilibrium biomass for the various fish groups:
(a) A minimum equilibrium biomass using the lowest estimated food requirements and the highest estimated growth rates of predators,
(b) A maximum equilibrium biomass using the highest estimated food requirements and lowest estimated growth rates of predators, and
(e) The mean equilibrium biomass using the most likely mean values for both requirements.
The estimated levels of minimum and maximum equilibrium biomass for the groundfish groups are shown in Table 2. These equilibria provide the lower and upper bound by which expected long-term effects of fishing can be gauged. The minimum equilibrium biomass for the major species taken in the commercial fishery totals about 19 million t. The maximum is about 32 million t, indicating a long-term average variation of about 13 million t.
On a long term basis, and given the present species composition in the ecosystem, pollock is by far the most abundant and makes up 63% of the total biomass of the groundfish complex. Atka mackerel is the next most abundant species, making up about 9–13% of the biomass. Pacific cod is third and is followed closely by yellowfin sole. Each species makes up about 5–6% of the groundfish biomass. The rest of the species groups make up the remaining 18–20%.
2 Laevastu, T., P. Livingston, and K. Niggol. 1980. Basic inputs to PROBUB model of the eastern Bering Sea and western Gulf of Alaska. Northwest and Alaska Fish. Cent., Natl. Mar. Fish. Serv., 2725 Montlake Blvd. E., Seattle, WA 98112.
| Species group | Minimum biomass (1,000 t) | Maximum biomass (1,000 t) |
|---|---|---|
| Pollack | 10,600 | 20,000 |
| Pacific cod | 1,160 | 1,700 |
| Yellowfin sole | 980 | 1,480 |
| Turbots | 540 | 765 |
| Other flatfish | 1,785 | 2,450 |
| Sablefish | 140 | 200 |
| Pacific Ocean perch | 420 | 720 |
| Other rockfish | 980 | 1,680 |
| Atka mackerel | 2,400 | 2,800 |
| Total Groundfish | 19,005 | 31,795 |
Experimental Simulations
After the ecosystem model has been simulated to reach the mean equilibrium state, experimental catch levels, equal to or close to ASP's, were incorporated into the simulations to determine their effects on groundfish biomass. As examples, three series of catch levels were simulated (Table 3). These series of catch levels correspond to first-guess acceptable catch estimates used by Laevastu and Larkins (1981). Series A depicts catch and discard levels in 1980. Series B and C depict alternative catch levels that were selected as possible catch quotas. It is obvious that virtually limitless combinations of catches may be simulated and the proper combination would have to be specifically picked. The model computes the changes to the biomass of each species group given the assumption that from the point of mean equilibrium, the fishery will take a constant catch of each species each year as depicted in Series A to C.
| Species group | Catch levels in thousand t | ||
|---|---|---|---|
| Series A | Series B | Series C | |
| Pollock | 1,500 | 1,560 | 1,135 |
| Pacific cod | 100 | 88 | 137 |
| Yellowfin sole | 86 | 120 | 137 |
| Turbots | 34 | 53 | 53 |
| Other flatfish | 66 | 86 | 105 |
| Sablefish | 9 | 9 | 9 |
| Pacific ocean perch | 9 | 9 | 9 |
| Other rockfish | 19 | 17 | 19 |
| Atka mackerel | 28 | 47 | 28 |
| Total groundfish | 1,851 | 1,989 | 1,632 |
The 10-year trend of the biomass level resulting from the simulations for each groundfish species and the entire groundfish complex are shown in Figures 2–11. The figures show the minimum and maximum equilibrium biomass for each species, the estimated ASP level for 1983 and how the biomass of each species would change if the resources are exploited at the three series of catch levels.
The manner in which the biomass fluctuates about the minimum and maximum equilibrium bounds may be used to evaluate whether a catch level is appropriate and likely to be sustained. Since the PROBUB model is structured to simulate the long-term average condition of the ecosystem, results shown in Figures 2–11 do not account for short-term anomalies which may cause unusually high or low recruitment levels. Interpretation of these figures should, therefore, take into account these anomalous factors and other population dynamics characteristics of the current populations. These short-term features are more readily revealed by single-species analyses.
In practice, therefore, catch quota regulations for groundfish resources in the Bering Sea have been determined largely from the species-by-species analyses depicted in Table 1, but modified by the longer term simulations depicted in Figures 2–11. A species- by-species interpretation of the figures and pertinent comments follows.
POLLOCK ASP= 1,300,000 t
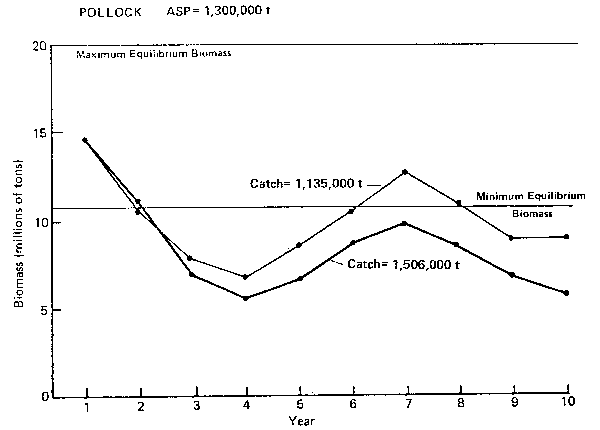
Figure 2. Annual surplus production (ASP) in 1983 and predicted trends in population biomass at selected catch levels for pollock.
Pollock (Figure 2): Abundance of pollock shows the widest level of fluctuation of all the groundfish species, largely due to its cannibalistic characteristics. The stock biomass is affected differently by simulated catch levels of 1.13 to 1.50 million t. At the higher catch level, the amplitude of variation is smaller and the population biomass is driven lower. The period of the cycle is 6 years, although Laevastu et al. (1982) have reported that temperature anomaly can shorten it to 5 years. Therefore, in a matter of 3 years, the stock biomass can drop from peak to trough by about 50% (Table 4).
| Species group | Period | Fluctuation |
|---|---|---|
| (years) | (percent) | |
| Pollock | 6 | 50 |
| Pacific cod | 5 | 8 |
| Yellowfin sole | 5–7 | 12 |
| Turbots | 7–9 | 20 |
| Other flatfish | 5 | 10 |
| sablefish | 6 | 20 |
| Pacific ocean perch | No apparent cycle | |
| Other rockfish | No apparent cycle | |
| Atka mackerel | 8–10 | 30 |
| Total groundfish | 5–6 | 22 |
The simulations show that the proper level of catch for the fishery should be set according to the current point on the biomass abundance cycle. Information from the single species analysis suggested that the biomass of pollock went through a period of biomass decline (1973–76), increased slightly from 1977–81 and may be on a down cycle near the peak. The 1983 ASP of 1.3 million t would, therefore, be on the high side, since biomass is expected to be driven even lower below the minimum equilibrium level for 3 years before the next up cycle. Therefore, it is suggested that the catch level for 1983 be set in the 1.1–1.2 million t range to help keep the population biomass at a higher level. This is a case example, where the model has suggested a catch level lower that derived by single species analyses.
PACIFIC COD ASP= 298,000 t
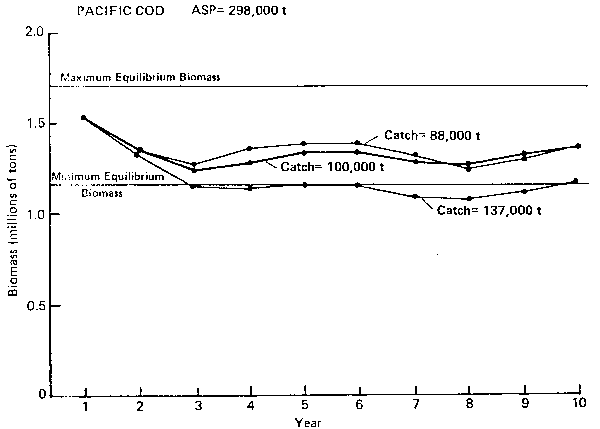
Figure 3. Annual surplus production (ASP) in 1983 and preicted trends in population biomass at selected catch levels for Pacific cod.
Pacific cod (Figure 3): In the case of cod, the long-term sustainable yield is about 100,000 t based on model simulations. Normal variations in cod biomass are not very large (± 8%) and has a weak periodicity of 5 years. However, based on single species analysis, it can be shown that variations can be much higher and that the current biomass of cod is at an unprecedented high level because of unusually strong year classes in the population. The ASP of 298,000 t is about 3 times larger than the long-term equilibrium yield. This ASP should provide a better basis for setting the 1983 catch level for the fishery because the strong year classes are expected to remain in the fishery for no more than another 3 years. It is, perhaps, more prudent to take advantage of the available surplus production now before it is lost to natural mortality.
YELLOWFIN SOLE ASP= 200,000 t
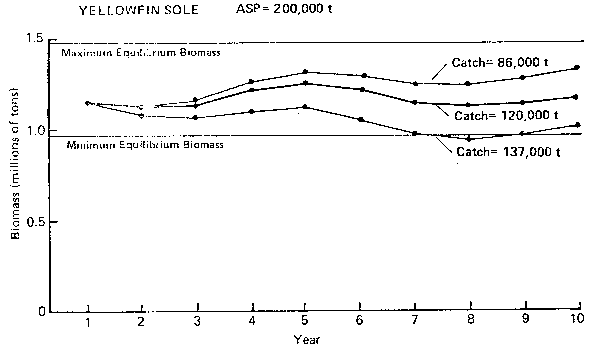
Figure 4. Annual surplus production (ASP) in 1983 and predicted trends in population biomass at selected catch levels for yellowfin sole.
Yellowfin sole (Figure 4): Model simulations show that the long-term equilibrium yield is about 130,000 t and natural fluctuations in biomass are not large (± 12%). The period of the cycle is about 5–7 years. From single species assessments, on the other hand, it was noted that the yellowfin sole population is at an historic high and has been increasing rapidly in abundance since 1979 (Bakkala and Low 1982) 1. The high level of abundance is attributed to a succession of strong year classes. The 1983 ASP is at least 200,000 t. Since population abundance is substantially above the long-term maximum equilibrium level, it is prudent to harvest at the ASP level to take advantage of high surplus production. The 200,000 t catch level may also be beneficial to the overall ecosystem since it would contribute to drive the population biomass down towards equilibrium.
TURBOT ASP= 85,000 t
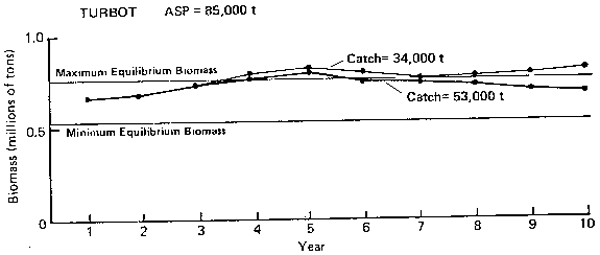
Figure 5. Annuall surplus production (ASP) in 1983 and predicted trends in population biomas at selected catch levels for turbot.
Turbots (Figure 5): Turbots are made up mainly of two species, arrowtooth flounder and Greenland turbot. The species-by-species analyses show that ASP for the group in 1983 is about 85,000 t. The PROBUB simulations show that this catch level appears sustainable and will keep the population biomass within its equilibrium bounds. The simulations also indicate that long-term fluctuations in abundance are ± 20% over a period of 7–9 years.
OTHER FLATFISH ASP = 120,000t
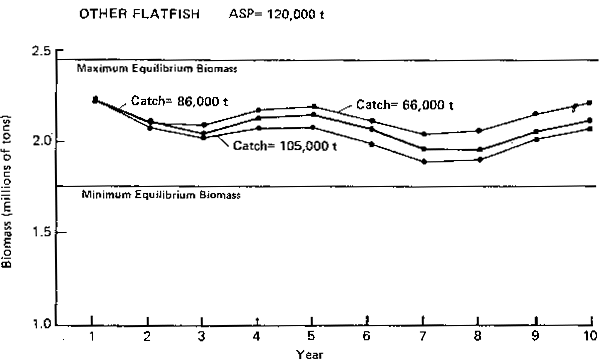
Figure 6. Annual surplus production (ASP) in 1983 and pridicted trends in population biomass at selected catch levels for other flatfish.
Other flatfish (Figure 6): This group is made up of smaller size flatfishes. The major species are Alaska plaice, rock sole, and flathead sole. The 1983 ASP for the group has been estimated to be 120,000 t (Table 1). The PROBUB simulations show that this catch level appears appropriate and sustainable since it should keep the population biomass within its equilibrium bounds. The simulations also indicate that long-term fluctuations in abundance are ± 10% over a period of 5 years.
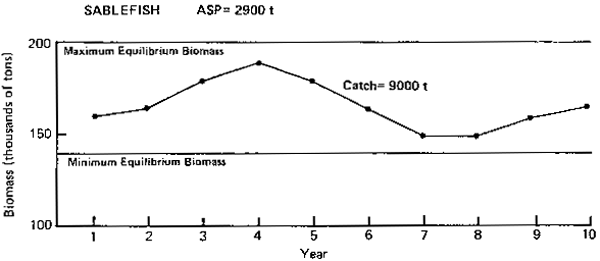
Figure 7. Annual surplus production (ASP) in 1983 and predicted tends in population biomass at selected catch levels for sablefish.
Sablefish (Figure 7): Single-species analysis shows that abundance of sablefish has declined from former levels of abundance in the 1960's to a relatively low and stable level in recent years. The ASP for 1983 has been estimated at 2,900 t. The PROBUB model, however, shows that the long-term abundance of sablefish is normally lower than the high levels encountered in the 1960's. It also shows that under mean equilibrium conditions, the long-term sustainable yield could be 9,000 t, or three times higher than the 1983 ASP. Since the present level of abundance may be close to the mean equilibrium condition, it is suspected that the 1983 ASP may have been too conservatively estimated and may be increased. The model also indicates fluctuations of ± 20% over a 6 year cycle.
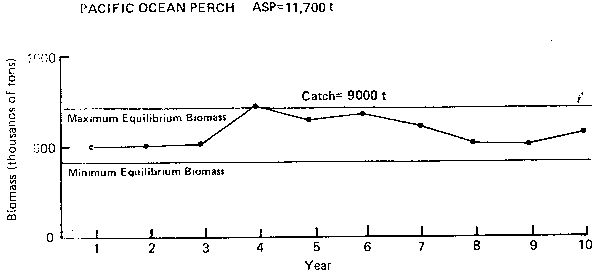
Figure 8. Annual surplus production (ASP) in 1983 and predicated trends in population biomass at selected catch levels for Pacific ocean perch.
Pacific Ocean perch (Figure 8): As in the case of sablefish, abundance of ocean perch has declined to a relatively low and stable level from former higher levels of abundance in the early and mid-1960's. The PROBUB model, however, shows that the present level of abundance is the normal long-term equilibrium trend. It also suggests that under long-term conditions, sustainable catches would be lower than the 11,700 t ASP estimated for ocean perch in 1983. Therefore, the catch quota for ocean perch should be set lower than 11,700 t and, perhaps, about 9,000 t as noted in the figure. The model does not show an apparent cycle of production.
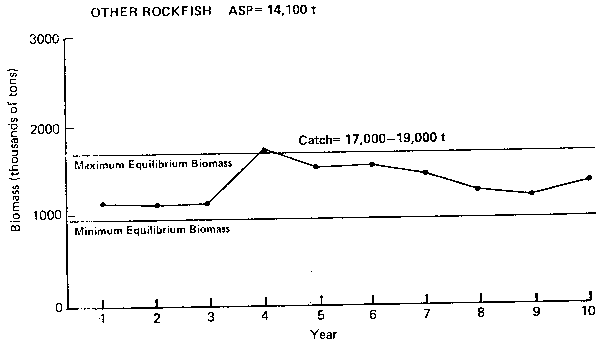
Figure 9. Annual surplus production (ASP) in 1983 and predicted trends in population biomass at selected catch levels for other rockfish.
Other rockfish (Figure 9): This group of rockfish is made up of more than 14 species of the Sebastes and Sebastolobus genera. The long-term abundance of this group is about twice that of Pacific ocean perch. The ASP for the group has been estimated to be 14,100 t (Table 1) which appears to be an appropriate catch level since the long-term equilibrium catch is likely not much higher. As in the case of ocean perch, the model does not show an apparent cycle of production.
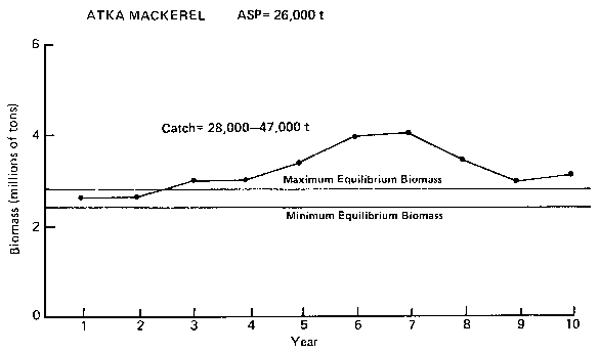
Figure 10. Annual surplus production (ASP) in 1983 and predicted trends in population biomass at selected catch levels for Atka mackerel.
Atka mackerel (Figure 10): The model suggests that Atka mackerel is the second-most abundant commercially important groundfish in the region. The resource makes up about 9–13% of the groundfish complex. Catch levels, however, are substantially lower than that of yellowfin sole because of its scattered semi-pelagic existence and lower commercial importance. Although the ASP level of 26,000 t was empirically estimated from catch trends and preliminary hydroacoustic estimations, the model shows that higher catches are sustainable. The simulations show that catches of 28,000–47,000 t have about the same effects on the overall biomass of the resource. Moreover, they also imply that catches in excess of 47,000 t may be sustainable since the population is above maximum equilibrium level. The simulations indicate biomass fluctuations of ± 30% over a period of 8–10 years.
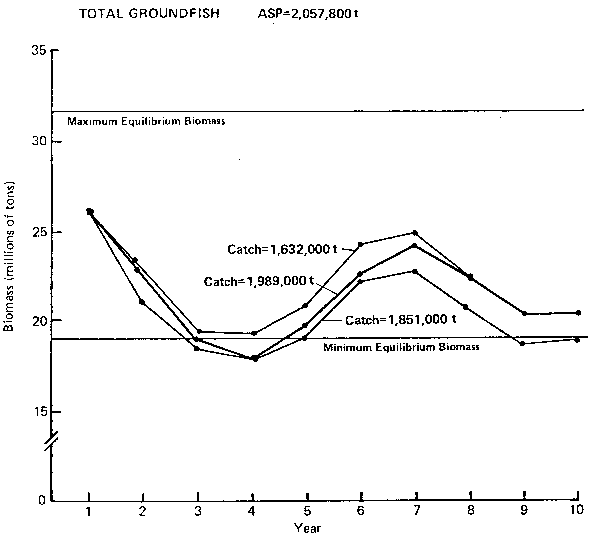
Figure 11. Annual surplus production (ASP) in 1983 and predicated trends in population biomass at selected catch levels for total groundfish.
Total groundfish (Figure 11): The PROBUB model shows that the overall biomass trend for the groundfish complex is strongly influenced by pollock. The total biomass would fluctuate by ± 22% over a distinct population cycle of 5–6 years. In general, an overall catch of 1.9–2.0 million t appears to be the long-term upper bound. A lower catch may allow the groundfish complex to achieve higher biomass levels and remain above the minimum equilibrium biomasslevel. The actual level of catch should take into account the relative position of the current level along the natural cycle of abundance. From single species analyses it is believed that abundance of the groundfish complex is very high and a catch of 2.0 million t or higher appears to be possible for 1983. However, the abundance of pollock is projected to be on the decline. Since pollock is the principal component species in the ecosystem, it is suggested that the total 1983 catch from the groundfish complex not exceed 1.9 million t.
DISCUSSION
The use of ecosystem models to complement traditional species-by-species analyses of data for development of management measures, particularly those on catch quotas, has taken on a more practical value for assessment and management of Bering Sea groundfish resources. This paper has illustrated that the PROBUB model may be used to evaluate long-term trends in biomass levels for the component species groups in the groundfish complex and can make a difference for setting regulations. The model, however, cannot yet replace the traditional species-by-species analyses for management since these analyses provide a great amount of essential details on current year-to-year changes to the trend, species composition, age structure, and other population dynamic characteristics of the stocks.
The use of the PROBUB model has resulted in considerations for setting catch quotas that are different from ASP's estimated for pollock, sablefish, Pacific ocean perch, and Atka mackerel. In the case of sablefish and ocean perch where abundances have stabilized at relatively low levels, it is an impulsive reaction to want to rebuild the stocks to former levels of abundance. However, the model has shown that the present low level of abundance may have been part of natural fluctuations about which little is known. Therefore, any desire for rebuilding the populations to former levels of higher abundance may not be realistic. It has reaffirmed that the estimated ASP's for turbots, other flat- fishes, and other rockfishes are appropriate catch levels.
The model also shows that short-term fluctuations in abundance of yellowfin sole and Pacific cod could not be detected without additional model development, particularly to reflect environmental anomalies and their effects on populations. As such, the estimated ASP's via single-species analytical techniques which reflected the presence of strong year classes, are better criteria for setting catch quotas.
The PROBUB model is perhaps most informative for indicating fluctuations in production under average conditions. The biomass of pollock follows a distinct cycle of 6 years and can vary by ± 50% over this short period (Table 4). Since pollock is the most dominant species in the groundfish complex, the overall trend for the complex is the same as pollock. However, the magnitude and period of fluctuations are dampened a little by the other component species to ± 22% in a 5–6 year cycle. The model also shows that biomass of Atka mackerel can fluctuate by 30%, sablefish and turbots by 20%. yellowfin sole by 12%, other flatfish by 10%, and Pacific cod by 8%. A biomass cycle is not apparent for rock- fishes, at least not over a 10-year period. Since rockfish are known to have substantially longer life spans, any cycle is expected to have a longer period.
Although the PROBUB model has been used to help set catch quotas, it may also be used to evaluate impacts of exploitation strategies that are not limited to catch quotas. For example, effects of proposed time-area closures to the fishery, changes in the species composition of the groundfish complex, and that of other trophic levels, such as those of marine mammals, can be evaluated through simulations. These simulations would be very useful for formulating management philosophies and policies.
REFERENCES
Bakkala, R., W. Hirschberger and K. King. 1979. The groundfish resources of the eastern Bering Sea and Aleutian Islands regions. Mar.Fish.Rev. 11:1–24.
Laevastu, T. and H.A. Larkins. 1981. Marine fisheries ecosystems: Its quantitative evaluation and management. Fishing News Books Ltd., Farnham.
Laevastu, T., R. Marasco and M.L. Hayes. 1982. Evaluation, harvesting, and management of fluctuating stocks. Oceans 9:761–765.
by
Gary D. Sharp, Jorge Csirke and Serge Garcia
Marine Resources Service
Fishery Resources and Environment Division
Fisheries Department
FAO, Via delle Terme di Caracalla
00100 Rome, Italy
Resumen
En este trabajo se revisa la evolución de los modelos matemáticos empleados en la evaluación y ordenación pesquera, tanto desde el punto de vista histórico como desde el punto de vista conceptual. Se subrayan los problemas que han sur- gido durante esta evolución tales como las variaciones en las capturas, la morta- lidad, distribución, crecimiento, reclutamiento, escalas de tiempo y estructuras por edades. Se pone también énfasis en problemas de particular importancia, tales como el muestreo, el origen y la estructuración de hipótesis, así como los conceptos relacionados con los stocks, incluyendo la caracterización de los mísmos. Todo esto lleva a la pregunta en forma de conclusión de ¿por qué modelar las pesquerías? Se revisa la evolución y las diferentes alternativas que se han presentado y se presentan en el campo del modelaje en pesquerías. Se revisa el esquema conceptual, se compara el enfoque tradicional en los modelos de pesque- rías y se dan algunas sugerencias en cómo pueden ser mejorados. Se hace una revisión de la perspectiva histórica y después se discuten algunos aspectos rela- cionados con los estados de no equilibrio de las poblaciones de peces, tales como las variaciones en las capturas, las variaciones en mortalidad, cambios en la distribución de las poblaciones, los cambios en crecimiento, la variabilidad del reclutamiento, las variaciones en las escalas de tiempo, variaciones en la estruc- tura por edades, cambios en la captura por unidad de esfuerzo, la abundancia, etc. Se dan algunos ejemplos de cómo la selección de un determinado modelo tiene una influencia grande en los resultados y conclusiones que se pueden obtener de un determinado conjunto de datos. Se agrega un anexo donde se discuten los métodos empleados en la caracterización de poblaciones de peces.
INTRODUCTION
The mathematical models used in conventional fisheries science are abstract, simplified representations of the processes governing growth, decay and reproduction of exploited populations as well as their reaction to exploitation. As all models, they are only special cases of particular scientific theories. At any point in time they reflect, at best, some part of the knowledge and understanding we have been able to reflect, at best, some part of the knowledge and understanding we have been able to integrate in order to try and answer a specific question. Models must, therefore, change and new dimensions must be captured in the representation when our knowledge improves or when a different problem must be addressed. Many aspects of the models available to us for the last 3 decades are more and more often said to be unsatisfactory and it is probably time to check their appropriateness in face of the knowledge accumulated since their inceptions, but it is also time to ask whether the model is being applied to a situation which corresponds to the special case for which the model was developed.
A model can be a useful tool only when the problem addressed is well formulated for numerical analysis. It is therefore, useful to consider classical formulations of each fishery problem in order to compare the advanced point of view with the previous ones. It is important to recognize that the scientists who developed the original production or yield per recruit models based their work on the observation that the economically and biologically depressed resources of the North Atlantic recovered noticeably during the 1st and 2nd World War periods when fishing effort declined considerably.
The rapid development of fishing effort in the fifties rapidly raised questions about inherent limitations of resources particularly about the necessity to regulate fishing mortality. It was largely accepted that unregulated fisheries lead to economic, and possibly, biological overfishing. The problem to be solved was therefore, that of optimizing captures while conserving the resources by regulating fishing. Essential questions had to be answered such as: How do growth and mortality interact in the development of population biomass and production? What is the effect of additional mortality by fishing at different ages? How much can be extracted from a given stock? What is the relation, if any, between parental and progeny abundance etc.? Therefore, models had to be designed to investigate these since experimentation was and remains hardly feasible.
The same problems have still to be faced today but with new knowledge additional dimensions need to be added. The climate driven environment has been recognized as an important causal factor of variability of pelagic stocks, with or without intensive fishing, through changes in both recruitment success and geographic distribution. This has created the need for more reliable predictive “tactical” models usable in the short term for deciding on management measures in order to cope with continuous environmental changes. Of course ecological perturbations due to fishing are also likely since the removals are often selective and can be large proportions of major populations.
In this paper we try to retrace the evolution of modelling from the historical as well as from the conceptual point of view, underlining the problems that have been raised during that evolution such as variations in catches, mortalities, distribution, growth, recruitment, time scales and age structures. Emphasis is given to problems of particular importance like sampling, causality and hypothesis structuring as well as stock concepts including stock characterization which then lead us to ask again as a conclusion: “Why model fisheries”.
FISHERIES MODELLING: EVOLUTION AND ALTERNATIVES
The Conceptual Modelling Framework
Our knowledge of species specific biology or understanding of ecological interactions has improved with time although most fisheries models basically rely on Russell's (1931) and Graham's (1935) original conceptions that the state of a single species population at any time was the result of growth, mortality, recruitment and migration processes. This has clearly been the basis for historical elaborations of all the presently available models including the most sophisticated ones. The different directions taken by fishery modelling stem from different perceptions about the key factors responsible for population changes, namely fishing effort, physical environment or predation.
The classical theory of fishery science has been based for a long time on a series of elementary models where natural mortality (M) is usually considered as an exponential process, constant after recruitment; growth (G) is represented by various continuous or discrete functions of size or age. Fishing mortality (F) is supposed to be proportional to fishing effort (f) and therefore catch per unit effort is equally taken as proportional to abundance. Biological production is mostly considered as a function of biomass and recruitment is often represented as a function of stock size.
Additional basic concepts or assumptions underlying the classical modelling of fisheries are those of steady state and homeostasis. It is believed that a given population is in permanent dynamic equilibrium with its environment and therefore, limited by some feature of it which defines a specific carrying capacity (K); that population abundance is significantly affected mainly by fishing; that a new steady state is reached when fishing is stabilized; and that the observed declines in productivity and economic conditions (attributed to excessive fishing) are almost certainly reversible (homeostasis).
The original conception of fishery systems models as closed.1 steady state systems was quite in line with the conceptual framework of other branches of ecology and science in general at that time.
Modern science is however discovering that systems (and more typically biological systems) are more often likely to be open and affected by non-linear, non-equilibrium and often non-reversible processes, characterized by threshold limits and discontinuities. As the available data base builds up it becomes more and more evident that recruitment variations cannot be considered as random noise, but that this variation is an essential signal, input into the adult population system (the only portion of populations usually modelled). Recruitment is the ‘output’ of an adjacent system (the nursery) about which our understanding has been poor and therefore, usually neglected in general fishery modelling. It becomes clear that, at least for quite a number of stocks, a drastic revision of the ‘original’ models is necessary and, for instance, that the concept of “carrying capacity” of the environment applies probably more often at the larval stages of a population than at adult stages, and that recruitment may be affected by stock size but is certainly affected at least as strongly by environment.
The Classical Modelling Approach
Within the above conceptual framework the precursors in biological fishery modelling integrated the elementary submodels on growth, mortality, production, etc. in the two most widely used surplus production and analytical approaches of fishery science in order to predict fisheries performance. These approaches have therefore the defects of the elementary models and assumptions employed, aggravated by the effects of their combination. The limitations of these higher order models are well known and the literature is crowded with theoretical considerations about the possible effects of density dependence of growth and mortality, or about the effect of seasonal or long term variability of the subpopulation component interactions as well as trophic factors. However, because such effects are difficult to isolate and measure, they have generally not been taken into account in modern decision-making processes related to fishery management.
1 In the sense that the species and its predator (the fishermen) were isolated conceptually from other species interaction or from the environment
These models were constructed to investigate steady states of self-contained mono- specific “unit” populations (as nearly closed systems) and their reactions to fishing. Density dependence was implicity accounted for in production modelling and could be introduced in analytical modelling as shown by Beverton and Holt (1957) themselves.
Despite their obvious limitations, the models traditionally used for management have had their indisputable usefulness. The Production Model was important in providing convincing arguments that resources are limited and that effort needs to be controlled if any conservation objective was to be reached on the long-term.
It can be said that production models should also have been strategic models (helping to identify possible objectives and ways of action). They have, however, been used with little success for year to year implementation of effort reduction policies or quota regulations and may be said to have often failed as tactical models.
Yield per recruit models gave clues about the puzzling problems of balance between growth and mortality and of the effects of ‘thinning’ of adult populations by fishing under different exploitation patterns. They have helped us to understand the effects of management measures as changes in mesh size and closed areas or seasons which, by the way, is what management has been about for decades. They finally showed, by ommission, that recruitment was crucial to understanding and prediction of year to year variations of stock sizes.
Towards Improved Modelling
Tersely stated, the problem is to either be simple or to be realistic. The situation described above is neither specific to fishery science nor to quantitative ecology in general. The fishery system is a very complex one. Modelling is necessary and, as always, compromise needs to be found between holism and practicality; between untractable sophistication and oversimplication. There will always be a need for partial analysis and modelling of the fishery systems, including in models the processes which are understood and documented, leaving out of the model (as externalities) the processes which are still beyond understanding and beyond control. The resulting simplification makes models more understandable but poses immediately the problem of interactions. As soon as it is recognized that the effect of externalities on the modelled sub-systems are far from negligible, it must be recognized that that specific approach is too narrow.
For example, in spite of the recent discovery and attention to relevant small time and space scales, proper modelling of recruitment will need to also involve observations of large time and space scales to take into account the phenomena of cross-oceanic or inter-oceanic teleconnections as well as low frequency signals like “sunspot cycles” and other related cosmic factors. However, it is quite evident that it is pointless to model recruitment without taking into account microscales (metres/days) in order to resolve particular time-space “survival windows” for the analysis of the mechanisms directly affecting larval survival.
In some cases a more comprehensive and realistic model would have to take into account species interactions as well, especially as far as food relationships are concerned, particularly when it is anticipated that predator-prey effects may be just as important, if not more so, than fishing effects.
Care must be however taken because there will also always be the tendency to develop as many holistic models as possible (energetic, trophic-dynamic, or self-generating models) trying to circumscribe in the model all the essential processes. The risk here is just as Hedgpeth (1977) states: “the construction of elaborate diagrams and mystic- mathematical representations of assumed relationships powered by selected values is a favourite pastime of many ecologists and environmental engineers and a model which is simply an elaborate mathematical summary of a textbook does not tell us more than we already know.” Erwick et al. (1979) remind us that a model can be made so sophisticated as to produce outputs resembling closely the real world. However, because the underlying assumptions are so complex and their interdependence so obscure the model may not be easier to understand than the real process was (Bonini's paradox).
The very complex models such as the trophic-dynamic (Andersen and Ursin 1977) ones have a high number of dimensions and therefore, their data requirements are high. They use numerous coefficients and involve quite a lot of measurement and estimation. As proposed by Fedra (1980), they will certainly have a role in the development of fishery science but their usefulness for management can be questioned because their complexity and number of dimensions prohibit analysis of their sensitivity to variation and hence evaluation of their ability to reflect biological responses.
The goals of model building, namely realism, precision, and generality seem therefore just as conflicting as management objectives generally are. A trade off has to be made when modelling fisheries. The energetic (Paloheimo and Plowright, 1979) and trophic- dynamic models being still too far from any practical application to fishery management, they will have to be further developed as experimental exercises, in order to try to gain some understanding on the mechanism of biological production of complex multispecific systems and to identify the most likely key factors responsible for year-to-year variations, and the most useful data to be possibly collected economically in the future.
This will be a long process and in the meantime we have to adopt a practical approach. We still need models but they have to be simple, (lower predictive value but lower risk of error) and enable the right choice be made between management options, within some tolerable limits of uncertainty. The precision required must be adapted to the objectives of management and unnecessary (overvalued) predictive reliability or precision should be avoided, because of the underlying costs or mistakes. For instance, it may be important to understand the mechanism of recruitment survival but it is essential only to know which parameters govern year class strength and supply predictive capabilities (see Csirke 1980 for the Peruvian anchoveta recruitment or Garcia and LeReste 1981 for predictive models in penaeid shrimp).
It is quite clear that, in contrast with the past, a strategy has to be adopted for management, under varying environmental conditions, for many stocks, but the cost of doing so in the future should be lower than the present losses by not doing so and the overall efficiency of management should increase. One obvious consequence of dropping the steady state hypothesis is the great increase in the need for monitoring (of species distribution, species composition, recruitment survival factors, etc.) in order to increase the short- term forecast capabilities of models. It will always cost a lot more to predict accurately next year's catch than to detect the most likely trend or sign of variation for a more or less near future.
Another consequence is the need to introduce the concept of risk management. It must be clear that the models cannot be deterministic and that there is no single answer to a given problem. It must also be clear that introducing a random factor (stocastic elements) into a model is not very useful for short-term management as most of the environmentally induced fluctuations are autocorrelated. It has been recently proposed to use decision theory (Lord 1976, Laurec and Maucorps 1981). Applied decision theory depends very much on the weighting of the various outcomes, and these will vary greatly among various interest groups. The uncertainty of the environment, biological responses, market fluctuations, and other often neglected control mechanisms make the first steps into a modern “decision theory” based management system difficult to implement, but this is an obvious requirement for progress toward proper system management.
The Historical Perspective
A brief review of past fisheries investigations helps to give some insights into possible trends and alternatives for fisheries sciences in the future, particularly in relation to the problem of modelling exploited fish populations.
The origins of fisheries modelling can be traced to the beginning of the century, and the available literature provides important landmarks in the history of fisheries sciences that will help in understanding why we are where we are now, what alternatives have been used and which ones have still to be explored.
The first landmarks in the history of fisheries science are those of Hjort (1914) and Baranov (1918). Both were compilers of the “pre-World War” knowledge in fisheries sciences and inspirers of the succeeding generations of fisheries scientists; and not surprisingly most of their basic concepts are still fully valid today.
It is not our intention to review the work of Hjort and Baranov but it is worth mentioning the main characteristics of their work and their general approach to fisheries sciences. This can probably be well-described by just the titles of their respective two main papers: “Fluctuations in the great fisheries of Northern Europe viewed in the light of biological research” and “The question of the biological basis of fisheries”. They both looked into the biology of fishes as the major issue when trying to solve fishery questions. Fishing itself was considered as an important factor, but the hydrographical and biological conditions were considered to be at least as important as fishing, with respect to the fluctuation of fisheries and of fish stocks. Already in 1914 Hjort concluded that, in the case of the few species he investigated, recruitment was highly variable and that this was the major cause of the fluctuation of fish stocks. While pointing out that variability of the strength of year-classes was the main cause of fluctuation of fish stocks, Hjort also indicated that these fluctuations were driven primarily by the conditions prevailing at the time at which the development of the early life history stages of fish were taking place.
If we are to make an inventory of the alternatives presented by Hjort (1914) and Baranov (1918) we will certainly find that these were quite ample. All factors that we consider important now were probably mentioned and proper justifications for investigating most of them were given. Moreover, in light of what we know now, we can probably conclude that the relative importance assigned by Hjort to each of these factors was probably well balanced. Great importance was assigned to the investigation of environmental and biological factors and of the effects of fishing, but what is most significant is that great importance was also assigned to the investigation of the relationship between these three groups of factors.
This was more or less the situation in the so-called “pre-World War” era, but then came the two World Wars which changed the course of civilization and also the course of fishery sciences.
The First World War (1914–18) caused fishing activities to be suspended for four years in the North Sea, and when fishing resumed in 1919, there were the results of the first large-scale non-voluntary experiment which demonstrated that fishing was in fact a major cause of fluctuation of exploited fish populations. These results were particularly striking in the case of some highly valuable fisheries that before the war were heavily exploited, such as cod, haddock, halibut, turbot, etc. Catch rates were higher, fish caught were bigger and total landings augmented during the few years after the war, but all these started to regress to pre-war levels as fishing intensity increased.
These series of events are the source of inspiration for another generation of fishery scientists, amongst whom the most representative are E.S. Russell and M. Graham. From this generation those classical papers that can also be considered as landmarks in fisheries literature are the one on “Some theoretical considerations on the overfishing problems” by Russell (1931) and the ones on “Modern theory of exploiting a fishery” and “The sigmoid curve and the overfishing problem” by Graham (1935 and 1939).
These were the first real attempts to represent the dynamics of an exploited fish population through a mathematical model, and it is noticeable that their subject matter was the same, the overfishing problem, although their approaches were different. Russell set up the foundations of what we commonly define now as analytical model, while Graham started another school of analysis based on the global production model. The works of these two authors also reflect the general concept that has lasted for many decades with regard to the most likely cause of fluctuation of exploited fish populations to be analysed and incorporated into fisheries models: the effects of fishing.
The Second World War (1939–45) gave a further large scale demonstration of the severe effects of fishing on exploited fish populations, and particularly demonstrated that the effects of fishing were reversible. This gave new impetus to the development of theories and models of population dynamics as well as new concepts of alternatives for exploited fish populations. The major factor remained fixed, i.e., the fishery.
The potential role of environmental and biological variability as major causes of fluctuations in fish populations was never completely ignored, but was conveniently releqated to just a potential role. In fact there were very few experimental observations to counterbalance the well accepted evidence that fisheries accounted for substantial amounts of the observed fishery and fish stock variablities.
The works by Beverton and Holt (1957), Ricker (1958) and Schaefer (1954) are probably the best examples of the bloom in the development of post-war fisheries modelling. The models proposed were based on the assumption that fishing or fishery related factors (fishing mortality, average size of individuals, spawner stock size, size at first capture, etc.) accounted for most, if not all, of the variability of fish populations. A well founded concept if we take into account the events that affected fish stocks and fisheries in Northern Europe in the first half of the century. If the Northern European fisheries had been hit by a series of “El Nino” type of short-term climatic events instead, it is certain that the evolution of fisheries science would have been completely different, but that would have been true for world history as well.
The developments introduced by Beverton and Holt (1957) and Ricker (1958) to analytical modelling of exploited fish populations, and the ones introduced by Schaefer (1954) to global production modelling contributed to building an entire theory about how fish populations behave and on how to manage fisheries. There have been some later refinements and descriptions of special applications of these two schools of analysing and modelling fisheries for the purposes of fisheries management, but in general terms these developments can be considered as the ultimate steps in modelling the effects of fishing on fish populations. These two types of models do provide a reasonable framework for the development of fisheries management strategies as they stand now, provided that (a) the effects of fishing on the population can be measured and controlled, and (b) it still holds that most of the variability of the population characteristics is due to fishing. Beyond what has been achieved so far probably little more can be added to fisheries modelling by further efforts to improve modelling the effects of fishing, unless the effects of hydrographical and biological factors are brought into the picture with considerable effort to inter-relate each to the others.
It is certainly not fair to say that the development of fisheries science and of fisheries modelling have been slow, but it is true that although it had developed in both theoretical and practical aspects, most of this development has been concentrated on only one of the three possible sets of alternatives that were identified almost 70 years ago. The investigation and modelling of fishing effects on fish populations have developed, but the investigation and subsequent modelling of environmental effects and biological reactions and interactions has been disregarded for a long time.
Major attention has been paid to factors other than fishing in relation to the fluctuation of fish stocks only after the collapse of several important pelagic fisheries. The collapse of the California sardine in the 1950's; the collapse of Japanese sardine in the 1940's and sudden recovery in the 1970's; the collapse of the North Atlantic herring in the late 1960's; the collapse of the Peruvian anchovy in the early 1970's and the associated increase of other pelagic stocks, all contributed to more attention being paid to “the other” possible causes of changes in the fish stocks, although in most cases the first act was still to blame the fishery for the collapse of the fisheries.
Although it is true that no fishery will collapse in the absence of fishing, in most cases it seems that other (i.e. environmental) factors had either favoured the over- development of fisheries prior to the collapse or had complemented the negative effects of heavy fishing by reducing the growth potential of the subject populations.
Changes in recruitment are the major cause of fluctuations in fishable populations. Although normally more attention tends to be paid to downward than to upward changes, in fact, attention should be paid to both. The successive failure of recruitment has been identified as the most immediate cause of reduced stock size and subsequent collapse of the fisheries, but in most cases these have been preceded by a series of increased recruitments, that increased both stock abundance and subsequent fishing activities, thus magnifying the downward changes in the fishery resources when or if more usual recruitment levels resume.
Although the potential effects of change in growth rate and natural mortality (e.g. the post-recruit stages) are not as great as those caused by changes in recruitment, these can still play an important role in fish stock variability and attempts should be made to model their fluctuations for incorporation into fishery models.
Probably the most striking example of recruitment failures and subsequent collapse of the fisheries is that of the Peruvian anchovy in the early 1970's. The collapse occurred in 1972, but the conditions for it started building up at least two years earlier. The Peruvian anchovy also provides a good example of the potential effect of changes in growth rate. In fact a good recruitment entered the fishable stock in 1976, raising the total biomass to 11 million tons at the beginning of the year. However, reduced growth rate (and heavy fishing) contributed to reductions in stock size by the end of the year to around 3 million tons, thus extinguishing the expected recovery of the stock. It is worth noting that both series of events were linked to changes in the environment that respectively affected either reproduction success or adult feeding success, hence lowered growth in numbers and biomass.
Efforts need to be made to model changes in recruitment, growth rates, natural mortality, etc. and to relate these with environmental factors, but for this to be successful further investigations are needed on the relationships between these factors and, of course, on the factors themselves, how they work, how to measure them, particularly since most data available to date was collected to answer the question of how fishing affects fisheries.
SOME ASPECTS OF NON STEADY STATES IN FISH POPULATIONS
When trying to internalize in the models more externalities than in the past, essential phenomenons have to be considered, like patterns of variations in catches, mortalities, distribution, growth, recruitment and time scales.
Catch Variations
Caddy (1983) has provided an assortment of examples of four classes of fishery resource behaviours: I) Steady state fisheries; II) those with regular periodic fluctuations; III) fluctuations with irregular periodicity; and IV) irregular or intermittent production. Many examples of each type are available. Classical production models and age structured models may suffice to portray types I and II adequately for most management purposes. However, types III and IV will certainly require far more extensive information pertaining to causal, environmental and ecosystem related effectors.
Mortality Variations
All mortality is “event” controlled; a series of hurdles or irregular filters imposed at each stage, differing drastically from point to point in space. Much as fish school sizes are often distributed in a negative binomial fashion, predators are also often “aggregated” this way. There are also density related thresholds of abundances of egg-larval fishes and their prey which are important for feeding, hence survival. The predator relying on egg-larva sized prey, or larvae feeding itself, requires encounter threshold densities in order to affect survival/mortality of their prey.
Much of the earlier modelling effort has been based on “average” information or probability estimates based upon over-integrated data sets rather than relevant scales of interaction such as those employed in the studies of Vlymen (1977) and Beyer and Laurence (1981). The hurdle concept evolved by Beyer (1976, 1980) has a very strong probabilistic component, but is at least intuitively satisfying in this regard as presented. This type of modelling needs to be merged with data on the distributional properties of predator fields, their sources, etc. But, quickly one arrives at the point that it is the universe needing described and modelled. What should be done? What is sufficient information, where should one start and when should one stop?
The search for precision has always had its advocates, and it should never be given up. However, in fishery related field research the available tools are simply too coarse to yield other than rough indices at present. The most productive use of day to day research resources would be on gathering basic information about where, when and what is caught by various gears; where and when reproduction appears, where and when young stages are observed, particularly if it is possible to have precise ages of these. Time series of climatic conditions on a daily basis would be useful over the region their fisheries occur. Ageing via daily growth rings in pelagic fishes; larval blooms and juvenile migrations in estuarine fisheries; and general age-specific geographic distribution time series probably tell more about the present status of a fishery than most other information.
For example, the hypothetical annual and interannual variability in eggs produced and subsequent recruits realized shown in Figure 1 gives far more information than, for instance, simple egg-recruits tables. Seasonal changes in survival rate of progeny are not unusual in many species. However, it would be even more useful to have the time series data on both the egg-larval distributions (a là Santander et al., Watanabe, this volume), and adults (à la Kondo, 1980) in order to better understand their trends and possible interactions (Csirke 1980, MacCall 1981).
Once a time series of data of these sorts is available it is quite obvious why the simplistic “stock-recruitment” relationships can be both incomplete and uninformative. Even in some cases poor knowledge of the whole system can lead to erroneous interpretation, for example, Figure 2 drawn from Garcia (1983) for depicting seasonal changes in recruitment survival in shrimps has been modified in order to show the kind of year-to-year changes which are often “projected” into artefactual stock recruitment curves, without regard to system variables.
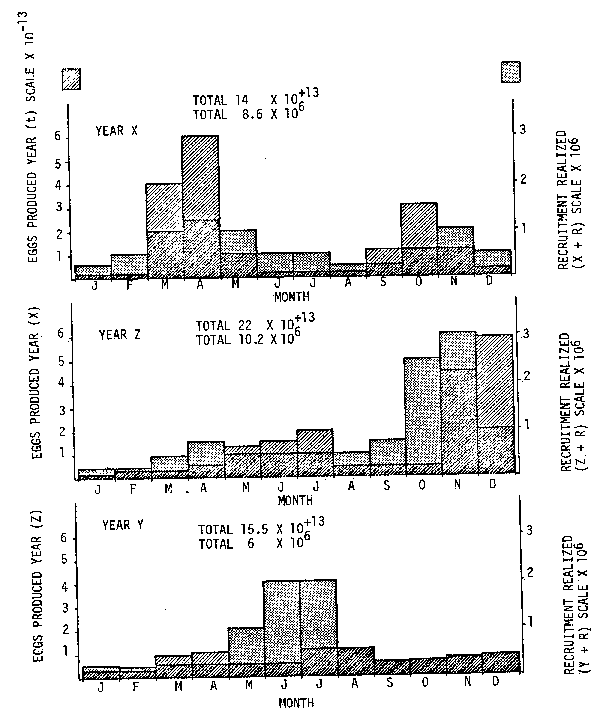
Fig. 1. Three hypothetical examples of monthly egg production and subsequent realized recruitments are shown. Note that although either total eggs produced or total recruitment may not be very different there is no suggested relation between either, or when survival, hence recruitment is determined.
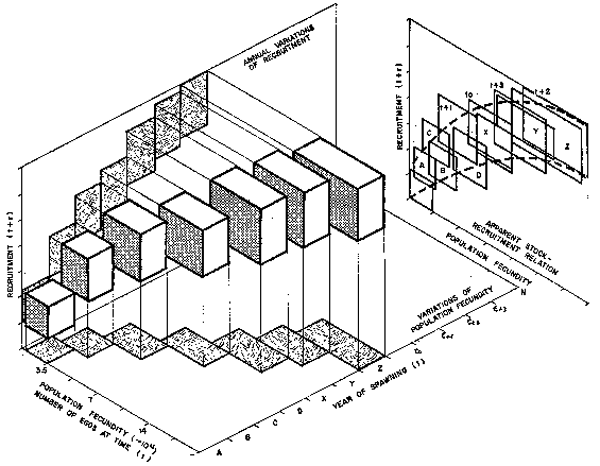
Fig. 2. Sequential changing population fecundity and recruitment are often compressed into presumed stock-recruitment relations by projection. One should recall the message by projection. One should recall the message in Figure 1 which suggest no apparent direct relation between eggs produced or recruitment in time. Artefactual stock-recruitment relations are often produced without necessary evidence (from Garcia, in press).
Modelling of non-equilibrium populations is slowly coming of age. Age structured models are commonly employed in which size-age data are arrayed in time-series such that from total catch and estimates of M and F, recruitment can be estimated. Assumptions are made about the relation between fishing intensity (f) and relative fishing mortality (F) i.e., fq = F, and either f or q (the so-called catchability coefficient) are held relatively constant. These models are appropriate for small regional fisheries with relatively direct concurrence between the population being exploited and fishing effort distribution. However, if there is emigration or migration such that the effort distribution is not concurrent with the resource, then knowledge of the In and Out proportions need to be known. If these estimates or assumptions are invalid then the model results are simply artefactual.
Species with diverse discontinuous fishing effort applied to them are certainly not easily modelled and this is a problem where population structure is vague and unknown. Age structure changes need to be evaluated from knowledge of both the distribution in time and space of the fishery and the age groups of the fish.
Distribution changes
Csirke (1980) was able to model the dynamics of recruitment in the Peruvian anchoveta utilizing catch and effort data as a measure of density and the relation between this and independent population size estimates as a measure of area, hence volume of the occupied habitat. The density dependent mechanism (density per unit volume) for recruitment modification can be attributed to density related cannibalism of the eggs and larvae by adult anchoveta (Santander et al, this volume).
Growth Changes
In many studies (e.g. Jones and Hislop, 1978; Zijlstra, Dapper and Witte 1982) there are clear examples showing modification of growth at various ages/stages, from early juvenile (prerecruit) to adults. In adult stages these changes in growth are related to changes in individual fecundity and often age of first spawning, all of which need careful analysis in order to understand the resulting reproduction success and subsequent recruitment. (Fontana 1981).
In the younger stages, the variables affecting growth reflect both biological distribution variations and also physical-chemical ones. Temperature, food particle distributions and predation appear to dominate larval growth and survival parameters (Theilacker and Dorsey 1981, and others - see IOC Workshop No. 28).
Changes in growth with time, associated with changes in either the overall abundance or the environmental conditions are also important and need to be given careful considera- tion when modelling non steady state fisheries. For instance, Zuta, Tsukayama and Villanueva (this volume) show that in the Peruvian anchoveta there were drastic losses in weight (of up to 36% for a given length) associated with abnormal environmental conditions and due to the high concentrations of the population that occurred in 1976– 1977 and 1982–1983 even though there were no indications of changes in growth in length (Pauly and Tsukayama, this volume). Changes in growth with time and space are also shown for the Pacific mackerel and anchovy of California (Parrish and Mallicoate, this volume).
The variable Recruitment Issue
As most population variation is explained by changes in natural mortality (M) at some stage in the life history, it should be asked where this might be accounted for. Of course, the usual mechanism involved is “a change in recruitment” which involves changes in either M or fecundity-related potential recruitment. Actually it is due to convenience of data availability that fishery models have to-date depended primarily on the post-age-at-entry portions of populations, and that these groups are assigned constant M values. This latter assumption is among the more troublesome in interpreting, for example, cohort analyses or other age structured models, and is simply blurred in the production models to such a degree that any changes in size-age structure for whatever reason and subsequent changes in “intrinsic rate of increase” (i.e. growth rate and mortality) are confounded.
There are several logical issues which should be examined in this set of “conventional explanations” before applying these models. These derive from a cursory understanding of fish life histories and an emerging literature on what comprises natural mortality at various stages. The fate of most dead fish is to be consumed by other organisms. The capture and engulfing of a fish is direct predation (Mp1). This is enhanced by the prey being debilitated through disease, poor nutritional condition, or senescense. However, good predators also consume healthy, viable prey, but may have to work harder to obtain their meals. There are secondary forms of predation (Mp2) such as scavenging, fungal or bacterial actions. Of course, this brings up the size hierarchy of direct predation, which often breaks down in secondary predation. That big fishes eat little fishes is no surprise, but it does appear that it is often forgotten that big fishes start out as little fishes, i.e. that the proportion of little fishes during their progression toward larger sizes is controlled by the amount of predation and therefore the abundance of predators in the system at various sizes.
Without fisheries operating, natural populations comprise a larger proportion of larger, older fish. The relative stability of such systems depends nearly entirely on the predation/consumption rates on smaller individuals somehow balancing losses at the larger sizes, i.e., lost due to senescense, disease or the limited predation by very large predators. The bulk of fecundity is usually bound up in large-old individuals in such systems. Once a fishery begins the older, larger individuals are preferentially harvested, and a flood of “uneaten” small fish is left to grow into older, larger individuals. However, under exploitation of the larger predators, not only do we expect a surge of survival in the intermediate sizes, but we also expect an increased predation upon the smaller individuals due to this removal of the larger, more ecologically efficient classes, i.e., fish generally have lower per-unit-weight nutritinal demands as they grow. Remembering that these are system processes, rather than single population characteristics, we should find that the prey species preferred by the juveniles or pre-recruits (intermediate sized fishes) of each exploited population should be subject to increased mortalities, while these intermediate sized fishes' natural mortality should actually decrease in the exploited system from which their predators are being removed.
In any situation where cannibalism plays a major role, i.e. filter feeders and many opportunists like tunas, gadoids, etc., the fishery related changing predation rates represent variable M at age-size. In complex ecosystems comprising vast species arrays and diverse behaviours it is not so easy to pin-point which fishery is affecting, secondarily, which age-size-species, but the obvious point is that M values are neither stable, nor only a “species specific” character, they will depend to a great extent upon the ecosystem and its exploitation modes.
By definition the recruitment (R) criteria are fishery and gear specific, but the fluctuations in R will also reflect system variation of the sort just described. Any population can be said to have a finite recruitment potential in the numbers of viable, fertilized eggs it introduces into the sea. Of this potential, only some very tiny fraction will reach maturity, and contribute to the potential of subsequent generations.
Time scale variations
The difficulties with estimating natural mortalities are immense. The recent approach by Csirke and Caddy (1983) to equilibrium systems would permit estimation of M if a low variance, steady state system could be identified. However, the probability of any fishery resource being in relative steady-state changes with the number of species it might interact with and their (the predator's) mobilities; the reciprocal of the distribution area compared to its potential habitat; as well as the reciprocal of the number of age classes within the population. This latter problem is particularly relevant in that for descriptive expressions employing differential equations (dx/dt) where t is time, the time frame needs careful consideration. Differential equations refer specifically to time intervals where dt is small, i.e. ≈ 0, whereas t is often expressed as months or year long periods in fishery formulations. The assumption of year-wide responses, to say decreased adult abundance in recruitment process applications, is that for each species, and population component, the expected response time t is a reproductive life-span. For example, a fish living up to 13 years with maturity at age 6 has eight years to “reproduce itself” whereas our fishery models often expect any responses to perturbation to occur “instaneously”, i.e. in one year. Simulation modelling has shown us that when employing simplistic density dependent self generating models there is a resonance period of several reproduction cycles before “steady-state” is approached. This implies that perhaps only short lived species can “live up to” the assumptions of such models, and long lived fish cannot be adequately portrayed using models with only annual or shorter response time increments. It does seem rather foolhardy to expect that a population whose age structure is being collapsed due to fishing pressure will respond in the same fashion as the unfished or lightly fished one might. The “survival insurance” of many age classes is being harvested also, from which it is obvious that any selection over time for a minimum longevity guaranteeing persistence beyond long term perturbation processes can be reversed, i.e. early maturation and shorter, slower growth may be selected for by removing individuals of above average sizes from the virgin populations. Certainly changes in growth rate and age structure need to be accounted for, while the significance of the time increment should be considered in regard to the intensity of fisheries operating on the population or in regard to the intensity and variations of seasonal and epochal anomalies affecting local hydrography, and the behavioural characteristics of the population, i.e., its distribution, aggregation and migration characteristics, among many other ecological details.
All of these variables can and do affect natural mortality in a population, leading to variations or oscillations throughout the ecosystem.
Ursin (1982) has provided an exceptionally insightful discussion of the relative stability of marine ecosystems, based primarily on North Sea studies. His studies of predation (starting with Andersen and Ursin, 1977) lead him to look for stabilizing mechanisms such as the “triangular meshes” of a food web which he describes in some detail. Ursin's Table 2 ( p.65) gives an interesting summary of Pauly's (1979) study of Gulf of Thailand trawl survey results, which can be contrasted with other systems.
Table 1: Changes in stock sizes of commercial species in the Gulf of Thailand, (Ursin 1982 - from Paulv 1979). Two significant digits retained.
| Ecological group | Units of 1000 metric tonnes | ||
|---|---|---|---|
| Virgin | Exploited | Exploited | |
| stock | stock | Virgin | |
| 1. Large benthos feeders | 150 | 1.3 | 0.0087 |
| 2. Small demersal prey | 830 | 21 | 0.025 |
| 3. Intermediate predators | 620 | 74 | 0.12 |
| 4. Large predators | 21 | 5.3 | 0.25 |
| 5. Pelagic fishes | 13 | 5.7 | 0.44 |
| 6. Flatfish | 3.5 | 7.6 | 2.2 |
| 7. Squid and crustaceans | 30 | 90 | 3.0 |
| Deplenished stocks (1–5) | 1600 | 110 | 0.066 |
| Replacing stocks (6–7) | 33 | 97 | 2.9 |
Ursin concludes: “Obvious collapses of exploited stocks are few and limited to heavily schooling pelagic stocks, mostly or exclusively clupeoids. Most other fishes of temperate seas and of subtropical upwelling systems appear to be to a large extent ubiquitists and ‘opportunists’. Contrary to this, the demersal fisheries of the tropical Pacific interfere with an extremely old and highly specialized ecosystem… In these, large longlived demersal feeders as well as shortlived pelagic feeders maintain large stock sizes in spite of heavy fishing…. In the demersal fisheries of the Gulf of Thailand… both of these groups collapsed quickly under fishing pressure whereas large pelagic predators were reduced only to half their former stock sizes.”
From Table 1, it is obvious that populations of flatfishes, squids and crustaceans grew in the face of the decreases in the other groups. One should wonder what the usual pattern of variation might be in the Gulf of Thailand, with or without fisheries. Assuming stability is certainly questionable, but it would be nice to know just how much fishery operations perturb or contribute to natural fluctuations.