by
Pairoj Sirimontaporn1
1. INTRODUCTION
Seabass, Lates calcarifer, is an economically important food fish in tropical countries. It is a species with catadromous habits within its areas of distribution. Studies on the biology and fisheries of the species are still in progress but the possibility of its culture has been established and techniques undergoing development.
2. TAXONOMY
The recently accepted taxonomic classification of seabass is as follows:
| Phylum: | Chordata |
| Class: | Pisces |
| Order: | Percomorphi |
| Family: | Centropomidae |
| Genus: | Lates |
| Species: | Calcarifer (Bloch, 1970) |
3. MORPHOMETRIC CHARACTERS OF SEABASS AND RELATED SPECIES (Table 1)
3.1 Lates calcarifer (FAO, 1974)
Body oblong-elongated, compressed with a deep caudal peduncle. Head pointed with concave dorsal profile becoming convex in front of dorsal fin. Mouth large, slightly oblique; lower jaw projecting, upper jaw reaching to behind eye. Teeth villiform, no canine. Lower edge of pre-operculum with strong spine; operculum with a small spine and with a serrated flap above the origin of lateral line. Dorsal fin with 7–9 spines and 10–11 soft rays; a very deep notch dividing spine from soft part of fin; dorsal and anal has have scaly sheaths; anal fin round with three spines and 7–8 soft rays; caudal fin round. Scales large, ctenoid.
Colour: adult with olive brown sides and a silvery belly; juvenile stage, body with 3–4 black transverse bars which disappear when the fish grow to adult.
3.2 Psanmoperca waigienses (Cavier)
Body oblong compressed. Mouth large, jaw equal; upper jaw reaching to below eye. Tongue with patch of small teeth; lower edge of operculum smooth.
| Item | Lates | Psanmoperca |
| Soft dorsal rays | 11 | 13–14 |
| Jaws | lower jaw | equal projecting |
| Lower margin of preoperculum | with three spines | smooth |
| Maxillary | beyond posterior margin of eye | extending to level of eye |
3.3 Distribution
The species is widely distributed in the tropical and subtropical areas of the western Pacific and Indian ocean. Its range of distribution includes the areas from Australia, Southeast Asia, the Philippines and countries bordering the Arabian sea.
1 Senior Fishery Biologist, National Institute of Coastal Aquaculture (NICA), Songkhla, Thailand.
3.4 Biology and life history
The euryhaline (can live in fresh, brackish and marine environments) and catadromous (grows to maturity in fresh or brackish waters and spawns in the sea) characteristics of the species result in a very interesting ecological distribution at various stages of its life history. The fish spend most of their life in a lagoon which connects to the sea. They spend two to three more years in estuarine areas until they mature, then migrate to the sea water around the mouth of a river or lagoon for spawning. Larvae and juveniles live in the sea grass bed in coastal areas for about six months, attaining a size of about 2 to 5 inches. The fish migrate to freshwater when they grow bigger. Spawners live in coastal rocky shores but some migrate to a freshwater body after the spawning is over.
3.5 Feeding habits
The adult fish is regarded as carnivorous, but juveniles are omnivorous. Analysis of stomach content of wild seabass (1 to 10 cm) found 20 percent phytoplankton and the rest are small fish and shrimp. Larger fish consists of 100 percent animals with 70 percent crustaceans and 30 percent small fish (Wongsomnuk, 1969 and Bhatia, 1971).
3.6 Fecundity and spawning
Fecundity of seabass is related to the weight of the fish (Table 2). Females with weight from 5.5 to 11 kgs gave 2.1 to 7.1 million eggs as indicated in Table 2 (Wongsomnuk and Maneewong, 1974).
| Total (cm) | Weight (kg) | No.of fish | No.of eggs range | Average |
|---|---|---|---|---|
| 70–75 | 5.5 | 3 | 2.7–3.3 | 3.1 |
| 76–80 | 8.1 | 5 | 3.1–3.8 | 3.2 |
| 81–85 | 9.1 | 4 | 5.8–8.1 | 7.2 |
| 86–90 | 10.5 | 3 | 7.9–8.3 | 8.1 |
| 91–95 | 11.0 | 3 | 4.8–7.1 | 5.9 |
Spawning of seabass in Thailand is year round, but the peak occurs during April-September in the mouth area of Songkhla Lake. A large number of fry can be collected during May-August along the coast and seagrass bed areas close to the lake entrance.
3.7 Sexual maturity
In Songkhla Lake, fully mature fish grow to 70–90 cm; fish over 100 cm seem to be overripe. Sex inversion has not been examined (Wongsomnuk, 1969).
by
Yongyut Predalumpaburt1
1. INTRODUCTION
Seabass spawn naturally in captivity during the hours of 2000 to 2100. Fertilized eggs need 12 to 15 hours in 29.5–31 degrees Celsius seawater for hatching. The spherical eggs range from 0.74 to 0.80 cm in diameter with a single oil globule from 0.20–0.28 mm in diameter (Maneewong and Watanabe, 1984).
Details of larvae and juvenile development based on the samples from hatchery rearing at the National Institute of Coastal Aquaculture (NICA) are as follows:
1.1 Larvae at 2.05 mm
(1 day after hatching)
Yolk absorbed. The single oil globule at the anterior end of yolk. Mouth still closed. Anus situated near the middle of body. Eyes are unpigmented. Pectoral fin appeared as bud (see illustration).
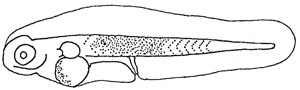
1.2 Larvae at 2.75 NL. mm
(4 days after hatching)
The mouth is open with differentiated upper and lower jaws. The nostrils are located on each side of snout. The pectoral fin developed in round shape was finfold. The digestive tract extended relatively in thickness. Melanophores present on many parts of body. Stellate and blotch melanophores scattered on mid-brain, lower jaw angle. The row of branches melanophores occurred on dorsal contour, ventral colour, body mid-line, the smaller ones on ventral of abdomen (see illustration).
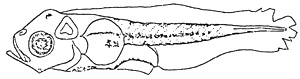
1.3 Larvae at 3.60 NL.
(10 days after hatching)
The larvae developed three preopercular spines on preopercle. The head is slightly round and the body depth extended with developing dorsal and anal fin base. The tip of notochord just flexed to develop the caudal fin. Melanophores increased densely on various parts of the body such as dorsal contour, ventral contour, body mid-line and also appeared on nasal, lower jaw angle, preopercular, dorsal and ventral of digestive tract, caudal fin base (see figure).
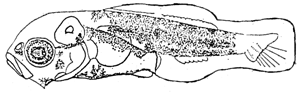
1.4 Larvae at 3.80 NL. mm
(13 days after hatching)
The number of preopercular spines increased to five and the spines on angle of preopercle was slightly bigger than the others. The nostrils are still not separated. The gut is fully extended. The dorsal and anal fins were developing with increasing fin ray. The medial fin folds behind the dorsal and anal fin were separated to form the dorsal and anal fins. The caudal fin is round and the fin rays are completely developed.
1 Senior Fishery Biologist, National Institute of Coastal Aquaculture (NICA), Songkhla, Thailand
The melanophores increased gradually on the tip of snout, lower jaw, otic capsule and body. The blotch melanophores on body such as dorsal and ventral contour, body mid-line extended to form three dark bands on body (see figure below).
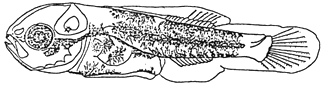
1.5 Juveniles at 5.5 mm SL
(18 days after hatching)
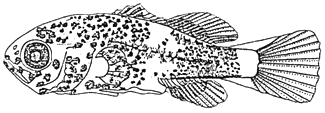
The nostrils are completely separated in two pores. Maxillary reached to the center of eye. The single opercular spine is presented on the upper part of opercle. Body depth is expanded relatively. The dorsal, anal and caudal fin are completely developed, spines and rays are as follows: D.VIII, 8; A.III, 8; C6, 5. The pectoral fin is developing fin ray while the pelvic fin appears as bud. The number of preopercular spines increased to six, but its size is relatively small. The blotch melanophores scattered on head and most of the body parts and the posterior end of body also remained branched melanophores on dorsal and ventral as well as body mid-line. The body has two vertical bands and divided by mid-point of body. There was no melanophore on caudal peduncle (see figure below).
2. FORMATION OF FIN
The seabass larvae formed dorsal, anal and caudal fins when the total length reached 3–4 mm or at seven days after hatching.
The first seven spines of dorsal fin were formed at 6 mm TL. The eight spine is still like a soft ray and became a spine at 8–12 mm TL. The number of fin rays and spines became constant (VIII, 11) when the larvae reached 8–12 mm TL. The segmentation of soft dorsal fin started from 5 mm TL; and completed at 6–16 mm in TL; the branching of soft dorsal fins started from 20 mm TL but was still not completed at 44 mm TL.
The first two spines of anal fin were formed at 6 mm TL while the third one was still like a soft ray. After the larvae reached the size of 8–11 mm TL, the anal fin was completely developed and its formula was A III, 8. The segmentation of anal fin was the same as the dorsal fin but branching was completed at 34–38 mm TL.
The pelvic fin developed rapidly from 5 mm TL and completed at 8 mm TL. The formula was P I, 5. Segmentation of the pelvic fin started at 12–16 mm TL.
The caudal fin developed after the notochord flexed. The total number of fin rays which contained rudimentary soft rays become constant (30–33) at 11–12 mm TL. The segmentation of the caudal fin started from 3–4 mm TL and completed at 12–15 mm TL. Branching started from 12–17 mm TL and completed at 31–35 mm TL.
The larvae of seabass become juveniles when the total length reached 8–12 mm or 20 days after hatching. Furthermore, branching of soft rays in all fins except the pectoral fin might be completed before 35–50 mm TL so that it seems that the juvenile may become a young fish at that size.
REFERENCES
Kosutarak, P. and T. Watanabe. Notes on the development of larval and juvenile stages of seabass. p. 36–45. Report of Thailand and Japan Joint Coastal Aquaculture Research Project. No. 1, JICA. FDT. 84–35. 189p.
Maneewong, S. and T. Watanabe. 1984 Record of spawning and hatching of egg of seabass, Lates calcarifer, in concrete tank. p. 46–57. Report of Thailand and Japan Joint Coastal Aquaculture Research Project. No. 1, JICA. FDT 84–35. 189p.
by
Pinij Kungvankij1
1. INTRODUCTION
The basic considerations in establishing a fish hatchery are: (a) which site is suitable; (b) the area of site and the facilities required in relation to the goals and objectives of the hatchery; and (c) how will the hatchery be managed.
It is of primary importance to conduct a feasibility study to determine the suitability of the site. This should be done prior to the establishment of the hatchery.
There are three factors that must be considered in designing a fish hatchery: (a) species; (b) production target; and (c) level of financial input. In addition, the facility requirements will depend on the nature of organization to run the hatchery. For government pilot projects, some laboratory support facilities are required. Otherwise, it may not be necessary as in commercial projects.
The design of the hatchery will also depend on its objectives. Experimental facilities or production-oriented system for commercial purposes or the combination of both may be incorporated into the design.
The hatchery can be an independent enterprise which is entirely self-sufficient in terms of facilities and manpower or as part of a bigger organization which utilizes its facilities and technical know-how. The hatchery can be an independent enterprise by itself or vertically integrated with other aquaculture enterprises in an organization.
2. CRITERIA FOR SELECTING SITES FOR SEABASS HATCHERY
2.1 Seawater supply
The seawater used in a hatchery should be clean, clear and relatively free from silt. The water quality should be good with minimal fluctuation in salinity all year round. Suitable sites are usually found near sandy or rocky shores. Sites which are not suitable for hatchery include areas which are heavily influenced by rain or turbulence. River mouths should be avoided as sudden salinity change occurs after a heavy rainfall. An added advantage of having a site on rocky shore is that good quality seawater is relatively near the shoreline. This reduces the cost of piping installation and pumping. The hatchery site should also be free from any inland water discharges containing agricultural or industrial wastes.
2.2 Accessibility
Ideally, a hatchery site should be selected in areas where there are active fish farming operations so that the fish larvae produced can be easily transported and distributed to the grow-out ponds and cages. The site chosen for a hatchery must have easy access to communication and transportation channels.
2.3 Availability of power source
A fish hatchery cannot be operated without electricity. Electricity is essential to provide the necessary power to run the equipment and other life support systems of the hatchery. Hence, the site must have a reliable source of power. Installation of a standby generator is absolutely necessary especially in areas with frequent and/or lengthy power failures and fluctuations.
2.4 Topography
The ideal site should be spacious, situated on flat to gently sloping grounds, well drained and not susceptible to floods, strong wave and tidal actions. It should also be on compact soil and accessible by paved road.
1 Former NACA Senior Aquaculturist
2.5 Acquisition
It is advisable to pay attention to land values early in the site selection phase to ensure that the site is available for purchase or lease and a price consistent with the project budget. Since land with the above characteristics is generally also desirable for other activities, it may be competitive for alternate land usage.
3. HATCHERY SIZE
Hatchery design is aimed at achieving certain production targets which in turn determine the size of the hatchery. The capacity is based on an approximate ratio between tank for production of natural food (algae and rotifer) and larval rearing tank. The spawning tank depends on the larval requirement which is based on the number of spawners.
Based on hatchery techniques and practices in Thailand for seabass fry production, the following assumptions are made and used for estimating tank capacities.
3.1 By means of environmental manipulation or induced spawning, the species spawns monthly for six months (whole spawning season).
3.2 Survival rate of larvae from day 1 to 50 is 15 percent. A 50-day-old larvae have an average length of 3 cm.
3.3 Production rate of 50-day-old larvae in larval rearing tank is 5 per liter.
3.4 Larval rearing period is 50 days. One larval rearing tank can be utilized only for three runs in a single spawning season. Since fish spawn monthly, there should be two sets of larval rearing tanks to accommodate monthly production of larvae.
3.5 All the 50-day-old larvae are stocked in earthen ponds and nursery cages.
3.6 The tank capacity for natural food production is the same as that for the larval rearing tank. The proportion of algal culture tank and rotifer culture tank is 2:1.
3.7 The tanks for conditioning of selected spawners, for spawning, natural food production and larval rearing are often located outdoors.
The following is an example of estimating roughly the tank capacity required for spawning, larval rearing and natural food production:
| Production target | 2 million (50-day-old larvae) |
| Spawning season period | 6 months |
| Spawning frequency | monthly |
| Production target/ month | 2 000 000 larvae/mo. = 340 000 |
| Production of 50-day-old larvae in the larval rearing tank | 5 000 larvae/ton |
| Total capacity of larval rearing tank needed | 340 000 larvae/ 5 000 larvae/ton = 68 tons |
| Larval rearing period | 50 days |
| Total capacity of larval rearing tank for monthly production | 68 tons × 2 sets = 136 tons |
| Use of natural food/crop | 15–20 days |
| Natural food culture tank | 68 tons |
| Ratio of algal culture tank and brachionus culture tank | 2:1 = 45:23 tons |
| Total number of newly hatched larvae needed | 2 000 000 larvae/ 15 percent survival = 14 000 000 larvae |
| Average hatching rate | 70 percent |
| Total eggs requirement | 14 000 000 larvae/ 70 percent hatching = 20 000 000 eggs |
| Average number of eggs/spawner | 1 000 000 eggs/spawner |
| Number of females needed | 20 000 000 eggs/ 1 000 000 eggs/ spawner = 20 female spawners |
| Stocking rate in spawning tank (1 male: 1 female) | 20 males + 20 females at 1 fish/5 tons = 200 tons |
4. HOLDING TANKS
The holding tanks in the seabass hatchery are used for various purposes such as broodstock conditioning and subsequent spawning, incubation, larval rearing and production of natural food. The design of various types of holding tanks is shown in Table 1 and Figures 1, 2 and 3.
| Stage | Facility | Stocking density | Volume needed (ton) | Unit volume (ton) | Number unit | Size, shape, construction material |
|---|---|---|---|---|---|---|
| Adult | spawning tank | 1 fish per 5 tons | 200 | 50 | 4 | square concrete tank 6 m × 6 m × 1.5 m capacity of 50 tons with water and aeration system (Figure 1). |
| Eggs | incubation tank | 100 eggs per liter | 14 | 1 | 14 | circular with flat or conical shape bottom; 1 000 liters capacity fiberglass tank (Figure 2). |
| Larvae | larval rearing tank | 20–50 larvae per liter | 150 | 15 | 10 | rectangular concrete tank (1 m × 1.5 m × 10 m) of hollow block cement with mild aeration (Figure 3). |
| Natural food | starter tank | 6 | 1 | 6 | circular tank flat bottom 1 000 liters fiberglass tank | |
| Phytoplankton | algal culture tank | 40 | 10 | 4 | square concrete tank (3 m × 3 m × 1.2 m) with aeration. | |
| Zooplankton | brachionus culture tank | 20 | 10 | 4 | rectangular concrete tank (1.5 m × 3 m × 1.2 m). |
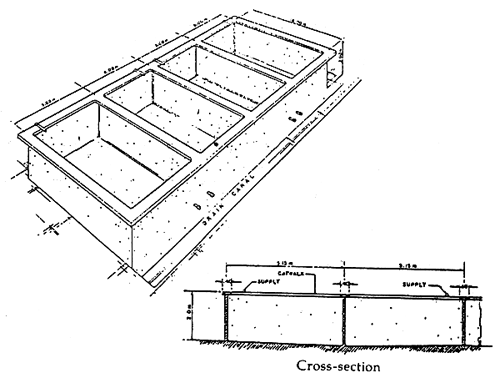
Figure 1. A broodstock development/spawning tank
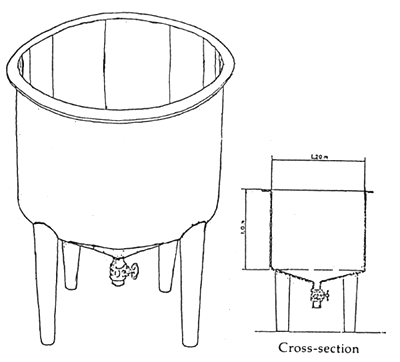
Figure 2. An incubation tank
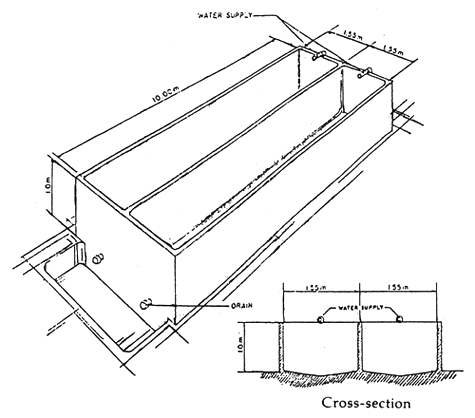
Figure 3. A larval rearing tank
5. FLOOR SPACE REQUIREMENT
Table 2 describes the space requirements of a production hatchery (Figure 4) with a capacity of 2 million 50-day-old larvae for a six-month operating season.
| Facility | Dimension (m) | Area (m2) |
|---|---|---|
| Staff office | 5 × 4 | 20 |
| Algal culture room | 5 × 4 | 20 |
| Wet laboratory | 8 × 10 | 80 |
| Spawning tank | 25 × 6 | 150 |
| Larval rearing tank | 17 × 10 | 170 |
| Phytoplankton culture | 12 × 3 | 36 |
| Zooplankton culture | 6 × 3 | 18 |
| Dry laboratory | 5 × 4 | 20 |
6. SEAWATER SYSTEM
Seawater can be drawn directly from the sea or from the sump pit. If the source of water is relatively clear, the water can be pumped directly into the overhead filter tank and stored in the reservoir or storage tank. Water is then gravity-fed to various culture tanks through delivery pipes. However, if the water is turbid and contains a high concentration of suspended solids, it must first be pumped into the filter tank. In some areas where the water source is far from the shoreline and during low tide where large quantity of water is needed continuously, the sump pit or tube well can be constructed inshore near the hatchery. The sump pit is connected to an underground pipe which is situated towards the water source. The water continuously enters the sump pit through the underground pipe even during low tide. Water is then pumped directly from the sump pit or tube well (Figure 5). Water from the sump pit or tube well is usually clear because the water is filtered naturally through a layer of sand before entering the pipe so that it can be used directly. However, if very clear and clean water is required, it should be pumped through the filter tank before use.
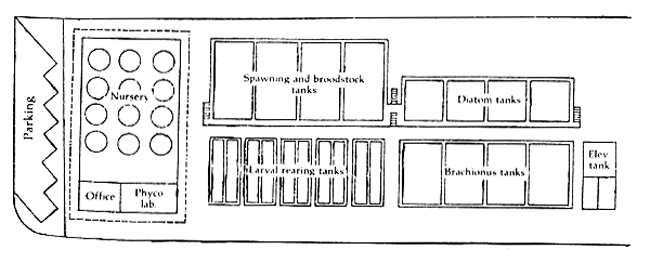
Figure 4. Layout of seabass hatchery
Pump specification must be decided on properly since the size of the pump depends on the total water requirement per day and maximum pumping time. Figure 6 indicates the total head suction pipe, discharge value and pump horsepower. With these data, pump specification required for such hatchery facilities can be derived.
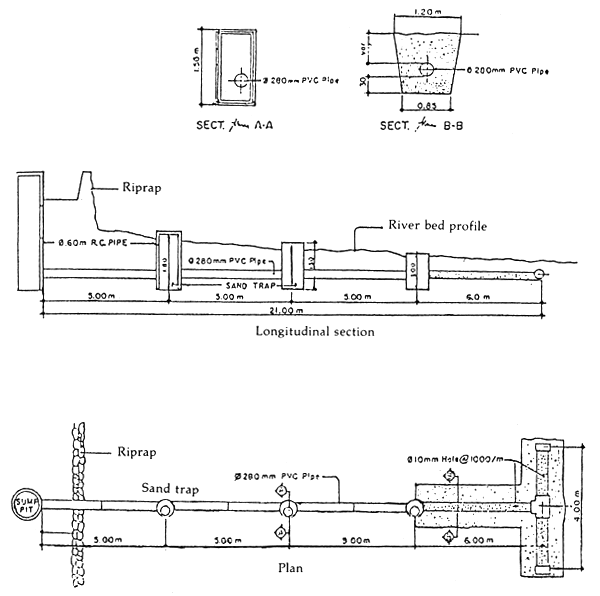
Figure 5. Seawater intake through sump pit
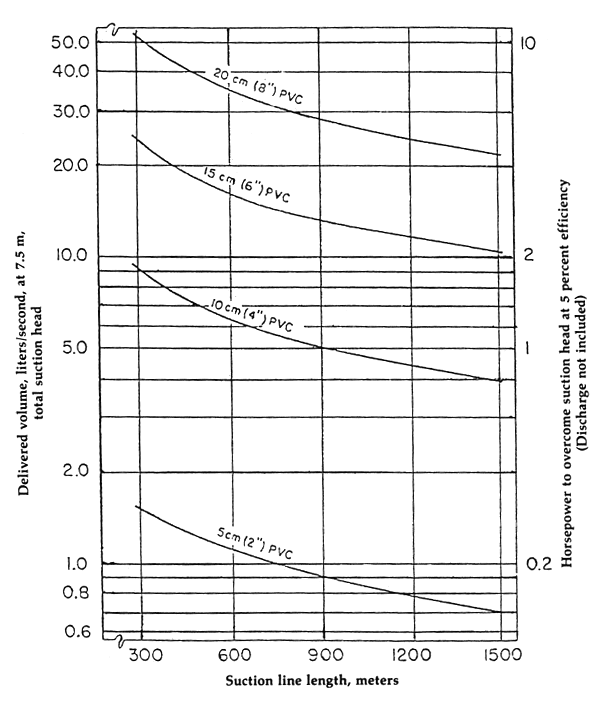
Figure 6. Waterflow (Q) and pump hp vs. size and length of intake line (After Nash, et. al., 1980)
by
T. Tattanon and S. Maneewongsa
1. COLLECTION AND SELECTION OF SEABASS SPAWNERS
1.1 Introduction
Before controlled spawning of seabass was discovered, we tried to produce fertilized eggs by using spawners from natural waters. We had to study and investigate about spawning seasons, spawning grounds and spawning time of seabass in the natural grounds. If we can get all of these information clearly, then success in seabass growing can be assured. This will be of prime importance in promoting the seabass culture industry.
Fortunately, this has been successful in Thailand with test trials which started in 1971. Success in producing consequential number of seabass fry (200 000) was first achieved in 1973. This triggered and encouraged the establishment of hatchery facilities.
1.2 Conditions of the spawning ground of seabass on Songkhla Lake
The spawning ground of seabass along Songkhla Lake is at the mouth of the lake. Spawning begins from April to last till October or before the rainy season. Salinity during the spawning season is high at about 28 to 32 ppt and water temperature ranges from 28 to 34 degrees Celsius. The spawning period depends on the phase of the moon, that is, it usually occurs from full moon to six days after full moon during low tide at 1800 to 2100 hours or in the evening.
1.3 Gears used to collect spawners
Along the mouth of Songkhla Lake, gears used to catch mature seabass are enumerated below:
1.3.1 Bottom gill nets
1.3.2 Hook and line
1.3.3 Cast nets, traps
1.4 Selection of ripe spawner
The wild spawners obtained from the natural spawning grounds should be selected. They should be of suitable size for stripping eggs and milt; too big or too small spawners should be avoided as the survival rate of larvae and fry from such spawners seem to be comparatively lower.
1.4.1 Distinction of male and female seabass adult fish
Male spawner is more slender and generally weighs less than the female spawner even with the same total length. Abdomen of the male is not bulging out like the female spawner and the depth of the body of male is less than that of the female. The scales around the anal area of male becomes thicker than the female. The female spawner has large quantities of eggs causing the abdomen to bulge out. With slight pressure by hand, the eggs will flow out. If the male is pressed on the abdomen, the milt will come out from the urogenital opening.
In general, male spawners for stripping range from 2 to 10 kg in body weight. The milt obtained from the male spawner should not be sticky and strongly concentrated. If one male spawner yields milt less than 2 ml in volume, this may not be very satisfactory. The male spawners that can give milt between 5 to 15 ml stripping would be more suitable.
Female spawners are more than 3 kg in weight and can yield 1.0 to 1.5 million eggs per stripping.
In the case of broodstock raised in nursing nets and later in grow-out cages, before they are brought into the tanks for natural spawning, careful selection is made for both the female and male spawners. The characteristics of good female and male spawners are the following:
2. CARE AND MAINTENANCE OF SEABASS BROODSTOCK
2.1 Introduction
The broodstock can be obtained from two sources: (a) broodstock collected from the spawning ground during the spawing; and (b) broodfish from grow-out cultured especially from netcages.
Broodfish collected from the spawning grounds are used only for artificial fertilization method by stripping. The fish taken from grow-out cages are usually used for spawning in captivity.
At present, seabass fry are in demand among the fish farmers in Thailand and also in some other countries such as Malaysia, Singapore and Taiwan. The production of seabass seedling should not be conducted without enough broodfish that are properly maintained.
2.2 Care of broodstock during their earlier age stages
Broodfish are raised from juvenile stages in cages. The healthy and fast growing fish with size 1.5 to 2.0 cm in total length are selected out from the rearing tank to be raised in netcages of 1 × 2 × 1 m in natural open water for use as future spawners.
About one month later, they become 3 to 3.5 cm in length and again approximately 50 percent of them are selected from those that appear healthy and fast growing to be kept for culture for up to two years. After the first year rearing period when the fish are within their third or fourth month, they are transferred from cage of 1 × 2 × 1 m to a bigger cage of 5 × 5 × 2 m until they are mature. After two years, another selection of 50 percent of this stock is made and the remaining stock are then raised up to three years. At this time, the fish will have attained 3.5 to 4 kg in their body weight and are ready to mature. The gradual reduction in number of selected broodstock is shown in Table 1.
| Age stage | Cage dimension (m) | Stock density |
|---|---|---|
| 1 month | 1 × 2 × 1 | 2 000 |
| 2 months | 1 × 2 × 1 | 1 000 |
| 1 year | 5 × 5 × 2 | 500 |
| 2–3 years | 5 × 5 × 2 | 250 |
During this period, the fish are fed approximately 5 to 10 percent of their body weight daily.
2.3 Care of maturing and mature broodstock
One month before the spawning season (April-September), broodfish are transferred from the cage net to the spawning tank. These are round-concrete tanks with 10 m diameter and 2 m depth, used as spawning tanks. Twenty-four fish are kept in each tank with female to male ratio of 1:1. The spawners are fed with sardines after the head portion and the intestines are removed. The feeding rate is approximately 1 percent of their body weight daily.
The water in the spawning tank should be in good quality, utilizing the running water system. Everyday, the total amount of water changed by this system is about 30 percent of the volume of water in the tank. Every few days, the tank should be cleaned and 80 percent of the water in the tank changed for a new fresh seawater. Enough aeration is always supplied in the spawning tank.
The fish spawn in the tanks without any inducement. After spawning, the fertilized eggs are scooped out from the spawning tank to the hatching-rearing tanks. Since the spawners are kept in the tank for a long time, they are sometimes damaged by bacteria that cause finrot disease. If this occurs, they should be treated with 1 ppm (KMnO4 solution for 10-15 minutes and antibiotics such as tetracycline hydrochloride at 50–60 milligrams per day for 3–4 days continually.
The age of the fish which one can continue to use as spawner each year is under study. At NICA 6-year old female fish is still usable as good spawner. Each year a number of seabass fingerlings should be selected for future spawners in order to replace the old one.
At the end of the spawning season, the broodfish are moved back from the tanks to the netcages in natural open water.
2.4 Further information needed on seabass broodstock
Studies on genetic selection and the use of artificial diets are needed in order to improve the technique and production of seabass fry.
by
T. Tattanon and S. Maneewongsa
1. SPAWNING OF SEABASS BY STRIPPING OF SEXUALLY MATURED SPAWNERS
1.1 Introduction
Induced spawning of seabass by hand stripping of eggs and milt from wild spawners collected from the natural spawning grounds is one of the best methods for collecting adequate amount of eggs for mass production of seabass fry. This method is suitable for the hatcheries that are near the spawning grounds and have no spawner in grow-out culture for broodstock.
1.2 Spawning techniques
The process of stripping the spawner from natural spawning grounds needs a fair amount of skill to avoid injuring the eggs as well as the fish.
1.2.1 Stripping ripe female spawners
This is usually done by two men working as a team. One man grasps the ripe female over a container while the other gently extrudes the eggs by applying gentle pressure on the abdomen from the anterior towards the posterior with the thumb and forefinger; the eggs will flow into the container. The characteristics or stage of eggs that are ready to be fertilized are enumerated below:
Eggs round and with distended surface (no wrinkles).
Yolk distributed in whole area of inner egg shell, no pervitelline space.
Eggs are separated and do not stick together in groups.
Eggs are transparent but when in groups they appear to be light yellow or golden in colour.
Eggs float in water at 28–32 ppt salinity.
Diameter of the egg averages 0.8 mm.
No yolk vesicle.
1.2.2 Stripping the male spawner for milt
For stripping the male spawner for milt, the same technique as with the female spawner is used. Good sperms must not be thick and stocky with plasma. When it is poured from a container, it should flow easily and on examination under the microscope, the sperms can be seen to be moving rapidly.
Sometimes the mature male spawner is encountered before you can find suitable running ripe female. In this case, the milt can be placed in glass vials or test tubes, stoppered and kept in the ice box. By this method, the sperms remain viable up to five days.
1.2.3 Method of fertilization
As soon as the female has been stripped, ripe male has to be stripped to the container with the eggs. The eggs and sperms are then gently mixed with chicken feather until well mixed. This is the dry method of egg fertilization.
After that, add some water and mix again. Then the eggs should be washed repeatedly with clean saline water.
1.3 Care of fertilized egg, embryo and early larvae
The fertilized eggs are put in vinyl plastic bags with some seawater with proper salinity (28–32 ppt) and transferred to the hatchery. Before putting the eggs into the hatching nursery tanks, the unfertilized eggs have to be taken out and the fertilized ones should be washed again. If necessary, the eggs are disinfected with acriflavine before being placed in the tanks.
If the hatchery is far from the spawning ground, aeration should be supplied to the bags of eggs and placed in low-temperature conditions. This will prevent development of egg which will appear to be damaged. At lower temperature (about 27 degrees Celsius) hatching will occur in about 17 hours. At higher temperature (30 degrees Celsius) the eggs hatch earlier or in about 12 hours. After hatching, care of the young larvae starts.
2. NATURAL SPAWNING OF SEABASS UNDER CONTROLLED ENVIRONMENT
2.1 Introduction
Artificial spawning of seabass was first discovered in 1971. Broodfish were caught from natural open water during the spawning season and in 1973, 200 000 fingerlings were produced and distributed to the fish farmers. However, commercial culture as practised at that time was of lower intensity. This was due to the unpredictable natural supply of broodfish.
Natural spawning of seabass in controlled environment was first successful in 1973. By this method much greater number of seedfish supply can be produced for commercial seabass culture. This operation has been conducted since that time.
2.2 Source, collection and selection of spawners
Parent fish are raised from the juvenile stage in cages. These can come from fry originally collected from natural waters or from larvae and fry spawned in the hatchery. There is not much selection at this stage except for the regular routine grading of the growing fry. When this stock attain the juvenile stage, the healthy ones that grow very fast are usually separated to be reared as future broodstock. Second selection is done after the first year and on the second year for the healthy and fast growing individuals. All these stages are still being reared in netcages in the sea.
After the second year, some of the male individuals can be identified as males when they would extrude milt with pressure on the belly. However, the females and other males cannot still be separated at this stage.
If during the elimination process for slow-growing individuals not enough stock is available, the deficiency is usually filled by selective purchase from private fishcage owners. Be sure that fish of the same age group are put together. The reared broodfish would be ready to start spawning at the end of their third year when they attain about 3.5 kg body weight. The best male milt is furnished by male 2–4 years old and female fish are not used before their third or fourth year. About one month before the spawning season, the parent fish are moved from cages into the spawning tanks.
Round tanks, 10 m in diameter and 2 m in depth are used as spawning tanks at NICA. Approximately, 24 spawners are kept in each tank at a male-female ratio of 1:1.
2.3 Care and maturation of spawners in the hatchery
Water supply in the spawning tanks is seawater with average salinity of 30 ppt. Every other day 80–100 percent of the total volume of water is drained for new clean seawater. Fish, sardine or anchovy with intestines and heads removed are used for feeding the spawners by chopping these food into bite-sized pieces. Spawners are fed once a day in the morning using food equivalent to 1 percent of their body weight. The excess food that accumulates at the bottom of the tank should be removed or siphoned out.
Generally, during spawning season the female will appear with their big abdominal portion and swim awkwardly. Approximately 1 to 2 weeks before spawning, the female fish separate from the school and their feeding activity decreases. The male fish still keeps on eating normally, schools and swims actively.
2.4 Method of spawning in the hatchery
Natural spawning in controlled tanks takes place at the same time as natural spawning in open waters. It starts from the beginning of April to the end of September. Spawning activity always occurs between 1900–2300 hours on the first to the eighth day of a full moon. As the female approaches fully maturity, there is an increase in spawning play activity. The ripe male and female swim together often turning laterally when swimming and then spawn. The fertilized eggs then float at the surface while the unfertilized eggs sink down to the bottom. The floating fertilized eggs are scooped out of the tank and placed in hatching/nursery tanks. The unfertilized eggs and other dirt at the bottom of the tank are cleaned out by siphoning.
2.5 Procedure followed after spawning
The spawners are free in their tank habitat while they are spawning. After the fertilized eggs are scooped out, the spawning tank should be cleaned and a volume of new seawater is supplied in preparation for repeated spawning activity in the future. It is also possible that different fish of the confined spawners in each tank spawn in different days within a spawning period.
As mentioned earlier, the fertilized eggs are placed in a separate tank for embryonic development and hatching. To safeguard proper development of the eggs, the salinity should be maintained at levels of 25–32 ppt. Salinities above or below this range can result in weak larvae. The water temperature of the big tanks is usually stable and does not fluctuate much, generally, at an average of 28 degrees Celsius. Radical temperature fluctuations which may occur in smaller tanks can harm the developing eggs and early larvae.
3. EXPERIENCES IN INDUCED SPAWNING OF SEABASS
3.1 Methods of inducing seabass to spawn
At present, spawning of seabass is very popular and it has been done among the fisheries stations located along the coast of Thailand. Two methods to induce seabass to spawn have been done in hatcheries:
3.1.1 Natural spawning in concrete tanks
a) Spawning without hormone injection
Two hatcheries in Songkhla and Satul are successful in spawning of seabass in concrete tanks without hormone injection. This is probably because these fisheries stations are located near the spawning grounds and the spawners are kept in the netcage before putting them in the concrete tanks during the spawning season.
b) Spawning with hormone injection
Many seabass hatcheries in Rayong, Chonburi, Phuket and Prachuap Kirikhan have to use hormone injection for seabass spawning in concrete tanks. Even stock previously obtained from Songkhla reared in Rayong failed, for unknown reasons to spawn naturally, but with hormone injection they were able to spawn. These hatcheries mentioned appear to have different environment for the rearing of the spawners. Although on the spawning trials, the female spawners have ripe eggs, they failed to spawn in the concrete tanks. Thereafter, use of hormone injection of one to two doses to the seabass spawner were done and the spawners easily spawn in concrete tanks.
3.1.2 Spawning by stripping
For this method, the spawners have to be collected from the spawning grounds. For the male spawner, there is usually no need to use hormone injection. In the case of the female spawner, the eggs have to be checked before stripping. If the eggs are good or in the running stage, there would be no need to use hormone injection. But if the eggs are not in running stage hormone injection has to be done on the female spawner. The dose of hormone is rather high compared with female spawner reared high compared with female spawner reared in concrete tanks.
3.2 Natural spawning in concrete tanks
3.2.1 Factors that may affect spawning
a) Food
Good quality and suitable amount for spawners should be given. Only about 1 percent of body weight using trash fish is fed to spawners. Overfeeding can result in failure to spawn.
b) Water quality
Good fresh running water supply with adequate dissolved oxygen should be used or water with not less than 6 ppm, DO and pH ranges of 7.5 to 8.5.
c) Salinity
Salinity in the concrete tanks should be 28–32 ppt and maintained at this range.
d) Clean tanks
Spawning concrete tanks should be clean.
e) Stress
Disturbances to spawners during spawning season in concrete tanks are not conducive for the spawning of the seabass spawners.
f) Water management
Usually changing of water or running water system have to be done in the spawning tanks.
g) Uniform size of spawners
The size of male and female spawners should not be very different.
h) Age
Age of spawners should also be suitable so that spawners can spawn readily.
i) Sex ratio
Sex ratio in spawning tank is usually 1:1, but the male spawners are often very much less in weight and their number should sometimes be increased.
3.2.2 Types and doses in using hormone for injection
Three types of hormones are used for injection to induce spawning:
The most popular hormone that is used is the HCG hormone. The dosage in using hormone for injection of seabass spawner in concrete tanks ranges from 50–100 IU/kg of body weight. For Puberogen, 200 IU/kg is used.
3.3 Spawning by stripping
The hormone that is used for injection is HCG, the same as that used in concrete tanks. But the dosage is higher, ranging between 50–1 000 IU/kg of body weight. The interval of injection is about 12 hours. Three spots on the body where hormone is usually injected are: (a) behind the pectoral fin; (b) on the lateral side; and (c) between the first and dorsal fins. The best spot for scaled fish such as the seabass is behind the pectoral fins.
3.4 Some results after injection
In the experiments on induced spawning of seabass by hormone injection carried out in 1974, the results are shown below.
The foregoing experiments show some of the experiences on induced spawning, by hormone of seabass at NICA. It should be noted that after spawning by stripping running ripe spawners and natural spawning in tanks were achieved at the Institute, these latter methods were found much more convenient with the facilities at hand.
| Specimen number | Body weight (kg) | Time | HCG (IU) | Pitituary (doses) | Remarks |
|---|---|---|---|---|---|
| 1 | 5.2 | 1000 | 2 500 | - | |
| 2200 | 2 500 | - | |||
| 1000 | 2 500 | - | |||
| 1100 | - | - | Dead, some parts showed ripe eggs. Sperm added and 10 percent fertilized. | ||
| 2 | 5.0 | 0830 | 2 800 | - | |
| 2030 | 2 800 | - | |||
| 08.30 | 3000 | - | - | ||
| 2030 | - | - | Killed, found some ripe eggs. | ||
| 3 | 5.0 | 0945 | 3 000+ | 2 doses | |
| 2145 | 3 500+ | 4 doses | |||
| 0000(midnight) | stripped | - | All running ripe eggs. Seventy percent fertilized eggs. Hatched out is 80 percent. |
by
T. Tattanon and S. Maneewongsa
1. NATURE OF EGGS, JUVENILES AND LARVAE OF THE SEABASS
1.1 Introduction
In the past before seabass became a cultured fish, no one studied about its eggs and larvae so that there was lack of knowledge on this subject. Only some general background on seabass was known, i.e. that the adult fish live in the sea and migrate to the mouth of rivers that are connected with the sea to spawn during the spawning season. The Songkhla Fisheries Station was established near the mouth of Songkhla Lake and it was observed that during April to September each year, adult seabass were caught at the mouth of Songkhla Lake. By dissecting fish, these were found to be spawners. This finding started the experiments on induced spawning of seabass by stripping eggs and milt in 1971. The trial that year was successful. Since then studies have been done and some knowledge gained on eggs, larvae and juveniles of this species.
1.2 The seabass egg
1.2.1 Immature eggs in the ovary
There are no specific studies on the matter made in Thailand at present, but aquaculturists in the country have more or less clear recognition of the immature eggs as found in the ovaries of the fish. Specific studies on this will be needed and should be made in the future.
1.2.2 The mature egg
As previously described, the mature egg is round with a shell membrane fully distended (no spaces nor distortions) measuring about 0.8 mm in diameter. They tend to stick together and while in groups, the eggs give a golden hue. It has one oil globule inside which measures about 0.2 mm in diameter.
1.2.3 The milt and sperm
Some milt will flow freely from a mature male spawner. It should be of good amount, preferably about 10 ml and not very sticky so that it would flow freely if poured from the container. If the milt is examined under the microscope, the sperms can be observed to move very rapidly. There is no detailed study yet of the seabass sperm in Thailand.
1.2.4 The fertilized egg and embryonic development
When the milt and eggs are mixed by the dry method, fertilization takes place. There appears to be no significant change on the egg from outside observations during the early stages. It was observed that it takes about 35 minutes after the mixing of the eggs and milt that the beginnings of embryonic development takes place (Figures 1 and 2). The approximate time and duration of the various embryonic stages of seabass is in Table 1.
1.2.5 Hatching (Figure 2)
The mechanics of hatching of the seabass embryo has not been studied in detail. However, since the hatching (newly hatched larvae) has by now free tail fin, it can move freely. The hatchlings tend to confine themselves at levels below the surface (about 0.5 m) and near parts of the water medium that have aeration or slight water movements.
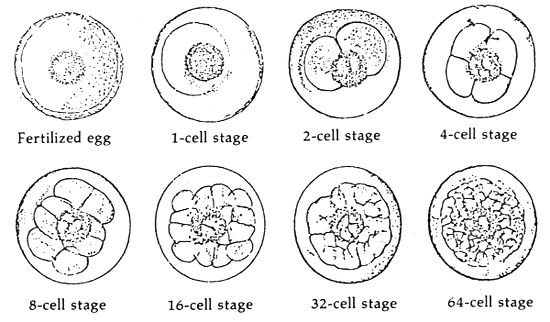
Figure 1. The fertilized egg and embryonic stages of seabass
| Embryonic stage | Period | ||
|---|---|---|---|
| Hours | Minutes | ||
| 1) | Fertilized egg | 0 | 0 |
| 2) | One-cell | 0 | 35 |
| 3) | Two-cell | 0 | 38 |
| 4) | Four-cell | 0 | 44 |
| 5) | Eight-cell | 1 | 3 |
| 6) | 32-cell | 2 | 12 |
| 7) | 64-cell | 2 | 43 |
| 8) | 128-cell | 2 | 55 |
| 9) | Pre-bastula | 3 | 11 |
| 10) | Blastula | 5 | 32 |
| 11) | Gastrula | 6 | 30 |
| 12) | Neurula | 8 | 32 |
| 13) | Embryo develops head, optic lobes and tailbuds | 11 | 20 |
| 14) | Heart starts functioning, tail free, body starts to move | 15 | 50 |
| 15) | Hatching | 17 | 30 |
The period from fertilization to hatching can be affected by temperature. As mentioned earlier at 27°C, the eggs can hatch in about 17 hours while if the temperature were 30–32°C, the eggs can hatch in 12–14 hours. Salinity on the other hand can affect the rate of hatching. This is shown in Table 2.
| Salinity (ppt) | Rate of hatching (%) |
|---|---|
| 0 | 0.00 |
| 5 | 2.86 |
| 10 | 58.56 |
| 15 | 75.03 |
| 20 | 82.35 |
| 25 | 83.36 |
| 30 | 80.78 |
| 35 | 46.90 |
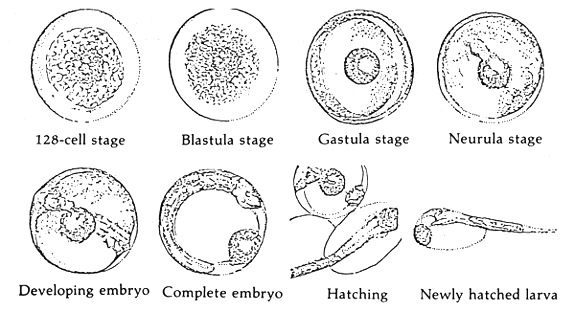
Figure 2. Advance embryonic stages, hatching and newly hatched larvae of seabass
1.3 The seabass larvae
1.3.1 Development of hatchlings with egg yolk
The early hatchling or newly hatched larvae is 1.5 mm long with a big yolk sac (Figure 2). The yolk sac has one big oil globule at its anterior. This enables the larvae to be set in the water with head raised up at 45 to 90° angle of the water surface. The body is slender and pale in colour with a distribution of pigments. The eyes, digestive tract, anus and caudal fins are distinctly seen but the mouth remains closed for a while or a period of about three days.
Under normal conditions, the rate of absorption of the yolk has been observed to be as follows (Table 3).
| Diameter* of yolk in yolk sac (mm) | Time (days) |
|---|---|
| 0.8800 | 0 |
| 0.3525 | 1 |
| 0.2752 | 2 |
| 0.1530 | 3 ** |
| 0.0050 | 4 |
| 0.00 | 5 *** |
* As the yolk is actually elongated, this is a measurement of length from anterior to posterior.
** Mouth opens.
*** Yolk completely absorbed.
1.3.2 Development of larvae, fry and juveniles
The mouth opens when the larvae get to about three days old and the yolk has been almost completely absorbed. This is a sign that the fry can start to feed. Up to seven days the larvae are pale in colour and from the age of seven days to metamorphosis at 18–20 days, they appear dark with distinct vertical stripes on certain parts of the body. After the 18th to 20th days, the larvae again assume pale brownish colour. This time, the vertical stripes can be more clearly distinguished. The stripes are three; one at the caudal peduncle, another at the level between the first or spinous dorsal fin and the second or soft dorsal fin, and the third over the head, all of which are particularly distinct.
In one month, the larvae metamorphose into the fry stage which has the appearance very close to the parent fish. the fry measures 1.5 to 2.0 cm. These further grow and develop into juveniles after the third to the fifth month when they attain 8–15 cm.
2. FOOD AND FEEDING OF SEABASS LARVAE AND JUVENILES
2.1 Introduction
Food and feeding are two of the most important factors affecting the survival rate of seabass larvae. The larvae cannot survive if there is inadequate supply of food which comprises various live organisms, and which varies with the development of the larvae.
2.2. Food and sources
Most of the food that seabass larvae feed on is composed of live zooplankton. The larvae first begin to feed on rotifer. Other kinds of food have also been tried with the early larvae but without success. The supply of live zooplankton is expensive and sometimes causes problems because zooplankton culture needs time, facilities and skills. Further, the different kinds of live food required must be prepared in time to satisfy the need of the fast-growing larvae. To maintain a high survival rate, the feeding schedule for the larvae must be closely adhered to.
2.2.1 Live food used at different age stage
The live food used for the different developmental stages of the seabass larvae is shown in Table 3.
| Food type | Days after hatching | |||||
|---|---|---|---|---|---|---|
| 1 | 3 | 8 | 16 | 25 | 30 | |
| Chlorella | ||||||
| Rotifer | ||||||
| Artemia | ||||||
| Moina/Daphnia | ||||||
| Acetes/Trash fish | ||||||
3. LARVAL REARING OF SEABASS
3.1 Introduction
Widespread demand for commercial scale culture of seabass is now encouraged by the government of most Southeast Asian countries including Indonesia, Hong Kong, Taiwan (China), Malaysia, Singapore and Thailand. Accordingly, the cost of seabass larvae has become very high and is increasing year after year. In Thailand particularly, it is estimated that at least 50 000 000 larvae are needed to supply the needs of the whole country. The advancement of technology on hatchery operation will be required to insure that the supply of fry for the grow-out ponds and cages can be met. The seabass seed supply problem is felt not only in Thailand but in the whole region.
Research on the propagation of seabass, Lates calcarifer commenced at Songkhla in 1969 under the direction of Mr. Sawasdi Wongsomnuk, who was later joined by Ms. Sujin Maneewongsa. The objective of the project was to develop methods of mass propagation of fry and to study propagation methods applicable to the physical and economic environment in Thailand.
The first successful mass production technique for seabass fry was achieved in 1973.
3.2 Care of fertilized eggs and hatching
Fertilized eggs are dipped into 5 ppm of acriflavine solution for one minute then washed for two to three times before placing in hatching-rearing tanks with the size of 3.5 × 4.75 × 1.20 m. Each tank is filled with filtered seawater at the salinity of 28–30 ppt and aerated from the bottom.
Approximately 800 000 to 1 000 000 eggs are stocked in each tank. The eggs hatch out in 17 hours at average water temperature of 27°C or at 12 hours at water temperature of 30°C. Various salinities of water give different hatching rates; the best salinity for hatching appears to be between 20–30 ppt (Table 5).
| Salinity (ppt) | Hatching rate (%) |
|---|---|
| 0 | 0.00 |
| 5 | 2.90 |
| 10 | 58.5 |
| 15 | 75.0 |
| 20 | 82.4 |
| 25 | 83.4 |
| 30 | 80.8 |
| 35 | 49.9 |
3.3 Care of newly hatched larvae before feeding
After hatching, aeration is stopped for a few minutes in the tank so that the sediments of undeveloped embryos and other dirts can be siphoned out. During this period, running water system is used in the rearing tank for two to three days before feeding.
3.4 Start of feeding and care of growing larvae
3.4.1 Feeding of various age stage
From three days old larvae, the yolk is almost completely absorbed, the mouth opens and the larvae start their feeding habit. By this time, rotifer (Brachionus) is required to feed the larvae. Enough rotifer is added or approximately 5–10 rotifers per ml are stocked into the larval tank. Larvae are fed with rotifer up to 10–14 days, later on they are fed with Artemia salina until 20 days. Daphnia or Moina is the last living organism that is fed on by 20–30 days old larvae. After this period, they are trained to change their predatory habit by feeding trash fish which are chopped or minced into bite-sized pieces.
3.4.2 Nursery tank management
The rearing tank should be cleaned up every-time before using. The rate of water replacement in the rearing tanks depend on feeding period of each age stage. In the period of rotifer feeding to prevent the loss of rotifer through the outlet, approximately 10–20 percent of the water in the rearing tank is drained out only for the replacement of rotifer supply each day. During Artemia feeding period, approximately 50 percent of water is changed while almost complete change is made during trash fish feeding period.
The sediment of dead organisms, larvae or leftover food are siphoned out everyday. The management of seabass nursery is shown in Figure 3.
3.4.3 Grading techniques
Due to cannibalistic nature of the fish, size selection or grading or sorting is of prime importance. The first sorting should start at the second week since during this period, the bigger fish can eat the smaller ones. The easiest way of sorting is to use screen with various mesh size so that the various sizes of fish can be separated easily. Stocking the same size of fish will reduce the rate of cannibalism, thus the survival rate will be increased and the growth rate of the fish could also be faster and more uniform.
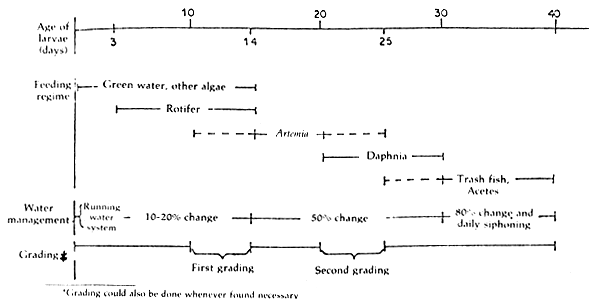
Figure 3. Chart showing management method for seabass nursery tank within the first 40-day period
3.4.4 Growth and care of larvae as they develop to fry and juveniles
Up to 40–45 days, the larvae develops to juvenile stage. They are moved from the rearing tanks for culture in netcages. The netcages are of 1 × 2 × 1 m in dimensions are usually set in open water. Stock of 2 000–3 000 fry are raised to the fingerling size in these cages.
3.4.5 Diseases
If hygienic conditions are maintained, the larvae are generally resistant to diseases. However, since the larvae are stocked in the tank for a long period, sometimes they show their abnormal swimming character, stop feeding and turn black. These are signs of disease or poor health so that if these occur, they should be treated with 1:2 000 parts formalin solution for 10–15 minutes for 2–3 days continuously.
3.4.6 Survival rate
The system of culture outlined above gives about 85 percent hatching rate and a survival rate of 1–7 days old larvae of 30 percent. For 8–15 days old larvae, the survival is 80 percent after which they can be maintained indefinitely with negligible mortality (Table 6).
| Age (days) | No. of larvae* per liter | Survival rate (%) |
|---|---|---|
| 1–7 | 30–40 | 37.2 |
| 8–15 | 15–20 | 80.9 |
| 16–23 | 5–10 | 70.0 |
| 24–30 | 2–5 | 85.3 |
* Normal stocking density used in nursery tanks.
4. GROWTH OF SEABASS LARVAE AND JUVENILES
4.1 Introduction
There is no information or previous studies made on the growth of seabass larvae and juveniles from the wild. It is commonly known that the seabass fry when collected from natural areas are big enough so that they can be suitable for stocking grow-out ponds and cages. Only approximate estimates of their possible age can be made.
As we are now able to spawn the fish and grow the larvae and juveniles under controlled conditions, we have better knowledge on their growth. We are also successful in nursing the seabass larvae and juveniles in controlled conditions are relatively high survival rates.
4.2 Growth of larvae and juveniles
4.2.1 Growth in relation to food
In the nursery of seabass larvae, the most important factor to consider is to have the right kind and amount of food prepared for the larvae. Various stages of larvae need different kinds of food. The food to be given to the first stage of the larvae when they start to feed is rotifer, Brachionus plicatilis. This kind of food is very suitable for the young larvae making them grow well and giving high survival rates. Therefore, adequate amount of rotifer is very necessary for nursing seabass larvae. The density of rotifer should be 5–10 rotifers per ml in the nursery tank. Feeding larvae with rotifer is started when the seabass larvae are three days old until their 14th day. Artemia is fed to seabass when they get to the 8th day until the 20th day. Daphnia or Moina is fed to the larvae on the 17th day until the 30th day. Acetes and minced fish are fed to the fry after the 21st day. These feeding stages are shown in Table 7.
| Percent of food given | ||||||
|---|---|---|---|---|---|---|
| Age (days) | Chlorella | Rotifer | Artemia | Daphnial Moina | Acetes | Minced fish |
| 3–7 | 10 | 90 | - | - | - | - |
| 8–15 | 10 | 75 | 15 | - | - | - |
| 16–20 | - | - | 50 | 50 | - | - |
| 21–30 | - | - | - | 80 | 10 | 10 |
| 31–40 | - | - | - | 50 | 25 | 25 |
| 41- | - | - | - | - | - | 100 |
Experiments were conducted on nursing of seabass larvae with three kinds of zoo-plankton, namely: rotifer, Artemia and Moina. The larvae were reared during their 3–15 days of age. The results are shown in Table 8.
| Type of food | Number of larvae | Number survived | Survival rate (%) |
|---|---|---|---|
| Rotifer | 2 000 | 808 | 40.40 |
| Rotifer + Artemia | 2 000 | 951 | 47.55 |
| Rotifer + Moina | 2 000 | 381 | 19.05 |
The results showed that rotifer and combination of rotifer and Artemia were the more suitable food of seabass larvae at this age stage.
4.2.2 Growth in relation to space
The tanks used as nursery for the seabass larvae measure 3.50 m × 4.75 × 1.20 m deep. This is filled with good fresh seawater 1.10 m deep and aerated at four points of the tank bottom. The density of stocking of this size of nursery facility ranges from 600 000 to 1 000 000 larvae/tank. Larval densities that are usually used in nursing tanks per ton is shown in Table 9.
| Age (days) | Density of larvae per ton | Survival rate (%) |
|---|---|---|
| 1–7 | 60 000–100 000 | 37.23 |
| 8–15 | 15 000–20 000 | 80.91 |
| 16–23 | 5 000–10 000 | 70.05 |
| 24–30 | 2 000–5 000 | 85.33 |
4.2.3 Growth in relation to water quality
Good fresh seawater has to be filtered by the phytoplankton net to prevent predators from entering the nursery tank. The salinity of water should be maintained at 10–30 ppt.
Experiments were conducted using various salinities of water for nursing larvae of 1–30 days of age. The results reveal that the salinity of 20 ppt gave the highest survival rate as shown in Table 10.
| Salinity (ppt) | Survival rate (%) |
|---|---|
| 0 | 0.0 |
| 5 | 24.0 |
| 10 | 28.0 |
| 15 | 28.0 |
| 20 | 68.0 |
| 25 | 22.0 |
| 30 | 18.0 |
| 35 | 10.0 |
The effects of other water quality factors (temperature, pH, DO, etc.) have not been fully studied.
4.3 Growth rate of seabass larvae
By nursing with enough food of the kind described above within 30 days, the larvae should normally attain a length of 1.205 cm. The normal growth within the first 30 days is shown in Table 11.
| Age (days) | Total length (mm) | Remarks |
|---|---|---|
| Fertilized egg | 0.870 | Diameter of fertilized egg |
| 0 | 1.5175 | |
| 1 | 2.1850 | Beginning to hatch out* |
| 7 | 3.5916 | |
| 14 | 4.3650 | |
| 20 | 8.10 | |
| 30 | 12.05 |
* Hatching is at 12th to 17th hour depending on temperature.
5. DISTRIBUTION AND TRANSPORT OF SEABASS FRY
5.1 Collection and conditioning of fry before transport
Fry are collected from the rearing tan and placed in smaller receptacles.
Fry are treated with 5 ppm of acriflavine solution or 0.5 ppm of copper sulfate solution for 5–10 minutes.
There should be no feeding within 1 hours before packing.
5.2 Packing and amounts that can be transported
Plastic bags of 40 × 60 cm of proper gauge are filled with 6–7 liters of fresh seawater and saturated with oxygen; 10–12 liters of oxygen gas are used for packing. The amount of transportable fry depends on size of fry, water temperature in plastic bags and duration of travel and handling from source of fry to its destination (Table 12).
5.3 Transport
In transporting by truck, a mixture of crushed ice and sawdust is needed to control the water temperature in the plastic bags during transport. The mixture is spread uniformly on the floor of the truck before the plastic bags are laid upon it. The proportion of crushed ice and sawdust is 1:1 for long period transport (12–16 hours) and 1:2 for short period (4–5 hours). Transportation should be carried out at night time. By this method, it is possible to control the water temperature between 19–23°C.
| Age stage (days) | Size (TL) (cm) | Number of fry per bag | Water temperature | Duration (hours) | Survival rate (%) |
|---|---|---|---|---|---|
| 7–15 | 0.2–0.3 | 10 000 | 19–23°C | 16 | 90 |
| 20–22 | 0.5 | 5 000 | -do- | 16 | 90 |
| 1 month | 1.0–1.5 | 1 000 | -do- | 16 | 90 |
| 2 months | 2.0–3.0 | 500 | -do- | 16 | 90 |
Figure 4 shows the observed fluctuation in temperature of the water in the plastic bags during transport. It was also observed that the dissolved oxygen starting initially at 5.3 to 5.0 ppm will drop to 2.3–2.6 ppm at destination.
However, another and more convenient way for land transport of live fish is by using refrigerated truck or air-conditioned bus where temperature of 20–22°C can be controlled during transport.
Air transport is of course a very convenient and fast but expensive way. In this case, the plastic bags are required to be placed in a receptacle. The bags can be covered with some crushed ice before loading. This method takes about 3–4 hours duration while it takes 13–16 hours to transport by truck over the same distance. The price of air transportation can vary from one country to another.
5.4 Distribution
Usually, 7–14-day-old larvae are distributed to the fish farmers who have their own nursery to continue nursing the larvae. After the larvae attained fry sizes of 1.5 to 2 cm, they are distributed to the local fishermen to be reared in grow-out cages or ponds.
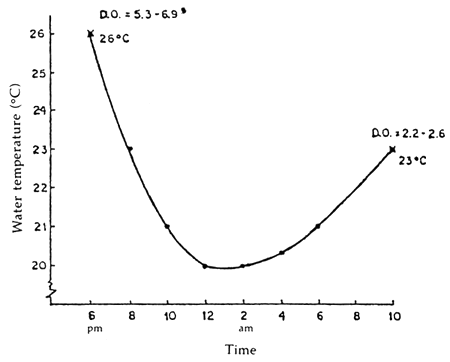
Figure 4. Fluctuation of water temperature under transportation