by
Tida Pechmanee
1. INTRODUCTION
The Songkhla Fisheries Station of the Department of Fisheries produced the first successful batch of artificially bred seabass fry in 1975. Until now, the availability of rotifer, (Brachionus plicatilis) and brine shrimp nauplius, (Artemia sp.) as larval feed is still the most important factor affecting growth and survival rate of seabass larvae. The larvae are first fed with rotifers until they attain 4 mm in total length when the diet is switched to brine shrimp nauplii until the larvae accept minced fish or formulated feed.
2. BRACHIONUS PLICATILIS
2.1 Taxonomic position
Phylum Trochelminthes
Class Rotifera
Order Monogononta
Family Brachionidae
Genus Brachionus Pallas
2.2 General information
The body of most rotifers is divided into three portions: head, trunk and foot. The head carries a corona which is surrounded by cilia (Figure 1). There are two types of Brachionus plicatilis. One is the S-type which has pointed anterior spines and short lorica (140–220 μm). The other is the L-type which has blunt anterior spines and long lorica (230–320 μm). The S-type grows well in temperature above 20°C, the L-type is less than 20°C. All the mass cultured rotifers in Thailand are of the S-type. They grow well when the salinity is 15 ppt and temperature is 28°C to 30°C.
2.3 Food for rotifer
In nature, rotifers feed on phytoplankton, yeast, bacteria and protozoa. In mass culture, they are usually fed Chlorella, Tetraselmis and yeast. It has been found that rotifers fed on baker's yeast lack n-3 highly unsaturated fatty acids (n-3 HUFA) which are essential fatty acids for marine fishes. Seabass larvae fed with rotifers raised only on yeast (yeast rotifer) show low survival rate. It is, therefore, necessary to enrich yeast rotifer with squid liver oil or marine fish oil providing the n-3 HUFA at least six hours.
2.4 Rotifer culture
Large-scale hatcheries usually adopt the partial harvest system using large tank. The volume of production tank is 10–100 tons. In small-scale hatcheries, the total harvest system is effective using one to 10-ton production tank.
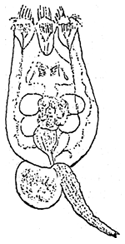
Figure 1. Rotifer (Brachionus spp.)
At the National Institute of Coastal Aquaculture (NICA), a 50-percent harvest system is employed for the mass culture of rotifer using a 26-ton production tank. Rotifers are fed mainly on Chlorella or Tetraselmis. Yeast is used as supplementary food when phytoplankton supply is not sufficient.
The process of culturing B. plicatilis is as follows:
First, 1–2 tons of freshwater is added to 11–10 tons of phytoplankton (Chlorella density, 10 × 10 cells/ml or Tetraselmis, 10 × 10 cells/ml), in order to adjust salinity to 25–30 ppt.
Rotifer seed is introduced at a density of 10–20/ml. After two days, this initial food supply is exhausted and the density of rotifer would have increased to 40–100/ml. At this point, an additional 13 tons of food and water mixture (same as initial batch) is added to the tank for a total volume of 26 tons.
The next day, the rotifer density would be about 40–100/ml. It is then ready for harvesting by draining the water from the rotifer tank through 63 μ mesh bag, leaving half of the original volume to serve as starter for the next batch.
Phytoplankton, or phytoplankton plus baker's yeast (0.2 g/10 rotifer) or marine yeast (2 × 10 cells/ml) is added to the rotifer tank to the 26-ton level in order to grow the next batch of rotifer.
The rotifers are harvested daily from the same tank for about 10 days at a constant rate of production.
2.5 Phytoplankton culture
Chlorella sp. and Tetraselmis sp. are cultured in 26-ton rectangular tanks. The kind and amount of fertilizer applied to the culture tanks are: 1 200 g of (NH4)2SO4, 120 g of calcium superphosphate or agriculture fertilizer formula 16–20-0, and 60 g of urea. It takes about 2–5 days for Tetraselmis and three days for Chlorella to grow to harvest level.
2.6 Marine yeast culture
Marine yeast seed is collected from fish culture water. The nutrient solution for growing marine yeast is 15 g sugar 3 g of (NH4)2SO4, and 1 g of KH4 PO2 dissolved in one liter of water. One ml of HCl concentrate is added to the solution to produce a pH level of 4. The yeast is reared for 2–3 days, then transferred to grow in a 10-liter vessel for 1–2 days. Add the same nutrient mix but withou the HCl. The yeast is then transferred to a 100 to 500-liter tank and the sugar supply reduced to 8 g/l. One day later it can be used for feeding rotifer.
2.7 Rotifer enrichment
Yeast rotifers should be enriched before using. To enrich, 100 ml of cod liver oil, 1 g of raw egg yolk and 1 liter of water are homogenated for 2–3 minutes by mixer then added to a 1-ton tank of rotifer with about 500–1 000 ml/for at least six hours. The rotifers are cleaned before using.
3. ARTEMIA SP.
3.1 Taxonomic position
Phylum Arthropoda
Class Crustacea
Order Anostraca
Family Artemiidae
Genus Artemia Leach
3.2 General information
Salt lakes and brine ponds with Artemia populations are found all over the world. At certain moments of the year, cysts of Artemia floating at the lake surface are washed ashore by wind and waves. The cysts are harvested, processed, packed and sold. Artemia nauplii can be produced easily from these cysts. The first larval stage of Artemia measures about 500 microns in length and has three pairs of appendages. An important criterion in selecting a suitable brand of Artemia cyst is the amount of nauplii that can be derived from a unit weight of cyst. This can be determined by the hatching efficiency, that is the number of hatched nauplii obtained from 1 g of cyst.
3.3 Hatching (Figure 2)
3.3.1 Soak the cysts for one hour in a 20 ppm hypochlorite solution in tap water with good aeration. After soaking, wash the cysts with tap water.
3.3.2 Add not more than 5 g of cyst for 1 liter of seawater. The hatching containers should be cylindrical tanks with conical bottom, aerated from the bottom. The hatching containers should be illuminated at least during the first hour of hatching. NaHCO is added to adjust the pH to 8–9 whenever necessary. Artemia cysts usually start to hatch after incubation at 25–30°C.
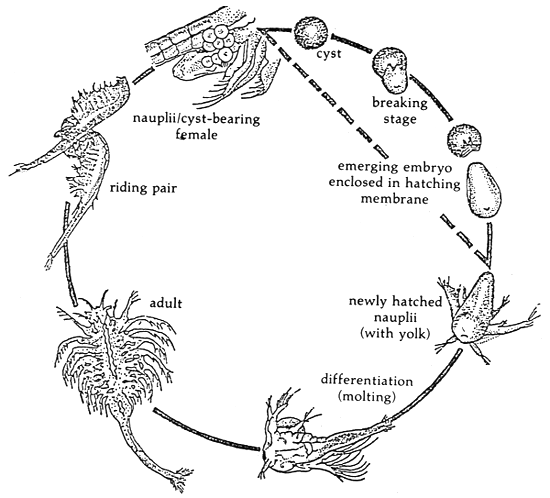
Figure 2. Life cycle of Artemia
3.4 Harvesting
Harvesting of Artemia nauplii is done after 5 to 10 minutes interruption of the aeration. Empty cyst shells float to the surface, while the nauplii concentrate in the lower part of the tank and the unhatched cysts accumulate underneath the nauplii. Since most nauplii are positively photostatic, their concentration can be hastened and increased by shading the upper part of the hatching container with a black plastic sheet so that light reaches the lower part of the container only. Remove the unhatched cysts for the second hatching, after which the nauplii can be collected. A second collection of nauplii may be done 5 to 10 minutes after the first.
by
Siri Tookwinas and Boonchu Charearnrid
1. INTRODUCTION
Lates calcarifer (Bloch) is commonly called the seabass or giant sea perch. It has been cultured in Southeast Asia for more than 10 years, in marine, brackish and fresh waters. The cage culture of seabass in coastal waters is the most popular in Thailand, Hong Kong, Taiwan, Malaysia, Singapore and Indonesia. The method is simple and highly profitable compared to pond culture. Thus, the cage culture of seabass has expanded very rapidly in the last five years in Thailand. However, the major constraints to rapid expansion are the disease outbreaks during culture period and insufficient supply of trash fish.
Despite some imperfections, the basic techniques of seabass cage culture have been developed and now considered economically viable.
1.1 Selecting a suitable site for cage culture
Criteria for selecting a suitable site for cage culture of seabass are the following:
1.1.1 Water salinity (which should range from 10–31 ppt).
1.1.2 Tide and water depth. Water depth should be more than 2–3 meters. This is due to the usual size of culture cage which is 5 m × 5 m deep. The tidal fluctuation should allow the water depth to be at least 2 m at the low water of spring tide.
1.1.3 Current and waves. Area should be protected from strong winds, waves and current. An ideal area would be in protected bays, sheltered coves and inland sea.
1.1.4 Water quality. The site should be relatively free from domestic, industrial and agricultural wastes and other environmental hazards.
1.1.5 Water circulation. The site should have enough water circulation to improve on poor water quality that could occur at some period in the culture due to the decomposition of waste material which often accumulate at the bottom under the net cage.
The water quality parameters which are considered of minimum range for cage culture of seabass is shown in Table 1.
| Parameters | Ranges |
|---|---|
| pH | 7.5–8.3 |
| Dissolved oxygen | 4.0–8.0 mg/L |
| Water salinity | 10–31 ppt |
| Water temperature | 26–32°C |
| Ammonia-nitrogen | less than 0.02 mg/L |
| Hydrogen sulfide | none |
1.2 Nursery
Seabass fry and fingerlings should be reared in concrete tanks up to the size 2.5 cm or 1 inch. After that they can be transferred for rearing in nylon net cages until they attain 25 cm or 10 inches in about two to three months of culture period.
The most convenient cage design is a rectangular cage made of synthetic netting attached to wooden, GI pipe or bamboo frames. It is either (a) kept afloat by styrofoam, plastic carbuoy; or (b) stationary by fastening to a wooden or bamboo pole at each corner. The size of cage varies from 0.9 × 2.0 m and a depth of 0.9 m to 1.0 × 2.0 m and a depth of 1.0 m (Figure 1). The mesh size of the nylon net is 1.0 mm. However, after a month of nursing they can be transferred to cages with nylon net with mesh size of 0.5 cm. This would allow water to pass through the cages more freely.
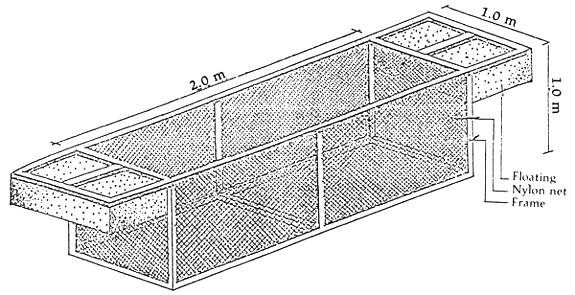
Figure 1. Nylon netcage for nursing seabass/fingerlings
The stocking density is approximately 1 000 fingerlings per cage. Grading of fingerlings has to be done at least once a week during the nursery period. Stocking is done separately for each size group. This would minimize the losses from cannibalism. Fingerlings of 2.5–5.0 cm should be fed with ground trash fish at 8–10 percent of body weight daily or about four to five times a day. After that, they can be fed with finely chopped trash fish.
The net cage should be checked daily to ensure that it is not damaged by crabs or clogged with fouling organisms. The cage should be cleaned every other day by soft brushing in order to allow water circulation in the cage.
The survival rate for the nursery period would be 50 to 80 percent. This would depend on feeding, aquatic environmental conditions, and the expertise of the fish farmers.
1.3 Rearing marketable fish
Fish are reared from juvenile to marketable size for another 5 to 20 months. The marketable size requirements of the seabass are between 700–900 g and 2 000 – 3 000 g. However, the size between 700–900 g is demanded by the local market and consumers in neighbouring countries.
There are two types of cages used in seabass culture in Thailand.
1.3.1 Floating cages
The net cages are hung on GI pipe, wooden or bamboo frames. The cage is kept afloat by styrofoam drum, plastic carbuoy or bamboo. The most convenient dimension for a cage is that of a rectangle and a volume of 50 cubic meters (5.5 m × 6 m × 2 m). The cage unit is stabilized with concrete weight at each bottom corner (Figure 2). The cage unit has to be anchored to the bottom. The cages might be rocked a little by strong wind and current. Floating cages can be set on coastal waters where tidal fluctuation is wide.
1.3.2 Stationary cages
This type is fastened to wooden poles installed at its four corners (Figure 3). Stationary cages are usually set in shallow bays where the tidal fluctuation is narrow.
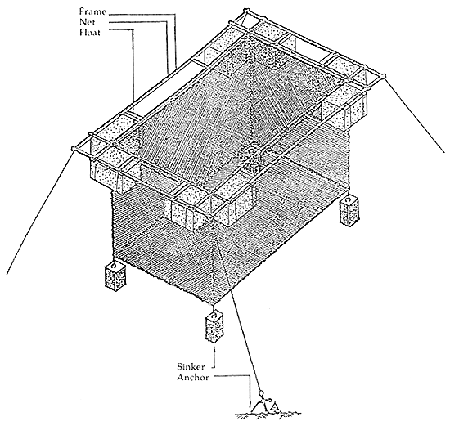
Figure 2. Floating cage
The mesh size of nylon net would depend on the size of fish as shown in Table 2. Firstly, fingerlings should be transferred to a nylon net (mesh size of 2.0 cm) for about two months of culture period. Then they are moved to a cage net of 4.0 cm mesh size until harvest.
Stocking density for marketable fish culture varies from 12 sq m to 300 sq m (Table 2), depending on water quality and the environmental conditions of the culture site. Floating cages can be stocked with more than stationary cages. This is because floating cages are usually set in sites with better aquatic environmental condition such as deeper water, narrower fluctuation of water salinity, more rapid circulation and further away from sources of pollution.
Trash fish is the main feed for seabass culture. Trash fish used in Thailand are sardines and other small marine fish. The trash fish should be chopped and fed twice a day, in the morning and afternoon. The size must be suitable for the size of the mouth of the fish. The farmers should give the feed slowly and watch the fish. Feeding should be stopped when the fish no longer come up to the surface; it shows that the amount of feed is enough for them.
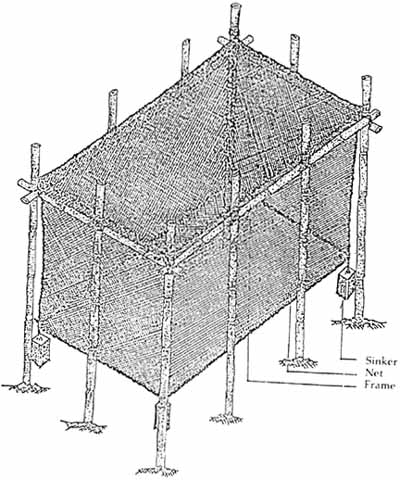
Figure 3. Stationary cage
| Culture period (days) | Stocking density | ||||
|---|---|---|---|---|---|
| 100/m3 (g) | 150/m3 (g) | 200/m3 (g) | 250/m3 (g) | 300/m3 (g) | |
| 30 (1 m) | 119.7 | 115.6 | 116.7 | 117.7 | 117.8 |
| 60 (2 m) | 222.7 | 218.4 | 208.6 | 212.4 | 208.1 |
| 90 (3 m) | 309.0 | 306.4 | 294.4 | 293.1 | 285.1 |
| 120 (4 m) | 380.0 | 361.2 | 368.0 | 353.0 | 345.7 |
| 150 (5 m) | 448.0 | 420.5 | 418.0 | 410.9 | 379.4 |
| 180 (6 m) | 523.4 | 495.8 | 463.3 | 449.9 | 436.5 |
| 210 (7 m) | 573.3 | 569.9 | 551.4 | 527.9 | 505.4 |
Food conversion rate of seabass culture in Thailand decreases with stocking density. They range from 3.0 to 10.0. It also depends on the quality and quantity of trash fish. Normally, seabass can grow at an average of 1 kg/yr.
Survival rates for marketable fish culture would be about 80–95 percent in normal culture conditions.
The cages should be checked bimonthly or monthly to ensure that they are not damaged by fouling organisms, crabs or floatsam. The cages should be cleaned or changed every month. Therefore, fish farmers should have spare nylon net cages. Changing cages also allows the farmer to check on the number and health of the fish.
1.4 Financial analysis for small-scale fish culture
Types of cages can be divided into two: (a) standard which is made of GI pipe frames; and (b) ordinary which makes use of wooden frames. The total cost is 12 700 baht per cage of the standard type and 4 400 baht per cage of the ordinary type. The cost per year is 4 051 for the standard type and 1 575 baht for the ordinary type (Tables 3 and 4).
A floating cage can culture 1 000 fish. The cost would be about 14 675 to 17 151 baht. The production or yield is about 350 kg/yr.
Thus, the fish farmer can realize a benefit of about 3 849 to 6 325 baht per cage per year (Table 5).
| Material | Number | Duration (yr) | Total cost (฿) | Cost per year (฿) | |
|---|---|---|---|---|---|
| 1. | GI pipe frame | 4 | 5–8 | 2 800 | 623 |
| 2. | Nursing net | 3 | 1–2 | 900 | 600 |
| 3. | Marketable net | ||||
| a) mesh size 2.0 cm | 1 | 3–5 | 3 600 | 1 028 | |
| b) mesh size 4.0 cm | 1 | 3–5 | 2 000 | 500 | |
| 4. | Styrofoam drum | 1 | 3–5 | 2 000 | 500 |
| 5. | Other materials | - | - | 1 000 | 500 |
| Total | 12 700 | 4 051 |
| Material | Number | Duration (yr) | Total cost (฿) | Cost per year (฿) |
|---|---|---|---|---|
| 1. Wooden frames | 4 | -2 | 800 | 400 |
| 2. Styrofoam drum | 4 | 2–4 | 1 200 | 400 |
| 3. Nursing net | 1 | 3–5 | 1 000 | 250 |
| 4. Marketable net | 1 | 3–5 | 900 | 225 |
| 5. Other material | - | - | 500 | 300 |
| Total | 4 400 | 1 575 |
| Standard type | Ordinary type | |
|---|---|---|
| 1. Cages | 4 051 | 1 575 |
| 2. Fingerlings | 2 500 | 2 500 |
| 3. Feed | 7 000 | 7 000 |
| 4. Labour | 3 600 | 3 600 |
| Total | 17 151 | 14 675 |
| 5. Yield (kg) | 350 | 350 |
| 6. Income (B) | 21 000 | 21 000 |
| 7. Benefit (B) | 3 849 | 6 325 |
REFERENCES
Kungvankij, P., et. al. Biology and culture 1986 of seabass (Lates calcarifer), NACA Training Manual Series No. 3. NACA/RLCP, Bangkok. 70p.
Sakaras, W. Optimum stocking density 1986 of seabass (Lates calcarifer) culture in cages. ACIAR Proceedings No. 20. 172–175 pp. Canberra Printing Co., Melbourne, Australia.
Tookwinas, S., et. al. Cage culture of 1987 brackishwater fish in Satul Province. Technical Paper, Brackishwater Fisheries Division, Department of Fisheries. 30p.
Tookwinas, S. Consideration aspects of 1985 coastal aquaculture survey. Thai Fisheries Gazette. 38(4): 243–249.
by
Siri Tookwinas
1. INTRODUCTION
Cage culture of seabass is one type of coastal aquaculture that can be undertaken in the sublittoral zone of the coastline. The coastal ecosystem or estuarine ecosystem is where freshwater as run-off from the land and seawater meet. Consequently, the estuarine environment is more extreme and undergoes more violent fluctuations than the open sea or freshwater habitats. Therefore, coastal aquatic organisms have to tolerate variations in the physico-chemical properties of the habitat.
This lesson describes some ecological aspects of seabass which have been cultured in cages in Thailand. Certain bio-physico-chemical parameters of the estuarine ecosystem which influence cage culture of seabass are summarized. Some methods for water quality management are also explained.
2. SUITABILITY OF THE AQUATIC ENVIRONMENT (Figure 1)
As mentioned previously, an estuarine ecosystem is subject to various changes in the aquatic environmental parameters. Those aquatic environmental factors that influence cage culture of seabass are summarized below:
2.1 Water salinity
Water salinity tends to fall with increasing distance from the open sea but this is not always true. The water salinity gradient depends upon the relative balance of three factors: (a) run-off from the land; (b) rainfall; and (c) evaporation from the estuary itself. The range of water salinity tends to be greater near the water surface than in the bottom. This is due to the specific gravity of sea water being greater than that of freshwater (Table 1).
| Chlorine (gm/l) | Salinity (S) (ppt) | |
|---|---|---|
| Freshwater | less than 0.1 | less than 0.21 |
| Oligohaline | 0.1–1.0 | 0.21–1.84 |
| Mesohaline | 1.0–10.0 | 1.84–18.0 |
| Polyhaline | 10.0–17.0 | 18.0–30.0 |
| Seawater | more than 17.0 | more than 30.0 |
When the water salinity decreases, aquatic organisms use up more dissolved oxygen for respiration indicating an increase in metabolic rate. Osmotic regulation has to occur, otherwise organisms would be killed by the reduced water salinity.
2.2 Dissolved oxygen
Fundamentally, the concentration of oxygen dissolved in seawater is inversely dependent upon salinity and temperature and is normally about 80 percent of the concentration in freshwater at the same temperature. Vertical gradients in oxygen concentration develop only in estuaries which have a vertical salinity stratification. In other words, the amount of oxygen at various water depths vary with the differences in salinity also at various water depths. Regeneration of oxygen in estuaries is brought about by mixing with well-oxygenated water from rivers or the sea, direct re-aeration from the air, and by the photosynthetic activity of plants. It should be noted that in an unpolluted estuary, decaying organic matter can produce localized oxygen deficiencies in bottom water. However, in a polluted estuary, the oxygen concentration drops and under extreme conditions, the water may become entirely lacking in dissolved oxygen (anoxic).
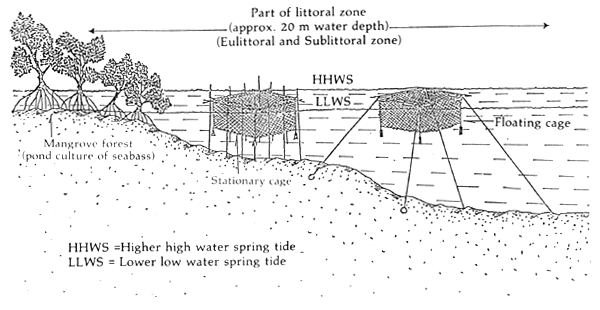
Figure 1. Suitable habitat for cage culture of seabass
The desirable range of dissolved oxygen for aquatic organisms is 5.9 mg/l and higher. A range of 5.0 mg/l to 1.0 mg/l may have a pronounced but sublethal effect on growth rate to fish and other aquatic organisms. A value of less than 1.0 mg/l would have a direct harmful effect on fish (Figure 2).
2.3 pH
The pH of estuarine water is more variable than that of the open sea. Under normal and unpolluted conditions, pH values from 6.8–9.25 have been recorded. In vertically stratified estuaries, the surface water generally have a higher pH than the bottom water. This is due to photosynthetic activities in the surface water taking up carbon compounds from the water column. The absorbed carbon compounds would affect pH balance in the surface water.
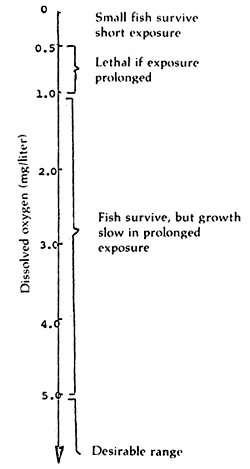
Figure 2. Effect of dissolved oxygen on aquatic organism (After Boyd, 1979)
The desirable range of pH for fish production is 6.5–9.0. Values lower than 6.5 and higher than 9.0 have direct effect on fish (Figure 3).
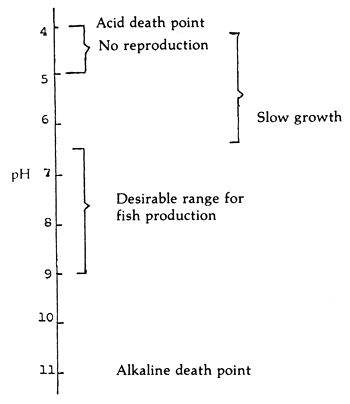
Figure 3. Desirable range of pH for aquatic organisms (After Boyd, 1979)
2.4 Turbidity
The penetration of light into estuarine water depends largely on the turbidity which is much more in estuaries than in the open sea. This turbidity is due to sedimentary materials from three sources: (a) the rivers flowing into the estuary; (b) transport into the estuary from the open sea; and (c) a reworking within the estuary.
In general, turbidity decreases and, therefore, the depth of light penetration increases as one gets nearer the open sea. The effect of turbidity and rapid absorption of light upon the photosynthetic processes which can take place in an estuary is considerable. Clearly, the phytoplankton will receive sufficient light for this vital process in the surface layers only, and will generally contribute little to primary production in turbid estuaries. Under these conditions, the shore-dwelling plants particularly the microflora take on a real importance as the primary source of food.
3. WATER QUALITY IN SEABASS CULTURE AREA
The physico-chemical properties of coastal aquaculture and seabass cage culture in lower west south of Thailand (Krabe, Trang and Satul Provinces) were surveyed between July 1980 and August 1982 in 19 survey stations. The methodology for water analysis was set along the standard line of APHA (1975), Lind (1974) and Strickland and Parsons (1969) as temperature, visibility, dissolved oxygen, pH, salinity, ammonia-nitrogen, nitrate-nitrogen, phosphate and silicate.
The water quality then were as follows (Table 2):
| Parameters | Mean | S.D. |
|---|---|---|
| Depth,m | 2.10 | 1.348 |
| Visibility,m | 0.87 | 0.324 |
| DO, mg/L | 5.24 | 0.495 |
| pH | 7.80 | 0.170 |
| Salinity, ppt | 26.10 | 3.923 |
| NH3-N, mgN/L | 0.01 | 0.01 |
| NO2-N, mgN/L | 0.0039 | 0.004 |
| PO4, mg PO4/L | 0.04 | 0.018 |
| Si, mg Si/L | 2.50 | 0.279 |
4. SUDDEN FISH KILLS IN SONGKHLA LAKE
4.1 Cage culture of seabass in Songkhla Lake
Songkhla Lake is the largest lagoon in Thailand and in Southeast Asia as well. It is located at latitude 7°8'N, 7°50'N and longitude 100°07'E, 100°37'E. Total area is approximately 98 680 hectares (616 370 rai). The eastern side of the lake (Thale Sap Tonnok) opens into the Gulf of Thailand).
The major aquaculture system in the lake is seabass culture in netcages. It was introduced 16 years ago (1972) by the Department of Fisheries. In 1986, seabass production from the 300 netcages of some 115 farmers in the lake was approximately 98.5 tons.
The annual average water values in the lake (Thale Sap Tonnok) are as follows: salinity, 13.68 ppt; dissolved oxygen, 7.17 mg/l and pH, 7.89. More details are shown in Table 3.
| Parameters | Mean | Ranges |
|---|---|---|
| Temperature (C) | 30.17 | 24.0–34.0 |
| Turbidity, (FTU/ NTU) | 19.34 | 6.90–33.50 |
| Conductivity (mm/hos/cm) | 22.24 | 0.10–58.70 |
| Salinity (ppt) | 13.68 | 0–34.00 |
| pH | 7.89 | 6.40–8.55 |
| Dissolved oxygen (mg/L) | 7.19 | 3.10–9.95 |
| COD (mg/L) | 2.46 | 0–9.50 |
| Orthophosphate (mgP/L) | 0.27 | 0–1.60 |
| Nitrate-nitrogen (mgN/L) | 0.01 | 0–0.09 |
| Alkalinity (mg/L) | 56.24 | 21.43–95.00 |
| Acidity (mg/L) | 2.79 | 0–5.96 |
4.2 Cause of sudden fish kills
The fish farmers in Songkhla Outer Lake always face the problem of fish kills in the summertime. Results of technical investigations have shown that the fish kills are caused by aquatic environmental pollution; the depth of water for cage culture was only 0.37–0.90 m (average 0.66 m). Dissolved oxygen was depleted to 1.30 mg/l at night time (Table 4 and Figure 4).
| Average from every culture area | |||
|---|---|---|---|
| Parameters | Mean | S.D. | The area of sudden fish killed |
| Depth (m) | 0.91 | 0.255 | 0.66 |
| Current (m/sec) | 0.04 | 0.077 | 0.0 |
| Salinity (ppt) | 23.7 | 4.029 | 24.0 |
| pH | 8.20 | 0.13 | 8.05 |
| Dissolved oxygen (mg/L) | 5.47 | 0.935 | 3.85 |
| NH3-N (mgN/L) | 0.019 | 0.000 | 0.014 |
| BOD (mg/L) | 0.89 | 0.315 | 1.40 |
| Soil COD (mgO2/L) | 1.350 | 0.626 | 2.381 |
Note: The water samples were collected between 0900–1100 hours.
The dead fish were examined and a bacterial plate count was conducted at the disease laboratory. The morphology of the fish and the bacterial check on dead and live fish showed normal conditions.
Other culture areas were also investigated. The bottom sediment under the netcage contained a high value of waste organic matter as shown in chemical oxygen demand (COD) value. The benthic organism found was a polychaete which can bloom in polluted condition. It can be noted that the decomposition process occurs at the bottom sediment which consumes a lot of dissolved oxygen in water column.
5. AQUATIC ENVIRONMENTAL IMPROVEMENT
Seabass culture in Songkhla Outer Lake has been going on for 8 to 10 years. The waste material from cage culture is directly deposited at the bottom. The cages are also set very close to each other so that the number of culture cages could have been more than the carrying capacity of the area. The fish farmers also stock densely at 42.8 kgs/cubic meter. The technical investigations suggest that the aquatic environment at the culture area must be improved by following these measures:
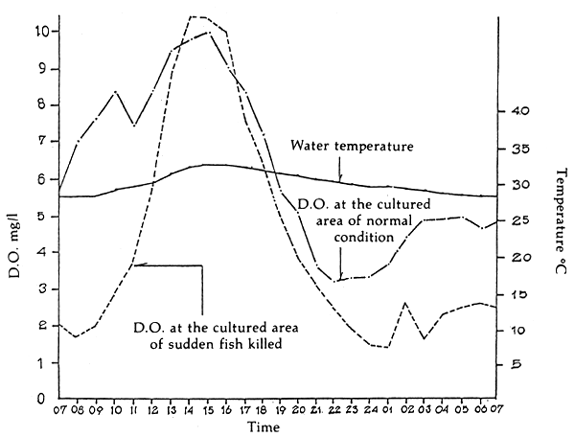
Figure 4. Dissolved oxygen in 24 hours of seabass culture in Songkhla Outer Lake (After Tookwinas, 1986)
5.1 The maximum stocking density of fish should be 300 fish per cage (cage size 7 ×8 × 2 m).
5.2 The dissolved oxygen can be increased by air pump especially at night from 0200–0800 hours.
5.3 For a long-term improvement measure:
The culture cages should be moved further away from one another and away from the village (about 300 m). This would avoid the effect of domestic waste materials.
The bottom sediment should be dredged. This would directly decrease the decomposition of waste materials.
REFERENCES
Boyd, C.E. 1979 Water quality in warm water fishponds. Auburn University, Alabama. 359p.
Perkins, E.J. 1974 The biology of estuaries and coastal waters. Academic Press Inc. Ltd., London. 667p.
Remane, A. and C. Schlieper. 1971 Physiology of brackishwater. Wiley Inter-science Div., New York. 365p.
Tookwinas, S., et. al. 1985 Investigation on water properties in coastal aquaculture areas in Krabe, Trang and Satul Provinces. Brackishwater Fisheries Division, Dept. of Fisheries. 35p.
Tookwinas, S., et. al. 1986 Study on aquatic environment of seabass cage culture at Songkhla Outer Lake: Investigation on the cause of sudden fish mortality. Thai Fisheries Gazette, 39(3):255–263.
Tookwinas, S. 1986 A general review of the hydro-physico-chemical properties of Songkhla Lake, Southern Thailand. Songklanakarin J. Sci. Technology 8(1):111–115.
by
Lila Ruangpan
1. INTRODUCTION
The recent emphasis on intensive fish culture has also brought a more acute concern for problems associated with pathogenic agents that cause diseases. The diseases whether by slow continuous attrition or by sudden catastrophic epizootics (a widespread outbreak of disease-causing microbes), can cause fish mortalities often resulting in great losses of the stocked fish. Generally, infectious diseases of fish and other aquatic animals are caused by parasites, bacteria, fungi and virus. But diseases and abnormalities due to environmental stresses and nutritional deficiencies have also been recognized. These will continue to be important until adequate and refined diets, as well as effective control of water quality are developed and fully implemented. These latter causes of mortality are not infectious diseases but they can be harmful to fish and result in secondary infection.
The technology of reducing the impact of diseases is evolving but most disease specialists feel at times like they were sorcerers or apprentices as newer and often more difficult problems emerge to replace the solved problems of yesterday. It would seem, therefore, that the biologists who have more experience and more interest in this work could get better results.
2. VIRUS
Viral diseases have not been considered to be a significant factor in marine and brackishwater culture, but there have been many reports indicating the existence of viral diseases in oysters and crustaceans which cause mortalities to their populations. However, such disease as lymphocystis has recently become one of the problems in seabass culture.
2.1 Lymphocystis disease
Lymphocystis disease is commonly found in seabass raised in cages especially among juveniles 4–7 cm in total length. It has been observed at all temperatures in rather high salinity. A gross sign of the disease is massive enlargement of the cell within the dermis layer of the fish skin which resembles the cauliflower disease (Figure 1). Transmission is from fish to fish. At present, no facilities for studying the virus are available so that the procedures or techniques for its diagnosis will not be discussed here.
2.2 Bacteria
Bacterial diseases in fish generally do not develop simply as the result of exposing a host to an infectious agent. Mostly, bacterial diseases occur as a result of the complex interactions between pathogen, fish and environmental stress which affects the susceptibility of the fish to diseases. Environmental stresses can affect the homoestatic mechanism of fish thus reducing their resistance to disease-causing organisms.
Fish reared in intensive culture conditions are exposed to extreme environmental fluctuations and they may be more sensitive to stress than wild populations.
2.2.1 Aeromonas bacteria
Aeromonas sp. is a common water-borne bacteria which may be present in the tissue of normal young or adult fishes. Whenever fishes are exposed to environmental stress or injury, Aeromonas causes serious outbreaks of haemorrhagic disease with high mortality. Temperature, pH, high CO and O depletion, decomposition products and free ammonia in the water, all of these can be considered as possible factors for Aeromonas infection.
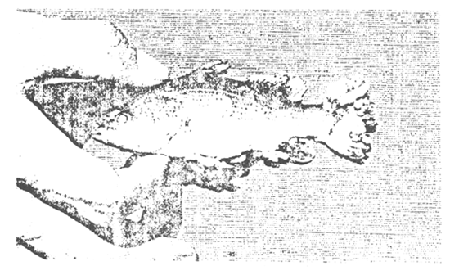
Figure 1. Lymphocystis disease
When seabass are overcrowded and water salinity is low for long periods, the diseased fish caused by A. punctata could be observed. Gross signs and behaviours are usually shown by hemorrhage on the fin and tail. In a heavy case, erosion of tail and fin can be clearly seen (Figure 2).
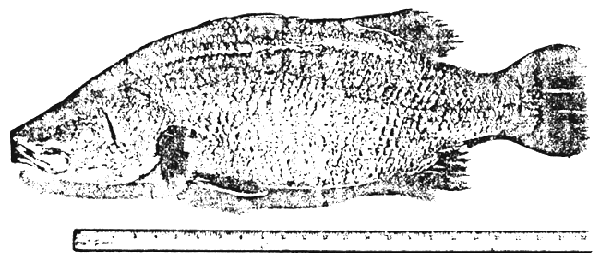
Figure 2. Vibrio bacterial disease
2.2.2 Vibrio bacteria
Diseases caused by Vibrio sp. typically appear as ulcerative haemorrhagic septecaemia. The typical symptoms of vibrio disease include congestion of the fins, eccymoses and petechiae on the body surface and frequently, haemorrhages and ulceration of the skin and muscle tissue. The tissues surrounding the infected anus (the vent) are usually reddened and inflamed. Internally, there is congestion and haemorrhagia of the liver, spleen and kidney, frequently accompanied by the presence of necrotic lesions. The gut and particularly the rectum may be distended and filled with a clear viscuous fluid (Figures 3 and 4).
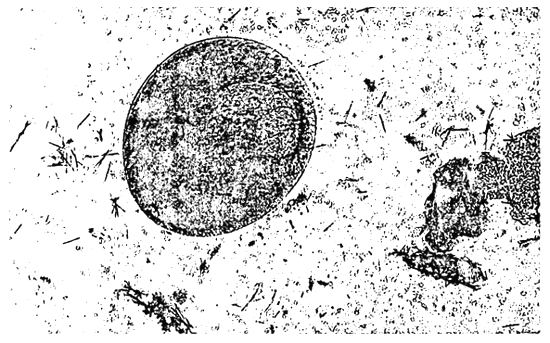
Figure 3. Cryptocaryon sp.; protozoa which cause the white spot disease in seabass
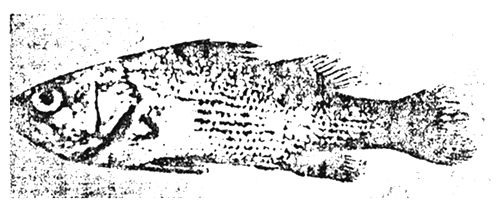
Figure 4. White spot disease
Vibrio disease in young fish has less welldefined clinical signs. The body is completely covered by a thick layer of mucus. Occasionally, small unbroken lesions are present. There may be a reddening of the caudal fins and vent. Internal organs appear normal. Young fish die more rapidly than adults.
The pathogenic vibrio which have been isolated from seabass include Vibrio parahaemolyticus, V. anguillarum and V. alginolyticus.
2.2.3 Columnaris disease
Columnaris disease caused by Flexibacter columnaris is one of the diseases commonly found in juvenile seabass which are raised in water of low salinity during rainy and winter seasons. Gross signs are observed by saddle-shaped lesions in the mid-body position about the dorsal fin of the fish. The bilaterally symmetrical lesion appears as a fuzzy, pale yellow white plaque, with dark margins, often eroding in the epidermis (Figure 5). Clinically, the condition may be chronic, acute or peracute. The gram negative, aerobic bacillus (about 12 um) can be isolated from the lesion of the diseased fish.
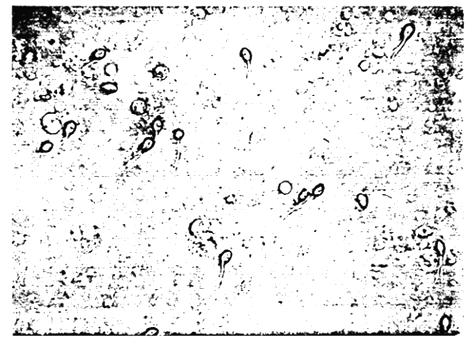
Figure 5. Henneguya sp., on the gills of seabass
2.3 Parasitic protozoa
Protozoans are probably the most important group of animal parasites affecting fish. Many reports from all over the world indicate great losses in fish culture caused by protozoans. Obligate parasites such as the ciliate ichthyophthirius and certain species of the cnidosporidians are responsible for many of these losses. Many species which are considered as commensal protozoans may become pathogenic under certain conditions. Environmental factors affect the susceptibility of fish to certain protozoans. Oxygen concentration and temperature are the factors affecting both hosts and parasites. Since many protozoans transfer from fish to fish through the water, fish population density is an important factor. Tremendous infestation of protozoans can occur in a relatively short time where fish populations are dense. Other factors such as host size, age, host specificity, immunity and the aforementioned influences of host condition also play an important part in the host reaction to invasion by protozoans. Most host reactions to invasion by protozoans are directed expelling or isolating the parasite.
Protozoans cause harm to fish mainly by mechanical damage, secretion of toxic substance, occlusion of the blood vessels, depriving the host of nutrition and rendering the host more susceptible to secondary infections. Some of the most common clinical signs are changes in swimming habits such as loss of equilibrium, flushing or scraping, loss of appetite, abnormal colouration, tissue erosion, excess mucus production, haemorrhage and swollen body or distended eyes.
2.3.1 Cryptocaryon sp.
Cryptocaryon sp. is a marine counterpart of the freshwater ichthyophthirius species and similarly causes the white spot diseases in marine fish. Its morphology and life cycle is quite similar to that of the “Ich”. The surface of invaded fish reveals white pustules or numerous minute, greyish vesicles which are nests of cilliates burrowing under the epidermis. They feed on the host's cells underneath the epithelium and cause heavy irritation resulting at first in excessive production of mucus and finally completely destroying the fine respiratory platelets of the gill filaments. On the skin, this parasitic protozoan causes considerable lesions resulting in destruction of large areas of the epidermis (Figure 6). Secondary infection may complicate the situation and the host dies. The incidence of Cryptocaryon sp. in seabass showed a distinct peak during low water temperature period, with a marked prevalence during February. This ciliated protozoan probably causes more damage to fish populations over the entire world than any other single parasite.

Figure 6. Epistylis sp., attached on the skin of seabass rearing in freshwater
2.3.2 Trichodina sp.
Members of the genus Trichodina with about 60 species described from marine fish and related peritrichous ciliates are the most common parasitic protozoans that are especially harmful to young fish. The species attach themselves to the gills of marine fish. More than half of juvenile seabass heavily infected with this parasite died. Trichodina also causes problems to crowded seabass in cages.
Clinical signs of trichodinosis include excess mucus production, flushing, debility and hyperplasia and necrosis of the epidermis. The fin may become badly frayed in heavily infected fish and this may be accompanied by sluggishness and loss of appetite. Excessive number of the gills of infected fish interferes with respiration (Figure 7).
2.3.3 Henneguya sp.
Henneguya sp. is a flaggelate found to attach mainly to the gills of seabass in cages. In heavy infections, it may be found in the skin (Figure 8). Gross signs are hyperplasia, bronchitis plus necrosis.
Life cycle involves a free-swimming dinospore which moves by means of two flagellae. It attaches to the host and transforms to a sac-like trophont which has elaborate attachment mechanism. It feeds and grows and detaches from host, sinks to substrate where it encysts and produces dinospores.
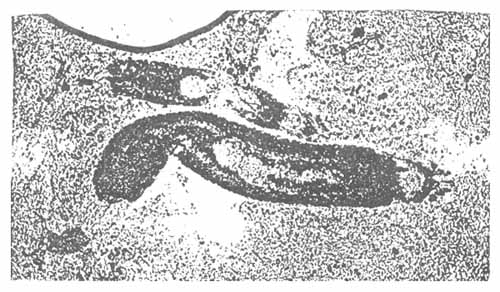
Figure 7. Monogenetic trematodes
2.3.4 Epistylia sp.
Epistylis is another protozoan found in seabass especially in freshwater environment. Epistylis belongs to the sub-class Peritrichia, and is a common ectocommensals; however, it occasionally turns pathogenic. It attaches to the fish with its stalks. This protozoan may be present at a variety of temperatures and its number may be large enough to cause a grey mat on the epithelial surface (Figure 8).
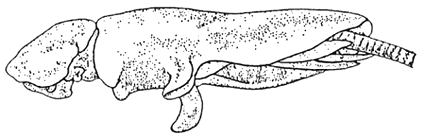
Figure 8. Lernanthropus sp.
2.4 Parasitic helminthes
Worm diseases with the possible exception of those produced by monogenetic trematodes have not yet appeared to be a serious problem in seabass culture. This is probably due in large part to their complex life cycle and the difficulty in completing such cycles in the culture system. Helminthes parasites which have been found in seabass include monogenetic trematode, digenetic trematide, nematide and acanthocephala.
2.4.1 Monogenetic trematodes
Monogenetic trematodes can be observed throughout the year. Abundance of these parasites and their seasonal distribution have not been studied. It has been reported that temperature apparently plays an important role in determining outbreaks of certain parasitic Monogenera. Peak infections of monogenetic trematodes usually occur among young susceptible fish. Such behaviour is advantageous for the spread of the organism in a fish population.
A monogenetic trematode which has been found to be a dominant species in the gills of seabass is Diplectanum latersi. Dactylogyrus sp. may be present but this has not been fully identified (Figure 7).
2.4.2 Digenetic trematodes
Lecithochirium sp. was found in the intestine of seabass especially in wild fish. Incidence of infection was 86.0 percent and average parasite burden was 5.5. Another digenetic trematode which was commonly found in the intestine of wild seabass is Pseudometadena celebesensis. Its incidence of infection and parasite burden were 100 percent and 9.3, respectively.
2.4.3 Nematodes
Although many species of nematodes are found either as adults or larvae in fish, few have been implicated as serious pathogens of their hosts. In seabass, nematode of the genus Cucullanus was found more common in the gut of larger fish than in that of young fish.
2.4.4 Acanthocephala
Acanthocephalid worms, despite their fearsome-looking proboscis with its rows of hooks have not been observed as serious pathogens of fish. The great majority of acanthocephalus in seabass are found as adults in the gut.
2.5 Parasitic copepods
The parasitic copepods are among the most devastating of fish parasites. The mature female usually attaches to the fish and feeds on the host. After copulation the female matures and produces egg sacs while the male dies.
2.5.1 Caligus sp.
Caligus sp. has caused big problems in cultured seabass. They attach to the gills, buccal and opercular cavities, occasionally on the skin and fins of the seabass. Heavy infections can cause mass mortalities especially in young fish.
2.5.2 Lernanthropus sp.
Lernanthropus sp. are found attached to the gill of seabass especially in cage cultured fish. Large numbers of this parasite can cause anaemia to the fish host.
2.6 Parasitic isopods
Isopods which closely resemble Aega sp. have been found abundant in cage-cultured seabass. The parasite always attaches to the gills of its host. Clinical signs of infected seabass are as follows: fish lose appetite, become anemic and grow very slowly. Quick death can occur in 2–3 days in heavily infected young fish.
2.7 Other causes of mortality
Diseases and abnormalities due to environmental contaminants and nutritional deficiencies have been recognized as important problems in fish culture whenever diets as well as control or water quality become inadequate.
2.7.1 Nutrition
Nutrition is vital for growth, maturation and reproduction even for fish life itself. In any environment, nutritional requirements of living organisms must include the various classes of nutrients to be furnished by the ration or available from the environment. Malnourishment or undernourishment of seabass under culture can result in slow growth, susceptibility to diseases or death.
2.7.2 Predators and pest organisms
Cultured fish must be protected from other animals which can be pests and predators or sometimes vectors of diseases such as leeches, water snakes, crabs, snails, water fowls and of course fish which might be either resident or accidentally introduced into the culture impoundment. The important enemies or predators of seabass in Thailand are Therapin jarbua and Pleuronema tetradactylum. Frequently, the bigger fish in this group by themselves can be predators. So the best way to reduce fish mortality is size grading. Usually culturists will grade their juvenile every three days. An important rule of fingerling production in a hatchery is not to rear or stock different age and size groups of fish in the same pond.
To eliminate other predators in the ponds, apply saponin in the form of teaseed cake (2 kg/rai with reduced depth of water of 10 cm) or rotenone (2–2.5 kg/rai with water depth of 10 cm).
2.7.3 Unfavourable environment and other stresses
Environmental causes of death also occur. These include lack of or very low oxygen content, extremes of salinities and temperature, etc. Other major problems are the stresses on fish that are in long-term contact with unsuitable environmental conditions such as temperature, oxygen, pH, salinity, light and other physical or chemical disturbances including various toxic materials.
3. IDENTIFICATION AND TREATMENT OF DISEASES AND PARASITES
Disease control depends on a combination of three factors: diagnosis, prevention and treatment. Effective control of diseases in seabass can be done by a combination of measures.
3.1 Diagnosis and prevention
3.1.1 Correct diagnosis (including understanding of the cycle and ecology of the pathogen) is obviously a critical step in any control programme.
3.1.2 Preventive measures constitute the core of disease control programme, and include:
3.2 Treatment
Treatment is usually in the form of chemotherapy, possible combined with some of the preventive measures listed above. Chemical control should be the “last resort” in disease control.
3.2.1 Chemical prophylaxis
The United States Food and Drug Administration (FDA) rules governing the use of drugs which can serve as useful guidelines are summarized below:
3.2.2 Use of chemicals for pond treatment
To treat the pond accurately, the volume of water in the pond must be known. To determine the volume of a pond, multiply the number of surface unit area of water by the average depth of the pond.
The following chemicals are often used in the treatment of various fish diseases:
3.2.3 How to apply the treatment
For small ponds, dilute the chemical in a bucket of water and distribute evenly over the pond using a dipper. In large ponds, the chemical should be mixed in a large drum with a spigot (faucet) connected to a 2 m hose. The chemical should be distributed evenly over the pond with a boat equipped with an outboard motor. A small but steady stream of the solution is delivered into the wake of the propeller while the boat cris-crosses the pond surface.
3.2.4 Conclusions on disease and parasite prevention
3.2.5 General treatments
When treating fish, it is advisable to know the quality of the water because such things as pH and temperature greatly affect treatment results. Treat a few fish first and see how they react before treating the entire group. Use only the drugs and chemicals that have been cleared by an authorized agency for use on food fish. Here are some general treatments for specific groups of pathogens:
a) Viruses
No treatment known. Avoidance and prophylactic measures are best to prevent viral diseases.
b) Bacteria
Treated best by injection or use of food additives or antibiotics.
Terramycin 2.5–3.0 g per 100 lb body weight (BW) per day for 10 to 12 days. (A withdrawal period of 21 days is necessary before the fish are marketed).
Sulfamerazine, Nitrofurans, Furazin, 10 g per 100 lb BW per day for 10 days. (Also Furozone and Furanace).
Erythromycin, 4.5 g per 100 lb BW per day for two weeks.
Potassium permanganate (KMnO), used as a wide-spectrum treatment at the rate of 2–3 ppm in ponds gives good results against bacterial infections.
c) External parasites
Formalin is the best treatment for protozoans and gill flukes. Effective rates are 15–25 ppm as a pond treatment and 100–250 ppm for one hour as a prolonged treatment. At temperature below 60°F (15.6°C) fish will tolerate a formalin concentration of 250 ppm for one hour. A rate of 100 ppm for one hour should be used at higher temperature. Fish should be closely watched during the treatment period; if they show distress, put them back into freshwater. Oxygen depletion may occur a few days after treatment with formalin. Malachite green has been used succesfully to treat cryptocaryon. A combination of 25 ppm formalin and 0.1 ppm malachite green can give excellent results against cryptocaryon. Malachite green has been used as a dip treatment at a concentration of 1:15 000 to control fungus on both fish and eggs. Acetic acid at a 1:5 concentration has been used for 1–2 minutes to control external parasites.
Dylox has been shown to be an effective control for anchor worm and crustaceans. It is also effective as a treatment for gill and body flukes but is not effective against protozoans. Concentration is 0.25 ppm. At high pH levels, treatment should be done with caution. Acriflavin has been used at 3–5 ppm to treat external parasites.
d) Internal parasites
Most parasites inhabiting the alimentary canal can be controlled by the use of antihelminthes Di-N-Butyl tin oxide. The tin compound can be mixed in the food at the rate of 1 percent and fed at 3 percent of BW for three days.
Remember the following precautions during treatment:
by
Siri Tookwinas
1. INTRODUCTION
Seabass (Lates calcarifer) has a great commercial value in Southeast Asia and the Pacific. Many countries have selected seabass culture for research and development. In Thailand, spawning of seabass had been successfully achieved which has made seabass fry readily available. Cage culture of the fish has been done mainly in the southern part of Thailand. However, culture has expanded to other parts of the country. Production is sold in the country or exported to neighbouring countries such as Malaysia, Singapore, Hong Kong and Australia.
2. CULTURE
Marine fish culture in Thailand has been practised in ponds and cages. Seabass can be cultured in a pond or a cage. In comparison, grouper can be cultured only in a cage. This is due to the water salinity and other habitat requirements of the species.
| Type of culture | No. of families | Area (rai)1 | Percent by area | |
|---|---|---|---|---|
| 1. | Fish culture | 1 579 | 3 698 | 1.58 |
| a) pond culture | 289 | 3 418 | 1.46 | |
| b) cage culture | 1 290 | 280 2 | 0.12 | |
| 2. | Shrimp culture | 4 480 | 217 574 | 92.98 |
| 3. | Crab culture | 122 | 369 | 0.16 |
| 4. | Oyster culture | 1 170 | 3 924 | 1.67 |
| 5. | Mussel culture | 257 | 1 456 | 0.62 |
| 6. | Cockle culture | 112 | 6 956 | 2.97 |
| 7. | Horse mussel culture | 6 | 13 | 0.005 |
| 8. | Others | 3 | 3 | 0.001 |
| Total | 7 720 | 233 993 | 100.00 | |
1 rai = 1 600 m2
2 280 rai = 17 920 cages (5 + 5 + 2m)
From the fishery census of 1985, some 1 579 families engage in marine fish culture in an area of 3 698 rai (6.25 rai = 1 ha) with around 17 920 cages (Table 1).
Some 87.02 percent of the marine fish culture is in the south (Table 2). Finally, the 1985 total production of seabass was about 512 tons, more than the combined production of grouper and mullet (Table 3).
| Zone | Number of families | Percent |
|---|---|---|
| Eastern part | 94 | 5.95 |
| Central part | 222 | 7.03 |
| Southern part | 1 374 | 87.02 |
| Total | 1 579 | 100.00 |
| Species | 1981 | 1982 | 1983 | 1984 | 1985 |
|---|---|---|---|---|---|
| Seabass | 215 | 145 | 1 059 | 473 | 512 |
| Grouper | - | - | 176 | 149 | 117 |
| Mullet | - | 1 | - | 4 | - |
| Total | 215 | 146 | 1 235 | 626 | 629 |
3. MARKETING
Seabass is more expensive than most other fish species. The demand is, therefore, rather limited to those who can afford it. The supply for the local market is already adequate and the prospect for markets abroad is being developed by local producers. The demand for specific processed types and various sizes of marketable fish will also influence the expansion of the industry and its foreign market.
At present, cultured marine fish especially seabass are sold in the local markets (in the provinces and in Bangkok). The product is also exported to neighbouring countries such as Malaysia, Singapore, Hong Kong and Australia (Figure 1). From the marine fishery census of 1985, most of the marine fish products were sold in the provincial areas (Table 4).
| Provincial area | Other provinces | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of culture | No. of family | Without sale | With sale | Sale to consumer | Sale to retailer | Sale to middleman | Sale to fish processor | Sale to Bangkok | Sale to1 others | Sale to fish processor | Not reported |
| Fish culture | |||||||||||
| 1. pond culture | 289 | 49 | 240 | 13 | 33 | 118 | - | 15 | 61 | - | - |
| 2. cage culture | 1 290 | 156 | 1 127 | 67 | 159 | 825 | 1 | 2 | 72 | 1 | 7 |
| Total | 1 579 | 205 | 1 367 | 80 | 192 | 943 | 1 | 17 | 133 | 1 | 7 |
1 Sale to provinces other than Bangkok
4. THE ECONOMICS OF SEABASS PRODUCTION
Seabass seed production and culture have been developed over the past 15 years. The technology has helped expand the industry and developed into a promising enterprise.
Figure 2 shows the typical techniques and flowchart of seabass culture as been developed and practised in Thailand.
The spawner can be collected from the culture area. Spawning is induced by hormone injection and water manipulation. Larval rearing technique developed in Thailand has been successful and the technology can be and has been transferred to private hatcheries and fishfarmers.
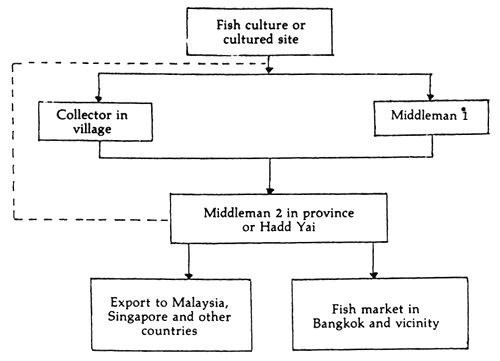
Figure 1. Marketing of cultured seabass in Southern Thailand
The financial analysis of seabass hatchery is shown in Table 5. It should be noted that the advantage of a hatchery in Thailand is that it can dispose of excess newly hatched larvae to farmers or to other hatcheries. The farmers can operate their own backyard hatchery to rear seabass fry to nursery stage. Therefore, it becomes quite convenient and economical to operate seabass hatchery. Table 5 shows that income from seabass hatchery is 51.34 percent over total cost.
The economics of marketable fishcage culture is shown in Table 5 of the lecture, “Cage Culture of Seabass in Thailand”.
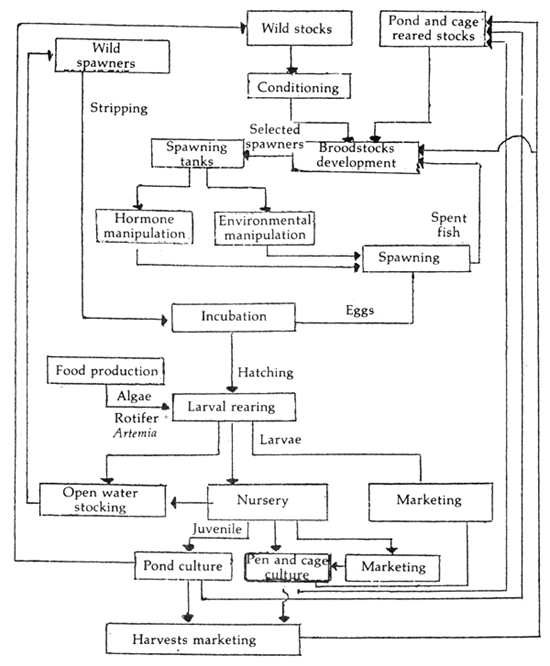
Figure 2. Flowchart of seabass culture (After Kungvankij, et. al., 1986)
| Item | Value | Percent | |
|---|---|---|---|
| A. | Income | ||
| Newly hatched larvae (1 day old) — 10 M (1 000/2 US$) | 20 000 | ||
| 0.5 cm larvae (15 days old) — 2 M (1 000/6 US$) | 12 000 | ||
| 2.5 cm larvae (40–50 days old) — 2 M (1 000/100 US$) | 200 000 | ||
| Sub-total A | 232 000 | ||
| B. | Fixed cost | ||
| Land cost (10 000, 18 percent interest) | 1 800 | (1.2) | |
| Hatchery construction (50 000, 10 percent depreciation) | 5 000 | (3.3) | |
| Equipment (20 000, 20 percent depreciation) | 5 000 | (2.6) | |
| Interest (200 000, 18 percent) | 36 000 | (23.7) | |
| Property tax (1.5 percent) | 150 | (0.1) | |
| Sales tax (1 percent) | 2 320 | (1.5) | |
| Sub-total B | 49 270 | ||
| C. | Operating cost | ||
| Broodstock | 2 500 | (1.6) | |
| Broodstock feed | 2 000 | (1.3) | |
| Artemia cyst | 40 000 | (26.3) | |
| Hormone | 2 000 | (1.3) | |
| Chemical/Fertilizer | 2 000 | (1.3) | |
| Larval feed | 5 000 | (3.3) | |
| Electricity (12 000/month) | 14 400 | (9.5) | |
| Fuel and oil | 1 000 | (0.7) | |
| Labour: chief technical — 400 × 12 = 4 800 | 22 800 | (15.0) | |
Technicians — 300 × 3 × 12 = 10 800 | |||
Workers, 100 × 2 × 12 = 7 200 | |||
| Materials and supply | 5 000 | (3.3) | |
| Maintenance | 4 000 | (1.3) | |
| Sundry | 2 000 | (1.3) | |
| Sub-total C | 102 700 | ||
| D. | Total cost (B = C) | 151 970 | |
| E. | Net operating cost (A — C) | 127 300 | |
| F. | Net income (A — B — C) | 78 030 | |
| G. | Income over total cost | (51.34) |
REFERENCES
Department of Fisheries. 1987 Fisheries record of Thailand 1985. No. 4/1987. Fish. Stat. Subdivision, Dept. of Fish. 94p.
Kungvankij, P., et. al. 1986 Biology and culture of seabass (Lates calcarifer), NACA Training Manual Series No. 3, NACA/RLCP, Bangkok. 70p.
National Statistical Office and Department 1985–1987 of Fisheries. Marine fishery census of Thailand. 329p.
Sirikul, B. 1982 Aquaculture for seabass in Thailand. SCS/GEN/82/39, South China Sea Fisheries Development and Coordinating Programme. 9–10p.
Sungkasem, P. 1982 The economics of seabass production. SCS/GEN/82/39, South China Sea Fisheries Development and Coordinating Programme. 53–58p.