2.1 Introduction
2.2 Physical Properties
2.3 Chemical Properties
Rozanov (1961) and Kurmangaliyev (1966b), who studied the gypsiferous soils of the Soviet Union, state that the physical and physico-chemical properties of gypsiferous soils are closely related to those of non-gypsiferous soils when the soils are developed under similar soil forming factors, and the gypsic layer is deep in the profile. However, where gypsum particles are present in the surface layer, their type, amount, and the degree of crystallization have a profound impact on the physical and physico-chemical properties of the soil as a medium for plant growth. Due to its solubility, gypsum is leached downward by rainfall or irrigation, and is moved upwards by capillary rise when the water-table is high. The gypsum leached from the upper soil layers tends to precipitate in lower horizons in the form of fine gypsum crystals, or as lumps consisting of soil particles cemented by gypsum, or as gypsum rosettes. In extreme cases it can form a hard horizontal crust. Where pedogenic gypsum accumulates in the subsurface horizons to the extent that the S-matrix becomes plugged, the growing gypsum crystals tend to interlock and indurate the horizon (Kulchitskii 1956), causing a serious impediment to root extension. The depth of the gypsum layer in the soil profile usually depends on the amount of water percolating through the soil. Summarizing, the physical properties of gypsiferous soils, as a medium for plant growth, depend on the surface gypsum content, the depth of the gypsic layer and its degree of induration.
2.2.1 Particle-size distribution
2.2.2 Structure
2.2.3 Soil-water relationships
Standard laboratory determination of the particle-size distribution (texture) of gypsiferous soils is a tedious and time-consuming process. Gypsum, which inhibits soil dispersion, is usually removed from soil samples before analysis by leaching the soil either with distilled water, or by extraction with a solution of ammonium oxalate (Coutinet 1965), or any concentrated chloride solution in which gypsum is more soluble than pure water. Because gypsum has marked effects on the physical properties of soils it is most desirable to determine the particle-size distribution without removing the gypsum fraction. Independently both Hesse (1974) and Matar and Douleimy (1978) developed tentative methods for the preparation of a stable suspension of gypsiferous soils without removing the gypsum fraction. Vieillefon (1979) gives a substantially improved method based on that of Hesse. Details of these procedures are discussed in Chapter 6.
At present no method for the determination of the particle-size distribution of gypsiferous soils is entirely satsifactory and the development of a reliable method without removing the gypsum fraction is needed.
The estimation of texture under field conditions is misleading because of the presence of gypsum crystals in various sand-sized fractions. The forms and degree of crystallization of gypsum particles influence the feel of the soil and as a result field estimates of texture are generally coarser than indicated by laboratory determinations.
A great range of textures has been observed in gypsiferous soils around the Mediterranean. Clay contents ranging between 2 and 50 percent have been recorded, depending upon the genesis of the soil (Mousli 1980; Van Alphen and de los Rios Romero 1971; Mardoud 1980; Dekkiche 1976; Barzanji 1973; Barzanji et al. 1975).
Experience of analysis of gypsiferous soils suggests the following relationships:
1. The sum of the various size-fractions of gypsiferous horizons may be less than 100 percent due to dehydration of CaSO4. 2H2O during oven drying at circa 105°C.2. The distribution of gypsum within the various size-fractions depends on the total amount of gypsum (Vieillefon 1979). The difference between the weight of such fractions obtained after treatment at 50°C and 105°C shows the following:
i. the fraction less than 20 µm is greatest when the total content of gypsum is about 10 percent and decreases with the gypsum content up to about 25 percent. It is constant and small when gypsum is between 25 and 45 percent and increases again when gypsum is more than 45 percent3. Gypsum is found in all size fractions, but it is mostly linked with the coarse and fine sand fractions (0.05 - 2 mm) followed by the silt fraction.ii. the amount of the fractions greater than 20 µm increases as the total amount of gypsum increases. For most samples with a gypsum content between 10 and 25 percent, the relationship is not significant.
Soil particles in gypsiferous soils are weakly aggregated as the cohesive forces attracting single soil particles are very weak. Gypsum particles have no cation exchange capacity. Erosion of gypsiferous soils can be very serious because of poor aggregation. Boyadgiev (1974) noticed that a gypsum content over 15 percent tends to give an unstable structure. There is however a tendency to increase in stability as the gypsum content exceeds 25 percent. He concludes from the results obtained in the Euphrates basin of Syria, that soils containing 10 to 35 percent gypsum are permeable, have poor structural stability and small water retention capacity, and are considered as dangerous for the construction of irrigation canals. He also notes that caverns, which will cause the collapse of irrigation canals, can be formed through the removal of fine soil particles by mechanical means, when gypsum is in a crystallized form and its content ranges from 10 to 35 percent.
Smith and Robertson (1962), working in Iraq, found that 3 to 10 percent of gypsum does not interfere significantly with soil characteristics such as structure, consistency and water holding capacity; while in soils containing 10 to 25 percent of gypsum, the gypsum crystals tend to break the continuity of the soil mass. This is also the case for the gypsiferous soils of the USSR. They observed that soils with more than 25 percent of gypsum do not provide a good medium for plant growth and the soil material lacks plasticity, cohesion and aggregation and becomes completely unstable in water.
The different observations by the various authors on the structural properties of gypsiferous soils could be due to the variation in the degree of hardness and crystallization of gypsum particles in the soils. Castroviejo and Porta (1975) found in the Giguela area that the A horizon of typic Gypsiorthids is brown in colour with good structure and organic matter content. The gypsic horizon however is structureless and poor in organic matter. The Giguela area is more humid and has a higher precipitation than the Euphrates basin and other gypsum-affected areas in Tunisia, Algeria, Saudi Arabia, Iran and elsewhere.
Most gypsiferous soils are poorly aggregated and consequently the structure of the surface layer is dominantly massive or fine platy. The gypsic layer can be strongly aggregated with the formation of hard crusts sometimes impeding the downward movement of water and the extension of roots. In field and laboratory studies, Abrukova and Isayev (1983) established qualitative and quantitative differences in the nature of structural bonds and also in the deformational behaviour of grey-brown gypsiferous soils studied as a function of their gypsum content.
The tolerance of various crops and land suitability for crop production depend on the depth and structure of the gypsic layer as well as the gypsum content of soils. Fruit and forest trees are most affected by the type of gypsic layer and its hardness.
Soil moisture retention
The energy of retention of soil moisture in gypsiferous soils is considered by many workers to be similar to that in non-gypsiferous soils and the available moisture to plants is considered to be that held between 0.3 and 15 bar. Van Alphen and de los Rios Romero (1971) discuss some of the published data on available moisture to plant growth. Minashina (1956) found that available moisture in the non-gypsic surface layer or the gypsic subsoil layer with 80 percent gypsum was in the range of 11 to 22 percent by volume. Similarly, Amami et al. (1967) found in the oasis of Gabès in Tunisia, where soils are coarse-textured with 10 percent of clay and about 20 percent of gypsum, that available moisture retained by these soils ranged from 10 to 12 percent. Van Alphen and de los Rios Romero (1971) mentioned that in the fine-textured soils of the Ebro Valley of Spain, with 40 percent clay and 1 to 9 percent gypsum, the available moisture was higher, ranging from 23 to 38 percent in the non-gypsic surface layers. These figures of available moisture are considered high even for non-gypsiferous soils.
Mousli (1980) mentioned that available moisture in the Balikh and Maskaneh basins of Syria ranged between 9 to 15 percent, depending upon the soil texture and gypsum content of soils. However, when gypsum occurs as crystals in the size of coarse to very coarse sand, available moisture becomes lower and could be as low as 5 percent by volume.
Recently, Heinze and Fielder (1984) found in a study on nine different gypsiferous soil profiles of various textures and degrees of maturity that gypsiferous soils have a large pore volume, especially the medium-size pores, and a water content comparable to calcareous or silicate soils of comparable texture and degree of maturity.
The results of soil moisture retention curves of certain gypsiferous soils conducted by Van Alphen and de los Rios Romero (1971) in the Euphrates Basin, found that an important part of soil water is retained in the low moisture tension part, between pF 1.5 and 2.7 (0.03 and 0.5 bar); and assumed that available moisture ranged between pF 2.0 and pF 4.2 (0.1 and 15 bar). The available moisture ranged between 13 and 22 percent by volume for the non-gypsic layer and 15 to 31 percent in the gypsic subsoil layer.
In a comparative study on the moisture characteristics of soils in the Hodna region (Algeria), Balikh basin (Syria), and Karak area (Jordan), Boyadgiev (1974) noted the following:
i. the retained and available water content in the soils is minimal when the gypsum content is about 25 percentIn the measurements of soil pF, the presence of gypsum interfered with the drying and wetting phase of the pF curve determination. A part of the saturation water was used to change the anhydrite (CaSO4) or semi-hydrated (CaSO4. 0.5H2O) into gypsum (CaSO4. 2H2O). Thus a correction in the calculation of water content of soil at various energy levels is required to get the correct pF curve (Vieillefon 1976) - or possibly the area of the hysteresis loop between drying and wetting curves can be used to indicate the extent of the effect in practice.ii. when the gypsum content is between 25 and 35 percent, the retained water content depends upon the forms of gypsum and the soil texture
iii. an increase in retained water was observed when the gypsum content increased from 35 percent and when gypsum content fell below 10 percent
iv. the marked irregularity of the distribution of water content is typical of gypsiferous soils. In the soils with the highest amounts of gypsum (more than 35-45 percent) the soil moisture content is uniform only in the first 10-15 cm from the surface. Below this depth the Water is localized in channels 10 to 20 cm in size and all other parts are dry. This irregularity of distribution of water is due to crevasses which are characteristic of hypergypsic horizons and which conduct the water through the soil profile (Figure 2.1).
Regarding the availability of soil moisture to plants, it was found by Hernando et al. (1963) that soil water in the range of pF 2.3 to 4.2 (0.2 to 15 bar) is not equally available to plants grown in gypsiferous soils. Yields of corn grown in soils containing 8 percent gypsum were greatest when the soil moisture content was 50 percent of field capacity. Consequently, it was found that the addition of gypsum to the soil decreases the yield but less so when the soil moisture was at a high or low moisture tension. Plant species were found to respond very differently to the soil moisture level at which irrigation application will give the maximum yields (Doorenbos and Pruitt 1976).
The interaction between the soil moisture energy and the effect of gypsum on plant growth is an important aspect of the management of gypsiferous soils which is relevant to the determination of optimum irrigation scheduling.
Water movement in soils
The internal water movement in most gypsiferous soils is normally moderate to rapid, except where gypsum incrusted layers impede the downward movement of water. Mousli (1980) states that hydraulic conductivity of gypsiferous soils in the Meskaneh Plain, Syria, ranges between 0.12 and 1.3 metres per day (m/d); and 1.6 to 8.2 m/d for the surface layers in the Euphrates Basin. Water permeability in the Granada experimental farm, Syria, ranged between 0.15 and 0.42 m/d for medium gypsiferous soils and 0.23 and 0.47 m/d for shallow gypsiferous soils (Mardoud 1980). Van Alphen and de los Rios Romero (1971) found that the hydraulic conductivity of the Kirovabad Massif (USSR) varies between 0.2 and 1.0 m/d for the gypsic subsoil and between 1.2 and 1.9 m/d for the non-gypsic surface layer. Amami et al. (1967) found that the hydraulic conductivity in the gypsum-bearing surface soil in the oasis of Gabès ranged between 2.0 to 2.5 m/d; and in the gypsum incrusted subsoil layer between 1.6 and 2.5 m/d. Porta Casanellas (1975) found that the permeability of many gypsiferous soils in the Margenes del Rio Giguella of Spain ranged between 0.8 and 1.0 m/d.
Figure 2.1 Diagram showing soil moisture content of gypsiferous soils
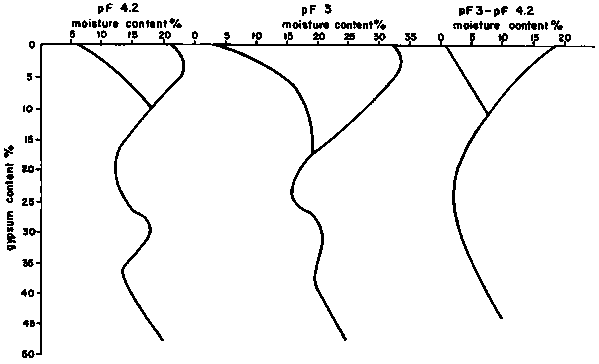
The water movement in gypsiferous soils is sometimes excessive, as can be seen from the above examples, so waterlogging is not a significant problem except where incrusted gypsic layers are found in the soil profiles. When gypsiferous soils are irrigated, the gypsum is leached and translocated and in some cases a gypsum layer is formed which reduces the hydraulic conductivity. Keren et al. (1980) suggest that the reduction in the hydraulic conductivity is due to the plugging of soil pores by the precipitation of the leached gypsum.
2.3.1 Solubility relationships
2.3.2 Gypsum and calcium carbonate interaction
2.3.3 Cation exchange properties and exchangeable cations
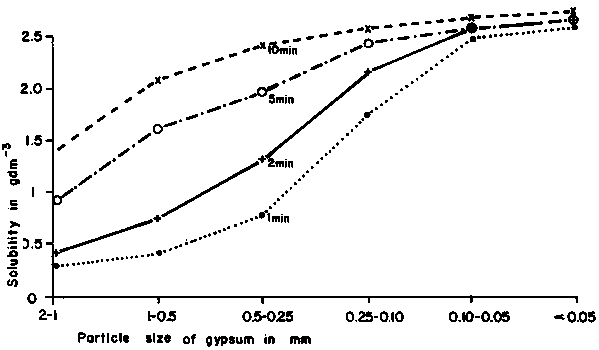
The solubility of gypsum depends on particle size (Figure 2.2), and several other factors.
Figure 2.2 The effect of particle size on the solubility of gypsum in water
In strictly thermodynamic terms the solubility of a sparingly soluble binary weak electrolyte such gypsum, is given by:
K = fA+[A+] × fB-[B-]
where K is a constant, the activity product; fA+ and fB- are the activity coefficients of the cation and anion respectively, and [A+] and [B+] are the cation and anion concentrations. For very dilute solutions, such as we are considering, the relationship:
f¥(1 - kÖc)
also holds, where c is concentration and k is a constant. Thus when c is very small f is approximately unity and:
K » [A+][B-]
K is then still the activity product. Since, in dilute solutions of weak electrolytes, the measured value for the molar concentration of a simple dissociated ion is close to its activity, we can write for gypsum:
Ksp = [Ca2+][SO42-]
where Ksp is the solubility product. Thus, the amount of gypsum that will dissolve is controlled by the molar concentrations of calcium and sulphate ions in solution. For a pure system containing only water and solid gypsum in excess, Glas et al. (1979) found that the calcium concentration varied only slightly between 14.9 and 15.8 millimoles dm-3. The results of Bennett and Adams (1972) show that activity coefficients must be about 0.5.
However, soil solutions are rarely simple and other factors need to be considered. One such, is that other more soluble minerals may supply calcium or sulphate ions to the soil solution. These common ions will help satisfy the solubility product relationship and hence depress the solubility of the solid gypsum phase.
Equally important is the concept of ionic strength (S) which is defined as:
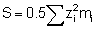
where zi is the valency of the ion and mi is its molar concentration. Ionic strength becomes important because in dilute solutions an increase in ( ) will cause a decrease in the activity coefficient of the sparingly soluble ions according to the relationship:
log fi = -0.505zi2. S05
where fi is the activity coefficient.
Thus in a solution of increased ionic strength, where there are no ions common with the sparingly soluble phase, the concentration of the ions of the latter will increase to compensate for the decrease in fi while Ksp remains constant. This means that, in practice, the amount of gypsum brought into solution will increase. These principles are demonstrated by the results of Bennett and Adams (1972) shown in Table 2.1.
Table 2.1 CALCIUM AND SULPHATE CONCENTRATION AND IONIC STRENGTH OF AQUEOUS SOLUTIONS SATURATED WITH CaSO4. 2H2O AT 25°C, WITHOUT AND WITH COMMON IONS
(Bennett and Adams 1972)
|
Solutions without common ions - |
Solutions with common ions |
||||||
|
Medium |
Ca2+ =
SO42 |
Ionic strength |
Medium |
Concentration |
Ca2+ |
SO42- |
Ionic strength |
|
|
mol m-3 |
M |
|
mol m-3 |
mol m-3 |
mol m-3 |
M |
|
KCl |
18.3 |
0.113 |
K2SO4 |
14 |
12.1 |
25.9 |
0.090 |
|
KCl |
26.2 |
0.345 |
K2SO4 |
83 |
10.1 |
93.2 |
0.290 |
|
NH4CI |
18.5 |
0.115 |
Ca(NO3)2 |
13 |
23.3 |
10.5 |
0.081 |
|
KH4CI |
26.6 |
0.315 |
Ca(NO3)2 |
79 |
90.6 |
9.0 |
0.279 |
|
KNO3 |
23.8 |
0.228 |
MgSO4 |
9.7 |
13.2 |
22.9 |
0.092 |
|
KNO3 |
27.0 |
0.373 |
MgSO4 |
58 |
11, 3 |
69.6 |
0.278 |
|
NH4NO3 |
17.4 |
0.108 |
nitrates1 |
27 |
20.7 |
16.8 |
0.104 |
|
NH4NO3 |
29.9 |
0.354 |
nitrates |
163 |
40.4 |
21.1 |
0.324 |
|
KH2PO4 |
19.4 |
0.117 |
chlorides1 |
26 |
19.6 |
17.2 |
0.107 |
|
KH2PO4 |
33.7 |
0.372 |
chlorides |
158 |
38.8 |
20.7 |
0.313 |
|
MgCl2 |
18.2 |
0.110 |
sulphates2 |
12 |
12.8 |
25.1 |
0.096 |
|
MgCl2 |
28.0 |
0.332 |
sulphates |
74 |
10.8 |
84.0 |
0.284 |
1 Nitrate and chloride solutions contained as cations: Ca, Mg, K and NH4The solubility of simple weak binary electrolytes is also influenced by the formation of electrically neutral ion-pairs of varying degrees of complexity. The effect of these ion-pairs can be considerable and may double the concentration of the sparingly soluble salt in solution. Quantitative estimates of ion-pair formation are usually made by solving simultaneous equations representing their formation, using iteration procedures. Readers are referred to Adams (1971) and Bennett and Adams (1972) for a fuller discussion of this topic.
2 Sulphate solution contained as cations: Mg, K and NH4
Thus the solubility of gypsum, its dissolution and transport in the soil profile, and to some extent its effect on the ionic nutrient composition of the soil solution, could be explained by its solubility-product relationships and ion-pair formation which in turn are dependent on the concentration and types of salts present in the soil.
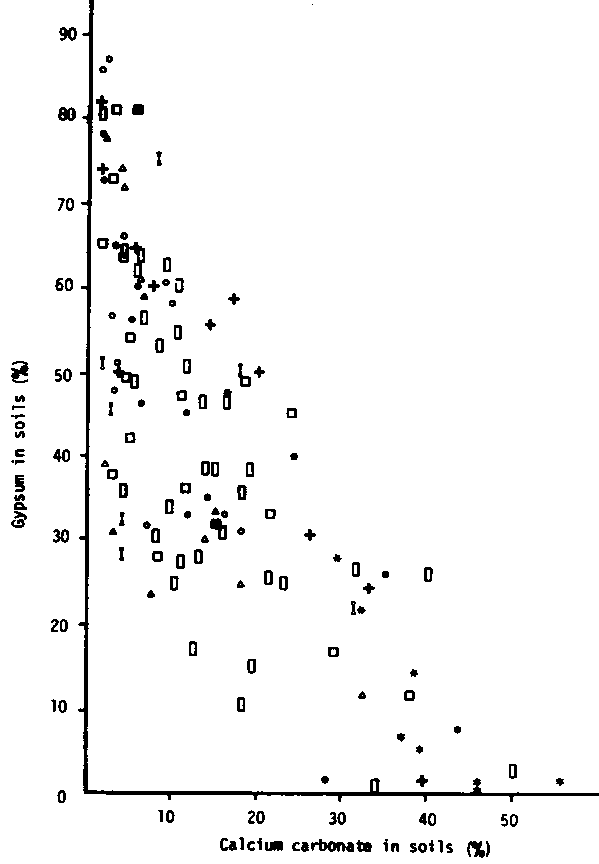
Most gypsiferous soils contain calcium carbonate in various amounts and forms. Gypsum and calcium carbonate in soils are not independent of each other except in soils with little profile development, such as Sierozems. It is also commonly observed that when the calcium carbonate content decreases, the gypsum content increases. Boyadgiev (1974) tried to explain this phenomenon on the basis of forms of calcium carbonate and gypsum and of the presence of soluble salts in the soils. He found the following in the Euphrates basin of Syria:
i. when the form of calcium carbonate and gypsum is soft and powdery, the relationship between the gypsum and calcium carbonate content was significant and expressed by the following equation:
Percentage gypsum = 100 - 1.281 CaCO3 percent
ii. when the gypsum is of sand grade and the calcium carbonate is present as nodules and crusts or when soluble salts are present, the relationship between gypsum and calcium carbonate contents becomes insignificant. In Tunisia, Vieillefon (1976) found a negative relationship between gypsum and calcium carbonate contents for soils with surface gypsiferous layers or gypsiferous incrustations (Fig .2.3).
The following paragraphs discuss the chemistry of the CaCO3. CaSO4. 2H2O system. Firstly, the solubility equilibria of pure calcium carbonate CaCO3(s) in soils is dealt with. Secondly, the solubility equilibria of the CaCO3. CaSO4. 2H2O present in soils is discussed. This helps to clarify the interaction between gypsum and calcium carbonate solid phases and their effect on the ionic composition of the soil solution, and consequently on the way their chemical properties affect the soil as a whole.
The CaCO3(s). CO2(g). H2O(1) system
Let us consider the system of pure water originally free of carbonate in solution and free of the gaseous phase but saturated with CaCO3 (solid phase). Under these conditions, the composition of the solution is governed by the solubility product of calcite as follows:
|
CaCO3(s) |
(1) |
|
where log Ksp (the solubility product) is equal to
-8.35 |
|
|
Because of the protonization of the
CO32- the following reactions take place: |
|
|
CO32- + H+
|
(2) |
|
with log K° (the thermodynamic equilibrium constant)
equal to 10.33 and |
|
|
HCO3- + H+
|
(3) |
|
with log K° equal -6.35 |
|
|
CO2 + H2O
|
(4) |
|
with log K° equal to -1.46 |
|
|
CaCO3(s) + 2H+
|
(5) |
|
where log K° is equal to 9.79. |
|
|
log Ca2+ = 9.79 - 2pH - log
PCO2 |
(6) |
The Ca2+ activity in solution could be determined by multiplying the measured concentration of Ca2+ in solution by the activity coefficient calculated either from the Debye-Hückel theory or the Davies equation (Bolt and Bruggenwert 1976).
From equations (5) or (6) it appears that the solubility of CaCO3 decreases at higher pH values and high CO2 partial pressure.
Under field conditions, the CO2 levels in the soil air is far above the 0.03 percent by volume of CO2 in the atmospheric air, due to biological activity of roots and micro-organisms.
A higher level of CO2 in the soil air would lead, on the one hand, to a drop in soil pH and a consequent increase in the solubility of CaCO3 but, on the other hand, this is counteracted by the higher partial pressure of CO2. As a result, the final solubility of CaCO3 will then be determined by the equilibrium conditions of equation (6).
The CaCO3(s). CaSO4. 2H2O. CO2 system:
In a system where both CaCO3 and gypsum co-exist with the Ca2+ ion in common, the solubility of both minerals and the ionic composition of the equilibrium solution will be governed by the simultaneous solubility products of both reactions:
|
CaCO3 + 2H+
|
(7) |
|
with log K° = 9.79 |
|
|
CaSO4. 2H2O
|
(8) |
|
with log K° = 4.64. |
|
1. The pH of calcareous-gypsiferous soils in equilibrium with CO2 is determined by the simultaneous solubility of CaCO3 and gypsum.From the plant nutrition point of view, the presence of gypsum in calcareous soils could have two opposite effects on nutrient mineral solubility and ion availability to plants. On the one hand, the presence of gypsum tends to lower the pH of calcareous soils and consequently to increase the solubility of several minerals, such as phosphates, oxides or hydroxides of iron, zinc and manganese. On the other hand, the increase in calcium activity of the soil solution, due to the presence of gypsum, could lead to a possible interaction between Ca and several nutrient cations such as zinc, iron and manganese and a drop in their availability to plants.2. The Ca2+ activity is much higher and the soil pH lower in the CaCO3-gypsum-CO2 system than in the CaCO3-CO2 system.
3. The effect of CO2 partial pressure on the Ca2+ activity is much less in the CaCO3-gypsum-CO2 system as compared to its effect on the CaCO3-CO2 system.
4. The solubility of CaCO3 should be much lower in the presence of gypsum due to the common ion effect.
From the soil genesis point of view, where soils contain a mixture of calcium carbonate and gypsum, several groups of soils could form under arid or semi-arid conditions. Typically, CaCO3 accumulates in the middle part of the profile in the form of calcareous concretions or nodules, with a gypsic horizon at depth. Soil profiles of this type are classified according to Soil Taxonomy as Calcic Gypsiorthids. This horizon sequence could be explained by the fact that gypsum, which is more soluble than calcite, is leached first to form a gypsic layer, to be followed above by lime nodules or a calcareous crust.
The interaction between gypsum and calcium carbonate thus has importance in soil genesis and also influences the soil medium for plant growth.
Gypsum particles have no negative charge and consequently it is expected that the total exchange capacity of gypsiferous soils decreases as the gypsum content of the soil increases. An example of the gypsiferous soils of Balikh basin of Syria is given in Table 2.2.
Table 2.2 RELATIONSHIP BETWEEN CATION EXCHANGE CAPACITY (CEC) OF SOILS AND GYPSUM CONTENT IN THE BALIKH VALLEY OF SYRIA
|
% gypsum |
0 |
10 |
25 |
50 |
75 |
|
CEC (mEq/100 g) |
24 |
21 |
18 |
11 |
5 |
The percentage clay in gypsiferous soils depends on the kind of soil developed. In the Typic Gypsiorthids of Iraq (Barzanji 1974), Algeria (Dekkiche 1976), Syria (Ilaiwi 1983) and elsewhere, the clay content rarely exceeds 20 percent by weight and the cation exchange capacity ranges between 7 and 14 mEq/100 g of soil. However, in Calcic Gypsiorthids with a calcic horizon overlying the gypsic layer and smaller amounts of gypsum, the clay content ranges between 20 and 50 percent by weight (Barzanji 1974) and cation exchange capacity ranges between 14 and 18 mEq/100 g. Porta Casanellas (1975) found, however, that the organic matter content of the A horizon in many of the gypsiferous soils of Spain ranged between 0.5 and 3.5 percent, with a cation exchange capacity as high as 50 mEq/100 g (see Appendices).
The types of clay mineral in a soil influence its physico-chemical properties, including the cation exchange capacity, structure, shrinkage and swelling, water-holding capacity, stickiness, and tillage properties.
In gypsiferous soils, many workers (Dekkiche 1976; Barzanji et al. 1975; Altaie 1968; Yahia 1971; Shadfan et al. 1984; Lee et al. 1983), have found that attapulgite is the main mineral in the fine fractions except in soils formed in recent deposits. Attapulgite is found mainly in gypsic and petrogypsic horizons. In addition to attapulgite (palygorskite) other minerals are found, such as chlorite, montmorillonite, illite, vermiculite, sepiolite and kaolinite. Attapulgite represents 40 to 80 percent of the clay minerals of the gypsic horizons in various areas of Iraqi soils and Barzanji et al. (1975) found a highly significant linear relationship between gypsum contents of soils (X) and the attapulgite content of the clay fraction (Y)
Y = 0.89X+ 15.85
with r = 0.963.
Barzanji et al. (1975) found some evidence, using a scanning electron microscope for the neoformation of attapulgite and its preferential occurrence on gypsum grains. He considers that attapulgite is formed later than the gypsum. In a later study in Iraq, he also found that with increasing rainfall attapulgite decreases and the montmorillonite content of soils increases.
However, Boyadgiev (1974) mentions that, in Calcic Gypsiorthids of Jordan, no attapulgite is found in the gypsic horizons, but a limited amount is found in the calcic horizon of the soil. Lee et al. (1983) from a study of gypsiferous soils of the eastern region of Saudi Arabia, concludes that palygorskite originates from the underlying Mio-Pliocene limestone in the basin and was distributed by alluvial and eolian processes. Aba-Hysayn and Sayegh (1977) report that attapulgite and illite were found as the most abundant and common clay minerals in the soil and strata of Al-Hasa soils of Saudi Arabia, and the origin of these minerals and their relative abundance is believed to be associated with the source of the sediment since minimum alteration seems to have taken place after deposition. From these and other observations, the relative roles of gypsiferous or calcareous environments in attapulgite formation cannot be separately estimated.
The dominant exchangeable cation in gypsiferous soils is calcium, followed by magnesium, potassium and sometimes sodium. The impact of cation exchange capacity and exchangeable cations on soil nutrient availability to plants will be discussed in the following chapter.