3.1 Introduction
3.2 Fertility and Use of Fertilizers
Surface layers of gypsiferous soils in many parts of the world are poor in nitrogen and phosphorus (Minashina 1956, Van Alphen and de los Rios Romero 1971). Total N as determined by the Kjeldahl method, ranges between 50 and 260 mg/100 g of soil; and total P extracted by 20 percent HCl ranges between 13 and 52 mg/100 g of soil. However, the extractable K determined using 20 percent hot concentrated HCl ranges between 200 and 700 mg/100 g of soil; and the water extractable K is generally between 4 and 5 mg/100 g, sometimes rising to 10 mg/100 g, a level considered by many workers as adequate for the growth of most crops. There is a wide difference of opinion about the need for potassium fertilizer on arid and semi-arid soils. The generally accepted concept, using soil analysis as a basis, is that the soils of the arid and semi-arid zones are rich in potassium. Soils of the arid and semi-arid zones are however mostly alluvial and heterogeneous, so it is not possible to make general statements. The existing routine laboratory tests for determining available potassium for plants do not always reflect the true situation under field conditions, because of the variations in the clay mineralogy of the soils. Furthermore the soils of the arid and semi-arid regions may contain minerals with a high K-content extractable under the routine laboratory tests but not extractable by plants under field conditions. Dobroval’skiy (1965) found that gypsiferous soils are poor in manganese, copper, zinc and molybdenum.
Rozanov (1961), Kurmangaliyev (1966b) and Van Alphen and de los Rios Romero (1971), using the limited available data, state that there is no essential difference between the soil fertility of gypsiferous and non-gypsiferous soils when found under the same pedogenic conditions. They however excluded soils with a gypsic layer at shallow depth, in which the volume of soil containing essential elements is limited.
Kovda (1954) considers that the accumulation of gypsum in soils results in very low fertility, and that their productivity remains low under irrigation, even with applications of fertilizers and organic manures. He found the potential productivity of gypsiferous soils to be related to the depth of the gypsic layer. In soils with a gypsic layer below 60 cm depth, the plant roots penetrate freely and the soil volume for nutrients is adequate. Fertilization of these soils improves plant growth and increases yield. In shallow soils, with a gypsic layer near the surface, the soil volume is limited and fertilization becomes of special importance for plant growth.
3.2.1 Phosphorus availability and immobilization
3.2.2. Nitrogen and organic matter
3.2.3 Calcium, magnesium and potassium
3.2.4 Micronutrients availability and problems
There is limited data available on the fertilization of gypsiferous soils under field conditions. Van Alphen and de los Rios Romero (1971) describe experiments in which nitrogen fertilizers in the form of ammonium sulphate and nitrate were added to irrigated cereals, cotton, sugar beet, fruit trees and other crops. Similarly, superphosphates and potassium were applied to cultivated crops under both rainfed and irrigated farming. Little attention was given to micronutrient deficiences in these experiments. Sayegh (1979) points out that soils in the Middle East are deficient mostly in nitrogen, phosphorus and micronutrients and to some extent in potassium.
Sayegh et al. (1981), discussing their fertility trials on both calcareous and gypsiferous soils, point out that the farmers in the Middle East face other limiting factors such as inadequate soil preparation, lack of irrigation, and weed control, which are prerequisites for crops to respond to fertilizer applications. Their experiments show that intensive cropping creates a demand for plant nutrients.
Hernando et al. (1963, 1965) who studied the interaction between gypsum content and available soil moisture on the growth of corn plants and their mineral uptake, note that water consumption by plants decreased with increasing gypsum content and that the uptake of phosphorus, potassium and magnesium decreased while nitrate, sulphate and calcium increased, with increasing gypsum content.
In general, plants react differently to the different gypsum content of soils. Plants tolerant to gypsum, for example alfalfa, usually maintain their ionic composition whatever the gypsum content of the soil (Matar, unpublished work).
Gypsiferous soils have been cultivated for centuries under traditional rotational rainfed farming systems in which wheat or barley is followed by leguminous grain crops or by fallow. Under rainfed farming conditions, yields depend mainly on rainfall and are usually low to moderate. Soil chemical properties are in a dynamic equilibrium. Gypsum and other salts are leached in the rainy season to deeper horizons and returned to the surface horizons during summer by capillary rise. When gypsiferous soils are irrigated changes in their chemical properties take place involving further movement of gypsum salts and nutrients.
The relative importance of organic and inorganic forms of phosphorus in soils depends upon the soil organic matter content. Organic phosphorus is usually low in the subsoil of temperate soils, as it is also in arid soils. Calcium, aluminium, iron and sometimes magnesium are the main metal ions that bind phosphorus in soils (Novozamsky and Beek 1978).
Phosphorus solubility in calcareous and gypsiferous soils
The solubility of phosphorus in soils depends upon the presence of other ions in the soil solutions which exist in equilibrium with their solid phases. The following is an illustration of a simple system (Novozamsky and Beek 1978):
|
Ca4H(PO4)3. 3H2O
|
log Ksp = -46.91 |
|
Octacalcium phosphate |
|
|
|
|
|
Ca10(OH)2(PO4)6
|
log Ksp = -113.7 |
|
Hydroxyapatite |
|
|
|
|
|
Ca10F2(PO4)6
|
log Ksp = -120.86 |
|
Fluoroapatite |
|
|
|
|
|
AlPO4. 2H20
|
log Ksp = -30.5 |
|
|
|
|
FePO4. 2H2O
|
log Ksp = -34.9 |
|
|
|
|
CaHPO4 |
log Ksp = -6.66 |
Simple phosphate-solubility diagrams could be developed whenever the solubilities of cation in soils form solid compounds with phosphates which are in equilibrium with the soil phases. When gypsum co-exists with calcite in soils, as is the case in most gypsiferous soils, the calcium activity in the soil solution is much higher when compared with soil in equilibrium with calcite only. The solubilities of calcium phosphate compounds in soils in the presence of gypsum are therefore lower, compared to that in the presence of CaCO3 alone. Figure 3.1 shows the solubility of tricalcium phosphate in the presence of CaCO3 or CaCO3 and gypsum combined at various levels of CO2 pressure. Consequently, it is expected that levels of soluble or available phosphorus are lower in gypsiferous soils than in calcareous soils.
When phosphate fertilizer is applied to land the immobilization rate of P varies significantly between soils. Larsen et al. (1965) suggested that the decrease in labile phosphate could be the result of the formation of a crystalline basic calcium phosphate at a rate that increases with soil pH (Figure 3.2). Matar (unpublished data) finds that, in general, the rate of P immobilization is greater in gypsiferous soils than in calcareous soils, as predicted from theoretical derivations (Figure 3.3). The P immobilization was significantly greater for soils with 40 percent of gypsum when compared with soils with 5 percent of gypsum, especially in the first period following the P application (Figure 3.4).
Figure 3.1 The solubility of calcium phosphate (calcium and hydrogen ion activities and inorganic phosphate concentrations) in the presence of calcite or calcite plus gypsum in an aqueous system at various levels of CO2 (Harmsen 1984)
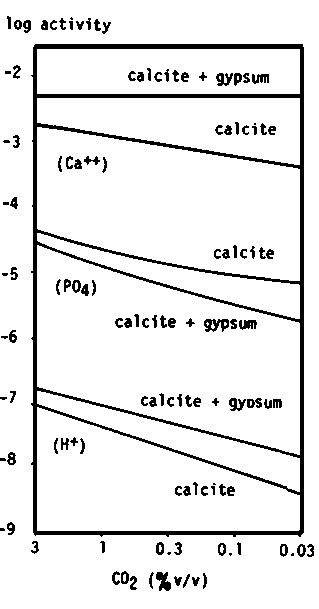
Figure 3.2 Relationship between half-life of labile phosphate and hydrogen-ion concentration of soils (Larsen et al. 1965)
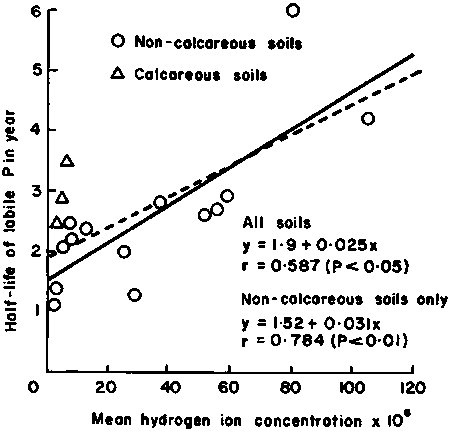
Figure 3.3 Average P-immobilization curve for calcareous versus gypsiferous soils
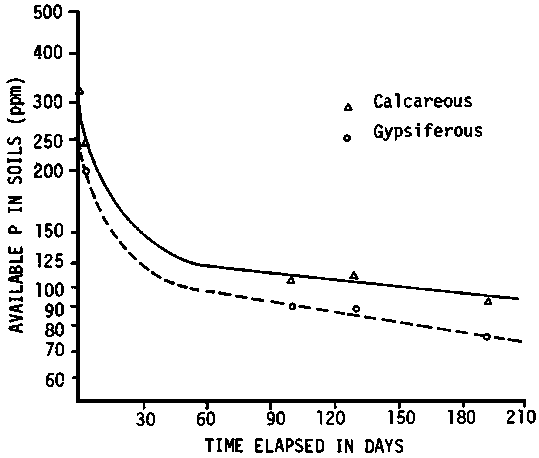
Figure 3.4 P-immobilization curve in soils with 5 percent and 40 percent of gypsum by weight
In general, gypsiferous soils are very low in phosphorus and need to be fertilized. They are buffered to a pH level of 7.5 to 8.4 resulting in low availability of natural soil phosphorus. Applied phosphorus quickly reverts to insoluble forms. Sayegh and Abdul Majid (1969) studied the fate of applied phosphorus in alkaline, calcareous and non-calcareous soils (Tables 3.1 and 3.2). It was found that, in the highly calcareous Bazourye soil with the effect of cropping, the applied P over a period of one year was mainly converted to forms of Ca-P and water soluble and easily replaceable P. In the slightly calcareous Innsar soils, the distribution of the applied P was mainly in the forms of Ca-P and Al-P, followed by organic P and soluble P. In the alkaline non-calcareous Zaouter soil, the applied P was distributed in a variety of forms in the following decreasing order: residual-P, Fe-P, Al-P, Ca-P, organic P. The retention of P was more rapid in the alkaline non-calcareous Zaouter soil which contained larger amounts of iron and aluminium oxides. The increase of Fe-P over a short period suggests that the chemical activity of hydrous oxides is appreciable, although the pH is alkaline. The above work has been carried out mainly in calcareous and non-calcareous soils. Phosphate retention would be more severe in gypsiferous soils, because of their higher calcium activity. Applied phosphorus fertilizer should be about 50 percent water-soluble to be effective on gypsiferous-calcareous soils. Atanasiu et al. (1978) found that combinations of water-soluble and citrate-soluble phosphates proved to be the most advantageous for the P supply for plants. Phosphorus in ordinary and concentrated superphosphates is about 100 percent water-soluble, while phosphorus in compound fertilizers varies in water solubility. Fertilizers that are wholly soluble have very reactive forms of phosphorus which in perfectly buffered soils, with a near neutral reaction, are not readily transformed into forms of soil phosphorus. Basically, the amount of the various discrete chemical fractions of P which are found in the soil determines the relative effectiveness of phosphate fertilizers on crop growth (Lindsay and De Ment 1961). The retention of P in soils is the result of chemical precipitation and physico-chemical sorption, and the subsequent availability to crops depends on the rate at which the P is released to the soil solution. In fact, soils do not have a well-defined capacity to retain phosphate. The extent and rate of immobilization are subject to several factors including the following:
i. the concentration of phosphate in the soil solution: an increase in phosphate concentration is accompanied by an increase in phosphate immobilizationTable 3.1 PHOSPHORUS RETENTION BY SOILS DEPENDING ON TIME AND CONCENTRATION OF ADDED PHOSPHORUSii. the time of reaction of phosphate with the soil: some of the reactions between the phosphate and the soil surfaces are completed quickly. In some tropical soils 95 percent of the applied P is immobilized within five minutes. In calcareous and gypsiferous soils, some reaction products are formed slowly so that the initial rapid stage in the reaction is followed by a slow decline in P solubility
iii. The mineralogical nature of the soil: the amount and forms of iron and aluminium oxides and the amount and types of CaCO3 present influence phosphorus retention.
|
Phosphorus retained |
||||||||||
|
Soil series
|
Clay |
Phosphorus |
After 1 day
|
After 4 days
|
After 10 days
|
After 30 days
|
||||
|
(%)
|
added |
|||||||||
|
(as ppm of soil) |
ppm1 |
%2 |
ppm1 |
%2 |
ppm1 |
%2 |
ppm1 |
%2 |
||
|
Bazourye
|
50.8
|
100 |
84 |
84 |
88 |
88 |
89 |
89 |
93 |
93 |
|
200 |
148 |
74 |
153 |
76 |
164 |
82 |
180 |
90 |
||
|
400 |
251 |
63 |
261 |
65 |
293 |
73 |
305 |
76 |
||
|
Zaoutar
|
46.8
|
100 |
94 |
94 |
96 |
96 |
97 |
97 |
99 |
99 |
|
200 |
190 |
95 |
191 |
95 |
195 |
97 |
198 |
99 |
||
|
400 |
349 |
87 |
364 |
91 |
378 |
94 |
386 |
96 |
||
|
Innsar
|
42.7
|
100 |
73 |
73 |
83 |
83 |
88 |
88 |
92 |
92 |
|
200 |
138 |
69 |
145 |
72 |
161 |
80 |
175 |
87 |
||
|
400 |
168 |
42 |
236 |
59 |
281 |
70 |
296 |
74 |
||
1 P retained in ppm of soilConcerning the availability of soil phosphorus to plants, it has been found experimentally, that there is a significant difference in the P uptake between corn and alfalfa in relation to the gypsum content of soils. The uptake of P by corn, from a soil well-fertilized with P, decreased very significantly as the gypsum content of soils increased. However, the uptake of P by alfalfa (a crop tolerant to gypsum) remained unaffected by the gypsum content of soils in spite of the greater Ca activity in the gypsiferous soils (Table 3.3). Apparently, the P level in crops tolerant to gypsum remained unaffected by P concentration in soils. Thus, the effect of gypsum on P uptake and P concentration in plants depends to a large extent on:
2 P retained as percent of P added
i. the plant species and its tolerance to gypsumTable 3.2 DIFFERENCES OF P IN THE SOIL SAMPLES RECEIVING LOW1 AND HIGH2 LEVELS OF P, EXPRESSED AS PERCENT OF THE DIFFERENCE BETWEEN THE TOTAL P OF THE TWO LEVELS
ii. the amount of phosphorus fertilizers added
iii. the percentage of gypsum and calcium carbonate in soils.
1 25 mg P per 2 kg of soil
2 400 mg P per 2 kg of soil
|
CaCO3 |
Soil series
|
Water soluble |
AI-P
|
Fe-P
|
Ca-P
|
Reductant soluble |
Occluded |
Residual |
Organic |
|
(%) |
P3 |
Fe-P |
AI-Fe-P |
P |
P |
||||
|
3.7 |
Innwar |
6.5 |
31.4 |
5.7 |
42.8 |
0.0 |
0.7 |
1.4 |
14.3 |
|
0.0 |
Zaoutar |
0.9 |
20.1 |
22.3 |
15.4 |
0.7 |
0.2 |
29.9 |
11.9 |
|
71.0 |
Bazourye |
20.6 |
4.5 |
0.0 |
67.2 |
0.0 |
0.0 |
5.5 |
2.2 |
3 Water soluble and easily replaceable PMatar and Jabbour (1982) reported that upon the application of P fertilizers the zinc content of corn leaves was reduced more significantly in gypsiferous than in calcareous soils. The application of phosphates particularly in low rainfall areas is found essential, and the response of cereals to P application was greater in the dry years (Matar 1977).
From this overview, it can be concluded that, in gypsiferous soils, higher rates of phosphorus applications than normal are essential because of their higher rate of P immobilization.
Table 3.3 EFFECT OF LEVEL OF GYPSUM ON UPTAKE OF P BY CORN AND ALFALFA PLANTED IN GYPSIFEROUS SOILS
|
Gypsum(%)
|
P Concentration in dry matter |
||
|
Corn leaves |
Alfalfa tops |
||
|
(% × 10) |
(% x 10) |
||
|
0 |
6.36 |
3.55 |
|
|
5 |
3.23 |
2.91 |
|
|
10 |
3.48 |
2.60 |
|
|
20 |
3.20 |
2.80 |
|
|
40 |
1.91 |
2.99 |
|
|
LSD
|
.05 |
.72 |
1.42 |
|
.01 |
1.05 |
2.00 |
|
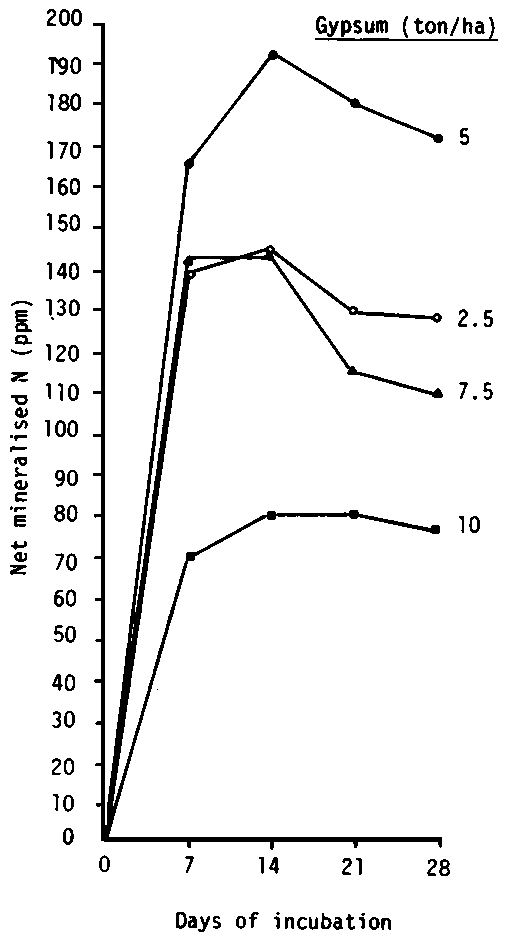
The organic matter content of most gypsiferous soils is relatively low and ranges between 0.4 and 1 percent for surface layers to less than 0.2 percent in the subsurface layers. The type of organic compounds and humus are affected by the presence of gypsum in soil. Velasco et al. (1980) found, from study of humification under different forest ecosystems in the province of Toledo, that gypsum in soils affects the humification process. The humus formed under Quercus woodlands on gypsum marls and limestones is a calcic mull with insoluble fulvic acids. Singh and Taneja (1977), report that the rate of nitrogen mineralization in soils is usually stimulated by the addition of gypsum to saline non-gypsiferous soils at a rate of 2.5 to 5 t/ha. However, the addition of higher rates of gypsum (7.5 to 10 t/ha) led to a lower level of N mineralization (Fig. 3.5). It is believed that the addition of gypsum at a low rate stimulates soil micro-organisms responsible for mineralization. Gupta and Salaran (1971) found that the addition of gypsum stimulates the fungus population in saline and sodic soils and decreases the actinomycetes count. The above discussion of the rate of nitrogen mineralization is mostly relevant to saline-sodic or sodic soils where gypsum is added as a soil ameliorant and to counteract the effect of toxic salts on plant growth. There is little published on the effect of gypsum on N mineralization and the activities of micro-organisms in gypsiferous soils.
Figure 3.5 Changes in mineral nitrogen status in gypsum-amended soil
Van Alphen and de los Rios Romero (1971) considering the nitrogen contents of gypsiferous soils note that the surface layers of soils in Spain contained generally less than 0.25 percent of total nitrogen (as determined by Kjeldahl); and that nitrogen contents in soils of the Kirovadad Massif, USSR, range between 0.07 and 0.26 percent. The surface layers of gypsiferous soils in the Euphrates Valley are relatively poor in total N, ranging between 0.07 and 0.15 percent.
Under dryland farming (average annual rainfall 200-300 mm), it is expected that significant and economic responses to nitrogen fertilization are unlikely, especially when wheat or barley crops are preceded by fallow. However, a significant response to N fertilizer is expected when wheat-wheat or barley-barley rotations are practised.
Under irrigated agriculture on gypsiferous soils with a low level of organic matter and total nitrogen, the regular application of N fertilizers is essential to secure adequate yields of most crops.
The problems associated with the application of nitrogen fertilizers are shown in Figure 3.6. To prevent the loss of ammonia, the nitrogen fertilizers should be incorporated well into soils with pH higher than 7. Little is known about the ammonium-fixing capacity of the soils and the possible economic implications where nitrogen fertilizer is added in ammonium forms. Sayegh and Rehman (1969) found that some soils with alkaline reaction can fix up to 46 percent of applied ammonium fertilizer. This corresponds to about 200 kg of nitrogen per hectare which is equivalent to the amount added by local farmers (Table 3.4). This property of fixing large quantities of ammonium may decrease yield.
Figure 3.6 Problems associated with the application of N-fertilizers
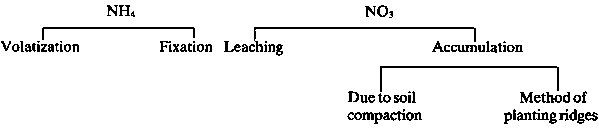
Table 3.4 AMMONIUM FIXATION IN DIFFERENT SOILS
|
Soil properties
|
Soil series |
||
|
Innsar |
Bazourye |
Zaoutar |
|
|
NH4 fixed (%) |
46 |
16 |
41 |
|
pH |
8.2 |
8.4 |
7.4 |
|
CEC (mEq/100 g) |
68.27 |
25.72 |
39.66 |
|
Nitrogen (%) |
0.03 |
0.01 |
0.02 |
|
CaCO3>3 (%) |
3.7 |
71.0 |
0 |
|
Clay (%) |
50.8 |
42.8 |
46.8 |
|
Silt (%) |
34.2 |
41.0 |
40.0 |
|
Sand (%) |
15.0 |
16.2 |
13.2 |
|
Textural class |
Clay |
Silty clay |
Clay |
|
Soil clay minerals |
|||
|
Montmorillonite |
Dominant |
Moderate |
Small |
|
Illite |
Small |
Moderate |
Small |
|
Kaolinite |
Small |
Trace |
Dominant |
1. The form of the N (Figure 3.6).Unfortunately, no work has been reported regarding the effect of gypsum content on ammonium fixation in gypsiferous soils.2. The clay content and clay mineralogy of the soil.
3. The amount and type of CaCO3 present.
As a rule of thumb, nitrogen needs to be applied at rates from 1.2 to 1.5 times the anticipated removal by the crop. Where generous amounts of N have been applied and where soils contain pans that impede leaching, large amounts of nitrate may accumulate in the soil. Some fields in Texas, USA, were found to have more than 600 kilograms per hectare of nitrogen in the form of nitrate, an amount far in excess of that needed for sugar beet production and resulting in a low sucrose percentage and high impurities. Where furrow irrigation is used, soil nitrate may move to the top of the ridges and accumulate as water evaporates, becoming unavailable to plants unless it is washed down into the root zone.
Where crops, for example wheat, are sown in the cold season when nitrification is inhibited, it is more profitable to apply complex fertilizers containing about half of their nitrogen content in the form of nitrate rather than applying ammonium or urea fertilizers, since the nitrate-N is the preferred form for uptake by wheat before the soil warms up.
The published results of field experiments conducted on gypsiferous soils to determine the optimum level of nitrogen fertilizers needed by various crops are very limited. The rate of nitrogen application adopted by most farmers for Mexican wheat, sugar beet and corn ranges between 70 and 140 kilograms per hectare. Cotton is fertilized with 50 to 120 kilograms of nitrogen per hectare, depending on the type of gypsiferous soils and the expected yields (Van Alphen and de los Rios Romero 1971, Mousli 1980). Usually one-third to one-half of N fertilizers is being applied before sowing, the rest is applied in one or two splits. Other crops such as sorghum (Sorghum vulgare), onion (Allium cepa), tomato (Lycopersicum esculentum) are fertilized with 100 to 120 kilograms of N per hectare (Bennett and Adams 1972). These applied rates of N fertilizer do not reflect the optimum rate for optimum yield.
It can be concluded that almost without exception, all calcareous and gypsiferous soils are low in nitrogen and need to be fertilized.
The inter-relation between the three macronutrients, calcium, magnesium and potassium are well known in plant nutrition studies (Ologunde and Sorensen 1982; Agboola and Corey 1973; Terman et al. 1975). The increase in calcium concentration in solution leads usually to a drop in potassium uptake by plants. Similarly, the increase in calcium concentration could lead to a drop in magnesium uptake. In general, the relationship between Ca, Mg and K is related to texture, organic matter and the type of clay in the soil.
Hernando et al. (1965) in a study on the mineral nutrition of corn on soils rich in gypsum, found in water culture, that increasing the concentration of Ca2+ and SO42- ions, by the addition of CaSO4, increased the uptake of Ca and S and decreased the uptake of K and Mg. Prasad and Paliwal (1976) report that application of gypsum to soils irrigated with water which has a high Mg: Ca ratio, equivalent to 16: 1, improves yields of crops and increases the Ca: Mg ratio in the plant tissues.
Poonia and Bhumbia (1973) show, on a sodic soil, that the application of labelled-gypsum (45CaSO4. 2H2O) to soils in pots, grown with barley, caused an increase in potassium content in tested plant tops. But, the application of labelled CaCO3 (45CaCO3) caused a slight increase in K uptake by crops. The application of either CaCO3 or gypsum increased the Ca content of the barley plants, but had no definite effect on the Mg content. The availability of Ca from applied gypsum was considerably more than that from applied calcium carbonate (Table 3.5).
Table 3.5 EFFECT OF DIFFERENT LEVELS OF 45CaSO4 AND CaCO3 ON THE YIELD AND CHEMICAL COMPOSITION OF BARLEY GROWN IN A NON-SALINE SODIC SOIL (ESP = 48.4)
|
Treatment % of gypsum requirement
|
Dry matter yield (g/pot)
|
mEq 100 g-1 |
% |
||||
|
Ca |
Mg |
Na |
K |
N |
P |
||
|
45CaSO4 |
|||||||
|
0 |
2.96 |
13.9 |
13.3 |
188.0 |
37.0 |
2.17 |
0.250 |
|
25 |
8.16 |
20.0 |
9.5 |
103.7 |
66.0 |
2.38 |
0.164 |
|
50 |
9.60 |
20.4 |
11.4 |
94.7 |
69.7 |
2.35 |
0.135 |
|
75 |
9.13 |
21.7 |
12.6 |
80.0 |
72.7 |
2.43 |
0.133 |
|
100 |
11.08 |
23.2 |
10.9 |
73.3 |
69.7 |
2.52 |
0.130 |
|
LSD (5%) |
1.29 |
1.1 |
1.9 |
6.7 |
3.6 |
0.15 |
0.013 |
|
45CaCO3 |
|||||||
|
0 |
3.16 |
13.9 |
12.7 |
166.7 |
45.7 |
2.98 |
0.238 |
|
25 |
2.76 |
16.9 |
12.9 |
168.7 |
52.7 |
3.23 |
0.224 |
|
50 |
3.76 |
22.4 |
16.0 |
165.3 |
42.3 |
3.13 |
0.229 |
|
75 |
4.26 |
21.6 |
14.1 |
176.3 |
41.8 |
3.10 |
0.219 |
|
100 |
4.06 |
22.0 |
17.9 |
193.3 |
53.5 |
3.03 |
0.217 |
|
LSD (5%) |
1.31 |
1.2 |
2.2 |
12.1 |
4.4 |
0.14 |
0.006 |
Accordingly, it becomes obvious that K and possibly Mg fertilization is of major importance to plant growth and for the maintenance of productivity of irrigated gypsiferous soils. Their application rates depend on local field conditions, the cropping system, the soil management practices used, and the degree of crop intensification.
The results of analyses of major cation concentrations on the adsorption complex and some other characteristics of some gypsiferous soils of the Balikh Valley in Syria are shown in Table 3.6.
Table 3.6 CHARACTERISTICS OF SOME GYPSIFEROUS SOILS IN THE BALIKH BASIN OF SYRIA (after Gibb et al. 1967)
|
Characteristic |
Shallow soils |
Medium deep soils |
||
|
Topsoil |
Subsoil |
Topsoil |
Subsoil |
|
|
% Gypsum |
10 |
54 |
0.5 |
16 |
|
% CaCO3 |
-1 |
- |
24 |
8 |
|
pH |
7.8-8.3 |
7.0-8.2 |
7.0-8.2 |
- |
|
% Organic matter |
0.8-1 |
0.2-0.8 |
0.6-1.2 |
0.4-0.5 |
|
Cation exchange capacity (mEq 100 g) |
9-11 |
4-7 |
10-14 |
- |
|
% Ca on the adsorption complex |
60-90 |
- |
15-16 |
- |
|
% Mg on the adsorption complex |
- |
- |
20-45 |
- |
|
% K on the adsorption complex |
5-10 |
- |
8-12 |
- |
|
% Na on the adsorption complex |
2-3 |
- |
- |
- |
1 Not measuredReview of the status of soluble and exchangeable forms of potassium and magnesium in naturally gypsiferous soils indicates that medium to high levels of both cations are usually present. The potassium content ranges between 1 to 2 mEq 100 g-1 of soil for those containing less than 25 percent gypsum (Barzanji 1973, Dekkiche 1976, Mardoud 1980, Mousli 1980, Osman 1982), and are less than 0.5 mEq 100 g-1 for soils with a higher gypsum content.
The debate on the need for potassium fertilizer on irrigated or non-irrigated calcareous and gypsiferous soils has been underway for some time. The generally accepted concept from soil analysis is that the soils of arid and semi-arid zones are rich in potassium. This is however a misconception because:
i. the gypsiferous soils of the arid and semi-arid zones are mostly alluvial and extremely heterogeneous, thus one cannot make general statements;Sayegh (1986) demonstrated the variation in the extractable amounts of K by the ammonium and sodium acetate extractants under different moisture conditions (Table 3.7).ii. the routine, laboratory tests for determining available potassium do not reflect the true situation under field conditions;
iii. there are marked variations in the type and amount of clay minerals, CaCO3, gypsum and the kind and amount of potassium minerals present in the soils.
Table 3.7 EXTRACTION OF K UNDER DIFFERENT MOISTURE CONDITIONS USING DIFFERENT EXTRACTING SOLUTIONS
|
Soil
|
Solution
|
Field |
50% Field Capacity |
Air drying |
||
|
Capacity1
|
Field drying2
|
Laboratory drying |
Field3
|
Laboratory
|
||
|
mEq/100 g of soil |
||||||
|
1. 5Y 7/2 |
NH4OAC |
0.77 |
0.40 |
0.53 |
0.65 |
0.96 |
|
Light grey |
NaOAC |
0.47 |
0.44 |
0.71 |
0.71 |
1.34 |
|
3. 10 YR 3/2 |
NH4OAC |
2.64 |
2.60 |
2.55 |
2.43 |
4.27 |
|
Very dark grey-brown |
NaOAC |
1.91 |
2.01 |
2.45 |
1.95 |
2.22 |
|
7. 10 YR 3/4 |
NH4OAC |
0.53 |
1.2 |
0.42 |
1.96 |
0.71 |
|
Dark brown |
NaOAC |
0.08 |
1.13 |
0.29 |
1.46 |
0.62 |
|
8. 10 YR 3/4 |
NH4OAC |
1.35 |
0.24 |
0.95 |
0.46 |
1.37 |
|
Dark brown |
NaOAC |
0.68 |
0.08 |
0.80 |
0.35 |
0.86 |
|
14. 5 YR 4/6 |
NH4OAC |
1.56 |
2.57 |
1.71 |
2.78 |
2.79 |
|
Yellowish red |
NaOAC |
0.47 |
0.64 |
0.41 |
1.03 |
0.82 |
1 Collected 25-26 February 1969Table 3.7 shows that the amount of extracted potassium depends on the moisture content in the soil (effect of seasonal sampling) and on whether the soil samples were dried in the field or in the laboratory. There is no general trend in the extractable amount of potassium, the variation is due to the different types of clay minerals present in the various calcareous soils used in this study.
2 Collected 8-9 April 1969
3 Collected 17-18 June 1969
This shows that the existing routine laboratory tests do not reflect the true availability of potassium for plant growth under field conditions. In most cases the extractable potassium is much exaggerated, that is, the extracted value corresponds to a "high" category yet the soil is K-deficient, while in other cases the value is "low", but the soil does not respond to applied potassium fertilizer. In general, at an equal content of exchangeable K, the concentration of potassium in the soil solution varies considerably depending on the pH, amount and type of CaCO3, amount and type of clay and amount and form of gypsum present. The K: Ca and Mg: Ca ratios in the soil solution are very low when the gypsum content is high resulting in a very low uptake of K and Mg from the soil solution which accounts for low crop yields (Van Alphen and de los Rios Romero 1971).
In conclusion, the application of potassium fertilizer is necessary on gypsiferous and on calcareous soils where vegetables, fruit trees and grasses are grown and where they are intensively cropped. Potassium increases the resistance to certain diseases, helps to overcome water stress, increases potassium uptake in clayey calcareous soils where aeration is poor and improves the quality of crops.
Under rainfed conditions, plants grown in gypsiferous soils may not suffer from any K or Mg deficiencies. Potassium and magnesium cations leached during the wet season, can return to the surface during the drier part of the year and become available to plants. However, the Mg levels on the adsorption complex of the soil and in plants should be monitored to prevent its deficiency.
In the past 25 years, the need for application of micronutrients has not been recognized except in special cases (Fritz et al. 1984). During this period however, substantial information has accumulated concerning the content of micronutrients in soils and plants, their availability to plants, the needs of the crop for micro-nutrients and the effects of their application on crop yields (Shorrocks 1984, Katyal and Randhawa 1983, Sillanpää 1982). This has been especially so in North Africa and the Middle East (Sillanpää and Vlek 1985, El-Fouly 1980, 1983). Further information and research however, are required to identify crop requirements and the need for micronutrients on various soil types and the varied agro-ecological conditions.
Availability of micronutrients to plants grown in soils is one of the major factors determining productivity. The high concentration of soluble calcium in gypsiferous soils, affects the availability of some macronutrients such as phosphorus, potassium, magnesium. The availability of micronutrients is also affected, on the one hand, by the presence of gypsum and its level and, on the other hand, by the effect of phosphorus and possibly potassium fertilizers added in large doses. The effect of gypsum on the solubility and availability of various micronutrients to plants is discussed below.
Molybdenum
Stout et al. (1951) found that, in contrast to phosphorus, the uptake of molybdenum by plants is reduced by sulphur application in the form of gypsum, as illustrated in Table 3.8. They explain that the effect of is possibly due to direct competition between two divalent anions of the same size SO42- and MoO42-.
Table 3.8 REDUCTION OF MOLYBDENUM CONTENT IN TOMATO AND PEA PLANTS BY APPLICATION OF CaSO4 2H2O TO SOIL
|
CaSO4. 2H2O
application |
Mo concentration |
|
|
(ppm) |
Tomato |
Peas |
|
0 |
5.25 |
12.80 |
|
100 |
3.52 |
8.05 |
|
400 |
2.45 |
5.70 |
Reisenauer (1963) also shows that S applied as gypsum reduces the yield and N concentration in leguminous crops such as peas on a soil low in molybdenum. The application of Mo overcame the depressive effect of S. Reisenauer suggests that S, in addition to its competitive effect on the adsorption of Mo, has an apparent inhibitive effect on Mo utilization within the plant at the low levels of Mo in plant tissues.
Molybdenum has long been known to be required for the normal assimilation of N in plants. Of the four enzymes found to contain Mo, nitrogenase and nitrate reductase are found in plants (Price et al. 1972). The Mo in these two enzymes appears to have similar catalytic functions judged from electromagnetic resonance studies. Nitrate reductase enzyme is essential for the assimilation of nitrates, since it is the catalyst in the first step in the reduction of NO3 to NH3. The other major molybdo-protein enzyme in plants is nitrogenase which reduces N2 to NH3, which is then assimilated by the plant.
The availability of Mo in gypsiferous soils should be considered when determining a suitable fertilization programme. Deficiency in Mo limits crop growth and especially that of legumes which require Mo for the functioning of nitrogenase. Berry and Reisenauer (1967) show that accumulation of Fe by tomato tops depends on the levels of Mo in the nutrient solution. Plants deficient in Mo show least uptake of Fe. However, Olsen and Watanabe (1979) found that the addition of gypsum to six sodic soils, planted with sorghum plants, decreased the Mo concentration from 2.33 to 1.26 ppm in the plants, while the Fe content was increased from 56 to 65 ppm.
Iron, Manganese and Copper
The effect of gypsum on the other micronutrients has not been so thoroughly investigated. Van Alphen and de los Rios Romero (1971) mention that chlorosis appears on many crops grown on gypsiferous soils in Spain, such as apricots and peaches. Recent work in Syria has shown that the effect of gypsum on the micro- nutrient contents of crops depends to a large extent on the plant species. Crops tolerant to gypsum, such as alfalfa, resist any significant changes in their macro- and micronutrient contents. However, for semi-tolerant crops such as corn, high levels of gypsum in soils (greater than 20 percent by weight) lead to a significant drop in Mn content (at the 1 percent level) from 79 to 57 ppm; while the same amount of CaCO3 in soils did not have any significant effect on the Mn content (Matar, unpublished work). Dobroval’skiy (1965) found that samples from gypsiferous layers contain low amounts of Mn, Cu, Zn and Mo. Soils from Spain showed a deficient level of Fe but Mn and Cu levels were found satisfactory (see Appendix 4).
Boron
Chaudhary et al. (1976) found that application of gypsum to sodic soil lowered the concentration of boron in oats and corn plants and the Ca:B ratio was increased several fold. They found in a laboratory experiment that the application of gypsum lowered the soluble B concentration in the soil solution. The change in the solubility of B compounds could be either due to the added gypsum which will increase the calcium concentration in the soil solution, or to the effect of gypsum on the soil pH. More investigations are needed on the chemistry of B in gypsiferous soils.
Zinc
Since phosphorus and potassium fertilizers are essential to maintain the productivity of gypsiferous soils, discussion of their effect on the availability of micronutrients to plants is very relevant. Though, it is now generally accepted that P and Zn interact in plants, the effect of the level of P in soils on Zn uptake remains controversial (Olsen 1972). In some cases, application of phosphorus decreases the total uptake of Zn in plants. Loneragan et al. (1979) consider that the effect of P in inducing Zn deficiency may be attributed to the effect of P in promoting growth and thus diluting the available Zn to values below those required for plant growth. Loneragan et al. (1979) consider that the effect of P cannot be attributed to any effect of the soil or the root in suppressing Zn adsorption or Zn transport since the plants grown in P-treated soils had more Zn in their tops than non-P fertilized plants.
The application of Zn compounds, in addition to P, to gypsiferous soils, improves the yield of corn tops and raises the Zn content of plants as well. It slightly reduces manganese uptake by corn. Safaya (1976) reports that the application of Zn to soils reduces the rates of Cu and Mn uptake more than that of Fe. In gypsiferous soils in Spain, Zn deficiences are more likely to appear on Zn-sensitive crops (Appendix 4).
In conclusion, the availability of micronutrients in gypsiferous soils could be the limiting factor for the growth of many plant species. Gypsum and calcium carbonate in soils, as well as other macronutrients added as fertilizers, could have significant effects on the availability of micronutrients to various plant species. More research is needed on the macro-and micronutrient status of gypsiferous soils.
The maintenance of the productivity of gypsiferous soils under irrigation, requires balanced use of both macro and micronutrients. Crops under irrigation show different fertilizer requirements to those under rainfed agriculture. Wallace (1980) makes the following recommendations for the use of micronutrients on arid soils to avoid toxicity:
1. The micronutrients should be applied after the need for them has been established by the following criteria: by visual symptoms; by leaf analysis with critical levels; by soil analysis with critical levels or by yield responses in test plots.2. The correction of micronutrient deficiencies is of little value if other factors are more limiting. For example, soil water management, pest and weed control and others. The effect of micronutrient applications on all crops in the rotation should be considered, and a written record kept of the quantities applied so a build up of toxicity can be avoided.
3. Nematodes, a high water-table, and imbalanced fertilizer application contribute to micronutrient deficiencies. Correction of such causes help avoid deficiencies.
4. Finally, the need to fertilize gypsiferous soils to maintain their productivity requires extensive field research programmes to determine the optimum level and kind of nutrients needed by different crops. It is rare that one micronutrient is the only factor limiting yield. The following are the major factors influencing the micronutrient status and needs: pH, calcium carbonate content, gypsum content, irrigation, water quality, amount and application system (Devaux 1980), soil texture, increasing use of NPK (Finck 1984, Fritz et al. 1984) use of high yielding varieties (Fritz et al. 1984, El-Fouly 1983) and climatic conditions (Sillanpää and Vlek 1985). Much more information is needed.