S.G.A. LEAK, K. AWOUME, C. COLARDELLE, W. DUFFERA, A. FERON B. MAHAMAT, K.
MAWUENA, M. MINENGU, M. MULUNGO, C. NANKODABA, G. ORDNER, M. PELO, M. SHERIA,
G. TIKUBET, M. TOURE and G. YANGARI
Introduction
Description of sites
Methods
Results
Discussion
Conclusion
References
African animal trypanosomiasis transmitted by tsetse flies, Glossina spp, is an important constraint on livestock production in sub-Saharan Africa. As it is well known that some breeds of cattle, sheep and goats thrive better than others when exposed to the same level of trypanosomiasis risk, one possible solution lies in the rearing of these breeds. However, in many parts of Africa, people are reluctant to make use of these trypanotolerant breeds as they are considered to be less productive than susceptible breeds. One of the aims of the ATLN is to determine the productivity of these trypanotolerant breeds under quantified levels of tsetse challenge and trypanosome prevalence. The determination of tsetse challenge at sites of the ATLN and its relationships with trypanosome prevalence are reported for the period 1984-1986.
The Network sites with trypanotolerant cattle or sheep are listed in Table 1 together with the tsetse species detected. The sites with cattle, for which a more complete description can be found elsewhere (ILCA, 1986a; article 3 of these Proceedings), are:
1. Boundiali and Tengrela in northern Cote d'Ivoire with N'Dama and Baoule cattle kept under village management;2. Avetonou in Togo, with N'Dama and Race Locale breeds under both village and research station management;
3. The Office Gabonais d'Amelioration et de Production de Viande experimental ranch in Gabon with locally adapted N'Dama Okouma and more recently introduced N'Dama Senegal;
4. The private commercial ranches of Kolo and Mushie in Zaire with N'Dama cattle; and
5. N'Dama cattle from the same ranch stock kept under village management in the plateau and forest areas of Idiofa in Zaire.
Sites with trypanotolerant sheep are Boundiali and Tengrela in Cote d'Ivoire, and Sokode and Avetonou in Togo, all with Djallonke sheep.
Table 1. Tsetse species, their distribution and principal habitats at nine Network sites with trypanotolerant livestock.
|
Group |
Species |
Principal habitat |
OGAPROV |
Mushie |
Kolo |
Avetonou |
Sokode |
Boundiali |
Tengrela |
Idiofa plateau |
Idiofa forest |
|
Palpalis |
G. fuscipes |
Gallery |
- |
+ |
- |
- |
- |
- |
- |
+ |
+ |
|
Palpalis |
G. tachinoides |
Gallery forest and forest relics in savanna areas |
- |
- |
- |
+ |
+ |
+ |
+ |
- |
- |
|
Palpalis |
G. Palpalis |
Gallery forest of stream and rivers |
+ |
|
+ |
+ |
+ |
+ |
+ |
- |
- |
|
Fusca |
G. tabaniformis |
Throughout rain forest and gallery forest* |
+ |
+ |
- |
- |
- |
- |
- |
+ |
+ |
|
Fusca |
G. nashi |
Gallery forest of streams and rivers |
+ |
- |
- |
- |
- |
- |
- |
- |
- |
|
Morsitans |
G. m. submorsitans |
Savanna** |
- |
- |
- |
- |
- |
+ |
- |
- |
- |
+ = Present- = Absent
* According to MacLennan (1974) G. tabaniformis is dependent upon the interior of rain forest unlike most other members of the Fusca group which are associated more with forest fringes. However, in the Network sites this where this species occurs it is captured at forest fringes bordering pastures.
** Only 5 G. m. submorsitans were captured at the Boundiali Network site during the period under review. Efforts to trap greater numbers of this species with box traps failed. It thus appears that this species occurs at very low densities at this northern limit of its range in Cote d'Ivoire and is not considered further.
Detailed protocols for the estimation of tsetse challenge and trypanosome prevalence in livestock within the ATLN have been reported previously (ILCA, 1986a, 1986b). Tsetse challenge is estimated as the product of the following three components.
Tsetse relative density was determined from catches in biconical traps (Challier and Laveisierre, 1973) expressed as the mean number of tsetse caught per trap per day. Biconical traps were chosen as the most convenient sampling method in Network situations and because probably more is known about the biases of this trap than those of other traps with equivalent efficiency. Trapping was carried out for 5-10 consecutive days per month, depending on the type of site and tsetse-population; traps were emptied daily.
Trypanosome infection rates in tsetse were determined by dissecting the proboscides, midguts and salivary glands from live tsetse and identifying the trypanosome species type, using the method of Lloyd and Johnson (1924).
The proportion of feeds taken by these tsetse from livestock was determined at each site by analysis of blood meals from tsetse residual undigested blood using the ELISA technique (Rurangirwa et al., 1986).
Trypanosome prevalence
Trypanosome prevalence in livestock was estimated from the results of blood examination by the dark ground/phase contrast method (DG) (Murray et al., 1977) and from stained blood smears. The same ear-tagged livestock were bled and examined monthly for trypanosomes.
Data analysis
Mean annual and monthly estimates of tsetse relative densities, trypanosome infection rates and tsetse challenge were obtained from the data, and standard errors for the estimates were calculated from the results of daily observations. The low tsetse density at several sites made the collection of sufficient tsetse blood meals for those sites impractical, and for these analyses the product of tsetse relative density and trypanosome infection rate were used to estimate tsetse challenge. The relationship between tsetse challenge and trypanosome prevalence was examined by linear regression analysis. An arcsin transformation of trypanosome prevalence was used to stabilize variance (Snedecor and Cochran, 1980) and a log10 + 1 transformation of tsetse challenge was carried out in order to make a presumed curvilinear relationship between the two parameters suitable for linear regression. As numbers of tsetse captured at some sites were too low for reliable mean monthly estimates of tsetse challenge to be made, only mean annual estimates were used for across-site analyses.
Tsetse species captured
Table 1 shows the species of tsetse captured at each Network site with trypanotolerant sheep and cattle. Six species representing the three sub-genera of tsetse were captured. Tsetse of the morsitans group have been considered the most important vectors of trypanosomiasis to livestock and species of the fusca group, including G. tabaniformis, among the least important (Page and Jordan, 1952; Jordan 1961, 1986; Glasgow, 1967).
Tsetse relative density
The tsetse relative densities, expressed as the mean number of flies caught per trap per day, at eight sites with trypanotolerant livestock are shown in Table 2. Although the data were collected separately for each tsetse species, they are presented here as a total for each site. The mean number of flies caught per trap per day was relatively low at all sites, ranging from 0.1 at Avetonou in Togo to 11.7 at Mushie in Zaire. No significant seasonal relationships between tsetse relative density and rainfall could be detected by regression analysis. There was a trend for tsetse density to increase during the rainy season (August-October) in Boundiali and Tengrela where rainfall data were obtained from the administrative centres. It is possible that rainfall data collected separately at each village study site in the region may have enabled relationships between rainfall and tsetse density to have been more readily detected.
Table 2. Mean tsetse relative density (flies/trap/day) at nine Network sites with trypanotolerant cattle or sheep 1984-1986.
|
Site |
Country |
1984 |
1985 |
1986 |
Overall |
|
Mushie |
Zaire |
2.3 |
11.7 |
3.7 |
5.9 |
|
Idiofa forest |
Zaire |
- |
- |
35 |
35 |
|
Idiofa plateau |
Zaire |
- |
- |
2.4 |
2.4 |
|
Boundiali |
Cote d'Ivoire |
0.7 |
1.4 |
4.3 |
2.1 |
|
Tengrela |
Cote d'Ivoire |
1.1 |
1.0 |
1.1 |
1.1 |
|
Sokode |
Togo |
0.9 |
0.6 |
1.5 |
1.0 |
|
Kolo |
Zaire |
0.5 |
0.9 |
1.2 |
0.9 |
|
OGAPROV |
Gabon |
0.5 |
0.6 |
0.3 |
0.5 |
|
Avetonou |
Togo |
0.03 |
0.1 |
0.1 |
0.1 |
Trypanosome infection rates and infection types in tsetse
The mean trypanosome infection rates observed in tsetse at each site are shown in Table 3. The infection rates detected in Glossina tabaniformis of the fusca group were higher than the rates previously reported for this species (Page and Jordan, 1952). Infection rates were relatively high at most sites where trypanosome prevalences in livestock were high. The high infection rates in tsetse at some of these sites may indicate that a high proportion of feeds were being taken from bovidae, as these two factors have previously been linked (Jordan, 1965). G. palpalis and G. tachinoides in northern Cote d'Ivoire took 24.2 and 70.8% of feeds on bovidae, respectively.
Table 3. Mean trypanosome infection rates (%) in tsetse at nine Network sites with trypanotolerant cattle or sheep, 19841986.
|
Site |
Country |
1984 |
1985 |
1986 |
|
OGAPROV |
Gabon |
14.8 |
15.2 |
18.2 |
|
Mushie |
Zaire |
9.1 |
14.5 |
12.4 |
|
Boundiali |
Cote d'Ivoire |
20.1 |
13.2 |
10.9 |
|
Sokode |
Togo |
- |
6.4 |
5.6 |
|
Idiofa Forest |
Zaire |
- |
- |
1.5 |
|
Avetonou |
Togo |
- |
1.2 |
1.0 |
|
Idiofa Plateau |
Zaire |
- |
- |
0.8 |
|
Tengrela |
Cote d'Ivoire |
7.5 |
1.1 |
0.4 |
|
Kolo |
Zaire |
0.9 |
0.0 |
0.3 |
Table 4 shows the frequency of "vivax-type" and "congolense-type" infections in five species of tsetse at eight study sites. These data help to define the type of challenge to which livestock are exposed; for example where only palpalis group tsetse were found, livestock were under a challenge of predominantly T. vivax. Table 5 shows that at Avetonou in Togo, where only palpalis group tsetse were found, 92% of trypanosome infections in cattle were of T. vivax; at the OGAPROV or Mushie ranches where the challenge was from fusca group tsetse, only 26% of infections in cattle were of T. vivax.
At least 75% of infections in tsetse of the palpalis group were of the "vivax-type". An exception was found in G. fuscipes in the plateau area of Idiofa, Zaire, in which the proportion of "congolense-type" infections was unusually high. However the infection rate in this species was very low, and 45% of the feeds taken by this tsetse were from wild suids which are reservoirs of T. vivax infections.
Table 4. Percentage of " vivax-type" and "congolense-type" infections in five tsetse species at eight Network sites.
|
Site |
Country |
Tsetse group |
Tsetse species |
"vivax -type" |
"congolense-type" |
|
OGAPROV |
Gabon |
Palpalis |
G. palpalis |
92 |
8 |
|
Boundiali |
Cote d'Ivoire |
Palpalis |
G. palpalis |
88 |
12 |
|
Tengrela |
Cote d'Ivoire |
Palpalis |
G. palpalis |
75 |
25 |
|
Sokode |
Togo |
Palpalis |
G. palpalis |
89 |
11 |
|
Avetonou |
Togo |
Palpalis |
G. palpalis |
100 |
0 |
|
Boundiali |
Cote d'Ivoire |
Palpalis |
G. tachinoides |
92 |
8 |
|
Tengrela |
Cote d'Ivoire |
Palpalis |
G. tachinoides |
81 |
19 |
|
Sokode |
Togo |
Palpalis |
G. tachinoides |
88 |
12 |
|
Avetonou |
Togo |
Palpalis |
G. tachinoides |
100 |
0 |
|
Idiofa Plateau |
Zaire |
Palpalis |
G. fuscipes |
29 |
71 |
|
Idiofa Forest |
Zaire |
Palpalis |
G. fuscipes |
79 |
21 |
|
Mushie |
Zaire |
Fusca |
G. tabaniformis |
65 |
35 |
|
Idiofa Forest |
Zaire |
Fusca |
G. tabaniformis |
83 |
17 |
|
OGAPROV |
Gabon |
Fusca |
G. tabaniformis |
69 |
31 |
|
OGAPROV |
Gabon |
Fusca |
G. nashi |
67 |
33 |
Table 5. Percentage T. vivax infection in cattle.
|
Site |
Tsetse group |
Percentage of T. vivax infection |
|
Avetonou |
Palpalis |
92 |
|
OGAPROV |
Fusca |
26 |
|
Mushie |
Fusca |
26 |
Table 6 shows the percentage of "vivax-type" infection in both G. tabaniformis and cattle at the OGAPROV ranch in Gabon from 1983 to 1986. "Vivax-type" infections increased in both cattle and tsetse during this period. G. tabaniformis has previously been found to feed predominantly on suids but at the OGAPROV ranch may take a higher proportion of feeds on bovidae.
Table 6. "Vivax-index" in N'Dama and G. tabaniformis at the OGAPROV ranch in Gabon.
|
Year |
G. tabaniformis |
Cattle |
|
1983 |
52 |
21 |
|
1984 |
66 |
28 |
|
1985 |
72 |
30 |
|
1986 |
82 |
35a |
a Data from August 1986-August 1987.
Tsetse challenge and trypanosome prevalence in trypanotolerant livestock
Tables 7 and 8 show the mean monthly estimates of trypanosome prevalence in trypanotolerant cattle and sheep, respectively, together with estimates of tsetse challenge at the same sites. Trypanosome prevalence in sheep was lower than that in cattle. This is likely to be due in part to differences in relative attractiveness of these livestock species to tsetse (Pilson et al., 1978; Boyt et al., 1978).
Table 7. Means and standard errors of tsetse challenge and of trypanosome prevalence in trypanotolerant cattle at eight Network sites, 1984-1986.
|
Site |
Country |
Tsetse challenge |
Trypanosome prevalence |
||
|
Mean |
s.e. |
Mean |
s.e. |
||
|
Mushie |
Zaire |
62.2 |
10.7 |
10.1 |
1.95 |
|
Boundiali |
Cote d'Ivoire |
26.2 |
4.24 |
13.9 |
2.16 |
|
OGAPROV |
Gabon |
9.3 |
2.75 |
4.6 |
3.26 |
|
Tengrela |
Cote d'Ivoire |
3.0 |
0.52 |
1.4 |
0.99 |
|
Idiofa Plateau |
Zaire |
1.9 |
0.34 |
1.0 |
0.53 |
|
Idiofa Forest |
Zaire |
4.5 |
1.01 |
5.1 |
1.37 |
|
Kolo |
Zaire |
0.2 |
0.05 |
0.1 |
0.22 |
|
Avetonou |
Togo |
0.2 |
0.03 |
4.7 |
1.44 |
Table 8. Means and standard errors for tsetse challenge and for trypanosome prevalence in trypanotolerant sheep at four Network sites, 1984-1986.
|
Site |
Country |
Tsetse challenge |
Trypanosome prevalence (%) |
||
|
Mean |
s.e. |
Mean |
s.e. |
||
|
Boundiali |
Cote d'Ivoire |
26.2 |
4.24 |
9.1 |
2.17 |
|
Sokode |
Togo |
6.2 |
2.25 |
10.0 |
3.70 |
|
Tengrela |
Cote d'Ivoire |
3.0 |
0.52 |
1.7 |
0.88 |
|
Avetonou |
Togo |
0.2 |
0.03 |
3.0 |
1.62 |
A linear regression analysis of an arcsin transformation of trypanosome prevalence in trypanotolerant cattle on log10+1 tsetse challenge showed that there was a significant relationship between these two parameters (P<0.001) across years (Figure 1). Using data obtained from the same sites in consecutive years leads to the possibility of autocorrelation and thus an artificially significant P value. Regression analyses for each year of study were therefore carried out giving results which showed a significant relationship in 1984 (P<0.05) and 1986 (P<0.005) but not in 1985 (P=0.188). Data from the research station at Avetonou, Togo were clearly anomalous. More than 90% of trypanosome infections in cattle at this site were of T. vivax. These were not treated since the management of this research station believe T. vivax infections to be non-pathogenic. This allows for the possibility of cumulative trypanosome prevalence. It has also been reported that mechanical transmission of T. vivax may occur at a significant rate at this site (Mawuena, 1981). The re-analysis of the data excluding the Avetonou site gave significant relationships between arcsin transformed trypanosome prevalence and log10 + 1 tsetse challenge in 1984 (P<0.05), in 1985 (P<0.05) and in 1986 (P<0.001). The data showed a remarkably close fit to the regression lines with r values of 0.94, 0.94 and 0.98, respectively.
Regression analysis of transformed data for trypanotolerant sheep similarly showed a significant relationship between the two parameters (P<0.05) as shown in Figure 2. As there were only 4 sites with trypanotolerant sheep, there were insufficient data for regression analyses to be carried out for each year of study.
Figure 3 shows the detransformed graph for the relationship between tsetse challenge and trypanosome prevalence in trypanotolerant cattle using the equations from the regression analysis carried out for all sites across years. The majority of Network sites fall in the first section of the curve illustrating the fact that there is an absence of sites with high tsetse challenge at which the interaction of factors affecting health and productivity could be more readily evaluated.
Figure 3. Overall relationship between tsetse challenge and trypanosome prevalence in trypanotolerant cattle.
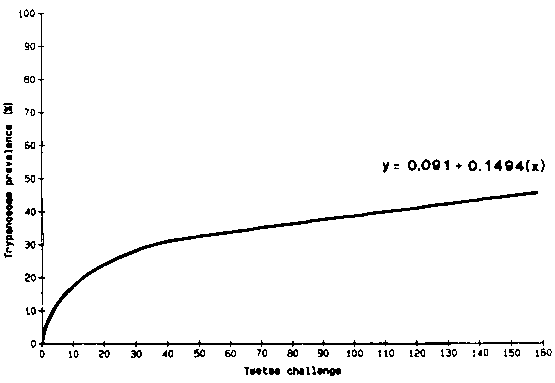
Although a significant relationship between tsetse challenge and trypanosome prevalence in livestock has been demonstrated, there remain several problems in the exact determination of tsetse challenge.
The occurrence of several different tsetse species in the various Network sites makes comparisons between sites difficult. Five tsetse species representing the three sub-genera of tsetse were detected at the Network sites with trypanotolerant livestock. These tsetse species each have different ecological preferences in terms of habitat and hosts. Morsitans group tsetse inhabit the savanna areas of Africa, which, in addition to being extensive, are more likely to be the habitat of domestic livestock and of game animals which serve as reservoirs of trypanosome infection. Fusca group tsetse inhabit the forest areas which are less suited to livestock production, whilst palpalis group tsetse are largely confined to gallery forests and forest relics where the degree of contact with livestock is limited. They are therefore likely to present a low trypanosomiasis risk. However watering places of livestock within the habitat of palpalis group tsetse may be important sites of trypanosomiasis transmission.
The method of estimating the density of these tsetse populations is somewhat imprecise as the number of tsetse caught in biconical traps depends not only upon the actual density of the tsetse populations but is also dependent upon the nutritional status of the population, the weather and the behavioural characteristics of different tsetse species. The efficiency of biconical traps is therefore likely to vary for different tsetse species. Some variation in the monthly estimates of relative density within the Network may be due to these factors, rather than reflecting real changes in tsetse density.
Sources of error may also be found in the determination of trypanosome infection rates in tsetse using the method of Lloyd and Johnson (1924), particularly where mixed infections occur. Infections of T. simiae cannot be distinguished from "congolense-type" infections using this method. Data obtained in The Gambia indicated that such infection may account for a significant proportion of Nannomonas infection in tsetse in some areas (Snow, 1987). Inaccuracies in the determination of infection rates and type in tsetse may be resolved to some extent by the use of DNA probes (Kukla, 1987; Gibson, in press), which could be particularly useful for identification of T. simiae infections. Finally the differing vectorial capacities of tsetse, for example as described for G. morsitans submorsitans and G. tachinoides as vectors of T. congolense, T. simian and T. vivax by Roberts and Gray (1971) and for G. morsitans, G. pallidipes and G. fuscipes as vectors of T. congolense by Harley and Wilson (1968), would need to be taken more fully into account.
The ultimate aim of describing the relationship between tsetse challenge and trypanosome prevalence would be to make realistic comparisons of productivity for trypanotolerant and susceptible breeds of livestock under various levels of tsetse challenge. This could assist livestock producers in making decisions concerning management, including those involving chemoprophylactic or therapeutic drug strategies. However, the relationship between tsetse challenge and trypanosome prevalence in livestock is a complex one involving at least thirteen factors (Whiteside, 1958; Jordan, 1986; Molyneux, 1977). As Ford (1964) pointed out, it is paradoxical that where tsetse are abundant there is little trypanosomiasis in livestock, but where trypanosomiasis is a serious problem there are few tsetse flies. The apparently simple linear relationship between log10+1 tsetse challenge and trypanosome prevalence in livestock described in this study confirms the importance of the parameters chosen for the determination of tsetse challenge. The establishment of a relationship between monthly estimates of the two parameters may require a more accurate determination both of tsetse challenge and of trypanosome prevalence. The latter is affected by factors such as the use of chemoprophylactic or therapeutic trypanocidal drugs, which may not be recorded, and phenomena in trypanotolerant livestock such as complete self-cure, or control of parasitaemia to undetectable levels.
In situations where livestock are kept commercially, tsetse densities are generally low although trypanosome infection rates in these tsetse may be high. An apparently simple linear relationship between trypanosome prevalence in trypanotolerant cattle or sheep, and log10+1 tsetse challenge has been observed when tsetse challenge is estimated as the simple product of tsetse relative density and the percentage of tsetse infected with trypanosomes.
Ninety-five percent upper and lower confidence intervals attached to these data indicate that a prediction of trypanosome prevalence in livestock made from a determination of tsetse challenge will fall into quite a wide range. In order to make such predictions more accurate, other factors such as vectorial capacity and behavioural characteristics of tsetse need to be taken more fully into account.
Challier, A. and C. Laveissiere. 1973. Un nouveau piege pour la capture des glossines, description et essais sur le terrain. Cah. Orstom Ser. Ent. med. Parasitol. 11: 251-262.
Boyt, W.P. P.K.I. Mackenzie and R.D. Pilson. 1978. The relative attractiveness of donkeys, cattle, sheep and goats to Glossina morsitans morsitans (Westwood) and G. pallidipes (Austen) (Diptera: Glossinidae) in a middle-veld area of Rhodesia. Bull. ent. Res. 68 (3): 497-500.
Ford, J. 1964. The geographical distribution of trypanosome infections in African cattle populations. Bull Epizoot. Dis. Afr. 12: 307-320.
Gibson, W. Species-specific DNA probes for the identification of African trypanosomes in tsetse flies. In press.
Glasgow, J.P. 1967. Recent fundamental work on tsetse flies. Ann. Rev. Ent. 12: 421-438.
Harley, J.M.B. and A.J. Wilson. 1968. Comparison between G. morsitans, G. pallidipes and G. fuscipes as vectors of trypanosomes of the T. congolense group. Ann. Trop. Med. and Parasitol. 62 (2): 178-187.
ILCA. 1986a. The ILCA/ILRAD Trypanotolerance Network: Situation Report, December, 1985. Proceedings of a Network Meeting held at ILCA, Nairobi. ILCA, Addis Ababa, Ethiopia.
ILCA. 1986b. The African Trypanotolerant Livestock Network, Indications from Results, 1983-1985, December 1986, ILCA, Addis Ababa, Ethiopia.
Jordan, A.M. 1961. An assessment of the economic importance of the tsetse species of southern Nigeria and the southern Cameroun based on their trypanosome infection rates and ecology. Bull Ent. Res. 52: 431-441.
Jordan, A.M. 1965. The hosts of Glossina as the main factor affecting trypanosome infection rates of tsetse flies in Nigeria. Trans. Roy. Soc. Trop. Med. Hyg. 59 (4): 423-431.
Jordan, A.M. 1986. Trypanosomiasis Control and African Rural Development. London: Longman.
Kukla, B.A., P.A.O. Majiwa, J.R. Young, S.K. Moloo and O.K. ole MoiYoi. 1987. Use of species-specific DNA probes for detection and identification of trypanosome infection in tsetse-flies. Parasitology 95: 1-16.
Lloyd, L.L. and W.B. Johnson. 1924. The trypanosome infections of tsetse flies in northern Nigeria and a new method of estimation. Bull. Ent. Res. 14: 265-288.
MacLennan, K.J.R. 1974. The epizootiology of tsetse transmitted trypanosomiasis in relation to livestock development and control measures. In Les Moyens de Lutte Bontre les Trypanosomes et Leurs Vecteurs, IEMVT, Paris, pp. 259-268.
Mawuena, R. 1981. Especes de tabanides existantes au CREAT. Trypanotolerance et Production Animale Publication No. 2, 1117. E. Karbe and E.K. Freitas, eds. CREAT; Togo.
Molyneux, D.M. 1977. Vector relationships in the trypanosomatidae. Adv. Parasitol. 15: 1-82.
Murray, M., P.R. Murray and W.I.M. McIntyre. 1977. An improved parasitological technique for the diagnosis of African trypanosomiasis. Trans. R. Soc. trop. Med. Hyg. 71: 325-326.
Page, W.A. and A.M. Jordan. 1952. The economic importance of some West African species of fusca group tsetse flies. Report of the West African Institute for Trypanosomiasis Research, Kaduna, Nigeria.
Pilson, R.D. W.P. Boyt and P.K.I. Mackenzie. 1978. The relative attractiveness of donkeys, cattle, sheep and goats to Glossina morsitans morsitans (Westwood) and G. pallidipes (Austen) (Diptera: Glossinidae) in a middle-veld area of Rhodesia. Bull. Ent. Res. 68 (3): 489-495.
Roberts, C.J. and A.R. Gray. 1972. A comparison of Glossina morsitans submorsitans (Newst) and G. tachinoides (Westwood) collected and maintained under similar conditions as vectors of Trypanosoma (Nannomonas) congolense, T. (N) simiae and T. (Duttonella) vivax. Ann. Trop. Med. Parasitol. 66 (1): 41-53.
Rurangirwa, F.R. S.H. Minja, A.J. Musoke, V.M. Nantulya, J. Grootenhuis and S.K. Moloo. 1986. Production and evaluation of specific antisera against sera of various vertebrate species for identification of blood meals of Glossina morsitans centralist Acta Tropica 43 (4): 379-389.
Snedecor, G.W. and W.G. Cochran. 1980. Statistical Methods. 7th edition. Iowa City: Iowa State University Press.
Snow, W. 1987. High incidences of Trypanosoma simiae obscuring the composition of trypanosome infections observed in tsetse: A neglected problem. ISCTRC, Lome, Togo.
Whiteside, E.F. 1958. The maintenance of cattle in tsetse-infested country. ISCTRC, Brussels, pp. 83-90.