D.J. GRAB and Y. VERJEE
International Laboratory for Research on Animal Diseases
P O Box 30709, Nairobi, Kenya
Methods
Results and Discussion
References
African trypanosomes, the causative agent of "nagana" in livestock and sleeping sickness in man, are protozoan parasites that are cyclically transmitted by tsetse flies (Glossina spp.). They have a 12-15 nm thick coat of a variable surface glycoprotein (VSG) covering the entire surface of the organism.¹ The generation of a new population of trypanosomes with a different surface coat has been shown to be the result of a clone-specific change in the expression of a particular VSG gene.2,3 It is clear that the ability of the parasite to alter its VSG2 has hampered the development of a conventional vaccine against trypanosomiasis. Therefore, a clearer understanding of the biochemistry of the VSG may suggest alternative targets for immunological intervention.
The VSGs from T. brucei are proteins known to be attached to the plasma membrane via a glycosyl-phosphatidylinositol (GPI) anchor.4 In addition, the presence of the GPI anchor is probably responsible for the immunological cross-reactivity observed between different VSGs.2,4,5 It has been postulated that the VSG is selectively released in vivo from the plasma membrane of the trypanosome as a result of the removal of the dimyristol-glycerol domain from the phosphatidylinositol via an endogenous phospholipase-C (PLC).6,7,8 The form containing the entire GPI anchor is commonly referred to as the membrane form of VSG (mfVSG) while the form lacking the dimyristolglycerol moiety is known as the soluble form of VSG (sVSG). Using subcellular fractions, we have that the VSG-specific PLC resided in the membrane of the flagellar pocket.9 However, we could not exclude the possibility that the mfVSG phospholipase-C activity could be in other membrane enclosed compartments: coated vesicles, endosomes, or other prelysosomal compartments. Internal localization of the mfVSG-PLC has also been postulated by others.4 The present report is an attempt to address this problem further.
Trypanosoma brucei clone MITat 1.52 was grown in lethally irradiated rats (600 red) and the organisms isolated from the infected blood using isopynic Percoll gradients followed by DEAE-cellulose chromatography supplemented with nucleosides.9 The cells were washed twice in ice cold SHKE (250 mM sucrose, 50 mM HEPES, 25 mM KCl, 1 mM EDTA, pH 7.4), and a membrane fraction prepared by the method of Grab et al..9 The fraction with the highest specific activity for mfVSG phospholipase-C (which banded on top of 14.4% Percoll; Fraction V) was then applied onto a 12.5-42.5% SHKE gradient and centrifuged (4 °C) for 16 hours in an SW-41 rotor. The gradient was collected and the fractions analysed for total protein,10 mfVSG-PLC,9 and adenyl cyclase.11 In one experiment the cells were resuspended in RPMI-1640 medium (108/ml) supplemented with 100 m m hypoxanthine and BSA (10 mg/ml) and incubated at 37 °C for 30 minutes before fractionation. The BSA content was determined by conventional liquid phase RIA using rabbit anti-BSA serum (Miles-Yeda, Ltd.) and 125I-BSA.
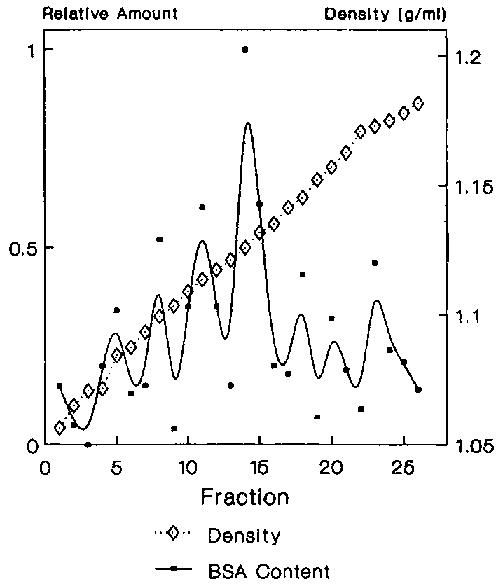
Data from several experiments showed that the mfVSG-PLC activity resided in two membrane fractions with densities of 1.13 and 1.14 gm/ml respectively (Figure 1), with equally highly specific activity in each. However, most of the enzyme was in the 1.14 gm/ml peak. Adenyl cyclase, a putative flagellar pocket membrane marker12, was found in high specific amounts in the 1.13 density fraction as well as in two broadly banding fractions with average densities of 1.11 (flagellar pocket membranes?13) and 1.09 gm/ml, with the bulk of the total activity residing in the lighter fractions (Figure 2). To clarify whether any of the mfVSG-PLC containing fractions could be of intracellular origin, the trypanosomes in one experiment were preincubated with BSA to load the endocytic compartments of the parasite. The 1.13 density fraction contained the highest specific amount of BSA (Figure 3).
Although preliminary, the data suggest that the 1.13 density fraction may be of endosomal origin resulting from the fusion of a relatively higher density mfVSG-PLC containing fraction (d = 1.14) with a lighter membrane component (density less than 1.12) of possible flagellar pocket origin. The intracellular localization of the mfVSG-PLC is also suspected by Bülow et al.14 The data and interpretation, although attractive, are still far from complete. One should be able to ascertain this by incubating the trypanosomes under conditions were BSA or horseradish peroxidase is known to load only the flagellar pocket region and other internal organelles. In addition, other fractionation methods using other physical parameters are being exploited: e.g., free-flow electrophoresis.
1. VICKERMAN, K. and A.G. LUCKINS. 1969. Nature 224: 1125-1127.
2. ENGLUND, P.T., S.L. HAJDUK and J.C. MARTINI. 1982. Ann. Rev. Biochem. 51: 695-726.
3. MAJIWA, P.A.O., J.R. YOUNG, R. HAMERS and G. MATTHYSSENS. 1986. Gene 41: 183-192.
4. FERGUSON, M.A.J. and A.F. WILLIAMS. 1988. Ann. Rev. Biochem. 57: 285-320.
5. FISH, W., C. MURIUKI, D. GRAB and J. LONSDALE-ECCLES. 1989. Biochem. 28: 5415-5421.
6. CARDOSO DE ALMEIDA, M.L., L.M. ALLAN and M. TURNER. 1984. Nature 302: 349-352.
7. FERGUSON, M.A.J., J.M. DUSZENKO, G.S. LAMONT, P. OVERATH and G.A.M. CROSS. 1986. J. Biol. Chem. 261: 356-362.
8. LOW, M.G., M.A.J. FERGUSON, A.H. FUTERMAN and I. SILMAN. 1986. Trends Biochem. Sci. 11: 212-215.
9. GRAB, D.J., P. WEBSTER, S. ITO, W.R. FISH, Y. VERJEE and J.D. LONSDALE ECCLES. 1988. J. Cell Biol. 105: 737-746.
10. VINCENT, R. and N. NADEAU. 1983. Anal. Biochem. 135: 355-362.
11. GRAB, D.J., U.A.K. KARA, S. ITO and L. ROVIS. 1984. J. Cell Biol. 99: 569-577.
12. WALTER, R.D. and F.R. OPPERDOES. 1982. Mol. Biochem. Parasitol. 6: 287-295.
13. MCLAUGHLIN, J. 1982. J. Immunol. 128: 2656-2663.
14. BÜLOW R., G. GRIFFITHS, P. WEBSTER, S. YORK-DIETER, F. OPPERDOES and P. OVERATH. 1989. J. Cell Science 93: 233-240.
Figure 1. Sucrose density gradient analysis of Fraction V. The relative (RSA) specific activity (RSA) and relative total activity (RTA) for mfVSG phospholipase-C
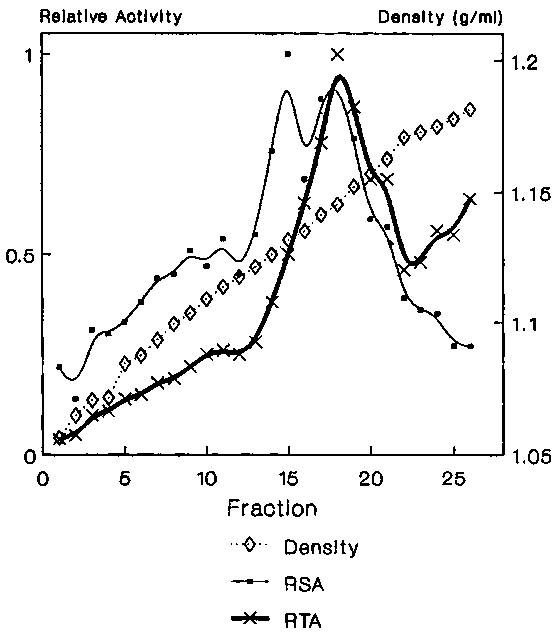
Figure 2. Sucrose density gradient analysis of Fraction V. RSA and RTA for adenyl cyclase activity.
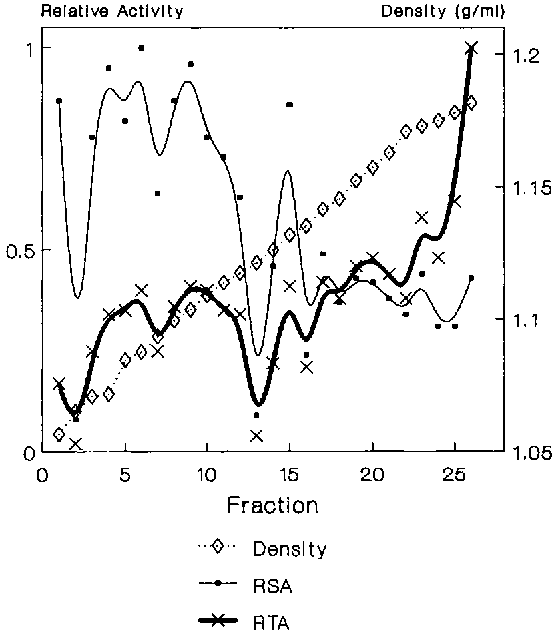
Figure 3. The relative specific content for BSA.