T.G. MORRISON and L.W. McGINNES
Department of Molecular Genetics and Microbiology
University of Massachusetts Medical School
Worcester, Massachusetts, USA
Intermolecular disulphide bonds form intracellularly
Oligomer formation and intracellular transport
Expression of the HN protein using a retroviral vector
Disulphide-linked oligomer formation early in infection
Conclusions
References
Plasma membrane glycoproteins are transported to the cell surface by cellular pathways that begin with the insertion of the nascent protein into the membrane of the rough endoplasmic reticulum (RER) and proceed with the transport of the protein through the Golgi membranes to the plasma membrane.1 During transport, glycoproteins are subjected to a variety of co- and post-translational modifications. In addition, the nascent protein must fold properly for full activity and possibly transport to the cell surface.2,3,4 One component of proper folding is the formation of intra- and, sometimes, intermolecular disulphide bonds.5,6,7 Current evidence suggests that disulphide bond formation is mediated by protein disulphide isomerase (PDI) located in the lumen of the rough endoplasmic reticulum.5,7
The glycoproteins of simple enveloped viruses have been useful in the study of the transport, modification and folding of plasma membrane glycoproteins. The HN protein of Newcastle disease virus (NDV), a paramyxovirus, has been used here to explore the formation of intermolecular disulphide bonds and the role this sort of post-translational modification plays in the intracellular processing of a glycoprotein. The HN glycoprotein is a type 2 glycoprotein,8 that is, the protein has no cleavable signal sequence but rather a bifunctional hydrophobic region near the amino terminus which serves as both a signal sequence and a membrane anchor. The amino terminus of the protein is the cytoplasmic tail while the carboxy terminus is transported across the membrane. It has been well documented that the HN glycoproteins of some paramyxomviruses, including the AV strain of NDV, form disulphide linked homo-oligomers.9 Indeed, as shown in Figure 1, virion associated HN protein is exclusively in the form of disulphide linked oligomers. The monomeric form of HN protein seen in the presence of reducing agent is absent when proteins are electrophoresed without reducing agent. Without reduction of virion proteins, HN is resolved as a high molecular weight band near the top of the gel (HNo). Evidence is presented here that the NDV HN disulphide linked oligomers form in the late stages of residence in the RER. However, not all nascent HN protein forms of oligomers and oligomer formation is not required for transport of the protein to the cell surface. Indeed, evidence is presented that the level of oligomer formation depends upon the concentration of the protein in cells.
To explore the formation of intermolecular disulphide bonds, infected cells were pulse labelled (5 min) or pulse labelled and then chased with non-radioactive methionine in the presence of cycloheximide. Proteins present in the cell extracts were resolved on polyacrylamide gels in the presence or absence of reducing agent (Figure 2). We have previously found that the HN protein is transported through the cell extremely slowly.10 Pulse labelled HN reaches the cell surface with a t½ of 78 min. We also found that 100% of cell associated pulse-labelled HN protein reaches the cell surface after approximately 2.5 hours of chase.10 Therefore, chase times were extended to four hours. It is clear that the oligomer does not begin to appear until 15 min of chase and continues to increase in amount up to 2 to 3 hours of chase.
To determine the intracellular location of the formation of the oligomer, use was made of inhibitors that block glycoprotein migration at various points in the cell. CCCP, an inhibitor of oxidative phosphorylation, blocks any energy-requiring process such as migration of protein from the RER.11 Figure 3A, lanes 5 and 6, shows that if CCCP is present during the non-radioactive chase, the disulphide linked oligomer does not form. Monensin, a sodium ionophore, is reported to interfere with the transport of most membrane glycoproteins by preventing their exit from the medial Golgi membranes.12 Clearly, the HN oligomer forms in the presence of monensin (Figure 3B, Lanes 6, 7 and 8).
These results argue that the formation of the disulphide-linked oligomer of the HN protein occurs postranslationally prior to the exit from the medial Golgi membranes. The results with CCCP suggest that the formation of the disulphide bonds either requires energy in the RER or that the oligomer forms after the protein leaves the RER. It has been shown that when cells are incubated at 15°C, glycoprotein transport from the RER is blocked.13 When pulse-labelled NDV-infected cells are subjected to a nonradioactive chase at 15°C, the disulphide-linked oligomer forms (not shown). Thus, it is likely that the formation of the HN oligomer is an energy-requiring process that occurs in the RER, a finding consistent with the proposed location of PDI in eucaryotic cells.15
A key to the understanding of the intermolecular disulphide bona formation in intracellular transport of the HN protein is the efficiency of this post-translational modification. If this modification is required for intracellular transport of the protein, all HN protein that reaches the cell surface should be in the form of disulphide-linked oligomers. It is clear from Figure 2 that not all pulse labelled HN protein is found in oligomers after prolonged chase periods. This finding was confirmed by immunoprecipitating the HN protein from these extracts with antibody specific for the HN protein (see Figure 7). Thus, we asked if the monomeric form of the protein can be found at the cell surface. We have previously devised an assay to isolate only cell surface glycoproteins using polyclonal antisera directed against NDV proteins.10 We have also adapted the assay for monoclonal antibody directed against the HN protein (Morrison and McGinnes, in preparation). Using this assay, and isolating the cell surface HN protein under non-reducing conditions, it is clear that a significant amount of cell surface HN is in the form of monomers (Figure 4).
It is possible that monomeric forms of the HN protein are less efficiently incorporated into the plasma membranes. To determine the kinetics of insertion into the cell surface of the two forms of HN protein, cell surface molecules were isolated at various times after the onset of the chase and resolved on gels. The amount of the two forms of the HN protein were quantitated with a densitometer. Figure 5 shows that the kinetics of appearance at the cell surface, as well as the release, of the monomer and the oligomer are very similar. Thus, the monomeric form of the HN protein is transported to the cell surface at a rate similar to that of the disulphide-linked form.
A cloned copy of the HN gene was expressed in chick cells to explore further the processing of this protein. The expression vector used for these studies is a retrovirus14,15 derived from Rous sarcoma virus. This virus is replication competent but with the sarc gene deleted. In the place of the sarc gene is a C1a I site for insertion of foreign DNA. Insertion in one direction allows for the expression of the sense version of the gene, while the opposite direction allows for the transcription of antisense sequences. The cDNA of the HN gene was inserted into a plasmid containing a DNA copy of the retrovirus and chick embryo fibroblasts were transfected with the DNA. Since the virus is replication competent, transfected cells will release virus, allowing for the spread of the infection throughout the entire culture. Thus all cells in the culture receive the HN gene. Figure 6 shows that the cells transfected with the retrovirus containing the sense version of the HN gene (RHN) express the HN protein, whereas cells that received DNA with the HN gene inserted in the antisense direction (R antiHN) do not contain the protein. The level of HN expression is approximately 5-10% that seen in NDV-infected cells 6 to 8 hours after infection.
The HN protein expressed from the retrovirus vector is transported to the cell surface. To assay for cell surface expression, two different assays were used. First, antibodies were used to isolate specifically cell surface molecules as described above. RHN cells contain HN at the cell surface while RSV or R antiHN cells are negative (not shown). A second assay for cell surface expression of the HN protein takes advantage of the ability of the HN protein to attach to red blood cells. Cells expressing HN on their surfaces will adsorb red blood cells.16,17 Table 1 shows a quantitation of haemadsorption using ND-infected cells. At various times after infection, red blood cells are added to intact cells, incubated once, and unbound blood cells washed away. Bound red blood cells are lysed and the amount of released haemoglobin determined in order to quantitate the amount of red blood cell binding. There is negligible binding early in infection, while binding increases with time, reflecting the presence of the HN protein on infected cell surfaces. Similar assays were done with the RHN cells. Significant binding of red blood cells (6 × 106) was observed while the antisense expressing cells were negative.
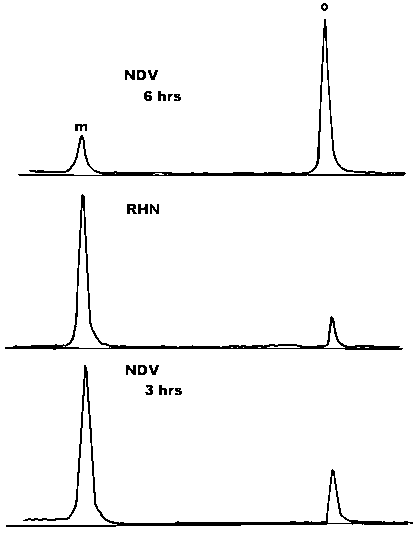
A surprising result was that the ratio of the monomeric form and disulphide-linked oligomers present in these HN expressing cells was quite different than that observed in infected cells (Figure 7, compare top and middle panels). In this experiment, NDV infected cells contained 70% of the HN protein in the form of disulphide-linked oligomer, whereas the retrovirus-infected cells contained 10% in the form of a disulphide-linked oligomer. In addition, the vast majority of cell surface HN protein is in the form of a monomer (not shown). There are two possible explanations for the reduced level of disulphide-linked oligomers in these cells. First, it is possible that the presence of another viral protein in the infected cells promotes the formation of disulphide-linked oligomers. A second possibility is suggested by the fact that the level of HN protein expression from the retrovirus vector is lower than that in an actively infected cell. Perhaps disulphide-linked oligomers form efficiently only after the HN proteins reach a critical concentration in the cell.
If it is the concentration of protein in a cell that promotes the formation of disulphide-linked oligomers, then monomer formation should be favoured very early in an infected cell when the concentration of viral proteins is relatively low. To test this possibility, infected cells were subjected to a pulse chase at 3 to 5 hours post-infection, in contrast to the usual time of 5 to 7 hours post-infection. The ratio of monomers and disulphide-linked oligomers was determined. Figure 7, bottom panel, shows a densitomer scan of the resulting auto-radiograph. At the early time point, most of the HN protein is in the form of a monomer (75%). However, later in infection, oligomer formation is favoured (Figure 7, top panel). This result argues that it is the concentration of the protein that determines the ratio of the monomer and the disulphide-linked oligomer.
One recently described post-translational modification of two viral glycoproteins, the influenza haemagglutinin protein HA and the vesicular stomatitis virus glycoprotein G, is oligomerization of the protein.2,3,4 The influenza haemagglutinin protein forms trimers and the vesicular stomatitis virus glycoprotein forms dimers and trimers soon after synthesis in the rough endoplasmic reticulum. Evidence has been presented that the formation of these homo-oligomers is essential for transport of the glycoproteins out of the RER. Thus, an obvious question is the universality of this requirement. It is clear from the results presented here that formation of the disulphide-linked oligomer of the NDV HN protein is not a prerequisite for transport from the RER. Significant amounts of the monomeric form of the protein can be detected at the cell surface. In addition, the amount of disulphide-linked oligomer in cells seems to depend upon the level of expression of the protein. Thus, the formation of oligomers may not be a universal requirement for the intracellular processing of viral glycoproteins.
While not all HN protein forms disulphide-linked oligomers, virion associated protein is exclusively in this form. Thus the fate of cell surface monomeric HN protein is of interest. It is possible that monomeric forms incorporated into virions rapidly form oligomers during budding. Alternatively, it is possible that monomers are not used in virion formation and may be endocytosed and degraded. This latter possibility raises interesting considerations for viral infections under conditions when gene expression is limited, such as persistent infections.
1. PFEFFER, S. and J. ROTHMAN. 1987. Ann. Rev. Biochem. 56: 829-852.
2. KREIS, T. and H. LODISH. 1986. Cell 46: 929-937.
3. COPELAND, C., R. DOMS, R. BOLZAN, R. WEBSTER and A. HELENIUS. 1986. J. Cell Biol. 103: 1170-1191.
4. GETHING, M.J., K. McCANNON and J. SAMBROOK. 1986. Cell 46: 938-952.
5. FREEDMAN, R. 1984. Trends in Biochem. Sci. 9: 438-441.
6. BERGMAN, L. and W. KUEHL. 1979. J. Biol. Chem. 254: 8869-8876.
7. BERGMAN, L. and W. KUEHL. 1979. J. Biol. Chem. 254: 5690-5694.
8. MORRISON, T. 1988. Virus Research 10: 113-136.
9. MARKWELL, M. and C. FOX. 1980. J. Virol. 33: 152-166.
10. MORRISON, T. and L. WARD. 1984. Virus Research 1: 225-239.
11. FRIES, E. and J. ROTHMAN. 1980. Proc. Natl. Acad. Sci. USA 77: 3870-3874.
12. TARTAKOFF, A. 1983. Cell 33: 1026-1028.
13. SARASTE, J. and E. KUISMANEN. 1984. Cell 38: 535-549.
14. HUGHES, S., J. GREENHOUSE, C. PETROPOULOS and P. SUTRAVE. 1987. J. Virol. 61: 3004-3012.
15. HUGHES, S. and E. KOSIK. 1984. Virology 136: 89-99.
16. MORRISON, T., P. CHATIS and D. SIMPSON. 1981. In The Replication of Negative Strand Viruses. D. Bishop and R. Compans, Eds.: 471-477. Elsevier, North Holland.
17. CHOPPIN, P. and R. COMPANS. 1975. In Comprehensive Virology, Vol. 4. H. Fraenkel-Conrat and R. Wagner, Eds.: 95-178. Plenum Publishing Co., New York.
Table 1. Haemadsorption of NDV-infected cells
|
NDV-infected cells | |
|
Time after infection (hrs) |
Red blood cells bound (×106) |
|
0.0 |
0.0 |
|
1.5 |
0.0 |
|
3.0 |
0.0 |
|
4.5 |
0.3 |
|
6.0 |
2.0 |
|
8.0 |
4.0 |
|
10.0 |
5.5 |
|
12.0 |
9.5 |
Figure 1. Virion proteins electrophoresed in the presence (+) or absence (-) of b mercaptoethanol.
Figure 5. The kinetics of appearance and release of cell surface HN monomers and oligomers. Cells pulse-labelled for 5 min were chased for various lengths of time and cell surface HN isolated and quantitated by densitometer scans of autoradiographs of polyacrylamide gels.
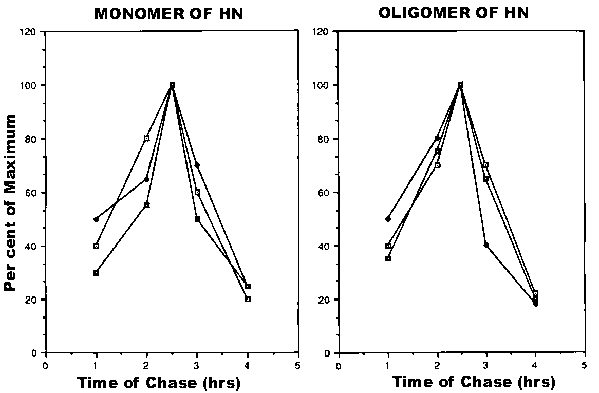
Figure 7. HN protein in NDV-infected cell extracts and retrovirus-infected cell extracts. Cells were pulse-labelled for 5 min and chased for 2 kits. HN protein present in the resulting cytoplasmic extracts was immunoprecipitated with monoclonal antibody against HN protein. The figure shows densitometer scans of autoradiographs of polyacrylamide gels of the immunoprecipitate. M, monomer; O, oligomer. Top panel: NDV-infected cells chased from 5 to 7 hours post infection. Middle panel: retrovirus containing the HN gene, 2 hour chase. Bottom panel: NDV-infected cells chased from 3 to 5 hours post-infection.