M.N. MOORE
Plymouth Marine Laboratory
Prospect Place, Plymouth, PL1 3DH, UK
Acknowledgement
References
A major function of lysosomes is the autophagic turnover of cytoplasmic components, including organelles and proteins.1,2 The precise mechanisms for the uptake of proteins are not clearly identified, although there are indications that both microautophagy and macroautophagy are involved.1,2 There is considerable but mainly circumstantial evidence that reduced lysosomal membrane stability results in enhanced catabolic activity and vacuolar fusion.3 In view, therefore, of the importance of the lysosomal system in turnover of cytoplasmic components, and its involvement in many pathological responses, it is important to establish the relationship between lysosomal stability and lysosomal function.
Structure-linked latency of lysosomal enzymes is a complex phenomenon involving several factors, including membrane permeability and the internal associations of the hydrolases with structural components.4 It is well established that many agents such as various disease conditions, stress, hormones and drugs can induce destabilizing alterations in lysosomes, resulting in reduced hydrolase latency.3,5 However, the relationship between altered physiological states or pathological conditions and lower order events involving hydrolase latency and protein catabolism is seldom clear, and it is often difficult to determine whether functional changes in lysosomes are a cause or an effect of cellular dysfunction.3
In our attempts to test this relationship we have used predominantly cytochemical studies on molluscan digestive cells from the hepatopancreas or digestive gland as a model system. Many molluscan cell types are particularly rich in lysosomes, which are involved in processes of digestion, excretion, immunity and autophagic mobilization of nutrient reserves.6 This relative abundance of lysosomes and the obvious dependence of these cells on lysosomal processes makes them suitable models for the investigation of functional relationships.
In this respect, singular attention has been paid to the digestive cells, which are in fact multifunctional.6 These cells are involved in the pinocytotic uptake of food and its subsequent digestion in secondary lysosomes.6 However, digestive cells also have a degree of functional analogy with vertebrate liver cells (hepatocytes) in that they store glycogen and lipid, and are the site of major physiological processes in the animal, including detoxication and excretion.6,7 As well as being of considerable metabolic significance, the digestive gland is a major interface between the organism and its environment Previous studies have clearly demonstrated that the cells of this organ are highly responsive to toxic chemical pollutants.6,7 Most of these responses apparently involve both structural and functional alterations in lysosomes, although it is probably artificial to discriminate between them. We have been particularly interested in the identification of causal relationships in the responses of marine molluscs to environmental stress and establishing a conceptual framework for the chain of events from environmental stimulus to consequences for the organism. Our approach has been to examine responses to stress and various drugs and xenobiotics at several levels of biological organization, namely from the biochemical and subcellular to that of the whole animal.
This report summarizes progress in this area, with particular emphasis on the cellular consequences of experimentally induced alterations in lysosomes. In attempting to establish functional links we have used experimental manipulation of digestive cells in order to characterize their lysosomal and cellular reactions. In this context, H.K. Hawkins has subdivided lysosomal reactions into three basic categories, namely, changes in membrane permeability, in vacuolar fusion events and in lysosomal contents.3
Lysosomal reactions in molluscs fall into these three classes, which often may have considerable overlap. We will now consider these reactions in more detail.
Changes in lysosomal stability are induced by a variety of in vivo experimental treatments of mussels. These include elevated temperature, hypoxia, salinity increase, certain sex steroids and lipophilic xenobiotics.6 All of these treatments result in a decrease in cytochemically determined stability of lysosomes, based on hydrolase latency, and this has been confirmed biochemically in selected experiments. These effects are believed to involve an increase in the permeability of the lysosomal membrane because they are frequently reversible by cortisol treatment, which is a recognized membrane stabilizer (Figure 1).8,9
Reductions in lysosomal stability in digestive cells have frequently been demonstrated to be reversible during the course of adaptation to particular stimuli such as salinity increase; this suggests that such changes may have an adaptive physiological role.10,11 The possible consequences of destabilizing alterations in lysosomal membranes have been investigated in a number of experimental conditions, for instance, treatment of mussels with 17 b -estradiol results in a rapid destabilization of digestive cell lysosomes (Figure 1).9 This is accompanied by an increase in the formation of tertiary lysosomes containing lipofuscin, which may be indicative of augmented autophagy.9
Salinity increase also results in a rapid decrease in lysosomal stability and evidence of enhanced autophagy.10,11,12 Mussels are known to osmoregulate intracellularly by increasing the concentration of cytosolic free amino acids.10,11 These are probably generated by enhanced protein degradation and this hypothesis is supported by evidence of increased amino acid concentrations in fractionated lysosomes.11 The implication is that decreased lysosomal stability is involved in the activation of lysosomes for the autophagic degradation of cytoplasmic proteins.
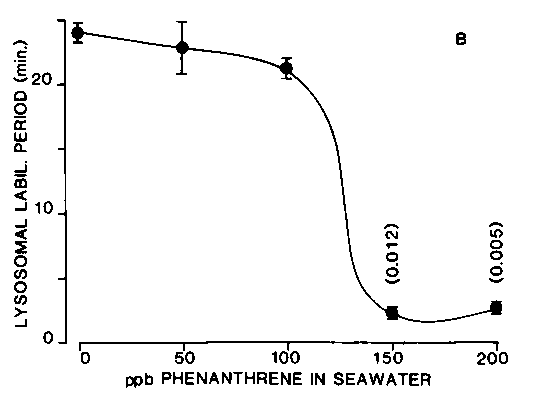
More recent studies have used the polycyclic aromatic hydrocarbon (PAH) phenanthrene as a model destabilizer of digestive cell lysosomes.13,14 Digestive cells have a relatively low cytochrome P-450 content, resulting in a low rate of metabolism of PAHs and other lipophilic drugs.7 The consequence is that PAHs accumulate in these cells and there is evidence that the lysosomes are a major site for their sequestration.15 The response relationship between tissue concentration of phenanthrene and lysosomal stability has been determined and this has a sigmoidal form indicating an "all or nothing" type of response.13,14 This finding suggests that at a particular threshold level of incorporation of phenanthrene into the lysosomes, there is a "catastrophic" alteration in membrane organization involving permeability/fluidity changes resulting in the reduced stability of latent b -N-acetylhexosaminidase and b -glucuronidase (Figure 2).13,14 Concomitant determinations were made of the loss of 14C-label from pre-labelled cytosolic proteins in the digestive gland cells over a concentration range of phenanthrene.13 The results indicated that there was an increased loss of 14C-label only from cells in which the lysosomes were destabilized (Figure 2).13 As the possibility of re-incorporation of 14C-label due to protein synthesis had been minimized, this finding was consistent with enhanced catabolism of cytosolic proteins. In fact, both lysosomal membrane stability and concentration of 14C-label cytosolic protein were significantly correlated (r = 5, p = 0.001, two-tailed test).14
Other changes in lysosomes frequently accompany the measured decrease in stability. These include increase fusion of vacuolar components, lysosomal swelling, increased neutral lipid and lipofuscin content and increased numbers of tertiary lysosomes (Figure 3).6,7,12,16 These changes are all consistent with the hypothesis of increased autophagy and catabolic activity resulting from increased lysosomal fragility. Ultrastructural evidence of increased architectural fragility of lysosomal membranes and enhanced fusion with other vesicular components lends further support to this hypothesis.12 Furthermore, evidence of enhanced microautophagy and leakage of hydrolase into the cytosol following treatment with PAHs has been tentatively linked with reduction of membrane stability.12,17
Additional support for the hypothesis that enchanced catabolic activity is linked with lysosomal fragility is provided by evidence of atrophic alterations in the digestive cells following lysosomal destabilization.
In conclusion, we can say that although the evidence of increased protein catabolism associated with lysosomal fragility is still correlational, there is considerable supportive evidence that increased membrane fragility does in fact result in enhanced autophagy leading to atrophy of the digestive cell.
This work is part of the research programme of the Plymouth Marine Laboratory, a component of the Natural Environment Research Council, UK.
1. MORTUNIRE, G.E., N.J. HUSTON and C.A. SURMACZ. 1983. Proc. Natl. Acad. Sci. USA. 80: 2179-2183.
2. MARZELLA, L., J. AHLBERG and H. GLAUMANN. 1980. Exp. Cell Res. 129: 460-466.
3. HAWKINS, H.K. 1980. In Pathobiology of Cell Membranes. B.F. Trump and A.V. Arstila, Eds.: Volume 2: 252-285. Academic Press, New York, San Francisco, London.
4. PFEIFER, U. 1987. In Lysosomes: Their Role in Protein Breakdown. H. Glauman and F.J. Ballard. Eds.: 3-59. Academic Press, New York, San Francisco, London.
5. SZEGO, C.M. and R.J. PIETRAS. 1984. Int. Rev. Cytol. 88: 1-302.
6. MOORE, M.N. 1985. Mar. Pollut. Bull. 16: 134-139.
7. MOORE, M.N., D.R. LIVINGSTONE, D.R. WIDDOWS, J. LOWE and R.K. PIPE. 1987. Phil. Trans. R. Soc. Lond. B316: 603-623.
8. MOORE, M.N. 1976. Cell Tiss. Res. 175: 279-287.
9. MOORE, M.N., D.M. LOWE and P.E.M. FIETH. 1978. Cell Tiss. Res. 188: 1-9.
10. MOORE, M.N., R.K. KOEHN and B.L. BAYNE. 1980. J. Exp. Zool. 214: 239-249.
11. BAYNE, B.L., M.N. MOORE and R.K. KOEHN. 1981. Mar. Biol. Letts. 2: 193-204.
12. PIPE, R.K. and M.N. MOORE. 1985. Marine Biology 87: 157-163.
13. MOORE, M.N. and A. VIARENGO. 1987. Experientia 43: 320-323.
14. NOTT, J.A. and M.N. MOORE. 1987. Histochem. J. 19: 357-368.
15. MOORE, M.N., R.K. PIPE and S.V. FARRAR. 1988. Mar. Environ. Res. 24: 352-353.
16. MOORE, M.N. 1988. Mar. Ecol. Prog. Ser. 46: 81-89.
17. PIPE, R.K. and M.N. MOORE. 1986. Aquat. Toxicol. 8: 65-76.
Figure 1. The effects of injections of sex steroids (100 m l 5 × 10-7 M) and cortisol (100 m l 10-2 M) on the labilisation period of lysosomal hexosaminidase after 2 h in Mytilus edulis (mean ± SE).9
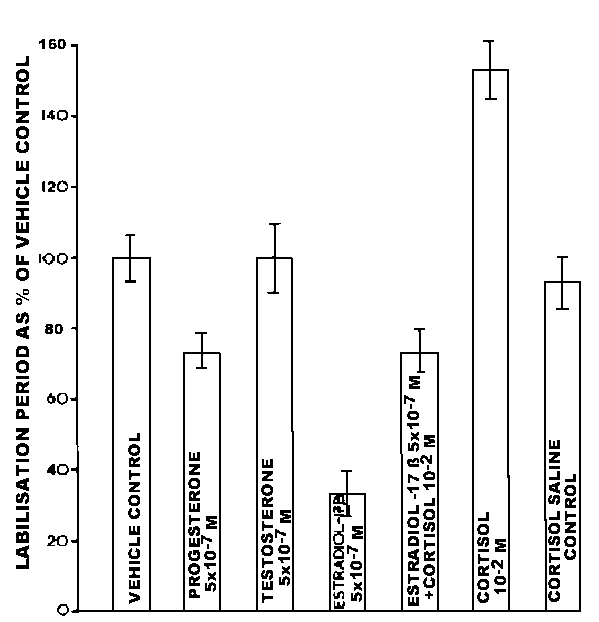
Figure 2A. The effect of phenanthrene concentration in seawater on specific activity of 14C-labelled cytosolic proteins (as % of control values) in the midgut gland (each point is the mean ± SE of at least five replicate experiments).
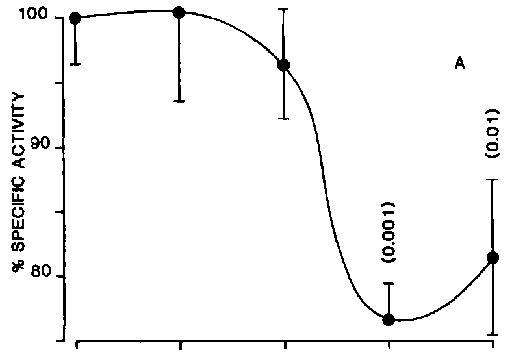
Figure 2B. The effect of phenanthrene concentration in seawater on lysosomal stability in digestive cells (each point is the mean ± SE of five replicate experiments, each experimental treatment contained five animals). Exact probability values are given in the figures where values differ significantly from those of the control. These are shown vertically above the appropriate data points.13