C. REDMAN
Lindsley F. Kimball Research Institute
New York Blood Center
New York, NY 10021, USA
Early studies
Intracellular forms of fibrinogen
Order of chain assembly
Enzymatic mechanism of chain assembly
Secretion of nascent fibrinogen
References
Fibrinogen is a large molecular weight (Mr 340,000) glycoprotein present in plasma. Its primary function is in blood clotting; it is cleaved by thrombin, and is transformed to fibrin clots and specifically binds to receptors on platelets and regulates their aggregation. Human plasma fibrinogen is a dimer with each half-molecule composed of three different polypeptides; Aa (Mr 66,000, 610 amino acids.), Bb (Mr 54,000, 461 amino acids) and t (Mr 48,000, 411 amino acids). The half-molecules of the dimer are held together by symmetrical disulphide bonds between two Aa chains (at Aa -28) and two t chains (at t -8 and t -9). In addition, fibrinogen contains a number of inter- and intrachain disulphide linkages. The 6 fibrinogen polypeptides are held together by 29 disulphide bonds and the protein does not have free sulphydyl groups.
The fibrinogen chains contain several prosthetic groups. Both the Bb and t chain contain asparagine-linked carbohydrates, ester-bound phosphate occurs in the Aa chain and tyrosine-O-sulfates in the Bb chain. There are two non-allelic forms of the t chain. In the t chain the 4 carboxy-terminal amino acids of the t chain are replaced by 20 amino acids.1,2,3
The complete molecule appears by electron microscopy to form a trinodal structure linked by slender rope-like strands. Studies in which both image processing of electron micrographs and analysis of low resolution X-ray crystallography were used extends this picture. It is thought that the central domain of fibrinogen contains the two amino-terminal portions of the half-molecule and the two end domains are composed of the carboxy-termini of the Ba and t chains. The carboxy-termini of the two larger Aa chains are believed to fold back to form an additional central domain.4
The fibrinogen genes have been localized in the long arm of chromosome 4 and the three fibrinogen genes are linked in the order of t,a,b with the Bb gene in the opposite orientation. Complete DNA sequences of the human t and partial sequences of the human Bb gene and rat Bb t genes are known. There is little similarity in the three genes. On stimulation of fibrinogen synthesis, during an acute phase response, there is a coordinate increase in levels of all three mRNAs and this is accompanied by changes in the transcription activity of each of the three fibrinogen genes. This indicates that similar mechanisms regulate the activity of the three fibrinogen genes.5
Fibrinogen is primarily synthesized by hepatocytes, but megakaryocytes are also capable of producing small amounts of fibrinogen. The production of fibrinogen by hepatocytes is probably controlled by humoral agents. One mechanism, which is attractive and has received much experimental attention, is that some degradation products of fibrinogen and fibrin elicit the formation of a small peptide (about 30,000 daltons) from monocytes and/or Kupffer cells, which in turn stimulates hepatocytes to produce more fibrinogen.6,7 The stimulatory agent, first termed hepatocyte stimulating factor, is probably identical to interferon b 2- B cell differentiation factor 2.8
Thus, fibrinogen is a multi-chain protein held together by an intricate array of disulphide bonds and the three chains are intertwined in a specific pattern giving fibrinogen its unique properties, which allow it to circulate in the blood awaiting signals to perform its haemostatic functions. How this complex multi-chain protein is assembled intracellularly and what regulates its production and secretion are the questions which our studies aim to answer.
The separate chains of bovine,9 dog10 and rat11 fibrinogen are synthesized by mRNA-dependent membrane-free translation systems as larger ("signal") precursors. This, together with molecular cloning of cDNA for the Aa, Bb and t chains of fibrinogen, showed that the three chains are synthesized from separate mRNAs. In vivo studies in dogs indicated that the dimeric molecule is quickly assembled in the rough endoplasmic reticulum (RER),12 but a time course study of secretion of rabbit fibrinogen suggested that the three chains are not immediately assembled prior to secretion. Newly secreted rabbit fibrinogen first contained nascent radioactive Bb chains, followed later by Aa and t chains.13 Pulse-chase experiments with Hep G2 cells confirmed that newly secreted fibrinogen contains higher specific radioactivity in the Bb chain than in Aa and t chains, again suggesting that pools of Aa and t chains exist intracellularly.14
Most of our knowledge of fibrinogen assembly relies on the results of experiments performed with a human hepatocellular carcinoma, the Hep-G2 cell.15 Hep-G2 cells were pulse-labelled with L-[35S] methionine and "chase" incubated with non-radioactive L-methionine. At various times the cells and the incubation medium were separated and fibrinogen and fibrinogen-related materials were isolated by immunoprecipitation with a polyclonal antibody that recognizes intact fibrinogen as well as its component chains. Prior to immunoprecipitation the cells were homogenized in the presence of 0.2 M iodo-acetamide to block free sulphydryl groups and minimize further disulphide interactions. The fibrinogen compounds were separated by SDS-PAGE and detected by autoradiography. In some experiments the fibrinogen-related compounds were excised from the gel and reduced and re-electrophoresed to determine the chain compositions and the specific radioactivity of the component chains.
In non-reducing gels, eight fibrinogen-related radioactive bands were obtained. The estimated molecular weight of these proteins, as deduced from their electrophoretic mobilities on SDS-PAGE and the chain composition of each is given in Table 1.
These studies confirm the presence of excess Aa and t chains and demonstrates that most of these "extra" Aa and t chains exist as Aa - t complexes. Free t chains are present, but very few free Aa or Bb chains.
The mechanisms by which excess Aa and t chains accumulate within Hep-G2 cells are poorly understood. Unequal synthesis of the three chains may contribute to these pools. We have determined that the initial rates of synthesis of Bb chain is less than that of Aa and t chains. This inbalance in the ratios of intracellular chains may also be due to small differences in the degradation rates of the three chains.
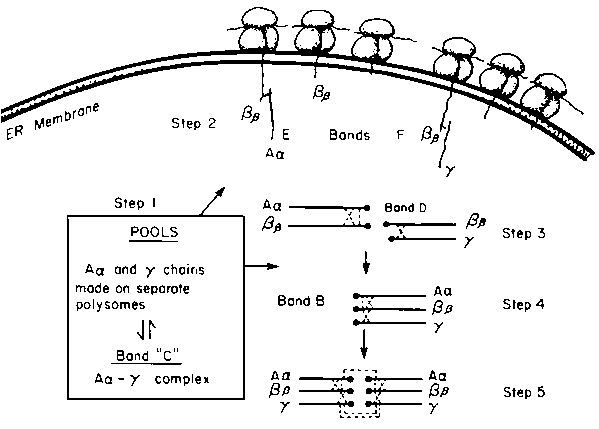
The sequence in which the three component chains of fibrinogen interact with each other to form fibrinogen was determined by following (1) the order in which the radioactivity of pulse-labelled forms of fibrinogen decrease during the "chase" incubation (Figure 1) and (2) the radioactivities of each of the chains in the various fibrinogen-related compounds at the end of the "pulse" period and at different "chase" times. An interpretation of the results is presented in Figure 2, and for convenience we have divided the intracellular assembly of fibrinogen into 5 steps.
Step 1. Unequal synthesis of the three chains, and perhaps unequal rates of degradation, leads to an accumulation of excess Aa and t chains. Aa and t chains combine to form an Aa -t complex (band C) which accounts for the major intracellular form of fibrinogen-related material. Hep-G2 cells also contain free t chains. These act as intracellular "pool" forms of Aa and t chains.Step 2. Fibrinogen assembly begins by the attachment of either Aa or t chains, drawn from the pool, with nascent Bb chains. This occurs while the Bb chain is not fully completed and is still attached to polysomes of the RER. For incomplete Bb chains to react with Aa and t chains the incomplete Bb chains, has to be of sufficient length to span the large ribosomal subunit and the width of the ER membrane and to expose N-terminal cysteine residues to the luminal surface of the ER. The earliest precursor forms of fibrinogen noted was "pulse" labelled incomplete Bb of between 27,000 and 32,000 daltons, complexed to either non-radioactive A or non-radioactive t chains. The size of the Bb polypeptide is sufficient to meet the above criteria.
Step 3. On completion of Bb chain synthesis, the Bb -Aa and Bb -t complexes are released into the luminal side of the ER and the remainder of the fibrinogen chain assembly occurs in the lumen of the RER.
Step 4. t chain is added to the nascent Bb -Aa complex and Aa is added to the nascent Bb -t, forming half-molecules of fibrinogen. The Aa and t chains are obtained from the intracellular pools. Analysis of these precursor forms from early "pulse-chase" periods show that they are composed of radioactive Bb and non-radioactive Aa and t chains.
Step 5. The final step in fibrinogen chain assembly is the joining of two half-molecules by the formation of symmetrical disulphide bonds between two Aa and two t chains.
All of the above steps occur in the RER. Fibrinogen at this stage is not fully glycosylated and has not yet attained its phosphate and sulphate moieties. These final touches are thought to occur in the Golgi complex, prior to secretion.
Other processing steps are probably involved in the final production of fibrinogen, cDNA cloning studies suggest that a precursor of Aa chain, with an extension at the carboxyl terminus, is a likely primary translation product,17 but our procedures do not allow us to distinguish between the secreted forms of Aa and the putative precursor.
That there is a pool of Aa and t chains and that Bb chains are directly inserted into fibrinogen precursors suggest that synthesis of the Bb chain may be the limiting factor in fibrinogen assembly.
Fibrinogen production may be easily regulated by modulating the rate of synthesis of the Bb chain. However, stimulation of fibrinogen synthesis in rats by defibrination results in an increase of all three fibrinogen mRNAs indicating control at the transcriptional level. Stimulation of synthesis in rabbits does not change the relative specific radioactivities of the component chains of secreted fibrinogen,13 indicating that increased fibrinogen synthesis does not alter the sequence of fibrinogen chain assembly.
An enzyme, a protein disulphide isomerase, is present on the inner surface of the ER membrane and is particularly abundant in secretory tissues. This enzyme may catalyze both intra- and interchain disulphide interactions and can rearrange disulphide bonds in an oxidized protein and also the formation of native disulphide bonds in reduced proteins.18 Therefore fibrinogen precursors may exist in either reduced or oxidized forms prior to further interactions with other fibrinogen chains. Incubation of Hep-G2 cells with 14C-iodoacetamide detected only substantial free sulphydyl groups in the Aa -t complex, with small amounts in the free t chain.
Ordered disulphide-bond formation of fibrinogen chains is not spontaneous and requires more than added enzyme. Attempts at in vitro assembly, in the presence or absence of disulphide isomerase, has led only to a haphazard assembly of chains. The chains probably also need to be held in proper juxtaposition. In this regard, it should be noted that a protein in the RER, termed IgG heavy chain binding protein (BiP), binds to nascent proteins that are incompletely assembled or with malformed proteins.19,20 Immunoprecipitation of nascent fibrinogen also co-precipitates a similar 78,000-dalton protein.
Only fully assembled fibrinogen is secreted into the medium by Hep-2 cells. None of the precursor forms of fibrinogen, the Aa -t complex or the free t chains, are secreted. Since free Bb chains do not occur intracellularly, but are complexed to other chains even before they are completed, we determined the fate of free Bb chains by transfecting a surrogate secretory cell (COS-1) with a shuttle vector pBc12BI containing full-length Bb cDNA. The Bb chain was expressed and not secreted.21 This suggests that the "signal" for secretion probably does not occur on any individual chain but resides on the intact dimeric molecule.
1. BLOMBACK, B. 1979. In Plasma Proteins. B. Blomback and H. Hanson, Eds.: 223: Wiley, New York.
2. HENSCHEN, A., F. LOTTSPEICH, M. KEHL and C. SOUTHAN. 1983. Ann. NY. Acad. Sci. 498: 28-43.
3. DOOLITTLE, R.F. 1984. Ann. Rev. Biochem. 53: 195-229.
4. WEISEL, J.W., C.V. STANFLACHER, E. BULLITT and C. COHEN. 1985. Science 230: 1388-1391.
5. CRABTREE, G.R., 1987. In The Molecular Basis of Blood Diseases. G. Stamatoyannopoulus, A.W. Nienhuis, P. Leder and P.W. Majerus, Eds.: 631-661. W.B. Saunders Co., Philadelphia.
6. RITCHIE, D.G., B.A. LEVY, M.A. ADAMS and G.M. FULLER. 1982. Proc. Natl. Acad. Sci. USA 79: 1530-1534.
7. NHAM, S. and G.M. FULLER. 1986. Thrombosis Res. 44: 467-475.
8. GAULDIE, J., C. RICHARDS, D. HAMISH, P. LANDSDORP and H. BAUMANN. 1987. Proc. Natl. Acad. Sci. USA 84: 7251-7255.
9. CHUNG, D.W., R.T.A. MACGILLIVRAY and E.Y. DAVIE. 1980. Ann. NY. Acad. Sci. 343: 210-217.
10. YU, S., C.M. REDMAN, J. GOLDSTEIN and B. BLOMBACK. 1980. Biochem. Biophys. Res. Commun. 96: 1032-1037.
11. NICKERSON, J.M. and G.M. FULLER. 1981. Proc. Natl. Acad. Sci. USA 78: 303-307.
12. KUDRYK, B., M. OKADA, C.M. REDMAN and B. BLOMBACK. 1982. Eur. J. Biochem. 125: 673-682.
13. ALVING, B.M., S. CHUNG, G. MURANO, D.B. TANG and J.S. FINLANGSON. 1982. Archives Biochem. Biophys. 217: 1-9.
14. YU, S., B. SHER, B. KUDRYK and C.M. REDMAN. 1983. J. Biol. Chem. 258: 13407-13410.
15. YU, S. B. SHER, B. KUDRYK and C.M. REDMAN. 1984. J. Biol. Chem. 259: 10574-10581.
16. YU, S., V. KONG, B. KUDRYK and C.M. REDMAN. 1987. Thrombosis Res. 46: 281-293.
17. RIXON, M.W., W.Y. CHAN, E.W. DAVIE and D.W. CHUNG. 1983. Biochemistry 21: 3237-3244.
18. FREEDMAN, R.B. 1984. Trends Biochem. Sci. 9: 438-441.
19. BOLE, D.B., L.M. HENDERSHO and J.F. KEARNEY. 1986. J. Cell Biol. 102: 1558-1566.
20. KASSENBROCK, C.K., P.D. GARCIA, P. WALTER and R.B. KELLY. 1988. Nature 333: 90-93.
21. DANISHEFSKY, K.J. and C.M. REDMAN. 1988. (Abstract) 4th International Congress on Cell Biology, Montreal, Canada.
Table 1. Size and chain composition of intracellular fibrinogen precursors
|
Bands on SDS-PAGE |
Estimated Mr |
Chain composition |
|
A |
340,000 |
Aa, Bb, t |
|
B |
214,000 |
Aa, Bb, t |
|
C |
140,000 |
Aa, t |
|
D |
125,000 |
mixtures Bb, t and Bb, Aa |
|
E |
113,000 |
Aa, incomplete Bb |
|
F |
102,000 |
t, incomplete Bb |
|
Aa |
65,000 |
Aa |
|
t |
45,000 |
t |
Table 2. Amount of endogenous fibrinogen-related protein detected by different procedures
|
|
% of total fibrinogen antigens |
||
|
Pulse-label |
Steady-state label |
Western Blot |
|
|
Fibrinogen |
4 |
33 |
13 |
|
Band B (half-molecule) |
10 |
8 |
0 |
|
Band C (Aa -t) |
18 |
30 |
53 |
|
Band D(Bb -a and t) |
15 |
0 |
0 |
|
Band E and F (incomplete + Aa or t) |
12 |
0 |
0 |
|
Aa (free chain) |
2 |
0 |
0 |
|
Bb (free chain) |
3 |
0 |
0 |
|
t (free chain) |
36 |
26 |
11 |
Hep-G2 cells were incubated with L-35S-methionine for either 3 mins (poise-label) or 15 h (steady state). The percentage of radioactivity in the various compounds was determined by scanning the autoradiograms with a laser densitometer and calculating the intensity profiles.
Figure 2. Scheme for the assembly of fibrinogen.