1. IDENTITY
2. DISTRIBUTION, ECOLOGY AND METABOLISM
3. LIFE HISTORY
4. POPULATION STRUCTURE AND MORTALITY
5. PRODUCTIVITY OF THE RESOURCE
6. METHOD OF HARVESTING AND HARVESTING CYCLE
7. EQUIPMENT USED FOR HARVESTING OF SEAWEED RESOURCES
8. PROTECTION AND MANAGEMENT OF THE RESOURCE
9. UTILIZATION
10. BIBLIOGRAPHY
by
A.R.O. Chapman
Department of Biology
Dalhousie University
Halifax, Nova Scotia
Canada B3H 4J1
|
ABSTRACT Laminaria longicruris was harvested as a source of alginate through the 1940's. Peak harvests were 5-6000 T/year. Since 1949 the species has been cut sporadically for human consumption with a peak harvest of 300 tons in 1979. The standing crop is at least 1,000,000 tons with a conservative yield estimate of about 300,000 T/year. Determinants of distribution, abundance and production are well studied for this under utilized species. |
The species binomial is Laminaris longicruris de la Pylaie (1824). De la Pylaie described the species from specimens collected on the island of Newfoundland. There are no published reports to show that a Type specimen has been designated in de la Pylaie's collection held in Paris (PC [see Stafleu and Cowan, 1976 for herbarium location]).
Recent taxonomic decisions (Chapman, 1974; Kain, 1976) indicate the following binomials may be regarded as synonyms of L. longicruris: L. agardhii Kjell.; L. faeroensis Borgesen.
Laminaria longicruris belongs to the family Laminariaceae (order Laminariales, Division Phaeophyta). The genus Laminaria is divided into sections Simplices and Digitatae which have undivided and divided laminae, respectively. L. longicruris belongs to the Simplices.
The North Atlantic species of the section Simplices were revised by Wilce (1965). He distinguished Laminaria longicruris from other members of the section by the presence of a long, hollow stipe. This definition reduces L. faeroensis to conspecificity with L. longicruris.
Since Wilce's (1965) revision, Laminaria longicruris has been the subject of a number of descriptive and experimental taxonomic studies which cast some doubt on it's taxonomic identity (Mann, 1971; Chapman, 1973, 1974, 1975; Kain, 1976; Luning et al., 1978; Bolton et al. 1983). In spite of this, the species is listed as a separate entity in the most recent checklist of marine algae of eastern Canada (South, 1984), with a footnote to indicate that L. longicruris may be conspecific with L. saccharina Lamour. According to Wilce (1965), L. longicruris can only be distinguished from L. saccharina on the basis of stipe length and the occurrence of hollowstipes. In Nova Scotia and in the Shetland Islands (to the north of Scotland) populations of plants with long, hollow stipes grade into populations with short, solid stipes (Chapman, 1973; Kain, 1976). There is no morphometric discontinuity. Furthermore, Bolton et al. (1983) were able to show that Nova Scotian populations of L. longicruris are interfertile with European populations of L. saccharina. The F1 hybrids became fertile in culture and produced healthy F2 gametophytes.
There is a significant genetic component in the expression of stipe length and hollowness in Nova Scotian populations of Laminaria, section Simplices, (Chapman, 1974) and it may be best to regard the long, hollow stiped members as a separate race of the species L. saccharina. Formal taxonomic decisions with nomenclatural changes have yet to be made and, for convenience, the race is considered here under the name Laminaris longicruris.
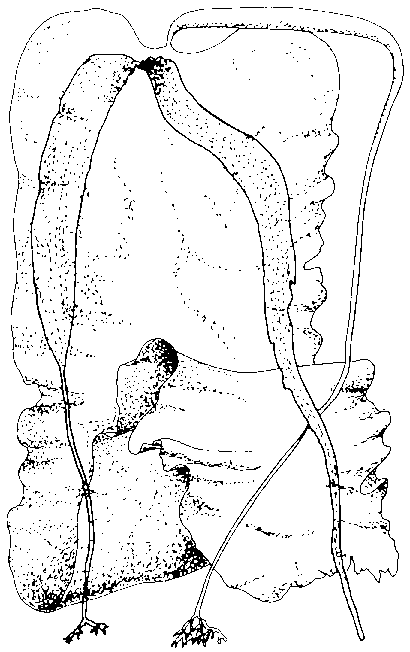
The morphological extremes found among the sporophytes of Laminaria longicruris are shown in Fig. 1. Very large plants with stipes up to 5 m and blades up to 7 m in length have been recorded from Seal Island off the southwestern shore of Nova Scotia (McPeak, 1980). More typically, populations contain plants with stipes and blades up to 2-3 m in length (Chapman, 1973; Kain, 1976).
Figure 1. Morphological form range in Laminaria longicruris (modified after Taylor, 1957, x 0.14.
The anatomy of Laminaria longicruris is typical for members of the genus as described by Pritsch (1945). The species has not been the subject of detailed fine structural analysis. The only aspect of the anatomy which has received special attention is the presence of mucilage ducts in the superficial tissues of the blade and stipe (Wilce, 1965; Chapman, 1975; Luning et al. 1978). At its southern limit in the NW Atlantic the species lacks mucilage ducts in the blade and in the stipe (Chapman, 1975). At the southern limit of the species in the eastern Atlantic (Shetland Islands, Kain, 1976) the blades have mucilage ducts, but the stipes do not. In more northerly locations in the NW Atlantic all combinations of duct occurrence in the blade and stipe may be found (Chapman, 1975).
2.1 Geographical extent
2.2 Local vertical and horizontal distribution
2.3 Effects of ecological determinants
2.4 Nutrition and growth
The species occurs widely throughout the NW Atlantic and eastern Arctic Oceans (South, 1984; Lee, 1980), mostly within Canadian territorial waters, and along the west coast of Greenland north of 62° N (Rosenvinge, 1899). The species is rare on the east coast of Greenland (Lund, 1959), but has been recorded from Iceland (Jonsson, 1912), the Faeroes (Borgesen, 1902) and as far south as Long Sound on the Shetland Islands at latitude 60°N (Kain, 1976). L. longicruris has not been recorded from the European mainland.
In the NW Atlantic Laminaria longicruris occurs as far south as Long Island Sound to about 41° N (Schneider et al., 1979). The species may occur further south on the New York side of Long Island Sound to Latitude 40°58N (Brinkhuis et al., 1984), but there is no indication that the plants at this site have hollow stipes.
North of New England Laminaria longicruris forms an important component of the sublittoral seaweed flora of the Canadian Maritime provinces (personal observations), Newfoundland and Labrador,(Wilce, 1959). In the eastern Arctic Ocean L. longicruris gives way to L. solidungula J. Ag. as the dominant component of the sublittoral seaweed vegetation (Chapman and Lindley, 1981). Nevertheless, populations of L. longicruris have been recorded to as far north as 82° off Ellesmere Island (Lee, 1980).
Laminaris longicruris is normally restricted to subtidal habitats. Very large plants often reach the surface at low water, but the holdfasts of the species are normally underwater at all times. Exceptions occur at very exposed sites where scattered small individuals may be found in the lower intertidal zone. Plants may also be found in intertidal pools on exposed coasts.
In Nova Scotia the species commonly forms a dense zone, in association with Laminaria digitata, through a depth range of about 4 to 18 m (Mann, 1972a). Members of the genus are commonly bounded by a zone of Chondrus crispus Stackh. at their upper limit and by a zone of the kelp Agarum cribrosum (Mert.) Bory at their lower limit. In very turbid waters (such as the upper reaches of the bay of Fundy) L. longicruris is restricted to shallow waters of less than about 3 m depth (Tremblay and Chapman, 1980).
In the High Arctic site of Turton Bay off Igloolik Island (69°21.9 N) Laminaria longicruris occurs to a depth of 20 m (personal observations).
Local horizontal distribution is determined to a large extent by substratum type and salinity. In very sheltered sites the species can occur on an unstable substratum of gravel and small stones, but it is absent from muddy and sandy bottoms. In exposed sites Laminaria longicruris is restricted to hard rock substrata. The species is absent from sites with salinities of less than about 20‰.
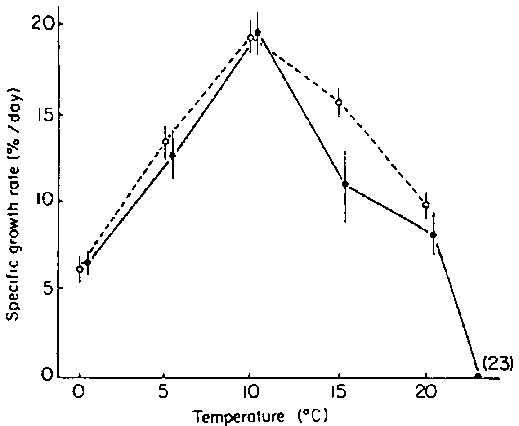
There is little information on the physiological determinants of geographical and vertical distribution of the species. Bolton and Luning (1982) have shown that above 22°C gametophytes of Laminaria longicruris suffer cell damage. All cells die in cultures maintained at 24°C. Bolton and Luning (1982) also tested the effects of temperature on the growth of sporophytes in culture (Fig. 2). Plants with parents from Nova Scotia and from Igloolik in the Canadian North-West Territories grew best at 10°C. Growth rate at 0°C was depressed by about 70% in both isolates. All plants disintegrated at 23°C.
There have been few laboratory studies on the effects of varying levels of irradiance on the photosynthesis and growth of Laminaria longicruris. Within the temperature range of 1-15°C photosynthetic saturation of tissue discs cut from adult blades occurs at photon flux densities between 75 and 90 m Em-2 s-1 (Fig. 3). The photosynthetic compensation photon flux density for tissue discs at temperatures between 2 and 5°C is about 4-5 m Em-2 s-1 (Chapman, unpublished data). Gagn'e and Mann (1981) have shown that in the middle of the depth range of L. longicruris in southwestern Nova Scotia, the average photon flux density in February, 1979, fell to about 7 m Em-2s-1. Presumably light levels were below photosynthetic compensation values at the lower limit of the kelp vegetation, at least during the month of February. The average photon flux density at about 8 m depth during the summer months at the 3 Nova Scotian sites studied by Gagne and Mann (1981) approached saturation.
Figure 2. Relationship between growth rate of juvenile sporangial plants and seawater temperature (modified after Bolton and Luning, 1982). Dashed line shows results for juveniles with parents collected in Nova Scotia, solid line for juveniles with parents collected in the Canadian High Arctic. Means and 95% confidence intervals are given.
The effects of salinity on the development of Laminaria longicruris is known from the work of H. Stegenga (unpublished study, 1973) and seem to explain the absence of this species from low salinity sites in the sea. In Stegenga's study plants were grown from spores in seawater diluted with distilled water. Below about 17 ‰ salinity the fertility of female gametophytes was reduced by nearly 50% in comparison with cultures maintained in full strength seawater. The eggs produced collapsed at salinities below about 17 ‰. The growth of young sporophytes was severely reduced at salinities below about 20 ‰. Maximum growth of sporophytes took place at salinities between 25 and 32 ‰.
2.4.1 Nutrition
There is information available on the carbon and nitrogen nutrition of Laminaria longicruris. Hatcher et al. (1977) carried out a study of photosynthetic carbon assimilation in a Nova Scotia population of the species. Rates of oxygen evolution by whole plants were measured over a 24 h period once every two weeks through 12 months. The apparatus used to make these in-situ measurements is described by Hatcher (1977). The daily net photosynthetic rates were extrapolated to 1 wk before, and 1 wk after the field measurements by reference to continuously measured, in-situ light measurements. In this way, daily net photosynthesis was estimated for every day of the study year. The weekly integrals of net production of tissue carbon are shown in Table 1. The carbon assimilation values were calculated from oxygen evolution rates using the photosynthetic quotients shown in Table 1. The photosynthetic quotients were derived from simultaneous in-situ measurements of O2 evolution and 14C bicarbonate uptake in plant tissue discs.
Hatcher et al. (1977) also calculated carbon accretion in this Laminaria from measurement of growth rates through the year in which photosynthetic assimilation rates were estimated. Increase in blade surface area was converted to carbon accretion by measurements of the total carbon per unit surface area. Net carbon assimilation by photosynthesis and the carbon content of new tissue are compared in Fig. 4. More than twice as much carbon was assimilated photosynthetically as was present in newly formed blade tissue. The fate of this excess carbon is unknown.
Table 1. Weekly integrals of (1) photosynthetic net production of carbon and (2) accretion of tissue carbon measured from growth rates in Laminaria longicruris in St. Margaret's Bay, Nova Scotia (modified after Hatcher et al., 1977).
|
Period |
Extrapolated net O2 production (m g O2 cm-2) |
Mean diel net O2 production (m g O2 cm-2) |
Photo-synthetic quotient |
Equivalent diel net carbon assimilation (m g C cm-2) |
Net production of tissue carbon: average of 2 plants in period (m g C cm-2 d-1) |
|
1975 |
|
|
|
|
|
|
May 1-7 |
311 |
42 |
1.03 |
17 |
4.3 |
|
May 8-21 |
845 |
60 |
1.33 |
17 |
6.4 |
|
May 22-June 4 |
491 |
35 |
1.33 |
10 |
5.2 |
|
June 5-18 |
1191 |
85 |
1.03 |
31 |
5.6 |
|
June 19-July 2 |
2506 |
179 |
1.03 |
65 |
6.2 |
|
July 3-16 |
3155 |
225 |
0.86 |
93 |
9.5 |
|
July 17-30 |
1892 |
135 |
1.29 |
39 |
7.5 |
|
July 31-Aug 13 |
1000 |
71 |
1.50 |
17 |
7.5 |
|
Aug 14-27 |
1132 |
81 |
1.11 |
25 |
11.4 |
|
Aug 28-Sept 11 |
957 |
68 |
1.14 |
23 |
10.2 |
|
Sep 12-25 |
717 |
51 |
1.17 |
17 |
8.3 |
|
Sep 26-Oct 9 |
344 |
25 |
1.11 |
8 |
5.9 |
|
Oct 10-23 |
250 |
18 |
1.04 |
6 |
17.0 |
|
Oct 24-Nov 7 |
62 |
4 |
0.98 |
2 |
15.6 |
|
Nov 8-21 |
-122 |
-9 |
0.91 |
-3 |
13.5 |
|
Nov 22-Dec 7 |
55 |
3 |
1.23 |
1 |
6.8 |
|
Dec 8-28 |
92 |
4 |
1.57 |
1 |
9.4 |
|
Dec 29-Jan 14 |
62 |
4 |
1.05 |
1 |
6.9 |
|
1976 |
|
|
|
|
|
|
Jan 15-28 |
987 |
70 |
1.05 |
25 |
9.0 |
|
Jan 29-Feb 11 |
569 |
41 |
0.77 |
18 |
8.8 |
|
Feb 12-25 |
370 |
26 |
1.06 |
9 |
9.2 |
|
Feb 26-Mar 11 |
457 |
30 |
1.04 |
11 |
8.1 |
|
Mar 12-25 |
853 |
61 |
1.01 |
23 |
6.3 |
|
Mar 22-Apr 8 |
266 |
19 |
0.84 |
8 |
7.0 |
|
Ap 9-30 |
889 |
40 |
0.67 |
19 |
4.2 |
Figure 4. Mean diel net photosynthetic assimilation by whole plant (open circles) and carbon content of new tissue formed at meristem (black circles), calculated for 2 wk intervals (modified after Hatcher et al., 1977).
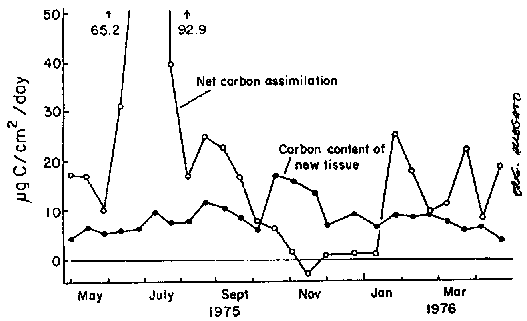
Although most photosynthetic carbon fixation occurs during the summer months (Fig. 4), the peak of carbon content in new tissue occurs during the winter. In the months between October and December there is insufficient photosynthetic carbon assimilation to support new growth. Chapman and Craigie (1978) have shown that storage carbohydrate reserves are exhausted through the same time period. Presumably the reserves are mobilized, translocated to the meristem and used to support the production of new tissue.
Information on nitrogen assimilation in Laminaria longicruris comes from the studies of Harlin and Craigie (1978) and Espinoza and Chapman (1983). Harlin and Craigie (1978) measured the rates at which tissue discs (2.3 cm diameter) removed nitrate nitrogen from 1 or 2 L of seawater spiked with NaNO3. Uptake rates were measured at various nitrogen concentrations, various temperatures, in light and in dark, in the presence or absence of oxygen, and in the presence or absence of NH4+. The results may be summarized as follows. An apparent Km (Michaelis-Menten half saturation constant) of 4- 6 m M NO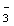 was estimated for winter and summer plants. A maximum uptake rate of 7- 10 m mol h-1g-1 dry wt. was recorded at 15°C at an irradiance of ca. 1800 m W cm-2. Uptake rates at 5°C were reduced by about one third, and those at 0°C were reduced by about two thirds of the rate at 15°C. The uptake rate of NO
was estimated for winter and summer plants. A maximum uptake rate of 7- 10 m mol h-1g-1 dry wt. was recorded at 15°C at an irradiance of ca. 1800 m W cm-2. Uptake rates at 5°C were reduced by about one third, and those at 0°C were reduced by about two thirds of the rate at 15°C. The uptake rate of NO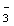 increased hyperbolically with NO
increased hyperbolically with NO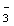 concentration (Table 2). Finally, Harlin and Craigie (1978) showed that the presence of ammonium did not inhibit nitrate uptake in tissue discs.
concentration (Table 2). Finally, Harlin and Craigie (1978) showed that the presence of ammonium did not inhibit nitrate uptake in tissue discs.
Table 2. Rate of nitrate uptake by Laminaria longicruris tissue discs at various substrate concentrations. Results are means (and standard deviations) at 15°C (modified after Harlin and Craigie, 1978).
|
Nitrate concentration (m M) |
Uptake rate (m mol h-1 g-1 |
No. of replicates |
|
1.2 |
2.1 (0.9) |
7 |
|
2.9 |
4.0 (1.3) |
7 |
|
6.8 |
6.7 (1.4) |
6 |
|
10.1 |
5.6 (1.3) |
3 |
|
11.7 |
6.7 (1.8) |
4 |
|
14.9 |
8.3 (1.8) |
6 |
|
17.2 |
8.2 (0.6) |
4 |
Nitrate uptake is energy demanding. When discs were pre-treated with 15 h of darkness, subsequent uptake rates of nitrate in the dark were between half and one third the rates of discs transferred to the light. Presumably the above dark treated discs were energy deficient. In the absence of oxygen, nitrate uptake in dark treated discs was reduced to zero. The uptake rates of light treated discs (which were presumably photosynthe-sizing) were not reduced in water from which the oxygen had been purged.
Espinoza and Chapman (1983) measured the nitrate uptake rates of small plants of Laminaria longicruris (<10 cm length) in a flow-through system (described by Probyn and Chapman, 1982). Harlin and Craigie (1978) used a batch mode system. In spite of the differences in the methods, the half saturation constants (Km) obtained by the two groups of researchers were quite similar. However, the uptake rates of young plants (measured by Espinoza and Chapman, 1983) were double those found in tissue discs cut from mature blades of plants from the same population (14.6 vs. 7. 0 m mol h-1g-1 dry wt.). Presumably the differences in uptake rates between juvenile and mature tissues can be related to their surface area to volume ratios (higher in younger tissues).
2.4.2 Growth
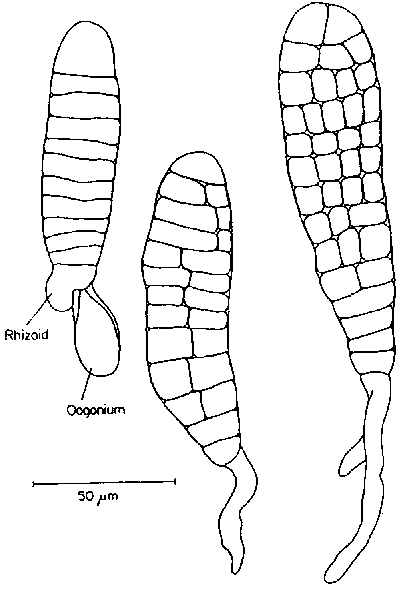
There is no information on the growth of microscopic juveniles of Laminaria longicruris in the sea. Stegenga (unpublished manuscript) has studied growth of juveniles in laboratory culture. Following fertilization the zygote undergoes a number of divisions producing a uniseriate filament of 5- 10 cells surmounting an attaching rhizoid (Fig. 5). Subsequently divisions occur in two planes to produce a monstromatic sheet of cells (Fig. 5). The thallus remains monstromatic until it reaches a surface area of 1- 2 mm². After this more cell layers are formed in the basal region of the plant and growth is gradually confined to a meristem between the base of the blade and the stipe that develop. During the first 28 days of development growth of plants was found to be exponential, with the average surface area doubling every 1.6 days.
Figure 5. Early stages in sporophyte development (after an unpublished MS by H. Stegenga).
Studies on the growth of Laminaria longicruris in the sea seem to have been restricted to adult sporophytes. Most workers have used the "punched-hole" technique of Parke (1948) to follow the elongation of blades. On the Atlantic coast of Nova Scotia blade elongation rates usually decline from a peak of 0.6 to 1.0 cm d-1 in May and June to a minimum of 0.2 to 0.4 cm d-1 in the late fall or early winter (Chapman and Craigie, 1977; Gerard and Mann, 1979). In the Bay of Fundy and in the Gulf of St. Lawrence maximum elongation rates are higher (2- 3 cm d-1) and occur later in the summer than on the Atlantic coast (Chapman and Gagne, 1980; Gagne and Mann, 1981; Anderson et al., 1982).
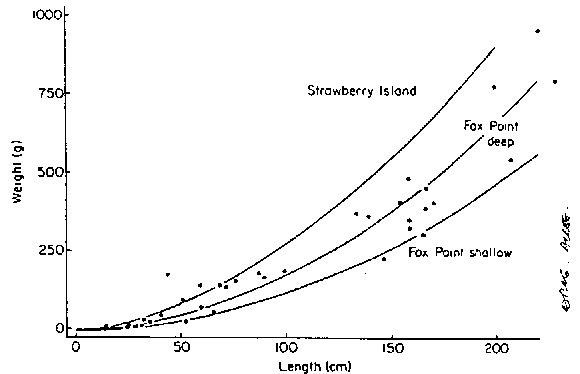
Mann (1972b) expressed monthly elongation rates as instan-taneous growth coefficients (logelength2-log2length1) for populations he studied on the Atlantic coast of Nova Scotia. The annual average in 1968/69 was 2.64 yr-1 (standard deviation = 1.2). In 1969/70 the annual average was 2.76 yr-1 (standard deviation = 0.84). The length increases were used to calculate biomass increases. To do these calculations, regressions of plant weights on plant lengths (power curve fits) were obtained (Fig. 6). It was found that in larger plants (>400 g initial weight) the annual biomass turnover was in the range of 5- 10 times. In smaller plants (<100 g initial weight) the annual turnover in weight approached 100 times in some populations. In the population at 10 m depth off Luke Island in St. Margaret's Bay a plant of 100 g increased it's biomass about 5 kg in one year, a 500 g plant about 10 kg, and a 2000 g plant about 20 kg.
Figure 6. Relationship between plant weight and plant length in 3 populations in St. Margaret's Bay, Nova Scotia (after Mann, 1972b). Regression lines are fitted power curves.
Laminaria longicruris has a life cycle that is typical of the order Laminariales. The large kelp plants found in the sea are sporangial and give rise to microscopic gametangial plants. Sporangial plants that are fertile may be recognized by the presence of sori on the distal median portions of the blade. A sorus is a dense aggregation of unilocular sporangia and associated sterile hairs (paraphyses) that form on the surfaces of the blade. There are between 2.8 and 4.4 x 105 sporangia cm-2 of sorus (Chapman, 1984). Sporangia are assumed to contain 32 spores each.
The spores released are biflagellate and about 5.5 m m in diameter. After a period in the plankton they settle and form germination tubes (Fig. 7) into which the cell contents move. The spore case remains empty. During further development male and female gametangial plants become distinguishable. Male plants are compact structures with cell dimensions that are not much different from those of the original spore. The narrow cells of the males are lightly pigmented. Conical antheridia may be formed (Fig.7) at the one-celled stage. After release of the sperm these plants usually die.
Female plants enlarge to diameters that are 2- 3 times those of the male cells. Females are also more darkly pigmented. Most one-celled plants transform directly into oogonia (Fig. 7) and form an egg which, in culture, remains seated on the top of the oogonium after extension. The egg cells reach diameters of about 25 m m. Fertilization has not been reported, but presumably occurs while the eggs are still attached to the oogonia. Young sporangial plants begin their development in the same position. The complete sequence from spore settlement to zygote formation occurs in less than 14 days under ideal culture conditions.
4.1 Age, weight and size composition
4.2 Sporophyte-gametophyte and sex composition
It is not possible to determine the age of individual plants in wild populations. Population age class structures are therefore unknown. Cohort analysis has shown that there is considerable variation in plant size at any given age (Chapman, unpublished observations). It is not possible to determine population age class structures from size class frequencies.
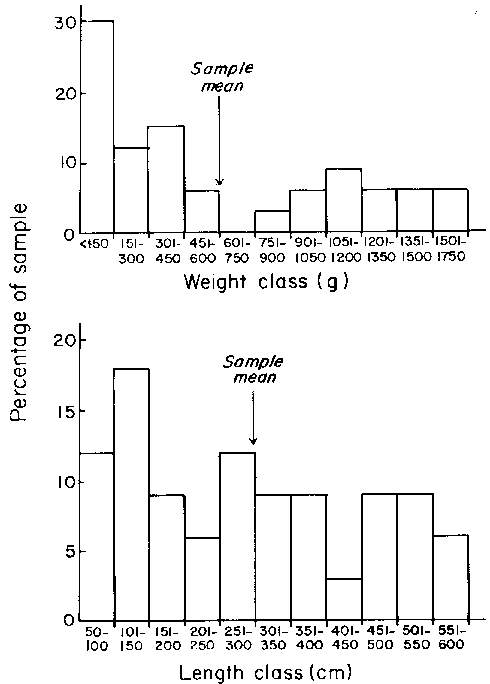
The weight and length class frequencies in 4 sample of Laminaria longicruris collected from Abbott Harbour (southwestern Nova Scotia) in August, 1981 are shown in Fig. 8.
Figure 7. Development of microscopic male and female gametangial phases from haploid spores. The final stage shown is a 3 celled sporangial plant growing attached to an empty oogonium (after an unpublished MS by H. Stegenga).
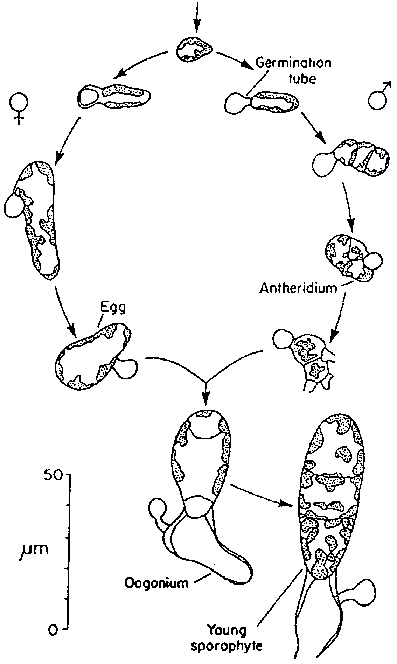
Figure 8. Size class frequency distributions for weight and plant length in a sample collected from Abbott Harbour in southwestern Nova Scotia.
On the shores of Nova Scotia closer to Halifax, Gerard and Mann (1979) showed that mean plant length varied with exposure to wave action. At an exposed site mean plant length (150 cm) was considerably shorter than in a nearby sheltered site (200.8 cm). The shorter stature of plants at exposed sites was seen as an adaptation to reducing form drag. In general, the populations of southwestern Nova Scotia are made up of longer individuals than those found in the eastern part of the province. The determining factors in this inter-population variability have not been identified. There is some evidence for genetic differentiation in total stipe length among sheltered and exposed coast populations (Chapman, 1974).
There are very few published data on population size class distributions in terms of plant mass. Some sample data are shown in Fig. 8 for a population in southwestern Nova Scotia. McPeak (1980) has reported that in 4 other populations sampled in that same general area the average weight of large (undefined) Laminaria longicruris plants varied between 1910 g and 550 g (mean = 670 g). The average weight of small plants (again undefined) varied between 60 and 160 g (mean = 70 g). Although it is difficult to interpret McPeak's data, since his "small" and "large" plant classes are undefined, it is clear that the population sample in Fig. 8 has a similar average plant mass (about 600 g) to those population sampled by McPeak. Average plant weights in sheltered and exposed sites close to Halifax were 549.0 and 228.5 g respectively (Gerard and Mann, 1979).
There is considerable variation in the densities of the Laminaria longicruris populations in Nova Scotia. These variations are not clearly related to the size class composition of the populations. In a population (close to Halifax) with the highest density recorded (24.0 m-2 SE = 4.0) the average plant mass was relatively low (228.5 g SE = 20.8, Gerard and Mann, 1979). In southwestern Nova Scotia, where average plant mass is higher, densities tend to be lower (McPeak, 1980; Sharp et al., 1981; Chapman, 1984). However, McPeak (1980) did not demonstrate any clear relationship between variations in plant density and average plant mass in 5 sites examined off the southwestern shore of the province.
Off Bon Portage Island on the southwestern shore of Nova Scotia densities of Laminaria longicruris varied between 3.8 and 14.2 m along a gradient of increasing shelter to wave action (Sharp et al., 1981). Through the same populations average plant mass varied very little (0.35 kg - 0.5 kg). The lack of any clear relationship between average plant mass and plant density might be expected in wild communities which contain several interacting species.
It seems likely that interactions with herbivores and competing plant species are major determinants of density variations in Laminaria longicruris populations (see Section 5.2 below). In addition, Sharp et al. (1981) have shown that density varies with depth and with exposure to wave action (Fig. 9, Table 3). At Bon Portage Island off southwestern Nova Scotia the maximum densities occurred in waters of about 5 m depth. Densities were inversely related to wave action.
Figure 9. Relationship between plant density and depth at a site in southwestern Nova Scotia (after Sharp et al., 1981).
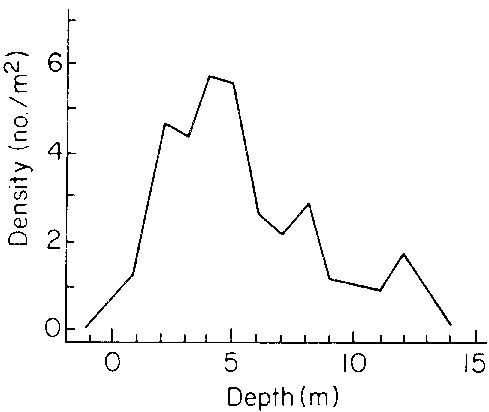
Table 3. Relationship between plant density (no./m²) and exposure to wave action. The wave exposure index is subjective and arranged from 2 (sheltered) to 6 (exposed). Data for plants of <1m length are displayed separately (modified after Sharp et al., 1981).
|
Exposure index |
|
Plant density |
|
|
< 1 m long |
> 1 m long |
|
2 |
4.6 |
9.6 |
|
3 |
1.7 |
3.7 |
|
5 |
1.3 |
3.6 |
|
6 |
2.3 |
2.5 |
Populations of Laminaria longicruris are made up of macroscopic sporangial plants (visible) and microscopic gametangial plants that are not visible and have never been identified in the wild (see Section 3.2). Chapman (1984) has used an indirect method to estimate the proportion of macroscopic to microscopic plants in a kelp bed off Abbott Harbour (southwestern Nova Scotia). The density of sporangial plants was counted directly by field observation and estimated at 1.24 m-2. Artificial ceramic surfaces were placed on the floor of the kelp beds at one month intervals through one year. Each set of ceramic surfaces was exposed for one month to spores descending from the plankton. At the end of each month the exposed surfaces were collected and transferred to showers of running seawater under enhanced light conditions. Microscopic stages that had been trapped in the field grew to a size that could be identified with a dissecting microscope within 6 weeks. The numbers of recruits collected in this way are shown in Table 4. Through one year almost ten million microscopic plants were recruited per meter squared.
Table 4. Benthic recruitment rate of microscopic stages of Laminaria longicruris (modified after Chapman, 1984).
|
Time period |
Number of recruits (no./m²[SE]) |
|
1981 |
|
|
Oct 6-Nov 5 |
5.11x105 [0.92x105] |
|
Nov 5-Jan 9 |
6.60x105 [0.59x105] |
|
1982 |
|
|
Jan 9-Feb 9 |
1.42x106[0.67x105] |
|
Feb 9-Mar 9 |
20.02x103 [5.84x103] |
|
Mar 9-28 April |
75.40x103 [18.28x103] |
|
April 28-May 18 |
1.62x105 [0.36x105] |
|
May 18-July 6 |
1.25x103[0.23x103] |
|
July 6-Aug 6 |
1.83x104[1.13x104] |
|
Aug 6-Sept 13 |
4.47x104 [2.59x104] |
|
Sept 13-Oct 13 |
3.48x104 [8.87x103] |
These microscopic sporangial plants were produced from female gametophytes. Normally, in culture, one zygote is produced from each female, and females comprise half of the gametangial population (Stegenga, unpublished data). Luning (1980) has shown directly that females of 3 European species of Laminaria (not including L. longicruris) produce only one egg each in the sea. It may be estimated, therefore, that about 20 million gametangial plants (male + female) develop per meter squared per year on the kelp forest floor at the site studied. The highest standing crop occurs in the winter months (November - January) with close to 13 million gametangial plants per meter squared. During these winter months there are at least 10 million gametangial plants per sporangial plant. Production of spores is minimal during the summer and this ratio falls to about 250/000:1.
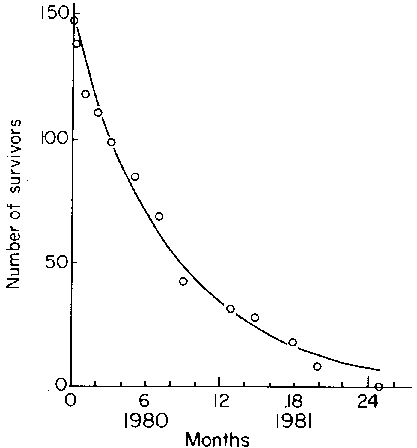
Chapman (1984) has studied some of the components of mortality in the kelp population at Abbott Harbour. A depletion curve for Laminaria longicruris is shown in Fig. 10. The individuals included were macrobenthic sporangial plants of mixed age and size classes. About 76% of the population was lost in a 12 month period. Since the population density remained stable during the same time period, the replacement rate was about 1m-2 yr-1. The decline in the numbers of tagged individuals was exponential with time and all plants were lost within 24 months. In a concurrent study of survivorship among a cohort of individuals recruited in 1980, sporangial plants were found to survive to a maximum age of 4 years (unpublished data).
Fig. 10. "Depletion" curve showing survivorship of a sample of plants of mixed age and size classes at Abbott Harbour, Nova Scotia. The curve is an exponential fit (r2=0.96). Modified after Chapman, 1984.
Replacement of lost individuals in the population studied by Chapman took place by spore production only (no vegetative regeneration). The number of spores produced by the population was estimated at 8.9 x 109 yr-1 of which half were assumed to produce one female gametangial plant and one zygote each. Since the rate of microbenthic recruitment was estimated (see above), the chances of a female spore surviving through the planktonic phase can be calculated as about one in 500. The chances of surviving the microbenthic to macrobenthic transition were much smaller (~ 1 in 9 x 106). The least risky phase of life was in the macrobenthos (chances of surviving one year ~ 1 in 4).
5.1 Standing Stocks and Harvestable Yields
5.2 Physical and biological determinants of productivity, growth and mortality
5.3 Potential for genetic improvement of stocks
5.4 Relative contributions of sexual and vegetative reproduction
5.5 Potential for environmental enhancement
The best estimates of standing stocks and harvestable yields are to be found in the review by Smith and Loucks (1980). In their work Smith and Loucks estimated K (the maximum total standing crop in the absence of harvesting) and msY (maximum sustainable yield). These two parameters were calculated as follows. K (tonnes) was estimated from data on standing biomass (kg m-2) and areas of seaweed beds (ha). msY is equal to R.K/4 where R is the instantaneous growth coefficient at low plant density. Smith and Loucks also provided a "conservative yield estimate" for the resource which is msY/2. The data available (Atlantic coast of Canada only) are presented in Table 5. The highest estimates of K are for the southwestern shore of Nova Scotia with 833,000 tonnes and a conservative yield estimate of 36,500 dry tonnes per year.
Table 5. Resource estimates for Laminaria longicruris in Atlantic Canada (modified after Smith and Loucks, 1980). K is the standing crop in the absence of harvesting, Y is the -conservative yield estimate". See Section 5.1 for calculation procedures.
|
Marine Plant District |
Standing crop bio-mass (kg m-2) |
Area of seaweed beds (ha) |
K (tonnes) |
ya (tonnes fresh) |
Ya (tonnes dry) |
|
New Brunswick |
0.7b |
1700b |
|
|
|
|
(Bay of Fundy) |
4.9c |
34c |
25800 |
9000 |
1100 |
|
Nova Scotia |
17.0 |
4860 |
833000 |
291400 |
36500 |
|
(Chebogue-Cape Sable) |
|
|
|
|
|
|
Newfoundland |
|
|
17800 |
6250 |
780 |
aAssumes an instantaneous growth coefficient of 2.8 yr-1
bHide frond population
c Narrow frond population
It should be pointed out that the kelp resource estimates of the southwestern shore of Nova Scotia of Smith and Loucks (1980) were based on standing crop and kelp bed area measurements of MacFarlane (1952). In 1977 McPeak (1980) resurveyed three of the sites examined by MacFarlane. McPeak found that, on the average; biomass (kg m-2) and kelp bed area (ha) were less than half of MacFarlane's earlier estimates. Accordingly, McPeak (1980) estimates that the total standing stock (K) for the 3 sites is only one quarter to one half of the earlier estimate. On this basis alone, it seems likely that the calculations of K and Y for Nova Scotia kelp beds (Smith and Loucks, 1980) are overestimates.
Biomass (kg m-2) has been estimated in several Nova Scotia kelp beds other than those examined by MacFarlane (1952) and McPeak (1980). In most recent studies (Neish, 1973; Gerard and Mann, 1979; Pringle and Sharp, 1980; Sharp et al. 1981; Chapman, 1981) the estimated biomass has consistently been within the range of 3.5 - 5.5 kg fresh weight/m² in stands that are dominated by Laminaria longicruris. An exception is found in the estimates of Mann (1972b) for St.Margaret's Bay on the Atlantic coast of the province (11.5 kg-2 m SE = 1.2).
At an arctic site (69°21.9'N 81°46.2'W) examined by Chapman and Lindley (1981) the biomass of Laminaria longicruris was only 0.097 kg m-2. At this site the kelp vegetation was dominated by the arctic species L. solidungula (0.87 kg m-2).
The productivity of Laminaria longicruris has been estimated several times since Mann's (1972b) pioneering study. According to Mann (1972b), the productivity of the species may reach 2000 g C m-2yr-1 in St. Margaret's Bay. This estimate was obtained from measurements of growth and biomass of plants in all size classes. In a later study of photosynthetic production of mature plants, Hatcher et al. (1977) estimated productivity as 143-428 gC m-2 yr-1 in the same bay. Using growth measurements and biomass data, Gerard and Mann estimated productivity as 540 g C m-2yr-1 for a sheltered site close to St. Margaret's Bay and 270g m-2yr-1 at an exposed site. The reasons for the discrepancies among these estimates are unknown.
The only information on harvested yield per unit area of a Laminaria longicruris forest comes from the work of Pringle and Sharp (1980). In 1979 a commercial dragrake was operated in southwestern Nova Scotia. The standing crop before harvesting was 4.0 kg m-2. The harvest removed 2.4 kg m-2. Assuming an instantaneous growth coefficient of 2.8 yr-1, a maximum sustain-able yield (msY) of 2.8 kg m-2, and a "conservative yield estimate" of 1.4 kg m-2 can be calculated. Within the swath of the dragrake the harvested yield is clearly close to the maximum sustainable yield. However, it should be pointed out that over the boulder terrain on which kelps grow substantial portions of the forest would remain unharvested.
5.2.1 Physical determinants
Nearly all information of the effects of physico-chemical determinants comes from studies in eastern Canada (especially Nova Scotia). Only two of these studies (Hatcher et al., 1977; Gerard and Mann, 1979) examined variations in productivity. Most other publications have been concerned with variations in linear growth rates.
During the year in which Hatcher et al. (1977) estimated the photosynthetic assimilation of whole mature plants of Laminaria longicruris, continuous measurements of photon flux density and temperature were also made. The data were subjected to multiple regression analysis (Table 6). Sixty percent of the variance in diel net photosynthesis was accounted for by variations in photon flux density. Twenty-seven percent of the variance in diel respiration rates were accounted for by temperature variations. The effect was significant (P<0.01) but hardly accounts for most of the variance in respiration rate.
Gerard and Mann (1979) examined the relationship between the rate of water movement (wave generated) and productivity of the species. At an exposed site the rate of diffusion of Plaster of Paris balls by water movement was 1.5 to 5 times greater than at a nearby sheltered site. Nutrient supply, temperature and irradiance were similar at the two sites. Biomass estimates were also similar at the two sites. Productivity at the sheltered site (1.8 kg dry wt m-2yr-1) was twice that at the exposed site (0.9 kg dry wt. m-2yr-1). Gerard and Mann (1979) suggest that the differences in productivity among the two sites may be related to differences in plant morphology. At the exposed site the laminae of the plants were narrow and thickened. At the sheltered site the blades were wider and thinner. These relationships mean that the surface area to volume ratios of plants in the sheltered site were higher than those at the exposed site. The consequences in respect to nutrient absorption rates are self evident.
Table 6. Multiple regression analysis of the relationship between (a) diel net photosynthesis and environmental variables (photon flux density and temperature), (b) diel respiration and environmental variables (modified after Hatcher et al., 1977).
|
Percent of variance accounted for | |||
|
Variables |
Photon flux density |
Temperature |
Both |
|
Diel net Photosynethesis |
61** |
6 ns |
63** |
|
Diel Respiration |
2 ns |
27** |
28* |
** Significant, P<0.01
* Significant, P<0.05
ns- Not significant
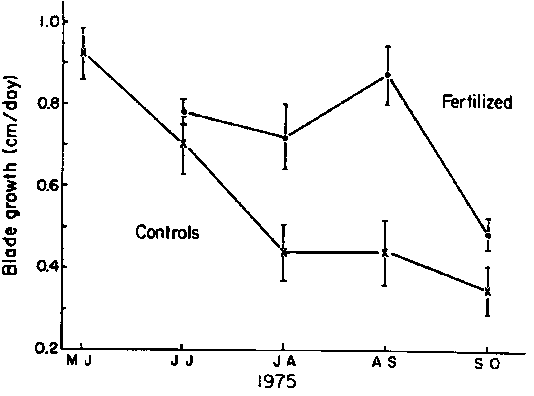
Experimental analyses of the relationships between linear growth rates and seawater nitrate concentration have been carried out by Chapman and Craigie (1977) and Espinoza and Chapman (1983). During the year 1974-75, Chapman and Craigie (1977) observed a declining rate of linear growth through the summer (Fig. 11) when there were very low nitrate concentrations.
Growth rates increased again in December as nitrate concentrations increased from undetectable levels to about 6 m M. During the winter internal tissue concentrations of nitrate increased to maximum values of about 150 m moles g-1 fresh wt. (~ 28,000 times ambient seawater concentrations). During the summer months tissue concentrations of nitrate fell to undetectable levels. To test the relationship between linear growth and seawater nitrate concentration, a portion of the kelp forest was fertilized with sodium nitrate at least once a week through the summer of 1975. Tissue nitrate concentrations were raised up to 20 fold in the experimental group of plants and linear growth rates increased back to maximum winter rates of about 1 cm d-1 (Fig. 12).
Figure 12. Effect of nitrate fertilization on the linear growth rate of plants through one summer. Means and standard errors are shown for treatment and control groups of 20 plants (after Chapman and Craigie, 1977).
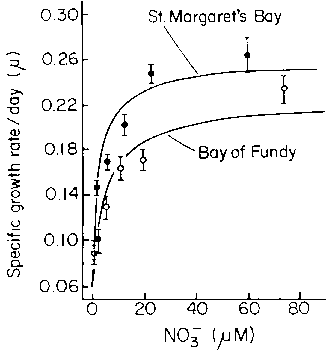
Espinoza and Chapman (1983) measured the linear growth rates and weight increases of small (2 cm long) plants in a flow-through culture system at various nitrate concentrations. They showed a hyperbolic relationship between specific growth rate (m) and substrate concentration (Fig. 13). The half saturation constant (KS = S at one half m max) at 9°C was 1.1 m M nitrate for linear growth and 1.8 m M nitrate for growth in fresh weight. Growth saturation occurred above 20 m M nitrate, which explains why the growth of the species is so limited during the summer months on the Atlantic coast of Nova Scotia.
Figure 13. Relationship between growth rate (weight increase) and nitrate concentration in juvenile sporangial plants collected from 2 sites in Nova Scotia. Means and 95% confidence limits are given. Curves fitted to the Monod equation (after Espinoza and Chapman, 1983).
The major effect of a summer depletion of available nitrogen in seawater is to uncouple seasonal growth patterns from seasonal variations in light availability and photosynthetic assimilatory rates (see Hatcher et al., 1977; Fig. 4). Anderson et al. (1981), Gagne and Mann (1981) and Gagne et al. (1982) have examined seasonal growth patterns at sites in eastern Canada that do not experience a major summer depletion of the nitrogen resource. It was shown in all cases that seasonal patterns of linear growth track seasonal variations in light availability when nitrogen supply is elevated during the summer months.
In populations which never experience a summer nitrogen depletion, winter growth rates are depressed in relation to populations that are nitrogen starved during the summer (Gagne and Mann, 1981; Gagne et al., 1982). The difference in winter growth rates may be attributed to differential carbohydrate reserve accumulation. In nitrogen depleted populations part of the photosynthetic assimilatory surplus that is built up during the summer is converted into reserves of laminaran (Chapman and Craigie, 1978; Gagne and Mann, 1981; Gagne et al., 1982). Laminaran was undetectable in the tissues of plants collected from the Bay of Fundy which has elevated nitrogen resources during the summer months. Carbohydrate reserves are used to support growth in late fall and early winter when the carbon demand for growth exceeds photosynthetic production (Hatcher et al., 1977; Chapman and Craigie, 1978).
It is interesting to note that, in one of two sites that experience a marked summer depletion of nitrogen, Gerard and Mann (1979) did not observe a corresponding decline in linear growth rates. In the second population, which was located at a site more exposed to wave action, the effects of the summer depletion of the nitrogen resource were clearly evident. Gerard and Mann (1979) had no obvious explanation for the growth rate difference among the two sites during the summer months.
5.2.2 Biological determinants
The standing stocks (and hence productivity) of Laminaria longicruris forests are regulated by a complex suite of biological interactions. Herbivory, carnivory and parasitism are all important structuring agents. Most of the experimental information available comes from work on the Atlantic shores of Nova Scotia (especially at St. Margaret's Bay).
The importance of herbivory to the maintenance of standing stocks of kelps was not apparent in an early study of energy flow through the marine benthos of St. Margaret's Bay (Miller et al., 1971). It was estimated that seaweed annual production exceeded the annual consumption by herbivores by a factor of >10. Furthermore, in a survey of the subtidal seaweed zone in 1968 sea urchins (Strongylocentrotus droebachiensis) were found in abundance in only 7 of 25 sites (Mann,1972a). Through the 1970s the patches of sea urchins expanded in area and eliminated the great bulk of the seaweed biomass (Breen and Mann, 1976a; Chapman, 1981). By the end of the decade about 90% of the kelp forest area had been removed by grazing (Chapman, 1981). Benthic primary production was reduced by about 2 orders of magnitude. After kelp bed destruction sea urchins were able to prevent the regrowth of perennial foliose macrophytes. The herbivore populations were maintained by a diet of microalgae and drift from the intertidal zone.
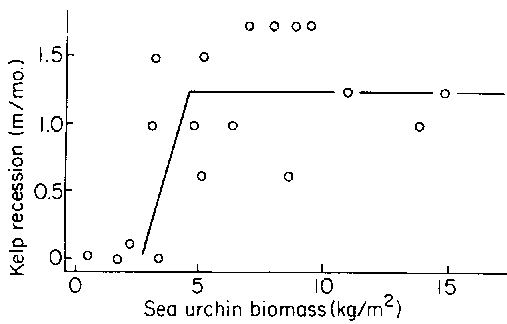
The relationship between kelp bed recession rate and sea urchin biomass was examined by Breen and Mann (1976a). The destruction of kelp appeared to be a threshold event (Fig. 14). Above a biomass of about 2 kg m sea urchins were able to effect kelp bed recession. Below this threshold kelp bed recession did not occur. However, once kelps had been removed, reforestation was prevented at sea urchin biomasses of only 150 g m-2.
Figure 14. Relationship between the rate at which a surveyed kelp bed receded and the biomass of sea urchins. Curve fitted by eye (after Breen and Mann, 1976b).
Experimental removal of sea urchins leads to reforestation by kelps (Breen and Mann, 1976a); Chapman, 1981). When Laminaria longicruris is rare in areas adjacent to experimental sea urchin removal areas, other kelp species may come to dominate the benthos (Himmelman et al., 1983; Keats et al., 1982).
Beginning in 1980, sea urchin populations of Atlantic Nova Scotia have been decimated each fall by a disease that has led to the loss of >99% of their biomass over nearly 1000 km of coastline (Miller and Colodey, 1983; Scheibling and Stephenson, 1984). Jones et al. (in press) have demonstrated that the pathogenic agent in this disease is closely similar to the genus Paramoeba. In absence of sea urchins the subtidal benthos has converted once more to a system dominated by foliose seaweeds (Johnson, 1984). At sites exposed to strong wave action Laminaria longicruris became the dominant component of the vegetation within 3 years (Scheibling, personal communication). In sheltered sites the benthos remains dominated by diminutive species of fleshy seaweeds more than 4 years after the destruction of the sea urchins (personal observation; Johnson, 1984).
The importance of sea urchins and their amoeboid disease causing agent as determinants of standing stocks of kelps is self evident. The reasons for the expansion of urchin populations are less fully understood. Breen and Mann (1976b) put forward evidence in support of the contention that a reduction of lobster (Homarus americanus) stocks led to an increase in sea urchin population densities. The evidence is as follows: (a) lobsters appear likely as key predators of sea urchins, (b) lobsters have declined in abundance and (c) sea urchins have increased in number and destroyed kelp beds at a high rate. Pringle et al. (1982) have difficulty in accepting the hypothesis that the lobster is a keystone predator of the kelp bed ecosystem and that overfishing of lobsters has caused Laminaria densities to decline. It is difficult to manipulate the densities of benthic and pelagic carnivores in order to observe their effects on community structure at lower trophic levels. In this respect the predation hypothesis of Breen and Mann (1976b) remains untested, but it needs to be pointed out there is no evidence in support of its rejection.
In a more recent graphical model (Fig. 15) of the predation hypothesis of benthic community organization Wharton and Mann (1981) have incorporated the effects of fishfish (plaice, wolffish etc.) along with the effects of lobsters. The model also takes into account the behavioural responses of urchins to their predators. The key to kelp bed destruction is the development of urchin biomasses of >2 kg m-2. Bernstein et al. (1981, 1983) believe that when urchin densities exceed 10 m-2 the presence of lobsters leads to the formation of aggregations which can overgraze kelp beds.
Johnson (1984) has studied the biological determinants that influenced the development of kelp stands following the disappearance of major sea urchin populations in St. Margaret's Bay. The interactions of major importance are (a) antifouling properties of crustose coralline algae and (b) grazing by the gastropod Lacuna vincta. At 2 sites in St. Margaret's Bay crustose corallines covered >90% of available primary rock substratum. The dominant genus in terms of cover was Phymatolithon. Johnson (1984) was able to demonstrate experimentally that the standing stock development of seaweeds growing on Phymatolithon was reduced by 46% in comparison with standing stock development on bare rock. The antifouling effect was not species specific; all seaweeds were equally affected.
The major grazer of Laminaria longicruris in the absence of sea urchins is Lacuna vincta (Johnson, 1984). Between February and September of 1980 the snails consumed only 0.05% of the blade biomass available to them. The major effect of snail grazing is to increase the ripping and loss of parts of the blade canopy. Johnson (1984) estimated that the standing biomass of L. longicruris in the bed he studied would have been about 28% higher in the absence of snails.
Chapman (1984) showed that in the kelp beds of the southwestern part of Nova Scotia, where sea urchins have never been recorded in high densities, interactions with perennial turf-forming red algal species are the major determinants of kelp density. Experimental removal of the red algal turf species (mostly Chondrus crispus, Phyllophora truncata and Ceramium rubrum) led to a ten fold increase in Laminaria recruitment.
There has been only one genetic study of a trait of commercial importance in Laminaria longicruris (Chapman and Doyle, 1979). Within a sample of 80 sporangial plants collected from a single population in St. Margaret's Bay, Nova Scotia there was found to be a three fold variation in percentage alginate of dry weight. Alginate is a structural cell wall carbohydrate of commercial value. Chapman and Doyle (1979) used a selective breeding program to quantify the genetic component of phenotypic variance in alginate content. The mean alginate content of the 80 plants tested was 23.8% of alcohol extracted dry weight (SE= 0.47). Five plants with a high alginate content (mean=30.1%) were allowed to interbreed. A second set of parents with a low alginate content (mean=14.4%) was also interbred. The alginate contents of the offspring off the 2 groups of families are shown in Table 7. After 6 months growth in the sea the alginate content of the two groups of offspring were not significantly different from one another or from the original 80 plants tested. Consequently the realized heritability values are very low. This result was confirmed in a separate breeding plan (offspring on mid-parent regression breeding scheme).
The work of Chapman and Doyle (1979) suggests that simple mass selection breeding for increased alginate content is unlikely to work. It should be pointed out, however, that in the People's Republic of China there has been considerable success in the production of strains of Laminaria japonica that are better suited to aquaculture than wild plants (Fang et al., 1962; Fang et al., 1963; Fang et al., 1978). The breeding program used involves selection of suitable phenotypes (with appropriate temperature tolerances, growth rates; etc.) followed by intensive inbreeding in which the gametangial plants from single parents are mated with one another. Fang et al. (1962) found considerable phenotypic variability within inbred lines indicating that a high degree of homozygosity had not been achieved. Complete homozygosity was achieved in later work (Fang et al. 1978) when sporangial plants were produced parthenogenetically from isolated female gametangial plants. Surprisingly, the partheno-sporophytes developed normally. Luning et al. (1978) showed that parthenosporophytes of L. longicruris were extremely stunted. However completely homozygous lines can be produced by isolating gametangiail plants and growing them vegetatively whilst preventing gamete formation through the use of red light (Luning and Dring, 1975). Large filamentous gametangial plants will become fertile on transfer to white light. Crosses of single large males and females in this way will lead to the production of hundreds of genetically identical offspring.
Table 7. Response of Laminaria longicruris to artificial selection. Results are means (standard errors) for % alginate of alcohol extracted dry weight.
|
|
Offspring |
|
|
Laboratory grown |
Field grown |
|
|
Mean of parent population = 23.8(0.47) |
|
|
|
Mean of 5 selected parents = 30.1 |
20.7(1.0) |
23.0(2.7) |
|
Mean of 5 selected parents = 14.4 |
26.6(1.25) |
25.6(1.0) |
Population replacement takes place entirely through the sexual life history of the species (Pringle and Sharp, 1980; Chapman, 1984).
There have been two experimental attempts at environmental enhancement of the kelp habitat by sea urchin removal (Bernstein and Welsford, 1982; Miller, 1985). Bernstein and Welsford used a diver to apply a quicklime slurry to reduce sea urchin densities. They found that an application of 1.02 kg of lime/m² resulted in a 73% reduction in urchin densities. There were no apparent ill effects on other organisms. Bernstein and Welsford estimated that liming 4 ha would require ca 40 t of lime (at CAN$60/t) and 60 man days of work.
Miller (1985) explored the possibility for biological control of sea urchins using a pathogenic disease (see Section 5.2). In the summer of 1983, 580 sea urchins exposed to the disease (and presumably infected) were transplanted into a natural population of urchins. A control group of uninfected animals was transplanted to another site. By October, 1983, diseased animals were found 50 m from the release site. The disease was not evident at the control site. There is therefore a potential for biological control of sea urchin densities by the virulent pathogen that occurs naturally in Nova Scotia.
6.1 Annual cycle of operations
6.2 Manpower productivity
6.3 Alternate employment
Laminaria longicruris was harvested in southwestern Nova Scotia between 1940 and 1949 (Sharp, 1980). Harvesting since 1949 has been sporadic and without any consistent annual cycle of operations. During the 1940s the main harvest began in the early summer and continued for many months into the early winter. At Clarks Harbour the operational period was 6 months/year through the first half of the decade declining to 3 months/year in the period 1946-49 (Sharp, 1980).
The annual cycle of harvesting operations was related to biological and environmental conditions in only one respect. During the early part of the 1940s a significant portion of the harvest came from beach cast kelp. The volume of beach cast increases during the stormy winter months and is related to the loss rate of larger plants. This portion of the harvest was clearly related to environmental conditions.
The only data available are for the Clarks Harbour operation during the 1940s. According to Sharp (1980) 20 - 30 boats each with a 2 man crew worked on the harvest operations around Clarks Harbour. MacFarlane (1952) estimated the total area of kelp beds on the Cape Sable Island ledges around Clarks Harbour as 11,700 ha. The manpower requirements to maintain the operation were therefore 0.0034 - 0.0051 men/ha. The seasonal variation in manpower requirements is unknown.
In an experimental harvest using a dragrake method (see Section 7.1 below) Pringle and Sharp (1980) demonstrated an economic CPUE (catch/unit effort) limit of 1.2 t h-1.
During the 1940's kelp harvesting was done from lobster fishing boats. The lobster fishing season in southwestern Nova Scotia extends through the winter months and provides alternate seasonal employment for the fishermen. After 1945 the Irish Moss (Chondrus crispus) harvesting industry developed into a major alternate form of employment. The dominant seaweed industry in eastern Canada is now based on Irish Moss.
7.1 The wild resource
7.2 Seaweed culture
7.3 Sources of credit and insurance of the crop
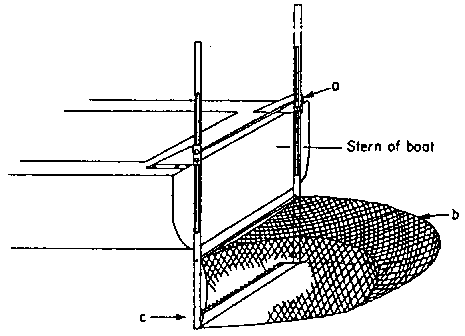
The equipment used to harvest Laminaria longicruris has been described by Sharp (1980). During the first 3 years of commercial harvesting plants were cut with sickles or scythes at low tide, using grapnels and gathering storm tossed drift. The kelp buyer improved harvest technology by the introduction of a large dragrake (Fig. 16) with which deeper water populations could be accessed. The rake consisted of a 1.5 - 1.8 m length of oak furnished with steel rods 30 - 45 cm long, spaced 7.5 - 12.5 cm apart as tines. This equipment was draggad behind a wooden lobster boat (9.2 - 12.3 m in length). Two men handled the equipment.
Harvested kelp was taken by boat to two holding tanks at Clarks Harbour and at Dog Island in the Tusket Islands. The holding tanks had dimensions of 4.6 x 4.6 x 1.2 m and could hold 40 - 60 t of kelp. During the harvest season the kelp was transported from the holding tanks to Rockland, Maine (for processing) twice a week in a 29 m boat.
Each of the harvest boats could hold 10 - 12 t of kelp and some harvesters made two collecting trips a day. The harvesting rate was therefore about 10 t per man per day. The price paid by the buyer peaked at CAN$8.00/t. Therefore the gross income was about CAN$80.00 per man per day. During the peak years of harvesting 5-6000 t of kelp were cut each year in southwestern Nova Scotia.
Pringle and Sharp (1980) reported on an experimental harvest of kelp with dragrakes identical to those used in the 1940s. Ninety eight per cent of the harvest was Laminaria longicruris. The plants were mostly whole so that regrowth from intact meri-stems was precluded. In 1946 a mechanical harvester was developed (Fig. 17). There were numerous problems with this mowing device, but it is interesting to note that kelp bed regrowth was judged to be faster than after dragraking.
Figure 16. Kelp dragrake used in southwestern Nova Scotia. a- towing point (flat steel bar), b- rake tines (mild steel rod), c- main beam (hardwood), d- trip point (after Sharp, 1980).
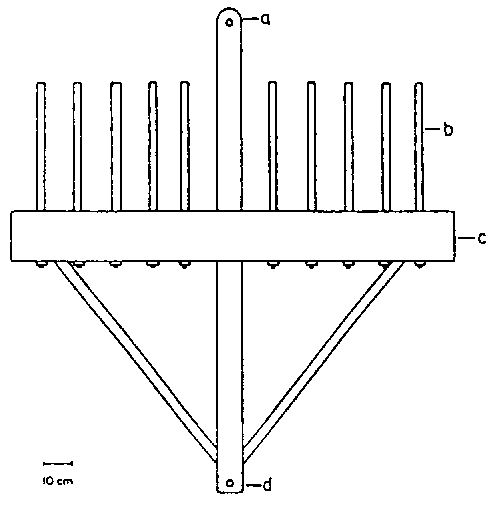
Figure 17. Mechanical harvester under development in 1946. a- spring loaded lifting device, b- removable net bag, c- mower blade chain driven from main engine (after Sharp, 1980).
The detailed economics of the 1940s kelp harvesting operation are unknown. According to Smith and Loucks (1980) the caloric advantage (caloric output [in cal/day]/caloric input [in cal/day]) of dragraking is 3 to 5. The caloric advantage of other harvesting methods are unknown, but cutting with sickles and scythes and collecting storm tossed drift are assumed to have high caloric advantages.
The plants harvested in the period 1940-49 were processed for alginate extraction in Rockland. Since 1949 there have been only minor harvests of 20 - 300 t per year and the product has been used mostly in the health food industry (kelp pills, kelp powder etc.). The technology of the later harvests was identical to that of the 1940s with similar catches per unit effort.
There is no aquaculture industry for Laminaria longicruris.
Within Nova Scotia the major source of credit for the industry is through the provincial Fishermen's Loan Board. Insurance for boats only is provided by an insurance program operated by the Canadian Federal Department of Fisheries and Oceans (a division of Environment Canada). This insurance program is to be discontinued in 1986.
8.1 Management of seaweed resources
8.2 Regulation of the harvest
Harvesting of Laminaria longicruris is restricted to two Canadian provinces, Nova Scotia and New Brunswick. The seaweed resource is managed in both provinces by the Department of Fisheries and Oceans, Environment Canada. This is the department of the Canadian federal government that is responsible for all marine biological resources. In addition to setting regulations of catch limits and techniques, the department conducts a research program into the effects of harvesting, not only on the seaweed resource, but also on associated fisheries (Pringle and Sharp, 1980)
The Canadian Department of Fisheries and Oceans interacts with the harvesting industry through a Marine Plants Advisory Committee. This committee has representatives from the industry, government and the academic communities. Members of the Department of Fisheries and Oceans also consult directly with the fishermen at local meetings arranged within the fishing communi-t ies.
The kelp harvest industry of 1940-49 was completely unregulated. Under present circumstances permits for harvesting are issued through the Canadian Department of Fisheries and Oceans. Because there is no viable ongoing kelp harvesting industry, permits are issued on an individual basis only. In 1979 the department issued an experimental permit for one boat with three crew members to harvest 1000 t of kelp using dragrakes.
All fisheries regulations governing marine resources of Canada are enforced by fisheries officers of the Department of Fisheries and Oceans. The officers may confiscate catches and equipment that contravene regulations.
9.1 Chemical and nutritional content
9.2 Human food
9.3 Animal fodder
9.4 Manure
9.5 Industrial products and processes
There is information available on the carbohydrate composition/amino acid composition, polyphenolic content and total organic nitrogen content of the species.
The annual variations in laminaran and mannitol contents in a range of plant portions in various depths of water in St. Margaret's Bay are shown in Fig. 18 (from Chapman and Craigie, 1978). The seasonal pattern for laminaran for this site was confirmed by Gagne and Mann (1981). In sites with enhanced seawater nitrate availability the laminaran content is reduced. At Centreville in the Bay of Fundy laminaran is undetectable throughout the year in L. longicruris (Gagne and Mann, 1981).
The annual variations in alginic acid and cellulose contents in a range of plant portions collected from St. Margaret's Bay are shown in Fig. 19 (from unpublished data of A.R.O. Chapman). The data are given as per cent of alcohol extracted dry weight. Variations in total organic nitrogen of extracted dry weight for the same site are given in Fig. 20 (from Chapman and Craigie, 1977). Fong (1978) measured seasonal variation in the amino acid composition of the species at Fox Point in the same Bay (Table 8).
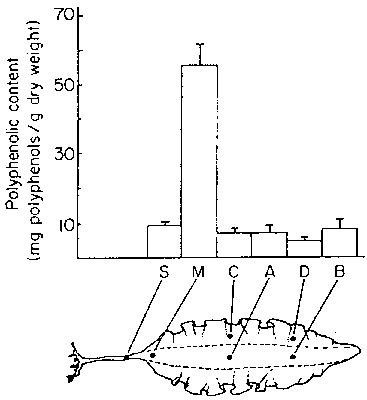
In a study of anti-herbivore defenses in Laminaria longicruris Johnson (1984) measured the polyphenolic content of various tissue portions (Fig. 21). The highest concentration of polyphenols occurred in the meristem region in the transition zone between blade and stipe. This zone is the least subject to grazing by Lacuna vincta.
Since 1949 most of the kelp harvest in southwestern Nova Scotia has been used for human consumption. The largest recent harvest (1979) of about 300 t was sold as kombu for consumption in Korea. In addition, a small amount of material is used for the production of health food products (kelp pills, kelp powder).
The harvest is not used for animal fodder.
The harvest is not used for commercial crop plant fertilizer although tests on its usefulness in this role were done during the late 1970s (P. Brock, personal communication).
The harvest of L. longicruris during the 1940s was organized by the Algin Corporation of America for the production of alginate (a cell wall carbohydrate). Alginate is used in a wide variety of industrial products. In the food industry it is used to increase viscosity or to stabilize emulsions (as in ice creams, canned soups etc.). In the textile industry it is used as a sizing agent. In addition, the product is used in the dental and pharmaceutical industries and in the production of coatings for high quality paper products.
Figure 20. Seasonal variations in total organic nitrogen content of 3 blade tissue portions in plants collected from St. Margaret's Bay. Means are from 5 plants (after Chapman and Craigie, 1977).
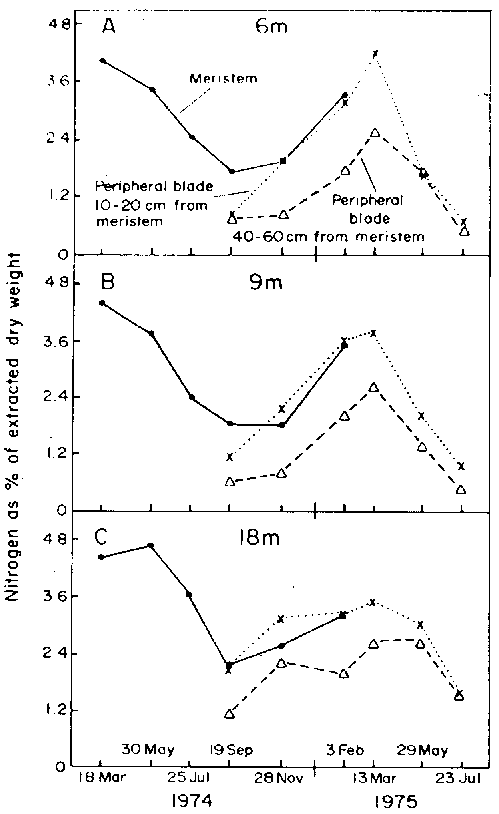
Table 8. Amino acid composition of Laminaria longicruris from St. Margaret's Bay, Nova Scotia. Values are means for 3 plants expressed as per cent by weight of total amino acid composition (after Fong, 1978).
|
Amino acid |
April 1977 |
June 1977 |
August 1977 |
October 1977 |
December 1977 |
February 1978 |
|
ASP |
10.07 |
12.99 |
10.39 |
10.44 |
11.01 |
12.95 |
|
THR |
4.44 |
4.38 |
3.86 |
4.33 |
4.68 |
4.96 |
|
SER |
3.05 |
2.45 |
2.40 |
2.53 |
3.03 |
3.14 |
|
GLU |
24.86 |
30.23 |
27.66 |
32.17 |
27.16 |
20.51 |
|
PRO |
9.90 |
9.22 |
5.95 |
4.52 |
5.78 |
6.20 |
|
GLY |
3.25 |
4.68 |
5.06 |
5.40 |
4.69 |
4.48 |
|
ALA |
19.54 |
9.49 |
7.28 |
9.91 |
13.48 |
16.15 |
|
VAL |
2.92 |
3.05 |
3.42 |
4.11 |
3.81 |
4.06 |
|
MET |
0.56 |
0.68 |
1.18 |
0.76 |
0.93 |
0.69 |
|
ILE |
1.94 |
2.97 |
3.93 |
3.36 |
3.72 |
3.84 |
|
LEU |
3.68 |
4.39 |
8.33 |
6.25 |
6.51 |
6.18 |
|
TYR |
1.72 |
2.37 |
3.96 |
3.12 |
3.18 |
2.79 |
|
PHE |
2.71 |
2.77 |
5.69 |
4.07 |
3.53 |
4.34 |
|
LYS |
5.69 |
5.08 |
5.21 |
4.01 |
4.03 |
4.81 |
|
HIS |
0.12 |
0.11 |
0.16 |
0.29 |
0.14 |
0.13 |
|
ARG |
5.37 |
5.16 |
5.54 |
4.74 |
4.33 |
4.77 |
Figure 21. Polyphenolic content of various plant portions (after Johnson, 1984).
Anderson, M.R., A. Cardinal and J. Larochelle, 1982 An alternate growth pattern for Laminaria longicruris. J.Phycol., 17:405-11
Bernstein, B.B., S.C. Schroeter and K.H. Mann, 1983 Sea urchin (Strongylocentrotus droebachiensis aggregating behaviour investigated by a subtidal multifactorial experiment. Can.J.Fish.Aquat.Sci., 40(11):1975-86
Bernstein, B.B., and R.W. Welsford, 1982 An assessment of feasibility of using high-calcium quicklime as an experimental tool for research into kelp bed/sea urchin ecosystems in Nova Scotia. Can.Tech.Rep.Fish.Aquat.Sci., (968):51 p.
Bernstein, B.B., B.E. Williams and K.H. Mann, 1981 The role of behavioral responses to predators in modifying urchins' (Strongylocentrotus droebachiensis) destructive grazing and seasonal foraging patterns. Mar.Biol., 63:39-49
Bolton, J.J., and K. Luning, 1982 Optimal growth and maximal survival temperatures of Atlantic Laminaria species (Phaeophyta) in culture. Mar.Biol., 66:89-94
Bolton, J.J., I. Germann and K. Luning, 1983 Hybridization between Atlantic and Pacific representatives of the Simplices section of Laminaria (Phaeophyta). Phycologia, 22:133-40
Borgesen, F., 1902 The marine algae of the Faeroes. In Botany of the Faeroes. Part II. Copenhagen, Det Nordiske Forlag, pp. 339-532
Breen, P.A., and K.H. Mann, 1976 Destructive grazing of kelp by sea urchins in eastern Canada. J.Fish.Res.Board Can., 33(6):1278-83
Breen, P.A., and K.H. Mann, 1976a Changing lobster abundance and the destruction of kelp beds by sea urchins. Mar.Biol., 34:137-42
Brinkhuis, B.H., et al., 1984 Cultivation of Laminaria saccharina in the New York Marine Biomass Program. Hydrobiologia, 116/117: 266-71
Chapman, A.R.O., 1973 Phenetic variability in stipe morphology in relation to season, exposure, and depth in the non-digitate complex of Laminaria Lamour. (Phaeophyta, Laminariales) in Nova Scotia. Phycologia, 12:53-7
Chapman, A.R.O., 1974 The genetic basis of morphological differentiation in some Laminaria populations. Mar.Biol., 24:85-91
Chapman, A.R.O , 1975 Inheritance of mucilage canals in Laminaria Lamour. (section Simplices) in eastern Canada. Br.Phycol.J., 10:219-23
Chapman, A.R.O, 1981 Stability of sea urchin dominated barren grounds following destructive grazing of kelp in St. Margaret's Bay, eastern Canada. Mar. Biol., 62:307-11
Chapman, A.R.O, 1984 Reproduction, recruitment and mortality in two species of Laminaria in southwest Nova Scotia. J.Exp. Mar.Biol.Ecol., 78:99-109
Chapman, A.R.O., and J.S. Craigie, 1978 Seasonal growth of Laminaria longicruris relations with reserve carbohydrate production and storage. Mar.Biol., 46; 209-13
Chapman, A.R.O., and R.W. Doyle, 1979 Genetic analysis of alginate content in Laminaria longicruris (Phaeophyta). Proc. Int.Seaweed Symp., 9:125-32
Chapman, A.R.O., and J. Gagne, 1980 Environmental control of kelp growth in St. Margaret's Bay and on the southwest shore of Nova Scotia. Can.Tech.Rep.Fish.Aquat.Sci., (954):194-207
Chapman, A.R.O., and J.E. Lindley, 1981 Productivity of Laminaria solidungula in the Canadian High Arctic: a year-round study. Proc.Int.Seaweed Symp., 10:247-52
Espinoza, J. and A.R.O. Chapman, 1983 Ecotypic differentiation of Laminaria longicruris in relation to seawater nitrate concentration. Mar.Biol., 74:213-8
Fang, T.C., D.Y. Wu and C.Z. Li, 1962 Increased adaptability to high temperature of gametophytes and sporelings of the Haiqing No. 1 breed of Laminaria japonica Aresch. Oceanol.Limnol.Sinica, 4:29-37 -
Fang, T.C., et al., 1963 The breeding of a new variety of Haidai (Laminaria japonica Aresch.). Scientia Sinica, 12:1011-8
Fang, T.C., et al, 1978 Some genetic observations on the monoploid breeding of Laminaria japonica. Scientia Sinica, 21:401-8
Fong, W-C., 1978. The role of gut flora in the transfer of amino acids through a marine food chain. Ph.d. Thesis, Dalhousie University. 157 p.
Fritsch, F.E., 1945 The structure and reproduction of the Algae. Vol. 2. Cambridge, Cambridge University Press, 939 p.
Gagne, J.A., and K.H. Mann, 1981 Comparison of growth strategy in Laminaria populations living under differing seasonal patterns of nutrient availability. Proc.Int.Seaweed Symp, 10; 297-302 -
Gagne, J.A., K.H. Mann and A.R.O. Chapman, 1982 Seasonal patterns of growth and storage in Laminaria longicruris in relation to differing patterns of availability of nitrogen in the water. Mar.Biol., 60:91-101
Gerard, V.A., and K.H. Mann, 1979 Growth and production of Laminaria longicruris (Phaeophyta) populations exposed to different intensities of water movement. J.Phycol., 15:33-41
Harlin, M.M., and J.S. Craigie, 1978 Nitrate uptake by Laminaria longicruris (Phaeophyceae). J.Phycol., 14:464-7
Hatcher, B.G., 1977 An apparatus for measuring photosynthesis and respiration of intact Large marine algae and comparison of results with those from experiments with tissue segments. Mar.Biol., 43:381-5
Hatcher, B.G., A.R.O. Chapman and K.H. Mann, 1977 An annual carbon budget for the kelp Laminaria longicruris. Mar.Biol., 44:85-96
Himmelman, J.H., A. Cardinal and E. Bourget, 1983 Community development following removal of urchins. Strongylocentrotus droebachiensis, from the rocky subtidal zone of the St. Lawrence Estuary, eastern Canada. Oecologia, 59; 27-39
Johnson, C.R., 1984 Ecology of the kelp Laminaria longicruris and its principal grazers in the rocky subtidal of Nova Scotia. Ph.D. Thesis, Dalhousie University, 280 p.
Jones, G.M., et al., 1986 Histopathology of the disease causing mass mortality of sea urchins (Strongylocentrotus dreobachiensis) in Nova Scotia. J.Invertebr.Pathol., 45:260-71
Jonsson, H., 1912 The marine algal vegetation of Iceland. In The botany of Iceland, edited by L.K. Rosenvinge and E. Warming. London, Wheldon, Vol. 1.
Kain, J.M., 1976 New and interesting algae from the Sheltland Islands. 2. Hollow and solid stiped Laminaria (Simplices). Br.Phycol.J., 11:1-11
Keats, D.w., G.R. South and D.H. Steele, 1982 Benthic algal succession following the experimental removal of urchins. In First International Phycological Congress, St. John's, Newfoundland, p. 24 (Abstr.)
Lee, R.K.S., 1980 A catalogue of the marine algae of the Canadian Arctic. Natl.Mus.Can.Publ.Bot., (9):82 p.
Lund, S., 1959 The marine algae of East Greenland. I. Taxnomic part. Meddel.Gronl., (156):247 p.
Luning, K., 1980 Critical levels of light and temperature regulating the gametogenesis of three, Laminaria species (Phaeophyceae). J.Phycol., 16:1-15
Luning, K., A.R.O. Chapman and K.H. Mann, 1978 Crossing experiments in the nondigitate complex of Laminaria from both sides of the Atlantic. Phycologia, 17:293-8
Luning, K., and M.J. Dring, 1975 Reproduction, growth and photosynthesis of gametophytes of Laminaria saccharina growth in blue and red light. Mar.Biol., 29:195-200
MacFarlane, C.I., 1952 A survey of certain seaweeds of commercial importance in southwest Nova Scotia. Can.J.Bot., 30: 78-97
Mann, K.H., 1971 Relation between stipe length, environment and the taxonomic characters of Laminaria. J.Fish.Res.Board Can., 28(5):778-80
Mann, K.H., 1972 Ecological energetics of the seaweed zone in a marine bay on the Atlantic coast of Canada. 1. Zonation and biomass of the seaweeds. Mar.Biol., 12: 1-10
Mann, K.H., 1972a Ecological energetics of the seaweed zone in a marine bay on the Atlantic coast of Canada. 2. Productivity of the seaweeds. Mar.Biol., 14:199-209
McPeak, R.H., 1980 A preliminary assessment of the Laminaria resource near Lower Woods Harbour, Nova Scotia, Canada during July, 1977. Can.Tech.Rep.Fish.Aquat.Sci., (954): 180-93
Miller, R.J., 1985 Sea urchin pathogen: a possible tool for biological control. Mar.Ecol.(Prog.Ser.), 21:169-74
Miller, R.J., and A.G. Colodey, 1983 Widespread mass mortalities of the green sea urchin in Nova Scotia, Canada. Mar. Biol., 73:263-7
Miller, R.J., K.H. Mann and D.J. Scarratt, 1971 Production potential of a seaweed-lobster community in eastern Canada. J. Fish.Res.Board Can., 28(11):1733-8
Neish, I.C., 1973 The distribution of kelp and other commercially useful marine algae in Charlotte County, New Brunswick. Report by Applied Marine Research Ltd. to New Brunswick Department of Fisheries and Environment, 46 p.
Parke, M., Studies on British Laminariaceae. I. Growth in Laminaria saccharina (L) Lamour. J.Mar.Biol.Assoc. U.K., 27:651-709
Pringle, J.D., G.J. Sharp and J.F. 1982 Caddy, Interactions in kelp bed ecosystems in the northwest Atlantic: review of a workshop. Can.Spec.PubL.Fish.Aquat. Sci., (59): 108-15
Probyn, T.A., and A.R.O. Chapman, 1982 Nitrogen uptake characteristics of Chordaria flagelliformis (Phaeophyta) in batch mode and continuous mode experiments. Mar.Biol., 71:129-33
Pylaie, M., de la, 1824 Quelques observations sur les productions de L'ile de Terre Neuve et sur quelques Algues de la cote de France, appartement au genre Laminaire. Ann. Sci.Nat.(Bot.Scr.1)Paris, 174-84
Rosenvinge, L.K., 1899 Deuxième memoire sur les algues marine du Groenland. Meddel.Gronl., (3):125 p.
Scheibling, R.E., and R.L. Stephenson, 1984 Mass mortality of Strongylocentrotus droebachiensis (Echindermata: Echinoidea) off Nova Scotia, Canada. Mar.Biol., 78: 153-64
Schneider, C.W., M.M. Suyemoto and C. Yarish, 1979 An annotated check List of Connecticut seaweeds. Bull.State Geol.Nat. Hist.Surv.Conn., (108):20 p.
Sharp, G.J., 1980 History of kelp harvesting in southwestern Nova Scotia. Can.Tech.Rep.Fish.Aquat.Sci., (954):170-9
Sharp, G.J. et al., 1981 The utilization of color aerial photography and ground truthing to assess subtidal kelp (Laminaria) resources in Nova Scotia, Canada. Tech.Pap.Am.Soc. Photogramm., (Fall.1981):57-67
Smith, R.E., and R.H. Loucks, 1980 A Literature and photo-assessment of the marine plant biomass of eastern Canada. Report by Loucks Oceanology to the Atlantic Research Laboratory, National Research Council of Canada, 235 p.
South, G.R., 1984 A checklist of marine algae of eastern Canada. Second revision. Can.J.Bot., 62:680-704
Stafleu, R.A., and R.S. Cowan, 1976 Taxonomic Literature, Vol. 1. Utrecht, Bohn, Scheltema and Holkema, 1135 p.
Taylor, W.R., 1957 Marine algae of the northeastern coast of North America. Ann Arbor, Michigan, University of Michigan Press, 509 p.
Wilce, R.T., 1965 Studies in the genus Laminaria. 3. A revision of the north Atlantic species of the Simplices section of Laminaria. Bot.Gothoburg, 3:247-56