Paper 4 - 1. Natural factors
Paper 4 - 2. Man-made factors
by
W.H. van der Molen
Agricultural University, Wageningen
1. INTRODUCTION
Soluble salts form a formidable menace to irrigated farming and so their occurrence and movements deserve a careful and intensive study. Among the various factors involved in soil salinization, the natural factors were clearly put forward several years ago by Kovda. He observed that salinity is not a phenomenon occurring at random, but is encountered in definite locations only, and that it is closely connected with the geomorphology of the area.
This is indeed what is to be expected, as soluble salts are easily moving substances. Salt movement, therefore, may be considered as the fundamental process behind all phenomena of salt accumulation and leaching, in nature as well as under conditions controlled by man.
2. MOVEMENT OF SALTS
Obviously soluble salts will move with the water in which they are dissolved. So we might use as a first approximation the principle that dissolved substances are carried along with soil moisture flown and groundwater currents. We might also use this principle in reverse; measuring the movement of dissolved substances allows an estimation of the displacement of soil moisture and groundwater. The latter hypothesis forms the background of most work concerning the application of tracers for measuring soil moisture flows or groundwater currents, in many cases these techniques yield excellent results.
However, there are many examples where soluble salts and water do not travel together, or at least not at the same velocity. The most obvious case is evaporation, where water is lost, but salts remain. Salts, therefore, tend to concentrate in places where water evaporates, a simple fact that is behind most of our considerations about salinity and salinization.
Less conspicuous is diffusion, by which salts may move whereas the water does not. Also dispersion has a tendency to uncouple the transport of water and salts, so that they may move at different rates.
The composition of salts is influenced by ion exchange, by precipitation of salts and perhaps also by osmotic phenomena. Moreover, large differences in salinity between different waters may cause density currents or layers of fresh water to float on top of saline water, as is often the case in coastal aquifers.
All these processes cause salt distributions which are often hard to explain.
3. TRANSPORT
3.1 Transport versus Dispersion and Diffusion
Dispersion and diffusion tend to blurr any sharp boundaries between waters of different quality. The vague transition zones produced by These processes will also be moved by groundwater currents. In many cases the times of residence can be estimated by considering transport only: this is especially true for transport in groundwater systems.
If transport only is active, a sudden breakthrough of “new” water at the end of a column will be observed. In reality this breakthrough is a more gradual process, but the time at which the outflow is composed of 50% “old” and 50% “new” water is still correct. This adds to the usefulness of calculations based on pure transport only. Pure transport is often denoted as “piston flow”.
3.2 Times of Residence in Simple Transport Systems
a. In a steady-state system, the time of residence is defined as:
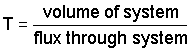
b. For a column of soil:
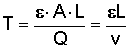
where v can be found from Darcy's Law:
v = -kÑh
|
T: time of residence |
s |
|
e: volume fraction of water |
- |
|
A: cross section |
m |
|
L: length of column |
mm |
|
Q: flow through column |
m3 s-1 |
|
v = Q/A: flux density |
m s-1 |
|
k: permeability |
m s-1 |
|
h: hydraulic head |
m |
V = v/e
|
V: average velocity of flow |
m s-1 |
|
v: flux density |
m s-1 |
|
e: volume fraction of water |
|
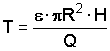
|
R: radius of island |
m |
|
H: thickness of aquifer |
m |
|
Q: yield of well |
m3 s-1 |
3.3 Times of Residence in More Complicated Transport Systems
In many systems T varies, even when diffusion and dispersion are neglected. If a well W near a canal is pumped, the shortest stream-lines (like AW) have by far the shortest times of residence; the travel time along BW is much longer.
Fig. 1 - TIMES OF RESIDENCE IN STREAM-LINES
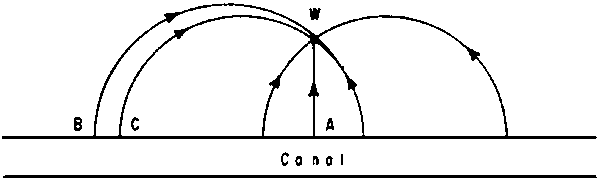
A simple method of solution is the following: from models or from calculations we derive the pattern of stream-lines and the corresponding stream functions (e.g. from 0 to 100% of the total flow). If CW and BW are two stream-lines of which the stream function differs 1%, 1% of the total flow towards the well occurs between CW and BW. For this area therefore:
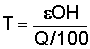
|
T: time of residence |
s |
|
e: volume fraction of water |
- |
|
O: area BCW (in reality!) |
m2 |
|
H: thickness of aquifer |
m |
|
Q: yield of well (positive) |
m3s-1 |
Example: scale of model 1:1000
0 (measured in model) 15 cm2A more direct method, is the use of hydraulic models with coloured solutions (e.g. a Hele-Shaw model) or electrical models with coloured ions like
1 cm in model = 1000 cm = 10 m in reality
1 cm2 in model = 10 x 10 m = 100 m2 in reality
0 = 15 cm2 in model = 1500 m2 in reality
If Q = 0,05 m3s-1; H = 10 cm; = 0.25 we have between BW and CE:
 .
For simple cases analytical solutions are available.
.
For simple cases analytical solutions are available.
Example: flow through aquifer towards a drain D
Fig. 2 - FLOW THROUGH AQUIFER TOWARDS A DRAIN, D
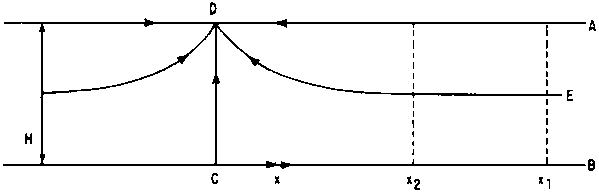
Q: total flow towards drain (positive, from both sides)
H: thickness of aquifer
x: horizontal distance from drain.
|
along AD |
|
|
along ED |
|
Between two distances x1 and x2 (x1 > x2) we have:
|
along AD |
|
|
along ED |
|
|
along BC |
|
Example: recharge, due to percolation of excess irrigation water is drained towards parallel canals (fully penetrating). We take x = 0 midway between two canals and suppose all flow to be horizontal.
Fig. 3 - TIMES OF RESIDENCE IN RECHARGE SYSTEM
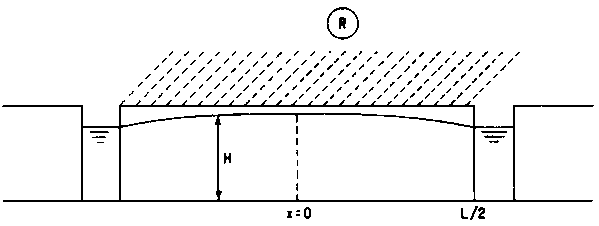
|
x: distance from divide |
(m) |
|
L: distance of canals |
(m) |
|
H: thickness of aquifer (» constant) |
(m) |
|
e: volume fraction of water |
(-) |
|
R: recharge rate |
(m s-1 or/day) |
|
Tx: time of residence between x and canal |
(s or days) |
|
Qx: flow at distance x from divide |
(m3 s-1 or m3/day) |
The flow from the left towards the canal at x = L/2 is RL/2
Total flow towards the canal (from both sides) is RL.
Between x and x + dx we have:
|
volume of system |
eH dx m3 |
|
flow through system |
Qx |
|
time of residence |
|
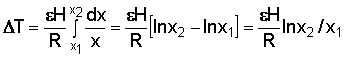
If x1 = 0 (midway between canals), the time of residence is infinite and stagnation occurs.
Between a point at distance x from the divide and the canal, the time of residence is:
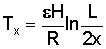
Example: In an irrigated area R = 1 mm/day; L = 200 mm; H = 5 m; e = 0.25.
|
x = 0 |
1 |
10 |
50 |
90 m |
|
Tx = ¥ |
5756 |
2878 |
886 |
132 days |
|
Tx = ¥ |
16 |
9.9 |
2.4 |
0.4 years |
DIFFUSION
Small particles are constantly moving. In liquids and gases, where particles are rather free. These random movements result in diffusion. If there is a difference in concentration, more particles will move from places of high concentration towards places of low concentration than in the reverse sense, because the probability of such a movement is greater.
Macroscopically diffusion is described by Fick's Law:
J = D Ñc
|
J: flux density |
kg m-2s-1 or kg m-2yr-1 |
|
D: diffusion coefficient |
m2s-1 or m2yr-1 |
|
c: concentration |
kg m-3 |
|
Ñc: concentration gradient |
kg m-4 |
|
x: distance |
m |
|
t: time |
s or year |
Fick's Law is equivalent to the Laws of Ohm (electricity) and Darcy (groundwater flow).
If the movement is in the x-direction only:
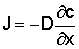
For NaCl in water: D = 1.52 x 10-9 m2s-1
For NaCl in soils: D = 0.57 x 10-9 m2s-1 = 0.018 m2yr-1
In soils D is lower, because the pathways (pores) are tortuous. For different soils (clay, silt, sand) the mutual differences are small, because D is not dependent on the size of the pores (unless they become of molecular dimensions), but on their tortuosity, which is not very different in different soils.
4.1 Diffusion in Soils, Flooded with Fresh Water
We assume a saline soil which is suddenly brought under a layer of fresh water. By renewing the water, its salinity is kept low and the soil continues to loose salt by diffusion. No movement of water through the soil is supposed to occur.
This situation arises in rice cultivation on saline soils and also at the bottom of salty lakes, which suddenly turned fresh by human interference.
For the salt concentration in the soil, we have:
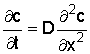
where x is depth below surface
In the cases mentioned, the solution appears to be:
c = c0 erf u with u x/2ÖDt
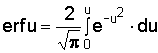
If t is expressed in years, D = 0.018 m2/year, x in metres we find c at any depth and time. Diffusion appears to be a slow process (see Figure 4).
Rice growing on saline soils is possible if a continuous stream of fresh water is maintained across the fields to remove the salts entering the water by diffusion. The topmost centimetres of the soil soon become fresh enough for root growth. The desalinization of deeper layers, however, is extremely slow; therefore rice growing is not a good measure for reclaiming saline soils.
An example is the Guadalquivir marshes in Spain, where rice on highly saline clay soils yielded 5 000 kg/ha if a continuous stream of fresh water was available. The salt content of deeper layers, however, was hardly changed.
Fig. 4 - THEORETICAL RELATIONS BETWEEN DEPTH AND SALINITY IN THE BOTTOM OF A LAKE WHICH TURNED FRESH AT t = 0
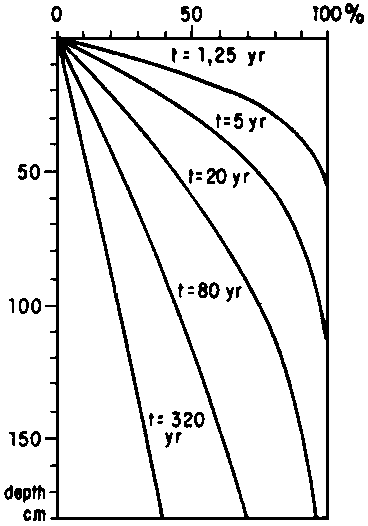
The diffusion process in the bottom of Lake Ijssel closely followed the laws mentioned above. This lake was separated from the sea in 1932 and turned fresh soon afterwards.
4.2 Diffusion from a Thick Clay Layer towards a Thin Sandy Aquifer
We may suppose a thick clay layer underlying a thin sandy aquifer. In the aquifer water is circulating, but the clay is considered impermeable. Both the sand and the clay are of marine origin.
Initially both the sand and the clay are filled with connate salt water of concentration c0, but at a time, t = 0, fresh water begins to enter the sand with c flux density v. Due to this recharge, the salt water in the sand is replaced by fresh water, but at the same time diffusion from the clay starts. Due to this process, the aquifer will continue to yield brackish water for a long time.
The water in the aquifer is supposed to be completely mixed over its cross-section, but no dispersion occurs in longitudinal direction. This is a good approximation for thin aquifers extending over large distances, but for aquifers of great thickness dispersion processes (transversal as well as longitudinal) must be taken into account.
Fig. 5 - DISPERSION IN AQUIFERS
The salt concentration in the aquifer is:
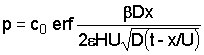
The salt concentration in the clay is:
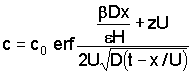
|
p: salt concentration sand |
kg m-3 |
|
c: salt concentration clay |
kg m-3 |
|
c0: salt concentration initial |
kg m-3 |
|
x: distance |
m |
|
z: depth in clay |
m |
|
t: time |
s or yr |
|
b: porosity clay |
- |
|
e: porosity sand |
- |
|
H: thickness sand1/ |
m |
|
D: diffusion coeff. clay |
m2 s-1 or m2/yr |
|
U: true velocity |
m s-1 |
|
v = eU: flux density |
m s-1 |
1/ See also point followingFor
H = 10 mthe salt concentrations were calculated. The results are given in the figure. For large distances and low velocity (x/U > 1000) the aquifer yields brackish water for very long periods.
D = 0.018 m2yr
e = 0.3
g = 0.5
Fig. 6 - DESALINIZATION OF AN AQUIFER IN CONTACT WITH SALINE CLAY OF GREAT THICKNESS
If the aquifer is confined between two initially saline clay layers of great thickness, H must be taken is half the thickness of the aquifer.
The theory explains the increasing salinity along the path of flow, as encountered in many aquifers. A similar theory has been developed in heat flow (Carslaw and Jaeger, p. 296).
5. DISPERSION
5.1 Mechanism of Dispersion
In porous media, like the soil, water moves through a complicated network of tortuous channels. In soils, the grains cause a frequent separation of water filaments which re-unite later on. The movement is very irregular on a microscopic scale, but on a macroscopic scale it shows a distinct direction and velocity of flow.
In places, where two or more pores unite to form a cavity, the water coming from different directions is mixed by molecular diffusion. It can be shown that in soils this process is rap enough to reduce any existing differences in concentration to less that 1% of their original value during the time that the water is present in such a cavity. Therefore, the entire movement can be considered as flow through a series of interconnected, small but well-mixed reservoirs with dimensions of the same order as the soil particles. From each reservoir smaller pores lead into different directions; a water particle from such a reservoir chooses one of these pathways, in the general direction of the macroscopic flow.
Another cause for dispersion is the irregular flow within the pores. In a capillary tube, water flows faster in the middle than near the walls, this leads to longitudinal dispersion.
In soils, the pores are of different size, and water flows faster through the larger pores. Later on, such fast filaments re-unite with slower ones and molecular diffusion again causes exchange. Also this mechanism is mainly operative in the direction of the macroscopic “stream lines”. Therefore longitudinal dispersion is larger (often 3-8 times as large) as transversal. This is in contrast with diffusion, which is equally active in all directions.
Elaborate models for dispersion have been worked out by Scheidegger, Bear, and others.
Dispersion is characterized by a dispersion coefficient Dx (m2s-1 or m2/yr), which plays the same part as the coefficient for molecular diffusion D. As dispersion is different in longitudinal and transversal direction, we must distinguish between:
DL (m s-1 or m/yr) for longitudinal dispersion
DT (m s-1 or m/yr) for transversal dispersion.
Both are approximately proportional to the average velocity of flow in the pores, V; they are often written as
|
DL = (l+2µ) V |
|
|
|
V = v/e |
|
DT = V |
|
|
l + µ: constants (lengths), of the
same order as the irregularities in the soil |
m |
|
V: average velocity in the pores in direction of flow |
m s-1 or m/yr |
|
v: flux density (“filtration velocity”) |
m s-1 or m/yr |
|
e: porosity (volume fraction) |
|
DL = (l+2µ) V and V = v/e we have:DL = k · v where
5.2 One-dimensional Dispersionis again a constant, the “characteristic length”.
If all changes occur in the direction of flow (x-direction), longitudinal dispersion only is operative. This is the case in the desalinization of a soil by rainfall or irrigation, where the direction of flow is vertical.
This leads to the differential equation
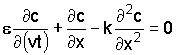
For desalinization of a semi-infinite soil column, the conditions are:
|
x > 0 |
t = 0 |
c = c0 |
(initial salt content) |
|
x = 0 |
t > 0 |
c = 0 |
(boundary condition) |
|
x ® ¥ |
t finite |
c = 0 |
(infinite depth) |
|
x finite |
t ® ¥ |
c = 0 |
(infinite time) |
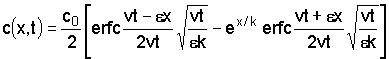
where
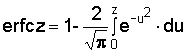
|
v: flux density |
(m s-1) |
|
t: time |
(s) |
|
c(x,t): salt concentration |
(kg m-3 of soil moisture) |
|
c0: initial salt concentration |
(kg m-3 of soil moisture) |
|
e: pore fraction filled with solution |
(-) |
|
k: characteristic length |
(m) |
|
x: depth |
(m) |
The solution mentioned applies only to substances which remain in solution: the Cl'-ion is a good example. For substances entering in ion exchange or precipitation reactions solutions have been worked out by Reiniger and Bolt (1972).
A simpler model consisting of a series of completely mixed reservoirs, each with a thickness 2k gives almost the same results; for practical cases this model is extremely useful (see section 6).
5.3 Two-dimensional Dispersion
If salts enter an aquifer, dispersion occurs in longitudinal as well as transversal directions. If an aquifer, overlying a salty clay layer (cf. 4.2) is thick, this dispersion cannot be neglected.
For certain cases, especially when a constant salinity is maintained at the clay surface, the problem of dispersion has been solved (Verruijt, 1973). For a combination of diffusion (from clay towards aquifer) and dispersion (within the aquifer) no exact solution has yet been found. Preliminary calculations, compared with observations in the thick Pleistocene aquifer of the Netherlands show that the irregularities (which govern the values of l and µ) are of the order of 1-2 metres. Obviously dispersion is governed by irregularities in the aquifer rather than by sand grain effects, which would cause values of l and µ of the order of one millimetre (Meinardi, 1973).
6. MIXED RESERVOIRS IS SERIES
6.1 Series of Reservoirs as a Model for Aquifer Behaviour
A simpler model is obtained by considering aquifers or soils as composed of a series of well-mixed compartments. Diffusion and dispersion act as mixing processes, whereas the dimensions of the compartments are a measure for their activity; with small compartments transport dominates, with larger ones mixing processes gain importance.
With compartments or reservoirs of equal size, originally saline and losing their salt by leaching, we have:
|
For the first compartment |
|
|
For the second compartment |
|
|
For the third compartment |
|
|
For the N-th compartment |
|
The results are almost the same as for the dispersion theory treated in 5.2, if the thickness of each compartment is taken equal to 2 k.
6.2 Reservoirs with Bypass
In irrigated soils, leaching is often not fully efficient. Such soils usually have cracks, root boles, etc., through which part of the water passes almost unchanged. The model for such cases is a mixed reservoir, provided with a bypass, through which part of the water flows unaltered towards the next compartment.
The leaching efficiency in irrigated soils is represented by the relative importance of such a bypass. It ranges from zero (all water passing through the bypass) to one (all water passing through the reservoir).
In practice, this leaching efficiency is lower for heavy soils than for light-textured ones: under sprinkling it is higher than under surface irrigation systems.
7. ORIGIN OF SALTS
7.1 Sources of Salts
The primary source of salt is the weathering of rocks. Rooks contain Na, K, Mg and Ca in the form of silicates. Chlorides are rare; sulphur is mainly present as insoluble sulphides, which may be transformed into soluble sulphates after oxidation.
Weathering occurs under the influence of CO2 from the air and especially CO2 produced by decay of organic matter. This process gives rise to soluble carbonates of Na, K, Mg and Ca. Also Cl and SO4 are removed by leaching and in the humid tropics dissolution of silicates is an important process.
The hydrological cycle transports the dissolved salt to the ocean, which is enriched with Na, K, Mg, Cl and SO4. In the sea, Ca is precipitated by organisms as shells and limestones; therefore the sea water has a low Ca content.
7.2 The Salt Cycle
The amount of soluble salts, carried by the rivers, is sufficient to give the ocean its present salt content within a geologically short time. As the ocean is much older, a salt cycle must exist, comparable to the hydrological cycle, but proceeding at a slower rate.
Rain and salt spray from the waves bring salt towards the continents. The amounts are considerable near the coast, but rapidly diminish further inland, as shown in the following table.
|
SALT CONTENT OF RAIN WATER IN GERMANY |
|||
|
Location |
Distance from coast |
Cl |
Na |
|
Westerland |
0.2 |
37.6 |
18.5 |
|
Schleswig |
50 |
4.9 |
2.4 |
|
Braunschweig |
450 |
1.9 |
0.8 |
|
Augustenberg |
800 |
0.9 |
0.5 |
|
Retz |
1250 |
0.3 |
0.1 |
Under special circumstances (bays in a dry climate, separated from the sea by a narrow and shallow entrance) evaporites are formed: gypsum, halite (NaCl), finally K and Mg salts. Such evaporites may reach a great thickness: in the Zechstein (Permain) hundreds of metres of evaporites were formed in N.W. Europe and similar formations of different geological age occur elsewhere.
Under high pressures (overburden of more than 1 000 m thickness) salt becomes plastic and starts to flow. Thick salt layers (of more than 300 m in thickness) form domes, which often rise another 1 000 m over the original position, forming slender pillars or walls. Sometimes such salt diapirs reach the surface, where they may form “salt glaciers” in a dry climate (Iran). In other cases the surface is not reached but the salt diapirs may become exposed by later erosion. Erosion of salt domes is a major source of salinization or rivers in mountainous areas (Karpathians and N. Africa, where Triassic salts influence the quality of many rivers).
If the salt diapirs remain buried, part of their salts wilt dissolve in the groundwater. The impurities in the salt are left behind as a “caprock” of clay or anhydrite (CaSO4), which is nearly watertight. Due to such caprocks, the salt domes of the northern Netherlands do not influence the recent groundwater, but in other areas (e.g. across the German border) highly saline groundwater is found near diapirs. Where such groundwaters rise to the surface, saline soils occur, characterized by a vegetation of halophytes.
Old marine sediments may still contain saline waters. The water pumped from coal mines in the Netherlands and Germany is often brackish; this is perhaps due either to marine transgressions during the Carboniferous or to later transgressions of the sea.
Permeable marine formations on the continents (sands, sandstones, limestones) often become fresh due to leaching by rainwater. Water from such formations may be used for domestic purposes or for irrigation water.
Examples:
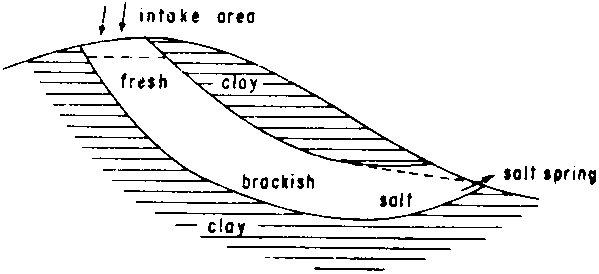
1. The Tilburg water supply draws water from a layer of marine shells dating from the Pliocene. In later periods this ancient “beach sand” has become fresh.However, if such permeable formations are in contact with marine clays, which are still salty, diffusion of salt from the clays often influences the water quality in the aquifer, a process which may continue for geological periods. As a consequence, the water in the aquifer becomes increasingly brackish in a downstream direction. In the long run the entire formation will become fresh, but this process may require geological time.2. Many limestones of marine origin are excellent sources for irrigation water.
Fig. 7 - INFLUENCE OF DIFFUSION OF SALT FROM MARINE CLAYS ON WATER QUALITY
7.3 Kinds of Salts
The chemical composition of the salts depends on the geochemistry of the region. In coastal areas, Cl usually dominates, due to cyclic salts carried with the rainfall and due to former marine transgressions.
In limestones and calcareous sands (many coastal dunes) Ca and HCO3 dominate, due to dissolution of CaCO3 in water containing CO2. Such waters are “hard”; they are useful for irrigation, but present problems in domestic use and especially in industry. Ion exchange may cause natural softening of such waters, converting them into waters of NaHCO3 type, which are less favourable for irrigation.
In some areas B is present. This element forms soluble borates, which may concentrate in borax lakes (Na2B4O7 · 10H2O). Boron is a minor element in plant nutrition, but excess leads to damage, especially in fruit trees. Such B toxicity is probably confined to areas of volcanic influence.
Other volcanic rocks are rich in Na (some basalts contain Na zeolites, some igneous rocks contain Na silicates) and give rise to waters high in Na, with a high SAR value.
In the centres of the continents ions other than Cl usually dominate: either SO4 or HCO3 - salinization occurs. In tropical regions HCO3 and silicates dominate, but the total concentration of the waters is extremely low. The few cations present in the soil are taken up by the tropical rain forest and return via falling leaves and dead wood. This cycle is almost closed and few cations escape by leaching. If the forest is cut, the elements are rapidly leached. Therefore, in regions with shifting cultivation the concentration of the river water is markedly higher than under natural conditions, where total dissolved solids are about 50 g m-3 (mg/l).
In other cases SO4 is the dominant anion, giving rise to sulphate salinization. In combination with Ca it leads to waters, rich in gypsum, which are of excellent quality for irrigation.
7.4 Pollution with Inorganic Compounds
In industrialized and densely populated regions pollution (both organic and inorganic) often dominates the natural water quality. An extreme example is the Rhine river, with a Cl content of about 250-300 mg/l, whereas the natural content is about 20 mg/l.
The following are examples of pollution with inorganic compounds:
- mining: the potash mines of the Elzas cause considerable pollution of the Rhine with NaCl, which is a useless by-product from these mines. In addition, coal mines pump salt water into the river.8. INFLUENCE OF GROUNDWATER CURRENTS AND CAPILLARY RISE- irrigation: drainage water of irrigated areas is often salty. If discharged into a river, it may damage other areas further downstream. In arid regions, this may become one of the major problems in stream basin development, especially in regions far from the sea.
- municipal waste water: contains several kinds of salts, like chlorides, nitrates and phosphates. Groundwater under old human settlements contains more Cl and NO3 than natural waters; in dry climates NaNO3, crystallizes around such old settlements.
- industry: soda industries convert NaCl into Na2CO3 according to the overall reaction:
2NaCl + CaCO3 ® Na2CO3 + CaCl2The calcium chloride in a useless by-product and is discharged.- drainage of polders causes seepage currents. In coastal areas the seepage water is often saline. In the Netherlands this is an important cause of salinization.
- cooling water, if pumped from great depth, may be saline. In Delfland district (Netherlands) a special pipeline has been constructed to convey saline cooling water from industries at Delft towards the sea.
8.1 Seepage Currents
From the foregoing it will be clear that movement of water causes movement of salts. Salts, therefore, are transported from places where water is infiltrating towards places where it evaporates. They accumulate in places where groundwater rises to the surface, a phenomenon denoted as seepage.
Seepage is common along the foot of a hill or natural terrace and in valleys. In humid climates, hydromorphic soils (gley soils) are found in such locations, in arid climates saline soils occur instead. If semi-permeable (semi-confining) layers are present in a valley near the surface, extended seepage of low intensity occurs over large areas; if they are absent seepage is concentrated near the foot of the slopes. (Fig. 8 A and B.)
In irrigated areas, leakage of water from unlined canals gives rise to seepage in their neighbourhood and often causes severe salinization of a strip along such a canal. (Fig. 8C.)
Extremely widespread is seepage from irrigated fields towards adjoining dry fields. Under irrigation, water tends to move downwards and there is little danger of acute salinization, but under non-irrigated fields water moves upward and evaporates, so that salts accumulate. Due to this movement, we often find a rim of highly saline soils around small irrigated areas or around villages. (Fig. 8.)
Fig. 8 - SEEPAGE PHENOMENA
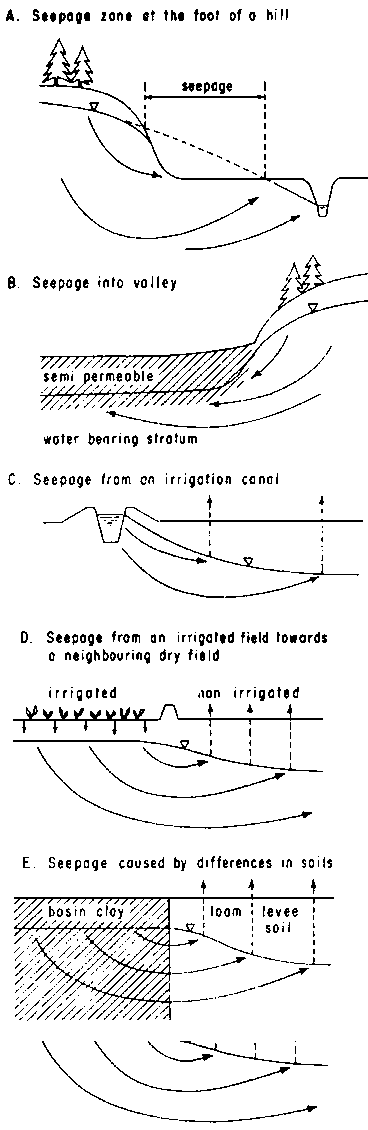
If soils with different capillary characteristics border each other, seepage currents may be pet up; directed towards soils of higher capillarity. This may cause natural differences in salinity, due to variations in soil texture. Slight differences in elevation may have similar effects. (Fig. 8E.)
8.2 Capillary Rise
Seepage currents are rarely so strong that the groundwater reaches the surface. Usually an unsaturated zone remains present, through which water rises by capillary action towards the soil surface or towards the root zone of plants. (Fig. 9).
Fig. 9 - WATER BALANCE OF AN IRRIGATED SOIL
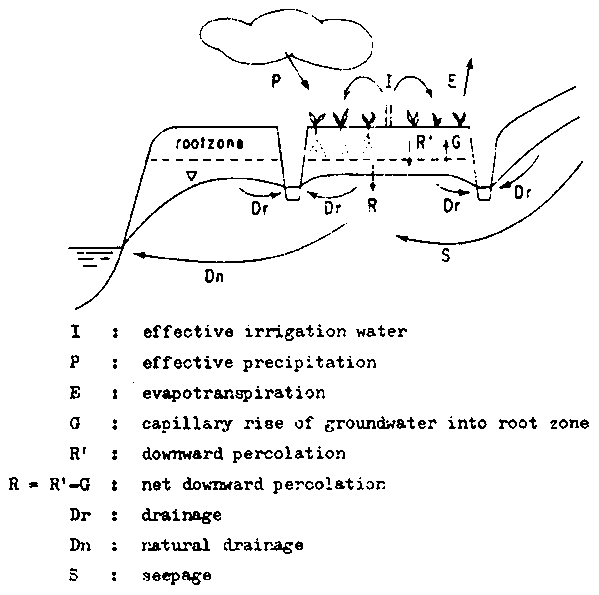
The nature of capillary rice is well understood and knowledge about the soil characteristics is increasing. Several models have been proposed for this process, among These the model of Rijtma-De Laat is useful for practical purposes. In this model the non-steady process is approached by a succession of steady states. Recently this model was applied to saline soils by Varallyay (1974). The model predicts that capillary rise will lead to a lowering of the groundwater table. This in turn, will cause a decrease in the upward flow and finally the process will come to a standstill. The depth of the groundwater after a prolonged dry period is a fair indication of the depth over which capillary flow is active and may be used as a check on the calculations.
The limiting groundwater depth, mentioned above, is only indicative if neutral groundwater conditions exist, i.e. if neither natural drainage nor seepage occur. If natural drainage is present, the groundwater will fall below this level and capillary rise will stop earlier. If seepage is present, the groundwater will be maintained at a higher level. In this case, an equilibrium is reached in which the capillary rise becomes equal to the amount of seepage. The amounts of salt transported by this process are usually considerable and may lead to severe salinization. Therefor, attention should be given to groundwater currents and especially to measures that prevent seepage water from reaching the surface, among these leaching and drainage are of moat importance.
9. SUMMARY AND CONCLUSIONS
Salts move with the water; this transport is modified but usually not profoundly altered by diffusion and dispersion.
Ion exchange, precipitation or solution may change the proportions of individual ions.
The occurrence of salinity under natural conditions can be explained from a study of the geo-hydrology of the area. Such investigations also form the base for predicting the changes introduced by irrigation.
Phenomena of seepage and capillary rise greatly influence salt transport and accumulation. For these processes, useful models have been developed; their application is hampered by a lack of knowledge about the soil constants, like saturated and unsaturated hydraulic conductivity. Work should be concentrated on methods to obtain such constant a rather than on further refinement of the models.
The “salt cycle” concept may be helpful in understanding phenomena of salinization and leaching.
REFERENCES
Bear, J. 1972. Dynamics of fluids in porous media. Ch. 10. New York
Carslaw, H.S. and Jaeger, J.E. 1959. Conduction of heat is solids. 2nd ed. Oxford University.
Kovda, V.A. FAO/Unesco. 1973. Irrigation, drainage and salinity. An International Source Book. Ch. 6 (155-176).
Meinardi, C.R. 1973. The origin of brackish groundwater in the lower parts of the Netherlands. Mededeling 74-6, Rijksinstituut Drinkwater-voorziening, 's Gravenhage.
Reiniger, R. and Bolt, G.H. 1972. Theory of chromatography and its application to cation exchange in soils. Neth. J. Agr. Sc., 20:301-303.
Frissel, M.J. and Reiniger, P. 1974. Simulation of accumulation and leaching soils in Wageningen.
Riehm, H. van and Quellmalz, E. 1959. (quoted in Schoeller, H. 1962.) Les eaux souterraines. Paris.
Scheidegger, A.E. 1957. The physics of flow through porous media. Macmillan, New York.
Várallyay, G. 1974. Hydrophysical aspects of salinization processes from the groundwater. Agrokémie es Talajtan 23:29-44.
Verruijt, A. 1971. Steady dispersion across an interfer in a porous medium. J. Hydrology 14:337-347.
Paper 5 - a. Water management and salinity
Paper 6 - b. Assessing the suitability of water for irrigation: theoretical and empirical approaches
Paper 7 - c. Soil management and agronomic practices
1/ Contribution from the Agricultural Research Service, USDA, U.S. Salinity Laboratory, Riverside, California.
by
Jan van Schilfgaarde
Director, U.S. Salinity Laboratory
1. INTRODUCTION
Permanent agriculture under conditions of insufficient precipitation depends on water management so that excessive salts do not accumulate in the root zone. When rainfall is insufficient to satisfy evapotranspiration and the plants must depend on drawing from a water table for part of their water supply, the only question is how soon the soil will salinize, not whether. When irrigation water is supplied, there is a twofold question: whether sufficient supplemental water is applied to provide the required leaching, and whether the drainage network (natural or man-made) has the capacity to remove sufficient water with its associated salts. In other words, to prevent salination, a net downward flux of water is mandatory.
In this discussion, I propose to distinguish between maintaining a favourable agriculture under irrigation and reclaiming excessively saline (or sodic) soils.
2. MAINTAINING A FAVOURABLE ROOT ENVIRONMENT
One of the criteria that is often applied to predict whether a soil will salinize is the so-called “critical water table depth”. As I understand it, the basic concept of a critical depth is rather simple, but its application extremely complex. As described by Talsma (1963), it was first introduced by Polynov in 1930 and defined as that maximum height above the water table to which the salts contained in the groundwater can rise under natural conditions both by capillary rise and diffusion. We understand better now than we did in the 1930s that the level to which water can rise in soils is essentially unlimited as long as we do not specify the rate of rise. Within the framework of this concept, however, numerous observations have been made, often associated with detailed field studies. They have led to estimates for critical depth varying from 1 to over 3 m, depending on soil morphology, climate, quality of the groundwater, cropping patterns and other factors. Kovda (1961), for example, makes clear and specific distinctions based on salt content of the groundwater. Talsma (1963) concluded, from his own field studies and data in the literature, that it was reasonable to specify that the upward flux should not exceed 0.1 cm/day. If the soil physical properties are known, this maximum flux permits, in theory, the calculation of a minimum depth to water table.
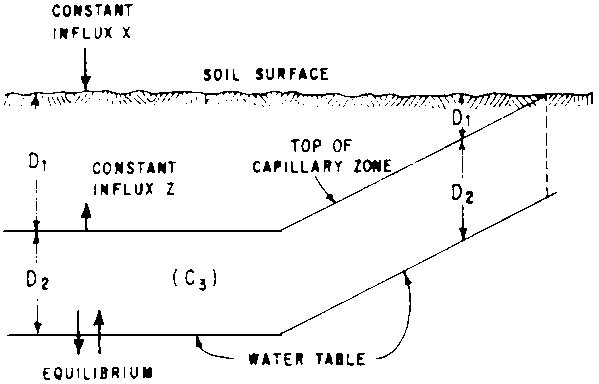
Even though numeric standards can be readily found in the literature, the determination of the critical depth remains a judgement, rather than a rational derivation, Szabolcs, Darab and Varallyay (1969) took an important step to rectify this shortcoming and proposed a method for calculating the critical depth from a salt balance model. Whereas their method has merit, it also suffers from a fundamental weakness. In effect, these authors assume that there is an influx, x, of salt to the root zone equal to the amount in the irrigation water, and an influx, z, from the capillary fringe. The salt concentration, C3, in the capillary fringe is derived from the groundwater, plus any residual salt. The influx z is determined by assuming a constant upward, water flux at concentration C3, independent of water table depth. Since the total salt flux into the root zone is assumed constant, it follows that the calculated concentration in the soil solution increases as the depth D1 of the root zone is decreased by a rise of the water table. These calculations completely ignore the dynamics of water flow (see Fig. 1). Another problem with the model is the assumption of a “salt regime coefficient”, or a natural, constant leaching rate expressed as a percent of soil per year. To the extent that such a rate can be estimated, it should be a function of the concentration of the soil solution near the lower boundary of the root zone and the water flux, rather than of the salt content of the soil and thus, the depth D1. Varallyay (1974), in recognition of this problem, carried the work further by determining, in the laboratory and by computer, the maximum height to which water could rise at prescribed rates for different arrangements of layers of soil with widely varying properties.
Fig. 1 - SCHEMATIC ILLUSTRATING SOME OF THE IMPLICATIONS OF CRITICAL WATER TABLE DEPTH MODEL BY SZABOLCS et al. (1969)
If we may look back to the concept advanced by Gardner (1958) and further developed by Raats and Gardner (1974), a constant upward flux from a water table can only be maintained as long as a limiting height is not exceeded. Starting with the steady state flow equation in the form
q = k(1 - dh/dz), ..... [1]where q(cm/day) is the flux, k(cm/day) the hydraulic conductivity, h(cm of water) the pressure head, and z the vertical coordinate, with positive direction downward, we may solve for z:
If the relation between k and h in known, the integration can be performed, be it sometimes with difficulty. For many soils, the k(h) relation can be adequately represented by (Raats and Gardner, 1974)
where K represents the conductivity at saturation, hK/2 the pressure head at which k = K/2 and n a constant varying from 1 to around 15. This relationship is equivalent to Gardner's (1958)
k = a/(Sn +b) ..... [3a]with S = - h, K = a/b and - hK/2 = b1/n. Another relationship, particularly convenient for analytical work, has the form
k = K exp (ah). ..... [4]Here a (cm-1) is a soil property that describes the relative rate of change in k with respect to the pressure head:
a = (1/k) dk/dh. ..... [4a]Gardner (1958) and before him Wind (1955) showed that there was a maximum upward flux from the water table that could be sustained over a given distance. This seems to be true wherever the integral
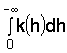 is finite, whatever equation is used to describe k(h). In Raats and Gardner's
(1974) notation, using Eq [3], one finds, for q << K,
is finite, whatever equation is used to describe k(h). In Raats and Gardner's
(1974) notation, using Eq [3], one finds, for q << K,
-qmax = K[hcr/(z-z0)]n ..... [5]or, exactly,
Here z0 is the elevation at which k = K (water table) and hcr = hK/2 p/n · sin(p/n) represents the critical pressure head introduced by Bouwer (1964).
Note that, because of our sign convection, qmax and z - z0 will be negative.
If Eq [4] is chosen instead of Eq [3] to represent k(h), one can integrate Eq [1] to obtain
az = ln [(k - q)/(K - q)]. ..... [7]This, in turn, yields the limiting value (with z = 0 at h = 0)
zmax = -a-1 ln(1-K/qmax). .....[8]For this case (Eqs [4] and [6]), hcr = -1/a.
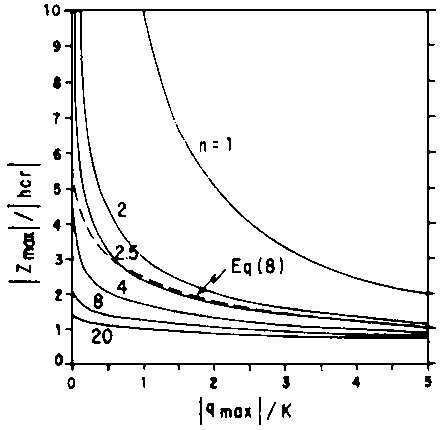
Thus Eqs [5] and [8] represent two relations that permit the determination of the minimum depth of the water table below a sink of given strength in a uniform soil.
Table 1 gives some zmax values for illustrative soil parameters taken from the literature. Note, that, in the range of q of importance for the present purpose, the approximate Eq [5] gives essentially the same result as the exact Eq [5a]. Eq [8], on the other hand, matches well only when n has a value close to 2.5, giving rather different results for n deviating substantially from that value. This latter point is especially clear in the more general comparison given in Fig. 2, showing the relationship between the dimensionless ratios zmax/hcr and -qmax/K for various values of n in Eq [5a] and for Eq [8].
Table 1 - CRITICAL DEPTH (zmax) CALCULATED FOR FOUR SOILS BY MEANS OF THREE EQUATIONS, ASSUMING -qmax = 0.1 cm/day
|
Soil type |
|
zmax-cm |
|
||||
|
K sat |
-hK/2 |
n |
Eq [5] |
Eq [5a] |
Eq [8] |
a1/ |
|
|
cm/day |
cm |
cm-1 |
|||||
|
Banno sand2/ |
26.5 |
6.6 |
1.5 |
659 |
658 |
89 |
0.0625 |
|
Yolo 13/ |
1.0 |
20 |
2 |
99 |
95 |
75 |
0.0318 |
|
Pachappa f.s.l.3/ |
12.3 |
30 |
3 |
180 |
179 |
175 |
0.0276 |
|
Sand4/ |
40.0 |
18 |
4 |
89 |
89 |
120 |
0.0500 |
1/ Calculated from -a-1 = hK/2 p/n · sin (p/n)
2/ From Talsma (sand overlying clay) (1963)
3/ From Gardner and Fireman (1958)
4/ From Wind (1961)
For non-uniform soils, thus in many field situations, the above approach is limited. Varallyay's (1974) numeric approach provides as yet the only available solution for layered soils. The other requirement of the theory, a given sink strength, raises two dilemmas: its magnitude and its location. Talsma (1966) advances several reasons for selecting -qmax = 0.1 cm/day, including that it seems to work satisfactorily in practice, that the nature of the k(h) curves often results in an abrupt change in required critical depth around this value, and that the potential evapotranspiration rate normally is higher. It appears indeed a reasonable choice, if the critical water table depth is used at all, and if it is kept in mind that over time any upward rate will salinize a soil. The question of the location of the sink is another matter. For bare soil, the sink is simply the evaporative flux from the surface, but for cropped soil, it becomes a distributed sink due to a variable root water uptake pattern. As a first approximation, one might estimate the depth of the rooting zone and use that value as the boundary for the upward flux calculations. This clearly is not a very satisfactory solution. The interaction between root uptake patterns and soil water and solute fluxes is a complicated subject in which only limited progress has been made to date, at least in the present context. One approach to an analytical evaluation will be deferred till later in this discussion. In any case, no matter how sophisticated the analysis, depth to water table cannot uniquely predict accumulation of salt in the soil profile.
Are there alternatives to the use of a critical water table depth as a criterion for maintaining a favourable root zone? The objective, achieved in whatever way, is to maintain a net downward flux sufficient in magnitude to prevent excessive accumulation of salts in the soil solution. Even with a high water table, upward flow will only occur when there is an upward gradient; an appropriate irrigation regime, in principle, can prevent such a gradient. Possibly more to the point, one must ask the question what factors cause the creation of a water table. In some cases, drainage from surrounding geologic formations causes a lateral inflow from natural sources; in others, excessive irrigation upstream may result in adverse conditions. Artesian pressure in a semi-confined aquifer may be caused by recharge hundreds of kilometres away, as is the case in the Red River Valley in North Dakota. In this valley, efforts at controlling salinity through cultural practices, tile drainage and soil management were only marginally effective because artesian pressure in a saline aquifer some 100 m deep caused a slow but continuous upward flow. Doering and Benz (1972) demonstrated, through theory and field tests, that a simple solution to the problem consisted of rather widely spaced wells, pumped at a low rate just sufficient to reverse the hydraulic gradient.
In most cases, however, irrigation excessive to the drainage capacity causes the rise in water table. To prevent this rise, one must either increase the drainage rate by artificial means, reduce the amount of irrigation water, or a combination of These. It is my view that the water table height should be viewed as a consequence of water management, rather than as the independent variable causing water management problems.
A clear example is offered by the Wellton-Mohawk region in Arizona, part of the Gila River drainage basin. Irrigation has been practised in the Valley since the 16th century by surface diversion and later groundwater pumping. Geologically a closed basin, only severely limited surface drainage is provided naturally. When upstream development reduced river flow, increased pumping lowered the water table, restricting drainage even more, and return flow salinized the groundwater. This in turn, led to importation of water from the Colorado River some twenty years ago, with a concomitant increase in area irrigated, often at excessive rates. Shortly thereafter, a rising water table caused severe problems, requiring the construction of over 100 drainage wells and a lined conveyance channel for disposal. From 1970-72, the irrigation efficiency (crop consumptive use/water delivered to farms) was estimated to be 56% (Adv. Comm. Irrig. Eff., 1974). Whatever the irrigation efficiency the same problems would have cropped up. However, the rate at which the problems developed, and the rate of drainage needed, thus its cost, depended strongly on the irrigation efficiency.
In most instances, drainage theory and practical experience are sufficiently well advanced to design a system for a given drainage requirement. This requirement must be established by consideration of i) the natural drainage rate, ii) the amount of water to be removed from extraneous sources, and iii) the amount of irrigation water in excess of crop needs that must be removed. The latter is strongly affected by management. Returning to the Wellton-Mohawk, it has been conservatively estimated that 10% of the water diverted for irrigation is lost in transit, an amount equal to about 24% of the total drainage water pumped. Also, using primarily current practices of the better farm operators as a guide, it was determined that the farm efficiency could be raised from 56% to 72%, without significant technological problems. Thus good management can have a substantial impact on reducing the drainage requirement. Canal lining, use of pressurized delivery systems and scheduling irrigation water delivery on demand can reduce distribution system losses as well as facilitate high on-farm efficiencies.
An important factor in determining the drainage requirement is the leaching requirement, or the amount of irrigation water needed in excess of crop consumptive use to maintain a favourable salt balance. The most common practice is probably to determine the leaching requirement (LR) from the relation (USSL Staff, 1954)
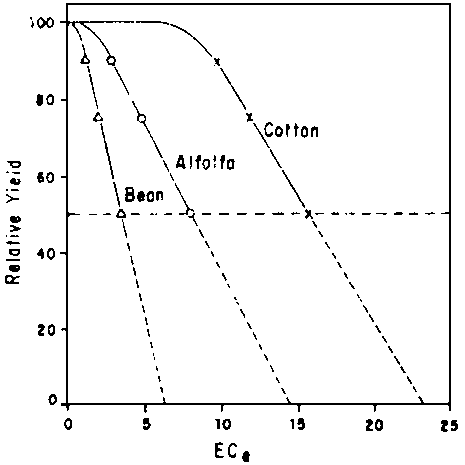
LR = ECiw/ECdw ..... [9]where ECiw and ECdw represent the electrical conductivities of the irrigation and drainage waters, respectively. Drainage water, in this context, means the water draining below the root zone. It is customary to select for ECdw in Eq [9] the conductivity, ECe-50, of the saturated soil extract at which a 50% reduction in crop yield was obtained in experiments with uniform salinity throughout the root zone. This usage results in a safe value that allows for relatively poor uniformity of water distribution in both time and space and may well be justified when water and energy are plentiful and drainage easily obtained. Recent findings (van Schilfgaarde et al., 1974) however, suggest that far lower LRs may suffice with precise water management. Based on extensive experiments with alfalfa by Bernstein and Francois (1973), it is hypothesized that the older data can be reinterpreted in the manner shown in Fig. 3. Extrapolating the yield reduction curves to zero yield leads to a value of EC beyond which roots are not able to extract water against the osmotic gradient. To convert the values in the figure from ECe to ECsw (from saturation to field water content) one must multiply ECe by the ratio qfc/qs, where qfc and qs are the volumetric water contents at field capacity and saturation. The EC thus obtained can be used in the denominator in Eq [9]. This interpretation of the data results in permissible salinity levels at the bottom of the root zone around 3 to 4 times those previously recommended. Whether or not the reasoning used here will stand the test of time in detail, there seems no question that far lower LRs than customarily recommended are entirely adequate, as long as proper management practices are used.
Fig. 3 - REINTERPRETATION OF CROP YIELD REDUCTION DATA AS A FUNCTION OF THE SALINITY OF THE SATURATION EXTRACT (ECe)
The importance of irrigation management, in this connection, has two aspects. First, if it is planned to operate an irrigation scheme at low leaching, it becomes especially important to obtain uniform water distribution over the field, because the margin of permissible error is far smaller; and it becomes desirable to maintain more nearly uniform conditions over time, because with long intervals between irrigation, the water and osmotic stress in the soil solution will build to excessively high levels. Secondly, in order to be able to apply water in quantities, or at rates, that are accurately enough known to assure a definite, but low, leaching fraction, the irrigation system must permit accurate control. Meeting both of these requirements is enhanced by frequent, low rate irrigation applications and especially, by means of pressurized systems.
When the rate of water application is less than the infiltrability - the potential rate of infiltration - the infiltration rate will be independent of soil properties. Even if water is applied periodically but frequently (say once a day), the fluctuations will be damped out at very shallow depth so that an essentially steady state condition prevails. Thus with frequent, low rate water applications, the control over water distribution in the soil passes from the soil to the irrigation system.
Raats (1974) has analysed a class of steady flows through soil profiles in the presence of plant roots. He solved the flow equation, Eq [1], for a k (h) distribution as in Eq [4], assuming that the rate of water uptake by the roots, could be described by
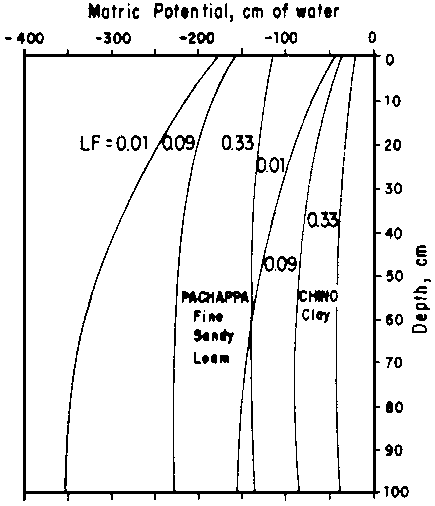
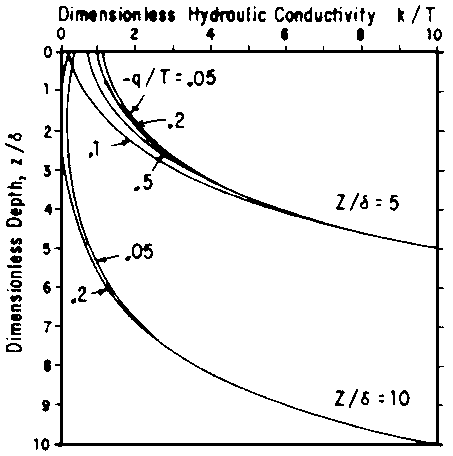
= (T/d) exp (-z/d), ..... [10]where T (cm/day) represents the total root uptake, or transpiration rate, and d (cm) is a characteristic length that corresponds to the depth above which 63% of the water is taken up. From this analysis, Fig. 4 was developed. It illustrates the point just made that, over a wide range of leaching fractions, the pressure head (or matric potential) does not vary much. The soil remains less than saturated and the pressure remains within the tensiometer range. Raats' analysis can also be used to evaluate the effect of a water table. In the limiting case without plant roots, the results reduce to Eqs [7] and [8], as they should. With roots present, his analysis leads to a relationship between k or h and z as a function of depth of water table, z, and the ratio q/T, representing the fraction of the water uptake supplied from the water table. This is illustrated in Fig. 5. Figure 6 further illustrates Raats' analysis by comparing various percentages of flow supplied from the water table. When - q/T = 1, all the plant need is satisfied from below; when it is zero, all is supplied from the surface. In this example, when the water table depth z = 10d, only 0.2 of the transpiration demand can be supplied from the water table. Furthermore, Raats interprets his analysis in terms of salt distribution, neglecting effects of precipitation or dissolution and of dispersion and diffusion. Accounting for These phenomena is a project now underway.
Fig. 4 - MATRIC POTENTIAL AS FUNCTION OF SOIL DEPTH FOR TWO SOILS AND THREE LEACHING FRACTIONS WITH T = 1 cm/DAY AND d = 15 cm (i.e., 91% OF WATER TAKEN UP ABOVE 36 cm)
RECLAMATION
Thus far we have considered maintaining a salt balance, or a quasi-steady state. Now consider the situation where a soil needs to be reclaimed, or where excessive salts are to be removed. The only reasonable way to accomplish reclamation seems to be by applying water to the surface for leaching, with or without chemical amendments, depending on soil chemistry.
The subject of amendments will not be discussed. Two other interrelated questions remain: the amounts of water to be applied and the manner of water application. Numerous field tests and detailed theoretical analyses have been reported in the literature. This experience verifies that leaching does not take place by simple piston flow displacement. If that were the case, one would need to apply only an amount equal to one pore volume displacement to obtain complete reclamation. Several factors cause the actual flow to be more complicated than simple displacement, including diffusion and variations in the localized flow velocities in the soil pores; they tend to reduce the leaching effectiveness. As suggested by Biggar and Nielsen (1967), leaching should be more efficient, in terms of amount of water needed, when it takes place at water contents below saturation.
Fig. 5 - HYDRAULIC CONDUCTIVITY AS A FUNCTION OF DEPTH FOR SOME UPWARD FLOWS. IN EACH CASE, THE WATER TABLE SUPPLIED 10% OF THE CROP TRANSPIRATION; THE CURVE PARAMETER INDICATES WATER TABLE DEPTH. TYPICAL VALUES FOR d MAY BE TAKEN AS d = 15 cm
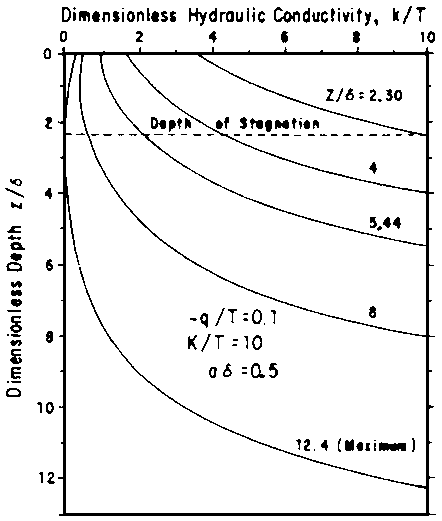
Fig. 6 - EFFECT ON HYDRAULIC CONDUCTIVITY DISTRIBUTION WITH VARYING IRRIGATION RATES (1 + q/T)T for K/T = 10 and da = 0.5. TYPICAL VALUES OF d are 10 and 20 cm
Comparing the results of several investigators, a relatively consistent picture emerges. Van der Molen (1956), in an excellent paper, adapted the theory of ion exchange columns to the removal of salt from soils and compared it to field observations in Holland after the seawater inundation during World War II. He showed that he could describe the changes over time of the chloride profiles very well with the error function relationship he derived. Similarly, Gardner and Brooks (1957) developed a somewhat different theory and compared it with data from laboratory column studies. In both cases quoted, the leaching took place at water contents less than saturation. Reeve et al. (1955), in a field experiment, compared the effect of different amounts of leaching water ponded on the surface, but since they applied the water in relatively small increments intermittently, they again were dealing with predominantly unsaturated flow.
Reeve et al. concluded that one unit depth of water was required per unit depth of soil to reduce total salinity by about 80%. [In their field experiments, adequate leaching of boron required substantially more water.] Since the volumetric water content, based on their data, was probably near 50%, this amounts to p = 2, where p is the number of pore volume displacements. Van der Molen's relationship requires that 50% of the choloride is removed when p = 1; assuming N = 10 in his expression (N = number of mixing layers), one obtains p » 1.9 for 80% salt removal. Gardner and Brooks' analysis yields, for 80% removal, about p = 1.5. Thus, for practical use, it seems entirely adequate to use Reeve et al's value p = 2, or their rule of thumb that one unit depth of water is required for every unit depth of soil to be reclaimed.
Oster et al. (1972) compared the effectiveness of continuous ponding, intermittent ponding and intermittent sprinkling for leaching under field conditions. They found that half as much water was required with intermittent flooding as with continuous flooding for the same reduction in salinity, with sprinkling intermediate. To reduce salinity by 70%, they applied 0.5 cm water per cm depth of soil on the intermittently flooded fields and 1.2 cm/cm on the continuously flooded.
As pointed out earlier, one reason for the greater effectiveness of intermittent or nonponding methods of leaching is the predominance of unsaturated flow and the consequent reduced “bypass”, or the “microgeometry” of flow. Another can be the “macrogeometry” of the flow pattern. Kirkham (1949) demonstrated that, for a ponded field with parallel underground drains, the surface infiltration rate near the drains is very much higher than near the centre between drains. Thus essentially no leaching takes place on a large section of the land, even if it is kept ponded for an extended period. Besides methods such as intermittent ponding or sprinkling, another possible approach to increasing the uniformity of infiltration is to modify the field surface geometry appropriately. Leaching by means of furrows or diked basins, if they are oriented parallel to the drains, should be highly advantageous.
Earlier reference was made to the leaching requirement. Boumans and van der Molen (1964) (see also van der Molen, 1973) have introduced a modification in determining the LR by considering that part of the water used for leaching bypasses the root zone without removing salt, the remainder being effective. Thus they introduce a leaching efficiency, f. When applied to the case of reclamation, or one-time salt removal, the use of the factor f has the same consequence as the use of a prescription based on displacement, such as p = 2. Although the physical models that led to the two methods may differ, the practical results are equivalent. I have serious reservations, however, about the suggestion, as interpreted by Bouwer (1969), that numeric values can be assigned to f based on soil texture. As pointed out by van der Molen (1973), f can be expected to depend significantly on the method of water application. More important is the need to distinguish between leaching for reclamation and leaching by over-irrigation.
When uncropped land is reclaimed by flooding or sprinkling, the theory of miscible displacement, verified in field and laboratory, predicts an efficiency well below 100%. The presence of root holes, cracks and animal burrows in the surface soil, combined with continuous large pores, also would lead one to predict substantial bypass in that superficial region. The use of an efficiency factor in such cases appears quite adequate for many practical situations. Even then, the example quoted above of the 2:1 ratio in water requirements for two methods of application indicates the need to take account of more than the soil texture in assigning a value to the efficiency.
On the other hand, when leaching is accomplished by continuous or intermittent over-irrigation of cropped land, an entirely different situation prevails. The average water content will be lower than in the previous case, and the activity of the roots should lead to substantially lower rates of downward flow. The extreme of piston displacement, by flooding, may now be contrasted with the opposite extreme under continuous trickle irrigation, of a steady state replenishment of the root zone water supply. While reclamation strives for deep percolation of 100% of the water applied, leaching for maintenance strives for, say 10%. I propose that, under such circumstances, the use of an efficiency factor is not warranted. Lysimeter, field plot and field-scale experience at our Laboratory tends to support this view.
The foregoing discussion has been devoted to physical and engineering aspects of water management for salt control. Aspects of soil and water chemistry have not been touched. The models of van der Molen and Raats, for example, can only be applied with integrity to simple systems with nonreacting salts such as NaCl. In principle, the concepts presented can also be applied to more complicated systems, containing, for example, carbonates. In general, such applications require numerical solution by computer. Other problems, such as boron removal, require different approaches. To keep this presentation within bounds, none of these will be discussed.
Suffice it to reiterate that the primary objective of water management for salinity control is to maintain a downward flux. Provision of adequate drainage together with precise irrigation management is the key to success.
ACKNOWLEDGMENT
The helpful cooperation of Dr. P.A.C. Raats, USSL, is gratefully acknowledged, both in formulating many of the ideas presented here and in preparing the material for the illustrations.
REFERENCES
Advisory Committee on Irrigation Efficiency. 1974. Measured for reducing return flows from the Wellton-Mohawk Irrigation and Drainage District. U.S. Dep. Interior Special Report. 109 p.
Bernstein, L. and Francois, L.E. 1973. Leaching requirement studies: Sensitivity of alfalfa to salinity of irrigation and drainage waters. Soil Sci. Soc. Amer. Proc. 37:931-943.
Biggar, J.W. and Nielsen, D.R. 1967. Miscible displacement and leaching phenomenon. In R.M. Hagan et al. (ed.) Irrigation of Agricultural Lands. Agronomy 11:254-274
Boumans, J.H. and van der Molen, W.H. 1964. Outwaterings-behoefte can bevloede gronden in verband met hun zouthuishouding. Landb. Tÿdschrift 76:880-887.
Bouwer, H. 1964. Unsaturated flow in groundwater hydraulics. ASCE Proc. 90(HY5):121-144.
Bouwer, H. 1969. Salt balance, irrigation efficiency and drainage design. ASCE Proc. 95(IRI): 153-170.
Doering, E.J. and Benz, L.C. 1972. Pumping an artesian source for water table control. ASCE Proc. 98(IR2):275-287.
Gardner, W.R. 1958. Some steady state solutions of the unsaturated moisture flow equation with application to evaporation from a water table. Soil Sci. 85:228-232.
Gardner, W.R. and Brooke, R.H. 1957. A descriptive theory of leaching. Soil Sci. 83:295-304.
Gardner, W.R. and Fireman, M. 1958. Laboratory studies of evaporation from soil columns in the presence of a water table. Soil Sci. 85: 244-249.
Kirkham, D. 1949. Flow of ponded water into drain tubes in soil overlying an impervious layer. Trans., Amer. Geophys. Union 30:369-385.
Kovda, V.A. 1961. Principles of the theory and practice of reclamation and utilization of saline soils in the arid zones. Proc. Teheran Symposium, Salinity Problems in the Arid Zones, Unesco, p.201-213.
Oster, J.D., Willardson, L.S. and Hoffman, G.J. 1972. Sprinkling and ponding techniques for reclaiming saline soils. Transactions of the ASAE, 15(6):1115-1117.
Raats, P.A.C. 1974. Steady flows of water and salt in uniform soil profiles with plant roots. Soil Sci. Soc. Amer. Proc. 38:717-722.
Raats, P.A.C. and Gardner, W.R. 1974. In Jan van Schilfgaarde (ed.) Drainage for Agriculture. Agronomy 17, p. 311-357.
Reeve, R.C., Pillsbury, A.F. and Wilcox, L.V. 1955. Reclamation of a saline and high boron soil in the Coachella Valley of California. Hilgardia 24:69-91.
Szabolcs, I., Darab, K. and Varallyay, C. 1969. Methods of predicting salinization and alkalinization processes due to irrigation on the Hungarian Plain. Proc. Yerevan Symposium, Reclamation of Sodic and Soda-Saline Soils, Agrokemia es Talajtan 18 (Suppl.), 351-376.
Talsma, T. 1963. The control of saline groundwater. Mededelingen Landbouwhogeschool, Wageningen, 63(10):1-68.
Talsma, T. 1966. The effect of soil physical conditions on reclamation of saline land. Sixth Congress ICID, New Delhi, 19.83-19.91.
United States Salinity Laboratory Staff. 1954. Diagnosis and improvement of saline and alkali soils. USDA Handbook 60. 160p.
Van der Molen, W.H. 1956. Desalinization of saline soils as a column process. Soil Sci. 81:19-27.
Van der Molen, W.H. 1973. Salt balance and leaching requirement. Chap. 9 in Drainage Principles and Applications, ILRI 16, Vol. II:59-102.
Van Schilfgaarde, J., Bernstein, L., Rhoades, J.D. and Rawlins, S.L. 1974. Irrigation management for salt control. J. Irrig. and Drainage Div., ASCE Proc. 100(IR3):321-338.
Varallyay, G. 1974. Unsaturated flow studies in layered soil profiles. Agrokemia es Talajtan 23(3-4):261-296.
Wind, G.P. 1955. A field experiment concerning capillary rise of moisture in a heavy clay soil. Netherlands J. Agr. Sci. 3:60-69.
Wind, G.P. 1961. Capillary rise and some applications of the theory of moisture movement in unsaturated soils. Inst. Land and Water Management Research, Tech. Bull. 22. 15 p.
1/ Contribution from the Agricultural Research Service, USDA, U.S. Salinity Laboratory, Riverside, California.
by
J.D. Rhoades and S.D. Merrill
Supervisory Soil Scientist and Physicist
U.S. Salinity Laboratory
1. INTRODUCTION
Numerous schemes have been proposed for classifying waters with respect to their suitabilities for irrigation. These have ranged from general schemes designed for average use conditions (USSL Staff, 1954; Rhoades and Bernstein, 1971; EPA, 1972) to specific schemes for restricted regional conditions (Thorne and Thorne, 1951; Handa, 1964). While some of These schemes may be useful for indicating the potentials of arbitrary waters for irrigation, the actual suitability of a given water for irrigation depends on the specific conditions of use. These specific conditions include the crop grown, various soil properties, irrigation management practices, especially leaching fraction (LF) (the fraction of infiltrated water that passes beyond the root zone), and frequency of irrigation, climatic conditions and certain cultural management practices.
The effects of irrigation frequency on water suitability have not been incorporated in any of the general water assessment schemes. Yet increasing irrigation frequency can markedly affect crop yield (Bernstein and Francois, 1973a; Mantell and Goldin, 1964), and one of the commonly used practical rules of salinity management is: when irrigating with saline waters, irrigate frequently. This recommendation implies that crop response is related to the sum of osmotic pressure, p, and matric suction, t, in the root zone and that this sum, Ø can be minimized by increasing the irrigation frequency. For a given quantity of salt in the soil, both p and t can be minimized by keeping the water content high by irrigating frequently. Wadleigh and Ayers (1945) and Richards and Wadleigh (1952) demonstrated that the effects of salinity and irrigation frequency on bean and “guayule” yield could be normalized by relating the crop response to the sum of p and t integrated over the root depth and the experimental period under conditions of general salinity where sodicity, nutrition and toxicity were negligible. Taylor (1952b) showed that alfalfa and sugar beet yields were significantly correlated with average seasonally integrated t with variable irrigation frequency. An increase of 1 atmosphere integrated t within the range 0.4 to 4 atmospheres reduced established alfalfa and sugar beet yield between 0.27 and 0,65 and 0.78 to 2.45 t/a/yr respectively. Although salinity was not included as a variable in this study, Taylor suggested that the integrated p could be obtained by measuring the p of the soil water as a function of time and depth and water content and that it could be added to t before integration. Yaron et al. (1972), Bresler and Yaron (1972), and Zur and Bresler (1973) have evaluated the interactions of irrigation regime, level of soil salinity, water and climatic conditions, absence of leaching and short-term use of variably salinized irrigation waters on grapefruit and groundnut yields by both statistical and computer simulation techniques. They concluded that, for non-steady state, short-term conditions and a given level of climatic stress, p is overwhelmingly dominant on fruit yield of these crops with condition of short irrigation intervals (3 days). For such intervals, the irrigated t was only 10 to 15 percent of the integrated Ø at longer irrigation intervals (about 20 to 30 days). They also found that irrigation water quality and initial level of soil salinity became less important as compared with t, on time-integrated Ø, as the irrigation interval increased becoming nearly negligible at the longest irrigation intervals. The integrated p achieved nearly a constant value - irrespective of the irrigation interval. From these observations they concluded that the pre-irrigation salt concentration of the soil water mainly determines the value of the time-integrated p under conditions of short-term irrigation with saline water without leaching. For this reason, they prescribed using an extra allotment to pre-leach and reduce the soil salinity level at the beginning of the crop season, rather than using such water for leaching during the irrigation season.
Under conditions of long-term use of waters for irrigation (steady state conditions)
with leaching, the interaction of salt concentration of the irrigation water
and the leaching fraction determines the level of soil salinity, as well as
the depth-integrated p, of the root zone soil
water. This latter conclusion can be deduced from the equation developed by
Bernstein and Francois (1973b) to describe the mean salinity against which water
is absorbed by the plant, 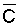 ,
,
where Viw, Vdw are volume of infiltrated and drainage water, respectively, and Ciw is the concentration of the irrigation water. Equation [1] applies only to the condition of conservation of mass, i.e., CiwViw = CdwVdw where Cdw is the concentration of drain water. Raats (1974) has shown that
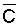 is
independent of the water uptake profile, for conditions of piston displacement,
but not where dispersion and diffusion affects the distribution of salinity in
the root zone appreciably. Under the assumption of piston flow,
is
independent of the water uptake profile, for conditions of piston displacement,
but not where dispersion and diffusion affects the distribution of salinity in
the root zone appreciably. Under the assumption of piston flow, 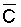 is also independent of frequency and time, because it is only the relation between
concentration and volume during evapotranspiration that affects
is also independent of frequency and time, because it is only the relation between
concentration and volume during evapotranspiration that affects 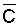 as the unit volume of applied water passes through the root zone. The degree of
volume reduction and concentration increase during this passage is determined
by the leaching fraction and in independent of time or the extent to which the
soil is dried between irrigations. This agrees with the observational and model
findings of Zur and Bresler (1973). Since concentration and osmotic potential
are closely related, Eq [1] can also be used to calculate p
weighted in proportion to water uptake. Ingvalson et al. (in press)
have modified Eq [1] to account for the effects of salt precipitation and dissolution.
as the unit volume of applied water passes through the root zone. The degree of
volume reduction and concentration increase during this passage is determined
by the leaching fraction and in independent of time or the extent to which the
soil is dried between irrigations. This agrees with the observational and model
findings of Zur and Bresler (1973). Since concentration and osmotic potential
are closely related, Eq [1] can also be used to calculate p
weighted in proportion to water uptake. Ingvalson et al. (in press)
have modified Eq [1] to account for the effects of salt precipitation and dissolution.
where a, b, and c are constants of the second-order polynomial equation describing the concentration of the water as a function of
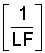 derived from the chemical model of Oster and Rhoades (1975). Thus, the water uptake
weighted p,
derived from the chemical model of Oster and Rhoades (1975). Thus, the water uptake
weighted p, 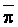 , may be estimated
as a function of Ciw and LF.
, may be estimated
as a function of Ciw and LF.
Since 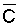 is more strongly a function
of Ciw than of LF, Bernstein and Francois (1973) concluded that crop
growth is very sensitive to ECiw and that high salinity levels in
the lower depths of the root zone have little effect on yield. This conclusion
overlooked the approximate additivity of effect that t
and p may have on crop response. In the case of
negligible t, like under conditions of high frequency
or trickle irrigation regimes,
is more strongly a function
of Ciw than of LF, Bernstein and Francois (1973) concluded that crop
growth is very sensitive to ECiw and that high salinity levels in
the lower depths of the root zone have little effect on yield. This conclusion
overlooked the approximate additivity of effect that t
and p may have on crop response. In the case of
negligible t, like under conditions of high frequency
or trickle irrigation regimes, 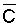 is
probably a better index of salinity for evaluating expected crop response than
under conditions of infrequent irrigation. As reviewed by Slatyer (1969) and
Rawlins and Raats (1975), time of exposure to salinity or salinity exceeding
some “critical” value may also affect crop response. Correlations
have been observed between such total water potential “stress days”
and crop yields. Such time of exposure to salinity stress is also ignored in
Eqs [1] or [2]. Bower et al. (1969, 1970) concluded that crop
response is primarily to average root zone salinity. Ingvalson et al.
(in press) correlated alfalfa yield obtained under conditions of non-uniform
root zone salinity to various indices of salinity including (i) irrigation water
salinities, (ii) space averaged, soil profile salinities, (iii) soil water salinities
weighted in accordance with water uptake by the crop, and (iv) time and space
integrated soil water salinities. The expressed reason for the latter experiment
was that “the assessment of irrigation water quality and the adoption of
appropriate irrigation management procedures require an adequate knowledge of
how crops respond to non-uniform soil salinity”. In this experiment alfalfa
yield correlated best with lower root zone depth, time-integrated soil water
EC (r = 0.89), although yield also correlated reasonably well with average root
zone soil salinity (r = 0.78) and
is
probably a better index of salinity for evaluating expected crop response than
under conditions of infrequent irrigation. As reviewed by Slatyer (1969) and
Rawlins and Raats (1975), time of exposure to salinity or salinity exceeding
some “critical” value may also affect crop response. Correlations
have been observed between such total water potential “stress days”
and crop yields. Such time of exposure to salinity stress is also ignored in
Eqs [1] or [2]. Bower et al. (1969, 1970) concluded that crop
response is primarily to average root zone salinity. Ingvalson et al.
(in press) correlated alfalfa yield obtained under conditions of non-uniform
root zone salinity to various indices of salinity including (i) irrigation water
salinities, (ii) space averaged, soil profile salinities, (iii) soil water salinities
weighted in accordance with water uptake by the crop, and (iv) time and space
integrated soil water salinities. The expressed reason for the latter experiment
was that “the assessment of irrigation water quality and the adoption of
appropriate irrigation management procedures require an adequate knowledge of
how crops respond to non-uniform soil salinity”. In this experiment alfalfa
yield correlated best with lower root zone depth, time-integrated soil water
EC (r = 0.89), although yield also correlated reasonably well with average root
zone soil salinity (r = 0.78) and 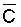 (r = 0.71). The chemistry model used to determine
(r = 0.71). The chemistry model used to determine 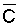 in this latter study was previously tested with respect to its ability to adequately
predict the drainage water composition of these same lysimeters resulting from
the use of eight widely varying waters for irrigation and leaching fractions
of 0.1, 0.2 and 0.3 (Oster and Rhoades, 1975). The predicted compositions and
salt loads agreed very closely with those determined experimentally (Rhoades
et al., 1973, 1974).
in this latter study was previously tested with respect to its ability to adequately
predict the drainage water composition of these same lysimeters resulting from
the use of eight widely varying waters for irrigation and leaching fractions
of 0.1, 0.2 and 0.3 (Oster and Rhoades, 1975). The predicted compositions and
salt loads agreed very closely with those determined experimentally (Rhoades
et al., 1973, 1974).
The ultimate method of assessing the suitability of waters for irrigation will require the attainment of our capability to: 1) predict the composition, the osmotic and matric potentials of the soil water and the corresponding exchangeable cation composition of the soil, both in time and space, resulting from the use of any arbitrary irrigation water under any given soil-crop-water management system and climatic conditions, and 2) interpret such information in terms of effect on crop response. The need for such an approach to assessing the suitabilities of waters for irrigation rather than the presently used tables of empirical values assumed to describe water suitability under hypothetical average-use conditions has been previously discussed (Rhoades, 1972). Obviously such a task would be very complex. Even if we could satisfy number 1, we doubt that our present knowledge would enable us to accomplish number 2. We especially do not understand the mechanisms by which salinity (as indicated in the preceding discussion), sodicity or toxicity affect crop response nor can we accurately predict, on the basis of any developed theory, the changes in soil hydraulic conductivity under field conditions, as affected by water and soil properties. Yet, there seems room for improvement of past conventional schemes of water assessment based on generalized empirical findings that have often proven insufficient in practice.
Because of the reasonably good correlations obtained between alfalfa yield
and average root zone salinity and 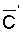 ,
and because of the ability of the chemistry model to predict either of the latter
two indices of salinity, apparently such a chemical model could serve as a basis
for assessing the suitabilities of waters for irrigation. It would be even better
if the model could be modified to predict soil water salinity compositions throughout
the root zone and if the effect of t and irrigation
frequency could somehow be incorporated with p
to estimate an adequate total water potential index of the crop root zone with
which to predict crop response.
,
and because of the ability of the chemistry model to predict either of the latter
two indices of salinity, apparently such a chemical model could serve as a basis
for assessing the suitabilities of waters for irrigation. It would be even better
if the model could be modified to predict soil water salinity compositions throughout
the root zone and if the effect of t and irrigation
frequency could somehow be incorporated with p
to estimate an adequate total water potential index of the crop root zone with
which to predict crop response.
With the above in mind, a water quality assessment model was developed and the concepts and utility of this model are discussed here. The chemistry part of the model will be recommended for adoption to aid in assessing sodicity hazards of irrigation waters and in the interim, (until a verified model capable of predicting the time and space varying total soil water potential is developed and information gained on how to quantitatively relate this to crop response) to aid in assessing salinity hazards of irrigation waters. The matric and total stress parts of the model are crude but do qualitatively demonstrate the effects of irrigation frequency, soil water retentivity characteristics, time leaching fraction and irrigation water salinity on time and space-integrated soil water potential. It also gives evidence to support our recommended method for assessing the salinity hazards of irrigation waters.
An improved model of this type, but more capable of calculating what this model can only approximate, is needed to advance our present, limited ability to assess water suitability for irrigation. For these reasons the matric and total potential parts of this model are included even though these parts of the model are not recommended for adoption in their present form.
2. CRITERIA FOR ASSESSING IRRIGATION WATER SUITABILITY
Criteria for assessing irrigation water suitability must include those chemicals physical and biological characteristics of soils, waters and crops which must be quantified and may have to be controlled for specific uses of irrigation waters. The suitability of waters for irrigation should be evaluated on the basis of criteria indicative of their potentials to create soil conditions hazardous to crop growth or crop use.
Besides the factors already discussed, many additional factors affect water suitability for irrigation. In this treatise we limit ourselves to salinity and sodicity hazards associated with water use for irrigation. Salinity is defined as the general effects of salts on crop growth thought to be largely osmotic in nature and related more to total salt concentration than to specific salt species. Sodicity is defined as the effect of an excessive amount of exchangeable sodium in the soil on soil permeability and structure deterioration, and a direct toxic effect of exchangeable sodium on plants specifically sensitive to sodium. The variables that affect water suitability for irrigation considered herein are: chemical composition of irrigation water, leaching fraction, irrigation frequency, soil water retentivity characteristics, crop tolerances to p, t and Ø, and allowable limits of EC-SAR with respect to soil permeability.
3. DESCRIPTIONS AND ASSUMPTIONS OF THE WATER SUITABILITY MODEL
3.1 Predicting Soil Chemistry Distribution
The chemistry model developed by Oster and McNeal (1971) and modified by Oster and Rhoades (1975) was used to estimate the distribution of salinity and sodicity in the soil profile based on an assumed water uptake pattern and a CO2 distribution in the crop root zone as described later. Electrical conductivity of the soil water, ECsw, or of the saturation paste extract, ECe, will be used as the index of salinity and the sodium-adsorption-ratio, SAR,1/ will be used as the index of sodicity. Briefly, the model calculates the resultant equilibrium chemical compositions of waters assumed to be concentrated from their initial compositions by the factor (1/LFa) appropriate for a given fractional interval of the root zone. The pH of the solution is assumed to be governed by soil carbonate equilibria. Soil CaCO3 is assumed to be present in sufficient quantity to saturate the soil solution before concentration begins. The model calculates equilibrium solute activities using the Debye-Hückel theory, taking into account ionic strength and ion-pair formation constants, and precipitation of CaCO3, MgCO3 and CaSO4 by iteration processes so that the final composition meets all the simultaneous equilibrium constants of the considered solid phases, ion-pairs and carbonate species for an assigned partial pressure of CO2. Briefly the iterative process involves repeated calculation of: (i) the ionic strength of the solution; (ii) single ion-activity coefficients; (iii) the molar concentrations of all 16 ion pairs and appropriate calculated ion activities, and (iv) the molar ion concentration of single ion species by difference between the analytical concentrations and the sum of their ion-pair concentrations. SAR is calculated from the total soluble concentrations of Na, Ca and Mg left in solution, including those in ion-pair forms, after the equilibrium composition is obtained. The electrical conductivity (EC) of the solution is then calculated by the third-order polynomial fit method (No. 2) of McNeal et al. (1970).
1/The input requirements for the model as used here include irrigation water concentrations of Ca, Mg, Na, K, CO3 + HCO3, Cl and sulphate, and, where the concentrations of Na, Ca and Mg are expressed in meq/litre.
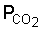 and
leaching fractions by depth through the profile, LFa. Leaching fractions
at any depth in the a root zone are calculated from known or estimated water uptake
patterns through the root zone from
and
leaching fractions by depth through the profile, LFa. Leaching fractions
at any depth in the a root zone are calculated from known or estimated water uptake
patterns through the root zone from
where f Vcu is that part of the total volume of consumptive use taken up above the depth in question. From an examination of the water uptake patterns of crops, we chose to use the values 40, 30, 20, 10, except as noted, as representative general percentages of total water uptake by fractional quarters of the root zone for use in Eq [3]. Such uptake patterns are affected by irrigation frequency and leaching fraction, as shown by Mantell and Goldin (1964) and Ingvalson et al. (in press). However, results obtained with the model using reasonable but different water uptake patterns show that variations in water uptake distribution do not appreciably change the resultant salinity (EC) or sodicity (SAR) profiles. Furthermore, as discussed earlier, water uptake pattern does not appreciably influence
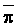 , unless dispersion and diffusion effects
are marked. Soil air
, unless dispersion and diffusion effects
are marked. Soil air 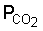 levels of 1.6,
3.3, 4.6 and 5.3 aim were used in the model, except as noted, for successively
deeper quarterly fractions of the root zone. Deviations from these
levels of 1.6,
3.3, 4.6 and 5.3 aim were used in the model, except as noted, for successively
deeper quarterly fractions of the root zone. Deviations from these 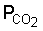 values are not expected to alter appreciably calculated EC and SAR values for
most water compositional types. Soil water composition was calculated at quarter
points in the root zone.
values are not expected to alter appreciably calculated EC and SAR values for
most water compositional types. Soil water composition was calculated at quarter
points in the root zone.
From the steady state water compositions through the crop root zone we calculate:
1) 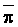 by the method discussed earlier
(Ingvalson et al., in press) using Eq [2] or by summing the products
of water uptake proportion and p for each depth increment and dividing by the sum of
the fractional water uptake in the root zone, using the calculation procedure
described later in Section 3.2, and 2) the various EC-SAR combinations through
the root zone. Comparisons of such latter combinations with the threshold EC-SAR
values of Quirk and Schofield (1955) or McNeal and Coleman (1965a), etc., or
preferably from known values established for the soil in question allow the
sodicity hazard of the water to be evaluated for the specific leaching fraction.
While not discussed here, other parameters of chemical composition can also
be predicted for suitability evaluation with this model, like Cl concentration,
Ca/Mg ratio, etc. (Oster and Rhoades, 1975).
by the method discussed earlier
(Ingvalson et al., in press) using Eq [2] or by summing the products
of water uptake proportion and p for each depth increment and dividing by the sum of
the fractional water uptake in the root zone, using the calculation procedure
described later in Section 3.2, and 2) the various EC-SAR combinations through
the root zone. Comparisons of such latter combinations with the threshold EC-SAR
values of Quirk and Schofield (1955) or McNeal and Coleman (1965a), etc., or
preferably from known values established for the soil in question allow the
sodicity hazard of the water to be evaluated for the specific leaching fraction.
While not discussed here, other parameters of chemical composition can also
be predicted for suitability evaluation with this model, like Cl concentration,
Ca/Mg ratio, etc. (Oster and Rhoades, 1975).
To test the applicability of this predictive chemistry part of our water quality model for the above-stated purposes, predicted and determined values of EC and SAR were compared where eight widely different waters (Table 1) were used to irrigate and alfalfa crop in soil filled lysimeters at leaching fractions of 0.1, 0.2 and 0.3. The experimental set-up has been previously described (Rhoades et al., 1973). Briefly, alfalfa (Medicago sativa L., var. Moapa) was grown in 36 outdoor lysimeters filled with Pachappa fine, sandy loam soil. The seeds were germinated and the plants were established while receiving small irrigations of deionized water each day for about 3 weeks. The lysimeters of one set of treatments (eight irrigation waters with three LFs) contained non-calcareous Pachappa soil, while those of the other set of treatments contained Pachappa soil to which CaCO3 was added to total 1% by weight. Salt sensors were installed at the 40 and 80 cm depths to monitor in situ soil salinity (Oster and Ingvalson, 1967). Acquarium airstones were installed at 40 and 80 cm depths to withdraw samples of soil air for CO2 determinations. Each lysimeter was irrigated individually when a tensiometer at 60 cm depth reached 0.5 bar. The water was flooded on the surface in approximately 20 litre increments and in a total amount which would achieve the target LF. Approximately 3 to 5 days were required, depending on the LF, to finish an irrigation. When it rained, the lysimeters were covered with sheet plastic. Upon completion of this experiment, soil samples were taken from the lysimeters at 30 cm intervals for analysis of soluble salts and exchangeable cations.
Table 1 - COMPOSITIONS OF RIVER WATERS USED FOR IRRIGATION OF ALFALFA
|
River |
EC1/ |
Ca |
Mg |
Na |
K |
cations |
HCO3 |
SO4 |
Cl |
SAR2/ |
|
Water type |
|
mmho/cm |
meq/liter |
|||||||||||
|
Feather |
0.10 |
0.45 |
0.36 |
0.20 |
0.04 |
1.05 |
0.86 |
0.16 |
0.03 |
0.3 |
8.6 |
Ca, Mg-HCO3 |
|
Grand |
0.94 |
2.00 |
0.79 |
7.08 |
0.19 |
10.06 |
6.29 |
3.43 |
0.34 |
6.0 |
7.3 |
Na-HCO3 |
|
Missouri |
0.91 |
4.06 |
1.92 |
3.02 |
0.10 |
9.10 |
3.24 |
4.05 |
1.81 |
1.8 |
7.3 |
mixed |
|
Salt |
1.56 |
3.15 |
1.35 |
9.62 |
0.17 |
14.29 |
3.21 |
0.89 |
10.19 |
6.4 |
7.5 |
Na-Cl |
|
Colorado |
1.27 |
6.95 |
3.63 |
3.35 |
0.22 |
14.15 |
3.73 |
9.31 |
1.11 |
1.5 |
7.0 |
Ca-SO4 |
|
Sevier |
2.03 |
3.71 |
6.05 |
10.62 |
0.15 |
20.53 |
5.21 |
5.96 |
9.36 |
4.8 |
6.9 |
Na > Mg > Ca-Cl |
|
Gila |
3.14 |
7.22 |
5.88 |
18.55 |
0.09 |
31.74 |
3.17 |
8.48 |
20.09 |
7.3 |
7.1 |
Na-Cl |
|
Pecos |
3.26 |
16.98 |
9.07 |
11.38 |
0.08 |
37.51 |
3.11 |
22.39 |
12.01 |
3.2 |
6.8 |
Ca-SO4 |
1/ Electrical conductivity.The water uptake pattern with depth wan determined from the soil profile Cl distribution (Table 2; Ingvalson et al., in press). The LF achieved past each increment in the profile was calculated from the ratio Cliw/Clsw. Here Clsw was calculated from Cle x (qe/qfc), where, e, fc, and refer to saturation extract, field capacity and gravimetric water content, respectively. Cle and qe were obtained from the soil analyses; qfc was obtained by sampling the lysimeters for gravimetric water content by depth 2 days after irrigation was stopped. The fraction of water consumed at any depth in the profile was obtained from (1 - LFa)/(1 - LF total), where LF total represents the LF obtained at the bottom of the root zone or soil profile as determined by Cliw/Cldw. The fractional water uptake for each soil profile interval (f) was then obtained as the difference in water consumed by appropriate successive depths in the root zone.2/
, where all concentrations are expressed in meq/l.
3/
, where p(Ca + Mg) and pAlk are the negative logarithms of the molar concentration of Ca + Mg and of the equivalent concentration of titratable base (CO3 + HCO3, respectively) and
and
are the respective logarithms of the second dissociation constant of H2CO3 and the solubility constant of calcite, respectively, both corrected for ionic strength (Bower et al., 1965).
Table 2 - DETERMINED LEACHING FRACTION, LFa, AND CARBON DIOXIDE LEVELS BY FRACTIONAL ROOT ZONE IN ALFALFA LYSIMETER EXPERIMENT
|
Fractional depth of root zone |
CO2 |
LFa |
||
|
% |
for leaching |
fraction |
treatment |
|
|
.1 |
.2 |
.3. |
||
|
1/4 |
3 |
.70 |
.74 |
.75 |
|
1/2 |
6 |
.49 |
.63 |
.70 |
|
3/4 |
9 |
.22 |
.37 |
.48 |
|
4/4 |
12 |
.13 |
.22 |
.32 |
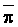 as a function of LF can be estimated as previously discussed. This latter value
is valuable in assessing the salinity hazard under conditions of low t.
It has less applicability for conditions of high t.
as a function of LF can be estimated as previously discussed. This latter value
is valuable in assessing the salinity hazard under conditions of low t.
It has less applicability for conditions of high t.
Fig. 1 - RELATION BETWEEN OBSERVED AND PREDICTED ECe
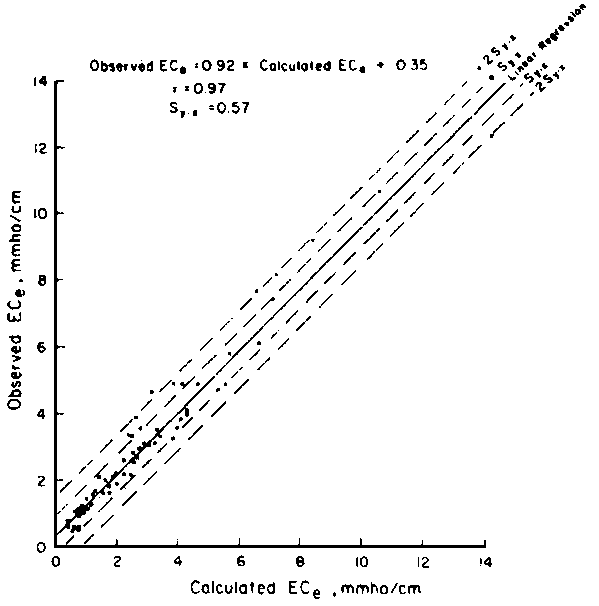
Fig. 2 - RELATION BETWEEN OBSERVED SARe and predicted SARsw
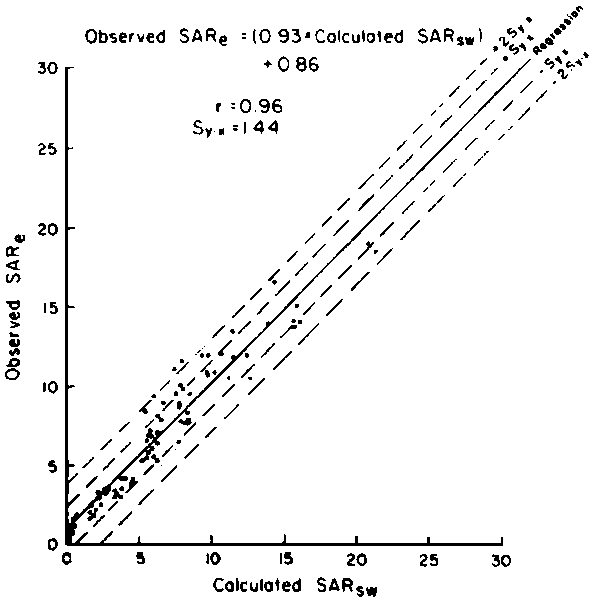
Fig. 3 - RELATION BETWEEN OBSERVED AND PREDICTED AVERAGE PROFILE ECe
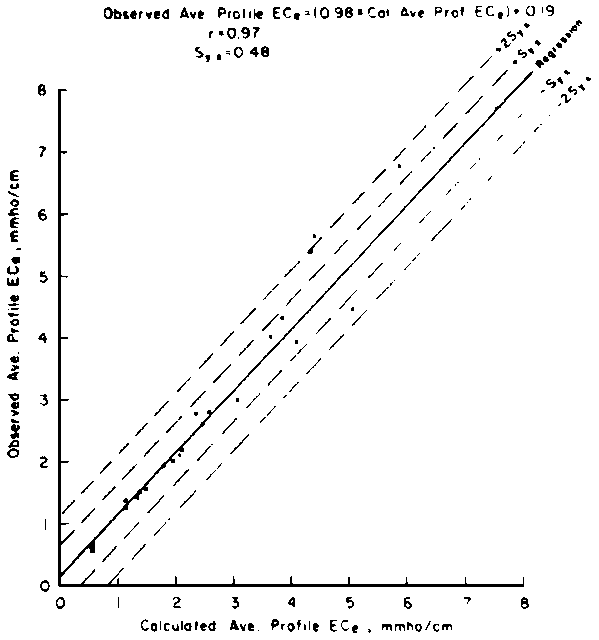
Fig. 4 - OBSERVED RELATIONS BETWEEN ECe AND SARe IN THE LYSIMETER SOIL PROFILES
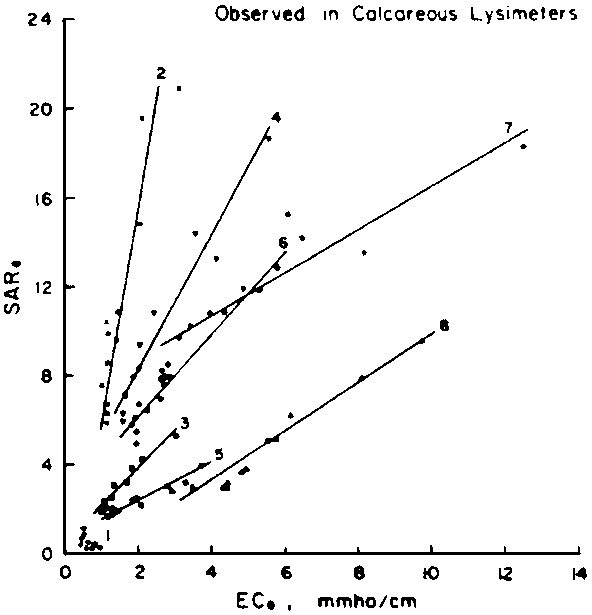
Fig. 5 - PREDICTED RELATIONS BETWEEN ECsw AND SARsw IN THE LYSIMETER SOIL PROFILES AND THRESHOLD VALUES ACCORDING TO QUIRK AND SCHOFIELD (1955), ANY COMBINATION OF SAR AND EC TO THE RIGHT OF THE “THRESHOLD” LIKE MOULD NOT BE EXPECTED TO RESULT IN SIGNIFICANTLY REDUCED HYDRAULIC CONDUCTIVITIES FOR SOILS LIKE THAT USED BY QUIRK AND SCHOFIELD
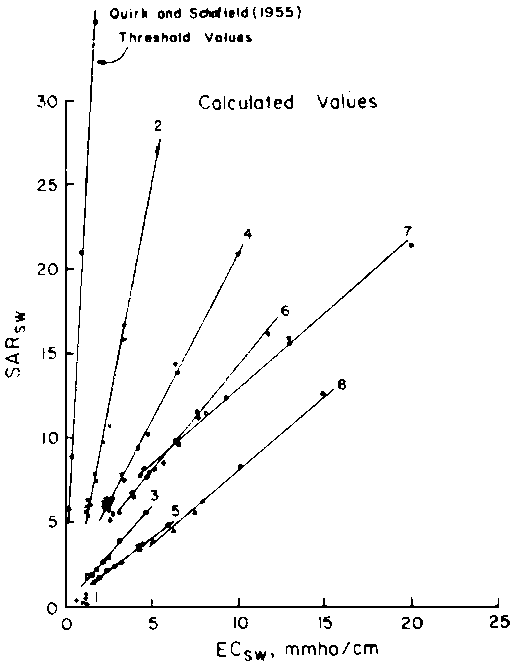
Fig. 6 - EFFECT OF LF ON ECsw DISTRIBUTION WITH DEPTH IN THE ROOT ZONE USING GILA RIVER MATER FOR IRRIGATION
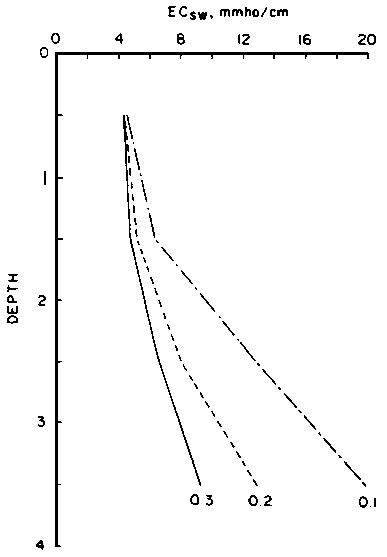
3.2 Predicting the Matric and Total Stress of the Soil Water
The degree of water depletion between irrigations and the soil water retentivity characteristics are assumed to be the dominating factors affecting t in irrigated soils. For this reason we developed a first approximation method of incorporating soil water retentivity effects on t with p to aid in the assessment of water suitability.
The calculations pertain to a crop, assumed to be at a constant rate of evapotranspiration, and for which the leaching fraction and soil salinity profile averaged over the irrigation cycle are at steady values. The purpose, then, of the calculations is to calculate osmotic and matric stresses in the crop root zone. The assumptions are:
1) At the end of an irrigation cycle, the total water stress, Øf, is at the same level throughout the root zone. Under conditions where the crop is extracting water from soil storage against significant osmotic and matric stresses simultaneously, Wadleigh et al. (1947) and Rawlins et al. (unpublished data, U.S. Salinity Laboratory) found that the total stress at the end of the irrigation cycle tends to be equal throughout the root zone.By the use of assumption No. 1, and the simple formulae given below, one avoids specifying the uptake pattern at q below the field capacity level, although when the calculations are completed, a new uptake pattern is given. The calculations proceed then as follows: the root zone is divided into 10 equal increments (we used a value of 10 cm for an increment, i, in our calculations). Next, one assumes a final total stress value, Øf. The greater the value of Øf1/ chosen, the longer will be the length of the irrigation cycle. This qf is, of course, equal to pfi where tfi (f.c. denotes field capacity). The components of Dpi and Dti, along with DØi, are given by:2) After an irrigation, the root zone is assumed to come to a “field capacity” water content, qfc, and vertical water flows are assumed to end for all practical purposes.
3) The salinity profile of the root zone at “field capacity” is, as a first approximation, taken as that calculated by the equilibrium soil chemistry model (discussed above) assuming uptake fractions of 0.4, 0.3, 0.2, 0.1 for the four quarters of the root zone in descending depths. The reader who wishes to improve this illustrative calculation scheme could well take the final osmotic pressure value pfc calculated by the model, and using the simplified salt flow model of Bresler (1967) arrive at a better field capacity profile, ps, (s denotes parameters at “field capacity”, f denotes parameters at the end of the irrigation cycle).
4) The water retentivity characteristics of the soil are represented as by Gardner et al. (1970), i.e., t = Aq-a.
DØi = Øf - (psi + tf.c.) = Dpi + Dti .....[4]and
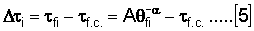
Dpi = pfi - psi = F [ECsi qf.c./qfi] - psi ..... [6]where 0,98 F = -0.0740 - 0.3470 EC + 0.0023 EC2 based on the data of Campbell et al. J. (1948). The only unknowns are qfi; they are obtained by iteration for each depth increment.
1/ If an Øf value that is less than some of the pfi values in the lower root zone is chosen, Øfi values for points i where Øf < (psi - tf.c.) are used to calculate uptake so that the differences (Øfi - psi) are in proportion to the distance from the bottom of the root zone, with the last (Øfn - psn) at the bottom “mesh” of the root zone set at some arbitrarily small value, of course, for points i where Øf > (psi + tf.c.), Øf is used as the final, total potential, adhering to the basic, generating assumption of the model (assumption no. 1).Once these calculations were made and values for each depth increment i obtained, several subsequent calculations and evaluations were made. These were as follows:
i. From the Dqi = qf.c. - qfi values, we estimated a time over which the crop is extracting water below qf.c. as4. INTERPRETATIONS AND USES OF WATER SUITABILITY CALCULATIONSDTu = DzSDqi/Et ..... [7]In our calculations, we used a value of 0.5 cm/day for the evapotranspiration, Et. Further, one can estimate the entire length of the irrigation cycle, DTt + DTu + DTp by using an approximate value of DTp, the time for irrigation water infiltration and redistribution to qf.c.. We chose a value of 5 days for DTp which we use for all irrigation waters and leaching fractions, based on experience with Pachappa f.s.l. soil (A = 2.53, a = 2.79) and some calculations using Gardner et al. (1970) redistribution formulae.ii. We prepared tables of times DTt for different chosen Øf values and water qualities at leaching fractions of 0.1, 0.2 and 0.3. This data makes apparent the number of days when the total stress is above some arbitrarily “critical” level, at which crop growth may be assumed to have ceased. With this part of the model, the effect of duration of stress is taken into account in the assessment, by comparing “stress days” for irrigation intervals of equal lengths.
iii. We calculated an “uptake weighted osmotic potential”,
, from the psi and the assumed uptake pattern at qf.c. as used in the soil chemistry model. This gives a volume integrated
, which, as discussed above, strictly applies to a system of absolute “piston displacement” water flow with no molecular diffusion.
iv. A root uptake weighted matric pressure,
, was calculated by
where t = Aqa 1/; thus
where the depth increments are of equal size.
1/ a = -a used by Gardner et al. (1970).
4.1 Sodicity Hazard Evaluation
There are two separate effects of sodium that must be considered in assessing water quality. The first is the effect of excessive sodium on soil permeability, infiltration and soil structure deterioration. The other is the direct effect of exchangeable sodium on plants specifically sensitive to sodium. The chemistry part of our water quality model can be used to evaluate both of these considerations.
The EC-SAR combinations predicted with the model for the eight irrigation waters, whose compositions are given in Table 1, for all combinations of profile depths and leaching fractions (0.1, 0.2 and 0.3) are given in Fig. 5. These data are summary forms of individual plots of EC and SAR like those of Figs. 6 and 7 for the Gila River. These EC-SAR combinations can be compared to determine if “threshold” values of SAR are exceeded either with respect to the soil hydraulic conductivity or ESP2/ tolerance of the crop. An example of such critical values for permeability is given in Fig. 5 according to the limits recommended by Quirk and Schofield (1955). In all instances, the EC-SAR values lie to the right of these limits indicating that no permeability problems would be expected with the use of these waters under these conditions of LF and soil type.
2/ ESP refers to exchangeable sodium percentage of the soils' cation exchange capacity. Since the SAR of the soil water is a good estimate of the ESP of soils, it can be used advantageously in place of ESP for diagnosing sodicity problems (USSL Staff, 1954).
Fig. 7 - EFFECT OF LF ON SARsw DISTRIBUTION WITH DEPTH IN THE ROOT ZONE USING GILA RIVER WATER FOR IRRIGATION
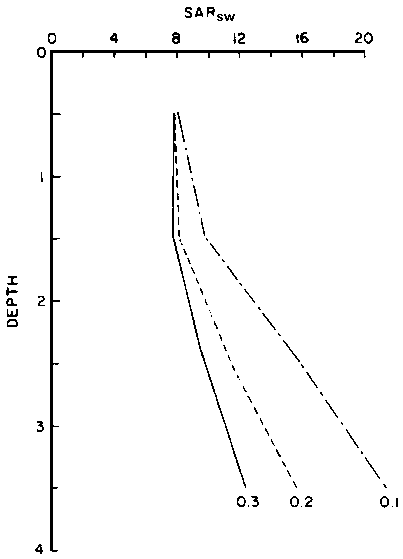
Fig. 8 - RELATIONSHIP BETWEEN 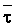 AND SET Øf VALUES CORRECTED AND UNCORRECTED FOR WATER UPTAKE
DURING INFILTRATION AND DRAINAGE TO “FIELD CAPACITY”, SEVIER RIVER,
LF = 0.2
AND SET Øf VALUES CORRECTED AND UNCORRECTED FOR WATER UPTAKE
DURING INFILTRATION AND DRAINAGE TO “FIELD CAPACITY”, SEVIER RIVER,
LF = 0.2
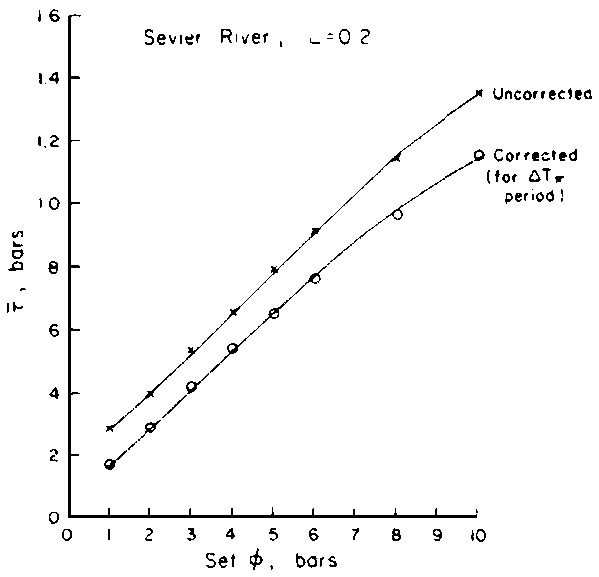
To complete the sodicity hazard evaluation, the question of effect of excessive exchangeable sodium, presuming the absence of poor structural problems, on crop yield, must also be considered. Tolerance of crops to sodicity under non-saline conditions varies widely (Pearson and Bernstein, 1958; Bernstein and Pearson, 1956; Pearson, 1960). The most sensitive crops (for example, beans) are affected at ESP levels of about 10. Most crops are moderately tolerant and are affected at ESPs of about 25. Highly tolerant crops, like tall wheatgrass, are not affected until ESPs reach or exceed 50. Tolerance reflects the species' ability to absorb nutritionally adequate levels of Ca and Mg and K from low concentrations of these elements in the soil solution.
Since the SAR distribution in the root zone, resulting from the use of a given water for irrigation and leaching fraction, can be predicted with the chemistry model (Fig. 7), it can be used to evaluate the likelihood of reduced crop yields because of excessive exchangeable sodium in the soil. Predicted ESP levels are compared with established crop tolerances to ESP for this evaluation. If exchangeable sodium is excessive for crop yield, possibly the ESP level associated with the use of any irrigation water can be reduced by increasing the LF. For such reasons, the concept of leaching requirement for exchangeable sodium control was introduced previously (Rhoades, 1968). The data of Fig. 7 illustrate the point. By increasing the LF from 0.1 to 0,3, the lower profile ESP level associated with the steady state use of Gila River water for irrigation can be reduced from 21 to 12. The surface ESP, however, is largely insensitive to LF, being only reduced from 8.1 to 7.7 for the same change in LF.
The chemistry model is of additional value in assessing the sodicity hazard because it can predict the concentrations and distributions of Ca and Mg as well as SAR (data not shown). This is important because whether a sodic soil condition upsets crop nutrition depends on the total salt concentration (Bernstein, 1974). As the total concentration increases into the saline range (> 4 mmho/cm ECe), even high ESPs are associated with nutritionally adequate levels of Ca and Mg (> 2 meq/l), and the nutritional levels of high exchangeable sodium decrease or even disappear Lagerwerff and Holland, 1960). The question of how crops are affected by non-uniform distributions of ESP in the root zone is not known; such information is needed before the sodicity hazard can be properly assessed.
4.2 Salinity Hazard Evaluation
Representative summary results of 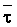 ,
,
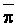 ,
, 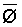 and SDq1 are given in Table 4 for the case of Pachappa
f.s.l. soil. Input data for this evaluation as obtained from the chemistry model
are given in Table 3. The effects of correcting
and SDq1 are given in Table 4 for the case of Pachappa
f.s.l. soil. Input data for this evaluation as obtained from the chemistry model
are given in Table 3. The effects of correcting 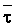 and
and 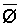 for the amount of water uptake
before the soil comes to field capacity, i.e.,
for the amount of water uptake
before the soil comes to field capacity, i.e., 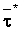 and
and 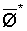 , are illustrated in Figs. 8 and
9. Because the correction made only about a 9 percent difference in
, are illustrated in Figs. 8 and
9. Because the correction made only about a 9 percent difference in 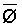 ,
we felt this correction was unnecessary, considering the other uncertainties
in the model. For this reason the remaining data presented here are uncorrected.
,
we felt this correction was unnecessary, considering the other uncertainties
in the model. For this reason the remaining data presented here are uncorrected.
The relations shown in the data of Table 4 are illustrated in Figs. 10 to 16. Briefly, these figures illustrate the following points:
i. The lower the EC of the irrigation water and the higher the LF used with the water, the lower is the resultant water uptake weighted osmotic pressure (Fig. 10) and total (Fig. 11) water stress. Such a lowering ofThe values ofwould expectedly increase crop yield in some cases.
ii. While
is not appreciably affected by LF, it does respond to ECiw (Fig. 12) and to Øf (Fig. 13). Thus,
increases with Øf and at any given level of Øf decreases with increasing ECiw as is expected. The drier the soil becomes between irrigations (i.e., the longer the irrigation interval and the greater Øf), the greater will be the degree of water depletion (Fig. 14) and hence
. Furthermore, the lower he ECiw, the lower the osmotic pressure in the upper part of the root zone where most of the water is absorbed and hence the greater the extent of water depletion for any fixed level of Øf (frequency) (Figs. 12, 13, 14). LF mainly affects the p level in the lower part of the root zone where there is water uptake, hence its minimal effect on
.
iii. Leaching fraction affects the need for increased frequency of irrigation more than it does
, for any given ECiw as shown in Figs. 15 and 16, because it decreases the availability of water more in the lower soil depths where p is high (Figs. 6 and 10) while having little effect on the upper root zone (Fig. 6) where most of the water uptake is absorbed; hence,
is not greatly affected, except under conditions of marked water depletion between irrigations, i.e., with very low frequency irrigation.
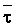 ,
, 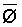 ,
and SDqi obtained with the water quality model for
Sevier River water and three soil types are given in Table 5. The effects of soil
retentivity characteristics for a fixed level of osmotic pressure on
,
and SDqi obtained with the water quality model for
Sevier River water and three soil types are given in Table 5. The effects of soil
retentivity characteristics for a fixed level of osmotic pressure on 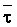 ,
,
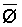 , and SDqi are illustrated in Figs. 17, 18, 19 for
the Sevier River water and LF = 0.1. These data clearly show the importance that
retentivity characteristics of different soil types may have on
, and SDqi are illustrated in Figs. 17, 18, 19 for
the Sevier River water and LF = 0.1. These data clearly show the importance that
retentivity characteristics of different soil types may have on 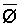 (Fig. 18) because of its effect on
(Fig. 18) because of its effect on 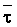 (Fig.
17). Its effect is much less, however, on the extent of water depletion (Fig.
19), especially under conditions of low set Ø (in high frequency irrigation).
This is so because with water uptake by the crop shortly after irrigation, a considerable
decrease in water content causes only a minor increase in total water stress;
however, later when a substantial fraction of the available moisture has been
used, then the further additional loss of moisture from the soil causes a large
increase in total water stress. Parameters for describing the moisture characteristics
of these soil types are given in the footnote to Table 5.
(Fig.
17). Its effect is much less, however, on the extent of water depletion (Fig.
19), especially under conditions of low set Ø (in high frequency irrigation).
This is so because with water uptake by the crop shortly after irrigation, a considerable
decrease in water content causes only a minor increase in total water stress;
however, later when a substantial fraction of the available moisture has been
used, then the further additional loss of moisture from the soil causes a large
increase in total water stress. Parameters for describing the moisture characteristics
of these soil types are given in the footnote to Table 5.
Table 1 - INPUT DATA FOR SALINITY HAZARD MODEL CALCULATIONS AS OBTAINED FROM CHEMISTRY MODEL SUBROUTINE
|
River |
LF |
Water uptake pattern1/ |
ECiw |
ECsw values1/ |
|||
|
Missouri |
.1 |
.40, .30, .20, .10 |
0.91 |
1.32 |
1.76 |
3.08 |
4.69 |
|
.2 |
| |
0.91 |
1.28 |
1.55 |
2.19 |
3.17 |
|
|
.3 |
| |
0.91 |
1.27 |
1.47 |
1.89 |
2.49 |
|
|
Salt |
.1 |
| |
1.56 |
2.35 |
3.26 |
6.43 |
10.00 |
|
.2 |
| |
1.56 |
2.25 |
2.70 |
4.18 |
6.53 |
|
|
.3 |
| |
1.56 |
2.23 |
2.51 |
3.42 |
4.80 |
|
|
Colorado |
.1 |
| |
1.27 |
1.69 |
2.29 |
4.27 |
5.98 |
|
.2 |
| |
1.27 |
1.63 |
1.95 |
2.89 |
4.35 |
|
|
.3 |
| |
1.27 |
1.61 |
1.83 |
2.43 |
3.30 |
|
|
Sevier |
.1 |
| |
2.03 |
2.79 |
3.88 |
7.67 |
11.85 |
|
.2 |
| |
2.03 |
2.67 |
3.19 |
5.00 |
7.80 |
|
|
.3 |
| |
2.03 |
2.64 |
3.20 |
4.06 |
5.76 |
|
|
Gila |
.1 |
| |
3.14 |
4.56 |
6.40 |
12.94 |
20.02 |
|
.2 |
| |
3.14 |
4.34 |
5.21 |
8.14 |
13.04 |
|
|
.3 |
| |
3.14 |
4.28 |
4.73 |
6.64 |
9.35 |
|
|
Pecos |
.1 |
| |
3.26 |
4.48 |
6.20 |
10.10 |
14.90 |
|
.2 |
| |
3.26 |
4.26 |
5.06 |
7.49 |
10.16 |
|
|
.3 |
| |
3.26 |
4.20 |
4.62 |
6.34 |
7.99 |
|
|
|
|
¯ |
|
|
|
|
|
1/ For successively deeper quarter fractions of the root zone.Table 4 - PARTIAL SUMMARY RESULTS OF WATER QUALITY MODEL FOR SALINITY HAZARD ASSESSMENT, PACHAPPA SOIL
|
River
|
LF
|
set Ø |
|
|
|
SDqi
|
|
bars |
bars |
bars |
bars |
|||
|
Missouri |
.1 |
1 |
0.34 |
0.54 |
0.88 |
0.32 |
|
| |
2 |
0.52 |
| |
1.06 |
0.69 |
|
|
| |
3 |
0.68 |
| |
1.22 |
0.92 |
|
|
| |
4 |
0.83 |
| |
1.36 |
1.05 |
|
|
| |
5 |
0.97 |
| |
1.50 |
1.16 |
|
|
| |
6 |
1.10 |
| |
1.64 |
1.20 |
|
|
| |
8 |
1.33 |
| |
1.87 |
1.29 |
|
|
¯ |
10 |
1.55 |
¯ |
2.09 |
1.34 |
|
|
|
.2 |
1 |
0.34 |
0.44 |
0.79 |
0.33 |
|
| |
2 |
0.54 |
| |
0.99 |
0.77 |
|
|
| |
3 |
0.72 |
| |
1.16 |
0.97 |
|
|
| |
4 |
0.86 |
| |
1.31 |
1.09 |
|
|
| |
5 |
1.01 |
| |
1.46 |
1.17 |
|
|
| |
6 |
1.15 |
| |
1.59 |
1.22 |
|
|
| |
8 |
1.39 |
| |
1.83 |
1.30 |
|
|
¯ |
10 |
1.61 |
¯ |
2.05 |
1.36 |
|
|
|
.3 |
1 |
0.35 |
0.41 |
0.76 |
0.34 |
|
| |
2 |
0.56 |
| |
0.97 |
0.80 |
|
|
| |
3 |
0.73 |
| |
1.14 |
0.99 |
|
|
| |
4 |
0.89 |
| |
1.30 |
1.10 |
|
|
| |
5 |
1.03 |
| |
1.44 |
1.18 |
|
|
| |
6 |
1.17 |
| |
1.58 |
1.23 |
|
|
| |
8 |
1.41 |
| |
1.82 |
1.31 |
|
|
¯ |
10 |
1.63 |
¯ |
2.04 |
1.36 |
|
|
Salt |
.1 |
1 |
0.29 |
1.11 |
1.40 |
0.17 |
|
| |
2 |
0.43 |
| |
1.54 |
0.45 |
|
|
| |
3 |
0.57 |
| |
1.68 |
0.64 |
|
|
| |
4 |
0.68 |
| |
1.79 |
0.82 |
|
|
| |
5 |
0.79 |
| |
1.90 |
0.96 |
|
|
| |
6 |
0.90 |
| |
2.01 |
1.05 |
|
|
| |
8 |
1.11 |
| |
2.22 |
1.18 |
|
|
¯ |
10 |
1.31 |
¯ |
2.42 |
1.26 |
|
|
|
.2 |
1 |
0.29 |
0.87 |
1.16 |
0.19 |
|
| |
2 |
0.44 |
| |
1.31 |
0.50 |
|
|
| |
3 |
0.58 |
| |
1.46 |
0.79 |
|
|
| |
4 |
0.72 |
| |
1.59 |
0.95 |
|
|
| |
5 |
0.85 |
| |
1.72 |
1.06 |
|
|
| |
6 |
0.98 |
| |
1.85 |
1.14 |
|
|
| |
8 |
1.21 |
| |
2.08 |
1.24 |
|
|
¯ |
10 |
1.42 |
¯ |
2.29 |
1.31 |
|
|
|
.3 |
1 |
0.29 |
0.79 |
1.08 |
0.20 |
|
| |
2 |
0.44 |
| |
1.23 |
0.60 |
|
|
| |
3 |
0.60 |
| |
1.39 |
0.84 |
|
|
| |
4 |
0.75 |
| |
1.54 |
1.00 |
|
|
| |
5 |
0.89 |
| |
1.67 |
1.10 |
|
|
| |
6 |
1.02 |
| |
1.81 |
1.17 |
|
|
| |
8 |
1.25 |
| |
2.04 |
1.26 |
|
|
¯ |
10 |
1.47 |
¯ |
2.26 |
1.32 |
|
|
Sevier |
.1 |
1 |
0.28 |
1.35 |
1.64 |
0.15 |
|
| |
2 |
0.39 |
| |
1.75 |
0.38 |
|
|
| |
3 |
0.53 |
| |
1.88 |
0.58 |
|
|
| |
4 |
0.65 |
| |
2.00 |
0.72 |
|
|
| |
5 |
0.74 |
| |
2.10 |
0.88 |
|
|
| |
6 |
0.84 |
| |
2.19 |
0.99 |
|
|
| |
8 |
1.04 |
| |
2.39 |
1.13 |
|
|
¯ |
10 |
1.24 |
¯ |
2.59 |
1.22 |
|
|
|
.2 |
1 |
0.29 |
1.07 |
1.35 |
0.17 |
|
| |
2 |
0.40 |
| |
1.47 |
0.42 |
|
|
| |
3 |
0.54 |
| |
1.61 |
0.69 |
|
|
| |
4 |
0.66 |
| |
1.73 |
0.89 |
|
|
| |
5 |
0.79 |
| |
1.86 |
1.01 |
|
|
| |
6 |
0.91 |
| |
1.98 |
1.10 |
|
|
| |
8 |
1.14 |
| |
2.21 |
1.21 |
|
|
¯ |
10 |
1.35 |
¯ |
2.42 |
1.29 |
|
|
|
.3 |
1 |
0.29 |
0.94 |
1.23 |
0.18 |
|
| |
2 |
0.41 |
| |
1.35 |
0.48 |
|
|
| |
3 |
0.56 |
| |
1.50 |
0.78 |
|
|
| |
4 |
0.70 |
| |
1.64 |
0.95 |
|
|
| |
5 |
0.83 |
| |
1.78 |
1.06 |
|
|
| |
6 |
0.95 |
| |
1.89 |
1.13 |
|
|
| |
8 |
1.18 |
| |
2.12 |
1.24 |
|
|
¯ |
10 |
1.40 |
¯ |
2.34 |
1.30 |
|
|
Gila |
.1 |
1 |
0.28 |
2.34 |
2.62 |
0.11 |
|
| |
2 |
0.32 |
| |
2.66 |
0.21 |
|
|
| |
3 |
0.42 |
| |
2.76 |
0.39 |
|
|
| |
4 |
0.52 |
| |
2.86 |
0.53 |
|
|
| |
5 |
0.62 |
| |
2.95 |
0.64 |
|
|
| |
6 |
0.71 |
| |
3.05 |
0.73 |
|
|
| |
8 |
0.87 |
| |
3.21 |
0.90 |
|
|
¯ |
10 |
1.01 |
¯ |
3.35 |
1.04 |
|
|
|
.2 |
1 |
0.28 |
1.80 |
2.08 |
0.14 |
|
| |
2 |
0.32 |
| |
2.12 |
0.23 |
|
|
| |
3 |
0.42 |
| |
2.23 |
0.44 |
|
|
| |
4 |
0.53 |
| |
2.34 |
0.61 |
|
|
| |
5 |
0.63 |
| |
2.43 |
0.78 |
|
|
| |
6 |
0.73 |
| |
2.53 |
0.91 |
|
|
| |
8 |
0.92 |
| |
2.72 |
1.08 |
|
|
¯ |
19 |
1.11 |
¯ |
2.91 |
1.18 |
|
|
|
.3 |
1 |
0.28 |
1.62 |
1.90 |
0.15 |
|
| |
2 |
0.32 |
| |
1.94 |
0.25 |
|
|
| |
3 |
0.43 |
| |
2.04 |
0.47 |
|
|
| |
4 |
0.54 |
| |
2.15 |
0.71 |
|
|
| |
5 |
0.65 |
| |
2.27 |
0.88 |
|
|
| |
6 |
0.76 |
| |
2.38 |
0.99 |
|
|
| |
8 |
0.97 |
| |
2.59 |
1.13 |
|
|
|
¯ |
10 |
1.17 |
¯ |
2.79 |
1.22 |
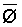 AND
SET Øf VALUES CORRECTED AND UNCORRECTED FOR WATER UPTAKE DURING
INFILTRATION AND DRAINAGE TO “FIELD CAPACITY”, SEVIER RIVER (ECiw
= 3.14 mmho/cm), LF = 0.2
AND
SET Øf VALUES CORRECTED AND UNCORRECTED FOR WATER UPTAKE DURING
INFILTRATION AND DRAINAGE TO “FIELD CAPACITY”, SEVIER RIVER (ECiw
= 3.14 mmho/cm), LF = 0.2
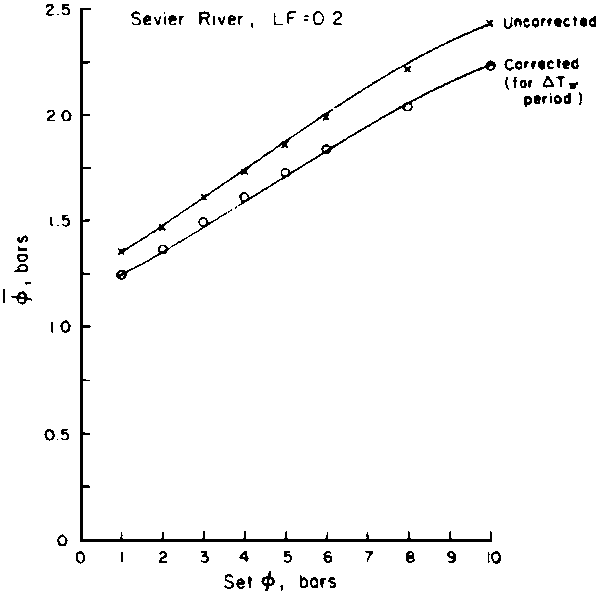
Fig. 10 - EFFECTS OF ECiw AND LF ON 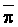
Fig. 11 - EFFECTS OF ECiw AND LF ON 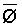
Fig. 12 - EFFECTS OF ECiw AND LF ON 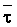 FOR SET Øf OF 2 BARS
FOR SET Øf OF 2 BARS
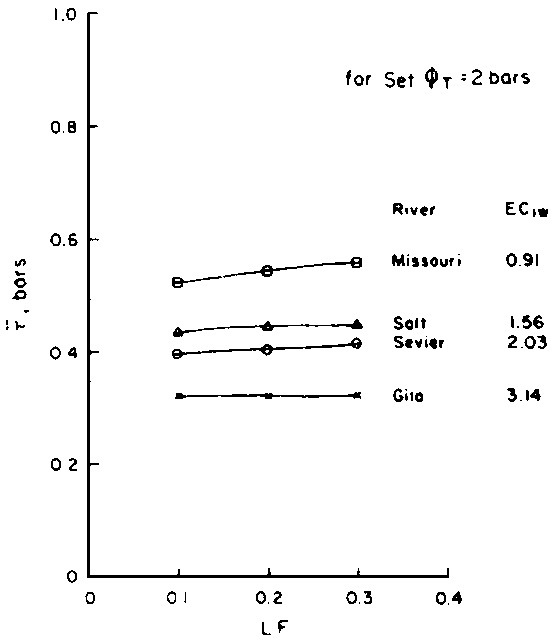
Fig. 13 - EFFECTS OF ECiw AND SET Øf
ON 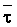 FOR LF OF 0.1
FOR LF OF 0.1
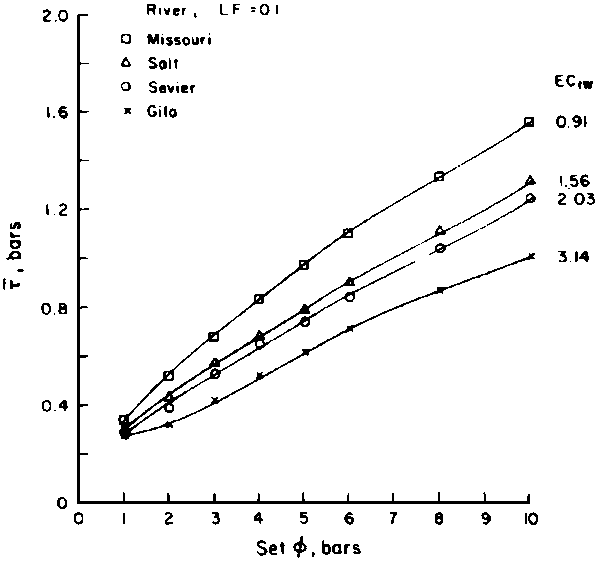
Fig. 14 - EFFECTS OF ECiw AND SET Øf ON SDq1 FOR LF OF 0.1
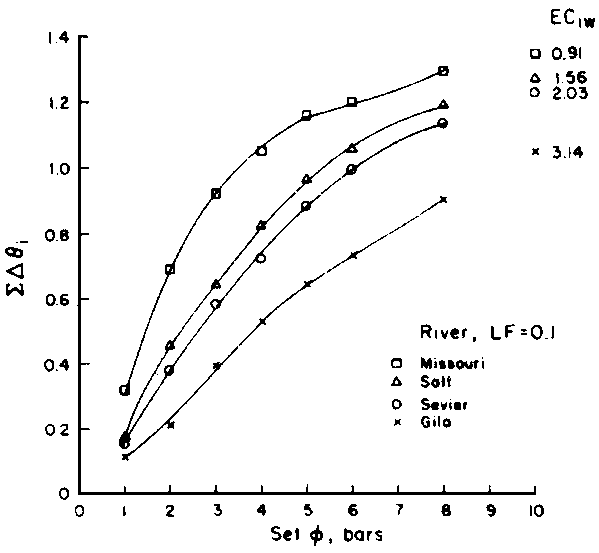
Fig. 15 - EFFECTS OF LF AND SET Øf ON SDqi FOR GILA RIVER (ECiw = 3.14 mmho/cm)
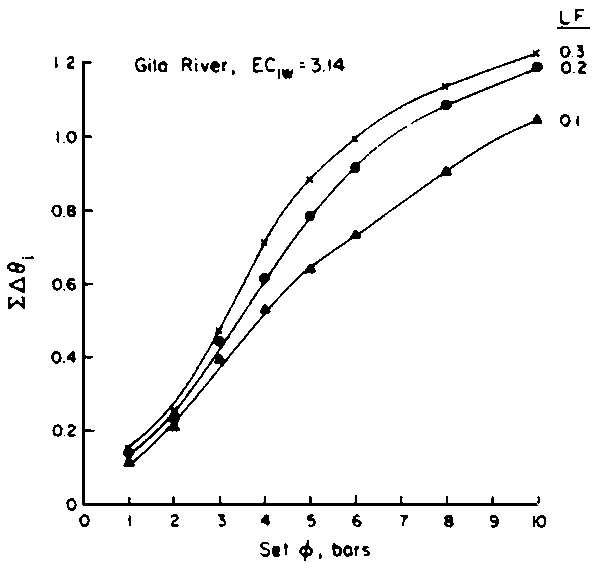
Fig. 16 - EFFECTS OF LF AND SET Øf ON
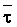 FOR GILA RIVER (ECiw = 3.14 mmho/cm)
FOR GILA RIVER (ECiw = 3.14 mmho/cm)
Table 5 - EFFECTS OF SOIL WATER CHARACTERISTICS ON 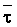 ,
,
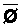 , AND SDqi, USING SEVIER RIVER WATER
, AND SDqi, USING SEVIER RIVER WATER
|
LF |
Set Øf |
|
|
SDqi |
||||||
|
bars
|
Pachappa1/ |
Gilat1/ |
Geary1/ |
Pachappa |
Gilat |
Geary |
Pachappa |
Gilat |
Geary |
|
|
.1 |
2 |
0.39 |
0.43 |
0.46 |
1.75 |
1.78 |
1.80 |
0.38 |
0.40 |
0.39 |
|
4 |
0.65 |
0.75 |
0.83 |
2.00 |
2.10 |
2.18 |
0.72 |
0.75 |
0.72 |
|
|
6 |
0.84 |
1.02 |
1.15 |
2.19 |
2.37 |
2.50 |
0.99 |
1.03 |
0.98 |
|
|
8 |
1.04 |
1.30 |
1.49 |
2.39 |
2.65 |
2.84 |
1.13 |
1.19 |
1.12 |
|
|
10 |
1.24 |
1.56 |
1.81 |
2.59 |
2.91 |
3.16 |
1.22 |
1.29 |
1.22 |
|
|
.2 |
2 |
0.40 |
0.44 |
0.47 |
1.47 |
1.51 |
1.54 |
0.42 |
0.43 |
0.42 |
|
4 |
0.66 |
0.78 |
0.87 |
1.73 |
1.85 |
1.94 |
0.89 |
0.91 |
0.86 |
|
|
6 |
0.91 |
1.11 |
1.25 |
1.98 |
2.18 |
2.32 |
1.10 |
1.13 |
1.06 |
|
|
8 |
1.14 |
1.41 |
1.60 |
2.21 |
2.48 |
2.67 |
1.21 |
1.25 |
1.17 |
|
|
10 |
1.35 |
1.68 |
1.93 |
2.42 |
2.75 |
3.00 |
1.29 |
1.33 |
1.25 |
|
|
.3 |
2 |
0.41 |
0.44 |
0.46 |
1.35 |
1.42 |
1.50 |
0.48 |
0.46 |
0.41 |
|
4 |
0.70 |
0.81 |
0.91 |
1.64 |
1.79 |
1.89 |
0.95 |
0.96 |
0.90 |
|
|
6 |
0.95 |
1.15 |
1.30 |
1.89 |
2.13 |
2.28 |
1.13 |
1.16 |
1.08 |
|
|
8 |
1.18 |
1.46 |
1.65 |
2.12 |
2.44 |
2.63 |
1.24 |
1.27 |
1.19 |
|
|
10 |
1.40 |
1.73 |
1.97 |
2.34 |
2.71 |
2.94 |
1.30 |
1.35 |
1.26 |
|
1/ In the relation t, cm H2O =Aq-a, the values of (A, a) are (0.108, 6.645), (0.63, 4.3), (2.53, 2.79) for Geary (Hanks and Bowers, 1962), Gilat (Gardner et al., 1970), and Pachappa (Wesseling, 1974) soils, respectively.
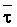 ,
SEVIER RIVER (ECiw = 2.03 mmho/cm), LF = 0.1
,
SEVIER RIVER (ECiw = 2.03 mmho/cm), LF = 0.1
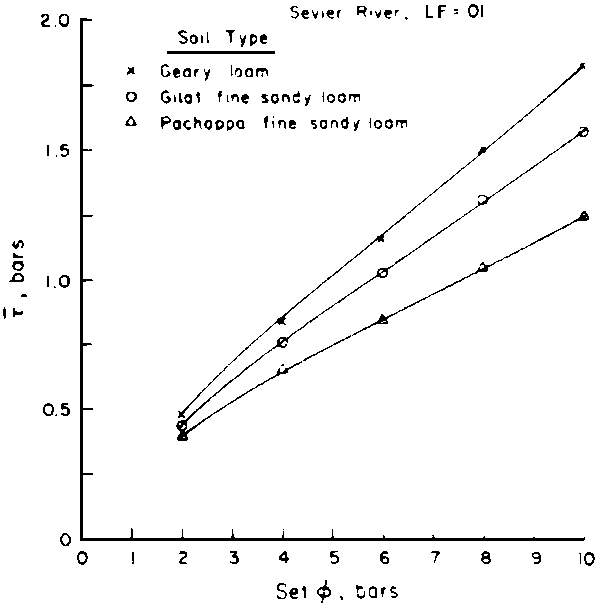
Fig. 18 - EFFECTS OF SOIL PROPERTIES AND SET Øf
ON 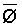 SEVIER RIVER (ECiw =
2.03 mmho/cm), LF = 0.1
SEVIER RIVER (ECiw =
2.03 mmho/cm), LF = 0.1
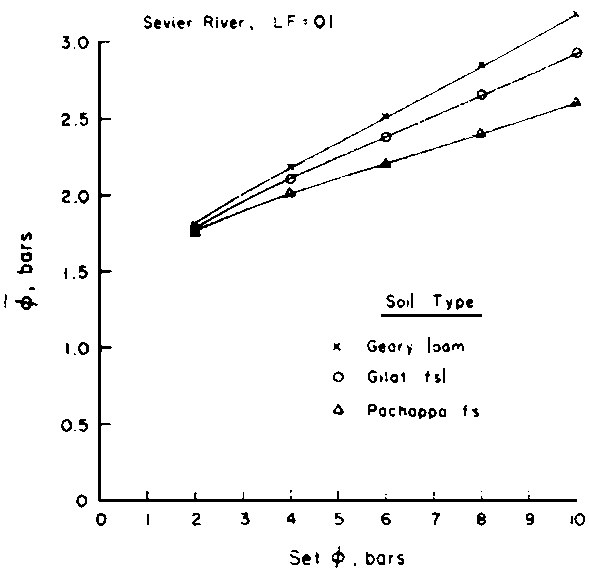
Fig. 19 - EFFECTS OF SOIL PROPERTIES AND SET Øf ON SDqi, SEVIER RIVER (ECiw = 2.03 mmho/cm, LF = 0.1
Results of calculated “stress days” are given in Table 6 for four different irrigation waters. These data show that for cases of infrequent irrigation (like 33 days here), the greater the salinity of the irrigation water, the longer the period the crop is exposed to total soil water potentials in excess of some arbitrary critical value. The data also show that the duration of this exposure to excessive stress can be appreciably reduced by increasing the LF with which saline irrigation waters are used. The benefit of LF is clearly illustrated here. These results support the value of increased LF for minimizing the deleterious consequences of saline irrigation waters.
5. DISCUSSION
5.1 Sodicity Hazard Evaluation
Examination of the soil permeability data now established in the literature shows that soils with similar texture and CEC may vary considerably in their susceptibility to the deleterious effects of exchangeable sodium and electrolyte concentration (Yaron and Shainberg, 1974; McNeal and Coleman, 1966a and b; Rhoades and Ingvalson, 1969). These findings demonstrate the inadequacy of using “average types of soils” for the basis of irrigation water suitability evaluations and the need of making such assessments with specific knowledge about the properties of the soil in question.
Table 6 - ILLUSTRATIVE CALCULATIONS: DAYS AT TOTAL SOIL WATER STRESS GREATER THAN ARBITRARY “CRITICAL” LEVELS
|
River Water |
Leaching Fraction |
Days for which Ø > Øc =
10 bars |
Days for which Ø > Øc =
8 bars |
|
Missouri |
0.1 |
1.12 |
2.28 |
|
0.2 |
0.82 |
1.90 |
|
|
0.3 |
0.72 |
1.77 |
|
|
Salt |
0.1 |
2.75 |
4.37 |
|
0.2 |
1.80 |
3.16 |
|
|
0.3 |
1.49 |
2.76 |
|
|
Sevier |
0.1 |
3.49 |
5.32 |
|
0.2 |
2.27 |
3.77 |
|
|
0.3 |
1.90 |
3.28 |
|
|
Gila |
0.1 |
7.09 |
9.92 |
|
0.2 |
4.38 |
6.28 |
|
|
0.3 |
3.50 |
5.35 |
Assumptions of calculations: 33-day irrigation cycle, assuming 5 days to reach tf.c. = 0.25 bar. Pachappa soil (t = Aq-a, A = 2.53, a = 2.79 for t in cm H2O). Et assumed constant at 0.5 cm/day.To illustrate the individuality of soils with regard to their stability of permeability to exchangeable sodium and electrolyte concentration, a brief review of pertinent studies follows. Quirk and Schofield (1955) found that the permeability of a Rothamsted Experimental Station soil, whose mineralogy was estimated to be 40% illite, 40% kaolin, and 20% vermiculite, was maintained (did not decrease more than 10 to 15%) as along as the electrolyte concentration, in meq/l of the infiltrating water exceeded the exchangeable sodium percentage (ESP) of the soil by a factor of two over the concentration range 0 to 20 meq/l and ESP range 0 to 40. On the basis of this study, the authors proposed the use of a graph (these data are shown in Fig. 5) to determine the suitability of waters for irrigation with respect to soil permeability problems. Doneen (1961) has similarly proposed a scheme for classifying waters into various “permeability classes” based on montmorillonitic Yolo soil whose permeability was reduced 25% when the electrolyte concentrations were less than 3.5, 6.5 and 10.0 at ESP values of 0.6, 3.4 and 8.0, respectively. McNeal and Coleman (1966a) presented detailed information on the effect of ESP and electrolyte concentration on the hydraulic conductivities (HC) of seven well characterized soils. In addition, studies of the swelling properties of these same soils have been made (McNeal and Coleman, 1966b). Five of the soils had exchange complexes which were predominantly montmorillonitic in nature, while one was kaolinitic and one was a mixture of montmorillonite and hydrobiotite. Four of the montmorillonitic soils and the montmorillonite-hydrobiotite soil demonstrated pronounced decreases in HC at ESP values of 25 but over a concentration range of 9 to 40 meq/l. Swelling properties of three of the montmorillonitic soils and of the montmorillonite-hydrobiotite soil were well correlated with their relative hydraulic conductivities. One montmorillonitic soil which underwent HC decreases apparently did not swell. Another of the montmorillonitic soils was stable (no decrease in HC) until ESP values over 50 were reached. This soil failed to swell appreciably. The HC of the kaolinitic soil was essentially independent of exchangeable sodium and electrolyte concentration.
Yaron and Thomas (1968) have reported on the effect of sodic waters on the HC properties of four Texas soils. At an electrolyte concentration of 11.3 meq/l reductions in HC were appreciable when ESP values exceeded approximately 7 to 8, 15 and 20 for soils whose mineralogy was predominantly montmorillonite, montmorillonite-illite, and illite-kaolin, respectively. At a higher electrolyte concentration of 34.5 meq/l analogous “threshold” ESP values were 8 to 10 (montmorillonite), 22 (illite kaolin) and 27 (montmorillonite-illite). Rhoades and Ingvalson (1969) investigated the relationship between hydraulic conductivity, ESP, and electrolyte concentration for vermiculite dominant soils and found that HC did not decrease at ESP values lower than 50 in the absence of mechanical and chemical disaggregation. After disaggregation, HC decreases in the ESP range of 10 to 40 at electrolyte concentrations of 5 to 10 meq/l. The apparent insensitivity of such soils to ESP were concluded to be related to the fact that most of the exchangeable sodium was sorbed on relatively large (silt-sized), semi-expanding vermiculite particles. Hence, swelling and dispersion processes are limited compared with montmorillonitic-type soils.
McNeal (1968) has proposed a procedure for predicting the relative HC of soils to mixed salt solutions using calculated swelling values of montmorillonite as a frame of reference. The procedure was tested with a group of soils of nearly constant clay mineralogy (42% montmorillonite, 29% mica, 16% quartz and feldspar, and 13% other) but varying in clay content from 5 to 49%. Yaron and Thomas (1968) have also proposed a semi-empirical method of predicting HC decreases expected from the use of sodic waters on the basis of their studies on four soils. Both of these procedures have given encouraging results. We do not yet know, however, how generally applicable these methods or those of Quirk and Schofield (1955) and Doneen (1961) will prove to be due to the limited number of soils so far studied. These attempts do, however, suggest the usefulness and potential application of such knowledge for evaluating the suitability of waters (with regards to soil permeability problems) for irrigation purposes.
With the above background, apparently our ability to evaluate the sodium hazard of waters will improve as our ability to predict the effect of exchangeable sodium and electrolyte concentration on the structural stability and permeability of soils improves. In the interim, however, we recommend that appropriate EC-SAR “threshold” relations for the particular soils in question be empirically determined under field conditions for this assessment. If field data are unavailable, we recommend that saturated hydraulic conductivity or swelling and dispersion sensitivities of the soils to anticipated EC-SAR levels be evaluated to guide in assessing the potential sodicity hazard of the water.
5.2 Salinity Hazard Evaluation
Before we can take advantage of the utility of any water quality model for evaluating the salinity hazard, it is necessary to know how crops respond to time and space varying p and t and critical values of p or Ø. At present, such information is not known and for this reason, any salinity hazard assessment of water quality is only an approximation at best. If crops respond to “stress days” of time and space integrated total water stress in excess of certain critical values, then such values must be established for important crop types and complicated dynamic types of models will be required to evaluate the suitabilities of waters for irrigation taking all of the crop, soil, water, atmosphere, irrigation management and time variables into account. Comprehensive models built upon those of the type described by Nimah and Hanks (1973), Bresler (1967), and Dutt et al. (1972) will be needed for such evaluations. At present, more information on how crops respond to salinity and more tests are needed to verify the abilities of comprehensive models to predict such crop responses.
If the concept of Bernstein and Francois (1975) that crop response is related
to water uptake, weighted salinity can be shown to apply in general, and not
just to high frequency irrigation, and if the concept of the additivity of osmotic
and matric stresses of Wadleigh and Ayers (1945) is verified by a broader range
of experimentation, then simpler models could be developed for assessing the
salinity hazards of irrigation waters. Specifically one based on the evaluation
of water uptake weighted total water stress as introduced herein. This would
have great appeal because the time factor is eliminated. Needed information
is not available to either prove or disprove the appropriateness of this concept.
For this purpose and that described in the preceding paragraph, we recommend
that experiments of the kind described by Ingvalson et al. (in
press) be carried out for many different crop species only with modification
to include frequency of irrigation and determination of time and depth integrated
t in addition to p.
Comparisons of correlations of yield with 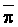 ,
,
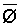 and analogous time and space integrated
values would demonstrate whether or not, and under what conditions, if any,
the time aspect of crop response to salinity and matric stresses can be eliminated
by weighting these parameters in proportion to water uptake.
and analogous time and space integrated
values would demonstrate whether or not, and under what conditions, if any,
the time aspect of crop response to salinity and matric stresses can be eliminated
by weighting these parameters in proportion to water uptake.
If it is found that crop response is suitably relatable to 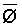 and that the effects of
and that the effects of 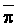 and
and 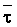 are additive, then appropriate critical values for salinity hazard assessment
could be established in one of four ways. Data on crop response to water stress
could be used. Richards and Wadleigh (1952) have reviewed and summarized much
of the early work and Slatyer (1969) some of the more recent work. Data on crop
response to osmotic pressure in water or sand culture studies could be used,
like that of Eaton (1942). Appropriate data could be collected using presently
available matric and osmotic sensors. The fourth way would be to estimate the
are additive, then appropriate critical values for salinity hazard assessment
could be established in one of four ways. Data on crop response to water stress
could be used. Richards and Wadleigh (1952) have reviewed and summarized much
of the early work and Slatyer (1969) some of the more recent work. Data on crop
response to osmotic pressure in water or sand culture studies could be used,
like that of Eaton (1942). Appropriate data could be collected using presently
available matric and osmotic sensors. The fourth way would be to estimate the
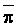 incurred for the experiments under
which the great majority of presently available salt tolerance data were collected.
This latter way would be the simplest and quickest. Most of the salt tolerance
data were determined under conditions of relatively uniform soil salinity levels
obtained by irrigating with highly saline waters and a leaching fraction of
about 50 percent (the range of salinity within the root zone was about ±
10 percent of the mean) (Bernstein, 1964). The ECiw values used to
establish the salinity levels in these studies, if known, could be used along
with knowledge that the LF was ~ 0.5 to calculate
incurred for the experiments under
which the great majority of presently available salt tolerance data were collected.
This latter way would be the simplest and quickest. Most of the salt tolerance
data were determined under conditions of relatively uniform soil salinity levels
obtained by irrigating with highly saline waters and a leaching fraction of
about 50 percent (the range of salinity within the root zone was about ±
10 percent of the mean) (Bernstein, 1964). The ECiw values used to
establish the salinity levels in these studies, if known, could be used along
with knowledge that the LF was ~ 0.5 to calculate 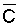 using Eq [1] and hence
using Eq [1] and hence 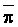 . These values
could then be related to corresponding determined crop yields to establish the
required crop response relations, since under these experimental conditions,
. These values
could then be related to corresponding determined crop yields to establish the
required crop response relations, since under these experimental conditions,
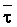 should have been relatively insignificant.
The
should have been relatively insignificant.
The 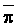 values could then be used as estimates
of
values could then be used as estimates
of 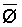 . Such values could also be used
to establish appropriate set point Ø values for guiding irrigation frequency
and determining appropriate LF values (i.e., establishing the leaching requirement)
to minimize crop-yield reductions (van Schilfgaarde et al., 1974).
. Such values could also be used
to establish appropriate set point Ø values for guiding irrigation frequency
and determining appropriate LF values (i.e., establishing the leaching requirement)
to minimize crop-yield reductions (van Schilfgaarde et al., 1974).
Since the present salt tolerance lists are summarized in terms of ECe
and corresponding yield decrements, it would be useful if this information could
be directly used for purposes of establishing 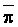 -
and
-
and 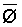 -crop response relations. This
can be estimated, given the above-mentioned assumptions, as follows: select
the ECe value (Ayers and Branson, 1975, have assembled a convenient
list) corresponding to no yield reduction,
-crop response relations. This
can be estimated, given the above-mentioned assumptions, as follows: select
the ECe value (Ayers and Branson, 1975, have assembled a convenient
list) corresponding to no yield reduction, 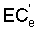 ,
(or some higher ECe value if some yield reduction can be tolerated).
Multiply this value by 2 to obtain the approximate soil water ECsw
(ECe
,
(or some higher ECe value if some yield reduction can be tolerated).
Multiply this value by 2 to obtain the approximate soil water ECsw
(ECe 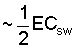 at near field capacity)
and divide by 1.5 to obtain the corresponding ECsw should be between
ECiw and 2 ECiw, hence ECsw ~ 1.5 ECiw).
This value is then multiplied by 0,36 to convert EC values to bars, i.e.,
at near field capacity)
and divide by 1.5 to obtain the corresponding ECsw should be between
ECiw and 2 ECiw, hence ECsw ~ 1.5 ECiw).
This value is then multiplied by 0,36 to convert EC values to bars, i.e., 
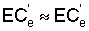 , then calculate maximum allowable
values of
, then calculate maximum allowable
values of 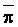 (or ~
(or ~ 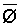 )
)
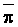 ' (or Ø') for use without yield
reduction for given crops from,
' (or Ø') for use without yield
reduction for given crops from,
If our assumptions are valid, results of Eq [10] show that the range of
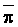 (or
(or 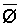 for most crops is 0.7-5 bars. Such
values seem to agree with experience and research findings.
for most crops is 0.7-5 bars. Such
values seem to agree with experience and research findings.
Speculation aside, our present state of knowledge does not allow us to adopt with any conclusive justification time integrated, stress day durations, or water uptake weighted indices for assessing the salinity hazards of irrigation waters. Each of the above, also assumes that crop response is only related to a water availability stress and ignores the consequences of nutritional or toxicity factors of salt damage. Yet a need exists now for some reasonable method for evaluating the salinity hazards of irrigation waters and therefore, some approach must be adopted based on best available information and logic. For this reason, we considered the results obtained with our illustrative model along with experimental observations discussed later to aid us to come to some decision as to what can we now recommend for this purpose.
In our example evaluations, we found the following: the ECiw and
LF combination establish the osmotic stress distribution in the root zone and
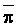 ; they also affect
; they also affect 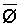 .
Leaching fraction has little effect on
.
Leaching fraction has little effect on 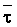 ,
but irrigation frequency, extent of water depletion between irrigations and
soil water retentivity characteristics do. The duration of “stress days”
is affected by irrigation water salinity, LF, frequency of irrigation and soil
water retentivity characteristics. The importance of these effects on crop response
will vary with crop tolerance and climatic stress conditions. On the basis of
this, where saline waters are used for irrigation, LF should be increased to
lower p and
,
but irrigation frequency, extent of water depletion between irrigations and
soil water retentivity characteristics do. The duration of “stress days”
is affected by irrigation water salinity, LF, frequency of irrigation and soil
water retentivity characteristics. The importance of these effects on crop response
will vary with crop tolerance and climatic stress conditions. On the basis of
this, where saline waters are used for irrigation, LF should be increased to
lower p and 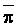 and frequency of irrigation be increased to lower t
and
and frequency of irrigation be increased to lower t
and 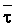 , the two combining to minimize
Ø and
, the two combining to minimize
Ø and 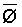 and duration of “stress
days”. We conclude on the basis of our results, especially Table 6, that
space averaged salinity should be a reasonably good index of crop response to
soil-water salinity in oases where matric stress is significant, such as with
infrequent irrigation, because of the marked dependence of duration of stress
days on LF. LF primarily affects the level of salinity in the lower depths of
the root zone; therefore, a parameter of salinity related to the space distribution
of salinity, and especially lower root zone salinity, for infrequent irrigation,
should be related to crop response. The data given in Table 7 support this conclusion.
In each of these studies, irrigations were given when tensiometers at about
the 60 cm depth reached 0.5 bar suctions. Under the outdoor atmospheric conditions,
this resulted in an irrigation interval of about several weeks. Under these
conditions crop response was well related to average root zone ECe
and ECdw values and poorly related to ECiw values. Duration
of stress is increased and less opportunity is allowed for growth “catch-up”
as the irrigation interval is extended. The increased osmotic pressure associated
with lower LFs and use of more saline irrigation waters becomes especially disadvantageous
then, because the “critical stress” level of Ø will be reached
quicker for a given amount of water use if the initial level of p
present at the start of water depletion is high, than if it is low. Under conditions
of more frequent irrigation, based on our model findings, we conclude that crop
response would become more responsive to ECiw and
and duration of “stress
days”. We conclude on the basis of our results, especially Table 6, that
space averaged salinity should be a reasonably good index of crop response to
soil-water salinity in oases where matric stress is significant, such as with
infrequent irrigation, because of the marked dependence of duration of stress
days on LF. LF primarily affects the level of salinity in the lower depths of
the root zone; therefore, a parameter of salinity related to the space distribution
of salinity, and especially lower root zone salinity, for infrequent irrigation,
should be related to crop response. The data given in Table 7 support this conclusion.
In each of these studies, irrigations were given when tensiometers at about
the 60 cm depth reached 0.5 bar suctions. Under the outdoor atmospheric conditions,
this resulted in an irrigation interval of about several weeks. Under these
conditions crop response was well related to average root zone ECe
and ECdw values and poorly related to ECiw values. Duration
of stress is increased and less opportunity is allowed for growth “catch-up”
as the irrigation interval is extended. The increased osmotic pressure associated
with lower LFs and use of more saline irrigation waters becomes especially disadvantageous
then, because the “critical stress” level of Ø will be reached
quicker for a given amount of water use if the initial level of p
present at the start of water depletion is high, than if it is low. Under conditions
of more frequent irrigation, based on our model findings, we conclude that crop
response would become more responsive to ECiw and 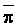 than to LF and irrigation interval.
than to LF and irrigation interval.
Table 7 - CORRELATION OF CROP RESPONSE WITH VARIOUS INDICES OF SALINITY UNDER CONDITIONS OF NON-UNIFORM ROOT ZONE SALINITY AND CONVENTIONAL IRRIGATION FREQUENCIES
|
Crop |
Reference |
Correlation coefficients |
||||
|
ECiw |
|
ECdw |
Ave. ECe |
|
||
|
Sudan grass |
Bower et al. (1970) |
0.19 |
0.57 |
0.88 |
0.84 |
- |
|
Tall fescue |
Bower et al. (1970) |
0.50 |
0.85 |
0.81 |
0.99 |
- |
|
Alfalfa |
Bower et al. (1969) |
0.31 |
0.84 |
0.89 |
0.98 |
- |
|
Alfalfa |
Ingvalson et al. (in press) |
0.53 |
0.71 |
0.80 |
0.78 |
0.89 |
1/ as calculated with Eq [2]6. RECOMMENDATIONS
2/ from time and space integrated in situ soil water salinity values.
6.1 Sodicity Hazard Evaluations
6.1.1 With computer facilities
We conclude that the soil-water chemistry information required to assess the sodicity hazard of any arbitrary irrigation water can be closely estimated with the “Oster” chemistry model, i.e., the EC and SAR levels in the soil-water by fractional depth throughout the root zone, and recommend its adoption for this purpose. With appropriate information on certain properties of the soil to be irrigated, like its critical limits of EC-SAR for maintaining hydraulic conductivity or preventing excessive swelling or dispersion or degradation of aggregate stability, etc., the suitability of a water for irrigation can then be assessed with respect to this soil related sodicity hazard. Similarly, the suitability of the water with reference to the crop related sodicity hazard can be assessed from the predicted SAR distribution in the profile, established ESP-SAR relations, and tables of crop tolerance to ESP. While data were not presented herein, the chemistry model also predicts the concentration of calcium and magnesium 30 that the likelihood of nutritional imbalance can be evaluated. This is important because, according to Bernstein (1974, in press), the deleterious effects of high ESP on crop response is moderated or eliminated for some crops at sufficiently high levels of calcium and magnesium (> ~2-3 meq/l).
6.1.2 Without computer facilities
For rough assessments of sodicity hazard, when computer facilities are unavailable, it is recommended that upper (u) and lower (l) root zone values of SAR be estimated as
and
SARl = k SARu ..... [12]respectively, after Rhoades (1972), where
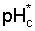 is as defined in the footnote of Table 1 and k is a factor dependent on LF and
soil properties. For many soils this factor has been found to be 2.06, 1.36 and
1.03 for LFs of 0.1, 0.2 and 0.3, respectively. Several studies have demonstrated
the utility of the above adjusted SAR procedure; these studies have been previously
reviewed (Rhoades, 1972). More recently, Oster and Rhoades (1975) obtained results
for a wide range of water types also supportive of this semiquantitative calculation
procedure.
is as defined in the footnote of Table 1 and k is a factor dependent on LF and
soil properties. For many soils this factor has been found to be 2.06, 1.36 and
1.03 for LFs of 0.1, 0.2 and 0.3, respectively. Several studies have demonstrated
the utility of the above adjusted SAR procedure; these studies have been previously
reviewed (Rhoades, 1972). More recently, Oster and Rhoades (1975) obtained results
for a wide range of water types also supportive of this semiquantitative calculation
procedure.
For soil related sodicity considerations, ECiw and SARu are recommended for use to assess the likelihood of significant permeability reductions for a soil of given EC-ESP (or SAR) tolerances as discussed in the proceeding section: SARu and SARl are recommended to predict the minimum, maximum and average root zone ESP values for comparison with crop tolerance - ESP tables to assess the likelihood of sodium toxicity problem of a given crop. Convenient ESP-tolerance lists are given in Bernstein (1974).
6.2 Salinity Hazard Evaluations
6.2.1 With computer facilities
For the reasons discussed in Section 5, in the interim, until more information
is available on how crops respond to time and space varying osmotic pressures
and matric stresses as a function of irrigation management, soil water retentivity
characteristics and atmospheric stresses, the following procedures are recommended
for evaluating the salinity hazards of irrigation waters. The assumption is
made that good irrigation management practices are followed, including uniform
application of water. For irrigation regimes in which significant matric stresses
are achieved, during the irrigation cycle, we recommend that the average root
zone ECe be estimated for any giver water and LF with the chemical
predictive model presented herein and that this value then be used to assess
the likelihood of yield reduction of any given crop by comparison with 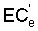 values given in standard tables of crop tolerance to salinity (like those of
Bernstein, 1974 or Ayers and Bran son, 1975).
values given in standard tables of crop tolerance to salinity (like those of
Bernstein, 1974 or Ayers and Bran son, 1975).
For conditions where significant matric stresses are avoided, like high frequency
irrigation, and osmotic pressure is the dominant factor affecting crop response
to saline irrigation waters, we also advise the use of the chemistry model for
assessing the salinity hazard. In this case, however, we recommend that  be calculated by Eq [2], and that the expected crop response evaluation be made
by comparison of such values with critical
be calculated by Eq [2], and that the expected crop response evaluation be made
by comparison of such values with critical  values obtained with Eq [10] and appropriate values of
values obtained with Eq [10] and appropriate values of 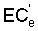 (such as those given in Ayers and Branson (1975) tables).
(such as those given in Ayers and Branson (1975) tables).
6.2.2 Without computer facilities
For rough assessments of salinity hazard, when computer facilities are unavailable
and for irrigation regimes where significant matric stresses are/achieved during
the irrigation cycle, we recommend that the maximum acceptable level of EC in
the irrigation water, 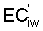 , for a crop
of given salt tolerance and for a given LF be estimated with
, for a crop
of given salt tolerance and for a given LF be estimated with
Equation [13] is obtained, by substitution of
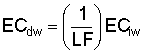 into
Eq [14].
into
Eq [14].
where
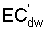 is the estimated maximum allowable
ECdw without crop yield reduction recommended for use in establishing
leaching requirements
is the estimated maximum allowable
ECdw without crop yield reduction recommended for use in establishing
leaching requirements 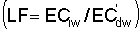 after Rhoades
(1974). The derivations and logic of Eqs. [13] and [14] are given in this latter
reference. The major assumption in these equations is that crops respond to average
root zone EC.
after Rhoades
(1974). The derivations and logic of Eqs. [13] and [14] are given in this latter
reference. The major assumption in these equations is that crops respond to average
root zone EC. 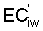 values obtained with Eq
[13] are higher and lower than those recommended by Ayers and Branson (1973) depending
on LF.
values obtained with Eq
[13] are higher and lower than those recommended by Ayers and Branson (1973) depending
on LF.
For irrigation regimes where significant matric stresses are avoided with appropriate
management procedures, such as high frequency irrigation (like drip irrigation),
we recommend that  values be estimated
with Eq [1] as
values be estimated
with Eq [1] as  . Such values then being
compared with
. Such values then being
compared with 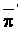 values obtained with
Eq [10] and appropriate
values obtained with
Eq [10] and appropriate  values as
previously discussed to assess the likelihood of crop yield reductions with
use of that water for that crop. This approach is the same as that recommended
previously for such irrigation regimes with use of computer facilities except
in this case corrections for salt precipitation and mineral weathering are ignored.
values as
previously discussed to assess the likelihood of crop yield reductions with
use of that water for that crop. This approach is the same as that recommended
previously for such irrigation regimes with use of computer facilities except
in this case corrections for salt precipitation and mineral weathering are ignored.
6.2.3 Research needs
We recommend that i) quantitative models be developed to predict the composition, the osmotic and matric potentials of the soil water, and the corresponding exchangeable-cation composition of the soil, both in time and space, that would result from the use of any arbitrary irrigation water under any given soil-crop-water management system and climatic conditions; ii) such models be verified by field testing, and iii) studies be carried out to determine how crops respond (rooting patterns, vegetative growth and yield) to time and space varying salinity and sodicity, as affected by LF, irrigation frequency, climatic stress and irrigation water composition, so that results of quantitative water suitability assessment models can be properly interpreted.
REFERENCES
Ayers, R.S. and Branson R.L. 1975. Guidelines for interpretation of water quality for agriculture. Univ. of Calif. Extension, mimeo. 13p.
Bernstein, L. 1964. Salt tolerance of plants. USDA Agr. Info. Bull. 283. 23 p.
Bernstein, L. 1974. Crop growth and salinity. In J. van Schilfgaarde (ed.) Drainage for Agriculture. Agronomy 17: 39-54.
Bernstein, L. 1975. Effects of salinity and sodicity on plant growth. Ann. Review of Phytopathology. (in press)
Bernstein, L. and Pearson, G.A. 1956. Influence of exchangeable sodium on the yield and chemical composition of plants. I. Green beans, garden beets, clover and alfalfa. Soil Sci. 82:247-258.
Bernstein, L. and Francois, L.E. 1973a. Comparisons of drip, furrow and sprinkler irrigation. Soil Sci. 115:73-86.
Bernstein, L. and Francois, L.E. 1973b. Leaching requirement studies: Sensitivity of alfalfa to salinity of irrigation and drainage waters. Soil Sci. Soc. Amer. Proc. 37:931-943.
Bernstein, L. and Francois, L.E. 1975. Effects of frequency of sprinkling with saline waters compared with daily drip irrigation. Agron. J. 67 (2):185-191.
Bernstein, L., Francois, L.E. and Clark, R.A. 1974. Minimal leaching with varying root depths of alfalfa. Soil Sci. Soc. Amer. Proc. 39:112-115.
Bower, C.A., Ogata, G. and Tucker, J.M. 1969. Root zone salt profiles and alfalfa growth as influenced by irrigation water quality. Agron. J. 61:783-785
Bower, C.A., Ogata, G., and Tucker, J.M. 1970. Growth of Sudan and tall fescue grasses as influenced by irrigation water salinity and leaching fraction. Agron. J. 62:793-794.
Bresler, E. 1967. A model for tracing salt distribution in the soil profile and estimating the efficient combination of water quality and quantity under varying field conditions. Soil Sci. 104:227-233.
Bresler, E. and Yaron, D. 1972. Soil water regime in economic evaluation of salinity in irrigation. Water Resources Res. 8:791-800.
Campbell, R.B., Bower, C.A. and Richards, L.A. 1948. Change of electrical conductivity with temperature and the relation of osmotic pressure to electrical conductivity and ion concentration for soil extracts. Soil Sci. Soc. Amer. Proc. 13:66-69.
Doneen, L.D. 1961. The influence of crop and soil on percolating waters. In: Leonard Schiff (ed.) Proc. Conference on Ground Water Recharge. Ground Water Recharge Laboratory, Southwest Branch, SWCRD, Fresno, CA. p. 1-70.
Dutt, G.R., Shaffer, M.J. and Moore, W.J. 1972. Computer simulation model of dynamic biophysicochemical processes in soil. Univ. of Arizona Tec. Bull. 196. 101 p.
Eaton, F.M. 1942. Toxicity and accumulation of chloride and sulphate salts in plants. J. Agr. Res. 64:357-399.
Environmental Protection Agency. 1972. Water Quality Criteria, 1972, Environmental Protection Agency, Washington, D.C. 594 p.
Gardner, R.W., Hillel, D. and Benyamini, Y. 1970. Post-irrigation movement of soil water. I. Redistribution. Water Resources Res. 6:851-861.
Handa, B.K. 1964. Modified classification procedure for rating irrigation waters. Soil Sci. 98:264-269.
Hanks, R.J. and Bowers, S.A. 1962. Numerical solution of the moisture flow equation for infiltration into layered soils. Soil Sci. Soc. Amer. Proc. 26:530-534.
Ingvalson, R.D., Rhoades, J.D. and Page, A.L. Correlation of alfalfa yield with various indices of salinity. Soil Sci. (In press)
Lagerwerff, J.V. and Holland, J.P. 1960. Growth and mineral content of carrots and beans as related to varying osmotic and ionic-composition effects in saline-sodic sand cultures. Agron. J. 52:603-608.
Mantell, A. and Goldin, E. 1964. The influence of irrigation frequency and intensity on the yield and quality of peanuts. Israel J. Agric. Res. 14: 203-210.
McNeal, B.L. 1968. Prediction of the effect of mixed-salt solutions on soil hydraulic conductivity. Soil Sci. Soc. Amer. Proc. 32:190-193.
McNeal, B.L. and Coleman, N.T. 1966a. Effect of solution composition on soil hydraulic conductivity. Soil Sci. Soc. Amer. Proc. 30:308-312.
McNeal, B.L. and Coleman, N.T. 1966b. Effect of solution composition on the swelling of extracted soil clays. Soil Sci. Soc. Amer. Proc. 30:313-317.
McNeal, B.L., Oster, J.D. and Hatcher, J.T. 1970. Calculation of electrical conductivity from solution composition data as an aid to in situ estimation of soil salinity. Soil Sci. 110:405-414.
Nimah, M.N. and Hanks, R.J. 1973. Model for estimating soil water, plant and atmosphere interrelations: I. Description and sensitivity; II. Field test of model. Soil Sci. Soc. Amer. Proc. 37:522-532.
Oster, J.D. and Ingvalson, R.D. 1967. In situ measurement of soil salinity with a sensor. Soil Sci, Soc. Amer. Proc. 31:572-574.
Oster, J.D. and McNeal, B.L. 1971. Computation of soil solution composition variation with water content for desaturated soils. Soil Sci. Soc. Amer. Proc. 35:436-442.
Oster, J.D. and Rhoades, J.D. 1975. Calculated drainage water compositions and salt burdens resulting from irrigation with river waters in the Western United States. J. Environ. Qual. 4:73-79.
Pearson, G.A. 1960. Tolerance of crops to exchangeable sodium. U.S. Dep. Agri. Inf. Bull, 216. 4p.
Pearson, G.A. and Bernstein, L. 1958. Influence of exchangeable sodium on yield and chemical composition of plants. II. Wheat, barley, oats, rice, tall fescue and tall wheatgrass. Soil Sci. 86:254-261
Quirk, J.P. and Schofield, R.K. 1955. The effect of electrolyte concentration on soil permeability. J. Soil Sci. 6:163-178.
Raats, P.A.C. 1974. Movement of water and salts under high frequency irrigation. Proc. 2nd Internatl. Drip Irrig. Cong., San Diego, CA 7-14 July. p. 222-227.
Rawlins, S.L. and Raats, P.A.C. 1975. Prospects for high-frequency irrigation. Science 188:604-610.
Rhoades, J.D. 1968. Leaching requirement for exchangeable-sodium control. Soil Sci. Soc. Amer. Proc. 32:652-656.
Rhoades, J.D. 1972. Quality of water for irrigation. Soil Sci. 113:277-284.
Rhoades, J.D. 1974. Drainage for salinity control. In: J. van Schilfgaarde (ed.) Drainage for Agriculture. Agronomy 17:433-461.
Rhoades, J.D. and Ingvalson, R.D. 1969. Macroscopic swelling and hydraulic conductivity properties of four vermiculitic soils. Soil. Sci. Soc. Amer. Proc. 33:364-369.
Rhoades, J.D. and Bernstein, L. 1971. Chemical, physical and biological characteristics of irrigation and soil water. In: Leonard L. Ciaccio (ed.) Water and Water Pollution Handbook, Marcel Dekker, Inc., New York, Vol. I: 141-222.
Rhoades, J.D., Ingvalson, R.D., Tucker, J.M. and Clark, M. 1973. Salts in irrigation drainage waters: I. Effects of irrigation water composition, leaching fraction and time of year on the salt compositions of irrigation drainage waters. Soil Sci. Soc. Amer. Proc. 37:770-774.
Rhoades, J.D., Oster, J.D., Ingvalson, R.D., Tucker, J.M. and Clark, M. 1974. Minimizing the salt burdens of irrigation drainage waters. J. Environ. Qual. 3: 311-316.
Richards, L.A. and Wadleigh, C.H. 1952. Soil water and plant growth. In: B.T. Shaw (ed.) Soil Physical Conditions and Plant Growth. Agronomy 2:73-251.
Slatyer, R.D. 1969. Physiological significance of internal water relations to crop yield. In Physiological Aspects of Crop Yield. Amer. Soc. of Agron., Madison, p. 53-38.
Taylor, A. 1952a. Estimating the integrated soil moisture tension in the root zone of growing crops. Soil Sci. 73:331-339.
Taylor, S.A. 1952b. Use of mean soil moisture tension to evaluate the effect of soil moisture on crop yields. Soil Sci. 74:217-226.
Thorne, J.P. and Thorne, D.W. 1951. The irrigation waters of Utah. Utah Agr. Expt. Sta. Bull. 346. 64 p.
United States Salinity Laboratory Staff. 1954. Diagnosis and improvement of saline and alkali soils. USDA Handbook 60, 160 p.
Van Schilfgaarde, J., Bernstein, L., Rhoades, J.D. and Rawlins, S.L. 1974. Irrigation management for salt control. J. Irrig. and Drainage Div., ASCE 100(IR3):321-338.
Wadleigh, C.H. and Ayers, A.D. 1945. Growth and biochemical composition of bean plants as conditioned by soil moisture tension and salt concentration. Plant Physiol. 20:106-132.
Wadleigh, C.H., Gauch, H.G. and Strong, D.G. 1947. Root penetration and moisture extraction in saline soil by crop plants. Soil Sci. 63:341-349.
Wesseling, J. 1974. Hydraulic conductivity of natural Pachappa soil columns. Soil Sci. 118:6-10.
Yaron, B. and Thomas, G.W. 1968. Soil hydraulic conductivity as affected by sodic water. Water Resources Res. 4:545-552.
Yaron, D., Bielorai, H., Shalhevet, J., and Gavish, Y. 1972. Estimation procedures for response functions of crops to soil water content and salinity. Water Resources Res. 8:291-300.
Yaron, B. and Shainberg, I. 1974. Electrolytes and soil hydraulic conductivity. In: B. Yaron, E. Danfors and Y. Vaadia (eds.) Arid Zone Irrigation, Springer-Verlag, New York, p. 189-199.
Zur, B. and Bresler, E. 1973. A model for the water and salt economy in irrigated agriculture. In: A. Hadas, D. Swartzendruber, P. E. Rijtema, M, Fuchs and B. Yaron (eds.) Physical Aspects of Soil Water and Salts in Ecosystems. Springer-Verlag, New York, p. 395-407.
by
Fathy I. Massoud
Technical Officer, Land Reclamation and Development
Land and Water Development Division
FAO, Rome
1. INTRODUCTION
The present need for more food and fibre entails reclamation and development of new land resources besides an increase in the agricultural inputs necessary for greater production. Irrigated agriculture plays, and will continue to play, a major role in increasing the food supply especially in arid and semi-arid regions. The contribution of irrigation to a substantial increase in quantity and quality of agricultural production, over that of rainfed farming, is an established fact in regions such as the Near East where 70% of the total crop production comes from irrigated lands. Consequently, heavy investments are being made in land reclamation and development of irrigated farming in countries such as Iraq, Iran, Syria, Egypt and Pakistan. In most of these development projects the quality of either the soil or the water, or sometimes both, is not good enough to yield an economic return without the addition of reclamation measures or special management practices. Salinity and alkalinity are amongst those unfavourable land characteristics responsible for this requirement. Moreover, the deterioration of agricultural production on previously productive lands in the arid and semi-arid regions can be directly attributed to the development of salinity and alkalinity. The decline of ancient civilizations in the Mesopotamian Plain was due to the spread of salinity over the agricultural lands.
The lack of awareness of the problems of salt affected soils where conditions permit their formation, and of necessary reclamation measures is one of the main factors for the failure of irrigated projects, while the early prediction of these hazards helps the timely setting up of land protection programmes. Although too much attention is being given to the study of diagnostic and improvement techniques, little is being paid to the study of prognosis of salt affected soils. Successful prognostic techniques should be based on knowledge of the factors involved in the formation and development of salt affected soils and the adoption of a sound methodology.
The factors that should be considered for prognosis could be broadly grouped under (a) natural and (b) man-made. This paper outlines some of the latter group related to soil management and cropping practices. It is not meant to be a detailed study, but rather to raise questions and to stimulate discussion as to how these factors or parameters will be considered in working out a methodology or guidelines for prognosis.
2. SOIL MANAGEMENT AND CROPPING PRACTICES
The main characteristics of these man-made factors are that:
a. they are dependent, by definition, on man's role where he can change their effects according to his management, to improve or worsen the situation;However, for the purpose of the present study those factors related to soil management and cropping practices are going to be presented as individual ones.b. they do not have the same importance or magnitude and consequently, should have different weighted values;
c. they are functionally interrelated and therefore, should not be evaluated separately but in an integrated approach.
3. LAND LEVELLING
This is an important operation, especially in newly developed irrigated lands, for efficient water distribution by surface methods and for the control of runoff. If land levelling is not done properly, or executed under certain conditions, the following may be expected:
a. creation of a micro-relief;Each of the above physical alterations may contribute to the formation of salt affected soils. Changes in the micro-relief in the order of less than 30 cm result in increasing salt content on the raised spots and better leaching in the dips, which explains the spotted nature of salinity observed in poorly levelled but otherwise normal fields. Early detection of variations in micro-relief either during irrigation or from the crop performance helps to give warning of future degradation. Fortunately, by repeated land shaping before cropping and as the development of the land proceeds, the micro-relief variations disappear and their effect becomes less obvious.
b. variation in depth and homogeneity of the soil profile;
c. pulverization of the fine soil material and changes in the soil structure.
The changes in depth and homogeneity of the soil profile as a result of land levelling depend on topography, original soil depth and nature of stratification and size of earth moving. Where shallow profiles are found or relatively less permeable layers are exposed close to the surface, the chances for development of salt affected soils become much greater than in the case of deep and homogeneous soils. Unlike the micro-relief, variations in soil depth are not easy to correct. Therefore, improper land levelling operations associated with shallow profile formation should be given more consideration in prognostic investigations than those with micro-relief.
Heavy land levelling machinery affects the texture and structure of certain soils, such as the calcareous ones and those high in silt content. Pulverizations of the soil material, breaking of aggregates and compaction alter the pore size distribution and decrease the soil permeability. Such alterations would slow down water movement, reduce leaching of salts, encourage waterlogging and consequently, the build up of salinity. Costly ameliorative measures have to be taken to correct these undesirable effects if improvement of aggregation and permeability can not be achieved by normal cropping practices.
Generally speaking, land levelling as a requirement for land reclamation and preparation of fields for surface irrigation is executed by technicians who may not be aware of the consequences of improper levelling on soils and crops. Under these circumstances not only technical training but also creation of awareness are needed in order to avoid land degradation. Since levelling is one of the earliest reclamation operations in newly irrigated farming projects it should be carefully evaluated at an early stage as a possible cause of secondary salinization.
4. TILLAGE
This is another mechanical operation that is usually carried out for numerous reasons including seed bed preparation and improvement of soil permeability. The relation between tillage and salinity depends on the following factors:
a. vertical distribution of salts;It is known that salts tend to accumulate closer to the soil surface in unirrigated dry lands and to move downward with reclamation and irrigation. So, tillage would speed up desalinization and dealkalization by mixing the easily soluble salts deeper in the soil, loosening the dense subsoil and mixing amendments, such as gypsum when applied to alkali soils. At an advanced stage of soil reclamation and under good management practices, where the salts are being pushed below the active root zone, care should be taken in order not to turn the soil by ploughing.
b. depth of tillage and soil stratification;
c. soil moisture at time of ploughing;
d. tillage machinery and implements;
e. timing of tillage.
Within the normal depth of seed bed preparation, which does not exceed 35 cm for most crops, a relatively less pervious layer might be formed as a result of repeated ploughing to the same depth, especially in heavy textured soils and at above adequate moisture content. This plough layer may cause temporary waterlogging followed by salinization that both affect seedling growth and crop stand. Variations in depth of ploughing, subsoiling, growing of crops with different rooting depth and growth habits, and thermal fallowing decrease the effect of this layer. For prognostic purposes, the depth and reversibility of the plough layer should be investigated.
Soil compaction induced by the use of heavy tillage machinery, especially at an unfavourable degree of wetness, increases the soil bulk density and decreases its infiltration rate and consequently, promotes secondary salinization. Therefore, the degree of soil compaction should not be overlooked in the prognostic investigations.
Soil ploughing can be done by various implements that not only affect differently the soil bulk density and aggregate stability but also the distribution and mixing of salts. A chisel plough will cause less disturbance than a mould-board and therefore, the former is preferable to the latter where turning over of the soil is to be avoided. So, the use of implements that enhance salt leaching, such as chisel and mould-board, has to be weighted differently from others, such as a disc harrow, in evaluating the role of tillage in salinization.
Tillage is mostly practised before sowing of each crop and if it is perfectly executed and followed by irrigation or heavy rainfall an effective leaching of salts can be achieved. Tillage after harvest may not be so effective, especially if the soil is tilted, dry or a long period lapses before sowing the next crop. In the latter case, salts may accumulate and affect seedling germination and growth. Therefore, the timing of tillage operations must be observed carefully as a factor in secondary salinization.
5. PLANTING
Planting techniques and positions vary with the type of crop and can be modified to overcome unfavourable conditions for germination and seedling growth. For saline soils and furrow row crops, in decreasing order, the effects of salinity on crop stand, due to planting position, are as follows:
a. planting on top of a single-row bed;Broadcasting or drilling of seeds on flat fields followed by heavy irrigation is also practised to overcome salinity effects on germination. However, where crusting is a problem, measures have to be taken to loosen the soil surface or break the crust to help seedling emergence. Under alkali conditions, characterized by low permeability and susceptibility to waterlogging, row crops are usually planted on high beds to reduce the harmful effects of waterlogging.
b. planting near edges of a double-row bed;
c. planting on side of a sloping bed;
d. planting in irrigation furrow, where crusting is not a problem.
The amount of seed required for planting a given crop on a salt affected soil is higher than on a normal one. Similarly, a decrease in the percentage of emerging seedlings and a delay in emergence are to be expected.
For prognostic purposes, observation of the above relations would help in the identification of the progress of salinity and alkalinity problems. In this regard, keeping records of the amount of seeds, percentage of emerging seedlings and time of emergence is a recommended practice.
6. MULCHING
Among the various objectives of this practice, the reduction of water losses by evaporation is closely related to the movement of salts. Disruption of capillary continuity hinders upward water movement to the soil surface and its loss as vapour, and consequently reduces salinity build up at the surface. Surface tillage, maintenance or surface application of crop residues and placement of gravel layers on the soil surface or slightly beneath it are among the various practices that are worth mentioning. Generally speaking the effectiveness of mulching depends on the depth to water table, pore size distribution, climatic conditions and crop cover.
Under a given circumstance, for surface tillage to be of value it must be done at an early stage while the evaporation rate is high and not after there has been considerable surface drying by evaporation which reduces the beneficial effects of mulching. The effectiveness of maintaining or spreading crop residues on the soil surface depends on the rate and method of application of mixing with the soil. Regarding the placement of a gravel layer for mulching, it is best suited for trees requiring minimum disturbance around the trunks, since the gravel layer creates a problem when cultivating the soil.
The practice of minimum or zero tillage as a measure for conserving water and controlling erosion should be carefully applied where the threat of salinization exists.
Considering the role of proper mulching in checking surface salinization and the various factors affecting it, evaluation of mulching should take into account these factors and the timing of this operation.
7. FALLOWING
Under conditions where water is a limiting factor for crop production the land may be left fallow for some time to increase the soil water reservoir to benefit subsequent crops as in dry fanning, or until an adequate water supply is available in irrigated farming. Measures are usually taken during fallowing to reduce evaporation and consequently, salinization. Mulching, as discussed before, weed control and shading of the soil surface are practised with fallowing to reduce salinity and increase subsequent crop production.
The effectiveness of fallowing in this regard depends on other factors including depth to water table, quality of groundwater, soil properties, climatic conditions, and length and frequency of fallowing. Soil salinity reductions attributed to fallowing are greater under deeper water table regimes and fresh circulating groundwater, compared to more shallow water table and groundwater of higher salt content. Theoretically speaking, evaporation from a dry surface of a fine sandy loam soil would proceed at a rate of about 8, 3 and 1 mm/day if the water table is kept at 90, 120 and 180 cm respectively. This indicates the importance of the water table depth factor and the danger of fallowing where a shallow water table exists.
Where the soil has favourable water transmitting properties and there is a high atmospheric evaporative demand, summer fallowing should be avoided or otherwise irrigated and fallow areas should be grouped and arranged in such a way to reduce unnecessary circulation and rise of groundwater. Temporary fallowing between cropping may not require special practices as would a longer one and more frequent ones.
Fallowing as a factor in salinity prognosis should be considered not only as a process for reducing water losses by evaporation and reducing salinity but also as a possible reverse process. In this respect, depth to and quality of groundwater are the most important parameters.
8. APPLICATION OF MANURES, FERTILIZERS AND AMENDMENTS
Manures and fertilizers are frequently added to the soil to improve its productivity, while amendments are applied in the first place to correct undesirable physical and chemical properties.
Manuring, beside its nutritional value, improves the physical condition of the soil, and therefore enhances leaching of salts and drainage of wet soils. In certain cases, the application of manures high in salt content may add to an existing problem and green manuring would be advantageous. Under arid conditions, manures do not have such a long lasting effect as in temperate or humid climates and frequent applications would be needed. Since the amount of manure normally applied is much higher than when using inorganic fertilizers, care should be taken in the preparation of the former to reduce its salt content. Evaluation of manuring with regard to salinity development should include the salt content, amount added and the effect of manures on the soil physical properties.
Fertilization is an important and essential input in present agricultural production. There is already much information on the characteristics, availability, methods of application, crop response to fertilizers and their effect on the environment. With regard to prognosis of salinity, consideration should be given to the chemical composition, solubility, rate of release and methods of placement, especially in the early stages of plant growth.
The application of amendment a is an ameliorative measure that has a positive effect on desalinization and dealkalization. Consequently, favourable consideration should be given to that practice in prognostic investigations. Other ameliorative techniques such as subsoiling, moling and sanding should be treated similarly.
9. CROP ROTATIONS
The selection of a given crop rotation is governed by the availability and adequacy of soil and water resources, suitability of crops to the prevailing climatic conditions and assurance of an economic return. While the latter factor may not be important in a reclamation rotation, the suitability of the crop to the soil and water qualities will be. Under conditions that encourage salinization crops should be selected on the basis of their salt tolerance and their effects on the salt balance. For alkali conditions, they are selected on the basis of their tolerance to the specific effects of the sodium ion and to the adverse physical conditions. Lists are available for the relative tolerance of crops to salinity and alkalinity.
Since plants not only differ in their tolerance to salts but also in their water needs in terms of quantity and frequency, it is to be expected that the salinity of the soil will be affected differently under various crop rotations. For example, the salinity will be higher after a rotation of cotton-cotton than after berseem-cotton-beans or cotton-berseem-rice. Crops with a long duration of evapotranspiration will cause, in the absence of proper leaching practices, accumulation of salts in the root zone, while crops such as berseem, rice and others requiring frequent irrigation reduce salinity effectively especially where there is adequate drainage.
For prognosis of salinity and alkalinity, an advance knowledge of the salt balance under various crop rotations is very important. Continuous monitoring of salinity and alkalinity after each crop or at least a rotation, not only creates awareness of a potential problem but also helps to re-evaluate the management practices associated with the cropping system. Crop performance and yield are good indices of the improvement or deterioration of the production inputs including soil conditions.
10. CONCLUDING REMARKS
The effects of the soil management and cropping practices on salinization and alkalization are closely related to water management, quality and use, and to climatic and other natural factors. For prognosis of salinity and alkalinity, factors having a long duration effect are land levelling, fallowing and crop rotations. The other factors, although important, generally have less Effect and are less significant than the above ones and could be corrected with less difficulty. Parameters such as depth of soil, depth to water table, quality of groundwater, atmospheric evaporative demand and tolerance of crops to salinity and alkalinity are important for prognostic investigations.