Paper 16 - 1. Interpretation of quality of water for irrigation
Paper 17 - 2. Predicting soil salinization, alkalization and waterlogging
by
R.S. Ayers
FAO Consultant and Water Quality Specialist
University of California, Davis
SUMMARY
1. Quality of water has meaning only in relation to a particular use. In agriculture water quality is related to effects on soils, crops and management necessary to compensate for problems linked to water quality.
Not all agricultural problems of salinity, soil permeability, toxicity, etc., can be related to quality of the water applied. Quality of water must be placed in proper perspective with all the other factors affecting crop production.
2. A water analysis is essential to water quality evaluation. Analyses or determinations needed are ECw, Ca+Mg, Na, CO3+HCO3, Cl, SO4, B, NO3-N, pH.
3. Water analyses can be evaluated in relation to four general problem areas - Salinity, Permeability, Toxicity and Miscellaneous.
4. “Guidelines for Interpretation of Quality of Water for Irrigation” have been prepared based on the above four problem areas and including three degrees of the problem expected - No Problem, Increasing Problems, Severe Problems. Guideline values for each degree of problem are given.
5. Crops vary greatly in their tolerance of poor quality waters and therefore the suitability of a water for irrigation will vary with the crop. Crop tolerance tables for salinity are presented.
6. Leaching Requirement (LR) is the minimum amount of water that must percolate through the active root zone to prevent excessive accumulation of salts. Leaching requirements for various crops for specific qualities of water are given in the Crop Tolerance Tables with the new method of calculation of LR now being discussed by the U.S. Salinity Laboratory.
7. Prevention of a potential problem is just as important as correction of a problem. By using the guidelines for evaluation of the problem potential, such as salinity, and the crop tolerance tables to select a crop compatible with the available quality of water, and by following other suggested corrective management alternatives in the guidelines, a water user may be able to understand the potential problems related to water quality and make good management decisions to adjust to existing water quality.
8. Permeability problems due to water quality are usually related to either extremely low salinity of the water or to an imbalance of sodium, calcium and magnesium (the sodium adsorption ratio). Guideline values are given at which permeability problems are expected from low salt waters or from waters with high sodium adsorption ratio (now modified by carbonate-bicarbonates and reported as adjusted SAR). Corrective action is suggested.
9. Toxicity problems are often very specific for a certain water constituent and crop. Guidelines recognize toxicities due to sodium, chloride, or boron due either to root absorption from soil solution or to foliar absorption from leaves wetted by sprinkler applied water. Management alternatives are suggested.
10. Miscellaneous problems include a mixed group of unrelated problems - excessive vegetative growth due to nitrogen, white deposits from sprinkler irrigation, etc. Corrective actions are suggested.
11. A few typical water analyses with brief guideline interpretations of the quality-related problem potential are given.
1. WHAT DOES WATER QUALITY MEAN?
Quality means different things to different people. Quality is difficult to evaluate except in terms related to the specific use. For household use, the taste, hardness and possible effects on health, food, drink, clothes and fixtures are all quality factors. For industrial use, corrosion, scaling, or certain other adverse effects on manufacturing or processing become the quality factors of paramount importance. For irrigation, water quality is related to its effects on soils and crops, and on the management that may be necessary to control or compensate for the water quality related problem.
2. HOW DOES IRRIGATION WATER QUALITY AFFECT AGRICULTURE?
Water diverted from springs or streams or pumped out of wells from under ground is never pure but always contains measurable quantities of soluble salts. These dissolved salts, if present in sufficient quantity, can adversely affect yield of crop or accumulate to affect productivity of soils. Significant adjustments and changes in farm practices may be required to attempt to compensate for these water quality related effects.
Crops and soils are affected in different ways by the several different types of dissolved salts. A water analysis is needed to determine what types of salts are present; then these must be evaluated in terms of their expected impact upon soils and crops.
3. WHAT SHOULD BE INCLUDED IN A WATER ANALYSIS?
The usual laboratory determinations needed to evaluate the suitability of an irrigation water should include 1) electrical conductance (a measure of the total salts present), 2) chemical analysis for sodium, calcium and magnesium, chlorides, sulphates, carbonates and bicarbonates, and 3) further determinations as may be needed to evaluate other specific chemical constituents or general quality factors (usually including boron, nitrates and pH and infrequently other solubles such as lithium, potassium, iron or ammonia).
4. HOW ARE WATER ANALYSES EVALUATED?
A general water quality evaluation can be made by interpreting the above listed laboratory determinations in relation to four general problem areas - Salinity, Permeability, Toxicity and Miscellaneous.
i. Salinity Problems5. GUIDELINES HELP IN WATER QUALITY EVALUATIONThese are associated with the total quantity of dissolved solids (salts) in the water and their effect upon crop growth. Salinity is usually measured and reported as electrical conductance (ECw) or total dissolved solids (TDS).
ii. Permeability Problems
Certain water constituents reduce soil permeability. The resultant poor permeability makes it more difficult to supply crops with needed water for good growth and may greatly add to cropping difficulties through waterlogging of surface soils accompanied by disease, salinity, weed and nutrition problems. The permeability problem potential is evaluated from a comparison of the quantity of sodium present in the water relative to the calcium and magnesium. Carbonates and bicarbonates will also effect soil permeability under certain conditions and must be evaluated.
iii. Specific Ion Toxicity
Certain specific solubles (ions) have a direct toxic effect on crop growth. Toxic solubles included are boron, chlorides and sodium.
iv. Miscellaneous Problems
In this special unrelated group are included such crop production problems as excessive plant vigour or excessive vegetative growth resulting from nitrogen in the water supply, white deposits on fruit or leaves due to sprinkler irrigation with high bicarbonate water and problems that may be related to pH such as high acidity or high alkalinity.
“Guidelines for Interpretation of Quality of Water for Irrigation” using the four problem areas mentioned were prepared by the University of California Committee of Consultants (1972). These “guidelines” (see Table 1) have evolved from a long line of former guidelines used over the years and are believed to correlate well with field conditions. Several assumptions regarding their preparation along with comments on their use are also given (see Table 2). Many of the recent research findings and reports from the U.S. Salinity Laboratory have been used in formulation of these “Guidelines”. (Refer to papers by Drs. Van Schilfgaarde and Rhoades presented at this Consultation.)
Each of the four problem areas - salinity, permeability, toxicity and miscellaneous - are further separated into three degrees of severity based on expected seriousness of the problem after several years of irrigation. “Water Quality Guideline” interpretive values are shown that are associated with a “No Problem”, “Increasing Problems” and “Severe Problems” potential. The “No Problem” potential indicates that for waters containing less than this listed value the water user would not ordinarily recognize a soil or cropping problem due to water quality. In contrast, the “Severe Problems” evaluation indicates the water user would ordinarily recognize there were some fairly severe soil or cropping problems associated with the continued use of this water. From “No Problem” to “Severe Problems” the water user should experience gradually “Increasing Problems”. In addition to evaluating the potential problem an indication of the corrective action to be taken is also presented. The water user should try to compensate for an indicated potential quality problem by adopting practices to prevent the problem from developing or try to correct or compensate for an existing problem.
Table 1 - GUIDELINES FOR INTERPRETATION OF QUALITY OF WATER FOR IRRIGATION
Table 2 - Assumptions and Comments on Guidelines for Interpretation of Quality of Water for Irrigation Developed by UC-Committee of Consultants.
1. These “guidelines” are flexible and intended for use in estimating the potential hazards to crop production associated with long term use of the particular water being evaluated. “Guidelines” should be modified when warranted by local experience and special conditions of crop, soil, method of irrigation or level of soil-water-crop management. Changes of 10 to 20 percent above or below an indicated guideline value may have little significance if considered in proper perspective along with all other variables that enter into a yield of crop.
2. It is assumed that the water will be used under average conditions - soil texture, internal drainage, total water use, climate, and salt tolerance of crop. Large deviations from the average might make it unsafe to use water which under average conditions, would be good, or might make it safe to use water, which under average conditions, would be of doubtful quality.
3. The divisions into “No problem - Increasing problem - Severe problem” is more-or-less arbitrary but based on large numbers of field studies and observations, as well as carefully controlled greenhouse and snail plot research conducted by various researchers over the past 40 years or more. Guidelines of one sort or another have been proposed by U.S. Geological Survey, University of California, U.S. Salinity Laboratory and many others starting as early as 1911. As new research and observations have developed additional information for assessing water quality, guidelines have been modified.
4. These “guidelines” apply to surface irrigation methods such as furrow, flood, basin, sprinklers, or any other which applies water on an “as-needed” basis and which allows for an extended dry-down period between irrigations during which the crop uses up a considerable portion of the available stored water.
5. The guidelines incorporate some of the newer concepts in soil-plant-water relationships as recently developed at U.S. Salinity Laboratory. Uptake of water occurs mostly from the upper two-thirds of the rooting depth of crop (the “more-active” part of the root zone). Each irrigation normally will leach this upper soil area and maintain it at relatively low salinity. Salts applied in the irrigation water under reasonable irrigation management concentrate in the soil water in this active root zone to about three times the concentration of the applied irrigation water and the salinity of this root area is representative of the salinity levels to which the plant responds. The salinity of the lower root zone is of less importance as long as plants are reasonably well supplied with moisture in the upper, “more-active”, root zone.
These guidelines represent the 1974 consensus of the UC-Committee of Consultants. It is recognized they are not perfect and it is expected they will be modified from time to time as further knowledge and experience dictate.
6. SALINITY PROBLEMS
Salinity problems are associated with the dissolved salts in the applied water. There are two components to the salinity problem. The first concerns the salinity of the applied irrigation water and its direct, rapid effect on the crop. The second concerns the salinity that may develop more slowly in the root zone over a period of time due to accumulation of salts and their effect on the crop. The two need to be considered separately.
Salinity accumulating within the root zone can be controlled, within limits, by application of extra water to satisfy an indicated leaching requirement. But, there is a limit to the correction that can be accomplished by leaching or improved management. This limit is closely related to the actual salinity of the applied irrigation water. If the salinity (ECw) of the applied water is excessive and exceeds the tolerance of the crop, less than a full potential yield is to be expected even though extra water is applied for leaching. Improved water management and extra leaching may produce better yields but only up to the yield limits imposed due to the salinity of the applied water.
Salinity problems and their management are often complicated by high or perched water tables. The usual corrective practices for salinity include use of extra water for leaching to remove the salts, and will very often include artificial or improved drainage (tile or open drains) to assure that leaching is effective. In some cases it may even be necessary to change the crop to one more tolerant of the salinity of soil or water.
7. SELECTION OF CROP TO MATCH IRRIGATION WATER QUALITY AND SOIL SALINITY
Cultural practices, crop selection and management to prevent salinity problems from developing are just as important as are corrective actions after problems develop. There are several management alternatives available to prevent or reduce salinity problems. One of the first alternatives relates to the choice of crop. Crops vary greatly in their salt tolerance and therefore the suitability of a relatively salty water for irrigation will also vary with the crop. For example, a water with ECw = 2 millimhos is poorly suited for irrigation of a salt sensitive crop such as field beans (tolerance of field beans at full yield potential is ECw = 0.7) but is excellent for corn (tolerance of corn at full yield potential is ECw = 2.2).
The crop tolerance tables (immediately ensuing pages) can be used to select crops to match the quality of the available water supply and the expected degree of soil salinity to which the crop will be exposed. These tables list tolerances of several representative field, vegetable, forage and fruit crops to both the salinity of the water applied (ECw) and to the salinity of the soil (ECe). Also shown are probable yield reductions due to various degrees of salinity of applied water (ECw) or salinity of soil (ECe).
Table 3 - CROP TOLERANCE AND LEACHING REQUIREMENT TABLES
Including Yield Decrement to be expected for Certain Crops Due to Salinity of Irrigation Water when Common Surface Irrigation Methods are Used 1/
FIELD CROPS
|
Crop |
0% |
10% |
25% |
50% |
MAXIMUM |
||||||||
|
ECe2/ |
ECw2/ |
LR3/ % |
ECe |
ECw |
LR% |
ECe |
ECw |
LR% |
ECe |
ECw |
LR% |
ECdw3/ |
|
|
Barley |
8 |
5.3 |
12 |
12 |
8 |
18 |
16 |
10.7 |
24 |
18 |
12 |
27 |
44 |
|
Sugarbeets |
6.74/ |
4.5 |
11 |
104/ |
6.7 |
16 |
13 |
8.7 |
21 |
16 |
10.7 |
26 |
42 |
|
Cotton |
6.7 |
4.5 |
11 |
10 |
6.7 |
16 |
12 |
8 |
19 |
16 |
10.7 |
26 |
42 |
|
Safflower |
5.3 |
3.5 |
12.5 |
8 |
5.3 |
19 |
11 |
7.3 |
26 |
14 |
8 |
28.5 |
28 |
|
Wheat |
4.74/ |
3.1 |
8 |
74/ |
4.7 |
12 |
10 |
6.7 |
17 |
14 |
9.3 |
23 |
40 |
|
Sorghum |
4 |
2.7 |
7.5 |
6 |
4 |
11 |
9 |
6 |
17 |
12 |
8 |
22 |
36 |
|
Soybean |
3.7 |
2.5 |
10 |
5.5 |
3.7 |
14 |
7 |
4.7 |
18 |
9 |
6 |
23 |
26 |
|
Rice (paddy) |
3.3 |
2.2 |
9 |
5 |
3.3 |
14 |
6 |
4 |
16 |
8 |
5.3 |
22 |
24 |
|
Corn (maize) |
3.3 |
2.2 |
12 |
5 |
3.3 |
18 |
6 |
4 |
22 |
7 |
4.7 |
26 |
18 |
|
Sesbania |
2.7 |
1.8 |
7 |
4 |
2.7 |
10 |
5.5 |
3.7 |
14 |
9 |
6 |
23 |
26 |
|
Broadbean |
2.3 |
1.5 |
8 |
3.5 |
2.3 |
13 |
4.5 |
3 |
17 |
6.5 |
4.3 |
24 |
18 |
|
Flax |
2 |
1.3 |
7 |
3 |
2 |
11 |
4.5 |
3 |
17 |
6.5 |
4.3 |
24 |
18 |
|
Beans (field) |
1 |
.7 |
6 |
1.5 |
1 |
8 |
2 |
1.3 |
11 |
3.5 |
2.3 |
19 |
12 |
1/ From USDA-Ag. Inf. Bull. 283 and personal communication from Dr. Leon Bernstein, U.S. Salinity Laboratory, Riverside, California.Table 4 - VEGETABLE CROPS2/ ECe means electrical conductivity of saturation extract in millimhos per centimetre (mmho/cm)
ECw means electrical conductivity of irrigation water in mmho/cm.

For an approximate conversion to TDS, mg/l, or ppm multiply mmho/cm by 640.
3/ ECdw is maximum concentration of salts that can occur in drainage water under crops due to ET (evapotranspiration). Use to calculate leaching requirement (LR = ECw/ECdw) to maintain needed ECe in more active root area. Leaching Requirement (LR) means that fraction of the irrigation water that must be leached through the active root zone to control soil salinity at a specified level. At 100% efficiency, applied water (needed to satisfy ET + LR) is equal to
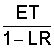
4/ Tolerance during germination (beets) or early seedling stage (wheat, barley) is limited to ECe = about 4 mmho/cm in the upper soil area where germination and early growth takes place.
Note: Conversion from ECw to ECe assumes that irrigation water salts increase 3 fold in salinity in becoming soil water salts (ECsw). This occurs in the more active part of ECw × 3 = ECsw; ECsw ÷ 2 = ECe.
|
Crop |
0% |
10% |
25% |
50% |
Maximum |
||||||||
|
ECe |
ECw |
LR% |
ECe |
ECw |
LR% |
ECe |
ECw |
LR% |
ECe |
ECw |
LR% |
ECdw |
|
|
Beets |
5.3 |
3.5 |
11 |
8 |
5.3 |
17 |
10 |
6.7 |
21 |
12 |
8 |
25 |
32 |
|
Spinach |
3.7 |
2.5 |
12.5 |
5.5 |
3.7 |
18.5 |
7 |
4.7 |
23.5 |
8 |
5.3 |
26.5 |
20 |
|
Tomato |
2.7 |
1.8 |
8 |
4 |
2.7 |
12 |
6.5 |
4.3 |
19.5 |
8 |
5.3 |
24 |
22 |
|
Broccoli |
2.7 |
1.8 |
7 |
4 |
2.7 |
10 |
6 |
4 |
15 |
8 |
5.3 |
20 |
26 |
|
Cabbage |
1.7 |
1.1 |
4 |
2.5 |
1.7 |
6.5 |
4 |
2.7 |
10 |
7 |
4.7 |
18 |
26 |
|
Potato |
1.7 |
1.1 |
5.5 |
2.5 |
1.7 |
8.5 |
4 |
2.7 |
13.5 |
6 |
4 |
20 |
20 |
|
Sweet Corn |
1.7 |
1.1 |
5.5 |
2.5 |
1.7 |
8.5 |
4 |
2.7 |
13.5 |
6 |
4 |
20 |
20 |
|
Sweet Potato |
1.7 |
1.1 |
5.5 |
2.5 |
1.7 |
8.5 |
3.5 |
2.3 |
11.5 |
6 |
4 |
20 |
20 |
|
Lettuce |
1.3 |
.9 |
5 |
2 |
1.3 |
7 |
3 |
2 |
11 |
5 |
3.3 |
18 |
18 |
|
Bell Pepper |
1.3 |
.9 |
5 |
2 |
1.3 |
7 |
3 |
2 |
11 |
5 |
3.3 |
18 |
18 |
|
Onion |
1.3 |
.9 |
7.5 |
2 |
1.3 |
11 |
3.5 |
2.3 |
19 |
4 |
2.7 |
22.5 |
12 |
|
Carrot |
1 |
.7 |
6 |
1.5 |
1 |
8 |
2.5 |
1.7 |
14 |
4 |
2.7 |
22.5 |
12 |
|
Beans |
1 |
.7 |
7 |
1.5 |
1 |
10 |
2 |
1.3 |
13 |
3.5 |
2.3 |
23 |
10 |
|
Cantaloupe1/ |
2.3 |
1.5 |
8 |
3.5 |
2.3 |
12 |
No Data |
No Data |
- |
||||
|
Watermelon1/ |
2 |
1.2 |
8 |
No Data |
No Data |
No Data |
- |
||||||
1/ Assumes ECdw = 20 similar to potato.Table 5 - FORAGE CROPS
|
Crop |
0% |
10% |
25% |
50% |
Maximum |
||||||||
|
ECe |
ECw |
LR% |
ECe |
ECw |
LR% |
ECe |
ECw |
LR% |
ECe |
ECw |
LR% |
ECdw |
|
|
Bermuda Grass |
8.7 |
5.8 |
13 |
13 |
8.7 |
20 |
16 |
10.7 |
24 |
18 |
12 |
27 |
44 |
|
Tall Wheat Grass |
7.3 |
4.9 |
11 |
11 |
7.3 |
17 |
15 |
10 |
23 |
18 |
12 |
27 |
44 |
|
Crested Wh. Grass |
4 |
2.7 |
6 |
6 |
4 |
9 |
11 |
7.3 |
17 |
18 |
12 |
27 |
44 |
|
Tall Fescue |
4.7 |
3.1 |
8 |
7 |
4.7 |
12 |
10.5 |
7 |
17.5 |
14.5 |
9.7 |
24 |
40 |
|
Barley (hay) |
5.3 |
3.5 |
10 |
8 |
5.3 |
15 |
11 |
7.3 |
20 |
13.5 |
9 |
25 |
36 |
|
Perennial Rye |
5.3 |
3.5 |
10 |
8 |
5.3 |
15 |
10 |
6.7 |
19 |
13 |
8.7 |
24 |
36 |
|
Harding Grass |
5.3 |
3.5 |
10 |
8 |
5.3 |
15 |
10 |
6.7 |
19 |
13 |
8.7 |
24 |
36 |
|
Birdsfoot Trefoil |
4 |
2.7 |
10 |
6 |
4 |
14 |
8 |
5.3 |
19 |
10 |
6.7 |
24 |
28 |
|
Beardless Wild Rye |
2.7 |
1.8 |
6 |
4 |
2.7 |
10 |
7 |
4.7 |
17 |
11 |
7.3 |
26 |
28 |
|
Alfalfa |
2 |
1.3 |
5 |
3 |
2 |
7 |
5 |
3.3 |
12 |
8 |
5.3 |
19 |
28 |
|
Orchard Grass |
1.7 |
1.1 |
4 |
2.5 |
1.7 |
6.5 |
4.5 |
3 |
11.5 |
8 |
5.3 |
20 |
26 |
|
Meadow Foxtail |
1.3 |
.9 |
4 |
2 |
1.3 |
5 |
3.5 |
2.3 |
10 |
6.5 |
4.3 |
18 |
24 |
|
Clover |
1.3 |
.9 |
6 |
2 |
1.3 |
9 |
2.5 |
1.7 |
12 |
4 |
2.7 |
19 |
14 |
|
Crop |
0% |
10% |
50%1/ |
Maximum |
||||||
|
ECe |
ECw |
LR% |
ECe |
ECw |
LR% |
ECe |
ECw |
LR% |
ECdw |
|
|
Date Palm |
5.3 |
3.5 |
7 |
8 |
5.3 |
11 |
16 |
10 |
21 |
48 |
|
Fig |
|
|
|
|
|
|
|
|
|
|
|
Olive |
2.7-4.0 |
1.8-2.7 |
6-10 |
4-6 |
2.7-4.0 |
10-14 |
9 |
6 |
21 |
28 |
|
Pomegranate |
|
|
|
|
|
|
|
|
|
|
|
Grape (Thompson) |
2.7 |
1.8 |
7.5 |
4 |
2.7 |
11 |
8 |
5.3 |
22 |
24 |
|
Grapefruit |
|
|
|
|
|
|
|
|
|
|
|
Orange |
1.7 |
1.1 |
7 |
2.5 |
1.7 |
11 |
5 |
3.3 |
33 |
16 |
|
Lemon |
|
|
|
|
|
|
|
|
|
|
|
Apple |
1.7 |
1.1 |
7 |
2.5 |
1.7 |
11 |
5 |
3.3 |
33 |
16 |
|
Pear |
|
|
|
|
|
|
|
|
|
|
|
Almond |
|
|
|
|
|
|
|
|
|
|
|
Apricot |
1.7 |
1.1 |
7 |
2.5 |
1.7 |
11 |
5 |
3.3 |
33 |
16 |
|
Peach |
|
|
|
|
|
|
|
|
|
|
|
Prune |
|
|
|
|
|
|
|
|
|
|
|
Walnut |
1.7 |
1.1 |
7 |
2.5 |
1.7 |
11 |
5 |
3.3 |
33 |
16 |
|
Blackberry |
|
|
|
|
|
|
|
|
|
|
|
Boysenberry |
1.0-1.7 |
0.7-1.1 |
5-8 |
1.5-2.5 |
1.0-1.7 |
7-12 |
4 |
2.7 |
19 |
14 |
|
Avocado |
1.3 |
0.9 |
7.5 |
2 |
1.3 |
11 |
4 |
2.7 |
22.5 |
12 |
|
Strawberry |
1.0 |
0.7 |
7 |
1.5 |
1.0 |
10 |
3 |
2.0 |
10 |
10 |
1/ Calculated values, assuming 50% decrease in yield results from doubling of salinity values for 10% yield decrement.After the evaluation of the water analysis according to the guidelines and after referring to the tolerance tables for the crop tolerance to salinity, the water user should be able to predict whether there is or is not a potential salinity problem and being forewarned may be able to take steps to prevent its developing.
8. ADDITIONAL WATER FOR LEACHING
The application of extra water for leaching is a second alternative that is normally used by good cultivators and farmers to control salt accumulation. The minimum amount of this extra water that must percolate through the active root zone to prevent excessive accumulation of salts is known as the leaching requirement (LR) and is reported as a percent of the normal water requirement of crop (see footnotes 2 and 3 of Crop Tolerance and Leaching Requirement Table 3). The water user will need to supply this extra water (the leaching requirement) for salt control in addition to the water he must supply to meet his crop water requirements. In many areas rainfall plus the usual inefficiencies in irrigation may supply this extra water required for salinity control.
Included as part of the crop tolerance tables are data on leaching requirements
(LR) and on the maximum allowable salinity of the drainage water (ECdw)
that can be tolerated by the crop at the bottom of the root zone with no more
than a 10 to 15% loss in yield. This ECdw is the specific drainage
water ECdw now being used to calculate leaching requirement (LR)
values for the quality of water (ECw) and crop grown, where 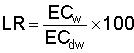 .
.
The satisfying of a leaching requirement to achieve adequate salt management is much more easily attained on sandy soils where water infiltrates and percolates more readily than on clays where percolation rates are slow to extremely slow and leaching is difficult. To be effective the leaching waters must percolate through the soil and out at the bottom of the lower root zone.
The presence of a high water table makes the satisfying of a leaching requirement much more difficult or even impossible. If salts are leached, a high water table may quickly allow resalinization to occur as salty subsurface waters rise to the soil surface where the water evaporates and leaves salts behind to accumulate again in the crop rooting zone. Where high water tables are a problem, artificial drainage to lower and stabilize the water table is usually a first essential to reclamation and salinity control.
9. PERMEABILITY PROBLEMS RELATED TO WATER QUALITY
Referring to the guidelines, permeability problems due to quality of water are usually, but not always, related to too little calcium and magnesium or to excesses of sodium. Permeability problems may also be related to very pure, low salt water (ECw less than 0.5 mmho/cm).
Low salt waters are corrosive and deplete surface soils of readily soluble minerals and all soluble salts. They often have a strong tendency to dissolve rapidly all sources of calcium from surface soils which then break-down, disperse and seal, resulting in poor water penetration (see Table 1 on guidelines). Soil and water amendments such as gypsum together with changes in cultural practices to promote improved water penetration are the usual preventive or corrective procedures used.
Permeability problems due to excesses of sodium or shortages of calcium are evaluated by a relatively new concept - the adjusted Sodium Adsorption Ratio (adj. SAR), formerly evaluated through the Sodium Adsorption Ratio (SAR) and the Residual Sodium Carbonate (RSC). This new concept adds to the older Sodium Adsorption Ratio (SAR), the effect of carbonate/bicarbonate through a theoretical and calculated pHc value added to the SAR. The pHc evaluates the tendency of the irrigation water to dissolve lime from the soil: adding to soluble calcium, or the tendency to precipitate lime: reducing the soluble calcium. The presence of appreciable carbonate/bicarbonate may markedly influence the calcium availability. This effect on SAR is evaluated by the new adjusted Sodium Adsorption Ratio equation (adj.SAR) as recently developed by the U.S. Salinity Laboratory as follows:
The values for sodium, calcium and magnesium (reported in milliequivalents per litre) are taken directly from the water analysis. The pHc is a calculated, theoretical value related 1) to the total salinity as measured by sodium + calcium + magnesium, 2) to the calcium + magnesium supply in the water, and 3) to the carbonate + bicarbonate present. Calculation of pHc is made by use of tables (see “Tables for calculating pHc Values of Haters” - Table 7). This particular adj.SAR procedure is most applicable at about 70% field efficiency of irrigation. At greater efficiency, these values may be a little low; at lower efficiency they may be a little high. Suggested guidelines for adj.SAR are given on Table 1.
There are still other permeability problems in agriculture that are unrelated to adj.SAR or to low salts which must be evaluated separately. These may include problems that are more closely related to soil texture, to compaction and plough pans, or to other soil physical as well as chemical properties.
Table 7 - TABLES FOR CALCULATING pHc VALUES OF WATERS
pHc can be calculated, using the table below; 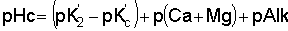
where
Tables for Calculating pHcis obtained from Ca + Mg + Na
p(Ca+Mg) is obtained from Ca + Mg
pAlk is obtained from CO3 + HCO3
|
Conct. Ca+Mg+Na (meq/l) |
|
|
Conct. Ca+Mg (meq/l) |
|
p(Ca+Mg) |
Conct. CO3+HCO3 p(Ca+Mg) (meq/l) |
|
pAlk |
|
.5 |
® |
2.11 |
.05 |
® |
4.60 |
.05 |
® |
4.30 |
|
.7 |
|
2.12 |
.10 |
|
4.30 |
.10 |
|
4.00 |
|
.9 |
|
2.13 |
.15 |
|
4.12 |
.15 |
|
3.82 |
|
1.2 |
|
2.14 |
.2 |
|
4.00 |
.20 |
|
3.70 |
|
1.6 |
|
2.15 |
.25 |
|
3.90 |
.25 |
|
3.60 |
|
1.9 |
|
2.16 |
.32 |
|
3.80 |
.31 |
|
3.51 |
|
2.4 |
|
2.17 |
.39 |
|
3.70 |
.40 |
|
3.40 |
|
2.8 |
|
2.18 |
.50 |
|
3.60 |
.50 |
|
3.30 |
|
3.3 |
|
2.19 |
.63 |
|
3.50 |
.63 |
|
3.20 |
|
3.9 |
|
2.20 |
.79 |
|
3.40 |
.79 |
|
3.10 |
|
4.5 |
|
2.21 |
1.00 |
|
3.30 |
.99 |
|
3.00 |
|
5.1 |
|
2.22 |
1.25 |
|
3.20 |
1.25 |
|
2.90 |
|
5.8 |
|
2.23 |
1.58 |
|
3.10 |
1.57 |
|
2.80 |
|
6.6 |
|
2.24 |
1.98 |
|
3.00 |
1.98 |
|
2.70 |
|
7.4 |
|
2.25 |
2.49 |
|
2.90 |
2.49 |
|
2.60 |
|
8.3. |
|
2.26 |
3.14 |
|
2.80 |
3.13 |
|
2.50 |
|
9.2 |
|
2.27 |
3.90 |
|
2.70 |
4.0 |
|
2.40 |
|
11 |
|
2.28 |
4.97 |
|
2.60 |
5.0 |
|
2.30 |
|
13 |
|
2.30 |
6.30 |
|
2.50 |
6.3 |
|
2.20 |
|
15 |
|
2.32 |
7.90 |
|
2.40 |
7.9 |
|
2.10 |
|
18 |
|
2.34 |
10.00 |
|
2.30 |
9.9 |
|
2.00 |
|
22 |
|
2.36 |
12.50 |
|
2.20 |
12.5 |
|
1.90 |
|
25 |
|
2.38 |
15.80 |
|
2.10 |
15.7 |
|
1.80 |
|
29 |
|
2.40 |
19.80 |
|
2.00 |
19.8 |
|
1.70 |
|
34 |
|
2.42 |
|
|
|
|
|
|
|
39 |
|
2.44 |
|
|
|
|
|
|
|
45 |
|
2.46 |
|
|
|
|
|
|
|
51 |
|
2.48 |
|
|
|
|
|
|
|
59 |
|
2.50 |
|
|
|
|
|
|
|
67 |
|
2.52 |
|
|
|
|
|
|
|
76 |
|
2.54 |
|
|
|
|
|
|
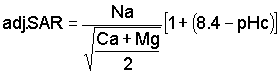
With report of water analysis
Na = 3.5 meq/l
Ca+Mg = 1.0 meq/l
Ca+Mg+Na = 4.5 meq/l
CO3+HCO3 = 3.0 meq/l
pHc = 2.21+3.30+2.5= 8.01 (from tables)
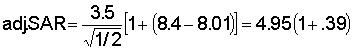
adj.SAR = 6.88
NOTE: Values of pHc above 8.4 indicate tendency to dissolve lime from soil through which the water moves; values below 8.4 indicate tendency to precipitate lime from waters applied.10. CORRECTIONS FOR PERMEABILITY PROBLEMS(ref: L.V. Wilcox, U.S. Salinity Laboratory, mimeo 30 December 1966).
Water quality problems related to soil permeability can often be prevented or corrected by either physical or chemical practices that will result in more water entering the soil.
Physical practices include:
- land grading for better water distribution and reduced run-off;Chemical amendments for correction of soil permeability problems are sometimes remarkably successful. Gypsum is the usual amendment and is added either to water or soil. Rates for addition of gypsum to water vary from about 200 to 1000 lb per acre foot of water. Rates for application to soil range from 2 to as high as 10 or 20 tons per acre. Soil tests aid in predicting amendment needs. Hater and soil analyses are essential in determining the rate of application as well as feasibility of adding gypsum.- holding the water on the field longer;
- decreasing the slope of land by land grading or by changing direction of irrigation to a lesser slope;
- collecting and recirculating tailwater runoff;
- with sprinklers, matching water application rate to less than the intake rate of soil;
- blending water supplies.
11. TOXICITY PROBLEMS
Toxicity problems due to quality of water are often very specific for a certain water constituent and for a certain crop. In general, tree crops and woody ornamentals are especially sensitive to rather low concentrations of sodium and chloride; annual crops do not exhibit this same degree of sodium or chloride sensitivity. Boron affects a wide range of crops. The interpretation of toxicity effects in the guidelines recognizes toxicities resulting from either root absorption of sodium, chloride, or boron from the soil solution or from sodium or chloride by foliar absorption through leaves wet by sprinkler irrigation.
The boron problem usually is associated with boron applied in the irrigation water but in some instances boron is present in the soil. It can be leached, but with difficulty. In general, correction of a boron problem is accomplished by changing water supplies or selecting a crop more compatible with the boron in the water supply (see table on Boron in Irrigation Waters).
Corrective or preventive action for toxicity problems due to root absorption, usually includes improved water management, leaching, and sometimes the addition of soil or water amendments. The foliar absorption problems (from overhead sprinkling) occur most generally during periods of high winds or low humidity. A change to night irrigation along with faster rotation of sprinkler nozzles to at least 1 rotation of the head per minute has sometimes greatly reduced the problem. Less frequent irrigations by sprinklers may also reduce opportunity for leaf absorption to occur.
Table 8 - BORON IN IRRIGATION WATERS
Boron toxicity in many areas is traceable to use of irrigation waters with boron content in excess of 1 ppm. The UC Ag. Extension laboratories are using the following interpretation as regards boron content of irrigation water:
|
Below 0.5 mg/l |
Satisfactory for all crops. |
|
0.5 - 1.0 mg/l |
Satisfactory for most crops; sensitive crops may show injury (may show
leaf injury but yields may not be affected). |
|
1.0 - 2.0 mg/l |
Satisfactory for semi-tolerant crops. Sensitive crops are usually reduced
in yield and vigour. |
|
2.0 - 10.0 mg/l |
Only tolerant crops produce satisfactory yields. |
RELATIVE TOLERANCE OF PLANTS TO BORON
(In each group the plants first named are considered as being more sensitive and the last named more tolerant)
|
SENSITIVE |
SEMI-TOLERANT |
TOLERANT |
|
0.5 mg/l |
1 mg/l |
2 mg/l |
|
Lemon |
Lima Bean |
Carrot |
|
Grapefruit |
Sweet Potato |
Lettuce |
|
Avocado |
Bell Pepper |
Cabbage |
|
Orange |
Tomato |
Turnip |
|
Thornless Blackberry |
Pumpkin |
Onion |
|
Apricot |
Zinnia |
Broad Bean |
|
Peach |
Oat |
Gladiolus |
|
Cherry |
Milo |
Alfalfa |
|
Persimmon |
Corn (maize) |
Garden Beet |
|
Kadota Fig |
Wheat |
Mangel |
|
Grape (Sultanina & Malaga) |
Barley |
Sugarbeet |
|
Apple |
Olive |
Palm (Phoenix canariensis) |
|
Pear |
Ragged Robin Rose |
Date Palm (dactylifera) |
|
Plum |
Field Pea |
Asparagus |
|
American Elm |
Radish |
Athel (Tamarix aphylla) |
|
Navy Bean |
Sweet Pea |
10 mg/l |
|
Jerusalem Artichoke |
Pima Cotton |
|
|
Persian (English) Walnut |
Acala Cotton |
|
|
Black Walnut |
Potato |
|
|
Pecan |
Sunflower (Native) |
|
|
1.0 mg/l |
2 mg/l |
|
Adopted from USDA Tech. Bull. No. 44812. MISCELLANEOUS PROBLEMS
As briefly mentioned previously, miscellaneous problems include a mixed group such as excessive nutrients, white deposits on fruit or leaves, and other occasional abnormalities that may be suspected to be caused by the water used for irrigation. Guideline values are given.
Nitrogen sometimes occurs in water supplies. Nitrogen is a recognized fertilizer nutrient which promotes growth of both crops and algae. If present, it may need to be considered, particularly in planning the crop fertilizer programme or in selection of an adapted crop. Certain crops such as sugar beets, grapes, apricots, citrus and a few others are sensitive to excessive nitrogen. The type of problem will depend on the crop but may include reduced yield, lower quality, or delayed maturity.
Bicarbonates in water applied by sprinklers has caused objectionable white deposits to form on fruit or leaves of some crops (grapes, leafy ornamentals, apples). The occurrence and correction is similar to that discussed for correction of foliar absorption problems - change to night irrigation, increase speed of rotation of sprinkler heads, decrease numbers of irrigation where possible.
Under this grouping of miscellaneous problems pH is included; pH values for waters normally fall within the range of 6.5 to 8.4 and if outside this range, may indicate that other problems may be present and should be studied.
13. TYPICAL WATER ANALYSES FROM WESTERN USA
For comparison purposes, several typical water supplies are evaluated in Table 9.
Table 9
|
|
|
Colorado River (imp. Dam-9/8/71) |
Pecos River (1945-46 USDA Handbook 60) |
San Joaquin River (Vernalis 9/14/71) |
Calif. Aqueduct (San Luis Res.-8/7/68) |
Sacramento River (Fremont weir 5/17/72) |
|
ECw |
(mmho/cm) |
1.3 |
3.2 |
0.9 |
0.5 |
0.19 |
|
Ca+Mg |
(meq/l) |
7.3 |
26.5 |
4.2 |
3.6 |
1.40 |
|
Na |
(meq/l) |
6.1 |
11.5 |
4.4 |
1.4 |
0.83 |
|
CO3+HCO3 |
(meq/l) |
3.1 |
3.2 |
3.1 |
1.4 |
1.51 |
|
Cl |
(meq/l) |
3.1 |
12.0 |
4.2 |
2.9 |
0.28 |
|
B |
(mg/l) |
0.2 |
- |
0.4 |
0.2 |
0.1 |
|
NO3-N |
(mg/l) |
- |
- |
0.8 |
0 |
0.3 |
|
pH |
|
8.1 |
- |
8.0 |
7.9 |
7.6 |
|
SAR |
|
3.2 |
3.2 |
3.0 |
1.0 |
1.0 |
|
adj.SAR |
|
6.5 |
7.8 |
5.8 |
1.6 |
1.2 |
|
|
|
Moderate salinity problem expected; low potential to develop permeability
problem; select crops for salinity and sodium tolerance; supply extra
water to meet LR for crop and water supply. |
Severe salinity problem expected. Permeability problem not expected since
high salinity should maintain permeability. Probable sodium and chloride
toxicities to sensitive crops. Select crops carefully, meet LR for crop
and water. Monitor crops and soil. |
Low to moderate salinity problem expected. Some slight toxicity effects
if crop leaves are wet by sprinklers. Select crops for moderate tolerance
to salinity. Monitor soil and crop. |
No potential water quality problems indicated. |
A moderately severe permeability problem expected due to low salt content.
Evaluate amendments. |
|
|
|
Friant-Kern Canal |
#1 |
#2 |
#3 |
#4 |
#5 |
|
(Friant Dam 5/9/65) |
Well Water |
Well Water |
Well Water |
Well Water |
Well Water |
||
|
ECw |
(mmho/cm) |
0.04 |
0.6 |
0.4 |
1.1 |
0.9 |
0.35 |
|
Ca+Mg |
(meq/l) |
0.22 |
2.59 |
0.66 |
1.7 |
6.4 |
0.2 |
|
Na |
(meq/l) |
0.17 |
3.90 |
3.46 |
9.4 |
2.6 |
3.3 |
|
CO3+HCO3 |
(meq/l) |
0.28 |
4.70 |
3.27 |
7.1 |
1.25 |
1.1 |
|
Cl |
(meq/l) |
0.06 |
0.83 |
0.59 |
2.0 |
2.0 |
1.0 |
|
B |
(mg/l) |
0 |
- |
- |
1.2 |
0.12 |
- |
|
NO3-N |
(mg/l) |
1.6 |
0.12 |
0.06 |
0 |
28.0 |
- |
|
pH |
|
7.2 |
- |
- |
8.1 |
6.8 |
8.8 |
|
SAR |
|
0.5 |
3.4 |
6.0 |
10.2 |
1.4 |
10.4 |
|
adj.SAR |
|
- |
6.4 |
7.3 |
14.9 |
2.5 |
3.2 |
|
|
|
A very severe permeability problem is expected due to extreme purity
of the water. Evaluate use of water amendments. |
No more than a slight permeability problem is expected due to adj.SAR.
Possible sodium toxicity for sensitive crops. |
Slight to moderate permeability problem is expected. Possible sodium
toxicity for sensitive crops. Evaluate need for amendments. |
Moderate potential for salinity problem. Strong probability of permeability
problem. Select crops carefully; meet LR for crop and water; monitor soil
and crop and evaluate need for amendments. |
Low to moderate potential for salinity problem. Very high nitrogen (76
lb N/acre ft of water). Select crops, especially for high N adaptability. |
Moderate permeability problem expected due to low salts and high SAR
(in absence of source of Ca). If sprinklers used, adjust application rate
and evaluate need for an amendment. |
Bernstein, L. 1964. Salt tolerance of plants. USDA Agric. Inf. Bull. No. 283.
Bernstein, L. and Francois, L.E. 1973. Leaching requirement studies; sensitivity of alfalfa to salinity of irrigation and drainage waters. Soil Sci. Soc. Amer. Proc. 37:931-943.
Bernstein, L. and Francois, L.E. 1975. Effects of frequency of sprinkling with saline waters compared with daily drip irrigation. U.S. Salinity Laboratory (in press).
Bower, C.A. et al. 1965. An index of the tendency of CaCO3 to precipitate from irrigation waters. Soil Sci. Soc. Amer. Proc. 29:91-92.
Bower, C.A., Ogata, G., and Tucker, J.M. 1967. Sodium hazard of irrigation waters as influenced by leaching fraction and by precipitation or solution of calcium carbonate. Soil Sci. 100:29-34.
Doneen, L.D. (ed.) 1958. Quality of water for irrigation. Proceedings of a Conference on Quality of Water for Irrigation, Water Resources Center Contribution No. 14, 208 p.
Doneen, L.D. 1964. Water quality in agriculture. Department of Irrig., Univ. of Calif., Davis, 48 p.
FAO/Unesco. 1973. Irrigation, Drainage and Salinity - An International Sourcebook. Paris, Unesco/Hutchinson, London, 510 p.
Lunt, O.R. (ed.) 1963. Agricultural Water Quality Research Conference Proceedings. University of California Water Resources Center Report No. 5, 69 p.
Rhoades, J.D. 1971. Quality of water for irrigation. Soil Science 113:277-284.
Rhoades, J.D. et al. 1973. Salts in irrigation drainage waters. II. Effects of irrigation water composition, leaching fraction and time of year on salt precipitation, soil mineral weathering and the salt burdens of irrigation drainage waters. Manuscript from U.S. Salinity Laboratory, Riverside, Calif., 20 p.
U.S. Salinity Laboratory Staff. 1954. Diagnosis and improvement of saline and alkali soils. USDA Handbook 60, 160 p.
Wilcox, L.V. 1966. Tables for calculating the pHc values of waters. Mimeo. of U.S. Salinity Laboratory, 8 p.
by
A. Monem Balba
Prof. of Soil and Water Science
University of Alexandria, Alexandria
1. INTRODUCTION
Soil salinization, alkalization and waterlogging are complex phenomena; they are closely related to water movement in the soil profile and affected by several factors such as:
i. climatic conditions;Predicting that a certain area is subject to salinization or alkalization is not difficult. The complexity of the problem arises when attempts are made to quantify the prediction. The role of the factors mentioned has been demonstrated, but the quantitative evaluation of their effect in relation to each other still needs further consideration.ii. soil properties including texture, topography, presence of indurated layers and hydraulic conductivity;
iii. groundwater characteristics including depth, slope, direction and salt concentration and composition;
iv. irrigation, including amount of water added at each irrigation, frequency and method of irrigation; surface or subsoil;
v. water quality; salt concentration, cation and anion composition;
vi. vegetative cover;
vii. human activities.
This report presents results and findings of interest in the processes of salinization, alkalization and waterlogging related to some of these factors; others, not discussed herein, have been aptly presented by members of this panel. Tentative guidelines for prognosis are suggested.
2. THE SALINIZATION PROCESS
2.1 Climatic Conditions
The components of the climatic conditions which are involved in the soil salinization phenomenon are those related to evaporation from bare soil surfaces and evapotranspiration from soils with vegetative cover. These components are air temperature, rainfall, relative humidity, wind velocity, relative duration of bright sunshine and solar radiation.
Using meteorological records, several investigators were able to evaluate evaporation from bare soils and evapotranspiration from cultivated soils. Among the methods used for predicting potential evapotranspiration are those of Blaney and Criddle, Penman and Rijtema. If the salt concentration in the soil solution is known, an estimation of the accumulated amounts of salts due to evaporation and evapotranspiration processes can be made. Use of sub-irrigation makes such calculation feasible since a regular flow of water from underground to the soil surface and/or the plants takes place.
In an attempt to express quantitatively the process of salinization due to capillary movement and subsequent evaporation, the writer (Balba and Soliman, 1969) used the following relationship:
DS = DWRCW + WECWwhere DS is the increase in soil column content of salts after a period of time, WR is the liquid water retained by the soil column, WE is the evaporated water in the period of time and CW is the salt concentration in the saline groundwater. If plants are growing in the soil, the problem is not so simple, as shown in an unpublished work by the writer and his co-workers (Balba and Soliman, 1975). When applying saline water in sub-irrigation, actual evapotranspiration decreased with the increase in the salt concentration of water. Accordingly, the term WECW does not equal the amount of salt accumulated in the soil due to evapotranspiration when the value of WE is calculated from any of the above-mentioned methods.
Calculated evapotranspiration of Sudan grass using the Rijtema equation (1973) was 5.72 mm/day or 4.378 l in the period of the experiment (25 days). The actual ET was 5.79 mm/day or 4.651 l when tap water was used for irrigation and 4.234 l and 3.886 l with the use of solutions containing 15 or 30 meq/l. Thus, the calculation of the term WECW is not correct under saline conditions.
Evaporation and evapotranspiration depend on climatic conditions. Balba and Soliman (1969) showed that evaporation from a loamy soil with a shallow water table was 0.43 mm/day in July and dropped to 0.11 mm/day in September. Rijtema and Aboukhaled (1973) prepared tables for maximum atmospheric evaporative demand in several agroclimatological regions in Egypt; the values varied each month and in each region.
2.2 Soil Properties related to Water Movement
It has been mentioned above that soil water movement is the basic criterion in salt accumulation in the soil. This movement is controlled by the potential differences between different points in the soil-water system. The water tends to move from a position of higher to one of lower potential. Several factors are involved in the capillary movement of water in soil among which are:
i. Soil texture2.3 Soil Salinization due to Irrigation with Saline WaterFrom a water table 50 cm deep, the water reached the soil surface after 1 day in columns of loamy soil, 16 days in sandy soils and 28 days in clay soils (Balba and Soliman, 1969). Balba and Soliman showed that rates of evaporation from loamy, sandy and clay soil columns in July were 0.43, 0.20 and 0.12 mm/day.
ii. Depth of groundwater table
The water content of the upper part of the loamy soil column (0-10 cm) decreased from 33 to 27 and 25 percent with the increase in the length of the soil columns above the water table from 50 cm to 80 cm and 110 cm, respectively. As the water decreases, the suction of the soil surface layers increases and the evaporation rate approaches a limiting value which cannot be exceeded no matter what the potential evaporation rate. After 100 days, the rates of evaporation from the loamy soil columns, 50, 80 and 110 cm long above the water table, were 0.35, 0.29 and 0.17 mm/day, respectively. The concentration of salts in the upper 0-2.5 cm layer in the soil columns was 112, 74 and 63 meq/l, respectively (Balba and Soliman, 1969). Elgabaly and Naguib (1965) studied the effect of depth and salt concentration of the groundwater table on the soil salinization process using lysimeters planted with cotton and with under surface irrigation. Their study showed that: when the groundwater table was kept at 50 cm from the soil surface, the increase in the salinity of the upper 20 cm was very pronounced. At a depth of 90 cm, the soil salinity was less than 1/3 of its level at a depth of 50 cm, and the effect of differences in the salinity of the groundwater was not very pronounced. They concluded that the depth of groundwater contributed more to the salinity of the soil surface than the salinity level of the groundwater.
If salt accumulation in the soil due to surface application of saline water is considered separately from other processes such as capillary movement of water, plant absorption or evaporation, the following equation represents the salt balance:
SF = Si + RC - S0where SF and Si are the final and initial salt content of a soil layer, R is the amount of water retained by the soil layer, C is the salt concentration of the applied water and S0 is the amount of salts removed from the soil layer with the water front during its passage from one layer to the other. Under free drainage conditions the writer and his co-worker (Balba, 1965) showed that:
i. the soils differ in the amount of salt retained according to the amount of water retained;2.4 Effect of Vegetative Soil Cover on Soil Salinizationii. using Na22, the amount of salts removed from the soil was found to decrease with the increase in the salt concentration of applied water;
iii. when the soil columns were leached with increasing amounts of the salt solution, the final salt content of the soil column did not materially vary with variations in the amount of added water;
iv. the final soil salt content after one leaching with saline water was almost the same after 2 or more leachings. An equilibrium state takes place in which the retained amount of salt from the applied water equals the removed amount in each application.
Several investigators have studied the role of soil vegetative cover on soil salinization. Unpublished work by Balba and Soliman shows that:
i. the amount of water lost to the atmosphere from bare soil drums with a shallow water table was almost doubled when Sudan grass was grown in August (2.172 vs. 4.651 l in 25 days);Salt accumulation as a result of the soil vegetative cover varies considerably according to all factors which affect plant growth since evapotranspiration is a biological process. Among these factors are: plant morphology especially the root system distribution, rate and pattern of planting, plant tolerance to salinity, water regime, water salinity, depth of groundwater table and the climatic conditions, and the method of irrigation: surface vs. subsurface.ii. the absorption of water by plants greatly exceeded the absorption of salts dissolved in the applied water - hence salts accumulated in the root-system zone of the soil;
iii. the increase in salt concentration of the applied water decreased the evapotranspired water (4.651 l of tap water vs. 3.886 l from a solution containing 30 meq/l of NaCl);
iv. typical salt distribution in bare soil profiles with a shallow, saline groundwater under arid conditions is characterized by a maximum concentration at the soil surface followed by a sharp drop in the subsoil and a gradual decrease with depth to a minimum concentration which is equal to the salt concentration of the groundwater. Under vegetative cover, the salt distribution in the soil profile changes. The following features characterize salt distribution in cultivated soil (as if in the case of subsurface irrigation), as compared with bare soil. It was noted that maximum salt accumulation was not at the soil surface, but rather at a depth of about 10 cm; actually it followed the intensity of the root system. The bare soil drums accumulated 181.6 meq of salts, of which 115.7 meq were in the upper 5 cm, while the cultivated soil drums accumulated 236.1 meq with 38.2 meq in the upper 5 cm after 25 days using a solution containing 30 meq NaCl/l.
THE ALKALIZATION PROCESS
The processes which result in an increase in the exchangeable sodium and sodium carbonate in the soil are:
i. desalination of saline sodic soils which do not contain a supply of calcium;WATERLOGGINGii. irrigation of soils with water containing residual
and high SAR values;
iii. groundwater rich in
and with high SAR values have the same effect as irrigation water of the same quality;
iv. the microbial processes of sulphate reduction under anaerobic conditions.
Soils saturated with water are said to be waterlogged. In waterlogged soils, anaerobic conditions, unfavourable to plant growth, may prevail. Salinization is usually due to the upward movement of water and subsequent evaporation as mentioned above. Alkalization also may occur because of waterlogging.
Conditions which cause waterlogging are:
i. seepage of water from irrigation canals;Egypt first suffered from waterlogging when irrigation was changed from basin to permanent irrigation depending on an elaborate network of canals. A drainage system had to be established. The problem also became acute in the newly reclaimed area (200 000 ha) west of the delta “west of the Nubaria Canal Project”, threatening the whole project and the adjacent low-lying area east of the Nubaria canal which has been under cultivation for a very long time.ii. rise of the groundwater table due to excessive irrigation;
iii. presence of impermeable layers in the soil profile;
iv. areas of low relief adjacent to or surrounded by areas of relatively higher relief which usually receive water from these surrounding areas.
The problems of salinization and waterlogging are related to each other. Wherever waterlogging takes place, salinization is a result.
5. TENTATIVE GUIDELINES
Predicting soil salinization, alkalization and waterlogging requires extensive investigations directed towards the groundwater, the soil, the irrigation system, the quality of irrigation water, the cropping system and the climatic conditions.
5.1 Predicting Waterlogging
To predict that waterlogging will take place in an area the following groups of investigations must be carried out. These investigations aim to analyse the problem in its various elements and to determine their individual characteristics. Thus there should be an understanding of how these elements - or groups - interrelate and function.
|
Group A |
Groundwater investigations |
|
|
These investigations should cover all aspects concerning the groundwater
such as: depth from soil surface, salt concentration and composition,
the hydraulic properties including slope, direction and rate of flow. |
|
Group B |
Soil investigations |
|
|
These include: the soil profile, the soil texture, soil-water constants,
presence of indurated layers and their composition, hydraulic conductivity
and exchangeable cations. |
|
Group C |
Irrigation investigations |
|
|
These include: the method of irrigation (surface or subsoil), design
of the network of canals, the amount of water to be delivered, and the
frequency of irrigation. |
|
Group D |
Agronomic investigations |
|
|
Include: the most suitable cropping system, the water consumptive use
of each crop and each rotation, and the water regime of each crop. |
i. from the irrigation method, the distribution network of the canals, the amount of water and frequency of irrigation, the amount of water seepage from the canals downward to the groundwater and through the canal borders to the adjacent land;5.2 Predicting Soil Salinization due to Upward Movement of Groundwater and its Subsequent Evaporationii. from the amount of water applied at each irrigation, the number of irrigations, the evapotranspiration of each crop and each crop rotation and the soil properties, the amount of excess water which replenishes the groundwater; in this group of calculations any artificial drainage system should be taken into consideration;
iii. of the water balance, since the inflow of water to the groundwater and its outflow are known.
Thus it is possible to know the rate of the groundwater table rise towards the soil surface.
The time after which the groundwater reaches the critical depth as well as the time it needs to saturate the soil can be calculated. If this study is not feasible, the alternative is to establish a pilot project and measure the necessary records.
The above-mentioned groups of investigations, which constitute the basic elements of the problem of waterlogging, are the same for predicting soil salinization. For the interrelationships between these elements from the standpoint of their effect on soil salinization, the following points should be considered:
i. The following conditions enhance the salinization process:Quantifying the prediction requires weighting these factors in relation to each other. A mathematical model which takes into consideration these weighted variables simultaneously might give an answer to this problem.
shallow groundwater table, 50-75 cm,ii. The following conditions retard the salinization process:
salt concentration in the groundwater,
medium soil texture,
arid warm climate,
long periods between each irrigation,
subsurface irrigation,
inefficient drainage system,
water consumptive use of crops,
water regime of crops (cereals vs. rice),
fallowing in dry seasons.
deep groundwater,
surface irrigation,
efficient drainage,
crops grown under ponded conditions,
humid, cold climates.
5.3 Predicting Soil Salinization due to Irrigation with Saline Water
Several systems were established in different countries for classifying the suitability of water for irrigation according to its salinity levels. According to these systems, predicting soil salinization should be possible. However, the writer points out that the potential salinization of soils irrigated with saline waters depends not only on the water salinity level but on several other conditions such as:
the irrigation regime, especially the period between irrigations,To predict quantitatively the salinity level of a soil irrigated with water of a certain level of salts, the following information is required:
the amount of applied water,
the method of irrigation,
the depth of the groundwater,
the hydraulic conductivity,
the efficiency of drainage systems,
the climatic conditions,
the soil texture,
the presence of impermeable layers and their depth,
the kind of vegetative cover,
the soil topography.
i. the amount of salts accumulated in the soil from each irrigation as a result of evapotranspiration by the growing crop under the prevailing conditions;The salt balance can be calculated, from which the net amount of accumulated salt will be known. Two elements constitute the salt balance:ii. the amount of salts displaced by the water of each irrigation and removed from the soil;
iii. the amount of salt which might be displaced and removed by the rainfall.
a. the gain of salts which can approximately be evaluated from the product of potential evapotranspiration times the salt concentration in water. Distribution of salts in the soil, however, remains to be studied;Studying the salt balance of irrigated soils, Szabolcs (1972) suggested the following equation:b. the loss of salts displaced with each irrigation or with rainfall. This element might approximately be determined by applying equations used for calculating the salt movement with water, such as that suggested by Gardner and Brooks (1957), Terkeltraub and Babcock (1971) or other equations.
where:
b = soluble salt content of the soil at the end of observations, mg/100 g soil.He stated that the salt regime gives the change that occurred in the salt content of the soil during the observation period. It gives the difference between the amount of salts leached from the soil and the amount of salts that got into the soil from sources other than the irrigation water.a = soluble salt content of the soil at the beginning of observation, mg/100 g soil.
c = salt concentration of the irrigation water g/l.
v = quantity of the irrigation water applied during the observation period, m3/ha.
M = thickness of the soil layer for which the salt balance was established, m.
tfs = bulk density of the soil.
d = salt regime coefficient of the soil, g/100 g soil.
5.4 Predicting Soil Alkalization
The conditions leading to soil alkalization have been described earlier. Thermodynamic
studies of the cation exchange reaction offer a means to calculate the exchangeable
sodium percentage when Na rich water is used for irrigation. The concept of
sodium adsorption ratio SAR, widely used in water classification according to
its sodium hazards, is a direct application of the thermodynamic approach (Richards,
1954). Also, recognizing the effect of 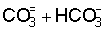 on the solubility of calcium, the residual
on the solubility of calcium, the residual 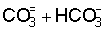 concept was used as a parameter for potential alkalization of the soil when
irrigated with waters containing residual
concept was used as a parameter for potential alkalization of the soil when
irrigated with waters containing residual 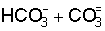 (Richards, 1954). Also in this regard, based on thermodynamic considerations
and experimental results, the writers (Balba and Balba, 1972) showed that
(Richards, 1954). Also in this regard, based on thermodynamic considerations
and experimental results, the writers (Balba and Balba, 1972) showed that 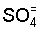 rich waters are more Na hazardous than Cl- rich waters. However,
the exchange reaction between the sodium of the water and the soil exchangeable
calcium takes place under flow conditions and not in a closed system under which
SAR standards are calculated.
rich waters are more Na hazardous than Cl- rich waters. However,
the exchange reaction between the sodium of the water and the soil exchangeable
calcium takes place under flow conditions and not in a closed system under which
SAR standards are calculated.
Brooks et al. (1957) showed the applicability of the following approximated equations for use in the field for irrigation waters containing a high proportion of sodium:
and,
where:
K' is the apparent second order equilibrium constant as approximated for exchange of cations having unequal valence, dimensionless.
|
C0: |
total cation concentration of the irrigated water in meq/ml. |
|
Q: |
cation exchange capacity of soil in meq/g. |
|
D: |
depth of irrigation water applied, cm. |
|
qNa: |
concentration of Na in the adsorbed phase at a specified depth (Z) in
the column, meq/g. |
|
CNa: |
concentration of Na in the solution phase at a specified depth (Z), meq/ml. |
|
Z: |
depth of soil, cm. |
|
b: |
dry bulk density of soil g/cc. |
|
f: |
ratio of void space occupied by dilution to total volume of column, dimensionless. |
The potential alkalization during the desalinization of saline sodic soil might be predicted by the direct determination of the soil content of gypsum and calcium carbonate. In the presence of neutral salts and excess water, the supply of Ca++ from the soil gypsum and lime prevents soil alkalization.
Balba and El Laithy (1968) suggested the following simplified test: the soluble Ca is determined in the soil paste extract and in a 1:100 soil-water extract. The increase of Ca++ in the latter extract, per 100 g soil, above Ca++ in the former, indicates that the soil contains Ca-compounds which dissolve in excess water giving additional Ca++. If otherwise, alkalization will develop in this soil during or after the completion of the leaching process.
REFERENCES
Balba, A.M. 1962a. Effect of waters with different sodium and carbonate concentrations on the chemical properties and the growth and composition of plants. J.S.S. UAR 1:85-97.
Balba, A.M. 1962b. The calculation of exchangeable sodium percentage from the cation composition of the soil extract. J.S.S. UAR 2:241-252.
Balba, A.M. 1965. A quantitative study of the salinization and desalinization processes of soil columns. Trans. Sodic Soils Symp. Bpst. Agrok. es Talaj. 14: 351-385.
Balba, A.M. and El Laithy, A. 1968. A laboratory study of the leaching process of saline alkali soil from the north of the Nile Delta. J.S.S. UAR 8:87-98.
Balba, A.M. and Soliman, M. 1969. Salinization of homogeneous and layered soil columns due to upward movement of saline groundwater. Agrochimica 13:542-550.
Balba, A.M. and Balba, Ali. 1972. Effect of anion composition of the solution phase on the cation exchange reaction. Intrnl. Symp. Salt-affected Soils, Cairo, 1972. Proc. pp. 361-368.
Balba, A.M. and Bassiuni, H. 1972. A study of soluble and exchangeable cations movement in soil columns using Na22. Presented in Arabic, Symp. on Use of Isotopes in Agric. Res. Damascus, 1972.
Balba, A.M. and Soliman, M. 1975. Effect of concentration and composition of shallow soil water table on evaporation and evapotranspiration. In preparation.
Bower, C.A. and Goertzen, J.O. 1958. Replacement of sodium in soils as a result of hydrolysis of calcium carbonate. S.S.S. A.P. 22:33-35.
Brooks, R.H., Goertzen, J.O. and Bower, C.A. 1958. Prediction of changes in the composition of the dissolved and exchangeable cations in soils upon irrigation with high sodium water. S.S.S. A.P. 22:122-124.
Elgabaly, M.M. and Naguib, H.M. 1965. Effect of depth and salt concentration of groundwater on salinization of soil. Int. Sodic Soils Symp. Bpst. Agrok es Talaj. 14:369-376.
Gardner, W.R. and Brooks, R.H. 1957. A descriptive theory of leaching. S.S. 83:293-304.
Richards, L.A. 1954. Diagnosis and improvement of saline and alkali soils. USDA. Agric. Handbook No. 60.
Rijtema, P.E. and Aboukhaled, A. 1973. A review of some experimental work on crop-water use in Egypt. FAO Report. In press.
Szabolcs, I. 1972. Salt balance in salt affected soils. Intrnl. Symp. Salt-affected Soils, Cairo, 1972. Proc. pp. 23-40.
Terkeltraub, R.T. and Babcock, K.L. 1971. Calculation of the leaching required to reduce the salinity of a particular soil depth beneath a special value. S.S.S. A.P. 35:411-414.