Appendix I. List of Participants
Appendix II. Draft Guidelines for Generic Official Certificate Formats and the Production and Issuance of Certificates
Appendix III. Proposed Draft Guidelines on the Judgement of Equivalence of Sanitary Measures Associated with Food Inspection and Certification Systems
Appendix IV. Proposed Draft Guidelines for Food Import Control Systems
LISTE
DES PARTICIPANTS
LISTA DE PARTICIPANTES
| Chairperson: |
Mr Digby Gascoine |
|
Président: | Technical
Advisor |
|
Presidente: | Australian
Quarantine & Inspection Service |
|
| Agriculture Fisheries &
Forestry -Australia |
|
| GPO Box 858 |
| |
Canberra ACT 2601 |
|
| Australia |
Dr
Alfredo Jorge Nader
Director Nacional de Alimentación
Secretaria
de Agricultura, Gañaderia
Pesca y Alimentación
2° Piso
of 238
Buenos Aires
Argentina
Phone: +54 11 4349 2054/2096
Fax: +54
11 4349 2097
Email: [email protected]
Ing. Fernando Lavaggi
Director
Funcionario
del Servicio Nacional de Sanidad
Agroalimentaria (SENASA)
Director de Fiscalización
Av
Paseo Colon 367 Piso 7
Capital Federal
Buenos Aires
Argentina
Phone:
+54 11 4902 3041
Fax: +54 11 4343 0644
Email: [email protected]
AUSTRALIA
AUSTRALIE
Dr
Bob Biddle
General Manager
Meat & Food Services Policy
Australian
Quarantine and Inspection Service
Agriculture, Fisheries and Forestry -Australia
GPO
Box 858
Canberra ACT 2601
Australia
Phone: +61 2 6272 5364
Fax: +61
2 6271 6522
Email: [email protected]
Ms Peggy Douglass
Senior Food
Technologist
Meat and Food Services Policy
Australian Quarantine and Inspection
Service
Agriculture, Fisheries and Forestry-Australia
GPO Box 858
Canberra
ACT 2601
Australia
Phone: +61 2 6272 5786
Fax: +61 2 6271 6522
Email:
[email protected]
Mr Peter Maple
Program Manager
Imported Foods
Australian
Quarantine and Inspection Service
Agriculture, Fisheries and Forestry - Australia
GPO
Box 858
Canberra ACT 2601
Australia
Phone: +61 2 6272 5419
Fax: +61
2 6272 3682
Email: [email protected]
Dr Paul Vitolovich
Manager
Trade
Policy
Market Access and Biosecurity
Agriculture, Fisheries and Forestry
-Australia
GPO Box 858
Canberra ACT 2601
Australia
Phone: +61 2 6272
5673
Fax: +61 2 6272 4600
Email: [email protected]
Mr Steve
Crossley
Program Manager, Monitoring and Evaluation
Australia New Zealand
Food Authority
PO Box 7186
Canberra Mail Centre
Canberra ACT 2610
Australia
Phone:
+61 2 6271 2624
Fax: +61 2 6271 2278
Email: [email protected]
Ms
Brigid Hardy
Senior Food Scientist
Food Safety
Australia New Zealand
Food Authority
PO Box 7186
Canberra Mail Centre
Canberra ACT 2610
Australia
Phone:
+61 2 6271 2612
Fax: +61 2 6271 2278
Email: [email protected]
Mr
Greg Roche
General Manager
Food Safety, Legal and Evaluation
Australia
New Zealand Food Authority
PO Box 7186
Canberra Mail Centre
Canberra
ACT 2610
Australia
Phone: +612 6272 2285
Fax: +61 2 6271 2278
Email:
[email protected]
Ms Nola Tomaska
Senior Policy Officer
Strategic
Development Program
Australia New Zealand Food Authority
PO Box 7186
Canberra
Mail Centre
Canberra ACT 2610
Australia
Phone: +61 2 6271 2602
Fax:
+61 2 6271 2278
Email: [email protected]
Mr Dave Jeffries
Quality
Assurance Coordinator
Agwest Trade and Development
Agriculture Western Australia
3
Baron-Hay Crt
South Perth WA 6151
Australia
Phone: +61 8 9368 3984
Fax:
+61 8 9367 7389
Email: [email protected]
Ms Christine Kershaw
Manager
Safe
Quality Food (SQF) Australia
Agriculture Western Australia
3 Baron-Hay Crt
South
Perth WA 6151
Australia
Phone: +61 8 9368 3203
Fax: +61 8 9367 7389
Email:
[email protected]
Mr Ian Longson
Executive Director
Program Coordination
Industries
Program
Agriculture Western Australia
3 Baron-Hay Court
South Perth WA
6151
Australia
Phone: +61 8 9368 3405
Fax: +61 8 9474 5974
Email:
[email protected]
Mr John Noonan
Operations Manager
Safe Quality
Food (SQF) Australia
Agriculture Western Australia
Locked Bag No. 4
Bentley
Delivery Centre WA 6983
Australia
Phone: +61 8 9368
Fax: +61 8 9474
Email:
[email protected]
Mr Don Nicholls
Project Manager
Western Australian
Seafood Quality Management
Initiative
Fisheries Western Australia
221
St Georges Tce
Perth WA 6000
Australia
Phone: +61 8 9424 3167
Fax:
+61 8 9322 7150
Email: [email protected]
Mr Peter Rutherford
Chemicals
Coordinator
Chemical Services
Agriculture Western Australia
3 Baron-Hay
Crt
South Perth WA 6151
Australia
Phone: +61 8 9368 3688
Fax: +61
8 9474 2408
Email: [email protected]
Mr Tony Downer
Assistant
Director, Scientific and Technical
Australian Food and Grocery Council
Locked
Bag 1
Kingston ACT 2604
Australia
Phone: +61 2 6273 1466
Fax: +61
2 6273 1477
Email: [email protected]
Mr Victor Hatch
State Secretary
(Western Australia)
Food Inspection Section CPSU
5260 Bunning Road
Gidgegannup
W A 6083
Australia
Phone: +61 8 9574 6162
Fax: +61 8 9574 6162
Email:
[email protected]
Ms Gae Pincus
81 Ferry Road
Glebe NSW 2037
Australia
Phone:
+61 2 9692 0097
Fax: +61 2 9692 0257
Email: [email protected]
Mr
Terrence Richards
Quality Assurance Manager
George Weston Foods Limited
PO
Box 539
Fremantle WA 6959
Australia
Phone: +61 8 9418 0738
Fax: +61
8 9418 5836
Email: [email protected]
Mr Phillip Richardson
Quality
Manager
Australian Dairy Corporation
Locked Bag 104
Flinders Lane VIC
8009
Australia
Phone: +61 3 9694 3785
Fax: +61 3 9694 3754
Email:
[email protected]
Miss Jennifer Smith
Manager Inspection
National Association
of Testing Authorities
(NATA)
71 - 73 Flemington Road
Nth Melbourne VIC
3051
Australia
Phone: +61 3 9329 1633
Fax: +61 3 9326 5148
Email:
[email protected]
Mr Bob Weston
Business Development Manager
Food
Safety System
Quality Assurance Services
Locked Bag 8
East Perth WA 6892
Australia
Phone:
+61 8 9221 6800
Fax: +61 8 9221 6900
Email: [email protected]
BELGIUM
BELGIQUE
BÉLGICA
Prof.
Dr Marc Cornelis
Director
Veterinary Policy
Institute for Veterinary
Inspection
Wetstraat 56
1040 Brussels
Belgium
Phone: +32 2 2870 253
Fax:
+32 2 2870 239
Email: [email protected]
BOTSWANA
Mrs
Barulaganye Machacha
Director
District Administration and Food Relief Services
Ministry
of Local Government
Private Bag 443
Gaborone
Botswana
Phone: +267
353 340
Fax: +267 373 483
Email: [email protected]
BRAZIL
BRÉSIL
BRASIL
Mr
Daniel Lins Menucci
Deputy Director
Ports, Airports, Borders and International
Affairs
Brazilian Health Surveillance Agency
SEPN 515 Bloco B Ed. Omega
3°Andar
Brasilia
- DF 70770-520
Brazil
Phone: + 5561448 1227
Fax: + 5561448 1107
Email:
[email protected]
Mr Alexandre Moreira Palma
Fiscal Federal Agropecuario
Posto
de Vigilancia Agropecuaria en el
Aeropuerto Internacional Salgado Filho
Ministerio
de Agricultura y Abastecimiento
Av. Loureiro da Silva, 515/710
Porto Alegre
- Rio Grande do Sul
Brazil CEP 90 010 -Y20
Phone: + 55 51 371 4177
Fax:
+ 55 51 371 4177
Email: [email protected]
Mr Carlos Eduardo Tedesco Silva
Fiscal
Federal Agropecuario
Ministerio de Agricultura y Abastecimiento
Brazil
Phone:
+ 5561 218 2684
Fax: + 55 61 218 2672
Email: [email protected]
BRUNEI
DARUSSALAM
BRUNÉI DARUSSALAM
Mr Jomari Haji Ahmad
Agriculture
Officer
Plant Quarantine Unit
Department of Agriculture
Ministry of Industry
and Primary Resources
Bandar Seri Begawan
Brunei Darussalam
Phone: +673
2 380 144
Fax: +673 2 382 226
Dr Dabeding Haji Dullah
Veterinary Officer
Animal
Quantine Unit
Department of Agriculture
Ministry of Industry and Primary
Resources
Bandar Seri Begawan
Brunei Darussalam
Phone: +673 2 380 144
Fax:
+673 2 382 226
CANADA
Dr Thomas Feltmate
Manager
Food Safety
Risk Analysis Unit
Science Division
Canadian Food Inspection Agency
3851
Fallowfield Road
Nepean, Ontario K2H 8P9
Canada
Phone: +1 613 228 6698
Ext. 5982
Fax: +1 613 228 6675
Email: [email protected]
Mr Glenn McGregor
National
Manager, Product Inspection
Fish Seafood and Production Division
Canadian
Food Inspection Agency
59 Camelot Drive
Nepean, Ontario K1A 0Y9
Canada
Phone:
+1 613 225 2342 Ext.4572
Fax: +1 613 228 6648
Email: [email protected]
Mr
Chris Palmer
Senior Advisor, International Programs
Food Directorate, Health
Products and Food
Branch
Health Canada
Tunney’s Pasture
Ottawa
Ontario K1A 0L2
Canada
Phone: +1 613 941 4616
Fax: +1 613 941 3537
Email:
[email protected]
Mr Peter Pauker
Trade Policy Officer
Technical
Barriers and Regulations Division
(EAS)
Ottawa Ontario KIA 0G2
Canada
Phone:
+613 992 0523
Fax: +613 943 0346
Email: [email protected]
Dr
Bertrand St-Arnaud
Chief
Export Programs
Canadian Food Inspection Agency
59
Camelot Drive
Nepean, Ontario K1A 0Y9
Canada
Phone: +1 613 225 2342
Fax:
+1 613 228 6636
Email: [email protected]
CHILE
CHILI
Mr
German Moya Rojas
Asesor
Departamento de Comercio Exterior-DECOEX
Ministerio
de Economia
Teatinos 120 Piso 11
Oficina 22
Santiago
Chile
Phone:
+56 2 698 8148
Fax: +56 2 697 4905
Email: [email protected]
CHINA
CHINE
CINA
Dr
Zhenghua Cheng
Deputy Director
State Administration for Entry-Exit Inspection
and
Quarantine of the People’s Republic of China
No. A10, Chaowaidajie
Beijing
100020
People’s Republic of China
Phone: +86 10 9599 4625
Fax: +86
10 9599 4570
Email: [email protected]
Mrs Feng Xu
Food Safety Officer
State
Administration for Entry-Exit Inspection and
Quarantine of the People’s
Republic of China
No. A10, Chaowaidajie
Beijing 100020
People’s
Republic of China
Phone: +86 10 6599 4541
Fax: +86 10 6599 4497
Email:
[email protected]
Mr Tian Lan
Staff Member
Native Produce and Animal By-products
China
Chamber of Commerce for Import and
Export of Foodstuffs
No. 21 Xitangzi
Bystreet
Dongcheng Diestrict 100006
People’s Republic of China
Phone:
+86 10 6513 2381
Fax: +86 10 6513 9069
Email: [email protected]
Prof.
Dalu Su
Food Safety Officer
Senior Engineer
Zhejiang Entry-Exit Inspection
and
Quarantine of the People’s Republic of China
No. 2, Wensan Street
Hangzhou
Zhejiang 310020
People’s Republic of China
Phone: +86 571 838 1111/60506
Fax:
+86 571 838 1500
Email: [email protected]
Dr Zhibiao Zhang
Vice Secretary
General
China Chamber of Commerce for Import &
Export of Foodstuffs
Native Produce and Animal
By-Products
No. 21 Xitangzi Bystreet
Dongcheng
District 100006
People’s Republic of China
Phone: +86 10 6513 2378
Fax:
+86 10 6522 7910
Email: [email protected]
CUBA
Mr Gabriel Lahens
Espinosa
Engineer
Funcionario del Ministerio del Comercio Exterior
Vedado,
Cuidad Havana
Cuba
Phone: +57 3 542025
Fax: +57 3 550376
Email: [email protected]
CZECH
REPUBLIC
RÉPUBLIQUE TCHÈQUE
REPÚBLICA
CHECA
Dr Jana Palackova
Deputy Director
Czech Agriculture and Food
Inspection
Ministry of Agriculture
Kvetna 15
603 00 Brno
Czech Republic
Phone:
+420 5 4354 0203
Fax: +420 5 4354 0202
Email: [email protected]
DENMARK
DANEMARK
DINAMARCA
Mr
Finn H Clemmensen
Head of Division
Danish Veterinary and Food Administration
Morkhoj
Bygade 19
DK 2860 Soborg
Denmark
Phone: +45 33 956 008
Fax: +45 33
956 001
Email: [email protected]
EGYPT
EGYPTE
EGIPTO
Prof.
Aly Rady
Professor, Nuclear Research Centre
Atomic Energy Authority
3
Ahmed El Zomor Street
El Zohoor District
Nasr City, Children’s Village
POB
Code 11787
Cairo
Egypt
Phone: +202 485 8062/287 5924
Fax: +202 287
6031
Email: [email protected]
Dr Ashraf Mahmoud Elmarsafy
Technical and
Quality Control Manager Deputy
Central Laboratory of Analysis of Pesticide
Residue
and Heavy Metal in Food
Agricultural Research Center
Minisistry of Agriculture
7
Nadi El Said St
Dokki Giza
Egypt
Phone: +202 360 1395/3611355
Fax:
+202 361 1216/3611106
Email: [email protected]
FINLAND
FINLANDE
FINLANDIA
Ms
Outi Tyni
Senior Veterinary Officer
Veterinary and Food Department
Ministry
of Agriculture and Forestry
PO Box 30 Fin-00023 Government
Helsinki
Finland
Phone:
+358 9 160 2432
Fax: +358 9 160 3338
Email: [email protected]
FRANCE
FRANCIA
Dr
Catherine Rogy
Veterinary Officer
Head, Import Section
General Division
for Food
International Coordination Unit
Ministère de l’Agriculture
et de la Pêche
251 rue de Vaugirard
75732 Cedex 15 Paris
France
Phone:
+33 01 4955 5835
Fax: +33 01 4955 8314
Email: [email protected]
Mr
François Falconnet
General Secretary
Alesial - CITPPM
44 Rue Alesia
F
75682 Cedex 14 Paris
France
Phone: +33 01 53 91 4464
Fax: +33 0 53 91
4470
Email: [email protected]
GERMANY
ALLEMAGNE
ALEMANIA
Dr
Hans Dieter Boehm
Head of Division
Food Hygiene and Food Trade
Federal
Ministry for Health
Am Propsthof 78 A
D-53121 Bonn
Germany
Phone:
+ 49 228 941 4220
Fax: +49 228 941 4944
Email: [email protected]
Dr
Luppo Ellerbroek
Director
Federal Institute for Protection of Consumer and
Veterinary
Medicine
Diedersdorfer Weg 1
12277 Berlin
Germany
Phone: +49 188 8412
2121
Fax: +49 188 8412 2966
Email: [email protected]
Dr Matthias Frost
German
Technical Cooperation GTZ
Postfach 5180
D-65726 Eschborn
Germany
Phone:
+49 619 679 1082
Fax: +49 619 679 7180
Email: [email protected]
GHANA
Mr
Theophilus Corquaye
Chief Executive
Food and Drugs Board
PO Box CT 2783
Cantonments
Accra
Ghana
Phone: +233 21 660489
Fax: +233 21 660389
Email:
[email protected]
ICELAND
ISLANDE
ISLANDÍA
Mr
Halldor Runolfsson
Chief Veterinary Officer
Ministry of Agriculture
Solvholsgata
7
150 Reykjavik
Iceland
Phone: +354 560 9750
Fax: +354 552 1160
Email:
[email protected]
INDIA
INDE
Ms Sareen Shashi
Director
Export
Inspection Council of India
Ministry of Commerce
Government of India
Pragati
Tower
11th Floor, 26 Rajendra Place
New Delhi 110008
India
Phone:
+91 11 571 8768
Fax: +91 11 573 0016
Email: [email protected]
Mr E.
K. Bharat Bhushan
Joint Secretary
Ministry of Commerce
Udyog Bhavan
New
Delhi 110011
Phone: +91 11 301 2526
INDONESIA
INDONÉSIE
Mr
Basrah Enie
Director
Institute for Research and Development of
Agrobased
Industry
Agency for Industrial and Trade R&D
Ministry of Industry and
Trade
JI Ir Juanda No 11
Bogor 16122
Indonesia
Phone: +62 251 324
068/323 339
Fax: +62 251 323 339
Email: [email protected]
Mr Agus Heryana
Vice
Consul
Consulate of the Republic of Indonesia
134 Adelaide Terrace
East
Perth 6000
Australia
Phone: +61 8 9221 5858
Fax: +61 8 9221 5688
IRELAND
IRLANDE
IRLANDA
Mr
Raymond Ellard
Chief Specialist in Environmental Health
Food Safety Authority
of Ireland
Abbey Court, Lower Abbey Street
Dublin 1
Ireland
Phone:
+353 1 817 1319
Fax: +353 1 817 1301
Email: [email protected]
JAPAN
JAPON
Dr
Satoru Matsubara
Director
Environmental Health Bureau
Food Sanitation
Division
Ministry of Health and Welfare
1-2-2 Kasumigaseki
Chiyoda-ku
Tokyo
100-8045
Japan
Phone: +81 3 3595 2326
Fax: +81 3 3505 7965
Email:
[email protected]
Mrs Mika Haruna
Standards and Labelling Division
Food
and Marketing Bureau
Ministry of Agriculture, Forestry and Fisheries
1-2-1
Kasumigaseki
Chiyoda-ku
Tokyo 100-8950
Japan
Phone: +81 3 3502 8111
Ext 4868
Fax: +81 3 3502 0438
Email: [email protected]
Dr Yoshiko
Saito
Assistant Director
Biotech Food Safety
Food Sanitation Division
Environmental
Health Bureau
Ministry of Health and Welfare Japan
1-2-2 Kasumigaseki
Chiyoda-ku
Tokyo
100-8045
Japan
Phone: +81 3 3595 2326
Fax: +81 3 3505 7965
Email:
[email protected]
Dr Hiroshi Umeda
Deputy Director
Food Sanitation Division
Environmental
Health Bureau
Ministry of Health and Welfare Japan
1-2-2 Kasumigaseki
Chiyoda-ku
Tokyo
100-8045
Japan
Phone: +81 3 3595 2326
Fax: +81 3 3505 7965
Email:
[email protected]
Dr Shinji Hisai
Technical Advisor
Japan Frozen Foods
Inspection Corporation
Shuwa Dai-2 Park Building
2-12-7 Siba-Daimon
Minato-Ku
Tokyo
105-0012
Japan
Phone: +81 3 3438 1981
Fax: +81 3 3438 1980
Email:
[email protected]
Mr Yutaka Satoh
Manager
Quality Division
Japan
Fisheries Association
Sankaido Building
1-19-13 Akasaka
Minato-Ku
Tokyo
107-0052
Japan
Phone: +81 3 3585 6985
Fax: +81 3 3582 2337
Email:
[email protected]
Dr Hiroshi Yoshikura
Director General Research Institute
International
Medical Center of Japan
1-21-1 Toyama
Shinjuku-ku
Tokyo
162-8655
Japan
Phone: +81 3 3202 7181 Ext 3000
Fax: +81 3 5273 4526
Email:[email protected]
Ms
Hinako Ishihara
Japanese Agricultural Standards Association
(JASA)
Aroma
Building 5F
3-5-2 Nihombashi Kayabcho
Chuo-Ku
Tokyo 103- 0025
Japan
Phone:
+81 3 3249 7120
Fax: +81 3 3249 9388
Email: [email protected]
Mr Yoshiharu
Toeda
Consul
Consulate-General of Japan
PO Box 7347
Cloisters Square
Perth
WA 6850
Australia
Phone: +61 8 9321 7816
Fax: +61 8 9321 2030
Email:
[email protected]
MALAYSIA
MALAISIE
MALASIA
Dr Zainal
Che Mee
Principle Assistant Director
Food Control Division
Ministry of
Health
4th Floor, Block E, Office Complex
Jalan Dungun, Bukit Damansara
50940
Kuala Lumpur
Malaysia
Phone: +03 254 0088
Fax: +03 253 7804
Email:
[email protected]
Mr Othman Semail
Assistant Secretary
Ministry of Primary
Industries
6-8 Menara Daya Bumi Jln Sultan Hishamuddin
50654 Kuala Lumpur
Malaysia
Phone:
+603 2275 6193
Fax: +603 2274 5014
Email: [email protected]
Dr Murugiah
Sivamoorthy
Veterinary Officer
Department of Veterinary Services
Ministry
of Agriculture
8 - 9th Floor Wisma Chase Perdana
Off Jalan Semantan
Bukit
Damansara
50630 Kuala Lumpur
Malaysia
Phone: +603 254 0077 Ext 168
Fax:
+603 253 5804
Email: [email protected]
Ms Mariam Abdul Latif
Assistant
Director
Food Quality Control Division
Ministry of Health Malaysia
3rd
Floor Block E
Jalan Dungun, Bukit Damansara
50490 Kuala Lumpur
Malaysia
Phone:
+603 254 0088
Fax: +603 253 7804
Email: [email protected]
Mr Wong Soo Khwan
Senior
Research Officer
Customer Technical Advisory Services
Malaysian Palm Oil
Board
PO Box 10620
50720 Kuala Lumpur
Malaysia
Phone: +03 89282521
Fax:
+03 892 59446
Email: [email protected]
MEXICO
MEXIQUE
Ms
Aida de Lourdes Albuerne
Director
Sanitary Compliance and Promotion
Dirección
General de Calidad Sanitaria de
Bienes y Servicios
Donceles 39 Col. Centro
Mexico
City D.F. 6010
Mexico
Phone: +525 521 9717
Fax: +525 521 9628
Email:
[email protected]
Mr José Luis Luna Flores
Director of Sanitary
Surveillance
Dirección General de Calidad Sanitaria de
Bienes y Servicios
Donceles
39 Col. Centro
Mexico D.F. 06010
Mexico
Phone: +525 521 1273
Fax:
+525 512 9628
Email: [email protected]
NETHERLANDS
PAYS
BAS
PAÍSES BAJOS
Dr Hans Jeuring
Senior Public Health
Officer for Food
Inspectorate for Health Protection and Veterinary
Public
Health
PO Box 16108
2500 BC The Hague
Netherlands
Phone: +31 70 340
5060
Fax: +31 70 340 5435
Email: [email protected]
Mr Otto Knottnerus
Main
Board of Arable Products
Post Box 29739
2502 LS The Hague
Netherlands
Phone:
+31 70 3708 343
Fax: +31 70 3708 444
Email: [email protected]
Dr
Wim A Ruiterkamp
Senior Policy Officer, Food Chains
Department of Veterinary
and Food Policy and
General Environmental Affairs
Ministry of Agriculture,
Nature Management and
Fisheries
Post Box 20401
2500 EK The Hague
Netherlands
Phone:
+31 70 378 5723
Fax: +31 70 378 6741
Email: [email protected]
Dr
Rijckert van der Flier
Coordinator of Veterinary Inspection and
Monitoring
Policy
Department of Veterinary and Food Policy and
General Environmental
Affairs
Ministry of Agriculture, Nature Management and
Fisheries
73 Bezuidenhoutseweg
PO
Box 20401
Netherlands
Phone: +31 70 3785 123
Fax: +31 70 3786141
Email:
[email protected]
Dr Aad van Sprang
National Inspection Service
for
Livestock and Meat
Ministry of Agriculture,
Nature Management and
Fisheries
PO Box 3000
2270 Voorburg
Netherlands
Phone: +31 70 357
8851
Fax: +31 70 387 6591
Email: [email protected]
Mr Koos Warmerhoven
Policy
Officer
Ministry of Health, Welfare and Sport
Parnassusplein 5
PO Box
20350
2500 EJ The Hague
Netherlands
Phone: +31 70 340 6942
Fax: +31
70 340 5554
Email: [email protected]
NEW ZEALAND
NOUVELLE
ZÉLANDE
NUEVA ZELANDIA
Dr Steve Hathaway
Director
Program
Development
Food Assurance Authority
Ministry of Agriculture and Forestry
PO
Box 646
Gisborne
New Zealand
Phone: +64 6 867 1144
Fax: +64 6 867
5207
Email: [email protected]
Mrs Cherie Flynn
Senior Policy Analyst
Food
and Animal Policy Group
Ministry of Agriculture and Forestry
PO Box 2526
Wellington
New
Zealand
Phone: +64 4 474 4169
Fax: +64 4 474 4265
Email: [email protected]
Ms
Judi Lee
National Manager
Food Assurance Authority
Ministry of Agriculture
and Forestry
95 McGregor Road
Papakura RD2
New Zealand
Phone: +64
9 292 9131
Fax: +64 9 292 9131
Email: [email protected]
Miss Debra Tuifao
Policy
Analyst
Ministry of Agriculture and Forestry
PO Box 2526
Wellington
New
Zealand
Phone: +64 4 478 9935
Fax: +64 4 474 4265
Email: [email protected]
Mr
R.A. (Bob) Martin
Market Access Manager
Zespri International Ltd
PO Box
9906
Auckland
New Zealand
Phone: +64 9 367 7538
Fax: +64 9 367 0222
Email:
[email protected]
NORWAY
NORVÈGE
NORUEGA
Mrs
Mette Solum Ruden
Head, Enforcement and Contingency
Department of Food Control
and Food Law
Enforcement
Norwegian Food Control Authority
PO Box 8187
Dep
N-0034 Oslo
Norway
Phone: +47 22 24 6650
Fax: +47 22 24 6793
Email:
[email protected]
Mrs Kari Bryhni
Head, Food Hygiene & Drinking Water
Food
Law and International Affairs
Norwegian Food Control Authority
PO Box 8187
Dep
N-0034 Oslo
Norway
Phone: +47 22 24 6650
Fax: +47 22 24 6699
Email:
[email protected]
Mr Lennart Johanson
Senior Advisor
Norwegian Ministry
of Fisheries
PO Box 8118 Dep
N - 0032 Oslo
Norway
Phone: +47 22 24
2665
Fax: +47 22 24 5678
Email: [email protected]
[email protected]
PAPUA
NEW GUINEA
PAPOUASIE-NOUVELLE-GUINÉE
PAPUA NUEVA GUINEA
Mr
Joseph Kerage
Export Program Manager
National Agriculture Quarantine and
Inspection
Authority (NAQIS)
PO Box 741
Port Moresby
Papua New Guinea
Phone:
+675 325 9977
Fax: +675 325 9310
Email: [email protected]
PHILIPPINES
FILIPINAS
Dr
Maria Araceli Albarece
Agricultural Attache
Embassy of the Philippines
1
Moonah Place
Yarralumla ACT 2600
Australia
Phone: +61 2 6273 2584
Fax:
+61 2 6273 2113
Email: [email protected]
REPUBLIC OF KOREA
CORÉE,
RÉPUBLIQUE DE
REPÚBLICA DE COREA
Mr Ok-Kyun Bang
Director
General
Bureau of Food Safety
Korea Food and Drug Administration
5 Nokbun-Dong,
Eunpyung-Gu
Seoul 122-204
Republic of Korea
Phone: +82 2 380 1652
Fax:
+82 2 388 6396
Email: [email protected]
Ms Mi-Young Cho
Senior Researcher
Food
Sanitation Council
Ministry of Health and Welfare
5 Nokbun-Dong, Eunpyung-bu,
Seoul
122-204
Republic of Korea
Phone: +82 2 380 1558
Fax: +82 2 388 8321
Email:
[email protected]
Dr Hyo-Ryong Kim
Deputy Director
Planning and Coordination
Division
National Veterinary Research and Quarantine
Service (NVRQS)
Ministry
of Agriculture and Forestry
480 Anyang-6-dong
Manan-gu
Anyang-City Gyeonggi-do
Republic
of Korea
Phone: +82 31 467 1923
Fax: +82 31 467 1938
Email: [email protected]
Dr
Jee-Woo Lee
Veterinary Officer
Planning and Coordination Division
National
Veterinary Research and Quarantine
Service (NVRQS)
Ministry of Agriculture
and Forestry
480 Anyang-6-dong
Manan-gu
Anyang-City Gyeonggi-do
Republic
of Korea
Phone: +82 31 467 1930
Fax: +82 31 467 1938
Email: [email protected]
Mr
Kwangha - Ha Lee
Deputy Director
Quality Management Division
National
Agriculture Products Quality
Management Service
Ministry of Agriculture
and Forestry
433-2 Anyang-6-dong
Anyang-City Kyonggi-Do 430-016
Republic
of Korea
Phone: +82 31 446 0126
Fax: +82 31 446 0903
Email: [email protected]
ROMANIA
ROUMANIE
RUMANIA
Mrs
Olimpia Vorovenci
Expert in Agro-Food Produce Standards
Romanian Standards
Association
21 - 25 Mendeleev Str
Sector 2 Bucharest
Romania
Phone:
+401 310 4309
Fax: +401 321 2928
Email: [email protected]
Miss Emilia Mihaela
Vorovenci
Director, Inspection and Certification Department
Bioagrocert
Consulting S.A.
25 Lebedei Str
Sector 1 Bucharest
Romania
Phone: +401
212 5832
Fax: +401 212 5998
Email: [email protected]
SINGAPORE
SINGAPOUR
SINGAPUR
Dr
Sin Bin Chua
Director
Veterinary Public Health and Food Supply Division
Agri-Food
and Veterinary Authority of Singapore
5 Maxwell Road
Singapore 069110
Phone:
+65 325 7622
Fax: +65 220 6068/+65 224 0601
Email: [email protected]
Ms
Huay Leng Seah
Head, Food Control Department
Ministry of the Environment
19th
Storey
Singapore 228231
Phone: +65 731 9819
Fax: +65 731 9843/731 9844
Email:
[email protected]
Dr Paul Chiew
Head, Food Inspection Services
Agri-Food
and Veterinary Authority Veterinary
Public Health & Food Supply Division
51
Jalan Buroh
Singapore 619495
Phone: +65 267 0820
Fax: +65 265 0784/266
4689
Email: [email protected]
SOUTH AFRICA
AFRIQUE DU SUD
SUDÁFRICA
Mr
A.W.J. Pretorius
Deputy Director
Food Control
Department of Health
Private
Bag X828
0001 Pretoria
South Africa
Phone: +27 12 312 0159
Fax: +27
12 326 4374
Email: [email protected]
SPAIN
ESPAGNE
ESPAÑA
Mrs
Margarita Garzon Rigau
Jefa de Servicio de Veterinaria Oficial
Subdir. Gral.
Sanidad
Exterior y Veterinaria
Ministerio de Sanidad y Consumo
C/Paseo
Del Prado 18 - 20
28071 Madrid
Spain
Phone: +34 91 596 1935
Fax: +34
91 596 2047
Email: [email protected]
Mr Javier Mate Caballero
Jefe de Servicio
de Inspección de Mercancias
Subdir. Gral. Sanidad
Exterior y Veterinaria
Ministerio
de Sanidad y Consumo
C/Paseo Del Prado 18 - 20
28071 Madrid
Spain
Phone:
+34 91 596 2050
Fax: +34 91 596 2047
Email: [email protected]
SWAZILAND
SWAZILANDIA
Mr
Richard Mamba
Senior Health Inspector
Ministry of Health and Welfare
PO
Box 5
Mbabane
Swaziland
Phone: +02 68 404 2431/3
Fax: +02 68 404 2092
SWEDEN
SUÈDE
SVECIA
Dr
Tor Bergman
Senior Veterinary Inspector
National Food Administration
Box
622
SE - 75126 Uppsala
Sweden
Phone: +46 18 175 587
Fax: +46 18 105
848
Email: [email protected]
Ms Ylva Wallen
Senior Administrative Officer
Ministry
of Agriculture
SE - 103 33 Stockholm
Sweden
Phone: +46 8 405 1106
Fax:
+46 8 405 4970
Email: [email protected]
SWITZERLAND
SUISSE
SUIZA
Mrs
Franziska Zimmermann
Non-Tariff Measures
State Secretariat for Economic
Affairs
Federal Department for Economic Affairs
Effingestr.
3003 Bern
Switzerland
Phone:
+41 31 324 0847
Fax: +41 31 324 0959
Email:[email protected]
THAILAND
THAÏLANDE
TAILANDIA
Mr
Somruay Harinasuta
Secretary - General
Thai Industrial Standards Institute
(TISI)
Ministry of Industry
Rama VI Road
Ratchathewi
Bangkok 10400
Thailand
Phone:
+662 202 3400
Fax: +662 246 4085
Email: [email protected]
Dr Kraingsak
Dangprom
Director
Veterinary Public Health (DLD)
Department of Livestock
Development
Ministry of Agriculture and Cooperative
Phayathai Road Rachataevee
Bangkok
10900
Thailand
Phone: +662 251 5646/5988
Fax: +662 251 7922
Dr Supranee
Impithuksa
Director
Coordination Office for Hygienic Fruit &
Vegetable
Department
of Agriculture
Ministry of Agriculture and Cooperatives
Chatuchak
Bangkok
10900
Thailand
Phone: +662 940 5503
Fax: +662 940 5503
Mr Montri Klitsaneephaiboon
Director
Fish
Inspection and Quality Control Division
Department of Fisheries
Kaset-Klang
Chatuchak
Bangkok
10900
Thailand
Phone: +662 579 7738
Fax: +662 579 6687
Email: [email protected]
Mr
Yuthana Norapoompipat
Food & Drug Specialist
Food Control Division
Food
& Drug Administration
Ministry of Public Health
Tiwanon Road
Nonthaburi
11000
Thailand
Phone: +662 590 7322
Fax: +662 591 8476
Email: [email protected]
Ms
Chatsiri Pinmuangngam
Standards Officer
Office of the National Codex Alimentarius
Committee
Thai
Industrial Standards Institute (TISI)
Ministry of Industry
Rama VI Road
Bankok
10400
Thailand
Phone: +66 2 202 3439
Fax: +66 2 248 7987
Email: [email protected]
Mrs
Nantapan Rattanatam
Director
Agricultural Chemistry Division
Department
of Agriculture
Ministry of Agriculture and Cooperatives
Chatuchak Bangkok
10900
Thailand
Phone: +662 579 7549
Fax: +662 561 5034
Email: [email protected]
Mrs
Duangporn Rodphaya
Director
Commodity Division
Department of Foreign
Trade
Ministry of Commerce
Sanam Bin Nam
Nonthaburi 11000
Thailand
Phone:
+662 547 4801
Fax: +662 547 4802
Mr Maris Sangiampongsa
Counsellor
Ministry
of Foreign Affairs
Bangkok
Thailand
Phone: +662 643 5000 Ext 4062
Fax:
+662 643 5247
Email: [email protected]
Mr Tanongpan Satjapala
Medical Scientist
Division
of Food-for-Export
Department of Medical Sciences
Muang District
Nonthaburi
11000
Thailand
Phone: +662 951 0000 - 11 ext. 9509
Fax: +662 951 1021
Email:
[email protected]
Dr Chumnarn Sirirugsa
Director
Office of Agricultural
Standards and Inspections
Ministry of Agriculture & Cooperatives
Bangkok
10200
Thailand
Phone: +662 628977
Fax: +662 6298978
Email: [email protected]
Mrs
Suchin Srikongsri
Director, Biological Science Division
Department of Science
Service
Ministry of Science, Technology and
Environment
Rama VI Road
Bangkok
10400
Thailand
Phone: +662 245 8993
Fax: +662 612 0967
Email: [email protected]
Mrs
Pranee Srisomboon
General Manager
Thai Food Processors’ Association
9th
Floor, Ocean Tower Building
170/22 New Ratchadapisek Road
Khet Klongtoey,
Bangkok 10110
Thailand
Phone: +662 2612684-6
Fax: +662 261 2996-7
Email:
[email protected]
Mrs Malinee Subvanich
Director & General Secretary
Thai
Food Processors’ Association
9th Floor, Ocean Tower 1 Building
170/22
New Ratchadapisek Road
Khet Klongtoey, Bangkok 10110
Thailand
Phone:
+662 261 2684-6
Fax: + 662 261 2996-7
Email: [email protected]
Mr
Lers Thisayakorn
Vice President
Thai Frozen Foods Association
160/194-7
13th
Floor ITF Building
Silom Road
Bangkok 10500
Thailand
Phone: +662 235
5622-4
Fax: +662 235 5625
Email: [email protected]
TONGA
Mr
Haniteli Fa’anunu
Director
Ministry of Agriculture and Forestry
PO
Box 14
Nuku’alofa
Tonga
Phone: +676 23 402
Fax: +676 24 271
Email:
[email protected]
Ms Lucy Lopeti
Principal Agriculture Officer
Ministry
of Agriculture and Forestry
PO Box 14
Nuku’alofa
Tonga
Phone:
+676 23 038
Fax: +676 24 271
Email: [email protected]
UNITED KINGDOM
ROYAUME-UNI
REINO
UNIDO
Dr Dorian Kennedy
Head of Branch
Food Labelling, Standards and
Consumer
Protection Division
Food Standards Agency
Ergon House
PO
Box 31037
Horseferry Road
London SW1P 3WG
United Kingdom
Phone: +44
20 7238 5574
Fax: +44 20 7238 6763
Email:[email protected]
Mr
David Taylor
Veterinary Director
Veterinary Public Health Unit
Food Standards
Agency
Ergon House
PO Box 31037
Horesferry Road
London SW1P 3WG
United
Kingdom
Phone: +44 20 7238 6421
Fax: +44 20 7238 6402
Email: [email protected]
UNITED
STATES OF AMERICA
ETATS-UNIS
ESTADOS UNIDOS
Mr L. Robert
Lake
Director
Office of Regulations and Policy
Center for Food Safety
and Applied Nutrition
Food and Drug Administration
Room 5807 C (HFS-4)
200
C Street SW
Washington DC 20204
USA
Phone: +1 202 205 4160
Fax: +1
202 401 7739
Email: [email protected]
Dr Catherine Carnevale
Director,
Office of Constituent Operations
Center for Food Safety and Applied Nutrition
Food
and Drug Administration
200 C Street SW
Room 5807 (HFS-550)
Washington
DC 20204
USA
Phone: +1 202 205 5032
Fax: +1 202 205 0165
Email: [email protected]
Mr
Mark G Manis
Director, International Policy Division
Food Safety and Inspection
Service
US Department of Agriculture
1400 Independence Ave SW
Room 2135-
South Building
Washington DC 20250
USA
Phone: +1 202 720 6400
Fax:
+1 202 720 7990
Email: [email protected]
Ms Audrey Talley
International
Marketing Specialist
Office of Food Safety and Technical Services
Foreign
Agricultural Service
United Stated Department of Agriculture
Room 5545 -
South Building
Washington DC 20250
USA
Phone: +1 202 720 9408
Fax:
+1 202 690 0677
Email: [email protected]
Dr H. Michael Wehr
Special Assistant
to the Director
Office of Constituent Operations
Center for Food Safety
and Applied Nutrition
Food and Drug Administration
Room 5818 (HFS-550) -
South Building
Washington DC 20204
USA
Phone: +1 202 260 2786
Fax:
+1 202 205 0165
Email: [email protected]
Mr Richard White
Director,
Sanitary and Phytosanitary Affairs
Office of the United States Trade Representative
600
17th Street, NW
Washington DC 20508
USA
Phone: +1 202 395 9582
Fax:
+1 202 395 4579
Email: [email protected]
Mr C.W. McMillan
President
C.W.
McMillan Co
PO Box 10009
Alexandria, VA22310
USA
Phone: +1 703 960
1982
Fax: +1 703 960 4976
Email: [email protected]
Mr Johnnie Nichols
Director
Technical
Services
National Milk Producers Federation
Suite 400
2101 Wilson Boulevard
Arlington,
VA 22201
USA
Phone: +1 703 243 6111
Fax: +1 202 841 9238
Email: [email protected]
Ms
Peggy Rochette
Director
International Affairs
National Food Processors
Association
1350 I Street
Washington DC 20005
USA
Phone: +1 202 639
5921
Fax: +1 202 639 5991
Email: [email protected]
URUGUAY
Dr
Hector J. Lazaneo
Director
Food Safety Division
Ministry of Livestock,
Agriculture and Fisheries
Constituyente 1476
Montevideo CP 11200
Uruguay
Phone:
+598 2 402 6346
Fax: +598 2 402 6317
Email: [email protected]
VANUATU
Ms
Emily Kalsakau
Senior Food Technologist
Food Technology Development Centre
Trade
Department
PMB 030
Port Vila
Vanuatu
Phone: +678 259, 78 24 278
Fax:
+678 256 40
VIETNAM
Mr Nhu Tiep Nguyen
Chief
Professional Affairs
Division
National Fisheries Inspection and Quality
Assurance Center
Ministry
of Fisheries
10-12 Nguyen Cong Hoan Street
Ba Dinh District Hanoi
Vietnam
Phone:
+84 4 831 0983/7715383
Fax: +84 4 831 7221
Email: [email protected]
INTERNATIONAL GOVERNMENTAL ORGANIZATIONS
EUROPEAN COMMISSION
Dr Henri Belveze
Deputy
Head of Unit
Health and Consumer Protection Directorate-
General
European
Commission
Rue de la Loi/Wetstraat 200
B-1049 Brussels
Belgium
Phone:
+32 2 296 2812
Fax: +32 2 296 2792
Email: [email protected]
Mr
Andrew John Wilson
Counsellor
Consumer Health Affairs
European Commission
140
Wireless Road
Bangkok 10330
Thailand
Phone: +66 2 255 9100 ext. 700
Fax:
+66 2 255 9114
Email: [email protected]
EUROPEAN UNION (COUNCIL OF MINISTERS)
Mr Klaus Skovsholm
Administrator
Secretariat General
of the Council of the
European Union
Rue de la Loi 175
Brussels B-1048
Belgium
Phone:
+32 2 285 8379
Fax: +32 2 285 7829
Email: [email protected]
INTERNATIONAL INSTITUTE OF REGRIGERATION (IIR)
Mr Keith Richardson
Liaison Officer
Food
Technology
Food Science Australia
PO Box 52
North Ryde NSW 1670
Australia
Phone:
+61 2 9490 8333
Fax: +61 2 9490 8499
Email: keith.richardson@ foodscience.afisc.csiro.au
OFFICE INTERNATIONAL DES EPIZOOTIES (OIE)
Dr Yoshiyuki Oketani
Chargé
de Mission
Information and International Trade Department
Office International
des Épizooties
12 rue de Prony
75017 Paris
France
Phone: +33
1 4415 1888
Fax: +33 1 4267 0987
Email: [email protected].
WORLD HEALTH ORGANIZATION (WHO)
Dr Yasuyuki Sahara
Scientist
Food Safety Programme
World
Health Organization
20 Avenue Appia
Geneva 271211
Switzerland
Phone:
+44 22 791 4324
Fax: +44 22 791 4807
Email: [email protected]
INTERNATIONAL NON-GOVERNMENTAL ORGANIZATIONS
COUNCIL FOR RESPONSIBLE NUTRITION
Dr
Janet E. Collins
Monsanto Company
600 13th Street NW
Suite 660
Washington
DC 20005
USA
Phone: +1 202 383 2861
Fax: +1 202 783 1924
Email: [email protected]
INSTITUTE OF FOOD TECHNOLOGISTS (IFT)
Ms Gloria Brooks-Ray
Adviser
Codex &
International Regulatory Affairs
Novigen Sciences Inc
PO Box 97
Mountain
Lakes, NJ 07046
USA
Phone: +1 973 334 4652
Fax: +1 973 334 4652
Email:
[email protected]
INTERNATIONAL ACCREDITATION FORUM (IAF)
Mr
Noel Matthews
Secretary
International Accreditation Forum Inc.
2 Marcus
Clarke Street #1801
Canberra City ACT 2601
Australia
Phone: +61 2 6257
1962
Fax: +61 2 6257 1965
Email: [email protected]
INTERNATIONAL ASSOCIATION
OF
CONSUMER FOOD ORGANIZATIONS
Mr Bruce Silverglade
President
International
Association of Consumer Food
Organizations
Suite 300
1875 Connecticut
Avenue NW
Washington DC 20009-5728
USA
Phone: +1 202 332 9110 ext. 337
Fax:
+1 202 265 4954
Email:[email protected]
INTERNATIONAL COUNCIL OF
GROCERY
MANUFACTURERS ASSOCIATION
(ICGMA)
Ms Sarah Geisert
Manager
Quality
and Regulatory Operations
General Mills
201 General Mills Boulevard
Minneapolis
Minnesota 55426 1350
USA
Phone: +1 763 764 2595
Fax: +1 763 764 2109
Email:
[email protected]
INTERNATIONAL DAIRY FEDERATION (IDF)
Mr
Philip Fawcet
National Manager
International Standards
Dairy and Plant
Products Group
Ministry of Agriculture and Forestry
BOX 2526
Wellington
New
Zealand
Phone: +64 4 4989 874
Fax: +64 4 4744 196
Email: [email protected]
Mrs
Slava Zeman
Manager
Processed Foods Policy Section
Australian Quarantine
& Inspection Service
Agriculture Fisheries and Forestry - Australia
GPO
Box 858
Canberra ACT 2601
Australia
Phone: +61 2 6272 5027
Fax: +61
2 6271 6522
Email: [email protected]
JOINT FAO/WHO SECRETARIAT
Dr
Alan W Randell
Secretary, Codex Alimentarius Commission
Joint FAO/WHO Food
Standards Programme
C/- FAO
Via delle Terme de Caracalla
Rome 00100
Italy
Phone:
+39 6 5705 4390
Fax: +39 6 5705 4593
Email: [email protected]
Mr David
H. Byron
Food Standards Officer
Food and Nutrition Division
Joint FAO/WHO
Food Standards Programme
Via delle Terme de Caracalla
Rome 00100
Italy
Phone:
+39 6 5705 4419
Fax: +39 6 5705 4593
Email: [email protected]
AUSTRALIAN SECRETARIAT
Ms Ruth Lovisolo
Manager
Codex Australia
Product Integrity
Animal and Plant Health
Agriculture Fisheries and Forestry - Australia
GPO
Box 858
Canberra ACT 260
Australia
Phone: +61 2 6272 5112
Fax: +61
2 6272 3103
Email: [email protected]
Ms Ann Backhouse
Assistant
Manager A/g
Codex Australia
Product Integrity Animal and Plant Health
Agriculture
Fisheries and Forestry - Australia
GPO Box 858
Canberra ACT 2601
Australia
Phone:
+61 2 6272 5692
Fax: +61 2 6272 3103
Email: [email protected]
CCFICS ADMINISTRATION
Ms Rose Hockham
Executive Officer A/g
Codex Australia
Product
Integrity Animal and Plant Health
Agriculture Fisheries and Forestry - Australia
GPO
Box 858
Canberra ACT 2601
Australia
Phone: +61 2 6272 5060
Fax: +61
2 6272 3103
Email: [email protected]
Mr Jeff Gilbert
Documentation
Officer
Codex Australia
Product Integrity Animal and Plant Health
Agriculture
Fisheries and Forestry - Australia
GPO Box 858
Canberra ACT 2601
Australia
Phone:
+61 2 6272 4542
Fax: +61 2 6272 3103
Email: [email protected]
(Advanced to Step 8 of the Codex Procedure)
SECTION 1 - PREAMBLE
1. These guidelines recognize that importing country authorities may, as a condition of clearance of consignments, require importers to present certification issued by, or with the authority of, exporting country authorities. These guidelines do not mandate a need to use such certification or in any way diminish the trade facilitatory role of commercial or other types of certificates, including third party certificates, not issued by, or with the authority of, exporting country authorities. These guidelines are based on the presumption that the commercial parties engaged in international trade in food are responsible for complying with the regulatory requirements of the exporting and importing country.
SECTION 2 - SCOPE
2. These guidelines concern the design and use of official and officially recognized certificates that attest to attributes of food presented for international trade. Hereafter, in these Guidelines, the term “certificates” means official and officially recognized certificates. Certificates should be required only where declarations are necessary relating to product safety or suitability for consumption, or to otherwise facilitate fair trade.
3. These guidelines do not deal with matters of animal and plant health unless directly related to food quality or safety. However, it is recognized that, in practice, a single certificate may contain information relevant to several matters.
4. These guidelines are equally applicable to the use of paper or electronic forms of certification.
SECTION 3 -OBJECTIVES
5. Certificates should contain essential information relating to food safety and the facilitation of trade. The level of information required should be adequate for the importing country’s purpose and not impose unnecessary burdens on the exporting country or exporter, nor should there be a requirement for the disclosure of information that is commercial-in-confidence unless it is of relevance to public health.
SECTION 4 - DEFINITIONS
Certificates are those paper or electronic documents which describe and attest to attributes of consignments of food moving in international trade.
Certification is the procedure by which official certification bodies or officially recognized certification bodies provide written or equivalent assurance that foods or food control systems conform to requirements. Certification of food may be, as appropriate, based on a range of inspection activities which may include continuous on-line inspection, auditing of quality assurance systems, and examination of finished products.[31]
Official certificates are certificates issued by an official certification body of an exporting country, in accordance with the requirements of an importing or exporting country.
Officially recognized certificates are certificates issued by an officially recognized certification body of an exporting country, in accordance with the conditions of that recognition and in accordance with the requirements of an importing or exporting country.
Certifying bodies are official certification bodies and officially recognized certification bodies[32].
Certifying officers are employees of certifying bodies authorized to complete and issue certificates.
SECTION 5 - PRINCIPLES
6. Certificates should be required only where declarations are necessary to provide information about product safety or suitability for consumption, or to otherwise facilitate fair trade. Multiple or redundant certificates should be avoided to the extent possible. The rationale and requirements for certification should be communicated in a transparent manner and consistently implemented in a non-discriminatory manner. Certificates should be designed and used in a manner that:
The government agency having jurisdiction shall take responsibility for any certificate issued by a certifying body.
SECTION 6 - CRITERIA
STANDARD FORMAT
7. Each certificate should contain a declaration by the official, or officially recognized certification body which relates to the consignment described on that certificate. The certificate should clearly identify the certifying body with letterhead and/or logo.
8. Each certificate should have a unique identification number and be presented in an unambiguous style in a language, or languages, fully understood by the certifying officers and by the receiving authority. A record of unique identification numbers assigned to certificates should be maintained by the competent authority and be able to be related to the distribution of the certificates.
9. Where certificates are produced as a paper document, the original certificate should be uniquely identifiable and be printed with at least one copy for the use of the certifying body and retention by that authority for an appropriate period of time. Further copies may be officially printed copies or photocopies. In all cases the status of the certificate should be clear, for example, marked “original” or “copy”, as appropriate.
10. Certificates should be designed so as to minimize the risk of fraud (for example, use of watermark paper, or other security measures for paper certificates; use of secure lines and systems for electronic certificates.)
11. Where certificates are produced in a physical form, they should occupy one sheet of paper or, where more than one page is required, in such a form that any two or more pages are part of an integrated whole and indivisible sheet of paper. Where this is not possible, each individual sheet should be separately initialed by the certifying officer and/or numbered so as to indicate it is a particular page in a finite sequence (for example page 2 of 4 pages) and should contain the unique identification number for that certificate.
12. The certificate should clearly describe the commodity and consignment to which it uniquely relates.
13. Certificates should contain a clear reference to any requirements to which the certified product is required to conform.
14. Certificates should be issued prior to the consignment, to which the certificate relates, leaving the control of the certifying body. Certificates may be issued while consignments are in transit to the country of destination only when appropriate systems of control are agreed by the competent authorities of the importing and exporting countries.
15. The use of electronic means for the issue or transfer of certificates should be accepted where the integrity of the certification system has been assured to the satisfaction of the relevant authorities of both the importing and exporting country. A hard copy form of an electronic certificate should be made available by the issuing authority on request of the importing country’s authorities. When electronic certificates are used, the importing country’s inspectors should have electronic access to the certification details.
DETAILS OF THE CONSIGNMENT
(NOTE: These details are not specific to food, as they constitute the normal field of information contained in any Bill of Lading for transport vessels carrying product between countries. The shipping data on the official certification documentation provides a means of verifying details about the product.)
16. The details of the product being certified should be clearly documented on the certificate, which should at least contain the following information:
Certificates may also contain information on relevant transport and handling requirements, including appropriate temperature controls.
STATEMENT OF ORIGIN
17. Where, in exceptional cases justified by immediate public health concern, the importing country requires a statement as to the origin of ingredients in a product, the certificate should specify the origin of ingredients sourced outside the exporting country.
ATTESTATIONS
18. The particular attestations to be included in a certificate will be determined by the requirements of the importing or exporting country. They should be clearly identified in the text of the certificate. Such attestations may include, but are not limited to:
RESPONSIBILITIES OF THE CERTIFYING BODY
19. The certifying body should be designated and adequately empowered by national legislation or regulation in a transparent manner to provide the particular attestations required in a certificate or officially recognized certificate. Such designation/empowerment should be recognized as sufficient by governments, alleviating requirements for further identity or authority.
20. The certifying bodies should ensure that their procedures allow for the issue of the certificate in a timely manner so as to avoid unnecessary disruptions to trade.
21. The certifying bodies should have in place an effective system to prevent, to the extent practicable, the fraudulent use of official and officially recognized certificates.
RESPONSIBILITIES OF CERTIFYING OFFICERS
22. Information and guidance notes to facilitate the correct completion of certificates should be available to all certifying officers and to the parties responsible for providing details for inclusion in a certificate.
23. The certifying officers should:
PRESENTATION OF ORIGINAL CERTIFICATES
24. The importer or consignee is responsible for ensuring that the product is presented to the importing country’s authorities with the original certificate in accordance with the importing country’s requirements. In the case of electronic certificates the consignee should supply the importing country authority with sufficient details concerning the consignment to allow the identity of goods to be established against the details contained in the certificate.
INSTRUCTIONS FOR COMPLETING PAPER CERTIFICATES
25. Certificates should always be issued and presented, to the exporter or their agent, as the original certificate (i.e., this is an original printed paper form of the original certificate issued once only).
26. A copy of the original certificate (clearly marked as such) should be kept by the certifying body in the exporting country and be provided to the competent authority in the importing country, on request.
27. When signing a certificate, the officer should ensure that:
INSTRUCTIONS FOR COMPLETING ELECTRONIC CERTIFICATES
28. The exporter or their agent should be notified when an electronic certificate has been authorized for a consignment.
29. Before authorizing an electronic certificate, the certifying officer should ensure that all steps and checks established for the secure operation of the electronic system have been satisfactorily completed.
REPLACEMENT CERTIFICATES
30. Where, for any good and sufficient reason (such as loss of or damage to the certificate in transit), a replacement certificate is issued by the certifying officer it must be clearly marked “REPLACEMENT” before being issued. A replacement certificate should reference the number of the original certificate that it supercedes.
REVOCATION OF A CERTIFICATE
31. When for good and sufficient reason there is cause to revoke a certificate, the certifying body should revoke the original certificate as soon as possible and notify the exporter or their agent in hard copy or by electronic means of the revocation. The notice should reference the number of the original certificate to which the revocation refers and provide all particulars regarding the consignment and the reason(s) for the revocation. A copy of the revocation should be provided to the appropriate food control authority of the importing country if the export of the consignment has occurred.
(Advanced to Step 5/8 of the Codex Procedure)
SECTION 1 - PREAMBLE
1. It is often the case that importing and exporting countries operate different food inspection and certification systems. The reasons for such differences include differences in prevalence of particular food safety hazards, national choice about management of food safety risks and differences in the historical development of food control systems.
2. In such circumstances, and in order to facilitate trade, there is a need to determine the effectiveness of sanitary measures of the exporting country in achieving the appropriate level of sanitary protection of the importing country. This has led to recognition of the principle of equivalence as provided for in the World Trade Organization Agreement on the Application of Sanitary and Phytosanitary Measures (WTO SPS Agreement).
3. Application of the principle of equivalence has mutual benefits for both exporting and importing countries.
SECTION 2 - SCOPE
4. This document provides guidelines on the judgement of the equivalence of sanitary measures associated with food inspection and certification systems. For the purpose of determining equivalence, these measures can be broadly characterized as: infrastructure; programme design, implementation and monitoring; and/or specific requirements (refer paragraph 7).
SECTION 3 - DEFINITIONS
5. The definitions presented in this document are derived from and consistent with those of the Codex Alimentarius Commission and the WTO SPS Agreement.
Sanitary measure: Any measure applied to protect human life or health within the territory of the country from risks arising from additives, contaminants, toxins or disease-causing organisms in food or feedstuffs, or from risks otherwise arising from diseases carried by foods which are animals, plants or products thereof.
Hazard: A biological, chemical or physical agent in, or condition of, food with the potential to cause an adverse health effect.[33]
Risk: A function of the probability of an adverse health effect and the severity of that effect, consequential to a hazard(s) in food.
Risk Assessment: A scientifically-based process consisting of the following steps: (i) hazard identification; (ii) hazard characterisation; (iii) exposure assessment; and (iv) risk characterisation.
Appropriate level of sanitary protection (ALOP): The level of protection deemed appropriate by the country establishing a sanitary measure to protect human life or health within its territory. (This concept may otherwise be referred to as the “acceptable level of risk”.)
Equivalence (of sanitary measures)[34]: Equivalence is the state wherein sanitary measures applied in an exporting country, though different from the measures applied in an importing country, achieve, as demonstrated by the exporting country, the importing country’s appropriate level of sanitary protection.
SECTION 4 - SANITARY MEASURES AND THE DETERMINATION OF EQUIVALENCE
6. To facilitate judgement of equivalence between countries and promote harmonisation of food safety standards, Codex members should base their sanitary measures on Codex standards and related texts.[35]
7. Sanitary measures include all relevant laws, decrees, regulations, requirements and procedures including, inter alia, end product criteria; processes and production methods; testing, inspection, certification and approval procedures; provisions on relevant statistical methods, sampling procedures and methods of risk assessment; and packaging and labelling requirements directly related to food safety. For the purposes of determining equivalence, the sanitary measures associated with a food inspection and certification system can be broadly categorised as:
a) infrastructure; including the legislative base (e.g., food and enforcement law), and administrative systems (e.g., organisation of national and regional authorities);8. A sanitary measure proposed for determination of equivalence may fall into one or more of these categories, which are not mutually exclusive. A single measure, however, on which an equivalence determination may be made, cannot be considered in a vacuum. In other words, whether the importing country’s ALOP is likely to be achieved can only be determined in most cases through an evaluation of all relevant components of an exporting country’s food inspection and certification system. For example, a determination of equivalence for a specific sanitary measure at the programme design, implementation and monitoring level will require in most cases a prior determination of an equivalent infrastructure. A determination of equivalence for a specific sanitary measure at the specific requirements level will require in most cases a prior determination of an equivalent infrastructure and equivalent programme design, implementation, and monitoring.b) programme design, implementation and monitoring; including documentation of systems, monitoring, performance, decision criteria and action, laboratory capability, transportation infrastructure and provisions for certification and audit; and/or
c) specific requirements; including individual facilities (e.g., premises design), equipment (e.g., design of food contact machinery), processes (e.g., HACCP plans), procedures (e.g., ante- and post-mortem inspection), tests (e.g., laboratory tests for microbiological and chemical hazards) and methods of sampling and inspection.
9. An objective basis for comparison of sanitary measures must be established to allow an equivalence determination to be made, and this may include the following elements:
a) the reason/purpose for the sanitary measure;b) the relationship of the sanitary measure to the ALOP, i.e., how the sanitary measure achieves or contributes to the achievement of the ALOP;
c) where appropriate, an expression of the level of control of the hazard in a food that is achieved by the sanitary measure;
d) the scientific basis for the sanitary measure under consideration, including risk assessment where appropriate.
SECTION 5 - GENERAL PRINCIPLES FOR THE DETERMINATION OF EQUIVALENCE
10. Determination of the equivalence of sanitary measures associated with food inspection and certification systems should be based on application of the following principles:
10.1 An importing country has the sovereign right to set a level of sanitary protection it deems appropriate in relation to the protection of human life and health.[36] The ALOP may be expressed in qualitative or quantitative terms.
10.2 An importing country should be able to describe how its sanitary measure achieves, or contributes to the achievement of, its ALOP.
10.3 An importing country should recognize that sanitary measures different from its own may be capable of achieving its ALOP, and can therefore be found to be equivalent.
10.4 The sanitary measures applied by the exporting country must achieve the importing country’s ALOP.
10.5 Countries should, upon request, enter into consultations with the aim of achieving bilateral or multilateral recognition of the equivalence of specified sanitary measures[37].
10.6 It is the responsibility of the exporting country to demonstrate that its sanitary measures can achieve the importing country’s ALOP.
10.7 The comparison of countries’ sanitary measures should be carried out in an objective manner.
10.8 Where risk assessment is used in the demonstration of equivalence, countries should strive to achieve consistency in the techniques applied so as to ensure that findings can be objectively compared.
10.9 When judging the equivalence of sanitary measures, the importing country should take into account any knowledge it has of the food inspection and certification systems in the exporting country and of the performance of those systems.
10.10 The exporting country should provide access to enable the inspection and certification systems which are the subject of the equivalence determination to be examined and evaluated upon request of the food control authorities of the importing country.
10.11 Countries should ensure transparency in both the demonstration and judgement of equivalence, consulting all interested parties to the extent practicable and reasonable.
SECTION 6 - PROCEDURE FOR THE DETERMINATION OF EQUIVALENCE
11. The importing country should make available details of its sanitary measures to the exporting country on request. The exporting country should review all applicable sanitary measures of the importing country for the food involved and identify those it will meet and those for which it seeks determination of equivalence. The importing and exporting countries should then use an agreed process for exchange of the relevant information to facilitate the determination of equivalence. This information should be limited to that which is necessary for this purpose.
12. The determination of equivalence is facilitated by both exporting and importing countries following a sequence of steps, such as those described below and illustrated in Figure 1:
12.1 The exporting country identifies the sanitary measure of the importing country for which it wishes to apply a different measure, and requests the reason/purpose for the measure.
12.2 The importing country provides the reason/purpose for the identified sanitary measure.
12.3 On the initiative of the exporting country, the importing and exporting countries should enter into a dialogue concerning an objective basis for comparison.
12.4 The exporting country develops the submission to demonstrate that the application of the different sanitary measure achieves or contributes to the achievement of the ALOP of the importing country, and presents it to the importing country.[38]
12.5 The importing country determines whether the exporting country’s measure achieves the importing country’s ALOP.
12.6 If the importing country has any concerns with the submission as presented, it should notify them to the exporting country at the earliest opportunity and should detail the reasons for concern. If possible, the importing country should suggest how the concerns might be addressed.
12.7 The exporting country should respond to such concerns by providing further information as appropriate.
12.8 The importing country notifies the exporting country of its judgement within a reasonable period of time and provides the reasoning for its decision, should the judgement be that the sanitary measure(s) is not equivalent.
12.9 An attempt should be made to resolve any differences of opinion over judgement of a submission, either interim or final.
SECTION 7 - JUDGEMENT
13. Judgement of equivalence by the importing country should be based on a transparent analytical process that is objective and consistent, and includes consultation with all interested parties to the extent practicable and reasonable.
14. Experience and detailed knowledge of an exporting country’s food inspection and certification systems may in itself be sufficient to allow an objective judgement of equivalence by the importing country. For example, a sanitary measure categorized as a specific requirement (refer paragraph 7) may be able to be judged equivalent without consideration of the supporting programme design, implementation and monitoring, and infrastructure.
15. Where countries have no previous history of significant trading in foods or detailed knowledge of each other’s food inspection and certification systems, the determination of equivalence may require a detailed side-by-side comparison of all relevant sanitary measures.
16. Judgement of equivalence should take into account those Codex texts relevant to the food safety matters under consideration.
17. Following any judgement of equivalence, exporting and importing countries should advise each other of significant changes in their supporting programmes and infrastructure that may affect the original determination of equivalence.
Figure I: Simplified flow chart for the determination of equivalence (individual steps may be iterated)
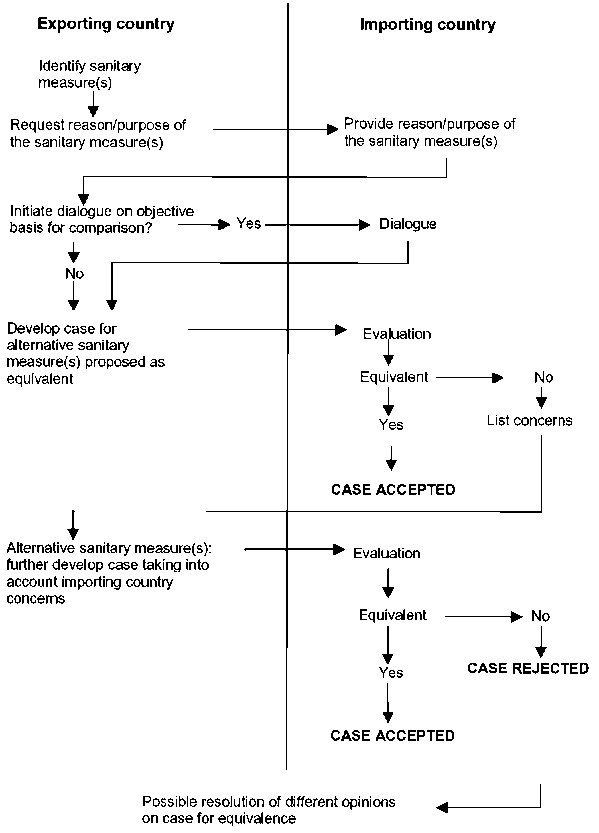
(Advanced to Step 5 of the Codex Procedure)
SECTION 1 -SCOPE
1. This document provides a framework for the development and operation of an import control system to protect consumers and facilitate fair practices in food trade while ensuring unjustified technical barriers to trade are not introduced. The Guideline is consistent with the Codex Principles for Food Import and Export Inspection and Certification[39] and provides specific information about imported food control that is an adjunct to the Guidelines for the Design, Operation, Assessment and Accreditation of Food Import and Export Inspection and Certification Systems[40].
SECTION 2 - DEFINITIONS[41]
Audit* is a systematic and functionally independent examination to determine whether activities and related results comply with planned objectives.
Certification* is the procedure by which official certification bodies and officially recognized bodies provide written or equivalent assurance that foods or food control systems conform to requirements. Certification of food may be, as appropriate, based on a range of inspection activities which may include continuous on-line inspection, auditing of quality assurance systems, and examination of finished products.
Inspection* is the examination of food or systems for control of food, raw materials, processing and distribution, including in-process and finished product testing, in order to verify that they conform to requirements.
Legislation* includes acts, regulations, requirements or procedures, issued by public authorities, related to foods and covering the protection of public health, the protection of consumers and conditions of fair trading.
Official accreditation* is the procedure by which a government agency having jurisdiction formally recognizes the competence of an inspection and/or certification body to provide inspection and certification services.
Official inspection systems and official certification systems* are systems administered by a government agency having jurisdiction empowered to perform a regulatory or enforcement function or both.
Officially recognized inspection systems and officially recognized certification systems* are systems which have been formally approved or recognized by a government agency having jurisdiction.
Requirements* are the criteria set down by the competent authorities relating to trade in foodstuffs covering the protection of public health, the protection of consumers and conditions of fair trading.
Risk assessment** A scientifically based process consisting of the following steps (i) hazard identification, (ii) hazard characterisation, (iii) exposure assessment, and (iv) risk characterisation.
Risk analysis** A process consisting of three components: risk assessment, risk management and risk communication.
SECTION 3 - GENERAL CHARACTERISTICS OF FOOD IMPORT CONTROL SYSTEMS
2. Food import control systems should have the following main characteristics:
REQUIREMENTS FOR IMPORTED FOOD THAT ARE CONSISTENT WITH REQUIREMENTS FOR DOMESTIC FOODS
3. Requirements are commonly expressed as end-point or limit value standards with complementary sampling regimes etc, or provisions concerning process controls, or a combination of these. In general, requirements should be applied equally to domestically produced and imported food. The extent and stringency of requirements applied in specific circumstances should be proportionate to risk, noting that risk may vary from one source to another because of factors such as technology employed, compliance history, etc. and/or examination of relevant attributes of a sample of products at import..
4. Where domestic requirements include process controls such as good manufacturing practice, compliance may be determined by auditing as appropriate, the systems, facilities and procedures in the exporting country.
CLEARLY DEFINED RESPONSIBILITIES OF IMPORTED FOOD CONTROL AUTHORITY OR AUTHORITIES.
5. The competent authority(ies) involved in any of the imported food inspection functions at the point or points of entry, during storage and distribution and/or at point of sale, should have clearly defined responsibilities and authority. When responsibility for determining compliance with requirements is shared among agencies of the importing country, multiple inspection and duplicative testing for the same analyte(s) on the same consignment by the different agencies should be avoided to the extent possible. In such situations, agencies having jurisdiction should share inspection, testing, and other information on the consignment.
6. Some countries, for example those that are part of a regional economic grouping, may rely on import controls implemented by another country. In such cases, the functions, responsibilities, and operating procedures undertaken by the country which conducts the imported food control should be clearly defined and accessible to authorities in the country or countries of final destination with the aim of delivering an efficient and transparent import control system that provides the appropriate level of protection.
7. Where the competent authorities of an importing country use third party providers as officially recognised inspection bodies and/or officially recognized certification bodies to implement controls, such arrangements should be conducted in the manner discussed in CAC/GL 26-1997, Section 8, Official Accreditation. The functions that can be conducted by such providers may include:
CLEARLY DEFINED AND TRANSPARENT LEGISLATION/REGULATIONS AND OPERATING PROCEDURES
8. The object of legislation/regulations is to provide the basis and the authority for operating a food import control system. The legal framework allows for the establishment of the competent authority(ies) and the processes and procedures required to verify the conformity of imported products against requirements.
9. Legislation/regulations should provide the competent authority with the ability to:
10. In addition the legal framework may make provisions for:
PRECEDENCE TO THE PROTECTION OF CONSUMERS
11. In the design and operation of food import control systems, precedence should be given to protecting the health of consumers and ensuring fair practices in food trade over economic or other trade considerations.
PROVISION FOR RECOGNITION OF THE FOOD CONTROL SYSTEM APPLIED BY AN EXPORTING COUNTRY’S COMPETENT AUTHORITY
12. Food import control systems should include provisions for recognition as appropriate of the food control system applied by an exporting country’s competent authority. Importing countries can recognise the food safety controls of an exporting country in a number of ways that facilitate the entry of goods, including the use of memoranda of understanding, mutual recognition agreements and equivalence agreements. Such recognition should, as appropriate, include controls applied during the production, manufacture, importation, processing, storage, and transportation of the food products, and verification of the export food control system applied.
UNIFORM NATION-WIDE IMPLEMENTATION
13. Uniformity of operational procedures is particularly important. Programmes and training manuals should be developed and implemented to assure uniform application at all points of entry and by all inspection staff.
IMPLEMENTATION THAT ENSURES THE LEVELS OF PROTECTION ACHIEVED ARE CONSISTENT WITH THOSE FOR DOMESTIC FOOD
14. As an importing country has no direct jurisdiction over process controls applied to food manufactured in another country, there may be a variation in approach to the compliance monitoring of domestic and imported food. Such differences in approach are justifiable on the basis that the objectives of the import controls are the same as those applied to domestically produced food.
SECTION 4 - IMPLEMENTATION OF THE CONTROL SYSTEM
15. Operational procedures should be developed and implemented to minimize undue delay at the point or points of entry without jeopardizing effectiveness of controls to ensure food safety. Implementation should take into account the factors listed in this section.
POINT OF CONTROL
16. Control of imported food by the importing country can be conducted at one or more points including:
17. The system should be structured to deliver the same outcomes regardless of the point or points of control.
18. The importing country can recognize controls implemented by the exporting country. The application of controls by the exporting country, during production, manufacture and subsequent transit should be encouraged, with the aim of identifying and correcting problems when and where they occur, and preferably before costly recalls of food already in distribution are required.
19. Pre-shipment clearance is a possible mechanism for ensuring compliance with requirements of, for example, valuable bulk packed products that if opened and sampled upon entry, would be seriously compromised, or for products that require rapid clearance to maintain safety and quality.
20. If the inspection system encompasses pre-shipment clearance then the authority to conduct the clearance should be determined and procedures defined. The importing authority may choose to accept pre-shipment clearance from an exporting country’s official certification system or from officially recognised third party certification bodies working to defined criteria.
INFORMATION ABOUT INCOMING FOOD
21. The efficacy of the control system in applying efficient targeted control measures depends upon information about shipments of food entering the jurisdiction. Details of shipments that may be obtained include:
FREQUENCY OF INSPECTION AND TESTING OF IMPORTED FOODS
22. The nature and frequency of inspection and testing of imported foods should be based on the risk to health presented by the product and the history of conformance to requirements. Control should be designed to account for factors such as:
23. Physical checks on imported product, using random statistically based sampling plans, are valid means of checking product compliance. Inspection procedures should be developed to include defined sampling frequencies or inspection intensities. The frequency of sampling should be proportionate to the assessed risk, which may take into account evidence of, or confirmed non-conformity for a particular product, processor, importer or country.
24. Sampling frequency of products supplied from a source for which there is no compliance history, should be set at a higher rate than for products from other sources. The sampling process enables a compliance history to be created. Similarly, food from suppliers or imported by parties with a known poor compliance history should be sampled at higher intensity. In these cases, every shipment may need to be physically inspected, until a defined number of consecutive shipments meets requirements. Alternatively the inspection procedures can be developed to automatically detain product from suppliers with a known poor compliance history and the importer may be required to prove the fitness of each consignment through use of an accredited laboratory until a satisfactory compliance rate is achieved.
SAMPLING AND ANALYSIS
25. The inspection system should have defined sampling procedures based on Codex sampling plans for the particular commodity/contaminant combination where available.
26. Where samples are selected for analysis standard methods of analysis, or methods validated through appropriate protocols, should be used. Analysis should be conducted in official or officially accredited laboratory facilities.
DECISION CRITERIA
27. Decision criteria should be developed that determine whether shipments are given
28. Results of inspection and, if required, laboratory analysis, should be carefully interpreted in making decisions relating to acceptance or rejection of a consignment. The inspection program should include decision-making rules for situations where results are borderline, or sampling indicates that only some lots within the consignment comply with requirements. Procedures may include further testing and examination of previous compliance history.
29. The system should include formal means to communicate decisions about results of analysis, clearance and status of shipments. Advice on decisions should be provided to importers without delay. There should be an appeal mechanism for review of rejections of consignments.
DEALING WITH EMERGENCY SITUATIONS
30. The responsible authority should have procedures that can respond appropriately to emergency situations. This will include holding suspect product upon arrival and recall procedures for suspect product already cleared and, if relevant, rapid notification of the problem to international authorities.
31. If the food control authorities in importing countries detect problems during import control of foodstuffs which they consider to be so serious as to indicate a food control emergency situation, they should inform the exporting country promptly by telecommunication.[42]
RECOGNITION OF EXPORT CONTROLS
32. Consistent with paragraph 11 of this guideline, the importing country should establish mechanisms to accept control systems in an exporting country where these system achieve the same level of protection required by the importing country. In this regard, the importing country should:
33. The competent authority of the importing country may, develop certification agreements with exporting country official certification bodies or officially recognized certification bodies, with the aim of ensuring requirements are met. Such agreements may be of particular value where, for example, there is limited access to sophisticated facilities such as laboratories and shipment tracking systems.
INFORMATION EXCHANGE
34. Imported food control systems involve information exchange between competent authorities and countries that are trading partners. The information may include:
35. Any changes to import protocols, which may affect trade, should be promptly communicated to trading partners, allowing a reasonable interval between the publication of regulations and their application taking into account the risk to the consumer and the urgency of the measure.
OTHER CONSIDERATIONS
36. The authority may consider developing alternative arrangements in lieu of routine inspection. This may include agreements where the inspection authority assesses the controls that importers implement over suppliers and the procedures that are in place to verify compliance of suppliers. Alternative arrangements may include some sampling of product by the authority as an audit, rather than routine inspection.
37. The inspection authority may consider developing a system where registration of importers is mandatory. Advantages include the ability to provide the importing and exporting community with information about their responsibilities and mechanisms to ensure imported food complies with requirements.
38. If a product registration system exists or is implemented, a clear rationale for such product registration (e.g. specific and documented food safety concerns) should exist. Such product registrations should treat imported and domestic product in the same or equivalent manner.
DOCUMENTING THE SYSTEM
39. A food import control system should be fully documented, including a description of its scope and operation, responsibilities and actions for staff, in order that all parties involved know precisely what is expected of them.
40. Documentation of an imported food control system should include
TRAINED INSPECTORATE
41. It is fundamental to have adequate, reliable, well trained and organised inspection staff, with supporting infrastructure, to deliver the imported food control system. Training, communication, and supervisory elements should be organised to provide consistent implementation of requirements by the inspectorate throughout the food import control system.
42. Where third parties are officially recognised to perform inspection work, or there are alternative arrangements in place, such as a quality assurance arrangement with the importing company, the qualifications of the auditors, or company inspection staff, should be at least the same for inspection staff of the competent authority.
43. The authority responsible for conducting assessment of food control systems of exporting countries should engage personnel with the qualifications and training expected of personnel assessing domestic food controls.
SYSTEM VERIFICATION
44. Consistent with Section 9 of the Guidelines for the Design, Operation, Assessment and Accreditation of Food Import and Export Inspection and Certification System (CAC/GL 26-1997) an imported food control system should be independently assessed on a regular basis.
SECTION 5 - FURTHER INFORMATION
45.
The Food and Agriculture Organization of the United Nations Manual of Food
Quality Control. Imported Food Inspection (Food and Nutrition Paper 14/15,
1993) and World Health Organization/Western Pacific Regional Center for the Promotion
of Environmental Planning and Applied Science (PEPAS): Manual for the Inspection
of Imported Food (1992) contribute valuable information for those engaged
in the design and re-design of imported food control systems.